Abstract
We have established a concise sub-typing system suitable for predicting the postoperative outcome in cases of stage I lung adenocarcinoma (ADC), using morphometric profiling. The association between postoperative disease recurrence and a variety of morphological features including histological architecture, cell type, cytoplasmic color/internal structure, nuclear shape/size, chromatin pattern, and nucleoli count/remarkableness, was analyzed. Histological architecture had the most prognostic value and could be subdivided into low-grade (bronchioloalveolar, papillary and tubular: “tubular” in this paper is defined as a tubular or glandular structure lined with single-layered neoplastic cells) and high-grade (acinar and solid: “acinar” is defined as a tubular or glandular structure lined with poly-layered neoplastic cells or as a fused glandular structure such as the cribriform pattern) components. The subgroups separated based on a cut-off value, 71.5% of the high-grade component comprised by a tumor, which was calculated according to a relative operating characteristic curve, exhibited a significant difference in disease recurrence [estimated 5-year disease-free survival rate, 95.3% in the low-grade group versus 66.7% in the high-grade group, hazard ratio 7.35, Log-rank test p = 0.002]. The sub-grouping system is concise and suitable for practical use. It will improve the histological classification of ADC.
Keywords: Lung adenocarcinoma, stage I, morphometric profiling, altered sub-typing system, prognostic value
Introduction
Lung cancer is a leading cause of cancer-related deaths in the developed world [1,2]. Adenocarcinoma (ADC) is the most common histological type and even tumors without nodal metastasis (stage I disease) can be fatal [2,3,4,5,6,7]. Recent studies reported a beneficial effect of adjuvant chemotherapy on survival in a poor prognostic group of patients with stage I ADC [8,6,9]. The accurate identification of tumors having the potential to recur and selection of an appropriate adjuvant therapy would improve postoperative performance. Histological appearance is one indicator of postoperative outcome [2,4,10]. The World Health Organization (WHO) has further classified ADC into five sub-types, bronchioloalveolar, acinar, papillary, solid, and mixed, the latter consisting different sub-types [2]. Although this sub-typing has been wildly adopted, it has a crucial flaw in that most ADCs (more than 80%) are inevitably categorized as mixed because they usually consist of highly heterogeneous components [2]. Actually, clinical course seems to vary among individuals with the mixed ADC even at an identical stage. Our recent study has established a novel system using morphometric profiling to subdivide mixed ADC and demonstrated its potential prognostic value [10]. However, the system was too complex in methodology to be widely adopted for clinical use [10]. The present study has tried to identify the morphometric element most closely associated with the postoperative out-come of ADC at stage I, and succeeded in establishing a concise sub-typing system suitable for practical use.
Materials and methods
Primary lung cancers
All 187 patients with ADC underwent radical surgical resection at Kanagawa Prefectural Cardiovascular and Respiratory Center Hospital (Yokohama, Japan) between January 2001 and December 2006. Every tumor examined was at stage I according to the international TNM classification system [11]. Among the 187 ADCs, 130 were at stage IA (T1N0M0; T1, tumor less than or equal to 30mm in the maximal diameter) and 57 were at stage IB (T2N0M0; T2, tumor more than 30mm in the maximal diameter or one with pleural invasion), respectively. One, 165, and 6 patients underwent a pneumonectomy, lobectomy, and segmentectomy, along with a lymphadenectomy extending to the hilar and mediastinal lymph nodes, respectively. Fifteen patients underwent a wedge resection along with lymph-node sampling. A follow-up evaluation was performed every 2 months for the first 2 years after the operation, every 3 months in the third year, and every 6 months thereafter. The evaluation included physical examinations and chest radiography. An examination using serum tumor markers, computed tomography of the thorax and upper abdomen, and magnetic resonance imaging of the brain were performed every 6 months for the first 3 years, and every 12 months thereafter. In the analyses for DFS, recurrence ratio and hazard ratio, SQCs were excluded. The median follow-up period was 35.9 (range, 1.1 to 82.5) months. The post-operative disease-free span was defined as the period ranging from the date of surgical operation to the date when the recurrence of disease was diagnosed. An observation was censored at the last follow-up if the patient was alive or had died of a cause other than lung cancer. The median disease-free span was 11.8 (range, 3.8 to 49.1) months for patients with recurrent disease, and 36.2 (range, 1.1 to 82.5) months for those without recurrent disease. None of the patients with stage IA disease, and 19 of 57 patients with stage IB disease received postoperative adjuvant chemotherapy (3 patients received cisplatin or carboplatin-based chemotherapy, and 16 patients received oral uracil-tegafur (UFT) chemotherapy). The 5-year disease-free survival (DFS) rate was 65.4% for the non-adjuvant stage IB group versus 66.0% for the adjuvant stage IB group (P=0.8057, 4 patients who could not continue oral UFT treatment for more than 6 months were excluded). Ethics committees of both Yokohama City University and Kanagawa Prefectural Cardiovascular and Respiratory Center Hospital approved the protocol of this work. Informed consent to use the resected materials for research was obtained from all the subjects.
