Abstract
We recently provided evidence suggesting a role for cytokine-mediated inhibition of Akt/Forkhead box O 1 (FOXO1) signalling in the induction of muscle atrophy and impairment of muscle carbohydrate oxidation during lipopolysaccharide (LPS)-induced endotoxaemia in rats. We hypothesized that a low-dose dexamethasone (Dex; anti-inflammatory agent) infusion during endotoxaemia would prevent the LPS-induced impairment of Akt/FOXO1 signalling, and therefore prevent the muscle atrophy and impairment of carbohydrate oxidation. Chronically instrumented Sprague–Dawley rats received a continuous intravenous infusion of LPS (15 μg kg−1 h−1), Dex (12.5 μg kg−1 h−1), Dex+LPS or saline for 24 h at 0.4 ml h−1. LPS infusion caused haemodynamic changes consistent with a hyperdynamic circulation and induced increases in muscle tumour necrosis factor-α (TNF-α; 10-fold, P < 0.001), interleukin-6 (IL-6; 14-fold, P < 0.001) and metallothionein-1A (MT-1A; 187-fold, P < 0.001) mRNA expression. Dex co-administration abolished most of the haemodynamic effects of LPS and reduced the increase in muscle TNF-α, IL-6 and MT-1A by 51% (P < 0.01), 85% (P < 0.001) and 58% (P < 0.01), respectively. Dex infusion during endotoxaemia also prevented the LPS-induced 40% reduction in the muscle protein:DNA ratio and decrease in Akt phosphorylation, and partially prevented the reduction in FOXO1 phosphorylation. However, Dex did not prevent the LPS-mediated increase in muscle atrophy F-box (MAFbx) and muscle RING finger 1 (MuRF1) mRNA expression, but did significantly reduce the LPS-mediated increase in cathepsin-L mRNA expression and enzyme activity by 43% (P < 0.001) and 53% (P < 0.05), respectively. Furthermore, Dex suppressed LPS-induced pyruvate dehydrogenase kinase 4 (PDK4) mRNA upregulation by ∼50% (P < 0.01), and prevented LPS-mediated muscle glycogen breakdown and lactate accumulation. Thus, low-dose Dex infusion during endotoxaemia prevented muscle atrophy and the impairment of carbohydrate oxidation, potentially through suppression of cytokine-mediated Akt/FOXO inhibition, and blunting of cathepsin-L-mediated lysosomal protein breakdown.
Introduction
Sepsis is a complex disorder that arises from an uncontrolled systemic inflammatory response to an infection, and is a major cause of mortality in critically ill patients (Angus et al. 2001). The rapid and marked loss of skeletal muscle mass (Hasselgren et al. 2005) and the development of muscle insulin resistance (Lang et al. 1990) are two metabolic consequences of sepsis. Increased muscle protein breakdown during sepsis is considered to be primarily mediated by ubiquitin–proteasome pathway (UPP) ATP-dependent protein degradation (Voisin et al. 1996; Lecker et al. 1999), although other systems contribute to muscle atrophy during sepsis, including lysosome- (Deval et al. 2001) and calpain-dependent pathways (Smith et al. 2008).
We have previously shown that during lipopolysaccharide (LPS)-induced endotoxaemia in rats, muscle atrophy and impaired muscle carbohydrate oxidation occurred concomitantly with the inhibition of muscle Akt, activation of Forkhead Box O (FOXO) transcription factor family members (FOXO1/FKHR, FOXO3a/FKHRL1, FOXO4/AFX and FOXO6), and transcriptional upregulation of FOXO gene targets, i.e. muscle atrophy F-box (MAFbx), muscle RING finger 1 (MuRF1) and pyruvate dehydrogenase kinase 4 (PDK4) (Crossland et al. 2008). Activation of FOXO, as a result of impaired Akt signalling, has been implicated in the induction of muscle atrophy, via upregulation of E3-ubiquitin ligases MAFbx and MuRF1 (Stitt et al. 2004), which are considered to be key regulators of muscle protein degradation via the UPP (Bodine et al. 2001). Impaired Akt/FOXO signalling has also been implicated in muscle insulin resistance, through FOXO-mediated upregulation of PDK4 (Furuyama et al. 2003; Kim et al. 2006). Furthermore, increased muscle PDK4 expression downregulates the activity of the pyruvate dehydrogenase complex (PDC), leading to an impairment of muscle carbohydrate oxidation at rest and during contraction (Constantin et al. 2007; Constantin-Teodosiu et al. 2009). Therefore, the findings from our previous study (Crossland et al. 2008) strongly suggest a role for Akt/FOXO signalling in the simultaneous induction of muscle atrophy and impairment of muscle carbohydrate oxidation during endotoxaemia.
With evidence supporting a role for pro-inflammatory cytokines in both loss of muscle mass (see Spate & Schulze, 2004) and the development of muscle insulin resistance (Plomgaard et al. 2005), and with observations of elevated levels of tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) during LPS-induced endotoxaemia (Crossland et al. 2008), we hypothesized that pro-inflammatory cytokines were, at least partly, responsible for driving the induction of muscle atrophy and the inhibition of carbohydrate oxidation during endotoxaemia, through alterations in the Akt/FOXO signalling pathway (Crossland et al. 2008). Therefore, strategies aimed at suppressing increases in pro-inflammatory cytokines during endotoxaemia could potentially be beneficial in facilitating the recovery of muscle mass and improving insulin sensitivity and/or carbohydrate oxidation under these conditions.
Glucocorticoids have been proposed to be useful in the treatment of systemic inflammatory diseases due to their anti-inflammatory properties (for review see Rhen & Cidlowski, 2005), but the potential benefit of glucocorticoid therapy in the treatment of sepsis is controversial, and may depend on dose (Bone et al. 1987; Briegel et al. 1994; Cronin et al. 1995; Annane, 2001; Keh & Sprung, 2004). Indeed, previous reports have indicated that glucocorticoids are important in regulating muscle protein breakdown during sepsis (Tiao et al. 1996). However, low-dose glucocorticoid therapy has been proposed to be beneficial in survival from severe sepsis (Annane, 2001), possibly due to its anti-inflammatory properties. The anti-inflammatory properties of glucocorticoids that may be beneficial in sepsis treatment include inhibition of nuclear factor-κB (NF-κB) activity, inhibition of the production of most pro-inflammatory cytokines (including TNF-α and IL-6) in inflammatory cells, and activation of anti-inflammatory proteins (Rhen & Cidlowski, 2005).
