Abstract
Synaptic activity in the medial prefrontal cortex (mPFC) is fundamental for higher cognitive functions such as working memory. The present study shows that small conductance (SK) calcium-activated potassium channels attenuate excitatory synaptic transmission at layer 2/3 and layer 5 inputs to layer 5 pyramidal neurons in the mPFC. SK channels are located postsynaptically at synapses where they are activated during synaptic transmission by calcium influx through NMDA receptors, L-type calcium channels, R-type calcium channels and by calcium release from IP3-sensitive stores. Removal of the SK channel-mediated shunt of synaptic transmission reveals significant NMDA receptor-mediated activation during basal synaptic transmission, which is greater at layer 5 inputs (approximately 30%) than at layer 2/3 inputs (approximately 20%). These findings show that interactions between NMDA receptors, SK channels and voltage-gated calcium channels play a critical role in regulating excitatory synaptic transmission in layer 5 pyramidal neurons in the mPFC.
Introduction
The medial prefrontal cortex (mPFC) is involved in higher cognitive, mnemonic and executive functions, such as planning and sequencing of actions, and attention (Fuster, 1973; Goldman-Rakic, 1995, 1999). One aspect of cognitive function is working memory, the ability to maintain information ‘in mind’ for a short period of time in the presence of distracting stimuli (Goldman-Rakic, 1995). Working memory is impaired in a number of neurological disorders, such as schizophrenia, attention deficit hyperactivity disorder and stress (Arnsten, 2007). The cellular basis for working memory is the persistent firing of networks of neurons within the PFC with shared stimulus properties. Reverberating synaptic activity, together with activation of intrinsic conductances within pyramidal neurons in the mPFC, are believed to underlie the persistent firing (Durstewitz et al. 2000; Wang, 2001). Thus, understanding how ion channels regulate synaptic excitability in the mPFC is fundamental to understanding the cellular basis of working memory, and for developing treatments for neurological disorders that involve disruption of working memory (Arnsten, 2007).
SK channels are small conductance calcium-activated potassium channels, of which there are three subtypes, SK1, SK2 and SK3 (Kohler et al. 1996). All three subtypes are expressed in the cortex (Stocker & Pedarzani, 2000; Sailer et al. 2002). SK channels were originally attributed with mediating the medium afterhyperpolarisation that follows action potentials (Faber & Sah, 2007). However, SK channels also play an important role in regulating synaptic transmission, synaptic plasticity and dendritic excitability (Faber & Sah, 2007). In amygdala and hippocampal pyramidal neurons, SK channels are located in dendritic spines, where they are activated by calcium influx through NMDA receptors and attenuate excitatory synaptic transmission and plasticity (Faber et al. 2005; Ngo-Anh et al. 2005). In prefrontal, visual and somatosensory cortical pyramidal neurons, SK channels regulate dendritic excitability by mediating a hyperpolarisation following calcium-induced calcium release, triggered by activation of muscarinic receptors (Yamada et al. 2004; Gulledge et al. 2007) or group 1 metabotropic glutamate receptors (Hagenston et al. 2008). Blockade of SK channels in the PFC also improves working memory performance (Brennan et al. 2008), suggesting an important functional role of SK channels in the PFC. To date, however, the role of SK channels in synaptic transmission in the PFC has not been investigated.
The current study aimed to examine the role of NMDA receptors and SK channels in excitatory synaptic transmission in the mPFC. The results show that SK channels are located at both layer 2/3 and layer 5 excitatory inputs to layer 5 pyramidal neurons. During basal synaptic transmission, calcium influx through NMDA receptors, L-type calcium channels, R-type calcium channels and calcium release from IP3 receptor-sensitive calcium stores activates synaptic SK channels, which are located postsynaptically within dendritic spines and attenuate the synaptic response. Furthermore, blocking SK channels reveals a larger activation of NMDA receptors at layer 5 inputs than at layer 2/3 inputs to layer 5 pyramidal neurons.
Methods
All experiments were performed on rat brain slices maintained in vitro. Wistar rats (total of 133 rats, of either sex, 21–28 days old) were anaesthetised with isofluorane and killed by decapitation. Isofluorane (1 ml) was added to a 10 l flask containing a rat, and was administered through inhalation. If animals were not anaesthetised by this dose, a further 1 ml isofluorane was added to the flask. Once anaesthetised, rats were killed by decapitation. These procedures were conducted in accordance with the guidelines of the University of Queensland Animal Ethics Committee, and comply with The Journal of Physiology and UK regulations on animal experimentation (Drummond, 2009). Brains were rapidly removed and placed in ice-cold artificial cerebral spinal fluid (aCSF) containing (mm): NaCl 118, KCl 2.5, NaHCO3 25, glucose 10, MgCl2 1.3, CaCl2 2.5 and NaH2PO4 1.2. Coronal brain slices (300 μm thick) containing the mPFC were prepared using standard techniques. Slices were allowed to recover in oxygenated (95% O2–5% CO2) aCSF at 35°C for 30 min, then kept at room temperature for a further 30 min before experiments commenced. Slices were transferred to the recording chamber as required and were continuously perfused with oxygenated aCSF maintained at 30–32°C.
