Abstract
Increased activity of the epithelial sodium channel (ENaC) in the respiratory airways contributes to the pathophysiology of cystic fibrosis (CF), a genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In some patients suffering from atypical CF a mutation can be identified in only one CFTR allele. We recently identified in this group of CF patients a heterozygous mutation (W493R) in the α-subunit of ENaC. Here, we investigate the functional effects of this mutation by expressing wild-type αβγENaC or mutant αW493RβγENaC in Xenopus oocytes. The αW493R mutation stimulated amiloride-sensitive whole-cell currents (ΔIami) by ∼4-fold without altering the single-channel conductance or surface expression of ENaC. As these data suggest that the open probability (Po) of the mutant channel is increased, we investigated the proteolytic activation of ENaC by chymotrypsin. Single-channel recordings revealed that chymotrypsin activated near-silent channels in outside-out membrane patches from oocytes expressing wild-type ENaC, but not in membrane patches from oocytes expressing the mutant channel. In addition, the αW493R mutation abolished Na+ self inhibition of ENaC, which might also contribute to its gain-of-function effects. We conclude that the αW493R mutation promotes constitutive activation of ENaC by reducing the inhibitory effect of extracellular Na+ and decreasing the pool of near-silent channels. The resulting gain-of-function phenotype of the mutant channel might contribute to the pathophysiology of CF in patients carrying this mutation.
Introduction
The epithelial sodium channel (ENaC) is a member of the ENaC/degenerin family of non-voltage gated ion channels. It is found in the apical membrane of sodium-absorbing epithelial cells e.g. in the respiratory tract, distal nephron, distal colon, sweat and salivary ducts. In these epithelia, ENaC is the rate-limiting step of sodium absorption (Garty & Palmer, 1997; Kellenberger & Schild, 2002). In the lung, fluid absorption critically depends on ENaC function (Hummler et al. 1996). ENaC forms a heteromeric channel composed of three homologous subunits α, β and γ (Canessa et al. 1994). Each subunit has two transmembrane domains, a large extracellular loop and cytosolic N- and C-termini. The recently published crystal structure of the related acid-sensing ion channel ASIC1 suggests that ENaC is probably a heterotrimer (Canessa, 2007; Jasti et al. 2007; Stockand et al. 2008).
The genetic disease cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (Riordan et al. 1989). The CFTR gene encodes a Cl− channel found in the apical membrane of many epithelial cells (Sheppard & Welsh, 1999). Thus, CF epithelia have a reduced apical Cl− conductance. Some patients may have all the classical symptoms of CF, while others have milder or even atypical disease manifestations. The CF phenotype is a continuum of symptoms and cannot be easily defined in two distinct disease categories (typical versus atypical CF) (De Boeck et al. 2006). The large variability of CF symptoms suggests that CF modifier genes exist. Interestingly, the genes encoding the β- and γ-subunits of ENaC have been reported to be potential modifier genes in CF (Stanke et al. 2006). Moreover, missense mutations in the β-subunit of ENaC causing abnormal channel function have been identified in two patients with CF-like syndromes without CFTR mutations (Sheridan et al. 2005). In addition, the tumour necrosis factor α (TNFα) receptor located next to the gene encoding the α-subunit of ENaC has been suggested as a potential modifier gene (Stanke et al. 2006).
In respiratory epithelia, a fine balance between Cl− secretion via CFTR and Na+ absorption via ENaC is necessary to maintain an appropriate airway surface liquid (ASL) volume which is important for pulmonary mucus clearance. CF airway epithelia are characterized by a combined defect of accelerated Na+ transport and the failure to secrete Cl− (Boucher, 2004, 2007). These abnormal ion transport properties are thought to cause ASL volume depletion leading to ‘thickened’ mucus plaques and plugs which become the nidus for bacterial infections.
In some patients with CF(-like) disease a mutation cannot be identified on both CFTR alleles. Mutations in the genes encoding ENaC may potentially explain disease in these atypical CF patients. Indeed, sodium hyper-absorption through ENaC is thought to contribute to CF pathophysiology (Boucher, 2004, 2007) and mice overexpressing βENaC in the airways present with CF-like pulmonary disease (Mall et al. 2004; Zhou et al. 2008). In a recent study, we investigated the possibility that atypical CF patients might harbour mutations in their ENaC genes (Azad et al. 2009). αW493R-ENaC, one of the identified mutations, stimulated ENaC currents when expressed in Xenopus oocytes. The size of this gain-of-function effect was similar to that described for an hereditary form of salt-sensitive arterial hypertension (Rossier & Schild, 2008). The latter mutations affect a PY-motif in the C-termini of the β- or γ-subunit of ENaC. The PY-motif is critical for the interaction of the channel with the regulatory protein Nedd4-2 which promotes channel internalization and degradation. Mutations affecting the PY-motifs lead to higher channel activity at the plasma membrane due to a rise in cell surface expression and an impaired Na+-dependent feedback inhibition of the mutant channel (Staub & Verrey, 2005). In contrast to the mutations causing Liddle's syndrome, the αW493R mutation is localized in the extracellular loop of the α-subunit. Thus, the gain-of-function effect of this mutation is likely to be mediated by a different mechanism. The aim of the present study was to investigate the mechanisms underlying the gain-of-function phenotype of the mutant channel.
Methods
Plasmids
Full length cDNAs for the wild-type α-, β- and γ-subunits and for the mutant αW493R subunit of human ENaC were in pcDNA3.1. The T663 polymorphism was present in the α-subunit, unless stated otherwise. Linearized plasmids were used as templates for cRNA synthesis using either T7 (αβγ-hENaC, αW493R-hENaC and αβγ-rENaC) or SP6 (β-FLAG-rENaC) RNA polymerases (mMessage mMachine, Ambion, Austin, TX, USA). To minimize the risk of expression artefacts that may arise from differences in cRNA quality, cRNAs for wild-type and mutant ENaC were synthesized in parallel and the experiments were performed using at least two different batches of cRNA. The αW493R, αW493A, αW493C and αW493E mutants were generated by site-directed mutagenesis (QuikChange Site-Directed Mutagenesis Kit, Stratagene, Amsterdam, the Netherlands). Mutations were confirmed by sequence analysis.
Isolation of oocytes and injection of cRNA
Oocytes were obtained from adult female Xenopus laevis in accordance with the principles of German legislation, with approval by the animal welfare officer for the University of Erlangen-Nürnberg, and under the governance of the state veterinary health inspectorate (permit no. 621-2531.32-5/02). The authors confirm that the experiments complied with the ethical guidelines of The Journal of Physiology (Drummond, 2009). Animals were anaesthetized in 0.2% MS222 (Sigma, Taufkirchen, Germany) and ovarian lobes were obtained through a small abdominal incision. After suture the animals were allowed to recover fully in a separate tank before returning to the frog colony 1 day later. A minimum of 8 weeks was obligatory before next surgery on the same animal. Oocytes were isolated from the ovarian lobes by enzymatic digestion at 19°C for 3–4 h with 600–700 U ml−1 type 2 collagenase from Cl. histolyticum (CLS 2, Worthington, Lakewood, NJ, USA) dissolved in a solution containing (in mm) NaCl 82.5, KCl 2, MgCl2 1, and Hepes 1 (pH 7.4 with Tris). Defolliculated stage V–VI oocytes were injected (Nanoject II automatic injector, Drummond, Broomall, PA, USA) with 0.5 ng cRNA per ENaC subunit, unless stated otherwise. The cRNAs were dissolved in RNase-free water and the total volume injected was 46 nl. Injected oocytes were stored at 19°C in high Na+ ND96 (NaCl 96, KCl 2, CaCl2 1.8, MgCl2 1, Hepes 5, pH 7.4 with Tris) or low Na+ solution (NMDG (N-methyl-d-glucamine)-Cl 87, NaCl 9, KCl 2, CaCl2 1.8, MgCl2 1, Hepes 5, pH 7.4 with Tris) supplemented with 100 units ml−1 penicillin and 100 μg ml−1 streptomycin.
