Abstract
Nitric oxide (NO) derived from endothelial NO synthase (eNOS) is an integral mediator of vascular control during muscle contractions. However, it is not known whether neuronal NOS (nNOS)-derived NO regulates tissue hyperaemia in healthy subjects, particularly during exercise. We tested the hypothesis that selective nNOS inhibition would reduce blood flow and vascular conductance (VC) in rat hindlimb locomotor muscle(s), kidneys and splanchnic organs at rest and during dynamic treadmill exercise (20 m min−1, 10% grade). Nineteen male Sprague–Dawley rats (555 ± 23 g) were assigned to either rest (n= 9) or exercise (n= 10) groups. Blood flow and VC were determined via radiolabelled microspheres before and after the intra-arterial administration of the selective nNOS inhibitor S-methyl-l-thiocitrulline (SMTC, 2.1 ± 0.1 μmol kg−1). Total hindlimb muscle blood flow (control: 20 ± 2 ml min−1 100g−1, SMTC: 12 ± 2 ml min−1 100g−1, P < 0.05) and VC (control: 0.16 ± 0.02 ml min−1 100 g−1 mmHg−1, SMTC: 0.09 ± 0.01 ml min−1 100 g−1 mmHg−1, P < 0.05) were reduced substantially at rest. Moreover, the magnitude of the absolute reduction in blood flow and VC correlated (P < 0.05) with the proportion of oxidative muscle fibres found in the individual muscles or muscle parts of the hindlimb. During exercise, total hindlimb blood flow (control: 108 ± 7 ml min−1 100 g−1, SMTC: 105 ± 8 ml min−1 100 g−1) and VC (control: 0.77 ± 0.06 ml min−1 100g−1 mmHg−1; SMTC: 0.70 ± 0.05 ml min−1 100g−1 mmHg−1) were not different (P > 0.05) between control and SMTC conditions. SMTC reduced (P < 0.05) blood flow and VC at rest and during exercise in the kidneys, adrenals and liver. These results enhance our understanding of the role of NO-mediated circulatory control by demonstrating that nNOS does not appear to subserve an obligatory role in the exercising muscle hyperaemic response in the rat.
Introduction
Nitric oxide (NO) is a low molecular weight, highly diffusible signalling molecule synthesized through the conversion of l-arginine to l-citrulline via the calcium-dependent enzyme NO synthase (NOS). In healthy subjects, NO derived from two constitutively expressed NOS isoforms, neuronal NOS (nNOS) and endothelial NOS (eNOS), exerts influences on a vast array of biological functions including, for example, the regulation of vascular control, facilitation of skeletal muscle glucose uptake, modulation of muscle contractile function and myoblast differentiation (for review, see Stamler & Meissner, 2001). Specifically in regards to vascular control, experimental pharmacological non-selective NOS inhibition in human and animal models provides the foundation for a substantial body of evidence that NO plays integral roles in regulating blood flow in skeletal muscle at rest and during exercise (for review, see Joyner & Dietz, 1997; Radegran & Hellsten, 2000) in the kidney (Ikenaga et al. 1996) and splanchnic organs (Hirai et al. 1994).
One consequence of non-selective NOS inhibition is the inability to differentiate the specific NOS isoform origin of vasoactive NO within various vascular beds and physiological conditions (i.e. rest or exercise). Given that vascular endothelial dysfunction commonly underlies peripheral circulatory derangements manifested in conditions such as chronic heart failure (CHF; Kubo et al. 1991), diabetes (Johnstone et al. 1993) and advancing age (Schrage et al. 2007), NO-mediated circulatory control has been ascribed principally to eNOS. However, the localization of nNOS near the sarcolemmal membrane (Lai et al. 2009) makes nNOS-derived NO a viable candidate in the regulation of the peripheral circulation, particularly during exercise. In this regard, emergent theoretical (Kavdia & Popel, 2004) and empirical (Ichihara et al. 1998; Thomas et al. 1998; Seddon et al. 2008, 2009; Lai et al. 2009) evidence has identified a more substantial role for nNOS-derived NO in vascular smooth muscle and blood flow regulation than considered previously. For example, nNOS-derived NO modulates vascular responses in isolated mouse hindlimb muscle (Lau et al. 2000; Grange et al. 2001) and modulates basal (i.e. resting) total forearm (Seddon et al. 2008) and coronary artery (Seddon et al. 2009) blood flow in humans.
Strong support for the importance of nNOS-derived NO in blood flow regulation is that sympathetic vascular modulation is impaired in nNOS-deficient skeletal muscle from both mice (Thomas et al. 1998, 2003) and humans (Sander et al. 2000). Consequently, skeletal muscle perfusion during exercise is compromised in nNOS-deficient mice (Lai et al. 2009). However, to our knowledge, obligatory participation of nNOS-derived NO in the hyperaemic response to active locomotor skeletal muscle in healthy individuals during physiological exercise has not been investigated. Given that many clinical populations are hallmarked by skeletal muscle blood flow decrements and resultant exercise intolerance, resolution of this issue would constitute a fundamental step in the development of pharmacological and non-pharmacological interventions to mitigate vascular dysfunction in affected individuals.
The aim of the present study was to investigate the effects of nNOS inhibition via the selective nNOS blocker S-methyl-l-thiocitrulline (SMTC; Furfine et al. 1994; Wakefield et al. 2003) on total and inter- and intramuscular hindlimb, kidney and splanchnic organ blood flow, and vascular conductance (VC) at rest and during submaximal dynamic treadmill exercise in healthy rats. Based on the evidence summarized above regarding the role of nNOS in peripheral circulatory control, we hypothesized that nNOS inhibition would significantly reduce blood flow and VC to these tissues at rest and during exercise.
Methods
Animal selection and assignment
A total of 19 young adult (age: 4–6 months) male Sprague–Dawley rats (body weight: 555 ± 23 g) were used. All animals were purchased from Charles River Laboratories (Boston, MA, USA) and, upon arrival at Kansas State University, were housed in approved facilities and maintained on a 12:12 h light–dark cycle with food and water available ad libitum. All experimental procedures described herein were conducted according to the guidelines established by The Journal of Physiology (Drummond, 2009) and the National Institutes of Health and were approved by Kansas State University's Institutional Animal Care and Use Committee. Initially, rats were assigned randomly to either a rest (n= 9) or exercise (n= 6) group with four additional rats added to the exercise group to increase statistical power. Prior to the initiation of the experimental protocol, the exercise group was familiarized with running on a custom-built motor-driven treadmill over a ∼2 week period in which rats ran for ∼5 min day−1 at a speed of 20 m min−1 up a 10% grade.
