Abstract
The activation characteristics of synaptic and extrasynaptic GABAA receptors are important for shaping the profile of phasic and tonic inhibition in the central nervous system, which will critically impact on the activity of neuronal networks. Here, we study in isolation the activity of three agonists, GABA, muscimol and 4,5,6,7-tetrahydoisoxazolo[5,4-c]pyridin-3(2H)-one (THIP), to further understand the activation profiles of α1β3γ2, α4β3γ2 and α4β3δ receptors that typify synaptic- and extrasynaptic-type receptors expressed in the hippocampus and thalamus. The agonists display an order of potency that is invariant between the three receptors, which is reliant mostly on the agonist dissociation constant. At δ subunit-containing extrasynaptic-type GABAA receptors, both THIP and muscimol additionally exhibited, to different degrees, superagonist behaviour. By comparing whole-cell and single channel currents induced by the agonists, we provide a molecular explanation for their different activation profiles. For THIP at high concentrations, the unusual superagonist behaviour on α4β3δ receptors is a consequence of its ability to increase the duration of longer channel openings and their frequency, resulting in longer burst durations. By contrast, for muscimol, moderate superagonist behaviour was caused by reduced desensitisation of the extrasynaptic-type receptors. The ability to specifically increase the efficacy of receptor activation, by selected exogenous agonists over that obtained with the natural transmitter, may prove to be of therapeutic benefit under circumstances when synaptic inhibition is compromised or dysfunctional.
Introduction
Synaptic and extrasynaptic GABAA receptors play important roles in controlling neuronal excitability under physiological and pathophysiological conditions. These receptors are considered to be pentamers assembled from at least two α and two β subunits that additionally include either a single γ2 or δ subunit (Luscher & Keller, 2004). Although expression patterns for individual GABAA receptor subunits vary across the central nervous system, γ2 subunit-containing receptors are predominantly located at synaptic and extrasynaptic sites, whilst δ subunit receptors are thought to populate only extrasynaptic sites (Fritschy & Brunig, 2003). Together with other less common populations of GABAA receptors (Sieghart & Sperk, 2002), the different subunit compositions of GABAA receptors shape their biophysical and pharmacological properties and influence the efficacies of synaptic and tonic inhibition.
From neuronal and heterologous expression studies, it is clear that synaptic-type GABAA receptors share several features that include the ability to rapidly respond to a brief GABA concentration transient followed by a prolonged phase of deactivation. By contrast, extrasynaptic-type receptors are usually exposed to low but persistent GABA concentrations. They are also noted for their increased sensitivity to GABA, a reduced propensity to desensitise, and a more rapid deactivation phase following removal of GABA compared to their synaptic counterparts (Gingrich et al. 1995; Banks & Pearce, 2000; Fischer et al. 2000; Yeung et al. 2003; Picton & Fisher, 2007; Lagrange et al. 2007).
Although synaptic and extrasynaptic receptors are differentially sensitive to some allosteric modulators (e.g. benzodiazepines and Zn2+) their activation properties remain less well understood, particularly when exposed to different receptor agonists (Mortensen et al. 2004). Some agonists have unique activation profiles and certainly their chemical structures will affect the binding affinity and efficacy when initiating GABAA receptor activation. Of course, the receptor subunit composition will also affect ligand affinity and efficacy.
GABA is often considered a potent full agonist at GABAA receptors, but there are extrasynaptic isoforms, particularly associated with δ subunit-containing receptors, e.g. α4β3δ (Adkins et al. 2001; Brown et al. 2002; You & Dunn, 2007), where it is a partial agonist by comparison with the high-efficacy behaviour of 4,5,6,7-tetrahydoisoxazolo[5,4-c]pyridin-3(2H)-one (THIP) (Krogsgaard-Larsen et al. 1994; Krogsgaard-Larsen et al. 2002). Interestingly, this high efficacy profile for THIP contrasts with its partial agonist activity on most other αβγ-type GABAA receptors (Ebert et al. 1994; Mortensen et al. 2004).
The fundamental reasons why synaptic-type receptors such as α1β3γ2 differ in their activation profile compared to extrasynaptic receptors such as α4β3δ, is poorly understood. In the present study, we have used GABA and two structurally related but conformationally constrained agonists, muscimol and THIP, to investigate the activation profiles of both synaptic and extrasynaptic receptors as well as that of the α4β3γ2 receptor, which represents a convenient intermediary. We selected α1 and α4 subunit-containing receptors as these are widely expressed in the hippocampus and thalamus (Wisden et al. 1992; Sur et al. 1999; Sieghart & Sperk, 2002), which are areas where synaptic and tonic inhibition could profoundly affect behaviour.
Methods
Stable cell lines
Mouse L-tk fibroblast cell lines providing stable expression of human α1β3γ2, α4β3γ2 and α4β3δ GABAA receptors were used. Expression of α, β and γ subunits was controlled by a dexamethasone inducible promoter (Hadingham et al. 1992; Sur et al. 1999; Brown et al. 2002), whilst δ subunits were expressed constitutively from a human CMV promoter (pcDNA3.1Zeo).
The L-tk cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with: 4.5 mg ml−1 glucose, 4 mm l-glutamine, 0.11 mg ml−1 sodium pyruvate, 10% fetal calf serum (FCS), and 1 mg ml−1 geneticin at 37°C in 95% air–5% CO2. The growth medium for α4β3δ expressing cells also contained 0.2 mg ml−1 of zeocin for cell selection. Prior to electrophysiology, cells were washed once in Hanks’ buffered salt solution, suspended with 2 ml 0.05% trypsin-EDTA, quenched with 10 ml medium, centrifuged for 3 min at 1000 g, triturated in 1 ml fresh medium with a polished glass pipette, and finally seeded at a suitable density onto poly d-lysine coated glass coverslips. The expression of the GABAA receptors was induced overnight in supplemented DMEM plus either 0.1 μm dexamethasone for α1β3γ2 and α4β3γ2 or 0.5 μm dexamethasone for α4β3δ. Electrophysiological recordings were performed within 48 h of inducing receptor expression.
Transient receptor expression in HEK cells
Human embryonic kidney 293 (HEK293) cells were maintained in DMEM supplemented with: 4.5 mg ml−1 glucose, 4 mm l-glutamine, 0.11 mg ml−1 sodium pyruvate, 10% FCS, 100 units ml−1 penicillin-G and 100 μg ml−1 streptomycin at 37°C in 95% air–5% CO2. Cells at ∼70% confluence were passaged at 1:10 usually every 2–4 days. Cells were plated onto poly d-lysine coated glass coverslips, and subsequently transfected using a calcium phosphate method for cDNAs encoding murine α1, α4, β3, γ2 and δ subunits and the reporter enhanced green fluorescent protein (eGFP). For a1β3γ2/eGFP and α4β3γ2/eGFP each dish was transfected with 4 μg of cDNA in a 1:1:1:1 ratio, whereas 7 μg of cDNA was used for α4β3δ/eGFP in a ratio of 1:1:4:1. Cells were incubated overnight and used for patch clamp recording the following day.
Electrophysiology
Agonist-activated whole-cell and single channel currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Cells were voltage clamped at −60 mV, whereas for single channels, outside-out patches were held at a holding potential of −70 mV. Whole-cell currents were filtered at 5 kHz and single channel currents at 3 kHz (−3 dB, 8 pole Bessel, 48 dB/octave). For whole-cell currents, the series resistance was 20 ± 10 MΩ and compensation was routinely applied up to approximately 75%. All currents were digitized at 50 kHz via a Digidata 1320A (Molecular Devices) and recorded straight to disk (Dell Pentium Dual Core – Optiplex GX745). Patch pipettes with a resistance of 3–5 MΩ for whole-cell and 10–15 MΩ for single channels were filled with an intracellular solution containing (mm): 120 CsCl, 1 MgCl2, 11 EGTA, 33 TEA-OH, 10 Hepes, 1 CaCl2 and 2 adenosine triphosphate; pH 7.10 (adjusted with 1 m HCl). Cells were continuously perfused with Krebs solution containing (mm): 140 NaCl, 4.7 KCl, 1.2 MgCl2, 2.52 CaCl2, 11 glucose and 5 Hepes; pH 7.4 (adjusted with 1 m NaOH).