Two-electrode voltage-clamp
Oocytes were routinely studied 1–2 days after injection using the two-electrode voltage-clamp technique (TEVC) as described previously (Rauh et al. 2006; Kraus et al. 2007; Wielpütz et al. 2007). The oocytes were placed in a small experimental chamber and constantly superfused at room temperature with ND96 supplemented with 2 μm amiloride (Sigma) at a rate of 2–3 ml min−1. Bath solution exchanges were controlled by a magnetic valve system (ALA BPS-8) in combination with a TIB14 interface (HEKA, Lambrecht, Germany). Voltage-clamp experiments were performed using an OC-725C amplifier (Warner Instruments Corp., Hamden, CT, USA) interfaced via a LIH-1600 (HEKA) to a PC with PULSE 8.67 software (HEKA) for data acquisition and analysis. Oocytes were voltage-clamped at −60 mV and amiloride-sensitive whole-cell currents (ΔIami) were obtained by washing out amiloride with amiloride-free ND96 and subtracting the whole-cell currents measured in the presence of amiloride (2 μm) from the corresponding whole-cell currents recorded in the absence of amiloride. To determine the concentration dependence of the amiloride effect, oocytes were continuously clamped at −60 mV and superfused with ND96 containing amiloride in increasing concentrations of 0.01, 0.1, 1, 10 and 100 μm. Current values were determined after a stable current plateau was reached. For the evaluation of the concentration–response curve the initial current value in the absence of amiloride was set to 0 and the minimum current value measured in the presence of 100 μm amiloride was set to 1. The IC50 value of amiloride and the Hill slope were determined by fitting the individual normalized current values to the Hill equation. For the detection of Na+ self inhibition, oocytes were continuously clamped at −100 mV and superfused at a rate of 8–10 ml min−1, initially with a 1 mm Na+ solution (in mm: NMDG-Cl 109, NaCl 1, KCl 2, CaCl2 1.8, MgCl2 1, Hepes 5, pH 7.4 with Tris) followed by a rapid change to a 110 mm Na+ solution (NaCl 110, KCl 2, CaCl2 1.8, MgCl2 1, Hepes 5, pH 7.4 with Tris). To quantify Na+ self inhibition, we used the model described by Chraibi & Horisberger (2002) in which the current It is given as a function of time by
| (1) |
where ka is the activation rate constant, ki is the inactivation rate constant, kdown is the rundown rate constant and Imax is the maximum current at t= 0. Rate constants were determined with eqn (1) and the non-linear regression function of GraphPad Prism 4.03 for Windows (GraphPad Software, Inc., San Diego, CA, USA), starting from the inflection point of the current declining phase of each trace (Chraibi & Horisberger, 2002). Imax was determined by extrapolation to t= 0.
Patch-clamp
Single-channel recordings in outside-out membrane patches were performed as described previously using oocytes kept in low sodium modified Barth's saline after cRNA injection to prevent Na+ overloading (Diakov & Korbmacher, 2004; Diakov et al. 2008). Patch pipettes were pulled from borosilicate glass capillaries (GC150–15, I.D. 0.86 mm, O.D. 1.5 mm, Harvard Apparatus Ltd, Edenbridge, UK) using a DMZ-Universal puller (Zeitz Instrumente, Munich, Germany) and had a tip diameter of about 1 μm after fire polishing. Pipettes were filled with potassium gluconate pipette solution (in mm: 90 potassium gluconate, 5 NaCl, 2 Mg-ATP, 2 EGTA and 10 mm Hepes adjusted to pH 7.28 with Tris). Seals were routinely formed in a low sodium NMDG-Cl bath solution (in mm: NMDG-Cl 95, NaCl 1, KCl 4, MgCl2 1, CaCl2 1 and Hepes 10, pH 7.4 with Tris). In this bath solution the pipette resistance averaged about 10 MΩ. In the NaCl and LiCl bath solutions, 95 mm NMDG-Cl was replaced with either 95 mm NaCl or 95 mm LiCl. Downward current deflections correspond to cell membrane inward currents, i.e. the movement of positive charge from the extra- to the intra-cellular side of the membrane. Membrane patches were voltage-clamped at −70 mV, close to the calculated reversal potential of Cl− (ECl=−77.2 mV) and K+ (EK=−79.4 mV) under our experimental conditions; experiments were performed at room temperature. Single-channel currents were filtered at 500 Hz and digitized at 2 kHz before refiltering at 16 Hz to calculate single-channel current amplitude and channel activity. The latter was derived from binned amplitude histograms as the product NPo, where N is the number of channels and Po is the open probability (Korbmacher et al. 1995; Diakov & Korbmacher, 2004; Diakov et al. 2008). Continuous current traces of 60–130 s were selected from different experimental periods to analyse changes in NPo.
Surface labelling
ENaC surface expression was determined using a chemiluminescence assay and a βENaC construct with a FLAG tag inserted into its extracellular loop (Firsov et al. 1996) as described previously (Zerangue et al. 1999; Konstas et al. 2001; Rauh et al. 2006; Wielputz et al. 2007). All steps were performed on ice and no glassware was used. Unspecific binding sites were blocked by 30 min incubation in ND96 supplemented with 1% bovine serum albumin (BSA, Sigma). Then oocytes were incubated for 1 h in primary mouse anti-FLAG M2 monoclonal antibody (Sigma), washed 6 × 3 min in ND96 + BSA, incubated for 45 min in secondary peroxidase-conjugated sheep anti-mouse IgG (Chemicon, Boronia Victoria, Australia), washed 6 × 3 min in ND96 + BSA and finally 6 × 3 min in ND96. Individual oocytes were placed in a white U-bottom 96-well plate and 50 μl of SuperSignal ELISA Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, USA) were added to each oocyte. Chemiluminescence was quantified with a Tecan GENios microplate reader (Tecan, Crailsheim, Germany). Results are given in relative light units (RLU).
Detection of ENaC cleavage products at the cell surface
Biotinylation experiments were performed essentially as described previously (Harris et al. 2007; Diakov et al. 2008) using 20 oocytes per group. All biotinylation steps were performed at 4°C. Oocytes were incubated in the biotinylation buffer (in mm: triethanolamine 10, NaCl 150, CaCl2 2, EZ-link sulfo-NHS-SSBiotin (Pierce) 1 mg ml−1, pH 9.5) for 15 min with gentle agitation. The biotinylation reaction was stopped by washing the oocytes twice for 5 min with quench buffer (in mm: glycine 192, Tris-Cl 25, pH 7.5). Oocytes were lysed by passing them 5 times through a 27-gauge needle in lysis buffer (in mm: NaCl 500, EDTA 5, Tris-HCl 50, pH 7.4) supplemented with protease inhibitor cocktail (Complete Mini EDTA-free protease inhibitor cocktail tablets, Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instruction. The lysates were centrifuged for 10 min at 1000 g. After addition of 1% Triton X-100 and 1% Igepal CA-630 (Sigma), supernatants were incubated for 20 min on ice and 100 μl of Immunopure immobilized Neutravidin beads (Pierce) were added. After overnight incubation at 4°C with overhead rotation, the tubes were centrifuged for 1 min at 1000 g. Supernatants were removed, and beads were washed 3 times with lysis buffer. A 100 μl aliquot of 2× SDS-PAGE sample buffer (Rotiload 1, Roth, Karlsruhe, Germany) was added to the beads. Samples were boiled for 5 min at 95°C and centrifuged for 3 min at 20,000 g before loading the supernatants on 10% SDS–PAGE. Monoclonal anti-V5 antibody (Invitrogen, Karlsruhe, Germany) was used at a dilution of 1:5000. Horseradish peroxidase labelled secondary sheep anti-mouse antibody (Sigma) was used at a dilution of 1:20,000. Absence of intracellular proteins was confirmed by absence of a β-actin signal. Densitometric analysis was done with ImageJ 1.38x (National Institutes of Health, Bethesda, MD, USA).