Surgical procedure
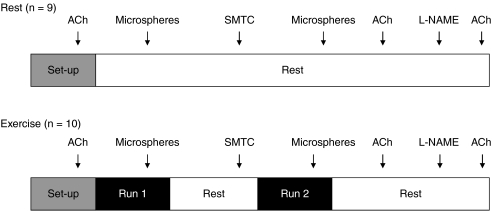
On the day of data collection, animals were anaesthetized with 5% isoflurane. Subsequently, while being maintained on a 2–3% isoflurane–oxygen mixture, one catheter (PE-10 connected to PE-50, Clay Adams Brand, Sparks, MD, USA) was placed in the ascending aorta via the right carotid artery and a second was placed in the caudal (tail) artery, as described previously (Musch & Terrell, 1992). Both catheters were tunnelled subcutaneously to the dorsal aspect of the cervical region, exteriorized through a puncture wound in the skin, and incisions were closed. Anaesthesia was then terminated and the animal was given 1–2 h to recover prior to the initiation of the final experimental protocol (Fig. 1).
Figure 1. Schematic representation of the experimental protocol for the rest and exercise groups.
See text for chronology.
Rest (n= 9)
Following recovery, the tail artery catheter was attached to a pressure transducer (Gould Statham P23ID, Valley View, OH, USA) which was connected to a recorder and 10 μg kg−1 of acetylcholine (ACh: Sigma Chemical, St Louis, MO, USA) was injected via the carotid artery catheter. The subsequent peak hypotensive response to ACh injections was measured and recorded. Following the normalization of mean arterial pressure (MAP), the tail artery catheter was connected to a 1 ml syringe and blood withdrawal was initiated at a rate of 0.25 ml min−1 via a Harvard infusion/withdrawal pump (model 907, Cambridge, MA, USA). Simultaneously, heart rate (HR) and MAP were measured and recorded via the carotid artery catheter for ∼10 s. Immediately after the HR and MAP recording, the carotid artery catheter was disconnected from the pressure transducer and 0.5–0.6 × 106 microspheres 15 μm in diameter (85Sr or 46Sc in random order: Perkin Elmer Life and Analytical Sciences, Waltham, MA, USA) were injected into the aortic arch to determine regional blood flows.
Following the initial microsphere injection, the animal remained in the resting condition for ∼10 min after which 2.1 ± 0.1 μmol kg−1 of SMTC (Sigma), an nNOS inhibitor with a 17-fold selectivity for nNOS over eNOS (Furfine et al. 1994), dissolved in 1.2 ml of saline, was infused into the tail artery catheter for 6 min. This dose of SMTC was selected based on previous investigations in which similar doses were used to inhibit nNOS (Ichihara et al. 1998; Wakefield et al. 2003; Seddon et al. 2008, 2009) and preliminary studies in our laboratory where we examined the highest possible SMTC dose that could be administered without affecting the hypotensive responses to ACh (authors’ unpublished observations). Once the infusion was complete, a second microsphere injection (differently labelled from the first injection) followed by a second ACh injection protocol were performed exactly as described above. Subsequent to the recovery of MAP after the second ACh injection (∼5 min), 10 mg kg−1 of the non-selective NOS inhibitor l-NAME (Sigma) was administered into the carotid artery catheter, and HR and MAP were monitored for 5 min. A third injection of 10 μg kg−1 ACh was then performed and the hypotensive response was again measured.
Exercise (n= 10)
Each rat was placed initially on the treadmill and, after a period of stabilization (∼2 h after instrumentation), 10 μg kg−1 of ACh was injected into carotid artery catheter. The peak hypotensive response was measured and recorded from the tail artery catheter. After the injection, the tail artery catheter was connected to a 1 ml plastic syringe and the Harvard infusion/withdrawal pump. Exercise was initiated, and the speed of the treadmill increased progressively over the next 30 s to a speed of 20 m min−1 (10% grade) which has been demonstrated previously to elicit ∼55–65% of maximum oxygen uptake 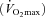 (Musch et al. 1988). The rat was then exercised steadily and after ∼3.5 min of total exercise time, blood withdrawal from the tail artery catheter was initiated at a rate of 0.25 ml min−1. HR and MAP were measured simultaneously and recorded via the carotid artery catheter for ∼10 s. Immediately afterwards, the carotid artery catheter was disconnected from the pressure transducer and 0.5–0.6 × 106 microspheres (85Sr or 46Sc in random order) were injected into the aortic arch. Approximately 15–30 s after microsphere injection, exercise was terminated and each rat was allowed a minimum of 30 min to recover.
(Musch et al. 1988). The rat was then exercised steadily and after ∼3.5 min of total exercise time, blood withdrawal from the tail artery catheter was initiated at a rate of 0.25 ml min−1. HR and MAP were measured simultaneously and recorded via the carotid artery catheter for ∼10 s. Immediately afterwards, the carotid artery catheter was disconnected from the pressure transducer and 0.5–0.6 × 106 microspheres (85Sr or 46Sc in random order) were injected into the aortic arch. Approximately 15–30 s after microsphere injection, exercise was terminated and each rat was allowed a minimum of 30 min to recover.
After recovery from this exercise bout, 2.1 ± 0.1 μmol kg−1 of SMTC dissolved in 1.2 ml of saline was infused via the tail catheter over a 6 min period. Following the infusion, the tail catheter was re-connected to a syringe and the Harvard pump for blood withdrawal. The second bout of exercise, microsphere injection (differently labelled from the first run), and ACh injection protocols were performed exactly as described above for the control (non-SMTC infusion) condition. Following recovery from the second ACh injection and as described at rest, l-NAME was administered and MAP and HR were monitored for 5 min. Subsequently, a third ACh injection was performed. The hypotensive responses from the rest and exercise groups were combined and the responses were then compared among conditions in order to determine the efficacy of selective (SMTC) and non-selective (l-NAME) NOS inhibition.
Determination of blood flow and vascular conductance
Following the final (third) ACh injection, each animal was killed with a pentobarbital overdose administered via the carotid artery catheter. The thorax was opened, and placement of the carotid artery catheter into the aortic arch was confirmed by anatomical dissection. Organs of the splanchnic region, the kidneys and principal locomotor muscles of both hindlimbs were identified and removed. The tissues were blotted, weighed and placed immediately into counting vials.