Morphometric features
Morphological elements were defined as in our previous study [10] but a few modifications. Histological architecture was subdivided into 10 patterns, (1) bronchioloalveolar (BAC), (2) tubular (TUB), (3) acinar (ACN), (4) ductular (DCT), (5) trabecular (TRB), (6) papillary (PAP), (7) solid (SOL), (8) single (SGR), (9) striated (STR), and (10) neuroendocrinal (NER). The BAC pattern was defined as an extension of neoplastic cells on proper alveolar septa (Figure 1), the TUB pattern as a tubular or glandular structure lined with single-layered epithelial cells (Figure 1), the ACN pattern was as a tubular or glandular structure lined with poly-layered epithelial cells or as a fused glandular structure such as the cribriform pattern (Figure 1), the TRB pattern as a line of single neoplastic cells connected like a train (Figure 1), the PAP pattern as an extension of neoplastic cells on fibrotic stalks like papillae (Figure 1), the SOL pattern as the formation of solid nests consisting of neoplastic cells (Figure 1), the SGL pattern as an extension of unconnected neoplastic cells (Figure 1), the STR pattern as the striation of neoplastic cells like the striated squamous epithelium (Figure 1), and the NER pattern as an architecture showing some neuroendocrinal features such as rosset and cord/ribbon-like structures (Figure 1). Cell type was classified as follows, (1) high columnar (HCL), (2) low columnar (LCL), (3) spheroid (SPH), (4) hobnail (HOB), (5) polygonal (PLG), (5) global (GLB), small round (SMR), and (8) spindle (SPN). HCL was defined as resembling bronchial surface epithelial cells (Figure 2), LCL as resembling non-ciliated bronchiolar epithelial cells (Figure 2), SPH as resembling type II pneumocytes (Figure 2), HOB as showing features intermediate between those of type II pneumocytes and non-ciliated bronchiolar epithelial cells (Figure 2), PLG as having a polygonal appearance with obvious cell-cell adherence (Figure 2), GLB as having a global appearance without obvious cell-cell adherence (Figure 2), SMR as having a small round or oat-like appearance with scant cytoplasm (Figure 2), and SPN as resembling spindle-shaped mesenchymal cells (Figure 2). Cytoplasmic color was classified as, (1) eosinophilic without keratinzation (EOS) (Figure 3A), (2) chromophobic (CHR) (Figure 3A), (3) basophilic (BAS) (Figure 3A), and (4) eosinophilic with keratinzation (KRT) (Figure3A). The cytoplasmic internal structure was classified as, (1) homogenous (HMG) (Figure 3B), (2) clear (CLR) (Figure 3B), (3) fine granular (FGR) (Figure 3B), (4) coarse granular (CGR) (Figure 3B), (5) fine vesicular (FVS) (Figure 3B), and (6) coarse vesicular (CVS) (Figure 3B). Nuclear outline was classified as, (1) smooth (SMT) (Figure 4A), (2) grooved (GRV) (Figure 4A), and (3) wrinkled (WKL) (Figure 4A). A grooved nucleus was one having grooves paralleling the long axis like a coffee bean. Chromatin pattern was defined as, (1) homogenous (HMG) (Figure 4B), (2) ground glass (GGL) (Figure 4B), (3) fine granular (FGR) (Figure 4B), (4) coarse granular (CGR) (Figure 4B), and (5) clear (CLR) (Figure 4B). A fine granular nucleus was one like that observed in non-tumoral bronchiolar epithelial cells. The count of nucleoli was classified as (1) 0 to 1 nucleoli/cell (NL1), (2) 2 to 3 nucleoli/cell (NL2), and (3) 4 or more nucleoli/cell (NL3). The remarkableness of nucleoli was classified as, (1) obscure (NLO) (Figure 4C), (2) modest (NLM) (Figure 4C), and (3) prominent (NLP) (Figure 4C). The largest tumor sections were examined under a light microscope. Proportions as percentages of each pattern were evaluated. Nuclear size, circularity, chromatin density (staining intensity of nucleus), and chromatin granularity (variance of staining intensity of nucleus) were measured in the area where atypical change seemed most prominent in the largest tumor section using NIH image (National Institute of Health, Bethesda, ML). Mean nuclear size and mean circularity were calculated from values for at least 50 nuclei. Variations in nuclear size and circularity were determined as variance values. The %mean and %variance of nuclear size, nuclear circularity, chromatin density, and chromatin granularity, were determined as a percentage relative to the maximum value.
Figure 1.