Agonists were applied to cells using a U-tube application system (Mortensen & Smart, 2007). Drugs were dissolved in the extracellular Krebs solution followed by pH correction. Only THIP changed the pH of the Krebs solution at the highest concentrations (10 mm; unadjusted ∼ pH 4.0). For those experiments that addressed the issue of pH on the single channel conductance, 10 mm GABA was also adjusted to pH 4.0 (with 1 m HCl).
Analysis of whole-cell currents
GABA, muscimol and THIP equilibrium concentration–response relationships were constructed for each receptor isoform by measuring agonist currents, which were normalized to the response induced by a maximal, saturating concentration of GABA (Imax) and subsequently fitted with the Hill equation:
| (1) |
where EC50 represents the concentration of the agonist ([A]) inducing 50% of the maximal current evoked by a saturating concentration of the agonist and n represents the Hill coefficient.
In order to obtain preliminary estimates of affinity, efficacy and desensitization, whole-cell concentration–response curves were also fitted to a function based on the receptor mechanism (Mortensen et al. 2004) shown in Scheme 1.
 |
where A represents an agonist molecule and R the GABAA receptor, with rate constants for binding (k1) and unbinding (k−1), and rate constants for channel opening (β) and closure (α). To reduce the number of variables in the fitting process, the binding rate constants were constrained to be identical for mono- and biliganded binding. This condition also applied to the unbinding rates. The channel gating rates (β, α) for mono- and biliganded receptors were also similarly constrained. The activated conducting forms of the receptor are represented by AR* and A2R*. A desensitised state of the receptor (D) has entry and exit rate constants denoted by δ1 and δ−1, respectively. The AR* state was considered rare and discounted from the fitting. Under these conditions, the general function derived for this mechanism, is:
| (2) |
where n signifies the number of ligand binding sites. As [A]→∞, Popen→Popen,max, which is given by E/(1 +E+D). The dissociation constant (K) is defined as k−1/k1; efficacy (E) is defined as β/α; and D is defined as δ1/δ−1. A non-linear least squares routine was used to fit the concentration–response curves (Mortensen et al. 2004).
Single channel current analysis
Single channel currents were recorded at −70 mV on the condition that there appeared to be only one active channel, or the number of simultaneous channel openings never exceeded 2% of all detected openings. SCAN, EKDIST (http://www.ucl.ac.uk/Pharmacology/dcpr95.html) and QuB (http://www.qub.buffalo.edu) software were used to analyse the single channel currents. The single channel current amplitude histograms were fitted with the sum of Gaussian components to define the single channel conductance states as described previously (Mortensen et al. 2004). Dwell time frequency distributions were constructed from the channel open and closed durations and analysed by fitting mixtures of exponential densities using the EKDIST program after imposing a minimum time resolution of 100 μs. The burst length analyses required a critical shut time (τcrit; usually between τC2 (denoting closures within a burst) and τC3 (closures outside bursts)), which was defined as described previously (Colquhoun & Sakmann, 1985; Mortensen et al. 2004; Mortensen & Smart, 2007).
Kinetic modelling of single channel currents
An extensive search for an optimal receptor model that could account for our experimental single channel data resulted in 30 different models being analysed using the HJCFIT program (Hatton et al. 2003) for GABA-activated channels on α1β2γ2 receptors (data not shown). Some of these models included extra desensitised states branching from the mono- and biliganded receptor states, as well as the inclusion of preactivation flip states which are closed agonist-bound states prior to channel activation (Supplemental Fig. 1F and G) and are capable of accounting for some GABA receptor single channel kinetic behaviour (Keramidas & Harrison, 2009). Despite these increasing levels of branching complexity, we found that none of the above models achieved a better description of the single channel data than our previously published six-state model (2 open, 4 closed; see above). Therefore, this model was adopted for the present study (Mortensen et al. 2004).
Clusters of channel openings for each isoform of the GABAA receptor, α1β3γ2, α4β3γ2 or α4β3δ, were idealised using the segmental-k-means method (SKM) in QuB (Qin et al. 1996; Qin, 2004). Multiple files of idealised single channel data from each of the three concentrations of the same agonist were used in QuB to determine the rate constants for the model that could describe the dwell time distributions. Using maximum interval likelihood rate estimation (MIL), which includes a correction for missed events, the determined optimal rate constants for transitions between states were robust, even when quite different starting estimates were used. We also used maximum likelihood fitting of our receptor mechanism to the single channel data, which included an exact correction for missed events, with the program HJCFIT (Hatton et al. 2003). For the single channel experiments, the binding and unbinding rate constants (k1, k−1, k2 and k−2), and the opening and shutting rate constants (β1, β2, α1 and α2) were no longer constrained in the receptor model (see below). The dwell time distributions and the fit of multiple exponential densities were exported to Origin v. 6 (OriginLab Corp., Northampton, MA, USA) to create the 3D dwell-time distributions (Scheme 2).
 |
Simulated whole-cell concentration response curves
The optimal single channel rate constants determined from fitting the single channel data with the receptor model using QuB were transferred to ChanneLab v. 2 (Synaptosoft, Decatur, GA, USA), where the linear/branched receptor model was recreated. Whole-cell responses were simulated by using a number of channels, set at 1000, a holding current of −60 mV, and 4 s agonist applications, to reproduce our original whole-cell experiments. The peak of the simulated whole-cell currents was measured (pA) for different agonist concentrations. These values were used to generate the theoretical agonist concentration–response curves using Origin 6.
Results
To understand the biophysical behaviour of different GABA agonists at synaptic and extrasynaptic GABAA receptors, we chose to compare the activity of GABA with muscimol and THIP. GABA was selected since it is the natural transmitter and would act as a ‘control’; muscimol was selected because of its greater potency at most GABAA receptors; and THIP was selected due to its superagonist activity at δ subunit-containing receptors, which are associated with extrasynaptic GABAA receptors. Although the structures of these three agonists appear quite similar, they display different degrees of conformational flexibility from the highly flexible GABA, to the constrained isoxazole ring structures of muscimol and also THIP, which is further constrained by a pyridine ring (Fig. 1A) (Krogsgaard-Larsen et al. 1977).
Figure 1. Activation of recombinant synaptic and extrasynaptic GABAA receptors by three agonists.
A, structures of the selective GABAA receptor agonists: GABA (square), muscimol (triangle) and THIP (circle). The agonists are labelled G (GABA), M (muscimol) and T (THIP) in this and subsequent figures. B, agonist concentration–response relationships for GABA, muscimol and THIP on recombinant α1β3γ2 (a), α4β3γ2 (b) and α4β3δ (c) receptors expressed in L-tk cells (n= 5–7; mean ±s.e.m.). C, whole cell currents induced by saturating concentrations of GABA, muscimol and THIP on recombinant α1β3γ2 (a), α4β3γ2 (b) and α4β3δ (c).
Agonist potencies and relative efficacies from whole-cell currents
We investigated the activity of the three agonists at synaptic- and extrasynaptic-type, α1β3γ2, α4β3γ2 and α4β3δ receptors, expressed in L-tk cells. Agonist concentration–response curves were constructed for GABA-, muscimol- and THIP-activated currents recorded under whole-cell voltage clamp conditions (VH−60 mV; Fig. 1B). The agonist-induced maximum currents were larger for α1β3γ2 and α4β3γ2 receptors than for α4β3δ GABAA receptors (α1β3γ2,Imax,GABA: 1742 ± 303 pA; α4β3γ2,Imax,GABA: 1767 ± 253 pA; α4β3δ,Imax,THIP: 851 ± 128 pA, n= 5–7; P < 0.05). Receptor desensitization was observed with high agonist concentrations, which was most evident for γ2 subunit-containing receptors, but also noticeable for the α4β3δ isoform (Fig. 1C).