The radioactivity of each tissue was determined on a gamma scintillation counter (Packard Auto Gamma Spectrometer, model 5230, Downers Grove, IL, USA). Accounting for cross-talk between isotopes (46Sc and 85Sr), blood flows to each tissue were determined using the reference sample method (Musch & Terrell, 1992) and expressed as milliliters per minute per 100 g of tissue (ml min−1 100 g−1). Adequate mixing of the microspheres was verified for each injection by demonstrating a <15% difference between blood flow to the right and left kidneys and/or to the right and left hindquarter musculature. All blood flow data were normalized to the MAP measured immediately prior to the microsphere injection and expressed as VC (ml min−1 100 g−1 mmHg−1).
Statistical analyses
Resting HR and MAP values as well as the hypotensive responses to ACh from the resting and exercising experimental groups were combined (n= 19) and compared among conditions (pre- and post-SMTC and post-l-NAME) via repeated measures one-way ANOVA. Where significant differences were found a Student–Newman–Keuls post hoc test was used to determine where differences existed. In the exercising group, HR and MAP measured during the two exercise bouts were compared via paired Student's t tests. Muscle blood flows and tissue VCs measured either at rest or during exercise before and after SMTC administration were compared using paired two-tailed Student's t tests. Pearson product moment correlations were performed to determine whether the absolute and relative reductions in resting blood flow (Δ blood flow) and VC (Δ VC) in the individual muscles or muscle parts of the hindlimb produced by SMTC administration were correlated with their estimated fibre type composition and/or relative blood flow and VC during the control condition. The fibre type composition of each muscle or muscle part was based on the percentage of type I and IIa fibres in the individual muscles and muscle parts of the rat hindlimb as described by Delp & Duan (1996). Results are presented as mean ±s.e.m. Significance was accepted at P < 0.05.
Results
Effects of SMTC on hindlimb muscle(s) blood flow and vascular conductance
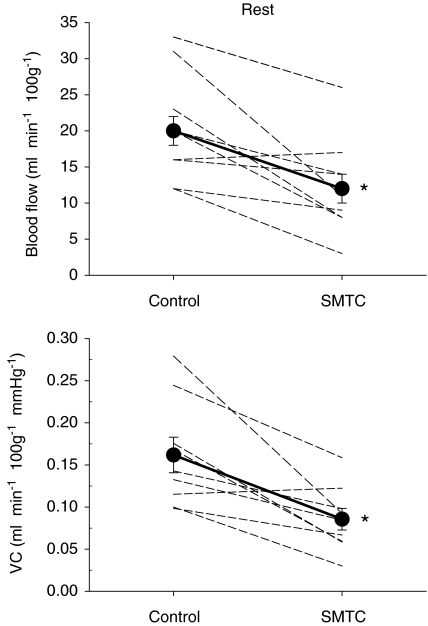
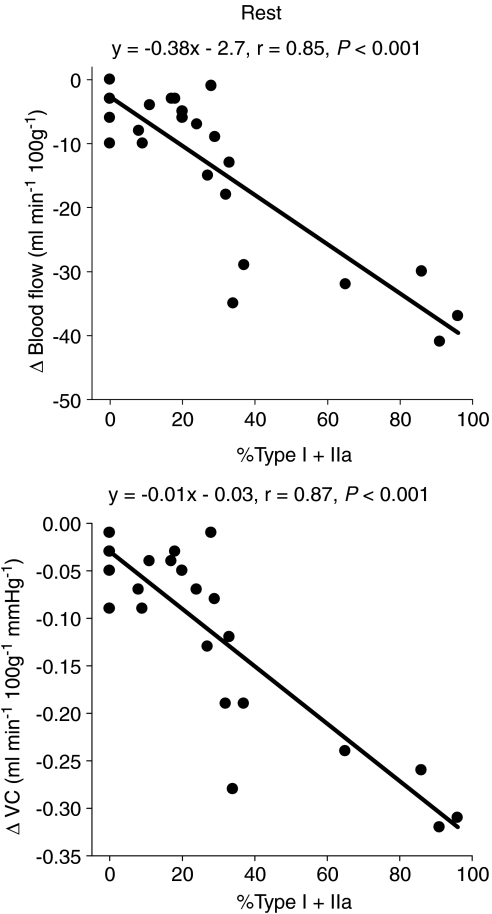
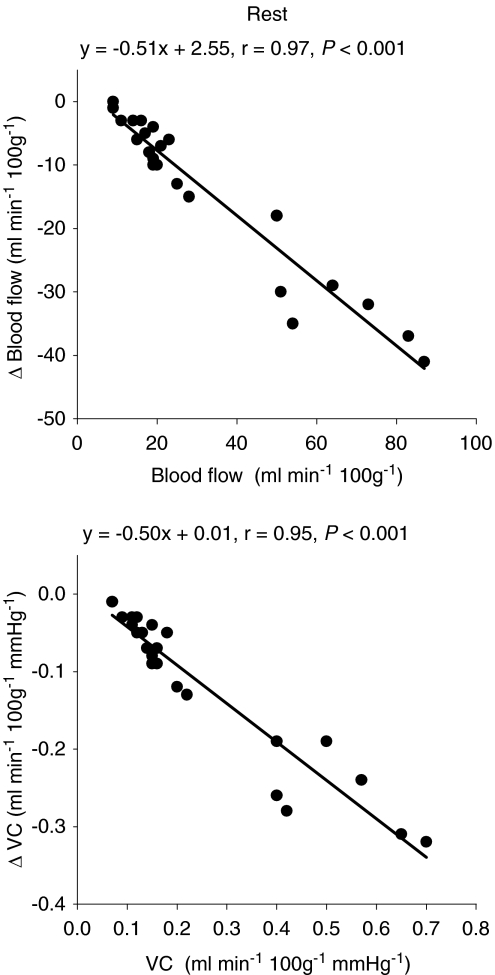
Total resting hindlimb muscle blood flow (control: 20 ± 2 ml min−1 100 g−1, SMTC: 12 ± 2 ml min−1 100 g−1, P < 0.05) and VC (control: 0.16 ± 0.02 ml min−1 100g−1 mmHg−1, SMTC: 0.09 ± 0.01 ml min−1 100g−1 mmHg−1, P < 0.05) were reduced following SMTC administration (Fig. 2). Specifically, blood flow was significantly reduced in 8 of 24 individual muscles or muscle parts whereas VC was reduced in 13 of 24 of these tissues (Table 1). The absolute Δ blood flow and Δ VC after SMTC were significantly correlated with the per cent sum of type I and IIa fibres found in the individual muscles or muscle parts (Δ blood flow: r= 0.85; Δ VC: r= 0.87, P < 0.001 for both, Fig. 3) such that the greatest effect of SMTC administration occurred in those muscles or muscle parts containing primarily oxidative muscle fibres. There were no significant correlations between the relative Δ blood flow and Δ VC after SMTC administration and the per cent sum of type I and IIa muscle fibres in the individual tissues examined. The absolute Δ blood flow and Δ VC after SMTC increased as a function of the relative resting blood flow (r= 0.97, P < 0.001) and VC (r= 0.95, P < 0.001) under control conditions, respectively, to the individual muscles or muscle parts of hindlimb (Fig. 4).