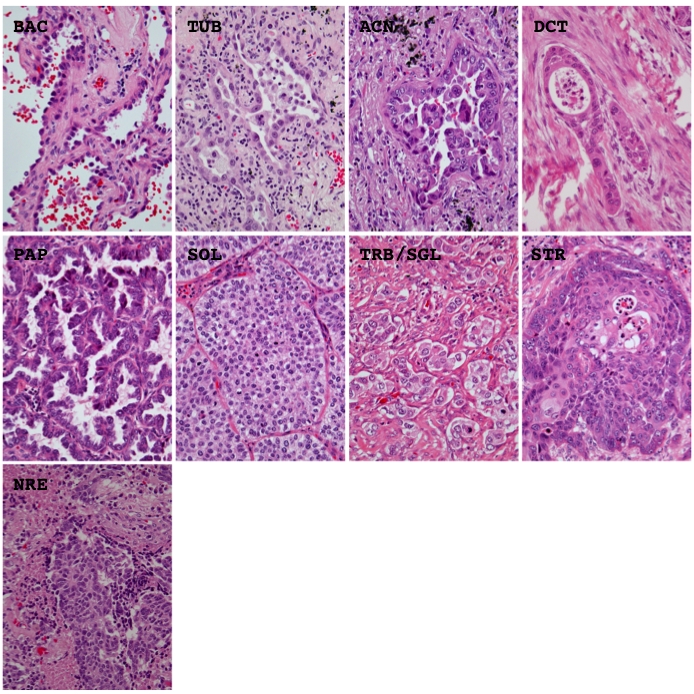
Representative histological appearance of the different architectural patterns. BAC, bronchioloalveolar; TUB, tubular; ACN, acinar; DCT, ductular; PAP, papillary; SOL, solid; TRB, trabecular; SGL, single; STR, striated; NRE, neuroendocrinal. Hematoxylin and eosin stain. Magnification is ×400.
Figure 2.
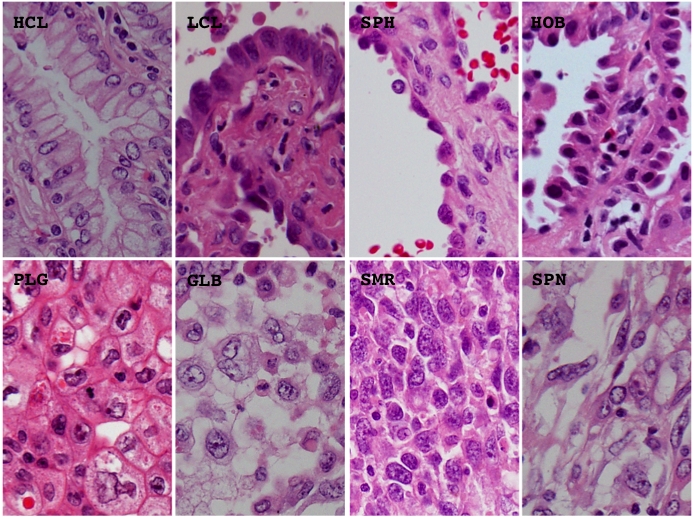
Representative histological appearance of the different cell types. BAC, bronchioloalveolar; HCL, high columnar; LCL, low columnar; SPH, spheroid; HOB, hobnail; PLG, polygonal; GLB, global; SMR, small round; SPN, spindle. Hematoxylin and eosin stain. Magnification is arbitrary.
Figure 3.
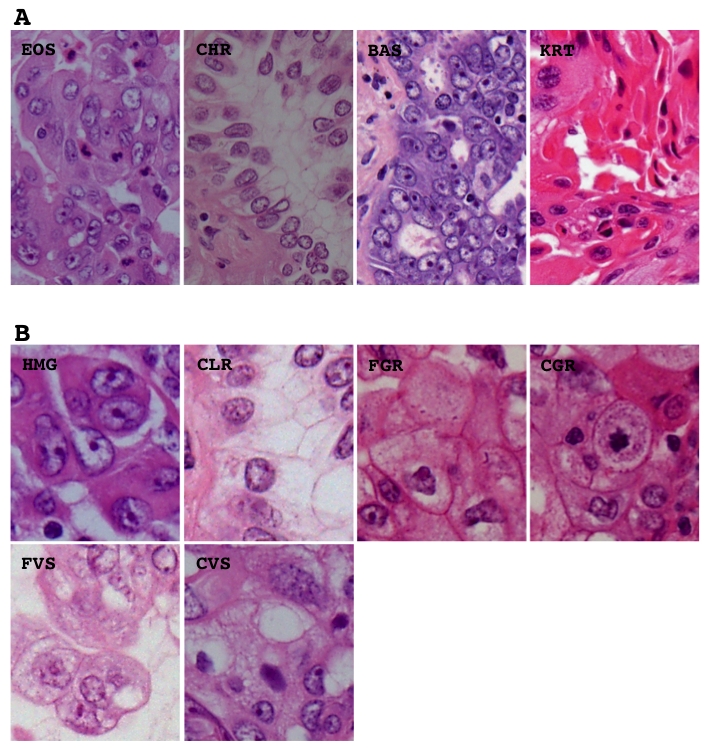
Representative histological appearance of the different cytoplasmic features including color (A) and internal structure (B). EOS, eosinophilic without keratinization: CHR, chromophobic; BAS, basophilic; KRT, eosinophilic with keratinization; HMG, homogenous; CLR, clear; FGR, fine granular; CGR, coarse granular; FVS, fine vesicular; CVS, coarse vesicular. Hematoxylin and eosin stain. Magnification is arbitrary.