Statistical methods
Data are presented as mean ±s.e.m. N indicates the number of different batches of oocytes, n the number of individual oocytes studied. Statistical significance was assessed by the appropriate version of Student's t test (i.e. paired test for the comparison of values before and after chymotrypsin application and for densitometric analysis; unpaired test for all other comparisons) with GraphPad Prism 4.03 for Windows (GraphPad Software, Inc.).
Results
αW493R stimulates ENaC-mediated whole-cell currents in Xenopus laevis oocytes
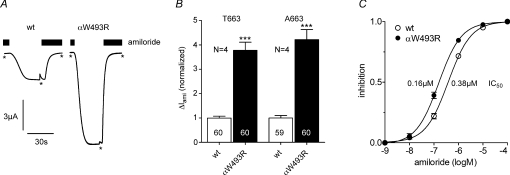
To investigate the effects of the αW493R mutation on ENaC function, we measured macroscopic amiloride-sensitive whole-cell currents (ΔIami) in wild-type αβγENaC or mutant αW493RβγENaC-expressing Xenopus laevis oocytes. Figure 1A shows representative whole-cell currents from oocytes expressing wild-type (left) and mutant ENaC (right). ΔIami was larger in oocytes expressing the mutant channel. With the common T663 polymorphism in the α-subunit, ΔIami was 3.8-fold larger in oocytes expressing αW493R compared to those expressing wild-type ENaC (Fig. 1B; T663). The stimulatory effect of the αW493R mutation was preserved (4.2-fold) when it was combined with the αT663A polymorphism (Fig. 1B; A663), a frequent variant that has been reported to increase ENaC activity in Xenopus oocytes (Tong et al. 2006). Since the αW493 residue is located 63 amino acids upstream of an important residue (αS556) for amiloride binding (Kellenberger & Schild, 2002), we also estimated the inhibitory constant (IC50) of amiloride in wild-type and mutant ENaC-expressing oocytes which averaged 0.38 ± 0.03 μm and 0.16 ± 0.01 μm (P < 0.001), respectively (Fig. 1C). The Hill slope was similar in both groups (wt: 0.95 ± 0.05, mutant: 0.93 ± 0.03). The apparent minor difference in the IC50 values cannot explain the larger ΔIami values observed in the oocytes expressing the mutant channel. Thus, other factors must be responsible for the observed ∼4-fold stimulation of ENaC whole-cell currents by the αW493R mutation.
Figure 1. αW493R mutation stimulates ENaC-mediated Na+ currents.
Oocytes were injected with 0.5 ng cRNA per subunit and incubated in ND96. Amiloride-sensitive whole-cell currents (ΔIami) were measured with the two-electrode voltage-clamp technique 2 days after cRNA injection. A, representative whole-cell current traces of a αβγENaC (wt) and a αW493RβγENaC (αW493R)-expressing oocyte at a holding potential of −60 mV. Amiloride (2 μm) was present in the bath to specifically inhibit ENaC as indicated by the black bars. Washout of amiloride revealed a sizeable inward current component which corresponds to the ENaC-mediated Na+ inward current. Reapplication of amiloride returned the whole-cell current toward the initial baseline level. At the time points indicated by asterisks current responses from voltage-step pulses were removed for clarity. B, summary of similar experiments as shown in A performed in wild-type and mutant ENaC-expressing oocytes on the wild-type αT663 and the polymorphic αA663 background. To pool data from different batches of oocytes, the individual ΔIami values were normalized to the mean ΔIami value of the corresponding wild-type ENaC-expressing control group. C, average amiloride concentration–response curves measured in wild-type and mutant ENaC-expressing oocytes (9 oocytes per group). Error bars are partly covered by symbols. IC50 inhibitory constants of amiloride are indicated. N indicates number of different batches of oocytes. ***P < 0.001.
Stimulatory effect of αW493R is independent of Na+ feedback inhibition
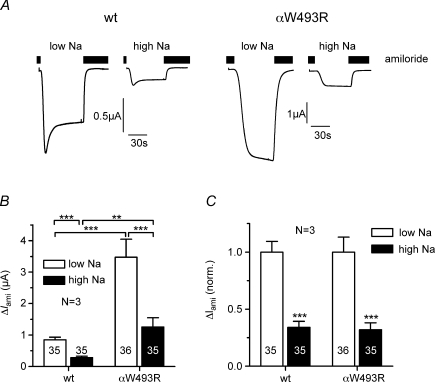
Since impaired Na+ feedback inhibition is a hallmark of the gain-of-function mutations causing Liddle's syndrome (Kellenberger et al. 1998), we tested whether impaired Na+ feedback inhibition also contributes to the stimulatory effect of the αW493R mutation. Oocytes heterologously expressing ENaC become severely sodium loaded when maintained in a bath solution containing a normal, i.e. high, extracellular Na+ concentration (Canessa et al. 1994). This activates Na+ feedback inhibition and promotes channel retrieval from the plasma membrane (Kellenberger et al. 1998; Konstas et al. 2002; Volk et al. 2004). Such Na+ feedback inhibition can be prevented by maintaining ENaC-expressing oocytes in a low Na+ solution. The stimulatory effect of ENaC mutations causing Liddle's syndrome is largely reduced in oocytes pre-incubated in low Na+ (Kellenberger et al. 1998). In contrast, as shown in Fig. 2, the stimulatory effect of the αW493R mutation was preserved in oocytes pre-incubated in low Na+. Pre-incubation in low Na+ increased ΔIami in wild-type and mutant ENaC-expressing oocytes to the same extent (Fig. 2A). Thus, the relative gain-of-function effect of the mutation was similar under high Na+ (4.4-fold) and low Na+ (4.1-fold) conditions (Fig. 2B). Moreover, the relative inhibitory effect of Na+ loading on ΔIami was similar in both groups of oocytes (Fig. 2C). This indicates that Na+ feedback inhibition is not reduced by the αW493R mutation and that impaired Na+ feedback inhibition does not contribute to the gain-of-function effect of the mutation.
Figure 2. The stimulatory effect of the αW493R mutation is independent of Na+ feedback inhibition.
αβγENaC (wt)- and αW493RβγENaC (αW493R)-expressing oocytes were incubated in parallel in high Na+ and low Na+ solution. A, representative whole-cell current traces of individual oocytes at a holding potential of −60 mV. Amiloride (2 μm) was present in the bath as indicated by the black bars. B, summary of similar experiments as shown in A performed in oocytes from 3 different batches. C, to illustrate the relative effect of Na+ feedback inhibition on wild-type and mutant ENaC the individual ΔIami values summarized in B were normalized for each batch to the mean ΔIami value measured in the corresponding group of low Na+ oocytes. Numbers in or above columns indicate individual number of oocytes, N indicates number of different batches of oocytes. **P < 0.01, ***P < 0.001.
Surface expression is similar for wild-type and mutant ENaC
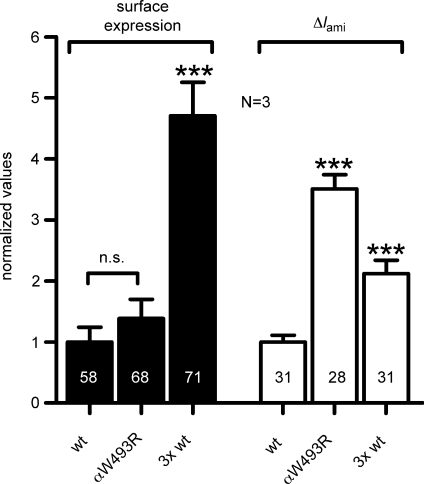
The stimulatory effect of the mutated α-subunit on ΔIami may be caused by an increase of the overall number of channels expressed at the cell surface. To assess channel surface expression, we inserted a FLAG epitope in the extracellular loop of the β-subunit and expressed αβFLAGγENaC and αW493RβFLAGγENaC in oocytes. We observed channel surface expression in oocytes expressing mutant ENaC to be similar to that in oocytes expressing wild-type ENaC. In contrast, a characteristic stimulatory effect of the mutated α-subunit on ΔIami was present in matched oocytes (Fig. 3). To confirm that the chemiluminescence assay can detect an increase in channel surface expression under our experimental conditions, we performed control experiments in which the amount of cRNA injected per oocyte was tripled. As expected, this increased both ΔIami and surface expression. We conclude that the stimulatory effect of the mutated α-subunit on ΔIami is not caused by an increase in ENaC surface expression.
