Abstract
The study of rhythmic electrical activity in slice preparations has generated important insights into neural network function. While the synaptic mechanisms involved in the generation of in vitro network oscillations have been studied widely, little is known about the modulatory influence exerted on rhythmic activity in neuronal networks by neuropeptides and biogenic amines. Gamma oscillations play an important role in cognitive processes and are altered or disrupted in disorders such as Alzheimer's disease (AD) and schizophrenia. Given the importance of gamma oscillations for learning, memory and cognition processes as well as the recent interest in histamine H3 receptors in the development of pro-cognitive drugs to treat disorders such as AD and schizophrenia, it is relevant to study the impact of histaminergic mechanisms on network gamma oscillations. Here we show for the first time a modulation of gamma oscillation by histaminergic mechanisms. Selective activation of the H3 receptor by R-α-methylhistamine significantly reduces the power of kainate-induced gamma oscillations, but not carbachol-induced gamma oscillations, in the rat hippocampal slice preparation without affecting oscillation frequency. This effect is neither caused by a decrease in excitatory or inhibitory postsynaptic currents, nor a decrease in cellular excitability. Instead, we find that the decrease in oscillation power following H3 receptor activation results from a desynchronization of pyramidal neuron action potential firing with regard to the local field potential oscillation cycle. Our data provide a possible mechanism of action for histamine in regulating gamma oscillations in the hippocampal network.
Introduction
Rhythmic electrical activity in neuronal networks is important for many higher-order processes in the brain, such as learning, memory and cognition. Of particular interest is rhythmic network activity in the gamma-frequency band (30–80 Hz, gamma oscillations), which plays a direct role in these brain processes (Gray & Singer, 1989). The power of gamma oscillations is correlated with the success of memory formation (Jensen et al. 2007) and they are altered in several brain disorders, such as Alzheimer's disease (AD), schizophrenia and epilepsy that result in decreased learning and memory performance as well as cognitive decline in patients (Spencer et al. 2004). Rhythmic electrical activity is based on the intrinsic characteristics of neuronal networks at different organizational levels, such as ion channels, synaptic connections and network interactions. Hence rhythmic electrical activity can be modulated on several distinct levels. Amongst the multitude of potential modulators of neuronal network activity, neuropeptides and biogenic amines have only recently begun to receive increased attention (Wójtowicz et al. 2009). Of particular interest are the histamine receptors and especially the H3 receptor (H3R) as potential targets for the development of pro-cognitive drugs to treat disorders such as AD, schizophrenia and attention deficit/hyperactivity disorder (ADHD) (Passani et al. 2004). Interestingly, the H3 receptor has been shown to modulate theta oscillations in vivo (Hájos et al. 2008; Masuoka & Kamei, 2009). Therefore, research into histamine receptor function in relation to network activity relevant for cognitive processes will provide physiological context to our understanding of brain disorders and probably accelerate relevant pharmaceutical developments.
Histamine in the central nervous system is associated with the regulation of sleep and arousal but has recently also been suggested to be important for learning and memory (Haas et al. 2008). Histaminergic neurons are hyperactive in schizophrenic patients (Prell et al. 1995), H3R antagonists have antipsychotic properties and several are currently undergoing clinical trials for use in cognitive and affective disorders (Sander et al. 2008). Antagonists of H3R enhance spatial learning, novel object recognition and reverses scopolamine-induced amnesia in rats (Medhurst et al. 2007). There are, however, conflicting results regarding the effect of H3R stimulation on learning and memory performance and as to whether H3R activation results in a direct effect or an indirect action via increased histamine release and stimulation of the H1R (Passani et al. 2000).
At the cellular level, histamine has been shown to elicit several effects such as excitation and bursting in pyramidal neurons, inhibition of slow afterhyperpolarization, facilitation of LTP, increase in frequency of spontaneous IPSPs, inhibition of glutamatergic transmission and modulation of acetylcholine release from septo-hippocampal projection fibres (Haas et al. 2008). Many of these effects result from the activation of H3R, which is strongly expressed in the pyramidal cell layer of the CA3 region of the ventral hippocampus (Pillot et al. 2002) and has been shown to regulate activity of histaminergic neurons (Haas et al. 2008). H3R activation inhibits the release of several major neurotransmitters (Haas et al. 2008) by interacting with N- and P/Q-type voltage-gated calcium channels (Takeshita et al. 1998) to reduce action potential-mediated influx of calcium. How these histamine effects might influence rhythmic activity in neuronal networks is currently unknown, but with knowledge of certain beneficial effects of histamine receptor antagonists on cognition, and the fact that H3R antagonists increased the power of theta oscillations in vivo, we wanted to investigate whether H3R activation might modulate cognition-relevant rhythmic network activity such as gamma oscillations. If this were so, we were interested in uncovering the cellular and synaptic mechanisms that underlie the H3R-mediated modulation of gamma oscillations. In this study we therefore investigated the effects of an H3R agonist (R-α-methylhistamine) on the cellular and synaptic mechanisms underlying kainate-induced gamma oscillations in the hippocampal slice preparation (Fisahn et al. 2004).