Whole cell recordings were made from layer 5 (L5) pyramidal neurons in the prelimbic region of the mPFC using infra-red differential interference contrast (IR/DIC) techniques. Electrodes (3–5 MΩ) were filled with a pipette solution containing (mm): KMeSO4 135, NaCl 8, Hepes 10, Mg2ATP 2, Na3GTP 0.3 and EGTA 0.3 (pH 7.3 with KOH, osmolarity 280 mosmol kg−1). On some occasions EGTA or BAPTA were diluted to the required concentration in this solution for intracellular dialysis, or a caesium-based solution was used containing (mm): CsMeSO4 135, NaCl 8, Hepes 10, Mg2ATP 2, Na3GTP 0.3 and phosphocreatine 7 (pH 7.3 with CsOH, osmolarity 280 mosmol kg−1). For experiments where drugs were added to the internal solution and synaptic responses were measured, cells were left for at least 20 min after achieving the whole cell configuration to allow diffusion to synapses. Signals were recorded using a patch clamp amplifier (Multiclamp 700A, Axon instruments). Responses were filtered at 4–8 kHz and digitised at 10 kHz (Instrutech, ITC-16). All data were acquired, stored and analysed on a Macintosh using Axograph X (Axograph). Only cells with a membrane potential greater than −55 mV were included in this study. Access resistance was 5–15 MΩ and was monitored throughout the experiment. Drugs were applied by diluting to the correct concentration in the superfusate.
For afferent stimulation, theta glass stimulating electrodes were placed in layer 2/3 (L2/3) and L5 within 100 μm from the cell body (for L5 stimulation) or apical dendrite (for L2/3 stimulation). All experiments were performed in the presence of picrotoxin (100 μm) to block GABAA receptors. Recordings from neurons in current clamp were performed from resting membrane potentials. Rise times of excitatory postsynaptic potentials (EPSPs) were measured between 20–80% of the peak. NMDA receptor-mediated EPSPs and currents (EPSCs) were evoked in aCSF containing 0.1 mm magnesium, NBQX (10 μm) and picrotoxin (100 μm). Responses evoked by synaptic stimulation were averages of 10 sweeps. The paired-pulse ratio was examined by evoking two EPSCs with a 60 ms delay between stimuli from a holding potential of −60 mV, and by dividing the second EPSC peak amplitude by the first EPSC peak amplitude. Trains of EPSPs were evoked by giving five stimuli at 50 Hz. Student's t tests were used for statistical comparisons between groups. Results are expressed as mean ±s.e.m. BAPTA, EGTA, nicardipine, apamin, and picrotoxin were obtained from Sigma. NBQX, bicuculline and AP5 were obtained from Tocris. SNX-482 was obtained from Sigma and Peptides International.
Results
SK channels attenuate synaptic inputs to layer 5 pyramidal neurons
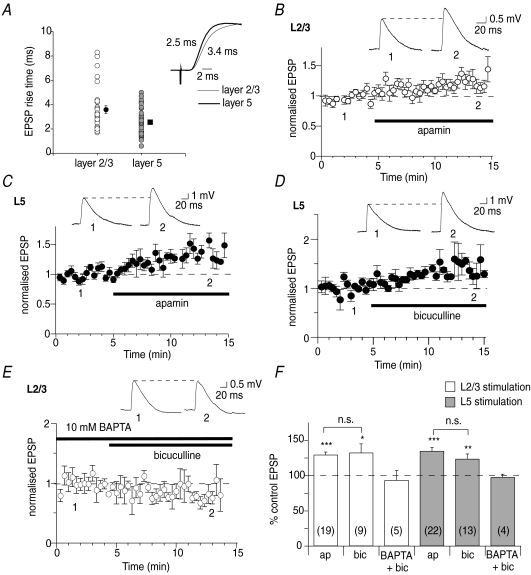
Whole cell recordings were made from L5 pyramidal neurons in the mPFC. EPSPs were evoked by stimulation of either L2/3 or L5. Synapses on pyramidal neurons at both inputs express both AMPA and NMDA receptors (Kang, 1995). EPSPs evoked following L2/3 stimulation had significantly slower rise times (3.5 ± 0.3 ms, n= 24) than EPSPs evoked by L5 stimulation (2.5 ± 0.2 ms, n= 27, P < 0.05, Fig. 1A), suggesting that L2/3 synapses are electrotonically more distal from the soma than L5 inputs, and are thus likely to be located on apical and basal dendrites, respectively (Letzkus et al. 2006; Sjostrom & Hausser, 2006). Blockade of SK channels with apamin (100 nm) significantly enhanced the amplitude of EPSPs evoked by stimulation of L2/3 (to 129 ± 4% of control, n= 19, P < 0.001, Fig. 1B and F), or L5 (to 134 ± 5% of control, n= 22, P < 0.001, Fig. 1C and F). Another SK channel blocker, bicuculline methiodide (10–50 μm; Johnson & Seutin, 1997), also potentiated EPSPs following stimulation of L2/3 (to 132 ± 12% of control, n= 9, P < 0.05) or L5 (to 123 ± 7%, n= 13, P < 0.05, Fig. 1D and F). These findings show that SK channels suppress basal synaptic transmission in the mPFC. Since bicuculline and apamin potentiate L2/3 and L5 inputs to a similar extent (P > 0.05), the two compounds were used interchangeably in subsequent experiments to block SK channels. SK channels are potassium channels that require a rise in intracellular calcium for activation (Lancaster et al. 1991; Kohler et al. 1996). Consistent with this, loading neurons with 10 mm BAPTA blocked potentiation of EPSPs by bicuculline (to 93 ± 14% of control at L2/3 inputs, n= 5, P > 0.05; to 97 ± 5% of control at L5 inputs, n= 4, P > 0.05, Fig. 1E and F). These findings show that SK channels reduce excitatory synaptic transmission at L2/3 and L5 inputs onto L5 pyramidal neurons, and require postsynaptic calcium for their action.
Figure 1. SK channels attenuate excitatory synaptic transmission in the mPFC.