Figure 3. The stimulatory effect of the αW493R mutation is not caused by an increase in channel surface expression.
Oocytes were injected with cRNA for αβFLAGγENaC (wt) or αW493RβFLAGγENaC (αW493R) and incubated for 2 days in a low Na+ solution. The insertion of a FLAG reporter epitope in the extracellular loop of the β-subunit enabled the detection of channel surface expression by a chemiluminescence assay (filled columns). For control experiments the amount of αβFLAGγENaC cRNA injected per oocyte was tripled (3× wt). ΔIami was determined in parallel in oocytes from the same experimental groups (open columns). To pool data from different batches of oocytes, individual current and surface expression values were normalized to the corresponding mean values of wild-type control oocytes. Numbers in columns indicate individual number of oocytes. N indicates number of different batches of oocytes. n.s., not significant. ***P < 0.001.
The αW493R mutation does not alter the single-channel conductance of ENaC in the presence of Na+ and Li+
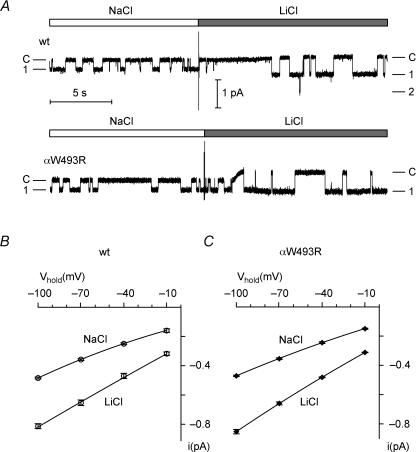
Since the αW493R mutation did not increase ENaC surface expression, we considered the possibility that it altered the single-channel conductance of ENaC. We therefore recorded single-channel currents in outside-out membrane patches from oocytes expressing wild-type or mutant ENaC. Figure 4A demonstrates that the single-channel current amplitudes of wild-type and mutant ENaC were comparable at −70 mV, while Fig. 4B and C reveals that their I–V relationships were the same. The single-channel conductance of mutant ENaC (5.02 ± 0.08 pS; Fig. 4C) was not significantly different from that of wild-type ENaC (4.87 ± 0.14 pS; Fig. 4B) and similar to previously reported single-channel conductance values for wild-type human ENaC (Waldmann et al. 1995; Ji et al. 2004). ENaC is known to have a larger single-channel conductance for Li+ than for Na+. Figure 4 demonstrates that the single-channel current amplitude and I–V relationship of wild-type and mutant ENaC remained comparable when Na+ was replaced with Li+ in the extracellular solution. Thus, increased single-channel conductance does not explain the gain-of-function phenotype of the mutant channel.
Figure 4. The αW493R mutation does not alter the single-channel conductance of ENaC.
A, representative single-channel current recordings obtained at a holding potential of −70 mV from an outside-out patch of an oocyte expressing wild-type (upper trace) or mutant ENaC (lower trace). NaCl or LiCl were present in the bath solution as indicated above the trace by white and grey bars, respectively. B and C, in similar experiments as shown in A single-channel current voltage (I–V) values were obtained at different holding potentials (Vhold) with NaCl or LiCl in the bath solution from outside-out patches of wt (B, n= 4, N= 2) and mutant ENaC (C, n= 6, N= 2)-expressing oocytes. The I–V data were fitted using the Goldman–Hodgkin–Katz equation.
Chymotrypsin activation is reduced in mutant ENaC
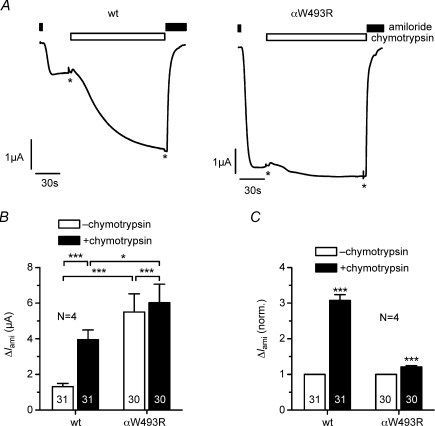
Since surface expression and single-channel conductance are similar for wild-type and mutant ENaC, the overall activity of the mutant channels in the plasma membrane must be increased to explain the larger ENaC whole-cell currents in oocytes expressing the mutant channel. ENaC can be activated by extracellular proteases without an increase in channel surface expression (Chraibi et al. 1998; Hughey et al. 2007; Diakov et al. 2008; Kleyman et al. 2009; Haerteis et al. 2009; Rossier & Stutts, 2009). Therefore, we tested whether the mutation alters the responsiveness of the channel to extracellular proteases. We used chymotrypsin as a prototypical protease, which has a robust stimulatory effect on ENaC. Unlike trypsin, it does not stimulate transiently Ca2+-activated Cl− currents in Xenopus oocytes (Durieux et al. 1994; Chraibi et al. 1998; Diakov et al. 2008). Chymotrypsin activated the steady state ΔIami in wild-type ENaC-expressing oocytes by ∼3-fold. In contrast, in oocytes expressing the mutant ENaC, chymotrypsin activated ΔIami only by ∼1.2-fold (Fig. 5). Thus, the αW493R mutation largely reduces the ability of chymotrypsin to activate ENaC. Such reduced activation by chymotrypsin supports the conclusion that the baseline activity of the mutant ENaC is increased.
Figure 5. The αW493R mutation reduces the stimulatory effect of chymotrypsin on ΔIami.
Oocytes were injected with cRNA (0.2 ng per subunit) for αβγENaC (wt) or αW493RβγENaC (αW493R) and incubated for 2 days in low Na+. A, representative current traces demonstrating the effect of chymotrypsin (2 μg ml−1) on wild-type and mutant ENaC. B, summary of ΔIami values determined before and after exposure to chymotrypsin in similar experiments as shown in A. C, same data as in B but with ΔIami values after chymotrypsin application normalized to the corresponding control value before chymotrypsin application. Numbers in columns indicate individual number of oocytes. N indicates number of different batches of oocytes. *P < 0.05, ***P < 0.001.
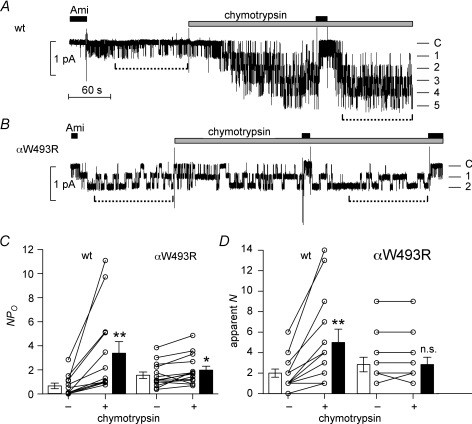
Chymotrypsin activates near-silent channels in outside-out patches from oocytes expressing wild-type ENaC but not in those from oocytes expressing the αW493R mutant channel
From the reduced stimulatory effect of chymotrypsin on mutant ENaC whole-cell currents we concluded that the baseline activity, i.e. the average baseline Po, of the αW493R mutant channel is higher than that of wild-type ENaC. To complicate matters, ENaC at the plasma membrane does not constitute a homogenous channel population with a uniform Po. Instead, ENaC populations with different Po values exist in the plasma membrane (Firsov et al. 1996; Rossier, 2002), including a pool of so-called near-silent channels (Caldwell et al. 2004, 2005) that can be acutely activated by extracellular proteases (Chraibi et al. 1998; Diakov et al. 2008). We hypothesized that the reduced stimulatory effect of chymotrypsin on ENaC whole-cell currents may be related to a reduced pool of near-silent channels in oocytes expressing the mutant channel. To test this possibility, we investigated the effect of chymotrypsin on wild-type and mutant ENaC in outside-out membrane patches. Figure 6A and B show representative recordings of wild-type and mutant ENaC, and Fig. 6C and D quantifies the number of apparent channels and their activity. In Fig. 6A, on washout of amiloride, a single wild-type ENaC channel was active. However, application of chymotrypsin activated at least four additional wild-type ENaC channels, consistent with the recruitment of near-silent channels (Caldwell et al. 2004, 2005; Diakov et al. 2008). Following chymotrypsin treatment, the single-channel current amplitude of wild-type ENaC was unchanged at −0.38 pA. However, chymotrypsin treatment increased the NPo of wild-type ENaC 4-fold (Fig. 6C) and the number of active channels 2.5-fold (Fig. 6D).