Given the evidence supporting a role for cytokines in the aetiology of muscle protein loss and impairment of carbohydrate oxidation during endotoxaemia (Crossland et al. 2008), we hypothesized in the present study that a low-dose infusion of dexamethasone (Dex), a synthetic glucocorticoid, may suppress pro-inflammatory cytokine levels during endotoxaemia, and therefore protect against LPS-mediated muscle atrophy and impairment of carbohydrate oxidation, possibly through maintenance of Akt/FOXO signalling. Since high doses of Dex (ranging from 700 to 1000 μg kg−1 day−1 in rats) have been shown to induce muscle atrophy (Wang et al. 1998; Frost et al. 2007; Zhao et al. 2008b), as well as muscle insulin resistance (Qi et al. 2004; Buren et al. 2008), we used a much lower dose (equivalent to 300 μg kg−1 day−1), in an attempt to suppress pro-inflammatory cytokine levels without producing the catabolic and insulin resistance effects that are associated with higher doses of glucocorticoids.
We aimed to determine the effects of Dex on LPS-induced alterations in Akt/FOXO signalling as well as downstream gene targets of FOXO (MAFbx, MuRF1 and PDK4). Additionally, we measured cathepsin-L mRNA expression and enzyme activity as indices of lysosomal proteolysis (Deval et al. 2001). Cathepsin-L is known to be activated by cytokines (Ebisui et al. 1995), and it has recently been shown that cathepsin-L appears to be a direct target of FOXO1 in muscle (Yamazaki et al. 2010). Thus, suppression of cytokines by Dex could potentially affect lysosomal proteolysis in sepsis, via changes in FOXO1 signalling. We also measured metallothionein-1A (MT-1A), a gene associated with oxidative stress that is markedly upregulated in atrophying muscle (Lecker et al. 2004; Mallinson et al. 2009). Oxidative stress has been proposed to play a role in FOXO activation, at least in cell lines (Nakamura & Sakamoto, 2008), and could be an important trigger for the signalling changes associated with muscle atrophy and insulin resistance during endotoxaemia. Finally, although the present study focused on the effects of Dex on muscle during endotoxaemia, we also assessed the effects of Dex on the regional haemodynamic responses to LPS infusion in a complementary study, as a measure of the systemic effects of Dex during endotoxaemia.
Methods
Ethical approval
All procedures were approved by the University of Nottingham Ethical Review Committee and were performed under Home Office Project and Personal Licence authority (Animal Scientific Procedures Act 1986), in accordance with the policies and regulations described by The Journal of Physiology (Drummond, 2009).
Animals
Male, Sprague–Dawley rats (350–450 g) were purchased from Charles River (Margate, UK) and housed in the Biomedical Services Unit, University of Nottingham for at least 10 days after delivery, with free access to standard rat chow (Teklad Global 18% protein rodent diet, Bicester, Oxon, UK) and water. Room temperatures were maintained at 21 ± 2°C, and lights were on from 06.00 h to 18.00 h.
Surgical preparation and experimental design for haemodynamic studies
Surgery was performed under general anaesthesia (fentanyl citrate (Janssen-Cilag, High Wycombe, UK) and medetomidine (Domitor; Pfizer, Sandwich, UK; 300 μg kg−1 of each i.p.)). Anaesthetic reversal and the provision of analgesia was achieved with s.c. atipamezole (Antisedan; Pfizer, Sandwich, UK; 1 mg kg−1) and buprenorphine (Vetergesic; Alstoe Animal Health, York, UK; 0.03 mg kg−1). Animals were chronically instrumented with miniature pulsed Doppler flow probes and intravascular catheters as described previously (Jolly et al. 2008). The arterial and venous catheters were connected to double channel fluid-filled swivels for overnight infusion of heparinised saline (15 U ml−1, 0.4 ml h−1) to maintain catheter patency.
Experiments began 24 h after catheterization and ran over four experimental days in two groups of rats (n= 8 in each). On Days 1–2, saline (0.4 ml h−1) or Dex (12.5 μg kg−1 h−1, Sigma-Aldrich, Poole, UK) was given for 1 h before and during a 24 h infusion of saline (0.4 ml h−1). On Days 3–4, saline or Dex was given (as above) before and during LPS infusion (15 μg kg−1 h−1, E. coli, serotype 0127: B8, Sigma-Aldrich, Poole, UK). Data acquisition was as previously described (Jolly et al. 2008).
Surgical preparation and experimental design for tissue collection
Using the same anaesthetic regime as above, rats were implanted with a catheter in the jugular vein and experiments began 24 h later. Prepared rats were divided into four groups (n= 8 in each), and given saline (0.4 ml h−1) or Dex (12.5 μg kg−1 h−1) for 1 h before and during a 24 h infusion of saline (0.4 ml h−1) or LPS (15 μg kg−1 h−1). After 24 h, animals were terminally anaesthetised with thiobutabarbital sodium (Inactin; Sigma-Aldrich; 80 mg kg−1i.v.), the extensor digitorum longus (EDL) muscle from both hindlimbs was removed, and samples were immediately snap-frozen in liquid nitrogen. The fast-twitch EDL muscle was chosen due to its higher susceptibility to sepsis than slow-twitch muscle (Tiao et al. 1997).
Muscle analyses
Protein and DNA measurements
A portion of frozen muscle was freeze-dried and powdered, and alkaline-soluble protein and DNA were extracted from approximately 3 mg powdered muscle, using perchloric acid (PCA), with the protein finally isolated by the addition of KOH. Muscle alkaline-soluble protein and DNA was quantified according to Forsberg et al. (1991).
Real-time PCR measurements
Total RNA was isolated from frozen wet EDL muscle (20–30 mg) using Tri Reagent (Sigma-Aldrich), according to the manufacturer's protocol. Total RNA quantification, first-strand cDNA synthesis and real-time PCR protocols were carried out as previously described (Constantin et al. 2007). Taqman primer/probe sets were obtained from Applied Biosystems (Foster City, USA): TNF-α, IL-6, MAFbx, MuRF1, PDK4, MT-1A and cathepsin-L. The housekeeping gene hydroxymethylbilane synthase (HMBS) was used as an internal control as it was unaffected by the treatments (data not shown).
Relative quantification of gene expression between groups was calculated using the  method. The saline control group was given a value of 1, and fold changes in mRNA expression for the LPS-treated, Dex-treated and Dex+LPS-treated groups were calculated, relative to the control group.
method. The saline control group was given a value of 1, and fold changes in mRNA expression for the LPS-treated, Dex-treated and Dex+LPS-treated groups were calculated, relative to the control group.