For both the α1β3γ2 and α4β3γ2 receptors, muscimol behaved as a full agonist, like GABA, with comparable maximal currents at saturating concentrations (101 ± 1.7% and 97 ± 3.7% of Imax,GABA, respectively). However, by comparing EC50 values, muscimol was more potent by approximately 3-fold (α1β3γ2: muscimol EC50= 0.92 ± 0.65 μm, GABA 3.4 ± 0.75 μm; P= 0.037; α4β3γ2: muscimol 0.69 ± 0.25 μm, GABA 2.1 ± 0.46 μm; P= 0.032, n= 5–7, Fig. 1B; Table 1). By contrast, THIP behaved as a partial agonist on both α1β3γ2 and α4β3γ2 receptors (85 ± 2.7% and 83 ± 4.7% of Imax,GABA, respectively), and was also significantly less potent than either GABA or muscimol (α1β3γ2: EC50= 107 ± 31 μm, P= 0.002; α4β3γ2: 69 ± 1.2 μm, P= 0.0001; ANOVA, n= 6).
Table 1.
Whole-cell current parameters for GABA, muscimol and THIP on α1β3γ2, α4β3γ2 and α4β3δ GABAA receptors
| Isoform | Agonist | Emax (%) | EC50 (μm) | nH | EClow,mid,high (μm) |
|---|---|---|---|---|---|
| α1β3γ2 | GABA | 100 ± 0.7 | 3.4 ± 1.0 | 1.4 ± 0.16 | 0.3, 3, 30 |
| Muscimol | 101 ± 1.7 | 0.92 ± 0.34 | 1.2 ± 0.17 | 0.03, 0.3, 3 | |
| THIP | 85 ± 2.7 | 107 ± 31 | 1.2 ± 0.07 | 3, 30, 300 | |
| α4β3γ2 | GABA | 98 ± 0.65 | 2.1 ± 0.56 | 1.5 ± 0.16 | 0.3, 3, 30 |
| Muscimol | 97 ± 3.7 | 0.69 ± 0.25 | 1.46 ± 0.22 | 0.03, 0.3, 3 | |
| THIP | 83 ± 4.7 | 69 ± 1.2 | 1.3 ± 0.08 | 3, 30, 300 | |
| α4β3δ | GABA | 98 ± 0.67 | 0.35 ± 0.03 | 1.1 ± 0.05 | 0.03, 0.3, 3 |
| Muscimol | 120 ± 5.1 | 0.20 ± 0.04 | 0.97 ± 0.15 | 0.03, 0.3, 3 | |
| THIP | 224 ± 9.3 | 13 ± 3.5 | 0.88 ± 0.05 | 3, 30, 300 |
The data were obtained from 5–7 experiments on L-tk cells stably expressing α1β3γ2, α4β3γ2 or α4β3δ GABAA receptors. The Hill equation was fitted to each individual data set and the mean parameter values are shown in the table as means ±s.e.m.Emax is the % maximum agonist-induced current. EC50 represents the agonist potency and nH, the Hill slope. The agonist concentrations selected for the single channel experiments were in the range of EC of ∼15 (EClow), ∼50 (ECmid), and ∼90 (EChigh).
For α4β3δ GABAA receptors, the increased maximum response to muscimol compared to GABA (120 ± 5.1%; P= 0.006, n= 6) implied a degree of superagonist activity. For THIP, this type of behaviour was clearly evident (224 ± 9.3% of Imax,GABA, P= 0.0001, n= 6). In comparison with the synaptic-type α1β3γ2 receptors, GABA, muscimol and THIP were more potent in activating the α4β3δ receptor (EC50= 0.35 ± 0.03 μm (P= 0.002), 0.20 ± 0.04 μm (P= 0.03) and 13 ± 3.5 μm (P= 0.01), respectively, n= 6–7; Table 1).
Next, all of the agonist concentration–response relationships were curve fitted using our linear/branched receptor model (see Methods; Mortensen et al. 2004) to obtain estimates of the agonist dissociation constant (K), its efficacy (E), and desensitisation (D), without discerning between monoliganded and biliganded receptor states so as to reduce the number of variables in the fitting routine (Table 2). These fits predicted that K is higher for THIP compared to GABA and muscimol at all three receptors; that the efficacy of THIP, which is low at γ subunit-containing receptors, is highest at the α4β3δ receptors; and finally, the extent of desensitisation of α4β3δ receptors is greater with THIP compared to either GABA or muscimol. Of course, we cannot guarantee accurate values for K, D and E by just fitting agonist concentration response relationships. In addition, it should be noted that the state function (eqn (2)) used to fit the concentration–response curve data, is most appropriate for receptors at equilibrium and this may not be the case for peak currents evoked by each of the three agonists, particularly at the α1β3γ2 receptor using a U-tube with a solution exchange time of 27 ± 6 ms (n= 5). Thus the determinants of K, D and E from dose–response curve data are only to be considered as estimates.
Table 2.
Efficacies, dissociation and desensitization constants and slope factors obtained for GABA, muscimol and THIP on α1β3γ2, α4β3γ2 and α4β3δ GABAA receptors
| Isoform | Agonist | E | D | K (μm) | n |
|---|---|---|---|---|---|
| α1β3γ2 | GABA | 8.3 ± 0.12 | 5.6 ± 0.07 | 16 ± 2 | 1.38 ± 0.06 |
| Muscimol | 8.8 ± 0.24 | 5.6 ± 0.14 | 10 ± 2 | 1.08 ± 0.08 | |
| THIP | 3.2 ± 0.32 | 2.6 ± 0.38 | 400 ± 8 | 1.18 ± 0.04 | |
| α4β3γ2 | GABA | 3.3 ± 0.05 | 1.7 ± 0.04 | 3.9 ± 0.6 | 1.60 ± 0.09 |
| Muscimol | 2.7 ± 0.07 | 1.2 ± 0.05 | 1.2 ± 0.3 | 1.57 ± 0.20 | |
| THIP | 3.2 ± 0.08 | 2.5 ± 0.09 | 250 ± 40 | 1.37 ± 0.09 | |
| α4β3δ | GABA | 1.8 ± 0.03 | 0.50 ± 0.02 | 0.84 ± 0.11 | 1.17 ± 0.07 |
| Muscimol | 1.9 ± 0.04 | 0.50 ± 0.03 | 0.44 ± 0.09 | 1.00 ± 0.09 | |
| THIP | 6.0 ± 0.24 | 4.0 ± 0.19 | 121 ± 14 | 0.99 ± 0.03 |
The agonist concentration–response data in Fig. 1B was fitted using our linear/branched receptor model (eqn (2) in Methods; Mortensen et al. 2004). A non-linear least squares fitting routine was used to obtain the estimates of E (efficacy), K (dissociation constant), D (desensitisation constant) and n (slope factor). Values are means ±s.e.m. from 5–7 experiments.
Therefore, to investigate further, we used the agonist concentration response curves to select three concentrations for each agonist, defined as low, mid and high (EClow (∼15% of max), ECmid (∼50%), EChigh (∼90%)), for single channel experiments (Table 1). This range enabled the kinetics of single GABA channels to be investigated at concentrations that resulted in superagonist activity as well as submaximal responses, enabling more precise determinations of K, D and E.
Single GABA channel conductances are unaffected by the agonist
Outside-out patches were pulled from L-tk cells and single channel activity induced by GABA, muscimol and THIP at −70 mV was recorded at three concentrations under equilibrium conditions from each receptor isoform. The recordings revealed no differences in the single channel current amplitudes between the agonists (Fig. 2A–C). Three different conductance levels were usually identified from amplitude histograms constructed for currents activated by GABA, THIP and muscimol. For all three receptor isoforms, the channel conductances equated to: 11–14 pS, 17–21 pS, and 26–31 pS (Fig. 2D and E). These conductance states did not vary with the receptor isoform, the activating agonist, or its concentration (Fig. 2E). By examining the frequencies for all the channel conductances, it was clear that openings to the highest conductance state dominated all the recordings (60–90%; Fig. 2F).
Figure 2. Single channels have three identical conductance levels for synaptic- and extrasynaptic-type GABAA receptors A–C, single channel currents induced by GABA.