Figure 4.
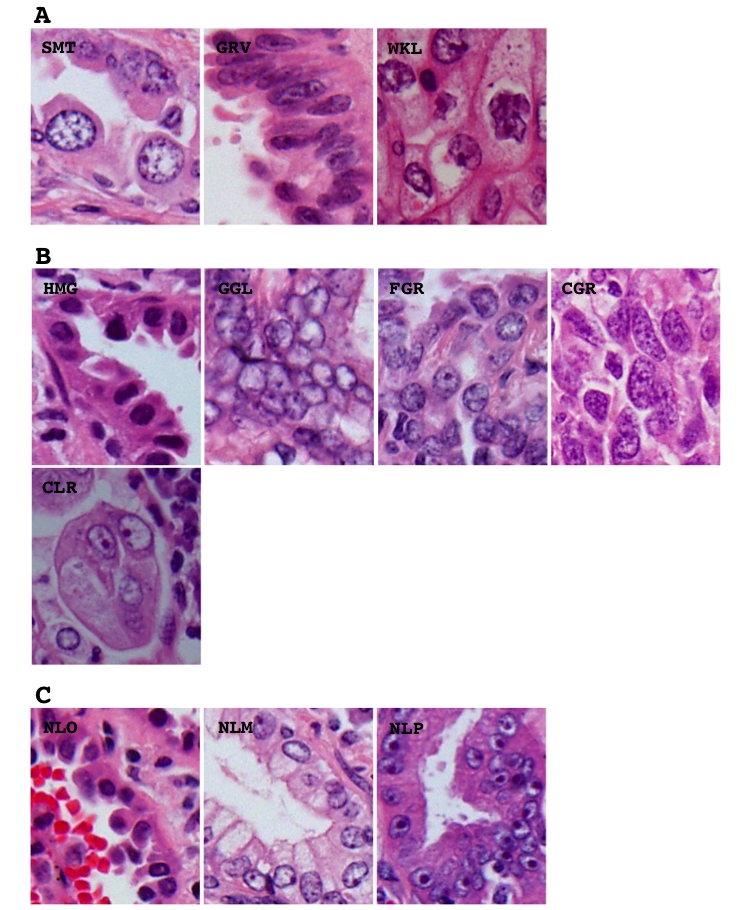
Representative histological appearance of the different nuclear features including nuclear outline (A), chromatin pattern (B) and remarkableness of nucleoli (C). SMT, smooth; GRV, grooved; WKL, wrinkled; HMG, homogenous; GGR, ground glass; FGR, fine granular; CGR, coarse granular; CLR, clear; NLO, obscure; NLM, modest; NLP, prominent. Hematoxylin and eosin stain. Magnification is arbitrary.
Morphometric profiling
Hierarchical clustering (Ward's method) based on the multiple morphometric parameters was performed with computer software (Mulcel, oms -publishing, Saitama, Japan).
Immunohistochemistry
The largest sections of tumors (4 μm thick) were cut from formalin-fixed, paraffin-embedded tissue blocks, and subjected to immunohistochemistry within 48 hours to avoid loss of antigenicity [12,13]. They were deparaffinized, rehy-drated, and incubated with 3% hydrogen peroxide, followed by 5% goat serum. The sections were boiled in citrate buffer (0.01 M, pH 6.0) for 15 minutes to retrieve masked epitopes and then incubated with a primary antibody against Ki-67 (MIB1, DAKO, Ely, UK). Reactivity was visualized using an Envision detection system (DAKO), and the nuclei were counterstained with hematoxylin. A Ki-67 labeling index (MIB1 index) was calculated as a proportion of positive cells by counting 500 or more neoplastic cells in an area where the immunolabeling seemed representative.
Statistical analysis
Disease-free survival curves were plotted using the Kaplan-Meier method, and used to estimate the absolute risk of recurrence at five years. Differences in the disease-free survival (DFS) span and rate were analyzed using the log-rank test. Differences in metric factors among the morphometric clusters were analyzed with Student's t test. Associations between the clusters and clinicopathologic features and genetic mutations were analyzed with Fisher's exact test. P values less than 0.0500 were considered significant. All the statistical analyses were performed using SPSS software (SPSS for Windows Version 10.0; SPSS; Chicago, IL).