Protein extraction and Western blotting measurements
Cytosolic and nuclear proteins were extracted from approximately 30 mg frozen wet tissue using a modification of the method by Blough et al. (1999). Extracted proteins were quantified using the Bradford assay (Bio-Rad, UK) and Western blotting was carried out, as previously described (Constantin et al. 2007). The antibodies used were purchased from Cell Signaling Technology (Danvers, MA, USA): total Akt (TAkt), phosphorylated Akt (serine473; pAkt), total FOXO 1 and 3 (TFOXO 1 and 3) and phosphorylated FOXO 1 and 3 (serine256 and serine253; pFOXO 1 and 3). The PDK4 antibody was a gift from AstraZeneca (Macclesfield, UK). Blots were incubated with ECL chemiluminescence reagent (Pierce, UK) and exposed to X-ray film. Bands were quantified by densitometry using GeneTools software (Syngene, Frederick, MD, USA). Values were adjusted by subtracting the background and normalized to an actin protein control for cytosolic proteins and lamin (New England BioLabs, Hitchin, UK) for nuclear proteins.
Muscle metabolite measurements
Freeze-dried and powdered EDL muscle samples (1–2 mg) were alkaline extracted and used for muscle glycogen determination according to Harris et al. (1974). Additionally, 5–10 mg of muscle powder was extracted with 0.5 mol l−1 PCA containing 1 mmol l−1 EDTA, then neutralised with 2.1 mol l−1 KHCO3. Muscle extracts were analysed for lactate concentrations, using a modification of the spectrophotometric methods of Harris et al. (1974).
Muscle enzyme activity measurements
Muscle 20S proteasome and cathepsin-L activity was assayed using muscle samples from the haemodynamics studies (see above), and hence no measurements were made from animals treated with Dex alone. Frozen muscle (15–25 mg) was homogenised, and the soluble muscle extract used to measure the chymotrypsin-like activity of the 20S proteasome using the fluorogenic substrate N-Suc-Leu-Leu-Val-Tyr-7-amido-4-methyl-coumarin (Sigma-Aldrich), according to Dawson et al. (1995). The same homogenate was used to determine cathepsin-L activity using the substrate Z-Phe-Arg-7-amido-4-methyl-coumarin (Sigma-Aldrich), according to Bergmeyer (1983).
Statistical analysis
Cardiovascular data are expressed as means ±s.e.m. Since not all data were normally distributed, within-group analyses were carried out by a non-parametric equivalent of analysis of variance (ANOVA) allowing for multiple comparisons (Friedman's test; Theodorsson-Norheim, 1987). P≤ 0.05 was taken as significant. Muscle data are presented as means ±s.e.m. and comparisons between treatment groups were performed using one-way ANOVA, with the Fisher's least significant difference post hoc test being used to locate any significant differences. Differences were considered statistically significant when P < 0.05.
Results
Effects of Dex on haemodynamic responses to LPS
In rats infused with saline–saline, the only significant haemodynamic change during the 25 h saline infusion was a fall in hindquarters vascular conductance at 24–25 h (Fig. 1).
Figure 1. Changes in cardiovascular variables during 25 h infusion of saline (left hand panel) or LPS (right hand panel) in rats pre-treated with saline (filled symbols) or dexamethasone (Dex; open symbols).
Values are means and vertical bars represent s.e.m. *P < 0.05 vs. baseline (Friedman's test).
In rats infused with Dex–saline, between 0 and 6 h after the onset of saline infusion, there was some bradycardia (significant at 3.5 and 6 h) and a modest increase in hindquarters vascular conductance (significant at 2.5 h and between 3.5 and 5 h), but no other haemodynamic changes. However, at 24–25 h, there was an increase in blood pressure, bradycardia and vasoconstriction in all three vascular beds (Fig. 1).
Infusion of LPS in saline-treated rats caused sustained tachycardia (significant from 0.5 h onwards), with a transient fall in blood pressure (at 1.25–1.5 h), but marked and persistent renal vasodilatation (from 0.75 h onwards), transient mesenteric vasoconstriction (between 1.5 and 5.5 h) and biphasic hindquarters vasodilatation (between 1 and 2 h and at 24–25 h; Fig. 1).
Infusion of LPS in Dex-treated rats caused tachycardia, which was significant between 0.25 and 6 h, but thereafter heart rate returned to baseline. This profile of heart rate change was different from that seen in saline-treated rats given LPS in which the tachycardia was sustained (see above). In Dex-treated rats given LPS, blood pressure showed an early modest rise (at 0.75–1 h) with a more substantial rise at 24–25 h. This differed from the blood pressure changes seen in saline-treated rats given LPS where there was an early fall and no delayed rise in blood pressure. In the renal vascular bed, vasodilatation occurred in Dex–LPS-treated rats, but the changes were slower in onset (significant from 1.75 h) than in the saline–LPS-treated rats (significant from 0.75 h) and not sustained at 24–25 h. Similarly, in Dex–LPS-treated rats, there was a transient mesenteric vasoconstriction (significant between 1.25–3 h) which was shorter in duration than in rats treated with saline–LPS. In the hindquarters vascular bed, rats treated with Dex–LPS showed a very modest and transient early rise in vascular conductance (at 1.25 h), which was followed by a fall (at 2.5–3.5 h) and then a return to baseline. These changes differed quite markedly from the biphasic hindquarters vasodilatation seen in rats given saline–LPS (see above).
Protein:DNA ratio
Twenty-four hours of LPS infusion resulted in a 40% reduction in the muscle protein:DNA ratio compared to saline (Fig. 2; P < 0.01), due to a reduction in alkaline-soluble protein concentration (Fig. 2A; P < 0.05) and no change in DNA content (Fig. 2B). There was no change in the protein:DNA ratio with infusion of Dex alone or Dex+LPS, compared to the saline control group (Fig. 2C).
Figure 2. Alkaline-soluble protein to DNA ratio in extensor digitorum longus (EDL) muscle following 24 h of dexamethasone (Dex), lipopolysaccharide (LPS) or Dex plus LPS infusion.
Alkaline-soluble protein (A) and DNA (B) concentration and protein:DNA ratio (C) in rat EDL muscle following 24 h Dex, LPS, Dex+LPS or saline infusion. Values represent means +s.e.m. Significantly different from saline group: *P < 0.05; **P < 0.01. Significantly different from Dex group: †P < 0.05; ††P < 0.01. Significantly different from LPS group: ‡P < 0.05; ‡‡P < 0.01.
Muscle mRNA expression
Figure 3 shows the fold changes in mRNA expression in muscles of Dex-, LPS- and Dex+LPS-treated animals compared to saline. Expression of TNF-α and IL-6 mRNA was not altered by Dex alone, whereas LPS infusion induced marked increases in both TNF-α and IL-6 mRNA relative to the saline control group (10-fold, P < 0.001 and 15-fold, P < 0.001 vs. saline, respectively). When Dex was co-administered with LPS, the LPS-mediated increase in TNF-α mRNA expression was markedly reduced, with a 5-fold increase compared to saline (P < 0.01). Dex+LPS administration completely suppressed the LPS-mediated increase in IL-6 mRNA expression.