Methods
Brain slice preparation
Experiments were carried out in horizontal hippocampal slices from a total number of 49 Sprague–Dawley rats of both sexes (p15-22, supplied by Scanbur, Sweden and Charles River, Germany). Rats were deeply anaesthetized with fluorethane (by inhalation), then decapitated and the brain rapidly removed. All procedures were performed in accordance with Swedish ethical regulations for animal procedures and comply with the policies laid out in Drummond (2009). Ethical permission was granted by northern Stockholm's animal research ethics committee (Norra Djurförsökningsnämnd). Slices (for extracellular recordings 400 μm thick; for patch-clamp recordings 300 μm thick) were prepared in ice-cold artificial cerebrospinal fluid (ACSF1, in mm: KCl 2.49, NaH2PO4 1.43, NaHCO3 26, glucose 10, sucrose 252, CaCl2 1, MgCl2 2) using a vibratome (Leica VT 1000S, Leica Microsystems, Sweden). Slices were maintained at room temperature at the interface between humidified carbogen gas (95% O2–5% CO2) and ACSF2 (henceforth referred to simply as ASCF) containing (in mm): NaCl 124, KCl 3.5, NaH2PO4 1.25, MgCl2 1.5, CaCl2 1.5, NaHCO3 30, glucose 10 for at least 1 h prior to recording.
Electrophysiology
A submerged recording chamber (32°C) was used for all experiments. Extracellular recordings were made in stratum pyramidale of area CA3 using glass microelectrodes containing ACSF (resistance 3–5 MΩ). Patch clamp recordings were made from visually identified CA3 pyramidal neurons (IR-DIC microscopy, Zeiss Axioskop, Germany) using glass microelectrodes (resistance 3–5 MΩ) containing (in mm): potassium gluconate 122, KCl 8, Hepes 10, MgATP 4, NaGTP 0.3 set to pH 7.3 with KOH, osmolarity 270–280 mosmol l−1. For IPSC recordings, microelectrodes were filled with a solution containing (in mm): CsMetSO4 140, Hepes 10, MgCl2 2, EGTA 0.6, ATPNa 2, GTPNa 0.3, set to pH 7.3 with CsOH, osmolarity 270–280 mosmol l−1. Data were recorded with a MultiClamp 700B amplifier, sampled at 10 kHz, low-pass filtered at 1 kHz, digitized (Digidata 1440A, Molecular Devices, CA, USA) and stored on a hard disc using pCLAMP 10.0 software (Molecular Devices). Chemical compounds were purchased from Sigma-Aldrich and Tocris (kainate, R-α-methylhistamine (RAMH), Clobenpropit, (±)-4-(4-aminophenyl)-1,2-dihydro-1-methyl-2-propyl-carbamoyl-6,7-methylenedioxyphthalazine (SYM2206), dl-2-amino-5-phosphonopentanoic acid (AP5), tetrodotoxin (TTX), picrotoxin (dissolved in 70% ethanol), carbachol). For all experiments, kainate (or carbachol) was perfused at 2–4 ml min−1 for 20 min to allow for stabilization of gamma oscillations before recording.
Data analysis
Fast Fourier transformations for power spectra were computed from 60 s long data traces using Clampfit 10.2 software (Molecular Devices). Power was calculated as the integrated power spectrum between 20 and 80 Hz. The low cut-off frequency of 20 Hz was chosen because of the lower-than-physiological recording temperature (32°C) used in our experiments. It has been demonstrated that the frequency of gamma oscillations decreases by 3.3 Hz for each 1°C temperature decrease (Dickinson et al. 2003; Leao et al. 2009). Synaptic events were analysed using the Mini Analysis program (Synaptosoft, NJ, USA). The phase of action potentials with regard to field oscillation cycle was analysed using a template-based algorithm for field cycles and a derivative threshold-based algorithm for detecting action potentials in Axograph 1.3 (Axograph Scientific, Sydney, Australia). In order to assess the rhythmicity of gamma oscillations before and after RAMH superfusion, the coefficient of rhythmicity (Cr) was calculated by computing autocorrelograms of recorded traces and then measuring the ratio Cr= (A− |B|)/(A+ |B|) were B is the amplitude of the first trough of the autocorrelogram and A is the amplitude of the second peak. The phase of action potentials with regard to field oscillation cycle was analysed using a template-based algorithm for field cycles and a derivative threshold-based algorithm for detecting action potentials. Action potential–phase histograms were fitted with 3rd order polynomial functions (y=ax3+bx2+cx+d) and the 3rd order coefficient a used as a measure of the histogram ‘peakedness’ (AP synchronicity or randomness). Preferred action potential phase angle in relation to the field oscillation cycle was calculated by aligning the average field oscillation cycles with the corresponding spike–phase histograms. The angle for each action potential was then calculated using the inverse sine function for the normalized field amplitudes (the trough of the cycle defined as −1, (−90 deg) and the peak defined as 1, (90 deg)) at 1 ms intervals (Leao et al. 2009). An average phase angle of all action potentials in each histogram was then taken as a measure as the preferred phase angle. Data values are reported as means ±s.e.m. Students t tests for pairs and Wilcoxon test were used for statistics.