Results
Essential morphometric elements to predict outcome
Hierarchical clustering of whole morphometric parameters was used to evaluate similarities among the tumors and to draw a dendrogram (Figure 5A). We tentatively set a threshold (Figure 5A, dashed line) to divide the cases into four clusters (Figure 5, cluster Ato D), because a number around five was considered convenient for a screening analysis. The results confirmed potential prognostic value of the sub-typing system based on morphometric profiling, as the estimated disease-free survival significantly differed among the four clusters (Figure 5B). To identify essential morphologic elements having prognostic value, possible associations between individual (or combination of) elements and postoperative outcome were analyzed. Tumors among clusters based on architectural element (ARC-CLSTs) showed the most significant difference in the crude recurrence rate and estimated disease-free survival, and the highest recurrence risk (hazard ratio) (Figure 6 and Table 1). (Dendrograms drawn from parameters of cellular features [cell type + cytoplasmic color + cytoplasmic internal structure] (CLL-CLST), nuclear features [nuclear outline + chromatin pattern + nucleoli count + nucleoli remarkableness + nuclear size + nuclear size variance + nuclear circularity + chromatin density + chromatin density variance + chromatin granularity + chromatin granularity variance] (NCL-CLST), a combination of architectural and cellular features (ARC+CLL-CLST), a combination of cellular and nuclear features (CLL+NCL-CLST), and a combination of architectural and nuclear features (ARC+NCL-CLST), are not shown. If readers request presentation of the data, the authors will provide as occasion demands.). The findings suggested histological architecture to be the most important, and an essential element for predicting postoperative outcome. Therefore, we focused on the ARC-CLSTs and further subdivided the clusters (Figure 6A). Significant differences in the estimated disease-free survival, crude recurrence ratio, and hazard rate were observed also among the six sub-clusters (Figure 6A [A1, A2, B, C1, C2, and D] and 6C, Table 2). Moreover, the sub-clusters provided an advantage over the original four clusters when it came to separating tumors, especially those with a better outcome (Figure 6A and 6C, Table 3). Similar findings were obtained when restricted in stage IA, but not stage IB, disease (Figure 6C, 6D, 6E and 6F, Table 2, and Table 3). Either tumor size or pleural invasion (determinants of stage IB disease) is very important in determining outcome [2,11]. The considerable influence of such factors can be a one of explanations why there was no association between the sub-clusters and outcome among stage IB disease.
Figure 5.

(A, see it in the previous page) Results of hierarchical clustering based on multiple morphological elements in the form of a dendrogram (upper panel). A threshold (upper panel, dashed line) was set to divide lung tumors into four clusters (A to D). Proportions of different patterns for each morphological element among individual tumors are plotted below the dendrogram. The morphological elements analyzed were as follows; histological architecture (second panel: BAC, bronchioloalveolar; TUB, tubular; ACN, acinar; DCT, ductular; TRB, trabecular; PAP, papillary; SOL, solid; SGR, single; SRT, striated; NER, neuroendocrinal), cell type (third panel: HCL, high columnar; LCL, low columnar; SPH, spheroid; HOB, hobnail; PLG, polygonal; GLB, global; SMR, small round; SPN, spindle), cytoplasmic color (fourth panel: EOS, eosinophilic without keratinization: CHR, chromophobic; BAS, basophilic; KRT, eosinophilic with keratinization), cytoplasmic internal structure (fifth panel: HMG, homogenous; CLR, clear; FGR, fine granular; CGR, coarse granular; FVS, fine vesicular; CVS, coarse vesicular), nuclear outline (sixth panel: SMT, smooth; GRV, groovy; WKL, winkled), chromatin pattern (seventh panel: HMG, homogenous; GGL, ground glass; FGR, fine granular; CGR, coarse granular; CLR, clear), count of nucleoli (eighth panel: NL1, 0 to 1 nucleoli/cell; NL2, 2 to 3 nucleoli/cell; NL3, more nucleoli/cell), remarkableness of nucleoli (ninth panel: NLO, obscure; NLM, modest; NLP, prominent), adjusted (%) nuclear size (tenth panel; maximal value on the Y axis is 100%), adjusted (%) nuclear size variance (eleventh panel; maximal value on the Y axis is 100%), adjusted (%) nuclear circularity (twelfth panel; maximal value on the Y axis is 100%), adjusted (%) nuclear circularity variance (thirteenth panel; maximal value on the Y axis is 100%), adjusted (%) chromatin density (fourteenth panel; maximal value on the Y axis is 100%), adjusted (%) chromatin density variance (fifteenth panel; maximal value on the Y axis is 100%), adjusted (%) chromatin granularity (sixteenth panel; maximal value on the Y axis is 100%), and adjusted (%) chromatin granularity variance (seventeenth panel; maximal value on the Y axis is 100%). (B) Five-year disease-free survival curves evaluated from a Kaplan-Meier analysis among the morphometric clusters. CLST means cluster. Values in parentheses are estimated five-year disease-free survival rates.
Figure 6.