Figure 6. Chymotrypsin fails to activate near-silent channels in outside-out patches expressing the mutant αW493RβγENaC.
A and B, representative single-channel current recordings obtained at a holding potential of −70 mV from an outside-out patch of oocytes expressing wild-type (A) or mutant ENaC (B). Amiloride (Ami, 2 μm) and chymotrypsin (2 μg ml−1) were present in the bath solution as indicated above the trace by black and grey bars, respectively. The current level at which all channels are closed (C) was determined in the presence of amiloride. The different channel open levels are indicated by horizontal lines. Time periods used to calculate NPo values are indicated by broken lines below the traces. C, amplitude histograms were used to calculate NPo values from similar outside-out patch-clamp recordings as shown in A and B. D, the maximal number of apparent channel levels was determined by visual inspection of single-channel current traces as shown in A and B. C and D summarize data from n= 13 and n= 14 outside-out patches with wild-type or mutant ENaC, respectively. Please note that some open circles and lines represent 2–3 individual experiments. *P < 0.05, **P < 0.01, n.s., not significant.
A minority of outside-out patches from oocytes expressing wild-type ENaC contained only a single-channel (online Supplementary Fig. S1). These patches were particularly instructive since they illustrate the variable baseline Po of ENaC which is a known feature of this channel (Garty & Palmer, 1997; Rossier, 2002). They confirm that proteolytic channel activation involves an increase in Po of channels that are already active in the membrane and the recruitment of near-silent channels (Diakov et al. 2008). This dual stimulatory effect is consistent with our observation that in multi-channel patches NPo of wild-type ENaC increased more than the number of apparent channels upon application of chymotrypsin (Fig. 6C and D).
Importantly, in patches from oocytes expressing the αW493R mutant channel we did not observe an increase in the number of apparent channel levels upon application of chymotrypsin (Fig. 6B and D). The average NPo was ∼3-fold larger in patches from oocytes expressing the mutant channel than in patches expressing wild-type ENaC (Fig. 6C). This suggests that more mutant channels than wild-type channels are active under baseline conditions and that the population of near-silent channels that can be proteolytically activated is reduced in oocytes expressing the mutant channel. Indeed, channels with very low Po were routinely observed in outside-out patches with wild-type but not with mutant ENaC (see Supplementary Fig. S2). As illustrated in the representative recording in Fig. 6B, chymotrypsin had a subtle effect on the gating kinetics of the mutant channel, which resulted in a small increase of NPo from 1.41 to 1.73. On average chymotrypsin increased NPo of mutant ENaC by ∼25% (Fig. 6C), which is in good agreement with the observed stimulatory effect of chymotrypsin on ENaC whole-cell currents in oocytes expressing the mutant channel (see Fig. 5). In the absence of an increase in the apparent number of active channels in the patch, this indicates that application of chymotrypsin slightly increased the Po of mutant channels that were already active in the patch at the time of chymotrypsin application. Importantly, our single-channel recordings in outside-out patches demonstrate that chymotrypsin fails to recruit additional near-silent channels in patches from oocytes expressing the mutant channel (Fig. 6D). Taken together, these findings indicate that the αW493R mutation causes a constitutive activation of the channel and reduces the pool of near-silent channels that can be activated by extracellular proteases.
αW493R does not alter constitutive channel cleavage
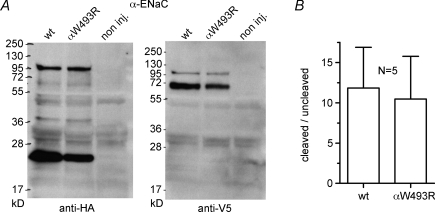
The reduced responsiveness of the mutant channel to chymotrypsin could be the result of an increased level of channel activation by enhanced constitutive channel cleavage (Diakov et al. 2008; Rossier & Stutts, 2009). To detect cleaved channel fragments at the plasma membrane, we tagged the α-subunit of ENaC with an N-terminal HA-tag and a C-terminal V5-tag. In the biotinylated cell surface fraction we detected the ∼95 kDa full length αENaC together with an expected N-terminal ∼24 kDa fragment and the corresponding C-terminal ∼68 kDa fragment (Fig. 7A), both representing typical αENaC cleavage products (Bruns et al. 2007; Harris et al. 2007; Rossier & Stutts, 2009). Importantly, there was no significant difference in the cleavage pattern between wild-type and mutant αENaC (Fig. 7B). We also failed to detect an effect of the αW493R mutation on the constitutive cleavage of the γ-subunit (data no shown), which is thought to be important for channel activation by extracellular proteases (Bruns et al. 2007; Harris et al. 2007; Carattino et al. 2008; Diakov et al. 2008). From these experiments we conclude that the stimulatory effect of the αW493R mutation is not caused by enhanced endogenous channel cleavage.
Figure 7. The αW493R mutation does not alter endogenous channel cleavage.
Oocytes were injected with 2 ng per subunit of cRNA for wt and mutant (αW493R) ENaC and incubated in low Na+ for 2 days. A, surface-expressed protein was isolated by biotinylation and separated by PAGE. Full length αENaC (at ∼95 kDa) and expected cleavage products (Rossier & Stutts, 2009) of the α-subunit were detected by using an N-terminal HA-tag (left blot) or a C-terminal V5-tag (right blot). Non-injected (non inj.) oocytes served as control. B, cleavage was quantified by densitometric analysis. The N-terminal ∼24 kDa and the ∼95 kDa bands were used to determine the amount of cleaved and non-cleaved ENaC, respectively. N represents number of blots from different batches of oocytes.
Sodium self inhibition is abolished and contributes to the gain-of-function effect of the W493R mutation
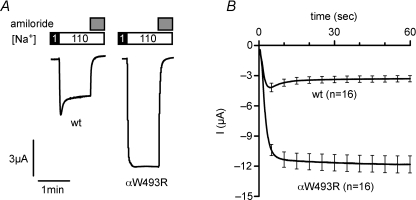
In Fig. 2, we excluded the possibility that impaired Na+ feedback inhibition contributes to the gain-of-function effect of the αW493R mutation. An alternative mechanism to repress ENaC activity is Na+ self inhibition, which attenuates the Po of ENaC within seconds when the extracellular Na+ concentration is acutely increased (Chraibi & Horisberger, 2002). To test whether the mutation impairs Na+ self inhibition, we performed experiments as illustrated in Fig. 8. The representative whole-cell current trace shown in Fig. 8A (left) demonstrates that in wild-type ENaC-expressing oocytes, changing the bath solution from a low (1 mm) to a high (110 mm) Na+ solution resulted in a transient inward current peak. The rapid current decay after the peak increase represents Na+ self inhibition (Chraibi & Horisberger, 2002). We noted that this initial peak response and the subsequent current decay were absent in oocytes expressing the mutant channel (Fig. 8A, right). Data from similar experiments as shown in Fig. 8A are summarized in Fig. 8B. These findings indicate that the mutation abolishes Na+ self inhibition. To investigate further how much this contributes to the gain-of-function effect of the mutation, we estimated the current component sensitive to Na+ self inhibition in oocytes expressing wild-type ENaC using a previously described model (Chraibi & Horisberger, 2002) (see eqn (1) in Methods). According to this model, the predicted amiloride-sensitive maximum current in the absence of Na+ self inhibition averaged 6.5 ± 0.7 μA, whereas steady state ΔIami averaged 3.0 ± 0.3 μA (n= 16). Thus, the current component sensitive to Na+ self inhibition is about 3.5 μA. From this we can conclude that the complete loss of Na+ self inhibition contributes about ∼40% to the observed increase of ΔIami to 11.3 ± 0.8 μA (n= 16) in oocytes expressing the mutant channel.