Figure 2. Effects of SMTC (2.1 ± 0.1 μmol kg−1) on resting total hindlimb blood flow (top panel) and vascular conductance (VC, bottom panel).
Individual animals (dashed lines, n= 9) and mean values (filled symbols) are plotted. *P < 0.05 vs. control.
Table 1.
Effects of SMTC (2.1 ± 0.1 μmol kg−1) on resting blood flow (ml min−1 100 g−1) and vascular conductance (ml min−1 100 g−1 mmHg−1) to the individual muscles or muscle parts of the rat hindlimb
| Blood flow |
Vascular conductance |
|||
|---|---|---|---|---|
| Control | SMTC | Control | SMTC | |
| Ankle extensors | ||||
| Soleus | 87 ± 14 | 46 ± 8* | 0.70 ± 0.11 | 0.38 ± 0.07* |
| Plantaris | 17 ± 3 | 12 ± 4 | 0.13 ± 0.02 | 0.08 ± 0.02 |
| Red gastrocnemius | 51 ± 12 | 20 ± 6* | 0.40 ± 0.09 | 0.15 ± 0.03* |
| White gastrocnemius | 15 ± 2 | 9 ± 2* | 0.12 ± 0.01 | 0.07 ± 0.02* |
| Mixed gastrocnemius | 19 ± 4 | 9 ± 2* | 0.15 ± 0.03 | 0.06 ± 0.01* |
| Tibialis posterior | 28 ± 7 | 14 ± 4 | 0.22 ± 0.05 | 0.10 ± 0.03* |
| Flexor digitorum longus | 50 ± 11 | 32 ± 15 | 0.40 ± 0.09 | 0.21 ± 0.11 |
| Flexor halicus longus | 19 ± 3 | 10 ± 3* | 0.15 ± 0.02 | 0.07 ± 0.02* |
| Ankle flexors | ||||
| Tibialis anterior, red | 64 ± 9 | 35 ± 14 | 0.50 ± 0.06 | 0.31 ± 0.04 |
| Tibialis anterior, white | 23 ± 3 | 16 ± 4 | 0.18 ± 0.02 | 0.13 ± 0.03 |
| Extensor digitorum longus | 21 ± 3 | 14 ± 5 | 0.16 ± 0.02 | 0.09 ± 0.03 |
| Peroneals | 25 ± 5 | 12 ± 4 | 0.20 ± 0.05 | 0.08 ± 0.02* |
| Knee extensors | ||||
| Vastus intermedius | 83 ± 13 | 46 ± 9* | 0.65 ± 0.09 | 0.34 ± 0.06* |
| Vastus medialis | 14 ± 3 | 11 ± 2 | 0.11 ± 0.02 | 0.08 ± 0.01 |
| Vastus lateralis, red | 73 ± 14 | 41 ± 14 | 0.57 ± 0.10 | 0.33 ± 0.12 |
| Vastus lateralis, white | 9 ± 1 | 9 ± 2 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| Vastus lateralis, mixed | 19 ± 3 | 15 ± 3 | 0.15 ± 0.02 | 0.10 ± 0.02 |
| Rectus femoris, red | 54 ± 14 | 18 ± 6* | 0.42 ± 0.10 | 0.14 ± 0.04* |
| Rectus femoris, white | 16 ± 2 | 13 ± 3 | 0.12 ± 0.01 | 0.09 ± 0.02 |
| Knee flexors | ||||
| Biceps femoris anterior | 11 ± 1 | 8 ± 1 | 0.09 ± 0.01 | 0.06 ± 0.01* |
| Biceps femoris posterior | 18 ± 3 | 10 ± 2 | 0.14 ± 0.03 | 0.07 ± 0.01* |
| Semitendinosus | 20 ± 3 | 10 ± 2* | 0.16 ± 0.03 | 0.07 ± 0.01* |
| Semimembranosus, red | 14 ± 1 | 11 ± 2 | 0.11 ± 0.01 | 0.07 ± 0.01* |
| Semimembranosus, white | 9 ± 1 | 8 ± 1 | 0.07 ± 0.01 | 0.06 ± 0.01 |
Data are mean ±s.e.m. n= 9
P < 0.05 vs. control.
Figure 3.
Correlations between the per cent sum of type I and IIa fibres in the individual muscles and muscle parts of the rat hindlimb and the absolute changes in resting blood flow (Δ blood flow) and vascular conductance (Δ VC) after SMTC infusion (2.1 ± 0.1 μmol kg−1).
Figure 4.
Correlations between the relative resting blood flow and vascular conductance (VC) to the individual muscles or muscle parts of the rat hindlimb and the absolute change in relative resting blood flow (Δ blood flow) and VC (Δ VC) after SMTC infusion (2.1 ± 0.1 μmol kg−1).