Figure 3. mRNA expression of selected genes in rat extensor digitorum longus muscle, following 24 h of dexamethasone (Dex), lipopolysaccharide (LPS) or Dex plus LPS infusion.
Relative mRNA expression of saline controls was set at 1. Values are means and vertical bars represent s.e.m. Significantly different from saline group: *P < 0.05; **P < 0.01; ***P < 0.001. Significantly different from Dex group: †P < 0.05; ††P < 0.01; †††P < 0.001. Significantly different from LPS group: ‡P < 0.05; ‡‡P < 0.01; ‡‡‡P < 0.001.
Dex infusion resulted in no significant change in MAFbx or MuRF1 mRNA levels from saline control (Fig. 3). LPS treatment elicited increases in both MAFbx and MuRF1 transcript levels (4.6-fold, P < 0.001 vs. saline and 13-fold, P < 0.001 vs. saline, respectively), but Dex+LPS did not significantly alter the LPS-induced increase in MAFbx and MuRF1 mRNA expression levels (3.5-fold, P < 0.001 and 12-fold, P < 0.001 vs. saline, respectively). PDK4 mRNA expression was significantly increased in the Dex (6.7-fold, P < 0.01), LPS (16-fold, P < 0.001) and Dex+LPS (8.6-fold, P < 0.01) treatment groups compared to control. The increase in PDK4 mRNA expression in the LPS-treated group was, however, markedly greater than in rats receiving Dex+LPS, in which the increase was similar to that in rats given Dex alone.
Cathepsin-L mRNA was not significantly altered by Dex infusion, but LPS infusion caused a marked increase in cathepsin-L relative to saline controls (9.7-fold, P < 0.001). When Dex was co-administered with LPS, the LPS-mediated increase in cathepsin-L mRNA expression was markedly reduced, with a 5.5-fold increase compared to control (P < 0.001). Compared to control, Dex infusion alone caused no significant change in MT-1A mRNA expression, whereas LPS infusion caused a marked increase in MT-1A transcript levels (187-fold, P < 0.001 vs. saline). Administration of Dex+LPS significantly reduced the LPS-mediated increase in MT-1A mRNA expression, with a 79-fold increase compared to saline.
Muscle protein expression
The expression of pAkt protein did not change in muscles of Dex-treated rats (Fig. 4A and C), but was decreased 2-fold in muscles of LPS-treated rats, compared to saline (P < 0.05; Fig. 4A and C). Dex+LPS treatment prevented the LPS-induced reduction in pAkt protein. There was no significant change overall in TAkt between all treatment groups (Fig. 4B and C).
Figure 4. Cytosolic Akt protein expression in rat extensor digitorum longus (EDL) muscle.
A, representative Western blots of phosphorylated Akt protein. B, representative blot of total Akt protein. C, density of Akt protein bands, measured by Western blotting, in EDL muscle from rats administered dexamethasone (Dex), lipopolysaccharide (LPS), Dex+LPS or saline for 24 h. D, ratio of phosphorylated to total cytosolic Akt protein in EDL muscle of Dex-, LPS-, Dex+LPS- or saline-treated rats. Significantly different from saline group: *P < 0.05. Significantly different from Dex group: †P < 0.05; ††P < 0.01. Significantly different from LPS group: ‡P < 0.05.
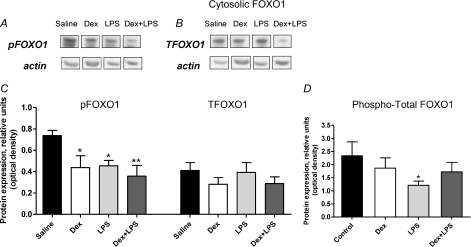
Compared to saline, Dex, LPS and Dex+LPS treatments all caused a reduction in cytosolic pFOXO1 (1.6-fold, P < 0.05; 2-fold, P < 0.01 and 1.6-fold, P < 0.05, respectively; Fig. 5A and C). There was no significant change in total cytosolic FOXO1 protein (Fig. 5B and C). Overall however, there was a significant reduction in the phosphorylated/total FOXO1 ratio within the cytosol of muscle from LPS-treated rats (P < 0.05 vs. saline; Fig. 5D), but in the Dex and Dex+LPS groups there was no significant change compared to the saline control group. Cytosolic FOXO3 protein expression was not significantly different across treatment groups, and there was no significant change in nuclear TFOXO1 across groups (data not shown).
Figure 5. Forkhead box O (FOXO) 1 protein expression in rat extensor digitorum longus (EDL) muscle.
A and B, representative Western blots of cytosolic phosphorylated and total FOXO1 protein. C, density of cytosolic FOXO1 protein bands in EDL muscle from rats administered dexamethasone (Dex), lipopolysaccharide (LPS), Dex+LPS or saline for 24 h. D, ratio of phosphorylated to total cytosolic FOXO1 protein in EDL muscle of Dex, LPS, Dex+LPS or saline-treated rats. Significantly different from control: *P < 0.05; **P < 0.01.
Analysis of PDK4 protein revealed no significant change in expression with Dex administration relative to the saline group, but there was a 2.6-fold increase in expression with LPS treatment compared to saline (P < 0.05; Fig. 6A and B). There was no significant change in PDK4 protein with Dex+LPS administration compared to the saline group (Fig. 6A and B).
Figure 6. Pyruvate dehydrogenase kinase 4 (PDK4) protein expression, and glycogen and lactate concentrations in rat extensor digitorum longus (EDL) muscle.
A, representative blot of PDK4 protein. B, density of PDK4 protein bands, measured by Western blotting, in EDL muscle of rats administered with dexamethasone (Dex), lipopolysaccharide (LPS), Dex+LPS or saline for 24 h. C and D, glycogen (C) and lactate (D) content in EDL muscle from rats administered with dexamethasone (Dex), lipopolysaccharide (LPS), Dex+LPS or saline for 24 h. Values are means and vertical bars represent s.e.m. Significantly different from saline control: *P < 0.05; **P < 0.01. Significantly different from Dex group: †P < 0.05; ††P < 0.01; †††P < 0.001. Significantly different from LPS group: ‡P < 0.05; ‡‡P < 0.01.