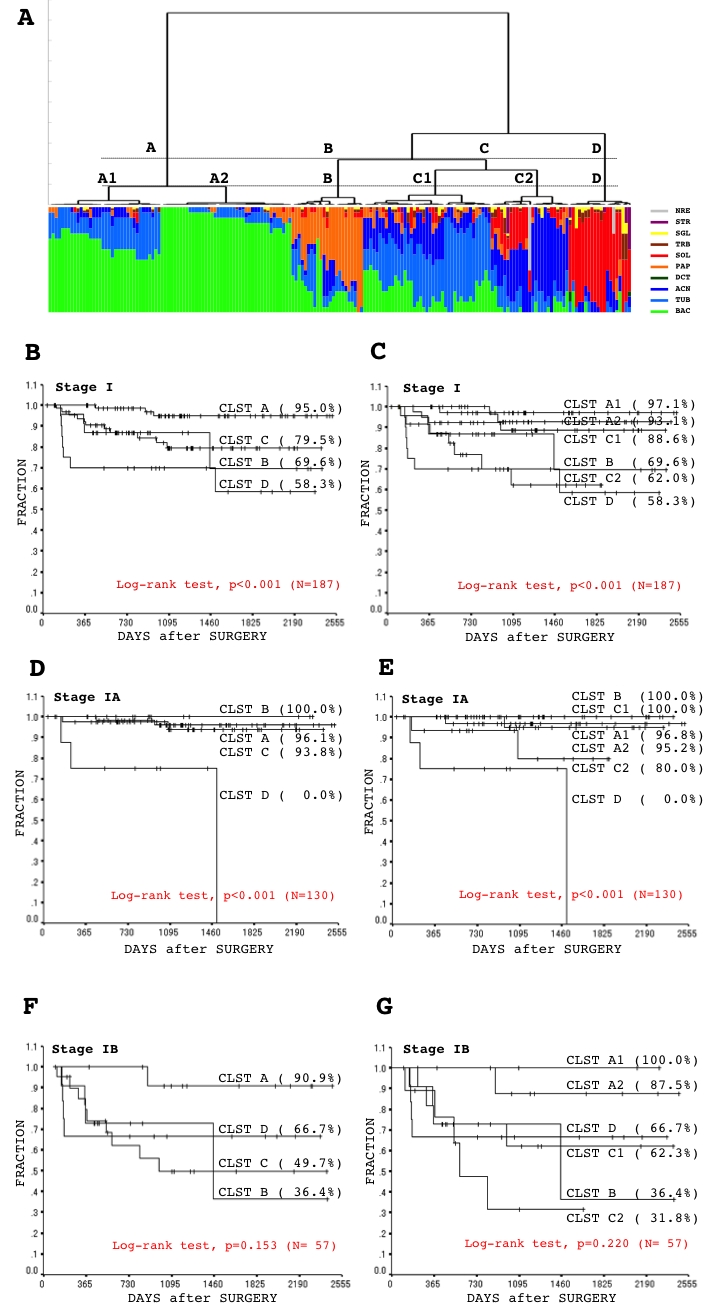
(see A-D in previous page) (A) Results of hierarchical clustering based on an element of histological architecture in the form of a dendrogram (upper panel). A threshold (upper panel, dashed lines) was set to divide lung tumors into four clusters (A to D) and six sub-clusters (A1, A2, B, C1, C2, and D). Proportions of different architectural elements among individual tumors are plotted below the dendrogram (lower panel: BAC, bronchioloalveolar; TUB, tubular; ACN, acinar; DCT, ductular; TRB, trabecular; PAP, papillary; SOL, solid; SGR, single; SRT, striated; NER, neuroendocrinal). (B to G) Five-year disease-free survival curves evaluated from a Kaplan-Meier analysis among the architectural clusters (stage I whole (B), stage IA (D), stage IB (F)) and sub-clusters (stage I whole (C), stage IA (E), stage IB (G)). CLST means cluster. Values in parentheses are estimated five-year disease-free survival rates.
Table 1.
Recurrence rates and hazard ratios among clusters based on different elements
| Crude recurrence rate % [No.] | Hazard ratio [95% CI] | |
|---|---|---|
| WHL | P = 0.177 | |
| CLST A [61] | 4.9 [3] | 1.00 [ref.] |
| B [73] | 13.7 [10] | 2.97 [0.82-10.79] |
| C [22] | 22.7 [5] | 5.59 [1.33-23.41] |
| D [31] | 22.6 [7] | 5.85 [1.51-22.66] |
| ARC | P = 0.012 | |
| CLST A [73] | 3.9 [3] | 1.00 [ref.] |
| B [23] | 17.4 [4] | 5.53 [1.23-24.77] |
| C [67] | 16.4 [11] | 4.90 [1.37-17.57] |
| D [20] | 35.0 [7] | 11.34 [2.93-43.88] |
| CLL | P = 0.153 | |
| CLST A [84] | 11.9 [10] | 1.00 [ref.] |
| B [30] | 3.3 [1] | 0.27 [0.04- 2.13] |
| C [36] | 25.0 [9] | 2.57 [1.04- 6.33] |
| D [37] | 13.5 [5] | 1.34 [0.46- 3.92] |
| NCL | P = 0.260 | |
| CLST A [43] | 7.0 [3] | 1.00 [ref.] |
| B [48] | 18.8 [9] | 2.94 [0.80-10.85] |
| C [58] | 10.3 [6] | 1.51 [0.38- 6.03] |
| D [38] | 18.4 [7] | 3.20 [0.83-12.39] |
| CLL-NCL | P = 0.642 | |
| CLST A [46] | 10.9 [5] | 1.00 [ref.] |
| B [29] | 6.9 [2] | 0.66 [0.13- 3.38] |
| C [29] | 13.8 [4] | 1.53 [0.41- 5.71] |
| D [83] | 16.9 [14] | 1.66 [0.60- 4.61] |
| ARC-CLL | P = 0.138 | |
| CLST A [54] | 3.7 [2] | 1.00 [ref.] |
| B [70] | 15.7 [11] | 4.43 [0.98-19.98] |
| C [31] | 16.1 [5] | 4.65 [0.90-23.99] |
| D [32] | 21.9 [7] | 7.53 [1.56-36.28] |
| ARC-NCL | P = 0.044 | |
| CLST A [66] | 6.1 [4] | 1.00 [ref.] |
| B [26] | 3.8 [1] | 0.65 [0.07- 5.77] |
| C [76] | 19.7 [15] | 3.88 [1.29-11.69] |
| D [19] | 26.3 [5] | 6.10 [1.63-22.80] |
No., number of cases; P, P value, calculated with Fisher's exact test; CI, confidence interval; ref., reference; CLST, cluster; WHL, whole elements; ARC, architectural element; CLL, cell type and cytoplasmic elements; NCL, nuclear element.