A, EPSP rise times are significantly (P < 0.05) slower at L2/3 inputs than at L5 inputs. Mean rise time at each input is shown by the symbols to the right. Inset shows an overlay of example EPSPs, normalised and with rise times indicated. Thin trace shows the beginning of a L2/3-evoked EPSP, while the thick trace shows the beginning of a L5-evoked EPSP. B, apamin (100 nm) enhances EPSPs evoked in L5 pyramidal neurons by L2/3 stimulation (from 3.2 ± 0.3 mV to 4.0 ± 0.4 mV, n= 19, P < 0.001). Time course showing enhancement of EPSPs by apamin at L2/3 inputs is shown. The drug application time is illustrated by the thick bar, and example EPSPs at time points are indicated in the inset in this and subsequent figures. C, EPSPs evoked by L5 stimulation are enhanced by apamin (from 3.1 ± 0.3 mV to 4.3 ± 0.4 mV, n= 22, P < 0.001). D, bicuculline (10–50 μm) enhances EPSPs evoked by L5 stimulation (from 2.5 ± 0.3 mV to 3.0 ± 0.3 mV, n= 13, P < 0.05). E, loading neurons with 10 mm BAPTA blocks enhancement of L2/3-evoked EPSPs by bicuculline (from 2.5 ± 0.3 mV to 2.2 ± 0.3 mV, n= 5, P > 0.05). F, summary data showing effects of apamin (ap) and bicuculline (bic) on L2/3 inputs (open bars in this and subsequent figures) and L5 inputs (light grey bars in this and subsequent figures). n numbers are shown within the bars for each group in this and subsequent figures. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
NMDA receptor activation is required for activation of SK channels
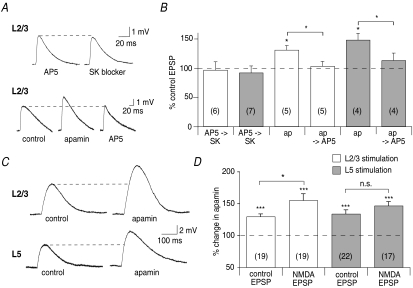
To investigate the source of calcium for SK channels in the mPFC, the role of NMDA receptors was examined. Blockade of NMDA receptors by d-AP5 (30 μm) prevented enhancement of EPSPs by SK channel blockade at L2/3 (to 96 ± 14% of control, n= 6, P > 0.05, Fig. 2A and B) and L5 inputs (to 92 ± 12% of control, n= 7, P > 0.05, Fig. 2B). Furthermore, AP5 reversed the enhancement of EPSPs by apamin evoked by L2/3 stimulation (from 130 ± 8% of control in apamin, n= 5, P < 0.05, to 103 ± 8% of the initial EPSP after subsequent application of AP5, n= 5; P < 0.01 compared with apamin; P > 0.05 compared with control, Fig. 2A and B) and by L5 stimulation (from 148 ± 12% of control in apamin, n= 4, P < 0.05 to 113 ± 13% in AP5, n= 4; P < 0.05 compared with apamin; P > 0.05 compared with control, Fig. 2B). Therefore, NMDA receptors provide a source of calcium for activation of SK channels during basal synaptic transmission. In view of this, if SK channels are not already maximally activated under basal conditions, increasing NMDA receptor activation by recording isolated NMDA receptor-mediated EPSPs (in the presence of 0.1 mm extracellular magnesium and 10 μm NBQX) should potentiate activation of SK channels, thereby increasing potentiation of EPSPs following SK channel blockade. Consistent with this, NMDA receptor-mediated EPSPs evoked by L2/3 stimulation were enhanced by apamin (155 ± 10%, n= 19, P < 0.001, Fig. 2C and D) to a significantly greater extent (P < 0.05) than control EPSPs (130 ± 4%, n= 18, Fig. 2D). In contrast, at L5 inputs NMDA receptor-mediated EPSPs were enhanced by a similar amount (to 147 ± 6%, n= 17, P < 0.001) as control EPSPs (134 ± 5%, n= 22, P > 0.05 vs. the NMDA receptor-mediated EPSP, Fig. 2C and D).
Figure 2. Calcium influx through NMDA receptors activates postsynaptic SK channels.
A, blockade of NMDA receptors by AP5 (30 μm) prevents enhancement of EPSPs by SK channel blockers at L2/3 inputs, given before (from 3.4 ± 0.6 mV to 3.3 ± 0.5 mV, n= 6, P > 0.05) or after the SK channel blocker (from 3.5 ± 0.5 mV in control to 4.4 ± 0.5 mV in apamin, to 3.6 ± 0.5 mV in apamin and AP5, n= 5). B, summary graph showing preclusion of the effect of SK channel blockers (SK) by AP5 at L2/3 and L5 inputs, and the reversal by AP5 of the enhancement of L2/3- and L5-evoked EPSPs by apamin (ap). C, isolated NMDA receptor-mediated EPSPs are enhanced by apamin at L2/3 inputs (top traces, from 2.3 ± 0.3 mV to 3.8 ± 0.6 mV, n= 19, P < 0.001) and at L5 inputs (bottom traces, from 2.6 ± 0.4 mV to 4.0 ± 0.7 mV, n= 17, P < 0.001). D, summary data showing that NMDA receptor-mediated EPSPs are enhanced significantly more by apamin than control EPSPs at L2/3 inputs. At L5 inputs enhancement of NMDA receptor-mediated EPSPs by apamin is similar to that of control EPSPs. *P < 0.05; ***P < 0.001; n.s., not significant.