Muscle metabolites and enzyme activity
Muscle glycogen content was 52% higher with Dex treatment compared to control (P < 0.001 vs. saline; Fig. 6C), and was 19% lower compared to control following LPS treatment (P < 0.05 vs. saline; Fig. 6C). Administration of Dex+LPS prevented the LPS-mediated reduction in muscle glycogen (Fig. 6C). Muscle lactate content in the Dex group was no different from the saline group; however in the LPS-treated group, muscle lactate concentration was 53% greater compared to saline (P < 0.01; Fig. 6D). Dex+LPS administration prevented the LPS-mediated increase in muscle lactate (Fig. 6D).
Muscle proteasome activity was increased in both groups treated with LPS, although the difference was of borderline significance (P= 0.057 vs. saline; Fig. 7A) in rats treated with LPS alone, whereas it was significant (P < 0.05; Fig. 7A) in rats treated with Dex+LPS. Muscle cathepsin-L activity was increased, albeit not significantly (P= 0.18), with LPS treatment compared to saline; however in rats treated with Dex+LPS, muscle cathepsin-L activity was suppressed (by 53%) compared to the LPS group (P < 0.05; Fig. 7B), and under those conditions, was not different from the control.
Figure 7. Proteasome and cathepsin-L activity in rat extensor digitorum longus (EDL) muscle, following 24 h of lipopolysaccharide (LPS) or dexamethasone (Dex) + LPS infusion.
Values are means and vertical bars represent s.e.m. Significantly different from saline control group: *P < 0.05. Significantly different from LPS group: ‡P < 0.05.
Discussion
We recently provided evidence of a role for the Akt/FOXO signalling pathway in the simultaneous induction of muscle atrophy and impairment of muscle carbohydrate oxidation in vivo during LPS-induced endotoxaemia, and suggested that the elevation of pro-inflammatory cytokines may have played an important role in initiating these events (Crossland et al. 2008). In the present study, we hypothesised that blunting the cytokine response to LPS using low-dose Dex, a potent synthetic glucocorticoid, would offset the dysregulation of Akt signalling, and prevent the LPS-induced loss of muscle mass and impairment of muscle carbohydrate oxidation.
Dexamethasone suppresses pro-inflammatory cytokines in muscle, abolishes the cardiovascular sequelae and prevents muscle atrophy during endotoxaemia
The results of this study clearly showed that a low dose of Dex during endotoxaemia reduced the rise in mRNA expression of pro-inflammatory cytokines TNF-α and IL-6 in EDL muscle, and substantially inhibited the early hypotensive and vasodilator effects of LPS infusion and the late-onset hindquarters vasodilatation, consistent with an anti-inflammatory effect (Gardiner et al. 1996). We have previously shown that many of the haemodynamic changes observed with LPS administration can be replicated with administration of cytokines including TNF and interleukins (Gardiner et al. 1998), and they are likely to be due to a cascade of events (induction of nitric oxide synthase, etc.) triggered by the cytokines. Dex infusion was previously shown to suppress LPS-induced increases in plasma cytokines in vivo (Myers et al. 2003), and although plasma cytokines were not measured in the present study, the present results are consistent with Dex-induced inhibition of the systemic inflammatory response. Nevertheless, the present results clearly indicate that Dex suppressed increases in local (muscle) cytokine mRNA expression during endotoxaemia. Protein expression of TNF-α and IL-6 should be measured in future studies to confirm that Dex suppressed muscle cytokine production during endotoxaemia.
Concomitantly, low-dose Dex administration protected the EDL muscle from LPS-mediated atrophy. The use of the protein:DNA ratio to assess changes in muscle protein mass comes from human studies (Gamrin et al. 1996), where reduced muscle protein content was consistently observed in critically ill patients. Moreover, this was observed in the face of increased extracellular muscle water content, implying that under conditions where fluid compartment changes occur, the protein:DNA ratio is a sensitive measure of protein mass. Therefore, the observed 40% reduction in the protein:DNA ratio confirmed that 24 h LPS administration caused EDL muscle atrophy. Furthermore, there was no reduction in the protein:DNA ratio with Dex treatment alone, indicating the present dose and infusion time for Dex administration did not induce atrophy as observed following high doses of Dex (Wang et al. 1998). More importantly, Dex prevented the LPS-induced reduction in the protein:DNA ratio, clearly showing that Dex was able to protect the EDL muscle from LPS-induced muscle atrophy. These observations are therefore in keeping with our aim of suppressing inflammation during endotoxaemia, without inducing the catabolic effects associated with high dose Dex. Since elevated pro-inflammatory cytokines have been suggested to trigger muscle wasting (Garcia-Martinez et al. 1993; Haddad et al. 2005), it is feasible that the Dex-mediated blunting of muscle TNF-α and IL-6 mRNA expression may have accounted for the prevention of muscle atrophy induced by LPS.
We have shown previously (Crossland et al. 2008), and also in the present study, that 24 h LPS infusion results in the marked upregulation of MAFbx and MuRF1 mRNA expression, suggesting that the loss of muscle protein observed during endotoxaemia may have been, at least partly, due to UPP-dependent muscle proteolysis (Voisin et al. 1996; Tawa et al. 1997). However, Dex did not suppress the upregulation of MAFbx and MuRF1 mRNA or the increase in 20S proteasome activity that was observed with LPS infusion alone. Therefore, it appears that the beneficial effect of Dex on sepsis-induced loss of muscle protein was not due to suppression of UPP-dependent protein breakdown (see Fig. 8 for schematic diagram).
Figure 8. Proposed mechanism of dexamethasone (Dex)-mediated prevention of muscle atrophy and impairment of carbohydrate oxidation during lipopolysaccharide (LPS)-induced endotoxaemia.
A, during LPS-induced endotoxaemia, cytokine-mediated impairment of Akt/Forkhead box O (FOXO) signalling is thought to be important in both the induction of muscle atrophy, via FOXO-mediated upregulation of muscle atrophy F-box (MAFbx) and muscle RING finger 1 (MuRF1), leading to an increase in ubiquitin–proteasome pathway (UPP)-dependent protein degradation, and the impairment of carbohydrate oxidation, through upregulation of pyruvate dehydrogenase kinase 4 (PDK4) and subsequent pyruvate dehydrogenase complex (PDC) inhibition. An increase in lysosome-mediated proteolysis is also thought to contribute to muscle protein loss, via cytokine-induced cathepsin-L upregulation. B, low-dose Dex infusion during endotoxaemia provides protection against muscle atrophy and the impairment of carbohydrate oxidation, most likely due to the blunting of cytokines e.g. tumour necrosis factor-α (TNF-α). Dex treatment prevents LPS-induced inhibition (i.e. reduced phosphorylation) of Akt and activation (i.e. reduced phosphorylation) of FOXO. Furthermore, LPS-induced upregulation of PDK4, and muscle glycogen breakdown and lactate accumulation, are suppressed by administration of Dex during endotoxaemia. In contrast, LPS-induced MAFbx and MuRF1 upregulation are not suppressed by Dex, and may be upregulated via an unknown, Akt/FOXO-independent mechanism. However, the LPS-induced increase in cathepsin-L mRNA expression is blunted by Dex.  and
and  arrows indicate upregulation/increased activity and downregulation/decreased activity, respectively, and arrow thickness represents the magnitude of upregulation/increased activity and downregulation/decreased activity.
arrows indicate upregulation/increased activity and downregulation/decreased activity, respectively, and arrow thickness represents the magnitude of upregulation/increased activity and downregulation/decreased activity.