Table 2.
Postoperative recurrence rates and hazard ratios in ARC-CLSTs among sub-stages
| Crude recurrence rate % [No.] | Hazard ratio [95% CI] | |
|---|---|---|
| Stage I | P = 0.002 | P < 0.001 |
| CLST A [78] | 3.8 [3] | 1.00 [ref.] |
| B [23] | 17.4 [4] | 5.59 [1.25- 25.06] |
| C [66] | 16.7 [11] | 4.96 [1.38- 17.78] |
| D [20] | 35.0 [7] | 11.47 [2.96- 44.39] |
| Stage IA | P = 0.007 | P < 0.001 |
| CLST A [65] | 3.1 [2] | 1.00 [ref.] |
| B [12] | 0.0 [0] | N.A. |
| C [45] | 4.4 [2] | 1.53 [0.22- 10.89] |
| D [8] | 37.5 [3] | 17.18 [2.82-104.69] |
| Stage IB | P = 0.191 | P = 0.153 |
| CLST A [13] | 7.7 [1] | 1.00 [ref.] |
| B [11] | 36.4 [4] | 7.45 [0.82- 67.44] |
| C [21] | 42.9 [9] | 7.82 [0.99- 61.94] |
| D [12] | 33.3 [4] | 5.77 [0.64- 51.67] |
ARC-CLSTs, clusters based on architectural elements; No., number of cases; CLST, cluster; P, P value for crude reccyrrence rate and harad ratio are calculated by Fisher's exact test and and Log-rank test, respectively; CI, confidence interval; ref., reference; N.A., not available Squamous cell carcinomas were excluded from analysis.
Table 3.
Postoperative recurrence rates and hazard ratios in ARC-subCLSTs among sub-stages
| Crude recurrence rate % [No.] | Hazard ratio [95% CI] | |
|---|---|---|
| Stage I | P = 0.002 | P < 0.001 |
| CLST A1 [36] | 2.8 [1] | 1.00 [ref.] |
| A2 [42] | 4.8 [2] | 1.84 [0.17- 20.26] |
| B [23] | 17.4 [4] | 8.07 [0.90- 72.41] |
| C1 [41] | 9.8 [4] | 3.90 [0.44- 34.92] |
| C2 [25] | 28.0 [7] | 13.51 [1.66-109.98] |
| D [20] | 35.0 [7] | 16.49 [2.03-134.14] |
| Stage IA | P = 0.003 | P < 0.001 |
| CLST A1 [32] | 3.1 [1] | 1.00 [ref.] |
| A2 [33] | 3.0 [1] | 1.14 [0.07- 18.30] |
| B [12] | 0.0 [0] | N.A. |
| C1 [29] | 0.0 [0] | N.A. |
| C2 [16] | 12.5 [2] | 5.14 [0.47- 56.86] |
| D [8] | 37.5 [3] | 18.44 [1.88-181.10] |
| Stage IB | P = 0.310 | P = 0.220 |
| CLST A1 [4] | 0.0 [0] | 1.00 [ref.] |
| A2 [9] | 11.1 [1] | N.A. |
| B [11] | 36.4 [4] | N.A. |
| C1 [12] | 33.3 [4] | N.A. |
| C2 [9] | 55.6 [5] | N.A. |
| D [12] | 33.3 [4] | N.A. |
ARC-subCLSTs, sub-clusters based on architectural elements; No., number of cases; CLST, cluster; P, P value for crude reccyrrence rate and harad ratio are calculated by Fisher's exact test and and Log-rank test, respectively; CI, confidence interval; ref., reference; N.A., not available;
*C3 (squamous cell carcinomas), excluded from anaysis.
Pathologic features among the sub-clusters
Tumors tended to be larger in sub-cluster D (Table 4). Pleural invasion occurred among tumors of all the sub-clusters except A1, and tended to be severe among tumors of sub-clusters B, C1, C2, and D (Table 4). Lymphatic canal and vascular involvement was observed for tumors of all sub-clusters, but tended to more frequent among tumors of sub-clusters C1, C2, and D (Table 4). Growth activity measured by Ki-67 immunolabeling (the MIB1 index) tended to be stronger in sub-clusters C2, and D. These findings were well consistent with postoperative outcome among the sub-clusters (Figure 6 and Table 3).