Results
Neuronal network oscillations
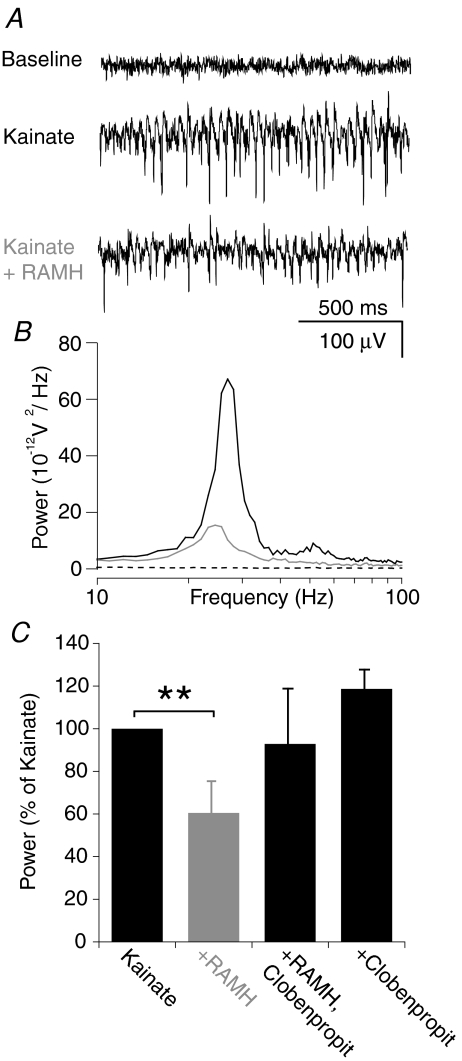
Network oscillations in the gamma-frequency range (30–80 Hz) were induced by perfusing hippocampus slices with 100 nm kainate (20 min, power: 2.31 ± 0.92 × 10−9 V2). Subsequent activation of the histamine H3 receptor (H3R) by its agonist R-α-methylhistamine (RAMH, 100 nm, 5 min) resulted in a significant decrease of gamma oscillation power (1.15 ± 0.4 × 10−9 V2) equivalent to 60.5 ± 14.0% of initial kainate oscillation (n= 17, P < 0.01; Wilcoxon test using absolute power values Fig. 1). When, after induction of gamma oscillations, RAMH was applied together with the H3R antagonist Clobenpropit (100 nm each, 5 min) the RAMH-induced decrease of oscillation power was blocked (92.8 ± 25.1% of initial kainate oscillation, n= 9; Fig. 1C), confirming the specificity of the H3R-mediated effect. Application of Clobenpropit alone did not have any significant effect on gamma oscillation power (118.7 ± 8.17% of initial kainate oscillation, n= 8; Fig. 1C). In contrast to power, the peak frequency of the oscillations (29.8 ± 0.64 Hz; n= 34) was not significantly affected by addition of RAMH (30.3 ± 1.35 Hz, n= 17) or Clobenpropit (28.3 ± 1.11 Hz, n= 8; Fig. 1A and B).
Figure 1. H3 receptor activation decreases power of kainate- but not carbachol-induced gamma oscillations.
A, kainate-induced (100 nm) gamma oscillations recorded in stratum pyramidale of CA3 are reduced 5 min after application of 100 nm RAMH. B, power spectra of the respective traces in A. C, results summary of H3 receptor agonists and antagonist action. The oscillation power after all treatments was normalized to the power recorded after 20 min kainate application.