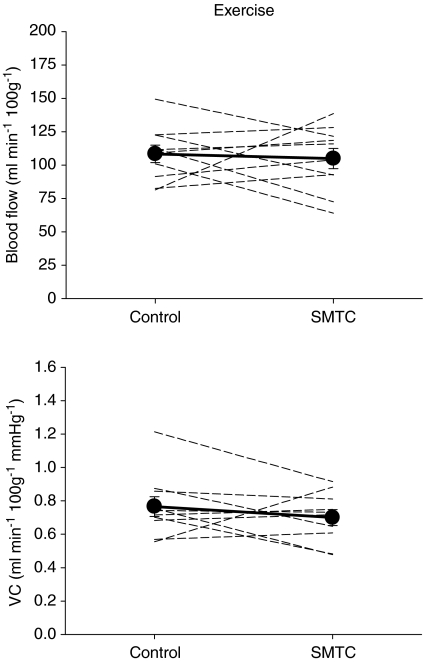
In marked contrast to the resting condition, total hindlimb muscle blood flow (control: 108 ± 7 ml min−1 100 g−1; SMTC: 105 ± 8 ml min−1 100 g−1, P > 0.05) and VC (control: 0.77 ± 0.06 ml min−1 100g−1 mmHg−1; SMTC: 0.70 ± 0.05 ml min−1 100g−1 mmHg−1, P > 0.05) during exercise were not different between control and SMTC conditions (Fig. 5). Moreover, blood flow and VC were not reduced (P > 0.05) in any of the 24 individual muscles or muscle parts of the hindlimb musculature.
Figure 5. Effects of SMTC (2.1 ± 0.1 μmol kg−1) on exercising total hindlimb blood flow (top panel) and vascular conductance (VC, bottom panel).
Individual animals (dashed lines, n= 10) and mean values (filled symbols) are plotted. There were no differences (P > 0.05) between control and SMTC for either measurement.
Effects of SMTC on kidney and splanchnic organ blood flow
During both rest and exercise SMTC administration reduced (P < 0.05) blood flow and VC in the right and left kidney, adrenal glands and liver but not in the small or large intestine, pancreas, spleen or stomach (Table 2).
Table 2.
Effects of SMTC (2.1 ± 0.1 μmol kg−1) on resting (n= 9) and exercising (n= 10) blood flow (ml min−1 100 g−1) and vascular conductance (ml min−1 100 g−1 mmHg−1) to the kidneys and organs of the splanchnic region
| At rest |
During exercise |
|||||||
|---|---|---|---|---|---|---|---|---|
| Blood flow |
Vascular conductance |
Blood flow |
Vascular conductance |
|||||
| Control | SMTC | Control | SMTC | Control | SMTC | Control | SMTC | |
| Right kidney | 871 ± 92 | 514 ± 45* | 6.92 ± 0.68 | 3.89 ± 0.40* | 666 ± 74 | 452 ± 30* | 4.73 ± 0.60 | 3.03 ± 0.18* |
| Left kidney | 880 ± 101 | 499 ± 65* | 6.98 ± 0.73 | 3.71 ± 0.50* | 666 ± 79 | 431 ± 31* | 4.75 ± 0.67 | 2.90 ± 0.20* |
| Stomach | 137 ± 24 | 111 ± 20 | 1.08 ± 0.18 | 0.80 ± 0.16 | 71 ± 12 | 61 ± 12 | 0.51 ± 0.10 | 0.42 ± 0.09 |
| Adrenals | 736 ± 103 | 443 ± 74* | 6.35 ± 0.60 | 3.71 ± 0.68* | 653 ± 85 | 261 ± 25* | 4.64 ± 0.69 | 1.77 ± 0.19* |
| Spleen | 269 ± 32 | 310 ± 30 | 2.13 ± 0.26 | 2.21 ± 0.26 | 60 ± 14 | 73 ± 19 | 0.43 ± 0.10 | 0.49 ± 0.12 |
| Pancreas | 240 ± 42 | 180 ± 29 | 1.88 ± 0.31 | 1.35 ± 0.21 | 132 ± 27 | 85 ± 20 | 0.94 ± 0.19 | 0.60 ± 0.15 |
| Small intestine | 539 ± 80 | 502 ± 76 | 4.24 ± 0.58 | 4.09 ± 0.55 | 366 ± 60 | 395 ± 55 | 2.61 ± 0.45 | 2.66 ± 0.38 |
| Large intestine | 309 ± 43 | 287 ± 34 | 2.47 ± 0.35 | 2.19 ± 0.25 | 194 ± 44 | 179 ± 43 | 1.71 ± 0.28 | 1.42 ± 0.21 |
| Liver | 26 ± 3 | 17 ± 3* | 0.22 ± 0.02 | 0.14 ± 0.03* | 30 ± 8 | 11 ± 1* | 0.21 ± 0.06 | 0.07 ± 0.01* |
Data are mean ±s.e.m.
P < 0.05 vs. control
Effects of SMTC and l-NAME on MAP, HR and hypotensive response to ACh injections
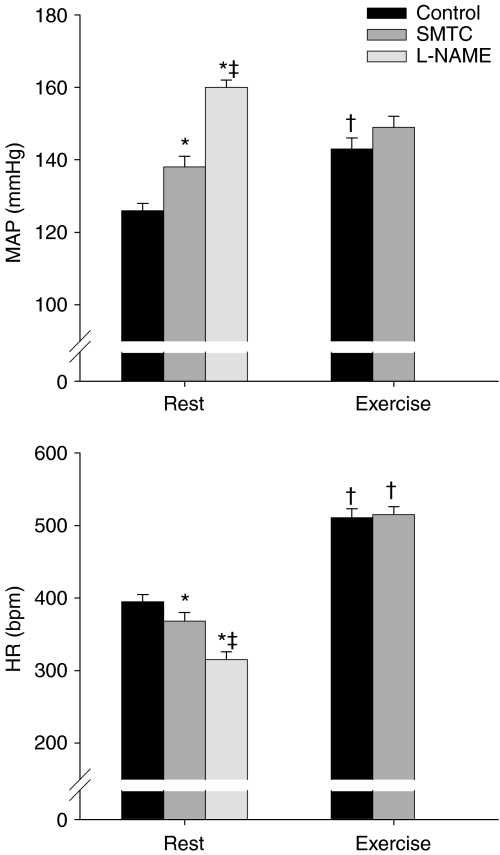
The effects of SMTC and l-NAME administration on resting and exercising MAP and HR are presented in Fig. 6. At rest, SMTC increased MAP and reduced HR compared to the control condition. The administration of l-NAME further elevated MAP and decreased HR such that the values attained were significantly different (P < 0.05) from both the control and SMTC conditions. In the exercising rats, MAP and HR measured during exercise immediately prior to microsphere injection were elevated above resting values in the control condition, whereas after SMTC administration exercising HR was significantly elevated (P < 0.05) above rest but MAP was not. More importantly, the MAP and HR measured during exercise were not different between control and SMTC conditions.