(A), muscimol (B) and THIP (C) on recombinant α1β3γ2 (a), α4β3γ2 (b) and α4β3δ (c) receptors. D, typical current amplitude histogram (α1β3γ2, 3 μm GABA) showing curve fits with three Gaussians each (dotted lines) and their sum (continuous line). E, three conductance levels (high, 26–31 pS (squares); intermediate, 17–22 pS (circles); low, 11–14 pS (triangles)) were resolved for all three agonists at three concentrations (low EC5-20 (white); intermediate EC40-60 (grey); and high EC80-100 (black)) (n= 5–7; mean ±s.e.m.). See Table 1 for agonist concentrations. F, relative areas of the single channel conductances for all three agonists at each receptor isoform, at three concentrations.
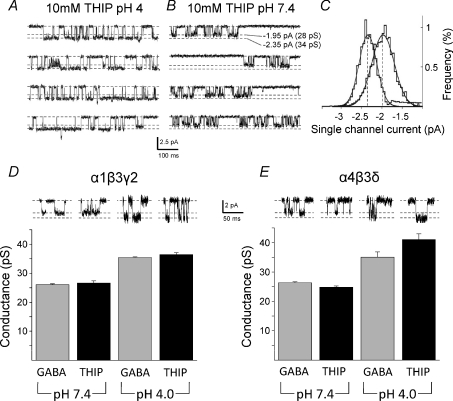
Low pH can modulate agonist-activated channels
The superagonist behaviour of THIP at only αβδ extrasynaptic-type receptors makes this a useful ligand for distinguishing δ from γ2 subunit-containing receptors. By using β2 subunit-containing receptors, part of THIP's superagonist effect was thought to be due to an increase in the single channel conductance from 25 to 36 pS at both α1β2γ2 and α1β2δ receptors (Keramidas & Harrison, 2008). Such an increase was not observed in our study of receptors containing β3 subunits. However, aqueous solutions of THIP (HCl salt) are very acidic, particularly at high concentrations (10 mm), which reduced the pH of the Krebs solution from 7.4 to ∼4.0 (Matsuda et al. 1996). During a previous study (Mortensen et al. 2004), using α1β2γ2 receptors, we noted that if THIP was applied to outside-out patches without pH correction to the normal physiological range, changes to the channel conductance were apparent.
To reaffirm, we applied without pH correction a saturating concentration of THIP (10 mm) to HEK cell outside-out patches containing either α1β3γ2 (Fig. 3A–D) or α4β3δ (Fig. 3E) murine receptors. The maximum single channel current was increased from 27 ± 0.7 pS and 25 ± 0.4 pS at pH 7.4 (n= 6–7) to 37 ± 0.7 pS and 41 ± 1.9 pS at pH 4, respectively (n= 6–9; Fig. 3D and E). These increments to 137% (α1β3γ2) and 164% (α4β3δ) in the most frequently occurring single channel current state, were not specific to THIP since GABA similarly increased channel current after lowering external pH from 7.4 to 4 (Fig. 3D and E). Moreover, whilst recording from the same patches expressing α1β3γ2 or α4β3δ receptors, readjusting the external pH from 4 to 7.4 or 7.4 to 4, caused the main conductance state activated by 10 mm THIP or 10 mm GABA to be either reduced or increased (Fig. 3A–C).
Figure 3. Low external pH affects the single channel conductance.
A and B, single channel currents induced by high concentrations of THIP at recombinant synaptic-type α1β3γ2 receptors exposed to pH 4 or 7.4 external solution. C, displacement of the single channel current amplitude histogram by pH 4 (left) from pH 7.4 (right). D and E, single channel main state conductances for synaptic-type α1β3γ2 and extrasynaptic-type α4β3δ GABAA receptors for both GABA (10 mm) and THIP (10 mm) at pH 4.0 and 7.4. Sample currents are shown in the insets.
THIP modulates channel open probability and mean open times
Another parameter that will affect the function of synaptic and extrasynaptic-type receptors is the open probability (PO). The number of channels in a patch will significantly affect PO, and although we cannot be sure of channel numbers, the occurrence of ‘channel current stacking’ was quite infrequent. Nevertheless, to avoid this common problem, we measured PO by only analysing clusters of channel activity. These cluster PO values increased with the agonist concentration as would be expected.
Interestingly, α4 subunit-containing receptors exhibited a smaller range for PO compared with that for α1β3γ2 receptors, indicating that α4β3γ2 receptors are likely to be less efficient at charge transfer (Fig. 4). For α1β3γ2 receptors activated by mid and high THIP concentrations, there was a tendency towards lower PO values compared with either GABA or muscimol. However, at the highest concentration of THIP, α4β3δ receptors had a tendency towards a higher mean cluster PO, which would contribute towards the superagonist activity of THIP at high concentrations (Fig. 4).
Figure 4. Channel open probability is affected by the receptor isoform and agonist concentration.
Open probability was measured by analysing clusters of channel activity induced by the three concentrations (see Table 1) of GABA (G), muscimol (M) and THIP (T). Data are means ±s.e.m. n= 5–7.
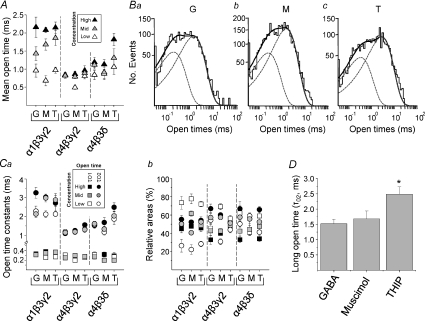
Next, we assessed the mean open times for channels activated by all three agonists at each receptor isoform. Overall, the mean open time increased with the agonist concentration. This was most pronounced for α1β3γ2 receptors compared to α4 subunit-containing receptors (Fig. 5A). Furthermore, the longest mean open times, at the highest agonist concentrations, were also consistently longer for α1β3γ2 compared with α4β3γ2 receptors. For the extrasynaptic-type α4β3δ receptors, the highest concentration of the superagonist THIP resulted in significantly longer mean open times (P < 0.05) compared with either GABA or muscimol (Fig. 5A).
Figure 5. Open times vary with the receptor isoform and agonist concentration.
A, mean open times for GABA (G), muscimol (M) and THIP (T) at three concentrations for α1β3γ2, α4β3γ2 or α4β3δ receptors. B, open time distributions for GABA (3 μm; a), muscimol (3 μm; b) and THIP (300 μm; c) on α4β3δ receptors, each showing two exponentials (dotted lines) and their sum (continuous line). C, open time constants (a; short τO1, squares; and long τO2, circles) and their relative areas (b) determined from exponential fits to the open time constant distributions. D, bar graph of the long open times (τO2) for THIP compared with GABA and muscimol on α4β3δ receptors (*P= 0.0136; ANOVA, n= 6–7).
As the mean open time is a weighted value of discrete open time constants, we analysed the underlying individual open time distributions. These were optimally fitted by two exponentials (based on an F test and visual evaluation of the fits), indicating that two populations of open time durations were present for all three receptors activated by all concentrations of the different agonists (Fig. 5Ba–c). The open time constant (τO1) describing the shortest open times was invariant at 0.2–0.4 ms, irrespective of the receptor isoform, the type of agonist, or its concentration. By contrast, the longer open time constant (τO2) was variable being longest for channel openings of α1β3γ2 receptors compared to the shorter values for α4β3γ2 receptors (Fig. 5Ca). With regard to the extrasynaptic-type α4β3δ receptors and the superagonist activity of THIP, it was notable that at the highest concentrations, THIP resulted in a significantly greater τO2 compared with that for either GABA or muscimol (P < 0.05; Fig. 5Ca and D).
By considering the relative frequencies of channel openings, events that can be assigned to the short open time population dominated (70–80%) over those of the longer open time population (20–30%) at low concentrations of all three agonists on α1β3γ2 receptors (Fig. 5Cb). However, at higher agonist concentrations, the frequencies of the two populations were similar. For the α4 subunit-containing receptors, the relative frequencies exhibited a narrower range. The long open time population tended to dominate (∼65%) at the highest concentrations of GABA and THIP on α4β3δ receptors (Fig. 5Cb).