The decrease of gamma oscillation power by activation of H3R is specific to kainate-induced gamma oscillations as no RAMH-induced effects were observed on carbachol-induced gamma oscillations. Hippocampal slices were perfused with 2 μm carbachol until stable gamma oscillations were achieved (20 min, power: 2.21 ± 0.48 × 10−9 V2; peak frequency: 33.06 ± 0.43 Hz). Addition of 100 nm RAMH had no significant effect on oscillation power or frequency (5 min, power: 2.08 ± 0.52 × 10−9 V2, n= 12, P= 0.97, Wilcoxon test; peak frequency: 33.21 ± 0.51 Hz, P= 0.65, Student's paired t test). In a second set of experiments, gamma oscillations were induced using 20 μm carbachol (20 min, power: 9.95 ± 3.86 × 10−9 V2; peak frequency: 31.28 ± 1.00 Hz). Again, addition of 100 nm RAMH had no significant effect on oscillation power (5 min, power: 9.26 ± 3.62 × 10−9 V2, n= 8, P= 0.11, Wilcoxon test), while the peak frequency increased slightly (32.05 ± 1.05 Hz, P= 0.049, n= 8, Student's paired t test).
Synaptic and cellular currents
In order to elucidate synaptic and cellular mechanisms, which could underlie the observed decrease in gamma oscillation power, we investigated (a) potential changes in synaptic input to pyramidal neurons, (b) potential changes in cellular excitability and (c) a potential desynchronization of pyramidal neuron action potential firing with regard to the field oscillation phase.
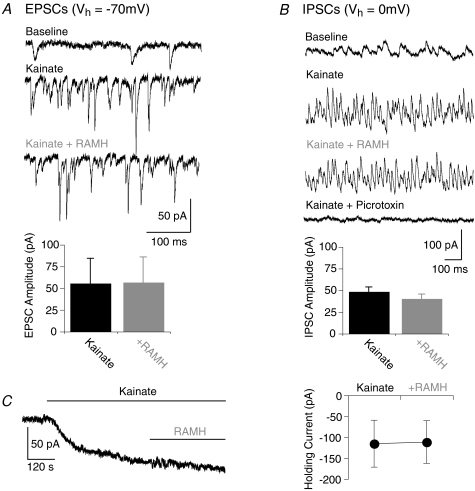
To investigate the effect of RAMH on excitatory and inhibitory synapses, postsynaptic currents (EPSCs and IPSCs) were recorded from pyramidal neurons (Vh=−70 mV for EPSCs and 0 mV for IPSCs; Fig. 2A and B). Adding 100 nm RAMH to the kainate-containing superfusate did not significantly affect either mean EPSC amplitude (kainate: 55.7 ± 28.9 pA; kainate + RAMH: 56.9 ± 29.2 pA, n= 5; Fig. 2A) or rate (kainate: 54.1 ± 21.4 s−1; kainate + RAMH: 54.0 ± 25.7 s−1, n= 5). Likewise, IPSC mean amplitude and rate were not significantly changed by RAMH (kainate: 48.6 ± 5.5 pA, 38.5 ± 5.6 s−1; kainate + RAMH: 40.4 ± 5.5 pA, 36.0 ± 6.8 s−1, n= 7 Fig. 2B). When measuring inhibitory currents, 50 μm picrotoxin was washed in for 5 min at the end of the experiment in order to block GABAA currents, thereby confirming the identity of the IPSCs. The fact that H3R activation did not alter the rate of synaptic events is consistent with the lack of gamma oscillation frequency changes observed in our extracellular recordings of gamma oscillations.
Figure 2. Synaptic and cellular currents in CA3 pyramidal cells are unaffected by H3 receptor activation.
A, EPSCs were recorded in standard ACSF in the absence of drugs, after kainate application, and activation of H3 receptors. Activation of kainate receptors (middle trace) shows the strong increase in number and amplitude of EPSCs typical during gamma oscillations. Activation of H3 receptors leaves EPSCs unaffected. Summary histogram is below. B, IPSCs were recorded in standard ACSF in the absence of drugs, after kainate application, and activation of H3 receptors. Activation of kainate receptors (middle trace) shows the strong increase in number and amplitude of IPSCs typical during gamma oscillations. Activation of H3 receptors leaves IPSCs unaffected. GABAA receptor block (lowest trace) removes the PSCs observed before and confirms their inhibitory nature. Summary histogram is below. C, example trace of cellular current recording (left; Vh=−60 mV). Observe a slowly developing inward current in response to kainate but no additional response to RAMH. The summary diagram (right) quantifies the cellular current experiments.
To further exclude synaptic changes in the neuronal network as a cause for the H3R-mediated decrease of gamma oscillation power we also examined the holding voltage of CA3 pyramidal neurons in the absence of synaptic blockers. Superfusion with 100 nm kainate resulted in a marked depolarization as described previously (Vm baseline: −58.3 ± 2.3 mV; Vm kainate: −48.0 ± 1.1 mV, P= 0.02; data not shown; Fisahn et al. 2004). Addition of 100 nm RAMH, however, did not result in a significant additional change of membrane voltage (−46.7 ± 1.6 mV, n= 6, P= 0.16; data shown as part of another experiment in Fig. 3A).