Figure 6. Mean arterial pressure (MAP, top panel) and heart rate (HR, bottom panel) during control and after SMTC (2.1 ± 0.1 μmol kg−1) and l-NAME (10 mg kg−1) administration.
Rest, n= 19; exercise, n= 10; *P < 0.05 vs. control, ‡P < 0.05 vs. SMTC, †P < 0.05 vs. same condition at rest.
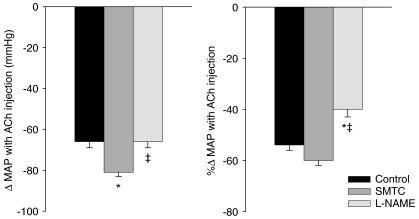
The absolute and relative hypotensive responses (Δ MAP) to ACH injection are presented in Fig. 7. The absolute Δ MAP was increased after SMTC administration compared to control whereas after l-NAME it was reduced compared to SMTC but not control. Conversely, there were no differences in the relative Δ MAP after ACh injections between control and SMTC conditions, and l-NAME resulted in significant blunting of relative Δ MAP compared to both control and SMTC.
Figure 7. Absolute and relative hypotensive responses to acetylcholine injection (10 μg kg−1) for control condition and after SMTC (2.1 ± 0.1 μmol kg−1) and l-NAME (10 mg kg−1) administration.
n= 19, *P < 0.05 vs. control, ‡P < 0.05 vs. SMTC.
Discussion
The present investigation is the first of its kind to investigate systematically the effects of selective nNOS inhibition on inter- and intramuscular hindlimb muscle(s) blood flow at rest and during submaximal treadmill exercise in the conscious rat. Consistent with our hypothesis, total hindlimb muscle blood flow and VC were reduced at rest after SMTC. The absolute, but not relative, Δ blood flow and Δ VC to the individual tissues after nNOS inhibition were correlated with muscle fibre type such that the greatest reductions occurred in individual muscles and muscle parts with the greatest proportion of oxidative fibres. However, contrary to our hypothesis, there was no effect of nNOS inhibition on exercising blood flow or VC in the total hindlimb or any of the individual muscles or muscle parts. In addition, nNOS inhibition reduced right and left kidney, adrenal and liver blood flow both at rest and during exercise. The present study demonstrates that nNOS-derived NO plays an integral role in regulating basal (i.e. resting) locomotory skeletal muscle blood flow but appears not to be obligatory for achieving the exercise hyperaemic response, at least during these submaximal running speeds (equivalent to ∼55–65% of 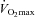 ) in the rat.
) in the rat.
Relationship with the literature
Presently, we found that SMTC administration reduced resting total hindlimb muscle blood flow and VC by 38% and 47%, respectively. These results are consistent with recent reports from Seddon and colleagues of 30% (2008) and 36% (2009) SMTC-induced blood flow reductions in the human forearm. In those same investigations from Seddon et al. infusion of the non-selective NOS inhibitor NG-monomethyl-l-arginine (l-NMMA) reduced blood flow by only marginally higher values of 37% (2008) and 40% (2009), respectively. Taken together, these data suggest that at rest nNOS may supply the bulk of the NO-mediated blood flow regulatory signal (Seddon et al. 2008, 2009). Furthermore, our data demonstrate that within the hindlimb musculature the greatest reductions in resting blood flow and VC occurred in the muscles composed of primarily oxidative muscle fibres. This was somewhat surprising given that previous in vitro (Roberts et al. 1999) and histochemical (Kobzik et al. 1994) data in rodents suggest that nNOS is the predominant NOS isoform in less-oxidative fast-twitch muscle but not in more oxidative muscle fibres. The different implications from those reports and our current study are probably due to our ability to measure specifically blood flow within an intact physiological model. For example, we demonstrate a strong correlation between the Δ blood flow and VC after SMTC and the relative blood flow and VC, respectively, measured during control conditions (Fig. 4). This may indicate that it is not fibre-type composition per se, but rather the relatively high resting muscle blood flows and VCs that drive the effects of SMTC in the primarily oxidative muscles.
Previously, our laboratory has utilized the conscious rat model of treadmill exercise in the presence of the non-selective NOS inhibitor l-NAME to demonstrate that NO plays an important role in the regulation of hindlimb muscle blood flow during locomotory exercise (Hirai et al. 1994; Musch et al. 2001; Copp et al. 2010). While those studies and others using non-selective NOS inhibition cannot identify the specific isoform from which the NO originates, eNOS-derived NO has traditionally been considered to be responsible for most, if not all, of the NO response. However, evidence in contracting mouse (Thomas et al. 1998; Grange et al. 2001; Fadel et al. 2003) and human (Sander et al. 2000) muscle in which nNOS is absent provides support for nNOS-derived NO as a key component in NO-mediated blood flow control during muscle contractions. Specifically, in healthy subjects, nNOS-derived NO attenuates noradrenaline (norepinephrine)-induced vasoconstriction in active muscle vascular beds whereas this sympathetic modulation is absent in skeletal muscle that lacks nNOS (Thomas et al. 1998; Sander et al. 2000). More recently, novel work in transgenic mdx mice (a model of Duchenne muscular dystrophy with transplanted nNOS anchoring proteins) indicates that these animals have greater active locomotor muscle blood flow and thus enhanced treadmill exercise performance compared to their non-transgenic mdx counterparts (in which nNOS is absent; Lai et al. 2009). While these observations have important clinical significance for a variety of chronic pathological conditions in which nNOS structure or function is altered, our current study is the first to test the hypothesis that, during physiological exercise, nNOS-derived NO contributes to the regulation of active muscle blood flow in healthy individuals. However, contrary to our hypothesis, our data demonstrate that nNOS inhibition does not alter the hyperaemic response to exercise.
The control of blood flow within active skeletal muscle is hallmarked by substantial redundancy and synergy (Clifford & Hellsten, 2004). For example, when one NOS isoform is chronically disrupted (i.e. eNOS or nNOS knockout models) compensatory mechanisms may increase NO production from existing isoforms (Huang et al. 2002; Kavdia & Popel, 2004; Talukder et al. 2004). Additionally, there is evidence that NO is not the sole mediator of sympathetic modulation within active skeletal muscle vascular beds (Dinenno & Joyner, 2003, 2004; Buckwalter et al. 2004) and the roles of other substances may increase when nNOS is inhibited. Until any such redundancy of the NO sympathetic-inhibitory and/or vasodilatory signal in healthy subjects is dissected systematically, it may be inappropriate to conclude that NO derived from nNOS does not contribute to locomotor skeletal muscle blood flow regulation during exercise under normal physiological conditions (i.e. in the absence of SMTC administration). Rather, the present investigation indicates that nNOS participation may not be obligatory for the exercise hyperaemic response.