Channel burst activity differs at α1 and α4 subunit-containing receptors
The closed times between openings are generally complex with the briefest closures appearing within bursts, and longer closures reflecting either sojourns of the receptor in unbound states (usually in the presence of lower agonist concentrations) or in desensitized states (common with higher agonist concentrations). By analysing the distribution of all closed states for each receptor, four populations of closed times were resolved at lower agonist concentrations, increasing to five at higher agonist concentrations, especially if very long desensitised periods were resolved (as for α1β3γ2 receptors; data not shown).
The two shortest closed time constants (τC1 and τC2) were unaffected by the agonist concentration, which is an indication that such closures most likely occur within bursts of channel activity (intraburst closures; Fig. 6A). The mean values for τC1 were noticeably greater for α4β3γ2 receptors, whereas τC2 was fractionally greater on α4β3δ receptors when compared with closed time constants for the other receptor isoforms (Fig. 6A). The longer closed times between bursts were not considered further as they are confounded by the uncertainty over the number of active channels present in the patch.
Figure 6. Intraburst closed time relationships for synaptic- and extrasynaptic-type GABAA receptors.
A, closed time constants determined from distributions of short closed times (τC1 (squares) and τC2 (circles)) representing closed times within bursts of channel activity induced by GABA (G), muscimol (M) and THIP (T) at low, mid and high concentrations. B, relative areas for the intraburst closed times, τC1 and τC2. n= 5–7 experiments.
By examining the closed time frequencies for the brief intraburst closures with high concentrations of GABA and muscimol on α1β3γ2 receptors, the most frequent closures (∼70%) were defined by the population described by τC1 (Fig. 6B). This reflected the apparent propensity of α1β3γ2 receptors to enter into long bursts of briefly spaced openings induced by the high efficacy agonists. The frequency of short closures was far less evident for the extrasynaptic-type α4β3δ receptors. This partly explained their shorter burst behaviour (Fig. 6B).
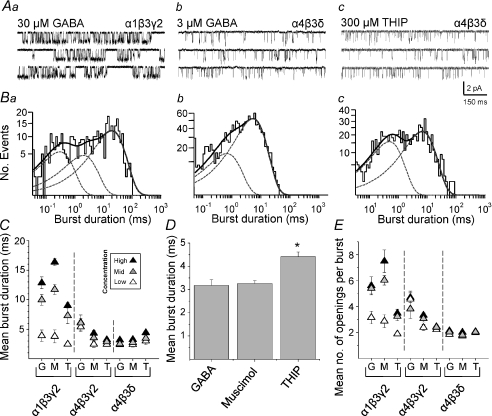
THIP critically increases burst durations of α4β3δ receptors
The steady-state activation of each receptor by GABA, muscimol and THIP produced clusters of channel openings with distinct features (Fig. 2A–C; 7A). For α1β3γ2 receptors, agonist activation produced long clusters of openings characterised by successive discrete bursts of channel activity (Fig. 7Aa). Such lengthy clusters were rarely observed following activation of either α4β3γ2 or α4β3δ receptors by GABA, muscimol or THIP (Fig. 2A–C; 7Ab and c) preventing a satisfactory cluster analysis. We therefore analysed burst durations having first defined bursts of activity using a critical closed time (τcrit) determined between τC2 and τC3. As closed times shorter than τcrit (τC1 and τC2) were unaffected by agonist concentration, these were assumed to represent intraburst closures, whereas the agonist concentration-dependent closed times longer than τcrit (τC3 to τC5) probably reflected closures between bursts.
Figure 7. Channel burst analysis for synaptic- and extrasynaptic-type GABAA receptors.
A, sample single channel recordings of bursts and single open events induced by GABA (30 μm) on α1β3γ2 (a), GABA (3 μm) on α4β3δ (b), and THIP (300 μm) on α4β3δ (c). B, burst duration distributions corresponding to the receptor isoforms shown in (A). Individual (dotted lines) and summed exponential fits (continuous line) are shown. C, mean burst durations for GABA (G), muscimol (M) and THIP (T) for three concentrations at each receptor isoform. D, bar graph of the mean burst durations for α4β3δ GABAA receptors with the highest concentrations of GABA, muscimol and THIP (*P= 0.0005, ANOVA, n= 6–7). E, mean number of openings per burst for three concentrations of GABA, muscimol and THIP on each receptor isoform. n= 5–7.
Analysis of mean burst durations showed that these increased with the concentration of the agonists as would be expected. Higher concentrations of GABA and especially muscimol resulted in longer bursts compared to those induced by THIP on α1β3γ2 receptors (Fig. 7C). However, mean burst durations for α4 subunit-containing receptors exhibited a much narrower range and were generally shorter than for α1β3γ2 receptors (Fig. 7C). For the α4β3γ2 receptors, GABA induced longer bursts compared to the other agonists, whilst for α4β3δ receptors, the highest concentration of THIP induced significantly longer bursts compared to either GABA or muscimol (P < 0.05; n= 7–8; Fig. 7C and D).
Fitting the distributions of all burst durations with exponential functions suggested that two or three populations of burst durations accounted for the activity of α1β3γ2 receptors. By contrast, two exponential densities were adequate for α4β3γ2 and α4β3δ receptors (Fig. 7Ba–c). The mean number of openings per burst for α1β3γ2 receptors was increased with higher concentrations of GABA and muscimol compared with α4β3γ2 or α4β3δ receptors (Fig. 7E). Interestingly, the mean number of openings per burst for α4β3δ receptors was particularly low (∼2) for all agonists, which explains the brevity of the bursts (Fig. 7Ac). Taken overall, THIP therefore prolongs the burst duration of α4β3δ receptors, compared to GABA and muscimol, without increasing the number of openings per burst.
Modelling synaptic- and extrasynaptic-type GABAA receptor behaviour
To evaluate the differences between the binding to and gating of α1β3γ2, α4β3γ2 and α4β3δ receptors by the agonists, we devised a number of kinetic models to account for their single channel behaviour. We investigated many different models concentrating mainly on those with two open states and at least three closed states. Overall, this extended analysis (data not shown) indicated that none of the more complex models provided fits to our data that were clearly superior to those provided by the linear-branched model used previously (Mortensen et al. 2004). This model allowed two agonist molecules to bind to the receptor with monoliganded and biliganded channel openings to a main state conductance. A desensitised state accessed from the bilganded state was also incorporated (Fig. 8A). We used this model, and initial values for the rate and conformation constants, based on our previous study, to begin accounting for the single channel behaviour of α1β3γ2 receptors, following activation by each agonist at each concentration. The final iterated rate constants were then used to generate fits to the open and closed time distributions for each agonist at all three concentrations (Supplemental Fig. 2Aa–Cb).
Figure 8. Binding and conformation constants for GABA, muscimol and THIP for synaptic- and extrasynaptic-type receptors.
These constants were determined from the single channel rate constants determined from fitting the linear/branched model to the single channel data for all three receptors: α1β3γ2, α4β3γ2 and α4β3δ receptors. A, GABAA receptor kinetic model. B, dissociation constants for monoliganded (K1; a) and bilganded (K2; b) receptor states. C, efficacies for the monoliganded (E1; a) and bilganded (E2;b) gating transitions. D, desensitisation equilibrium constants (D) calculated for GABA, muscimol and THIP.
For GABA and muscimol, the model predicts little change in the gating of the α1β3γ2 channel, or its entry into and exit from desensitisation. The greater potency of muscimol, evident from the concentration–response curves, must therefore depend upon the faster forward rate constants for agonist binding (Table 3; Supplemental Fig. 2Ac, Bc). For THIP activation of α1β3γ2 receptors, the rates of channel opening (β1 and β2), are considerably slower compared with those for GABA and muscimol and the binding rate constants (k1, k2) are also reduced (Table 3; Supplemental Fig. 2Cc) resulting in the displaced concentration–response curve for THIP towards higher agonist concentrations and the slightly reduced maximum response (Fig. 1Ba).
Table 3.