SK channels are present postsynaptically in L5 mPFC pyramidal neurons
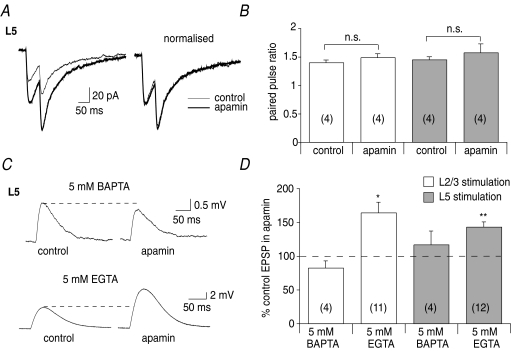
The above findings show that SK channels are activated during synaptic transmission following calcium influx through NMDA receptors. To investigate where SK channels are localised, the effect of apamin on the paired-pulse ratio of NMDA receptor-mediated EPSCs was examined since activation of SK channels is blocked when recording EPSCs from a holding potential of −60 mV (Faber et al. 2008). NMDA receptor-mediated EPSCs recorded with a potassium-based internal solution were potentiated by apamin following stimulation of L2/3 (to 168 ± 14% of control, n= 7, P < 0.05) and L5 (to 187 ± 5% of control, n= 5, P < 0.01, Fig. 3A). However, the paired-pulse ratio of NMDA receptor-mediated EPSCs was unaffected by apamin at both L2/3 inputs (107 ± 6% of control, n= 4, P > 0.05) and L5 inputs (109 ± 12% of control, n= 4 P > 0.05, Fig. 3A and B). Furthermore, NMDA receptor-mediated EPSCs recorded with a caesium-based internal solution were not potentiated by apamin at L2/3 (82 ± 12% of control, n= 4, P > 0.05) or L5 inputs (87 ± 9%, n= 5, P > 0.05, not shown), confirming a postsynaptic locus for SK channels. Next, neurons were loaded with equimolar concentrations of the fast binding calcium buffer BAPTA or the slower binding calcium buffer EGTA, and the effect of apamin on NMDA receptor-mediated EPSPs was examined, in order to maximise the effects of apamin. Loading neurons with 5 mM BAPTA blocked enhancement of EPSPs by apamin at L2/3 (82 ± 10% of control, n= 4, P > 0.05) and L5 inputs (117 ± 20% of control, n= 4, P > 0.05, Fig. 3C and D). In contrast, loading neurons with 5 mm EGTA had no effect on potentiation of EPSPs by apamin following stimulation of L2/3 (164 ± 15% of control, n= 11, P < 0.05) or L5 (143 ± 7%, n= 12, P < 0.01, Fig. 3C and D). These findings show that postsynaptic SK channels are located in close vicinity to their calcium source and are therefore likely to be located within the spine (Faber et al. 2005).
Figure 3. SK channels are located postsynaptically in spines.
A, NMDA receptor-mediated EPSCs are enhanced by apamin at L5 inputs (from 35 ± 7 pA to 66 ± 12 pA, n= 5, P < 0.01), but the paired-pulse ratio is unaffected (from 1.5 ± 0.1 to 1.6 ± 0.1, n= 4, P > 0.05). Overlaid raw traces are shown on the left and normalised traces on the right. B, graph summarising the effect of apamin on the paired-pulse ratio at L2/3 and L5 inputs. C, SK channels are located in spines. Loading neurons with 5 mm BAPTA blocks enhancement of NMDA receptor-mediated EPSPs by apamin at L5 inputs (upper traces, from 1.0 ± 0.2 mV to 1.1 ± 0.2 mV, n= 4, P > 0.05), whereas 5 mm EGTA has no effect on enhancement of L5 NMDA receptor-mediated EPSPs by apamin (lower traces, from 2.9 ± 0.5 ± 4.3 ± 0.8 mV, n= 12, P < 0.01). D, summary data showing that 5 mm BAPTA blocks the effect of apamin on NMDA receptor-mediated EPSPs at L2/3 and L5 inputs, whereas 5 mm EGTA has no effect. *P < 0.05; **P < 0.01; n.s., not significant.
NMDA receptors are activated more during basal synaptic transmission at layer 5 inputs than at layer 2/3 inputs
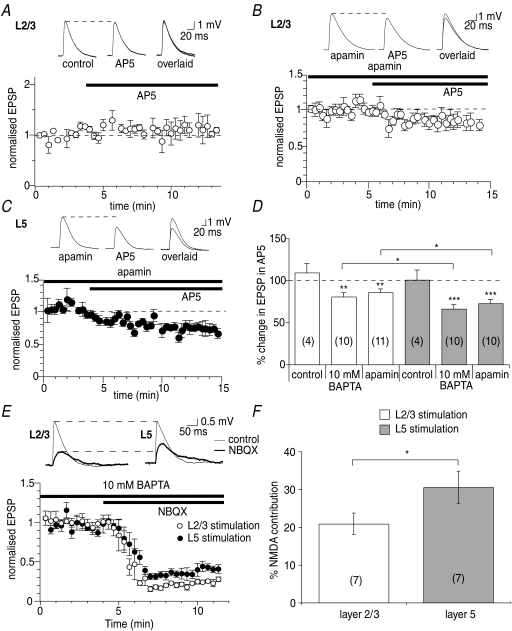
The similarity in the enhancement of isolated NMDA receptor-mediated EPSPs to control EPSPs by apamin at L5 inputs suggests that calcium influx through NMDA receptors during basal synaptic transmission is sufficient at these inputs to maximally activate SK channels. To investigate this, the extent of activation of NMDA receptors during basal synaptic transmission at L2/3 and L5 inputs was examined. Since activation of SK channels in conjunction with NMDA receptors masks the direct actions of AP5 on EPSP amplitude (Faber et al. 2005), blockade of NMDA receptors with AP5 (30 μm) had no effect on control EPSPs evoked by stimulation of L2/3 (109 ± 11%, n= 4, P > 0.05, Fig. 4A and D) or L5 (101 ± 11%, n= 4, P > 0.05, Fig. 4D). Therefore, to exclude the SK channel contribution to EPSPs, experiments were repeated in the presence of apamin. Under these conditions, a pronounced depression of the EPSP amplitude by AP5 was observed, to 86 ± 4% of control at L2/3 inputs (n= 11, P < 0.01, Fig. 4B and D) and to 73 ± 5% of control at L5 inputs (n= 10, P < 0.001, Fig. 4C and D). Depression of EPSPs by AP5 under these conditions was significantly greater at L5 inputs than at L2/3 inputs (P < 0.05). Similarly, when activation of SK channels was blocked by loading neurons with 10 mm BAPTA, EPSPs evoked at L2/3 inputs were reduced significantly (P < 0.05) less by AP5 (to 81 ± 4% of control, n= 10, P < 0.005) than EPSPs evoked at L5 inputs (to 66 ± 5% of control, n= 10, P < 0.001, Fig. 4D). To directly examine whether the NMDA receptor activation during synaptic transmission at L5 inputs is greater than at L2/3 inputs, neurons were loaded with 10 mm BAPTA, and EPSPs evoked by L2/3 and L5 stimulation were simultaneously monitored in the same cells. Blockade of AMPA receptors with NBQX (10 μm) revealed the slower NMDA receptor component of the EPSP, which was significantly greater at L5 inputs (31 ± 4% of the control EPSP, n= 7) than at L2/3 inputs (21 ± 3% of the control EPSP, n= 7, P > 0.05, Fig. 4E and F).