At rest, the splanchnic and renal circulations may account for nearly 50% of the total cardiac output and thus serve as important controllers of MAP, and constitute a vast blood volume reservoir for the active skeletal muscle to draw upon during dynamic exercise (Rowell, 1993). Previous investigations have indicated that NO is an important blood flow regulator in these circulations (Rockey & Chung, 1998; Jankord et al. 2009). The present investigation identifies nNOS-derived NO as an integral mediator of kidney, adrenal and liver blood flow at rest and during exercise which is consistent with in vitro (Ichihara et al. 1998) and anaesthetized (Jansson et al. 2005) rodent models after SMTC administration. The ∼35% reduction in liver blood flow and VC found herein is in agreement with the presence of nNOS in the liver vasculature of the rat (Wei et al. 2002). However, it is important to note that the human liver is thought to lack nNOS (McNaughton et al. 2002). Collectively, the literature supports a crucial role of nNOS in the regulation of the kidney, adrenal and liver circulations and, accordingly, diseases affecting these circulations have been associated with impaired nNOS function (Rockey & Chung, 1998; Kwon et al. 2009).
Experimental considerations
The interpretation of the present results depends highly on the efficacy of the current dose of SMTC (2.1 ± 0.1 μmol kg−1 or 0.56 ± 0.02 mg kg−1) to block nNOS without affecting eNOS-mediated function. We believe that the selected dose was appropriate for the following reasons: First, the SMTC dose used presently was selected based on previous investigations in rats reporting selective nNOS inhibition with SMTC in vivo (Ichihara et al. 1998; Komers et al. 2000; Wakefield et al. 2003) and preliminary studies from our laboratory which attempted to elucidate the highest SMTC dose that did not affect endothelial-mediated NO function (authors’ unpublished observations). Indeed, the SMTC dose utilized in the present investigation is slightly greater than the highest in vivo doses of SMTC that achieved selective nNOS inhibition in rats as reported by Wakefield et al. (2003; 0.3 mg kg−1) and Komers et al. (2000; 0.5 mg kg−1). Second, it is important to note the similar reductions in blood flow and VC observed in the kidneys, adrenals and liver both at rest and during exercise. This suggests that the SMTC dose was sufficient to inhibit nNOS under both conditions, and the lack of an effect observed during exercise in the hindlimb musculature results from the non-obligatory role of nNOS-derived NO during exercise in contrast to rest. Third, to confirm that the SMTC dose used presently did not affect endothelial-mediated NO function, we examined the hypotensive responses to ACh injections before and after SMTC, and after l-NAME administration (which is expected to inhibit endothelial-mediated vasodilatation). We considered that if SMTC did not affect eNOS function, the hypotensive response to ACh would not be attenuated when compared to control conditions. Conversely, we anticipated that eNOS function and, therefore, the hypotensive response to ACh, would be attenuated after l-NAME. As anticipated, SMTC did not attenuate the absolute or relative hypotensive responses to ACh compared to control. Furthermore, the absolute hypotensive response after l-NAME was attenuated compared to SMTC but not control, and the relative hypotensive response was attenuated compared to both control and SMTC conditions. Taking into account the primary comparison of interest (control vs. SMTC) and the considerations presented above, our results support that SMTC administration in the present study effectively inhibited nNOS but did not inhibit eNOS.
NO has many diverse roles in intact biological systems. For example, NO derived from nNOS has been implicated in the metabolic inhibition of sympathetically mediated vasoconstriction (i.e. functional sympatholysis; Thomas et al. 1998) as well as regulation of regional sympathetic nerve activity (Hirai et al. 1995), the modulation of skeletal muscle force production (Hirschfield et al. 2000), and the direct relaxation of vascular smooth muscle (Grange et al. 2001). Given that the same absolute treadmill speed was used before and after SMTC, the power output necessary to sustain running was the same during the two exercise bouts. Therefore, muscle force production probably changed little, if at all, between control and SMTC conditions. In addition, given that SMTC increased MAP at rest, we report both resting blood flow and VC. The fact that SMTC reduced blood flow and VC at rest provides convincing evidence that nNOS-derived NO is an obligatory controller of local resting muscle perfusion. However, the present in vivo model does not allow for the determination of the specific mechanisms by which bioavailable nNOS-derived NO exerts its influence on the regulation of blood flow and VC. Notwithstanding this model limitation, a major strength of the present study is that it presents the first evaluation of the effects of selective nNOS inhibition on circulatory control in a healthy, intact biological system performing locomotory exercise.
Summary and conclusions
This study investigated the effects of selective nNOS inhibition via SMTC on hindlimb muscle(s) blood flow and VC at rest and during submaximal treadmill exercise in the healthy conscious rat. We have identified that nNOS inhibition reduces blood flow and VC in the skeletal muscle of the hindlimb at rest; however, nNOS inhibition had no effect on hindlimb musculature blood flow and VC during exercise. These results enhance our understanding of the role of nNOS in circulatory control by demonstrating that nNOS-derived NO is not obligatory for achieving the hyperaemic response in healthy rats performing whole-body dynamic exercise. Moreover, our data may be directly relevant to a growing number of clinical populations in which impaired vascular control and reduced NO bioavailability are hallmarks of disease pathology.
Acknowledgments
The authors would like to thank K. Sue Hageman and Robert T. Davis for their valuable technical help in conducting this study. This work was supported by a Kansas State University SMILE grant to D.C.P. and American Heart Association Heartland Affiliate Grant 070090Z to T.I.M.
Glossary
Abbreviations
- ACh
acetylcholine
- eNOS
endothelial nitric oxide synthase
- HR
heart rate
- MAP
mean arterial pressure
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- SMTC
S-methyl-l-thiocitrulline
- l-NAME
NG-nitro- l-arginine-methyl-ester
- VC
vascular conductance
Author contributions
All authors contributed to the conception and design of the experimental protocol, analysis and interpretation of data, and revision of the manuscript. S.W.C. wrote the initial draft of the manuscript. D.C.P. and T.I.M. secured funding for the project. All authors have read and approved the final version of the manuscript. Experiments were conducted at Kansas State University, Manhattan, KS, USA.