The protective effects of Dex on muscle protein mass during endotoxaemia appeared to have been, at least partly, attributable to a reduction of lysosome-mediated proteolysis, since Dex blunted (by 43%) the LPS-induced increase in cathepsin-L mRNA expression, which is a lysosomal protease that is upregulated by cytokines (Ebisui et al. 1995), and is a reliable marker of lysosomal proteolysis (Deval et al. 2001). Moreover, Dex significantly reduced the increase in cathepsin-L activity during endotoxaemia compared to LPS infusion alone, although the increase in cathepsin-L activity with LPS treatment was not significantly different from control. FOXO transcription factors may also be important in mediating the activation of lysosomal proteolysis and autophagy in muscle (Mammucari et al. 2008; Zhao et al. 2008a), and in a recent paper, cathepsin-L was identified as a direct target of FOXO1 in skeletal muscle (Yamazaki et al. 2010). Therefore, the findings of the present study strongly suggest that the Dex-induced prevention of muscle atrophy during endotoxaemia may have occurred through suppression of cathepsin-L-mediated proteolysis, as a result of the blunting of cytokines and the partial prevention of FOXO activation (see below). However, it cannot be ruled out that other, perhaps rate-limiting, proteolytic pathways activated during sepsis such as the calpain-dependent proteolytic pathway (Smith et al. 2008) were also suppressed by Dex co-administration.
Dexamethasone prevents Akt inhibition and partially prevents FOXO activation in muscle during LPS-induced endotoxaemia
In catabolic states, suppression of Akt signalling has been implicated in the induction of muscle atrophy, through activation of FOXO and its target genes MAFbx and MuRF1 (Wang et al. 2006). In the present study, we hypothesized that Dex may prevent muscle protein loss via maintenance of the Akt/FOXO pathway. Co-administration of Dex with LPS prevented the LPS-induced reduction in muscle pAkt, and partially prevented the LPS-induced reduction in cytosolic phosphorylated/total or ‘active’ FOXO1 in EDL muscle. These findings suggest that Dex administration appears to have protected against muscle atrophy partly through prevention of LPS-induced inhibition of Akt phosphorylation and activation of FOXO signalling, possibly following the suppression of cytokines. It should be noted, however, that reduced levels of pFOXO1 protein were observed in the Dex and Dex+LPS groups, where pAkt levels were no different from control, indicating that FOXO1 phosphorylation may, at least partially, be independent of Akt. Despite not detecting an increase in total nuclear FOXO with LPS infusion, which might be predicted following the reduction in cytosolic phosphorylated FOXO1 (Brunet et al. 1999), observations of a marked reduction in FOXO1 phosphorylation, along with reduced Akt phosphorylation and upregulation of genes targeted by FOXO, are collectively strong evidence that LPS induces activation of FOXO1.
Although the findings are consistent with an important role for FOXO1 in MAFbx and MuRF1 upregulation, the results also suggest that the upregulation of MAFbx and MuRF1 mRNA with Dex+LPS treatment may have occurred via an Akt/FOXO-independent mechanism. For example, it has been proposed that MAFbx may be induced by TNF-α via FOXO4, independent of Akt (Moylan et al. 2008), and although TNF-α was reduced compared to LPS, it was still ∼5-fold higher than control. NF-κB activation has also been shown to result in MuRF1 upregulation (Cai et al. 2004).
Dexamethasone infusion during endotoxaemia blunts LPS-induced increases in muscle gene expression associated with oxidative stress
There is evidence for a high degree of oxidative stress in patients with sepsis (Macdonald et al. 2003). Metallothioneins (MTs) have been proposed to participate in the cellular defence against free radicals, and can be activated by glucocorticoids, oxygen free radicals and cytokines (Nath et al. 2000) and thus are used as a marker for oxidative stress. In the model of endotoxaemia used in the present study, MT-1A was upregulated 187-fold, strongly indicating the presence of oxidative stress. With evidence that reactive oxygen species may be involved in FOXO activation, at least in cell lines (Nakamura & Sakamoto, 2008), it is possible that oxidative stress had a regulatory role in the pathways leading to muscle atrophy in sepsis. Indeed, the LPS-induced increase in MT-1A expression was reduced by 58% with Dex co-administration, which may reflect decreased oxidative stress levels, possibly due to the direct blunting effect on cytokine levels by Dex, and may be important in the protective effects of Dex against muscle protein loss under these conditions. However, since only MT-1A mRNA was measured in this study as a marker of oxidative stress, which may not thoroughly reflect the extent of oxidative stress, additional measures are required to elucidate the role of oxidative stress in LPS-induced muscle atrophy.
Dexamethasone prevents impairment of muscle carbohydrate oxidation during LPS-induced endotoxaemia
The upregulation of PDK4 mRNA and protein during endotoxaemia in the present study, along with the accumulation of muscle lactate and increased glycogen breakdown, is in agreement with previous observations (Alamdari et al. 2008; Crossland et al. 2008) indicating inhibition of muscle PDC and thereby an impairment of pyruvate oxidation. In the present study, the LPS-mediated increase in PDK4 mRNA expression was suppressed by ∼50% during co-administration with Dex. Furthermore, PDK4 protein levels appeared slightly reduced in the Dex+LPS group compared to LPS. Expression of PDK4 mRNA in the Dex+LPS group was similar to Dex treatment alone, suggesting that the upregulation of PDK4 with Dex+LPS treatment may have been mediated by the Dex, rather than the LPS per se.