Figure 4. NMDA receptors contribute more to synaptic transmission at L5 inputs than at L2/3 inputs.
A, AP5 has no effect on synaptic transmission under control conditions at L2/3 inputs (from 3.8 ± 0.3 mV to 4.1 ± 0.4 mV, n= 4, P > 0.05). Example traces are shown in the insets and traces are overlaid on the right in this and subsequent panels. In the presence of apamin, AP5 depresses EPSPs at L2/3 inputs (B, from 3.8 ± 0.4 mV to 3.3 ± 0.4 mV, n= 11, P < 0.01) and at L5 inputs (C, from 4.0 ± 0.5 mV to 3.0 ± 0.5 mV, n= 10, P < 0.001). D, summary graph showing effects of AP5 on EPSPs evoked under control conditions, and when SK channels are blocked by apamin or after loading cells with 10 mm BAPTA. E, in cells loaded with 10 mm BAPTA, blocking AMPA receptors with NBQX reveals greater NMDA receptor activation at L5 inputs than at L2/3 inputs (from 4.7 ± 1.1 mV in control to 1.0 ± 0.3 mV in NBQX at L2.3 inputs, P < 0.01, n= 7 and from 4.4 ± 1.1 mV in control to 1.1 ± 0.2 mV in NBQX at L5 inputs, P < 0.05, n= 7). Example EPSPs from the same cell are shown above; time course of the effect at the two inputs is shown below. F, summary graph showing the contribution of NMDA receptors to basal synaptic transmission at L2/3 and L5 inputs to L5 pyramidal neurons.*P < 0.05; **P < 0.01; ***P < 0.001.
Calcium influx through L- and R-type calcium channels contributes to activation of synaptic SK channels
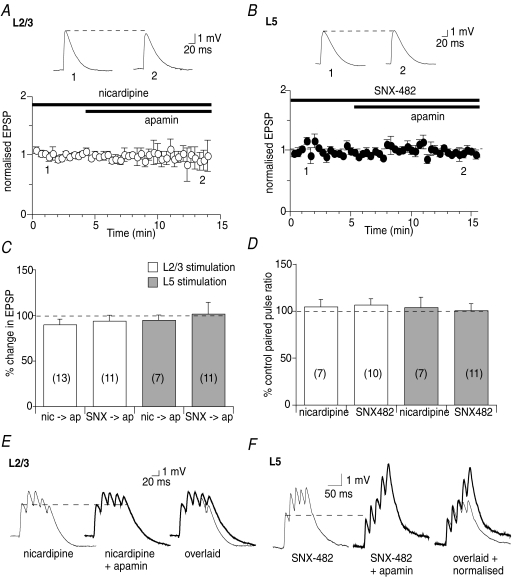
Activation of voltage-gated calcium channels has been shown to contribute to calcium rises in pyramidal neuron spines (Sabatini & Svoboda, 2000; Tsay & Yuste, 2004; Bloodgood & Sabatini, 2007; Meredith et al. 2007). To determine whether voltage-gated calcium channels also contribute to the activation of synaptic SK channels, a number of channel blockers were used. The ability of P/Q-, N- and T-type voltage-gated calcium channels to postsynaptically modulate SK channels could not be examined due to presynaptic actions (unpublished observations, Faber, E.S.L.). However, in the presence of the L-type calcium channel blocker nicardipine (5 μm), potentiation of EPSPs by apamin was blocked at L2/3 (to 90 ± 6% of the EPSP in the presence of nicardipine, n= 13, P > 0.05, Fig. 5A and C) and L5 inputs (to 95 ± 5%, n= 12, P > 0.05, Fig. 5C). Nicardipine had no effect on the paired-pulse ratio at L2/3 inputs (105 ± 8%, n= 7, P > 0.05) or L5 inputs (104 ± 11%, n= 7, P > 0.05, Fig. 5D). Furthermore, blockade of R-type calcium channels with SNX-482 (300 nm) also blocked potentiation of EPSPs by apamin at L2/3 inputs (94 ± 6%, n= 11, P > 0.05, Fig. 5C) and L5 inputs (101 ± 13%, n= 11, P > 0.05, Fig. 5B and C). SNX-482 (30–300 nm) had no effect on the paired-pulse ratio at either L2/3 (106 ± 6%, n= 10, P > 0.05) or L5 inputs (101 ± 7%, n= 11, P > 0.05, Fig. 5D).
Figure 5. Calcium influx through voltage-gated calcium channels activates SK channels.