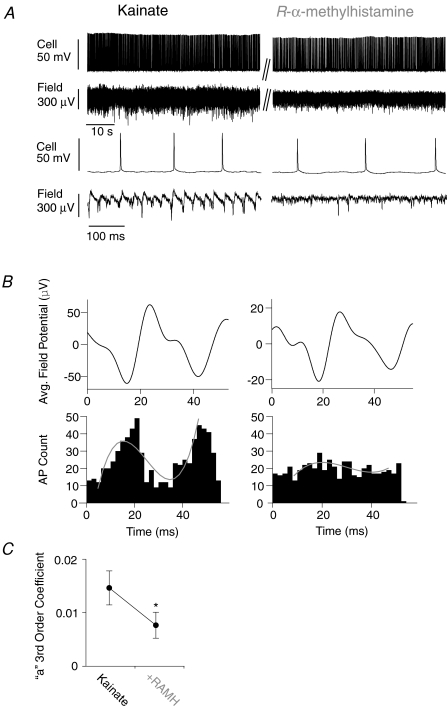
Figure 3. H3 receptor activation desynchronizes action potential firing in CA3 pyramidal cells.
A, concomitant recordings of cellular action potential firing and extracellular gamma oscillations. Lower traces are excerpts from the upper traces at increased temporal resolution. H3 receptor activation results in a decreased gamma oscillation amplitude. B, the average oscillation cycles of the traces in A are shown in the diagrams. The histograms show 2 ms bin counts of action potentials occurring during the oscillation cycles. The detected number of cycles are roughly the same before and after RAMH. Fitted to the histograms are 3rd order polynomial functions (see Methods). In kainate-only conditions action potentials show a clear synchronization and phase preference. H3 receptor activation causes action potential firing to become desynchronized, which is reflected in the flatter profile of the histogram and a lower ‘a’ coefficient. C, summary diagram showing the ‘a’ coefficients before and after H3 receptor activation.
With the above experiments suggesting that the H3R-mediated decrease of gamma oscillation power is probably not caused by a network effect, we next examined the intrinsic excitability of synaptically isolated pyramidal neurons by measuring the amount of current injected to hold the cells at −60 mV (drugs preset in bath (in μm); SYM2206 50, AP5 50, TTX 1, picrotoxin 50). Consistent with the current-clamp experiments, kainate superfusion resulted in a slow inward current as reported previously (−116 ± 56 pA, n= 4; (Castillo et al. 1997; Fisahn et al. 2004). The additional application of RAMH (100 nm) did not significantly change the amount of slow current injected (−112 ± 51 pA, n= 4; Fig. 2C).
Action potential phasing and network synchrony
With synaptic effects and changes in intrinsic excitability ruled out we proceeded to examine action potential desynchronization as a possible cause for the observed decrease of gamma oscillation power by H3R activation. Concomitant recordings of extracellular field potentials and intracellular action potentials were carried out to analyse the synchronization of the action potentials with regard to the phase of the network oscillation (Fig. 3). The mean spike rate did not change significantly after application of RAMH (kainate: 10.9 ± 3.5 s−1; kainate + RAMH: 9.9 ± 3.0 s−1, n= 9; Fig. 3A and B). A template-based detection method was used to identify oscillation cycles and determine spike phase (kainate: 3036 ± 227 cycles detected of which 0.32 ± 0.12 cycles showed a concomitant action potential in the pyramidal cell recording; kainate + RAMH: 2623 ± 280 cycles detected of which 0.31 ± 0.07 cycles showed a concomitant action potential in the pyramidal cell recording, n= 9, P= 0.18 (for cycles), P= 0.95 (for APs per detected cycle)). In the presence of kainate alone, the clear majority of action potentials occurred phase-synchronized around the negative peak or late in the falling phase of the field oscillation. The average of the detected cycles for each recording were aligned with the corresponding action potential firing histogram with the trough of the average oscillation cycle defined as −90 deg and the peak defined as 90 deg (full period was then −180 to 180 deg) (Leao et al. 2009). The preferred firing phase angle after gamma oscillation induction for kainate was −170.63 ± 20.76 deg. Additional application of 100 nm RAMH did not result in a significant change of preferred phase angle (−174.65 ± 19.31 deg, n= 9, P= 0.89, paired t test; Fig. 3B). There were no significant changes in the variability of the field potential period as a result of RAMH superfusion (coefficient of rhythmicity 0.26 ± 0.06 before and 0.27 ± 0.11 after RAMH, n= 9, P= 0.91, Wilcoxon test; this shows that the oscillations are still stable even after the application of RAMH, in spite of the decrease in power). Instead, application of RAMH caused action potential generation to desynchronize significantly throughout the oscillation cycle (Fig. 3B). In order to quantify the synchrony of action potential occurrence in relation to the network oscillation cycle, 3rd order polynomial functions (f(x) =ax3+bx2+cx+d) were fitted to the action potential histograms. The 3rd order coefficient a was used as an estimate of the ‘peakedness’ of the histogram (i.e. synchronization of action potentials (Leao et al. 2009). In the presence of kainate alone the a coefficient was 0.0147 ± 0.00318 while after H3R activation by 100 nm RAMH it decreased significantly to 0.00768 ± 0.00243 (n= 9, P= 0.02; Fig. 3C). This shows that H3R activation results in a substantial desynchronization of action potential generation in the pyramidal neuron population, which is a plausible mechanism for the observed reduction of gamma oscillation power in response to RAMH superfusion.