Co-administration of Dex with LPS also completely prevented LPS-mediated muscle glycogen breakdown and lactate accumulation. Sepsis is associated with an increase in circulating adrenaline (Jones & Romano, 1989) and this is known to activate muscle glycolysis (Raz et al. 1991). However, we previously demonstrated that whilst elevated muscle lactate following 2 h and 6 h LPS infusion was most likely attributable to an adrenaline-mediated increase in glycolytic flux, the response at 24 h is in all probability due to PDK4-mediated PDC inhibition (Alamdari et al. 2008). In keeping with our collective observations to date, it is likely that Dex reduced the LPS-mediated increase in lactate accumulation by suppressing PDK4-mediated inhibition of PDC, although we cannot rule out that a Dex-mediated reduction in circulating adrenaline as having also played a part. TNF-α was previously shown to impair Akt signalling and whole body glucose uptake in humans (Plomgaard et al. 2005), which is in line with our proposed model whereby LPS-induced increases in muscle TNF-α impair Akt signalling and oxidative carbohydrate utilisation. Interestingly, Dex had not only a sparing effect, but also induced muscle glycogen super-compensation when administered alone. There is no immediate explanation for this finding, although high doses of Dex have been previously shown to induce muscle glycogen synthesis (Coderre et al. 1992; Puthanveetil et al. 2008). Collectively, the negative effects of LPS on muscle glycogen utilisation and carbohydrate oxidation appear to have been alleviated by Dex infusion.
In summary, low-dose Dex infusion during LPS administration suppressed the LPS-induced increase in pro-inflammatory cytokines, as well as that of a marker of oxidative stress, MT-1A, within the EDL muscle. Concomitantly, Dex administration prevented LPS-induced EDL muscle atrophy, prevented the dephosphorylation of muscle Akt, and partially prevented the reduction in FOXO1 phosphorylation. Dex administration did not, however, suppress the LPS-induced increase in MAFbx and MuRF1 mRNA expression, but did significantly reduce the LPS-induced increase in cathepsin-L mRNA expression and enzyme activity. Furthermore, Dex suppressed LPS-induced PDK4 upregulation, and completely prevented LPS-induced muscle glycogen breakdown and lactate accumulation. It is likely that the beneficial effects of low-dose Dex on muscle protein and carbohydrate dysregulation in endotoxaemia are secondary to blunting muscle pro-inflammatory cytokine production (see Fig. 8 for schematic diagram).
Acknowledgments
We would like to thank Julie March and Philip Kemp for their excellent technical assistance. This study was funded by the Biotechnology and Biological Sciences Research Council (BBSRC).
Glossary
Abbreviations
- Dex
dexamethasone
- EDL
extensor digitorum longus
- FOXO
Forkhead box O
- HMBS
hydroxymethylbilane synthase
- IL-6
interleukin-6
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- MAFbx
muscle atrophy F-box
- MT-1A
metallothionein-1A
- MuRF1
muscle RING finger 1
- PDC
pyruvate dehydrogenase complex
- PDK4
pyruvate dehydrogenase kinase 4
- TNF-α
tumour necrosis factor-α
- UPP
ubiquitin–proteasome pathway
Author contributions
The experiments were conducted in the School of Biomedical Sciences, University of Nottingham. All authors approved the final version of the manuscript to be published, and all authors contributed to drafting the article and revising it critically for important intellectual content. All authors contributed to the conception and design, or analysis and interpretation of the data.
References
- Alamdari N, Constantin-Teodosiu D, Murton AJ, Gardiner SM, Bennett T, Layfield R, Greenhaff PL. Temporal changes in the involvement of pyruvate dehydrogenase complex in muscle lactate accumulation during lipopolysaccharide infusion in rats. J Physiol. 2008;586:1767–1775. doi: 10.1113/jphysiol.2007.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Annane D. Corticosteroids for septic shock. Crit Care Med. 2001;29:S117–120. doi: 10.1097/00003246-200107001-00036. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU. Methods in Enzymatic Analysis. 3rd edn. V. John Wiley & Sons; 1983. pp. 207–209. [Google Scholar]
- Blough E, Dineen B, Esser K. Extraction of nuclear proteins from striated muscle tissue. Biotechniques. 1999;26:202–204. 206. doi: 10.2144/99262bm05. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann GE, Buchler M, Uhl W, Peter K. Low-dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. The Phospholipase A2 Study Group. Clin Investig. 1994;72:782–787. doi: 10.1007/BF00180547. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Buren J, Lai YC, Lundgren M, Eriksson JW, Jensen J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch Biochem Biophys. 2008;474:91–101. doi: 10.1016/j.abb.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Coderre L, Srivastava AK, Chiasson JL. Effect of hypercorticism on regulation of skeletal muscle glycogen metabolism by insulin. Am J Physiol Endocrinol Metab. 1992;262:E427–433. doi: 10.1152/ajpendo.1992.262.4.E427. [DOI] [PubMed] [Google Scholar]
- Constantin D, Constantin-Teodosiu D, Layfield R, Tsintzas K, Bennett AJ, Greenhaff PL. PPARδ agonism induces a change in fuel metabolism and activation of an atrophy programme, but does not impair mitochondrial function in rat skeletal muscle. J Physiol. 2007;583:381–390. doi: 10.1113/jphysiol.2007.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Baker DJ, Constantin D, Greenhaff PL. PPARδ agonism inhibits skeletal muscle PDC activity, mitochondrial ATP production and force generation during prolonged contraction. J Physiol. 2009;587:231–239. doi: 10.1113/jphysiol.2008.164210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, Fisher CJ., Jr Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–1439. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- Crossland H, Constantin-Teodosiu D, Gardiner SM, Constantin D, Greenhaff PL. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J Physiol. 2008;586:5589–5600. doi: 10.1113/jphysiol.2008.160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SP, Arnold JE, Mayer NJ, Reynolds SE, Billett MA, Gordon C, Colleaux L, Kloetzel PM, Tanaka K, Mayer RJ. Developmental changes of the 26 S proteasome in abdominal intersegmental muscles of Manduca sexta during programmed cell death. J Biol Chem. 1995;270:1850–1858. doi: 10.1074/jbc.270.4.1850. [DOI] [PubMed] [Google Scholar]
- Deval C, Mordier S, Obled C, Bechet D, Combaret L, Attaix D, Ferrara M. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J. 2001;360:143–150. doi: 10.1042/0264-6021:3600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K, Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci (Lond) 1995;89:431–439. doi: 10.1042/cs0890431. [DOI] [PubMed] [Google Scholar]