A, summary graph showing that nicardipine (5 μm) occludes the enhancement of EPSPs by apamin at L2/3 inputs (from 3.1 ± 0.3 mV in nicardipine to 3.8 ± 1.3 mV in apamin, n= 13, P > 0.05). Example traces showing this effect are illustrated above. B, SNX-482 prevents enhancement of EPSPs by apamin at L5 inputs (from 2.7 ± 0.5 mV in SNX-482 to 2.7 ± 0.5 mV in apamin, n= 11, P > 0.05). C, summary graph showing that nicardipine and SNX-482 block enhancement of EPSPs by apamin at L2/3 and L5 inputs (nic, nicardipine; ap, apamin; SNX, SNX-482). D, summary graph showing that L-type and R-type calcium channels are located postsynaptically since they have no effect on the paired-pulse ratio at L2/3 and L5 inputs. E and F, SK channel activation is rescued during trains of EPSPs when voltage-gated calcium channels are blocked. E, while the first EPSP in a train of EPSPs in unaffected by apamin in the presence of nicardipine, the amplitude of the fifth EPSP in the train is enhanced at L2/3 inputs (EPSP5:EPSP1 ratio 1.5 ± 0.2 in nicardipine and 1.9 ± 0.3 in nicardipine and apamin, n= 6, P < 0.05). Individual traces are shown in the left and middle panels and overlaid on the right. F, in the presence of SNX-482, apamin has no significant effect on the first EPSP in a train but potentiates the fifth EPSP at L5 inputs (EPSP5:EPSP1 ratio 2.1 ± 0.5 in SNX-482 and 3.6 ± 0.8 in SNX-482 and apamin, n= 4, P < 0.05). Individual traces are shown in the left and middle panels, with the traces overlaid and normalised on the right.
These findings show that L-type and R-type calcium channels are located postsynaptically on L5 pyramidal neurons and, together with NMDA receptors, contribute to the calcium influx that activates synaptic SK channels. Moreover, these data suggest that activation of SK channels by calcium is finely tuned within dendritic spines, and that blockade of one of the calcium sources is sufficient to prevent activation of SK channels. Potentiation of the calcium rise mediated by other calcium sources within the spine, therefore, should ‘rescue’ SK channel activation. To examine this, a train of five stimuli was applied to enhance calcium influx through NMDA receptors, due to the relief of the magnesium block with repetitive stimulation (Mayer et al. 1984), in the presence of nicardipine or SNX-482. Under these conditions, apamin increased the amplitude of EPSPs later in the train, where calcium influx through NMDA receptors would be most potentiated. Thus, in the presence of nicardipine, the ratio of EPSP5:EPSP1 was enhanced by apamin at L2/3 inputs (to 127 ± 10%, n= 6, P < 0.05, Fig. 5E) and at L5 inputs (to 124 ± 8%, n= 5, P < 0.05). Similarly, in the presence of SNX-482, the EPSP5:EPSP1 ratio was also increased by apamin at L2/3 inputs (to 133 ± 7%, n= 5, P < 0.05) and at L5 inputs (to 176 ± 8%, n= 4, P < 0.05, Fig. 5F). These findings show that NMDA receptors, L-type calcium channels and R-type calcium channels each contribute to the calcium rise that activates synaptic SK channels in L5 pyramidal neurons in the mPFC.
Role of calcium release from intracellular stores in activation of synaptic SK channels
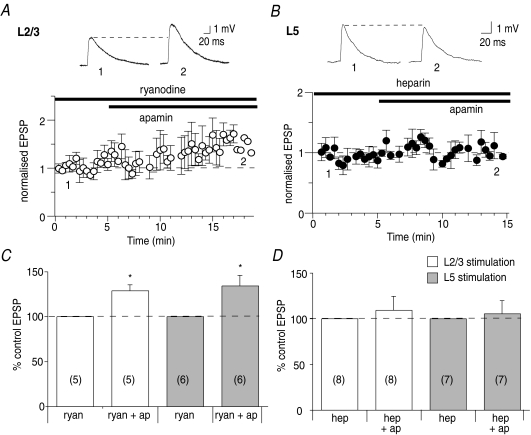
Finally, to test whether calcium-induced calcium release also provides calcium for SK channel activation during synaptic transmission, the effect of intracellular calcium store blockers was examined. Blocking calcium release from ryanodine receptor-sensitive stores with ryanodine (10 μm) had no effect on potentiation of EPSPs by apamin, at L2/3 (129 ± 6%, n= 5, P < 0.05, Fig. 6A and C) or L5 inputs (134 ± 12% of control, n= 6, P < 0.05, Fig. 6C). Since some calcium store blockers have presynaptic actions (Simkus & Stricker, 2002), the effect of postsynaptically loading neurons with the membrane-impermeant IP3 receptor blocker heparin was examined. Heparin (500 μg ml−1) blocked the enhancement of EPSPs by apamin at both L2/3 inputs (109 ± 15%, n= 8, P < 0.05, Fig. 6D) and L5 inputs (105 ± 14%, n= 7, P > 0.05, Fig. 6B and D). This suggests that release of calcium from IP3-sensitive calcium stores also contributes to activation of synaptic SK channels.
Figure 6. SK channels are also activated by release of calcium from IP3-sensitive intracellular stores.
A, ryanodine (10 μm) does not affect the enhancement of L2/3-evoked EPSPs by apamin (from 4.0 ± 0.7 mV to 5.1 ± 0.9 mV, n= 5, P < 0.05). B, loading neurons with heparin (500 μg ml−1) blocks the enhancement of EPSPs by apamin at L5 inputs (from 3.1 ± 0.5 mV in heparin to 3.1 ± 0.5 mV in apamin and heparin, n= 7, P > 0.05). Summary graphs showing the lack of effect of ryanodine on apamin's action at L2/3 and L5 inputs (C; ryan, ryanodine; ap, apamin), and the blockade of apamin's action by heparin at L2/3 and L5 inputs (D; hep, heparin). *P < 0.05.