Rate constants for transitions between states of α1β3γ2, α4β3γ2 and α4β3δ GABAA receptors activated by GABA, muscimol and THIP
| α1β3γ2 |
α4β3γ2 |
α4β3δ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Constant | Unit | GABA | Muscimol | THIP | GABA | Muscimol | THIP | GABA | Muscimol | THIP |
| k1 | m−1* s−1 | 51 × 106 | 453 × 106 | 1.6 × 106 | 3.5 × 106 | 7.1 × 106 | 3.9 × 106 | 32 × 106 | 15 × 106 | 5.7 × 106 |
| k−1 | s−1 | 399 | 245 | 3 | 6 | 6 | 6 | 7 | 1.3 | 56 |
| k2 | m−1* s−1 | 28 × 106 | 186 × 106 | 2.6 × 106 | 3.2 × 106 | 5.9 × 106 | 0.8 × 106 | 47 × 106 | 21 × 106 | 0.15 × 106 |
| k−2 | s−1 | 360 | 428 | 842 | 51 | 28 | 285 | 12 | 7 | 53 |
| δ | s−1 | 219 | 217 | 110 | 2 | 5 | 6 | 6 | 6 | 7 |
| δ−1 | s−1 | 17 | 21 | 15 | 3 | 11 | 15 | 3 | 6 | 2 |
| β1 | s−1 | 570 | 427 | 40 | 57 | 45 | 39 | 20 | 22 | 142 |
| α1 | s−1 | 2268 | 2471 | 2695 | 2972 | 2887 | 1625 | 465 | 1324 | 956 |
| β2 | s−1 | 2556 | 2690 | 1543 | 936 | 887 | 676 | 255 | 325 | 1362 |
| α2 | s−1 | 356 | 324 | 394 | 970 | 1023 | 812 | 1500 | 1729 | 348 |
| K1 | μm | 7.9 | 0.5 | 1.7 | 1.8 | 0.8 | 1.5 | 0.2 | 0.1 | 9.8 |
| K2 | μm | 12.8 | 2.3 | 330 | 16.0 | 4.7 | 357 | 0.3 | 0.3 | 359 |
| D | 13 | 10 | 7 | 0.62 | 0.45 | 0.40 | 2.0 | 1.0 | 3.5 | |
| E1 | 0.25 | 0.17 | 0.01 | 0.019 | 0.016 | 0.024 | 0.04 | 0.02 | 0.15 | |
| E2 | 7.2 | 8.3 | 3.9 | 1.0 | 0.9 | 0.8 | 0.17 | 0.19 | 3.9 | |
The rate constants were determined by optimally fitting the single channel data for each receptor (α1β3γ2, α4β3γ2 and α4β3δ) in the presence of each agonist (GABA, muscimol and THIP) at each of the three concentrations with the linear/branched receptor model using QuB. Values are accrued from n= 13–20 patches.
By exchanging the α1 subunit for α4, the forward and backward rate constants for agonist binding/unbinding at α4β3γ2 receptors are generally reduced for all the agonists (Table 3; Supplemental Fig. 3A–C). This caused either a decrease or no change in the agonist dissociation constants for monoliganded receptors (K1) whilst K2 (for biliganded receptors) was generally increased for each agonist (Fig. 8B). As for α1β3γ2 receptors, THIP exhibited the highest value for K2 compared to GABA and muscimol. With regard to channel gating, generally, β was lower and α was higher for α4β3γ2 compared to α1β3γ2 with little distinction between the agonists (Table 3; Supplemental Fig. 3Ac–Cc). Consequently, agonist efficacy for the biliganded α4β3γ2 receptor (E2) was less than 1 and much smaller compared with E2 for α1β3γ2 (Table 3; Fig. 8Cb). Finally, the entry and exit from desensitisation was also noticeably slower for α4β3γ2 compared to α1β3γ2 receptors such that D (δ1/δ−1) was less than 1 (Fig. 8D).
We next considered the consequences for the model rate constants by exchanging the γ2 subunit for δ forming the α4β3δ receptor (Supplemental Fig. 4). The forward binding rate constant (k1) for GABA is similar to that for the α1β3γ2 receptor, whilst for muscimol the binding rate is reduced. By comparison, the unbinding rate constants (k−1) are smaller, causing K1 and K2 to be substantially reduced (Table 3; Fig. 8B) contributing to the leftward displacement of the GABA and muscimol concentration–response curves (Fig. 1Bc).
However, for THIP, the binding rates are slower but the unbinding is much faster causing K1 and K2 to be much larger compared to values for GABA and muscimol (Fig. 8B). Thus, the concentration–response curve is displaced to higher concentrations as observed (Fig. 1Bc). Despite the differential binding rates to α4β3δ receptors, the most significant difference between the agonists concerns the gating of the channel. Here the opening rate (β) is far higher for THIP for both monoliganded and bilganded receptors, and generally, the closing rate (α) is also slower (Table 3). This significantly increases E1 and E2 for THIP compared with the efficacies for either GABA or muscimol (Fig. 8C; Supplemental Fig. 4).
As noted for the α4β3γ2 receptor, the entry into and exit from desensitisation for α4β3δ receptors are again slow for all the agonists. The estimates of D are slightly higher than for α4β3γ2, but much less than for α1β3γ2 receptors (Fig. 8D). Interestingly, THIP caused greater desensitisation of these extrasynaptic-type receptors than either GABA or muscimol. Although direct comparisons are difficult, the similarity is striking between the single channel predictions for the dissociation constants for THIP (particularly K2), its efficacy (E1 and E2) and its ability to desensitise (D) the α4β3δ receptors compared with the estimated constants, K, E and D, determined from the whole-cell concentration–response curve fits (Fig. 1B; Table 2).
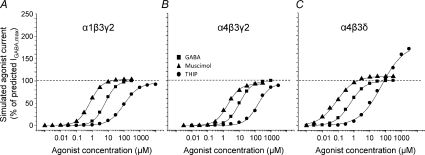
Finally, we determined the adequacy of the rate constants, determined from our single channel records, to account for the behaviour of the three agonists at each receptor isoform. This was accomplished by using the rate constants to predict the peak current amplitudes that would be apparent following the activation of α1β3γ2, α4β3γ2 and α4β3δ receptors with a concentration range for each agonist. The adequacy of our receptor model was then assessed by constructing theoretical agonist concentration–response curves entirely from the single channel data (Fig. 9). These curves accurately reproduced the concentration–response curves generated from the whole-cell current data (Fig. 1) including the lower potency of THIP at all three receptors, the higher potency of muscimol compared to GABA, and, of importance, the superagonist effect of THIP, and of muscimol, on the α4β3δ receptors (Fig. 9C).
Figure 9. Theoretical whole-cell agonist concentration–response curves.
The curves were generated from the state function for the linear/branched receptor model. The transition constants in the model were determined from the single channel rate constants following the predictive fitting of the single channel data with the linear/branched receptor model using QuB. The data points represent the peaks of simulated currents that were subsequently generated in ChanneLab using the linear/branched model and the best fit single channel rate constants for each agonist. Simulated concentration–response curves for GABA (squares), muscimol (triangles) and THIP (circles) are shown for α1β3γ2 (A), α4β3γ2 (B) and α4β3δ (C) GABAA receptors.
Discussion
This study examined the biophysical properties of typical synaptic- (α1β3γ2) and extrasynaptic-type (α4β3δ) GABAA receptors plus α4β3γ2, whose cellular location is less certain, by using three agonists that are known to have distinct effects: the natural transmitter GABA, the potent agonist muscimol, and THIP, which displays superagonist behaviour at δ subunit-containing receptors.