Figure 8. The αW493R mutation abolishes Na+ self inhibition.
Oocytes were injected with 0.5 ng per subunit of cRNA for αβγENaC (wt) or αW493RβγENaC (αW493R). After 2 days of incubation in low Na+, oocytes were clamped at −100 mV. The bath solution initially contained 1 mm Na+ and was subsequently changed to a bath solution containing 110 mm Na+ as indicated. After 60 s, amiloride (2 μm) was applied to determine the ENaC-mediated current component. A, representative current traces of a wild-type (left) and a mutant ENaC (right)-expressing oocyte. Downward deflections represent inward currents. B, averaged current traces from similar experiments as shown in A, starting from the change to 110 mm Na+. Lines represent mean, error bars s.e.m. n indicate number of oocytes.
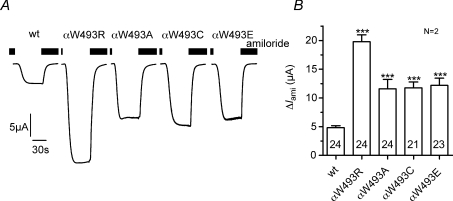
Substitution of αW493 by alanine, cysteine or glutamate also stimulates ENaC
Arginine carries a positive charge whereas aromatic tryptophan is uncharged. Therefore, the stimulatory effect of the αW493R mutation may depend on the charge of the amino acid that replaces the tryptophan residue in this position. To test this, we replaced tryptophan by the negatively charged amino acid glutamate (αW493E), by the uncharged amino acid alanine (αW493A), or by the uncharged but polar amino acid cysteine (αW493C). All three substitutions resulted in a ∼2.5-fold stimulation of ΔIami (Fig. 9). This stimulatory effect is smaller than that observed with the αW493R mutation. However, these results suggest that the gain-of-function effect of the mutation might not critically depend on the introduction of a positively charged arginine residue at this site, but instead that the loss of the tryptophan residue per se leads to ENaC activation. It should be noted that this tryptophan residue is highly conserved across species (see Supplementary Fig. S3A). In an additional set of experiments we demonstrated that a corresponding mutation in the α-subunit of rat ENaC (αW520R) had a similar stimulatory effect as the human αW493R mutation (see Supplementary Fig. S3B). Taken together these results confirm the functional importance of this highly conserved tryptophan residue in the α-subunit of ENaC.
Figure 9. Amino acid substitution at residue α493 stimulates ENaC.
Oocytes were injected with cRNA for αβγENaC (wt), αW493RβγENaC (αW493R), αW493AβγENaC (αW493A), αW493CβγENaC (αW493C) or αW493EβγENaC (αW493E). A, representative whole-cell current traces of individual oocytes at a holding potential of −60 mV. Amiloride (2 μm) was present in the bath as indicated by the black bars. B, summary of similar experiments as shown in A. To pool data from different batches of oocytes, individual ΔIami values were normalized to the mean ΔIami of the corresponding wild-type ENaC-expressing control oocytes. N indicates number of different batches of oocytes. Numbers in columns indicate total number of oocytes. ***P < 0.001.
Discussion
The present study demonstrates that the ENaC mutation αW493R associated with atypical CF stimulates ENaC currents when heterologously expressed in Xenopus oocytes. The magnitude of this gain-of-function effect is similar to that described for ENaC mutations causing Liddle's syndrome (Schild et al. 1995; Schild, 1996). However, the mechanisms responsible for the gain-of-function effect of the αW493R mutation are distinct from those of the Liddle's syndrome mutations. Liddle's syndrome mutations prevent ENaC retrieval from the cell surface and impair Na+ feedback inhibition (Rossier & Schild, 2008, 2009). By contrast, our data demonstrate that the gain-of-function effect of the αW493R mutation is caused by an increase in average channel Po.
For ENaC the single-channel Po values observed in patch-clamp experiments are known to be highly variable with a rather wide range and an average single-channel Po of ∼0.5 (Waldmann et al. 1995; Garty & Palmer, 1997). However, an average Po of ∼0.5 in the absence of an increase in channel surface expression could explain a doubling of ΔIami but not the ∼4-fold increase observed with the αW493R mutant channel. Thus, we have to assume that the average Po of wild-type ENaC is much lower than ∼0.5. This could be explained by the presence of a pool of so called near-silent channels which can be activated by extracellular proteases (Caldwell et al. 2004, 2005; Diakov et al. 2008; Rossier & Stutts, 2009). The concept that a pool of near-silent channels is present in the plasma membrane is supported by our finding that additional channel levels appear after chymotrypsin application to outside-out patches from wild-type ENaC-expressing oocytes. In contrast, chymotrypsin failed to activate near-silent channels in outside-out patches with the mutant channel. This suggests that the mutation reduces the pool of near-silent channels and mimics proteolytic activation of ENaC. Interestingly, constitutive activation of the mutant channel was not associated with increased endogenous channel cleavage at the cell surface. Thus, the mutation seems to convert near-silent channels into active channels by an alternative mechanism. The residual effect of chymotrypsin on the mutant channel indicates that the mutant channel is not completely unresponsive to proteolytic activation. However, the failure of chymotrypsin to activate near-silent channels in outside-out patches from oocytes expressing the αW493R mutant channel clearly distinguishes the mutant from the wild-type channel.
The αW493R mutation introduces a positive charge (arginine) at a site where normally an uncharged aromatic amino acid (tryptophan) is present. The introduction of a negatively charged (glutamate), a polar (cysteine) or an uncharged amino acid (alanine) also resulted in a stimulation of ENaC. Therefore, the introduction of a positive charge does not appear to be essential for the stimulatory effect. Similarly, the length of the substituting amino acids does not seem to be critical for mediating the stimulatory effect, since tryptophan and arginine are of similar size, whereas alanine, cysteine and glutamate are much shorter. This suggests that the W493 residue in wild-type αENaC mediates an inhibitory effect on the channel and is essential to maintain the channel in a near-silent state. In this context our observation that the αW493R mutation leads to a loss of Na+ self inhibition is of interest. The loss of the tryptophan reduces the channel's ability to decrease its activity within seconds following a sudden increase of the extracellular [Na+]. This suggests a role of this residue in mediating Na+ self inhibition.
In the absence of a crystal structure for ENaC it is difficult to predict a structural effect of the W493R mutation on ENaC. However, the recently published crystal structure of the related acid-sensing ion channel ASIC1 can be used to generate a homologous ENaC model (Jasti et al. 2007; Stockand et al. 2008). Using this modelling approach, we found that W493 is located in a region of the ENaC structure which binds a monovalent cation in the ASIC channel (Gonzales et al. 2009) (Supplementary Fig. S4). The structural similarity between ASIC and ENaC suggests that this region represents an ion binding site in ENaC and may play a role as sodium sensor or as a site for sodium entry. Mutation of W493 to other amino acids will probably alter the structure and, hence, channel properties. The fact that the type of amino acid substitution appears to be rather unimportant suggests that the tryptophan side chain might itself play a key role in the structural integrity of this region.