Discussion
In this study we show that the selective activation of H3Rs can cause a significant reduction in the power of neuronal network activity in the gamma-frequency range in the hippocampus in vitro (Fig. 1). This effect elicited by RAMH on kainate-induced oscillations is H3R-specific as the reduction is abolished by H3R antagonists. Our work demonstrates that this decrease is not due to overall decreased synaptic activity, since neither synaptic event amplitude nor frequency is decreased (Fig. 2). The slow inward current elicited by kainate as well as the frequency of action potential firing are also unaffected. Instead, a substantial desynchronization of action potential firing in hippocampal pyramidal neurons in relation to the gamma cycle underlies this reduction in network activity (Fig. 3).
The slice preparation is a highly useful tool to understand the cellular and synaptic mechanisms underlying in vivo phenomena such as rhythmic network activity. However, some care has to be taken when relating in vitro to in vivo findings. Gamma oscillations can arise spontaneously in some hippocampal slice preparations (Pietersen et al. 2009), but more robust and persistent oscillations result from various pharmacological induction protocols. Such pharmacologically induced oscillations differ from the spontanous oscillations in aspects such as modulation profile and spatial distribution. Gamma oscillations resulting from various pharmacological induction methods share some basic mechanisms but also show differences (Fisahn et al. 2004; Pálhalmi et al. 2004). Presumably because of those differences they often respond differently to neuromodulators (Fisahn et al. 2009; Pietersen et al. 2009; Wójtowicz et al. 2009) as was also the case in our study when, in a separate set of experiments, we found that stimulating H3Rs did not significantly reduce the power of carbachol-induced gamma oscillations. Again, this indicates that not all gamma oscillation protocols result in exactly the same activity. We do not yet have sufficient data to speculate about the mechanistic reasons for this and to our knowledge there are no extensive studies published yet comparing carbachol/muscarine-induced versus kainate-induced gamma oscillations. Nevertheless, our data provide one possible mechanism for how activation of the H3R affects gamma oscillations in an in vitro preparation, which could now be tested under more physiological conditions in vivo.
The physiological relevance of the H3R-mediated reduction of kainate-induced oscillations is, however, emphasized by two studies concerned with histamine modulation of theta oscillations in the hippocampus in vivo. In the first study, blocking the H3R resulted in higher power of theta oscillations (Hájos et al. 2008). This effect could be explained by local histamine action such as a direct effect of H3R on the network or by H3R acting in the capacity of an autoinhibitory receptor on histaminergic fibres, where H3R blockade increases tonic histamine release. In addition, there could also be histaminergic effects outside the hippocampal network that have an indirect effect on the hippocampus. In this scenario, the increased level of histamine would stimulate the histamine receptors (i.e. H1, H2 and H4). These receptors would then have an increased capability of sustaining or increasing the power of theta (and possibly other) oscillations. The net result would be the same for both scenarios: block of H3R, whether it exerts its effect directly or indirectly, would increase the power of theta oscillations. A second study also concerned with theta oscillations in the hippocampus in vivo demonstrated that H1R blockade produces memory deficits with a concomitant decrease in theta power (Masuoka & Kamei, 2009). These deficits were ameliorated by NMDA receptor agonists but exacerbated again with the addition of a H3R agonist. The results presented in our report do not differentiate between a direct action of H3R or an indirect action via H3R autoinhibition with subsequent reduction of histamine release and activity of the other histamine receptors. In situ hybridization shows that the H3R is strongly expressed in the ventral hippocampal complex of rats (Drutel et al. 2001; Pillot et al. 2002), but the subcellular localization in this area has not yet been determined; therefore, available histological data cannot exclude one possibility or the other.