Discussion
The results of this study show that SK channels reduce excitatory synaptic transmission at L2/3 and L5 inputs to L5 pyramidal neurons in the mPFC. SK channels are located postsynaptically, since they require postsynaptic calcium for their activation, and blocking SK channels has no effect on the paired-pulse ratio. SK channels are likely to be located within spines, since their activation is blocked by an equimolar concentration of BAPTA but not EGTA. During basal synaptic transmission SK channels are activated by calcium influx through NMDA receptors, L-type calcium channels, R-type calcium channels, and mobilisation of calcium from IP3-sensitive calcium stores, because AP5, nicardipine, SNX-482 or heparin all block the effect of apamin, showing that the spine calcium signalling involved in SK channel activation is finely tuned by a number of different sources. Activation of synaptic SK channels leads to a potassium efflux, which attenuates synaptic transmission at both L2/3 inputs and L5 inputs.
NMDA receptor-mediated EPSPs are enhanced by SK channel blockade significantly more than control EPSPs at L2/3 inputs, but not at L5 inputs. This is due to a larger activation of NMDA receptors during basal synaptic transmission at L5 inputs compared to L2/3 inputs; approximately 30% compared to 20%, which saturates activation of SK channels at L5 inputs. The NMDA receptor-mediated component of synaptic transmission is substantially larger than that measured in the same way in hippocampal and amygdala pyramidal neurons (approximately 10%; Faber et al. 2005; Ngo-Anh et al. 2005), suggesting that a large NMDA receptor component is a feature of synapses on L5 pyramidal neurons in the mPFC. This is consistent with the higher levels of NMDA receptor mRNA that are found in the human mPFC (Scherzer et al. 1998). The substantial NMDA receptor contribution at L5 inputs could result from one or more of the following. (1) A higher expression of NMDA receptors on synapses at L5 inputs. (2) A greater depolarisation achieved at L5 inputs during synaptic transmission, due to a higher number of synaptic AMPA receptors or a higher impedance spine, leading to greater alleviation of the NMDA receptor magnesium block. (3) Differential modulation of NMDA receptors, for example, by phosphorylation, at L2/3 versus L5 inputs. A recent study showed that NMDA receptors at L5 synapses in the mPFC have significantly more NR2B subunits than L5 synapses in the primary visual cortex, or in L2/3 of the mPFC (Wang et al. 2008). However, the slower decay of NR2B-containing NMDA receptors at L5 synapses is unlikely to contribute to the larger NMDA receptor activation observed in the current study because responses were measured at the initial peak amplitude of the EPSP. Irrespective of the cause, these findings are the first experimental findings to show a substantial contribution of NMDA receptors to basal synaptic transmission in the cortex, and are in good agreement with modelling studies that show that a large NMDA receptor contribution is required for persistent network activity in the mPFC, observed during working memory tasks (Wang, 2001).
If there is a greater activation of NMDA receptor at L5 inputs, why isn't the potentiation of EPSPs by apamin greater at L5 inputs than at L2/3 inputs? One explanation could be that there is lower expression of SK channels at synapses at L5 inputs. Future experiments using immunolabelling of SK channels, and visualisation at the electron microscopy level across a large number of synapses located in the apical versus basal dendrites, are required to determine this.
In addition to calcium influx through NMDA receptors, L-type calcium channels also contribute to activation of synaptic SK channels in the mPFC, since nicardipine blocks enhancement of EPSPs by apamin. This is a feature unique to the mPFC, since in hippocampal and amygdala pyramidal neurons L-type calcium channels do not contribute to SK channel activation during basal synaptic transmission (Faber et al. 2005; Bloodgood & Sabatini, 2007). L-type calcium channels mediate a significant calcium rise in spines of L5 mPFC pyramidal neurons in response to action potentials evoked at high frequencies (Meredith et al. 2007). However, the contribution of L-type calcium channels to spine calcium transients during synaptic transmission has not been examined. While L-type calcium channels have traditionally been considered high voltage-activated calcium channels (Ertel et al. 2000), L-type calcium channels containing the CaV1.3 (α1D) subunit with low voltage-activating properties have recently been identified (Lipscombe, 2002). Furthermore, physiological activation of L-type calcium channels at resting membrane potentials has been demonstrated in pyramidal neurons in the hippocampus (Avery & Johnston, 1996; Magee et al. 1996) and amygdala (Power & Sah, 2005). This suggests that CaV1.3-containing L-type calcium channels may mediate the effects of nicardipine on EPSPs evoked in L5 pyramidal neurons in the mPFC. In accordance with this, CaV1.3 mRNA is expressed in the cortex (Ludwig et al. 1997). Calcium influx through R-type calcium channels also contributes to activation of synaptic SK channels in mPFC pyramidal neurons, since SNX-482 prevents enhancement of EPSPs by apamin. This is consistent with what has been shown in hippocampal pyramidal neurons following uncaging of glutamate at single spines (Bloodgood & Sabatini, 2007). These findings suggest that calcium rises following synaptic stimulation are critically controlled within the dendritic spines of L5 pyramidal neurons in the mPFC, such that NMDA receptors, L-type calcium channels and R-type calcium channels each contribute to activation of synaptic SK channels. Blockade of one of these calcium sources can prevent activation of SK channels and enhancement of EPSPs by apamin, but this can be rescued by enhancing calcium influx through NMDA receptors with repetitive stimulation.