In the oocyte expression system the overall stimulatory effect of the αW493R mutation is similar to that of mutations identified in patients with Liddle's syndrome. However, in the latter patients this increased channel function results in a severe form of salt-sensitive arterial hypertension, while to our knowledge pulmonary symptoms have not been described in patients with Liddle's syndrome. In contrast, the αW493R mutation has been identified in patients with atypical CF, but as far as we know, these patients do not suffer from arterial hypertension. What could be the reason for the different pathophysiological consequences of the αW493R mutation?
It is noteworthy that after chymotrypsin stimulation ΔIami reached similar levels in wild-type and in αW493R mutant ENaC-expressing oocytes. Assuming that chymotrypsin leads to a maximal stimulation of the channels in the plasma membrane, this is in good agreement with our finding that channel surface expression was similar in wild-type and mutant ENaC-expressing oocytes. Interestingly, the constitutive level of proteolytic ENaC activation appears to be high in the native kidney (Nesterov et al. 2008). Thus, in the kidney, the stimulatory effect of the αW493R mutation may be limited. Moreover, it may be compensated by an appropriate down-regulation of the renin–angiotensin–aldosterone system. In contrast, protease inhibitors are normally present in the ASL and tonically inhibit the proteolytic activation of ENaC in the lung (Myerburg et al. 2006). Thus, in the lung, the αW493R mutation, which mimics proteolytic channel activation, might have a bigger stimulatory effect on ENaC function than in the kidney. This may explain why the αW493R mutation is associated with a pulmonary rather than a renal phenotype.
It also remains an unresolved issue why certain conditions with enhanced ENaC function lead to pulmonary symptoms while others do not. A possible explanation is the different molecular mechanism by which ENaC mutations in patients with Liddle's syndrome lead to a gain-of-function effect. In patients with Liddle's syndrome, increased surface expression of ENaC may not cause pulmonary symptoms as long as ENaC activation by endogenous proteases in the ASL is maintained at a low level (Myerburg et al. 2006). Moreover, mechanisms may exist in the respiratory epithelium to compensate for the retrieval defect of ENaC with Liddle's syndrome mutation by reducing channel insertion into the plasma membrane. Finally, we cannot rule out the possibility that patients with Liddle's syndrome have a minor pulmonary phenotype which so far has escaped clinical diagnosis. Indeed, it has been reported that alveolar fluid absorption is increased and the severity of hydrostatic pulmonary oedema is reduced in a mouse model for Liddle's syndrome (Randrianarison et al. 2007). Like patients with Liddle's syndrome, these animals have no apparent pulmonary symptoms under normal conditions. In contrast, overexpression of the β-subunit of ENaC in airway epithelia leads to CF-like pulmonary symptoms in mice (Mall et al. 2004).
It has been speculated that proteolytic activation of ENaC may aggravate symptoms of cystic fibrosis during acute respiratory infections associated with the generation of local proteases, e.g. neutrophil elastase (Boucher, 2004; Caldwell et al. 2005; Harris et al. 2007). Moreover, in CF the protease–protease inhibitor balance appears to be altered which may favour proteolytic ENaC activation in the lung. Consistent with this, increased proteolytic processing of the α-subunit of ENaC has been demonstrated in human CF airway epithelial cells (Myerburg et al. 2006). Thus, the gain-of-function effect of the αW493R mutation, in particular in association with one mutated CFTR allele, may be pathophysiologically relevant in patients with atypical CF carrying this mutation (Azad et al. 2009).
The concept of sodium hyperabsorption via hyperactive ENaC as a pathophysiological basis for CF-like lung symptoms is intriguing. However, in patients with atypical CF, stimulatory as well as inhibitory ENaC mutations have been identified (Sheridan et al. 2005; Azad et al. 2009; Huber et al. 2010). CF-like pulmonary symptoms with chronic airway infections have also been described in patients with pseudohypoaldosteronism type 1 caused by loss-of-function ENaC mutations (Kerem et al. 1999). The rhinorrhea and increased mucociliary clearance observed in these patients is consistent with reduced ENaC-mediated sodium absorption in the airways (Kerem et al. 1999). These findings illustrate the complex effects that ENaC might exert in the pathophysiology of CF or CF-like pulmonary disease.
In summary, we have shown that the stimulatory effect of the αW493R mutation is based on constitutively high Po of the mutant channel with a reduced pool of near-silent channels. Thus, the mutation functionally mimics proteolytic channel activation without changing the cleavage pattern of the mutant channel. This interpretation is supported by the reduced stimulatory effect of chymotrypsin on the mutant channel, by the failure of chymotrypsin to recruit near-silent channels in outside-out patches containing the mutant channel, and by the finding that the mutant channel lacks Na+ self inhibition. Thus, our results suggest an important role of the highly conserved αW493 residue for ENaC function and regulation of channel Po. Our results also suggest that this gain-of-function mutation may contribute to the pathophysiology of atypical CF.
Acknowledgments
We acknowledge the expert technical assistance of Ralf Rinke, Jessica Ott, Sonja Mayer and Céline Harlay. We thank Dr Gerhard Giebisch (Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT, USA) for carefully reading the manuscript. This study was supported by grants from the ELAN-Fonds and the Johannes and Frieda Marohn-Stiftung (both University Erlangen-Nürnberg), the Deutsche Forschungsgemeinschaft (DFG, SFB423: Kidney injury: Pathogenesis and Regenerative Mechanism), the Alphonse and Jean Forton Fund – Koning Boudewijn Stichting (2005 04 R7 115 BO and 2008-R10150-002) and from the Het Fonds voor Wetenschappelijk Onderzoek Vlaanderen (G.0521.06 and 1.5.111.07). Part of this work has been published in abstract form (Rauh et al. 2007, 2008).
Glossary
Abbreviations
- ASL
airway surface liquid
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- E
equilibrium potential
- ENaC
epithelial sodium channel
- ΔIami
amiloride-sensitive current
- Po
open probability
Author contributions
R.R., A.K.A., H.C., J.J.C., J.D. and C.K. participated in the conception of the study; R.R., A.D., A.T. and C.K. designed the experiments; R.R., A.D., A.T. and J.K. performed the experiments and data analysis; R.R., A.D. and C.K. interpreted the data; H.S. did the modelling. R.R. and C.K. wrote the manuscript; all authors were involved in critically revising the manuscirpt and approved its final version. Experiments were performed at the Department of Cellular and Molecular Physiology of the Friedrich-Alexander University of Erlangen-Nürnberg, Erlangen, Germany.