Several cellular and synaptic mechanisms can explain a decrease in field oscillation power. Oscillatory network activity is highly dependent upon concerted and balanced excitatory and inhibitory synaptic activity, a basic level of depolarization to pyramidal neurons and synchronized action potential discharge (Fisahn et al. 1998, 2004; Robbe et al. 2006; Leao et al. 2009). Disruption of any of these parameters can result in decreased gamma oscillation power. In the present study, activation of H3R did not result in modulation of active excitatory or inhibitory synapses contacting pyramidal cells (Fig. 2A and B), nor did it lead to a change in slow cellular currents (Fig. 2C). Therefore, taken together with the observed effects on the pyramidal cell action potential firing, we conclude that the decrease in gamma oscillation power reported here is brought about by the disruption of action potential synchrony in the pyramidal neuron population with respect to the local field potential oscillation (Fig. 3). The specific mechanism underlying the H3R-induced desynchronization of action potential firing in pyramidal neurons remains to be further investigated. At least two possible modes of regulating action potential phasing can be envisioned: either a direct modulation of the pyramidal neurons themselves, or an indirect effect causing changes in other cells of the network, which then affect pyramidal neurons. It is possible that H3R agonists, acting directly on pyramidal neurons, reduce their ability to follow the network oscillation rhythm. Histamine, acting through the H2 receptor, has been shown to regulate afterhyperpolarization amplitude in CA3 pyramidal neurons, thereby controlling inter-spike intervals (Haas et al. 2008). It is conceivable that a similar mechanism may be activated by H3Rs and other G-protein-coupled receptors (GPCRs), allowing them to modulate conductances underlying the afterhyperpolarization and thereby controlling the timing of action potential generation. This mechanism of modulation has been shown for the metabotropic actions of GluR6-containing kainate receptors (Melyan et al. 2002).
Another possible mode of inhibition of rhythmic network activity was demonstrated in a study on cannabinoids (Robbe et al. 2006), which showed a correlation between reduced synchronous discharge of action potentials among CA1 pyramidal neurons and reduced power of network oscillations as a result of stimulating the cannabinoid CB1 receptor (CB1R). In addition, CB1R agonists were also shown to inhibit kainate-induced gamma oscillations in the hippocampal slice preparation (Hájos et al. 2000), possibly through presynaptic inhibition of cholecystokinin (CCK)-expressing interneurons. Similar to what has been reported for H3Rs (Takeshita et al. 1998), CB1Rs are presynaptic inhibitory heteroreceptors (Hoffman & Lupica, 2000) and inhibit N- and P/Q-type calcium channels. Our data only show a slight decrease in the mean amplitudes of IPSCs during RAMH treatment (Fig. 2B, not significant) suggesting that H3Rs do not have a strong influence on general IPSC strength. However, our experiments do not differentiate between IPSCs from the various sub-types of interneurons active during gamma oscillations. One can speculate that a subpopulation of interneurons exhibits decreased transmitter release in response to H3R blockage yet we cannot distinguish such a potential effect in our experiments since it is masked by the activity of unaffected GABAergic synapses. Such a mechanism would rely on H3R-expressing interneurons having a diminished capability to coordinate pyramidal neuron firing in phase-locked synchrony with the ongoing gamma oscillation in the presence of RAMH. H3R expression in the ventral hippocampus seems to be highly concentrated in the pyramidal cell layers of CA1 and CA3 and almost absent in other layers (Pillot et al. 2002). Therefore we will, in future studies, determine whether H3R-mediated inhibition of gamma (and theta) oscillations is caused by H3R in the role of a presynaptic inhibitory heteroreceptor or autoreceptor, or if the effect is directly brought about by conductances controlling action potential synchronization in pyramidal neurons or, possibly, another as yet unknown mechanism.
Acknowledgments
The authors wish to thank Dr. R. Leão for help with the analysis. This work was supported by the Karolinska Institute (R.A., A.F.), the Swedish Research Council (M.L., A.F.), the Wenner-Gren Foundation, the Swedish Foundation for Strategic Research (M.L.) and the European Commission Coordination Action ENINET (contract number LSHM-CT-2005-19063, A.F.).