References
- Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Role of nitric oxide in exercise sympatholysis. J Appl Physiol. 2004;97:417–423. doi: 10.1152/japplphysiol.01181.2003. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Hageman KS, Poole DC, Musch TI. Nitric oxide synthase inhibition during treadmill exercise reveals fibre-type specific vascular control in the rat hindlimb. Am J Physiol Regul Integr Comp Physiol. 2010;298:R478–R485. doi: 10.1152/ajpregu.00631.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibres and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory. J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Zhao W, Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol. 2003;549:243–253. doi: 10.1113/jphysiol.2003.038828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfine ES, Harmon MF, Paith JE, Knowles RG, Salter M, Kiff RJ, Duffy C, Hazelwood R, Oplinger JA, Garvey EP. Potent and selective inhibition of human nitric oxide synthasesSelective inhibition of neuronal nitric oxide synthase by S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline. J Biol Chem. 1994;269:26677–26683. [PubMed] [Google Scholar]
- Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics. 2001;5:35–44. doi: 10.1152/physiolgenomics.2001.5.1.35. [DOI] [PubMed] [Google Scholar]
- Hirai T, Musch TI, Morgan DA, Kregel KC, Claassen DE, Pickar JG, Lewis SJ, Kenney MJ. Differential sympathetic nerve responses to nitric oxide synthase inhibition in anaesthetized rats. Am J Physiol Regul Integr Comp Physiol. 1995;269:R807–R813. doi: 10.1152/ajpregu.1995.269.4.R807. [DOI] [PubMed] [Google Scholar]
- Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol. 1994;77:1288–1293. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- Hirschfield W, Moody MR, O’Brien WE, Gregg AR, Bryan RM, Jr, Reid MB. Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am J Physiol Regul Integr Comp Physiol. 2000;278:R95–R100. doi: 10.1152/ajpregu.2000.278.1.R95. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Shesely EG, Levee EM, Koller A, Kaley G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2002;282:H429–H436. doi: 10.1152/ajpheart.00501.2001. [DOI] [PubMed] [Google Scholar]
- Ichihara A, Inscho EW, Imig JD, Navar LG. Neuronal nitric oxide synthase modulates rat renal microvascular function. Am J Physiol Renal Physiol. 1998;274:F516–F524. doi: 10.1152/ajprenal.1998.274.3.F516. [DOI] [PubMed] [Google Scholar]
- Ikenaga H, Fallet RW, Carmines PK. Basal nitric oxide production curtails arteriolar vasoconstrictor responses to ANG II in rat kidney. Am J Physiol Renal Physiol. 1996;271:F365–F373. doi: 10.1152/ajprenal.1996.271.2.F365. [DOI] [PubMed] [Google Scholar]
- Jankord R, McAllister RM, Ganjam VK, Laughlin MH. Chronic inhibition of nitric oxide synthase augments the ACTH response to exercise. Am J Physiol Regul Integr Comp Physiol. 2009;296:R728–R734. doi: 10.1152/ajpregu.90709.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson L, Carlsson PO, Bodin B, Andersson A, Kallskog O. Neuronal nitric oxide synthase and splanchnic blood flow in anaesthetized rats. Acta Physiol Scand. 2005;183:257–262. doi: 10.1111/j.1365-201X.2004.01396.x. [DOI] [PubMed] [Google Scholar]
- Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol. 2004;97:293–301. doi: 10.1152/japplphysiol.00049.2004. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Komers R, Oyama TT, Chapman JG, Allison KM, Anderson S. Effects of systemic inhibition of neuronal nitric oxide synthase in diabetic rats. Hypertension. 2000;35:655–661. doi: 10.1161/01.hyp.35.2.655. [DOI] [PubMed] [Google Scholar]
- Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- Kwon O, Hong SM, Ramesh G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am J Physiol Renal Physiol. 2009;296:F25–F33. doi: 10.1152/ajprenal.90531.2008. [DOI] [PubMed] [Google Scholar]
- Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics. 2000;2:21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci U S A. 2002;99:17161–17166. doi: 10.1073/pnas.0134112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol. 1988;65:964–970. doi: 10.1152/jappl.1988.65.2.964. [DOI] [PubMed] [Google Scholar]
- Musch TI, McAllister RM, Symons JD, Stebbins CL, Hirai T, Hageman KS, Poole DC. Effects of nitric oxide synthase inhibition on vascular conductance during high speed treadmill exercise in rats. Exp Physiol. 2001;86:749–757. doi: 10.1111/j.1469-445x.2001.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol. 1992;262:H411–H419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- Radegran G, Hellsten Y. Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2000;168:575–591. doi: 10.1046/j.1365-201x.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, Jasman A, Balon TW. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol Endocrinol Metab. 1999;277:E390–E394. doi: 10.1152/ajpendo.1999.277.2.E390. [DOI] [PubMed] [Google Scholar]
- Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- Rowell L. Human Cardiovascular Control. New York: Oxford University Press, Inc.; 1993. [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117:1991–1996. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Talukder MA, Fujiki T, Morikawa K, Motoishi M, Kubota H, Morishita T, Tsutsui M, Takeshita A, Shimokawa H. Up-regulated neuronal nitric oxide synthase compensates coronary flow response to bradykinin in endothelial nitric oxide synthase-deficient mice. J Cardiovasc Pharmacol. 2004;44:437–445. doi: 10.1097/01.fjc.0000139450.64337.cd. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of α-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires α-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res. 2003;92:554–560. doi: 10.1161/01.RES.0000061570.83105.52. [DOI] [PubMed] [Google Scholar]
- Wakefield ID, March JE, Kemp PA, Valentin JP, Bennett T, Gardiner SM. Comparative regional haemodynamic effects of the nitric oxide synthase inhibitors, S-methyl-L-thiocitrulline and L-NAME, in conscious rats. Br J Pharmacol. 2003;139:1235–1243. doi: 10.1038/sj.bjp.0705351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CL, Khoo HE, Lee KH, Hon WM. Differential expression and localization of nitric oxide synthases in cirrhotic livers of bile duct-ligated rats. Nitric Oxide. 2002;7:91–102. doi: 10.1016/s1089-8603(02)00103-9. [DOI] [PubMed] [Google Scholar]