Agonist potency at synaptic and extrasynaptic-type receptors
The order of potency for the agonists is independent of the GABAA receptor subunit composition studied here, with muscimol > GABA >> THIP. Agonist potencies (EC50 values) were increased by 25–38% after replacing α1 with α4 subunits in αβ3γ2 receptors; and further increased by 78–90% after exchanging γ2 for the δ subunit in α4β3δ receptors. Increased agonist potency has been noted previously with α4 and δ subunit-containing GABAA receptors (Whittemore et al. 1996; Brown et al. 2002), although others report no difference (Wafford et al. 1996; Lagrange et al. 2007). A major contributory factor affecting potency at synaptic- and extrasynaptic-type receptors is the agonist dissociation constant (K), which for the biliganded receptors (K2), has the following order: THIP > GABA > muscimol. The potency of agonists can be affected by their efficacies (E; Supplemental Fig. 5A), but for α4β3γ2 and α4β3δ, E1 and E2 for GABA and muscimol are much lower than corresponding values for α1β3γ2 receptors, which would tend to lower the potency, contrary to what we observed. Thus, the agonist dissociation constant rather than efficacy seems to dominate the potency rank order. Nevertheless, increased efficacy (E1 and E2) may contribute to the increased potency for THIP at α4β3δ compared to α1β2γ2 receptors. THIP's conformationally constrained double ring structure underlies its partial agonist behaviour at most synaptic-type αβγ GABAA receptors due to a suboptimal fit at the binding site compared with the more flexible GABA molecule (Krogsgaard-Larsen, 1988). However, this does not appear to be significant for α4β3δ receptors, where the increased potency depends on a domain in the δ subunit, stretching from M1 through the M1–M2 linker and just into M2 (You & Dunn, 2007).
Our results concur with the notion that synaptic γ2 subunit-containing receptors (α1β3γ2) are less sensitive to agonists than their extrasynaptic counterparts (α4β3δ). Native receptors will also experience different GABA concentrations. At synapses, receptors are briefly exposed to low millimolar concentrations following phasic GABA release, whilst extrasynaptic receptors experience an ambient overspill from synapses attaining 0.2–2 μm GABA (Stell & Mody, 2002; Caraiscos et al. 2004; Mortensen & Smart, 2006; Mtchedlishvili & Kapur, 2006).
Although α1β3γ2 receptors showed significant desensitisation, our single channel recordings suggested this was not so apparent for α4β3γ2 receptors. Previously, α4 subunit-containing receptors have been reported to desensitise either more slowly (Picton & Fisher, 2007) or more rapidly (Lagrange et al. 2007) compared to α1 subunit-containing receptors. As we could only accurately assess closed times within bursts we would have missed long desensitised periods in our channel analyses leading to an underestimation of the degree of desensitisation for α4 receptors. Replacing the γ2 subunit with δ in the α4β3δ receptors reintroduced desensitisation in our single channel experiments. This was surprising given that extrasynaptic GABA receptors containing the δ subunit (e.g. α1β3δ) are considered to desensitise at a slow rate (Haas & MacDonald, 1999; Bianchi et al. 2001; Bianchi et al. 2001). The model rate constants determined for the α4β3δ receptor suggested that at low agonist concentrations (<1 μm), the receptor could already be partly desensitised. Simulating agonist-activated currents for GABA (0.3 μm) and muscimol (0.3 μm) indicated the level of desensitisation can be considerable (Fig. 10). Thus, from this study, we would predict that the extrasynaptic α4β3δ receptor population that supports tonic inhibition, which is exposed to low submicromolar basal concentrations of GABA, will be substantially desensitised.
Figure 10. Extrasynaptic-type α4β3δ receptors are desensitised by low GABA concentrations.
Simulated agonist-activated currents are presented for the linear/branched receptor model. The transition constants used in the model to generate the currents were determined after predictive fitting of the single channel data for GABA and muscimol. Two simulations were used; one in which access to the desensitised state (D) was allowed (+D state) and one in which desensitisation was absent (–D state). Notably, the receptor is substantially desensitised by low concentrations of GABA (A) or muscimol (B) that do not cause overt fading of the current response.
Examination of the whole-cell current decays during agonist application did reveal the prospect of multiple phases of desensitisation. In our receptor model, the state ‘D’ represents a composite state that may include more than one discrete desensitised state. The reason for choosing this approach is that our single channel cluster analyses will mostly reflect only rapid desensitised states. The long desensitised periods that typify the longer tails of the whole-cell currents are better correlated with long closed periods between channel clusters; however, these inter-cluster closures may become contaminated by the appearance of more than one channel in the patch. Finally, we employed extended branching of the receptor model to include additional D states, but this did not improve the quality of the data fits. For these reasons a composite D state was preferred.
A consistent feature with all three receptors was the rapid deactivation observed with THIP induced currents, contrasting with the slowly deactivating muscimol currents, a difference also noted for these agonists activating α1β1γ2 receptors (Bianchi et al. 2007). This characteristic is likely to depend principally on agonist dissociation from the receptor and our results demonstrate that this is generally more rapid for THIP compared to GABA and muscimol at all three receptors.
Low pH can increase GABA channel current
The superagonist activity of THIP could arise from an increase in the channel current and/or modulation of channel kinetics. Our single channel recordings demonstrated that THIP does not affect the single channel current. Nevertheless, an increased conductance has been reported for α1β2γ2 and α4β2γ2 receptors, but only with saturating THIP concentrations (10 mm) (Keramidas & Harrison, 2008). Significantly, the increased channel conductance was evident for both γ2 and δ subunit-containing receptors. Alone, this would not account for the superagonist effect of THIP at α4βδ receptors, but is it likely to be a contributory factor? We can emulate the increased conductance by THIP, but only by allowing the 10 mm THIP solution to remain acidified at pH 4. This will significantly increase the cationic form of THIP (Krogsgaard-Larsen et al. 1994) at the expense of its zwitterion and could conceivably affect THIP's orientation at the ligand binding site, altering transduction and increasing channel conductance. An increased channel conductance was also induced by GABA, at pH 4, where its cationic form predominates (Krishek et al. 1996). Thus, a change in conductance is unlikely to contribute to THIP's unusual superagonist behaviour. Interestingly, previous studies of neuronal GABAA receptor channels have not observed an increased channel conductance at acidic pH (Huang & Dillon, 1999; Krishek & Smart, 2001), but the highest concentration of protons examined was only 2.5 μm (pH 5.6) compared to the 100 μm achieved at pH 4.
High efficacy superagonist behaviour of THIP
The constant nature of the single channel current indicates that THIP's superagonist activity must reside in the kinetic properties associated with δ subunit-containing receptors. The α4 subunit is probably not important for superagonism since THIP did not display this activity at α4β3γ2 receptors. Superagonism might originate from the agonist binding site, but the δ subunit is considered an unlikely component for binding, since the agonist site resides at the β–α subunit interface (Sigel et al. 1992; Amin & Weiss, 1993; Wagner & Czajkowski, 2001; Baumann et al. 2003; Wagner et al. 2004). However, co-expression of subunit-constrained GABAA receptors, using α1–β3–α1 and β3–δ concatamers, suggested that the δ-subunit may contribute to or affect the agonist binding site (Kaur et al. 2009), a feature that could then influence THIP activation of αβδ receptors. Nevertheless, the low potency of THIP compared to GABA and muscimol suggested that an alternative explanation of superagonism is required which does not involve ligand binding and probably derives from altered signal transduction from the binding site to the channel.
A comparison of the activation profiles of the three receptors proved instructive. Agonist activation of α1β3γ2 receptors produced channel openings with longer mean open times and longer bursts of channel activity, containing on average more openings per burst, resulting in higher open probabilities. By comparison, α4β3γ2 and α4β3δ receptors were compromised in terms of mean open times, the mean number of openings per burst, and therefore their mean burst durations. Notably, long bursts and the presence of clusters were far less prominent with α4 subunit-containing receptors, a feature noted previously for α4β2γ2 receptors (Akk et al. 2004). For the superagonist activity of THIP on α4β3δ GABAA receptors, the most important change was the increase in the long open time (τO2) by high concentrations of THIP, when compared with high concentrations of GABA and muscimol. By contrast, the mean number of openings per burst was similar for the three agonists on α4β3δ receptors, so the longer burst durations caused by THIP must follow the induction of longer mean open times. Using our kinetic model, THIP substantially increased both E1 and E2 at α4β3δ receptors and this is the fundamental reason for its superagonist behaviour even though THIP has low potency and caused more desensitisation of these receptors.