Glossary
Abbreviations
- AD
Alzheimer's disease
- ADHD
attention deficit/hyperactivity disorder
- AP5
dl-2-amino-5-phosphonopentanoic acid
- CA
cornu ammonis
- CB1R
cannabinoid CB1 receptor
- CCK
cholecystokinin
- GPCR
G-protein coupled receptor
- H3R
histamine H3 receptor
- IR-DIC
infra-red differential interference contrast
- RAMH
R-α-methylhistamine
Author contributions
R.A.: conception and design of the experiments included in Fig. 1; collection, analysis and interpretation of all electrophysiological data; drafting and revising the manuscript; final approval of version to be published. M.L.: conception and design of the study, interpretation of data; revising and final approval of the version to be published. A.F.: conception of the study and design of experiments included in Figs 2 and 3; interpretation of data; revising and final approval of the manuscript version to be published.
References
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Dickinson R, Awaiz S, Whittington MA, Lieb WR, Franks NP. The effects of general anaesthetics on carbachol-evoked gamma oscillations in the rat hippocampus in vitro. Neuropharmacology. 2003;44:864–872. doi: 10.1016/s0028-3908(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutel G, Peitsaro N, Karlstedt K, Wieland K, Smit MJ, Timmerman H, Panula P, Leurs R. Identification of rat H3 receptor isoforms with different brain expression and signalling properties. Mol Pharmacol. 2001;59:1–8. [PubMed] [Google Scholar]
- Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci. 2004;24:9658–9668. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hájos M, Siok CJ, Hoffmann WE, Li S, Kocsis B. Modulation of hippocampal theta oscillation by histamine H3 receptors. J Pharmacol Exp Ther. 2008;324:391–398. doi: 10.1124/jpet.107.130070. [DOI] [PubMed] [Google Scholar]
- Hájos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux J-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Leao R, Tan HM, Fisahn A. Kv7/KCNQ channels control action potential phasing of pyramidal neurons during hippocampal gamma oscillations in vitro. J Neurosci. 2009;29:13353–13364. doi: 10.1523/JNEUROSCI.1463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka T, Kamei C. The ameliorating effects of NMDA receptor agonists on histamine H1 antagonist-induced memory and hippocampal theta disruptions are prevented by the H3 receptor agonist in rats. Brain Res Bull. 2009;79:422–425. doi: 10.1016/j.brainresbull.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Atkins AR, Beresford IJ, Brackenborough K, Briggs MA, Calver AR, et al. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer's disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther. 2007;321:1032–1045. doi: 10.1124/jpet.107.120311. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron. 2002;34:107–114. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- Pálhalmi J, Paulsen O, Freund TF, Hájos N. Distinct properties of carbachol- and DHPG-induced network oscillations in hippocampal slices. Neuropharmacology. 2004;47:381–389. doi: 10.1016/j.neuropharm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Passani MB, Bacciottini L, Mannaioni PF, Blandina P. Central histaminergic system and cognition. Neurosci Biobehav Rev. 2000;24:107–113. doi: 10.1016/s0149-7634(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Passani MB, Lin J-S, Hancock A, Crochet S, Blandina P. The histamine H3 receptor as a novel therapeutic target for cognitive and sleep disorders. Trends Pharmacol Sci. 2004;25:618–625. doi: 10.1016/j.tips.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pietersen AN, Patel N, Jefferys JG, Vreugdenhil M. Comparison between spontaneous and kainate-induced gamma oscillations in the mouse hippocampus in vitro. Eur J Neurosci. 2009;29:2145–2156. doi: 10.1111/j.1460-9568.2009.06771.x. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz J-C, Arrang J-M. A detailed mapping of the histamine H3 receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Prell GD, Green JP, Kaufmann CA, Khandelwal JK, Morrishow AM, Kirch DG, Linnoila M, Wyatt RJ. Histamine metabolites in cerebrospinal fluid of patients with chronic schizophrenia: their relationships to levels of other aminergic transmitters and ratings of symptoms. Schizophrenia Res. 1995;14:93–104. doi: 10.1016/0920-9964(94)00034-6. [DOI] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Sander K, Kottke T, Stark H. Histamine H3 receptor antagonists go to clinics. Biol Pharm Bull. 2008;31:2163–2181. doi: 10.1248/bpb.31.2163. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita Y, Watanabe T, Sakata T, Munakata M, Ishibashi H, Akaike N. Histamine modulates high-voltage-activated calcium channels in neurons dissociated from the rat tuberomammillary nucleus. Neuroscience. 1998;87:797–805. doi: 10.1016/s0306-4522(98)00152-3. [DOI] [PubMed] [Google Scholar]
- Wójtowicz AM, Boom Lvd, Chakrabarty A, Maggio N, Haq Ru, Behrens CJ, Heinemann U. Monoamines block kainate- and carbachol-induced gamma-oscillations but augment stimulus-induced gamma-oscillations in rat hippocampus in vitro. Hippocampus. 2009;19:273–288. doi: 10.1002/hipo.20508. [DOI] [PubMed] [Google Scholar]