Supplemental material
Figure S1
Figure S2
Figure S3
Figure S4
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, et al. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat. 2009;30:1093–1103. doi: 10.1002/humu.21011. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from theγ-subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–C194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol. 2005;288:L813–L819. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- Canessa CM. Structural biology: unexpected opening. Nature. 2007;449:293–294. doi: 10.1038/449293a. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channelγ subunit has a dominant role in channel activation. J Biol Chem. 2008;283:25290–25295. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chraibi A, Horisberger JD. Na self inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol. 2002;120:133–145. doi: 10.1085/jgp.20028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol. 1998;111:127–138. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boeck K, Wilschanski M, Castellani C, Taylor C, Cuppens H, Dodge J, Sinaasappel M. Cystic fibrosis: terminology and diagnostic algorithms. Thorax. 2006;61:627–635. doi: 10.1136/thx.2005.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakov A, Bera K, Mokrushina M, Krueger B, Korbmacher C. Cleavage in the γ-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels. J Physiol. 2008;586:4587–4608. doi: 10.1113/jphysiol.2008.154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakov A, Korbmacher C. A novel pathway of epithelial sodium channel activation involves a serum- and glucocorticoid-inducible kinase consensus motif in the C terminus of the channel's α-subunit. J Biol Chem. 2004;279:38134–38142. doi: 10.1074/jbc.M403260200. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux ME, Salafranca MN, Lynch KR. Trypsin induces Ca2+-activated Cl− currents in X. laevis oocytes. FEBS Lett. 1994;337:235–238. doi: 10.1016/0014-5793(94)80198-3. [DOI] [PubMed] [Google Scholar]
- Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci U S A. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerteis S, Krueger B, Korbmacher C, Rauh R. The δ-subunit of the epithelial sodium channel (ENaC) enhances channel activity and alters proteolytic ENaC activation. J Biol Chem. 2009;284:29024–29040. doi: 10.1074/jbc.M109.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem. 2007;282:58–64. doi: 10.1074/jbc.M605125200. [DOI] [PubMed] [Google Scholar]
- Huber R, Krueger B, Diakov A, Korbmacher J, Haerteis S, Einsiedel J, et al. Functional characterization of a partial loss-of-function mutation of the epithelial sodium channel (ENaC) associated with atypical cystic fibrosis. Cell Physiol Biochem. 2010;25:145–158. doi: 10.1159/000272059. [DOI] [PubMed] [Google Scholar]
- Hughey RP, Carattino MD, Kleyman TR. Role of proteolysis in the activation of epithelial sodium channels. Curr Opin Nephrol Hypertens. 2007;16:444–450. doi: 10.1097/MNH.0b013e32821f6072. [DOI] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in α-ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Ji HL, Bishop LR, Anderson SJ, Fuller CM, Benos DJ. The role of Pre-H2 domains of α- and δ-epithelial Na+ channels in ion permeation, conductance, and amiloride sensitivity. J Biol Chem. 2004;279:8428–8440. doi: 10.1074/jbc.M312012200. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Gautschi I, Rossier BC, Schild L. Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J Clin Invest. 1998;101:2741–2750. doi: 10.1172/JCI2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, et al. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med. 1999;341:156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of the epithelial sodium channels by proteases. J Biol Chem. 2009;284:20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstas AA, Bielfeld-Ackermann A, Korbmacher C. Sulfonylurea receptors inhibit the epithelial sodium channel (ENaC) by reducing surface expression. Pflugers Arch. 2001;442:752–761. doi: 10.1007/s004240100597. [DOI] [PubMed] [Google Scholar]
- Konstas AA, Shearwin-Whyatt LM, Fotia AB, Degger B, Riccardi D, Cook DI, Korbmacher C, Kumar S. Regulation of the epithelial sodium channel by N4WBP5A, a novel Nedd4/Nedd4-2-interacting protein. J Biol Chem. 2002;277:29406–29416. doi: 10.1074/jbc.M203018200. [DOI] [PubMed] [Google Scholar]
- Korbmacher C, Volk T, Segal AS, Boulpaep EL, Fromter E. A calcium-activated and nucleotide-sensitive nonselective cation channel in M-1 mouse cortical collecting duct cells. J Membr Biol. 1995;146:29–45. doi: 10.1007/BF00232678. [DOI] [PubMed] [Google Scholar]
- Kraus C, Reis A, Naehrlich L, Dötsch J, Korbmacher C, Rauh R. Functional characterization of a novel CFTR mutation P67S identified in a patient with atypical cystic fibrosis. Cell Physiol Biochem. 2007;19:239–248. doi: 10.1159/000100643. [DOI] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem. 2006;281:27942–27949. doi: 10.1074/jbc.M606449200. [DOI] [PubMed] [Google Scholar]
- Nesterov V, Dahlmann A, Bertog M, Korbmacher C. Trypsin can activate the epithelial sodium channel (ENaC) in microdissected mouse distal nephron. Am J Physiol Renal Physiol. 2008;295:F1052–F1062. doi: 10.1152/ajprenal.00031.2008. [DOI] [PubMed] [Google Scholar]
- Randrianarison N, Escoubet B, Ferreira C, Fontayne A, Fowler-Jaeger N, Clerici C, Hummler E, Rossier BC, Planes C. β-Liddle mutation of the epithelial sodium channel increases alveolar fluid clearance and reduces the severity of hydrostatic pulmonary oedema in mice. J Physiol. 2007;582:777–788. doi: 10.1113/jphysiol.2007.131078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh R, Dinudom A, Fotia AB, Paulides M, Kumar S, Korbmacher C, Cook DI. Stimulation of the epithelial sodium channel (ENaC) by the serum- and glucocorticoid-inducible kinase (Sgk) involves the PY motifs of the channel but is independent of sodium feedback inhibition. Pflugers Arch. 2006;452:290–299. doi: 10.1007/s00424-005-0026-5. [DOI] [PubMed] [Google Scholar]
- Rauh R, Korbmacher J, Cuppens H, Cassiman J-J, Korbmacher C. A heterozygous ENaC mutation (αW493R) identified in patients with atypical CF increases amiloride-sensitive currents in Xenopus laevis oocytes. Acta Physiol. 2007;192(Suppl 663):P08-L06-06. [Google Scholar]
- Rauh R, Stuppy A, Diakov A, Korbmacher J, Azad AK, Doetsch J, Cuppens H, Cassiman J-J, Korbmacher C. Functional characterisation of a gain-of-function mutation of ENaC associated with atypical CF. Acta Physiol. 2008;192(Suppl 663):OM2-3-5. [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rossier BC. Hormonal regulation of the epithelial sodium channel ENaC: N or Po? J Gen Physiol. 2002;120:67–70. doi: 10.1085/jgp.20028638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier BC, Schild L. Epithelial sodium channel: mendelianversus essential hypertension. Hypertension. 2008;52:595–600. doi: 10.1161/HYPERTENSIONAHA.107.097147. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2009;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- Schild L. The ENaC channel as the primary determinant of two human diseases: Liddle syndrome and pseudohypoaldosteronism. Nephrologie. 1996;17:395–400. [PubMed] [Google Scholar]
- Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci U S A. 1995;92:5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Sheridan MB, Fong P, Groman JD, Conrad C, Flume P, Diaz R, et al. Mutations in the β-subunit of the epithelial Na+ channel in patients with a cystic fibrosis-like syndrome. Hum Mol Genet. 2005;14:3493–3498. doi: 10.1093/hmg/ddi374. [DOI] [PubMed] [Google Scholar]
- Stanke F, Becker T, Cuppens H, Kumar V, Cassiman JJ, Jansen S, et al. The TNFα receptor TNFRSF1A and genes encoding the amiloride-sensitive sodium channel ENaC as modulators in cystic fibrosis. Hum Genet. 2006;119:331–343. doi: 10.1007/s00439-006-0140-2. [DOI] [PubMed] [Google Scholar]
- Staub O, Verrey F. Impact of Nedd4 proteins and serum and glucocorticoid-induced kinases on epithelial Na+ transport in the distal nephron. J Am Soc Nephrol. 2005;16:3167–3174. doi: 10.1681/ASN.2005050454. [DOI] [PubMed] [Google Scholar]
- Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life. 2008;60:620–628. doi: 10.1002/iub.89. [DOI] [PubMed] [Google Scholar]
- Tong Q, Menon AG, Stockand JD. Functional polymorphisms in the α-subunit of the human epithelial Na+ channel increase activity. Am J Physiol Renal Physiol. 2006;290:F821–F827. doi: 10.1152/ajprenal.00312.2005. [DOI] [PubMed] [Google Scholar]
- Volk T, Konstas AA, Bassalay P, Ehmke H, Korbmacher C. Extracellular Na+ removal attenuates rundown of the epithelial Na+-channel (ENaC) by reducing the rate of channel retrieval. Pflugers Arch. 2004;447:884–894. doi: 10.1007/s00424-003-1193-x. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem. 1995;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- Wielpütz MO, Lee IH, Dinudom A, Boulkroun S, Farman N, Cook DI, Korbmacher C, Rauh R. NDRG2 stimulates amiloride-sensitive Na+ currents in Xenopus laevis oocytes and Fisher rat thyroid cells. J Biol Chem. 2007;282:28264–28273. doi: 10.1074/jbc.M702168200. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Treis D, Schubert SC, Harm M, Schatterny J, Hirtz S, Duerr J, Boucher RC, Mall MA. Preventive but not late amiloride therapy reduces morbidity and mortality of lung disease in βENaC-overexpressing mice. Am J Respir Crit Care Med. 2008;178:1245–1256. doi: 10.1164/rccm.200803-442OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.