- Forsberg AM, Nilsson E, Werneman J, Bergstrom J, Hultman E. Muscle composition in relation to age and sex. Clin Sci (Lond) 1991;81:249–256. doi: 10.1042/cs0810249. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Jefferson LS, Lang CH. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E501–512. doi: 10.1152/ajpendo.00359.2006. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J. 2003;375:365–371. doi: 10.1042/BJ20030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamrin L, Essen P, Forsberg AM, Hultman E, Wernerman J. A descriptive study of skeletal muscle metabolism in critically ill patients: free amino acids, energy-rich phosphates, protein, nucleic acids, fat, water, and electrolytes. Crit Care Med. 1996;24:575–583. doi: 10.1097/00003246-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez C, Lopez-Soriano FJ, Argiles JM. Acute treatment with tumour necrosis factor-α induces changes in protein metabolism in rat skeletal muscle. Mol Cell Biochem. 1993;125:11–18. doi: 10.1007/BF00926829. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, March JE, Bennett T. Effects of dexamethasone and SB 209670 on the regional haemodynamic responses to lipopolysaccharide in conscious rats. Br J Pharmacol. 1996;118:141–149. doi: 10.1111/j.1476-5381.1996.tb15377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, March JE, Woolley J, Bennett T. The influence of antibodies to TNF-α and IL-1β on haemodynamic responses to the cytokines, and to lipopolysaccharide, in conscious rats. Br J Pharmacol. 1998;125:1543–1550. doi: 10.1038/sj.bjp.0702250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Hasselgren PO, Menconi MJ, Fareed MU, Yang H, Wei W, Evenson A. Novel aspects on the regulation of muscle wasting in sepsis. Int J Biochem Cell Biol. 2005;37:2156–2168. doi: 10.1016/j.biocel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Jolly L, March JE, Kemp PA, Bennett T, Gardiner SM. Regional haemodynamic responses to adenosine receptor activation vary across time following lipopolysaccharide treatment in conscious rats. Br J Pharmacol. 2008;154:1600–1610. doi: 10.1038/bjp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SB, Romano FD. Dose- and time-dependent changes in plasma catecholamines in response to endotoxin in conscious rats. Circ Shock. 1989;28:59–68. [PubMed] [Google Scholar]
- Keh D, Sprung CL. Use of corticosteroid therapy in patients with sepsis and septic shock: an evidence-based review. Crit Care Med. 2004;32:S527–533. doi: 10.1097/01.ccm.0000142983.15421.11. [DOI] [PubMed] [Google Scholar]
- Kim YI, Lee FN, Choi WS, Lee S, Youn JH. Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin-resistant states. Diabetes. 2006;55:2311–2317. doi: 10.2337/db05-1606. [DOI] [PubMed] [Google Scholar]
- Lang CH, Dobrescu C, Meszaros K. Insulin-mediated glucose uptake by individual tissues during sepsis. Metabolism. 1990;39:1096–1107. doi: 10.1016/0026-0495(90)90172-9. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- Mallinson JE, Constantin-Teodosiu D, Sidaway J, Westwood FR, Greenhaff PL. Blunted Akt/FOXO signalling and activation of genes controlling atrophy and fuel use in statin myopathy. J Physiol. 2009;587:219–230. doi: 10.1113/jphysiol.2008.164699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- Moylan JS, Smith JD, Chambers MA, McLoughlin TJ, Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signalling. Am J Physiol Cell Physiol. 2008;295:C986–993. doi: 10.1152/ajpcell.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MJ, Farrell DE, Palmer DC, Post LO. Inflammatory mediator production in swine following endotoxin challenge with or without co-administration of dexamethasone. Int Immunopharmacol. 2003;3:571–579. doi: 10.1016/S1567-5769(03)00048-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2008;281:47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Nath R, Kumar D, Li T, Singal PK. Metallothioneins, oxidative stress and the cardiovascular system. Toxicology. 2000;155:17–26. doi: 10.1016/s0300-483x(00)00273-0. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- Puthanveetil P, Wang F, Kewalramani G, Kim MS, Hosseini-Beheshti E, Ng N, Lau W, Pulinilkunnil T, Allard M, Abrahani A, Rodrigues B. Cardiac glycogen accumulation after dexamethasone is regulated by AMPK. Am J Physiol Heart Circ Physiol. 2008;295:H1753–1762. doi: 10.1152/ajpheart.518.2008. [DOI] [PubMed] [Google Scholar]
- Qi D, Pulinilkunnil T, An D, Ghosh S, Abrahani A, Pospisilik JA, Brownsey R, Wambolt R, Allard M, Rodrigues B. Single-dose dexamethasone induces whole-body insulin resistance and alters both cardiac fatty acid and carbohydrate metabolism. Diabetes. 2004;53:1790–1797. doi: 10.2337/diabetes.53.7.1790. [DOI] [PubMed] [Google Scholar]
- Raz I, Katz A, Spencer MK. Epinephrine inhibits insulin-mediated glycogenesis but enhances glycolysis in human skeletal muscle. Am J Physiol Endocrinol Metab. 1991;260:E430–435. doi: 10.1152/ajpendo.1991.260.3.E430. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids: new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Smith IJ, Lecker SH, Hasselgren PO. Calpain activity and muscle wasting in sepsis. Am J Physiol Endocrinol Metab. 2008;295:E762–771. doi: 10.1152/ajpendo.90226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spate U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:265–269. doi: 10.1097/00075197-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Tawa NE, Jr, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest. 1997;100:197–203. doi: 10.1172/JCI119513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorsson-Norheim E. Friedman and Quade tests: BASIC computer program to perform nonparametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput Biol Med. 1987;17:85–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest. 1996;97:339–348. doi: 10.1172/JCI118421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiao G, Lieberman M, Fischer JE, Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am J Physiol Regul Integr Comp Physiol. 1997;272:R849–856. doi: 10.1152/ajpregu.1997.272.3.R849. [DOI] [PubMed] [Google Scholar]
- Voisin L, Breuille D, Combaret L, Pouyet C, Taillandier D, Aurousseau E, Obled C, Attaix D. Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+-activated, and ubiquitin-proteasome proteolytic pathways. J Clin Invest. 1996;97:1610–1617. doi: 10.1172/JCI118586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luo GJ, Wang JJ, Hasselgren PO. Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock. 1998;10:298–306. doi: 10.1097/00024382-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signalling. Endocrinology. 2006;147:4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Kamei Y, Sugita S, Akaike F, Kanai S, Miura S, Hirata Y, Troen BR, Kitamura T, Nishino I, Suganami T, Ezaki O, Ogawa Y. The cathepsin L gene is a direct target of FOXO1 in the skeletal muscle. Biochem J. 2010 doi: 10.1042/BJ20091346. in press. [DOI] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, Goldberg AL. Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy. 2008a;4:378–380. doi: 10.4161/auto.5633. [DOI] [PubMed] [Google Scholar]
- Zhao W, Pan J, Zhao Z, Wu Y, Bauman WA, Cardozo CP. Testosterone protects against dexamethasone-induced muscle atrophy, protein degradation and MAFbx upregulation. J Steroid Biochem Mol Biol. 2008b;110:125–129. doi: 10.1016/j.jsbmb.2008.03.024. [DOI] [PubMed] [Google Scholar]