Whilst the superagonist activity of THIP at α4β3δ receptors (Adkins et al. 2001; Brown et al. 2002) can be largely ascribed to increased efficacy, this does not explain the superagonist behaviour displayed by muscimol (Storustovu & Ebert, 2006). Muscimol has much lower dissociation constants at α4β3δ receptors compared to THIP and thus will bind more tightly, but this also does not explain its superagonist behaviour. Indeed, our model predicts that muscimol's efficacy at α4β3δ receptors is lower than that for THIP and on a par with that for GABA (E2) or even lower (E1). Therefore, the only plausible explanation for muscimol's superagonist activity is that it causes less desensitisation at α4β3δ receptors compared to both GABA and THIP. Reducing the conformational constant D in the model would manifest as an increase in apparent efficacy (Supplemental Fig. 5B) by scaling the concentration–response curve but without causing any shift in agonist potency, unlike changes to E.
In conclusion, the potency of the agonists at the three receptor isoforms is largely determined by their dissociation constants, and thus is a function of agonist binding. In comparison, the superagonist behaviour of THIP results from increased efficacy at extrasynaptic receptors whilst the superagonist behaviour of muscimol relies on reduced desensitisation of extrasynaptic receptors. Thus, there are two principle mechanisms for promoting superagonist behaviour at extrasynaptic receptors and this ability to selectively increase the efficacy and/or reduce the desensitisation of extrasynaptic GABAA receptor activation may prove to be a useful therapeutic avenue under circumstances when synaptic inhibition becomes dysfunctional.
Acknowledgments
This work was supported by the MRC. We are grateful to Damian Bright and Philip Thomas for helpful comments on the manuscript.
Glossary
Abbreviations:
- D
desensitisation constant
- E
efficacy
- EC50
effective concentration causing a 50% of maximum response
- eGFP
enhanced green fluorescent protein
- GABA
γ-aminobutyric acid
- Imax
maximum current induced by GABA
- M
transmembrane domain
- PO
open probability
- THIP
4,5,6,7-tetrahydoisoxazolo[5,4-c]pyridin-3(2H)-one
- τ
time constant
Author contributions
All authors contributed to the conception and design of the experiments. Data collection, analysis, interpretation and writing the paper was carried out by M.M. and T.G.S. All authors revised and approved of the final version of the manuscript.
Supplemental material
Supplemental Fig. 1
Supplemental Fig. 2
Supplemental Fig. 3
Supplemental Fig. 4
Supplemental Fig. 5
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Activation of GABAA receptors containing the α4 subunit by GABA and pentobarbital. J Physiol. 2004;556:387–399. doi: 10.1113/jphysiol.2003.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABAA receptors in CA1 pyramidal cells. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABAA receptors. J Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, MacDonald RL. Structural determinants of fast desensitization and desensitization- deactivation coupling in GABAA receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Botzolakis EJ, Haas KF, Fisher JL, Macdonald RL. Microscopic kinetic determinants of macroscopic currents: insights from coupling and uncoupling of GABAA receptor desensitization and deactivation. J Physiol. 2007;584:769–787. doi: 10.1113/jphysiol.2007.142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzomato V, Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. Single-channel behaviour of heteromeric α1β glycine receptors: an attempt to detect a conformational change before the channel opens. J Neurosci. 2004;24:10924–10940. doi: 10.1523/JNEUROSCI.3424-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You T, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of γ-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different α, β, and γ receptor subunit combinations. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- Fischer F, Kneussel M, Tintrup H, Haverkamp S, Rauen T, Betz H, Wassle H. Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. J Comp Neurol. 2000;427:634–648. doi: 10.1002/1096-9861(20001127)427:4<634::aid-cne10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the α-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, MacDonald RL. GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol. 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadingham KL, Harkness PC, McKernan RM, Quirk K, Le Bourdelles B, Horne AL, Kemp JA, Barnard EA, Ragan CI, Whiting PJ. Stable expression of mammalian type A γ-aminobutyric acid receptors in mouse cells: Demonstration of functional assembly of benzodiazepine-responsive sites. Proc Natl Acad Sci U S A. 1992;89:6378–6382. doi: 10.1073/pnas.89.14.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ, Shelley C, Brydson M, Beeson D, Colquhoun D. Properties of the human muscle nicotinic receptor, and of the slow-channel myasthenic syndrome mutant ɛL221F, inferred from maximum likelihood fits. J Physiol. 2003;547:729–760. doi: 10.1113/jphysiol.2002.034173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Effect of extracellular pH on GABA-activated current in rat recombinant receptors and thin hypothalamic slices. J Neurophysiol. 1999;82:1233–1243. doi: 10.1152/jn.1999.82.3.1233. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of δ-subunit-containing GABAA receptors. J Biol Chem. 2009;284:7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramidas A, Harrison NL. Agonist-dependent single channel current and gating in α4β2δ and α1β2γ2S GABAA receptors. J Gen Physiol. 2008;131:163–181. doi: 10.1085/jgp.200709871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramidas A, Harrison NL. The activation mechanism of α1β2γ2S and α3β3γ2S GABAA receptors. J Gen Physiol. 2009;135:59–75. doi: 10.1085/jgp.200910317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABAA receptor is associated with the receptor subunit composition. J Physiol. 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Smart TG. Proton sensitivity of rat cerebellar granule cell GABAA receptors: dependence on neuronal development. J Physiol. 2001;530:219–233. doi: 10.1111/j.1469-7793.2001.0219l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P. GABA synaptic mechanisms: stereochemical and conformational requirements. Med Res Rev. 1988;8:27–56. doi: 10.1002/med.2610080103. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Frolund B, Jorgensen FS, Schousboe A. GABAA receptor agonists, partial agonists, and antagonists: Design and therapeutic prospects. J Med Chem. 1994;37:2489–2505. doi: 10.1021/jm00042a001. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Frolund B, Liljefors T. Specific GABAA agonists and partial agonists. Chem Rec. 2002;2:419–430. doi: 10.1002/tcr.10040. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Johnston GA, Lodge D, Curtis DR. A new class of GABA agonist. Nature. 1977;268:53–55. doi: 10.1038/268053a0. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol. 2007;578:655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Hosie AM, Buckingham SD, Squire MD, Baylis HA, Sattelle DB. pH-dependent actions of THIP and ZAPA on an ionotropic Drosophila melanogaster GABA receptor. Brain Res. 1996;739:335–338. doi: 10.1016/s0006-8993(96)00998-5. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Kristiansen U, Ebert B, Frolund B, Krogsgaard-Larsen P, Smart TG. Activation of single heteromeric GABAA receptor ion channels by full and partial agonists. J Physiol. 2004;557:389–413. doi: 10.1113/jphysiol.2003.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic α subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Single-channel recording of ligand-gated ion channels. Nat Protocols. 2007;2:2826–2841. doi: 10.1038/nprot.2007.403. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Picton AJ, Fisher JL. Effect of the α subunit subtype on the macroscopic kinetic properties of recombinant GABAA receptors. Brain Res. 2007;1165:40–49. doi: 10.1016/j.brainres.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modelling. Biophys J. 2004;86:1488–1501. doi: 10.1016/S0006-3495(04)74217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Kellenberger S, Malherbe P. Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J. 1992;11:2017–2023. doi: 10.1002/j.1460-2075.1992.tb05258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storustovu S, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human y-aminobutyric acidA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wagner DA, Czajkowski C. Structure and dynamics of the GABA binding pocket: a narrowing cleft that constricts during activation. J Neurosci. 2001;21:67–74. doi: 10.1523/JNEUROSCI.21-01-00067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DA, Czajkowski C, Jones MV. An arginine involved in GABA binding and unbinding but not gating of the GABAA receptor. J Neurosci. 2004;24:2733–2741. doi: 10.1523/JNEUROSCI.4316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM. Pharmacology of the human γ-aminobutyric acidA receptor α4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol. 1996;50:1364–1375. [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- You H, Dunn SM. Identification of a domain in the subunit (S238-V264) of the α4β3δ GABAA receptor that confers high agonist sensitivity. J Neurochem. 2007;103:1092–1101. doi: 10.1111/j.1471-4159.2007.04817.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.