SK channels have also been shown to be activated by IP3-mediated calcium release in pyramidal neurons in the mPFC, triggered during calcium waves evoked by high-frequency synaptic activation or by application of group 1 mGluR agonists (Hagenston et al. 2008). In this study, the SK channel-mediated outward current was only observed when waves propagated into, or near, the soma, suggesting that SK channels are only present on the proximal dendrites and soma. However, the present findings show that SK channels are also located in synapses of L5 pyramidal neurons in the mPFC. SK channels are activated during synaptic transmission, and their activation is blocked by equimolar concentrations of BAPTA but not EGTA. This suggests that, as in amygdala and hippocampal neurons (Faber et al. 2005, 2008; Ngo-Anh et al. 2005; Bloodgood & Sabatini, 2007; Lin et al. 2008), SK channels in the mPFC are located in spines of mPFC neurons, in close vicinity to their calcium source. Thus, the localised activation of SK channels by calcium waves (Hagenston et al. 2008) may be due to SK channels only being sufficiently close to the calcium released from intracellular calcium stores during calcium waves at the soma and proximal dendrite. However, the present results show that IP3 receptor-sensitive intracellular calcium stores also provide calcium for activation of SK channels during synaptic transmission, because the effects of apamin were blocked by heparin. Since the results indicate that SK channels are located within spines, this suggests that calcium stores are also located within the spines of mPFC pyramidal neurons, as has been shown for other cortical neurons (Gray, 1959; Spacek & Hartmann, 1983), and that release of calcium from these stores is triggered during synaptic transmission.
The PFC is essential for executive function. A fundamental aspect of this is working memory, the ability to internally represent information in the absence of external input. In humans this is essential for, amongst other things, language, performing music and conceptual thinking. The persistent firing that underlies working memory is partly generated by recurrent activity in networks of L5 pyramidal neurons in the mPFC (Wang, 2001). The current findings show that SK channels play an important role in dampening down excitability during synaptic transmission in L5 pyramidal neurons in the mPFC. Removal of the SK channel-mediated shunt, for example, by phosphorylation by protein kinase A following neuromodulation or synaptic plasticity (Faber et al. 2008; Lin et al. 2008), could potentiate synaptic transmission L2/3 or L5 inputs, optimising activity within the mPFC network.
Acknowledgments
I would like to thank Pankaj Sah and Rowan Tweedale for comments on the manuscript. This work was supported by a National Health and Medical Research Council of Australia R. D. Wright Fellowship (ID404739) and a NARSAD Young Investigator Award.
Glossary
Abbreviations
- L2/3
layer 2/3
- L5
layer 5
- mGluR
metabotropic glutamate receptor
- mPFC
medial prefrontal cortex
- SK channels
small conductance calcium-activated potassium channels
Author contributions
The author performed all of the experiments, analysis and preparation of the manuscript. All experiments were performed at the Queensland Brain Institute, University of Queensland.
References
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of ‘representational knowledge’: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17:i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci. 1996;16:5567–5582. doi: 10.1523/JNEUROSCI.16-18-05567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV2.3 voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Brennan AR, Dolinsky B, Vu MA, Stanley M, Yeckel MF, Arnsten AF. Blockade of IP3-mediated SK channel signalling in the rat medial prefrontal cortex improves spatial working memory. Learn Mem. 2008;15:93–96. doi: 10.1101/lm.767408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Faber ESL, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. Modulation of SK channel trafficking by β adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci. 2008;28:10803–10813. doi: 10.1523/JNEUROSCI.1796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Faber ESL, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol. 2007;34:1077–1083. doi: 10.1111/j.1440-1681.2007.04725.x. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The ‘psychic’ neuron of the cerebral cortex. Ann N Y Acad Sci. 1999;868:13–26. doi: 10.1111/j.1749-6632.1999.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signalling in neocortical neurons. J Neurophysiol. 2007;97:2215–2229. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- Hagenston AM, Fitzpatrick JS, Yeckel MF. mGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- Kang Y. Differential paired pulse depression of non-NMDA and NMDA currents in pyramidal cells of the rat frontal cortex. J Neurosci. 1995;15:8268–8280. doi: 10.1523/JNEUROSCI.15-12-08268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA, Perkel DJ. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci. 1991;11:23–32. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci. 2006;26:10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D. L-type calcium channels: highs and new lows. Circ Res. 2002;90:933–935. doi: 10.1161/01.res.0000019740.52306.92. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Avery RB, Christie BR, Johnston D. Dihydropyridine-sensitive, voltage-gated Ca2+ channels contribute to the resting intracellular Ca2+ concentration of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1996;76:3460–3470. doi: 10.1152/jn.1996.76.5.3460. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signalling in mice lacking fragile X gene FMR1. Neuron. 2007;54:627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Power JM, Sah P. Intracellular calcium store filling by an L-type calcium current in the basolateral amygdala at subthreshold membrane potentials. J Physiol. 2005;562:439–453. doi: 10.1113/jphysiol.2004.076711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Svoboda K. Analysis of calcium channels in single spines using optical fluctuation analysis. Nature. 2000;408:589–593. doi: 10.1038/35046076. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22:9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM, Standaert DG, Daggett LP, Velicelebi G, Penney JB, Young AB. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. J Comp Neurol. 1998;390:75–90. doi: 10.1002/(sici)1096-9861(19980105)390:1<75::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Simkus CR, Stricker C. The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol. 2002;545:521–535. doi: 10.1113/jphysiol.2002.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Hausser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron. 2006;51:227–238. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J, Hartmann M. Three-dimensional analysis of dendritic spines. I. Quantitative observations related to dendritic spine and synaptic morphology in cerebral and cerebellar cortices. Anat Embryol (Berl) 1983;167:289–310. doi: 10.1007/BF00298517. [DOI] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Tsay D, Yuste R. On the electrical function of dendritic spines. Trends Neurosci. 2004;27:77–83. doi: 10.1016/j.tins.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- Yamada S, Takechi H, Kanchiku I, Kita T, Kato N. Small-conductance Ca2+-dependent K+ channels are the target of spike-induced Ca2+ release in a feedback regulation of pyramidal cell excitability. J Neurophysiol. 2004;91:2322–2329. doi: 10.1152/jn.01049.2003. [DOI] [PubMed] [Google Scholar]