Table 4.
Association between ARC-subCLST and pathobiological features
| Cluster | A1 | A2 | B | C1 | C2 | D |
|---|---|---|---|---|---|---|
| Stage I [191] | [36] | [42] | [23] | [41] | [25] | [20] |
| Size [mean ± SD mm] [24.8 ± 16.7 (7.0 to 120.0)] (Student's test, p < 0.0100: [A1,A2] vs [D]) | ||||||
| 19.3 ± 7.6 | 21.6 ± 13.8 | 26.4 ± 15.1 | 26.5 ± 19.2 | 26.7 ±17.7 | 35.2 ± 23.5 | |
| Pleural invasion (Fisher's exact test, p = 0.0002) | ||||||
| 0 [158] | 36 [100.0%] | 41 [97.6%] | 17 [74.0%] | 29 [70.7%] | 18 [72.0%] | 18 [72.0%] |
| 1[23] | 0 [0.0%] | 1 [2.4%] | 3 [13.0%] | 9 [22.0%] | 7 [28.0%] | 2 [22.0%] |
| 2 [10] | 0 [0.0%] | 0 [0.0%] | 3 [13.0%] | 3 [7.3%] | 0 [0.0%] | 4 [20.0%] |
| Lymphatic canal involvement (Fisher's exact test, p < 0.0001) | ||||||
| 0[61] | 12 [33.3%] | 31 [73.8%] | 8 [34.8%] | 6 [14.6%] | 2 [8.0%] | 2 [10.0%] |
| 1 [110] | 23 [63.9%] | 11 [26.2%] | 13 [56.5%] | 31 [75.6%] | 19 [76.0%] | 11 [55.0%] |
| 2 [18] | 1 [2.8%] | 0 [0.0%] | 2 [8.7%] | 4 [9.8%] | 4 [16.0%] | 5 [25.0%] |
| 3 [2] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 2 [10.0%] |
| Vascular involvement (Fisher's exact test, p < 0.0001) | ||||||
| 0 [129] | 29 [80.6%] | 41 [97.6%] | 13 [56.5%] | 28 [68.3%] | 10 [40.0%] | 6 [30.0%] |
| 1[46] | 6 [16.7%] | 0 [0.0%] | 8 [34.8%] | 12 [29.3%] | 11 [44.4%] | 7 [35.0%] |
| 2 [14] | 1 [2.7%] | 1 [2.4%] | 2 [8.7%] | 1 [2.4%] | 4 [16.0%] | 5 [25.0%] |
| 3 [2] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 2 [10.0%] |
| Grwoth activity [MIB1 index, mean ± SD %] [15.4 ± 17.5 (0.1 to 80.7)] (Student's t test, p < 0.0001: [A1,A2] vs [C2,D]) | ||||||
| 7.6 ± 12.7 | 4.4 ± 8.7 | 11.8 ± 13.0 | 15.1 ± 13.2 | 31.2 ± 21.1 | 33.5 ± 15.8 | |
| Stage IA [133] | [32] | [133] | [29] | [29] | [16] | [8] |
| Size [mean ± SD mm] [19.3 ± 7.6 (7.0 to 30.0)] (Student's t test, no significant difference) | ||||||
| 18.0 ± 6.7 | 15.7 ± 6.8 | 16.7 ± 7.7 | 18.0 ± 6.2 | 16.8 ± 4.9 | 14.8 ± 5.6 | |
| Pleural invasion (Fisher's exact test, p = 0.0188) | ||||||
| 0 [122] | 32 [100.0%] | 33 [100.0%] | 10 [83.3%] | 24 [82.8%] | 13 [81.3%] | 8 [100.0%] |
| 1[11] | 0 [0.0%] | 0 [0.0%] | 2 [16.7%] | 5 [17.2%] | 3 [18.7%] | 0 [0.0%] |
| Lymphatic canal involvement (Fisher's exact test, p < 0.0001) | ||||||
| 0[52] | 11 [34.4%] | 28 [84.8%] | 5 [36.4%] | 5 [17.2%] | 2 [12.5%] | 1 [12.5%] |
| 1[74] | 21 [65.6%] | 5 [15.2%] | 7 [63.6%] | 23 [79.4%] | 12 [75.0%] | 4 [50.0%] |
| 2 [6] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 1 [3.4%] | 2 [12.5%] | 2 [25.0%] |
| 3[1] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 1 [12.5%] |
| Vascular involvement (Fisher's exact test, not suitable for analysis) | ||||||
| 0[99] | 26 [81.3%] | 33 [100.0%] | 7 [58.3%] | 22 [75.9%] | 7 [43.8%] | 3 [37.5%] |
| 1[29] | 6 [18.7%] | 0 [0.0%] | 4 [33.3%] | 7 [24.1%] | 8 [50.0%] | 2 [25.0%] |
| 2 [5] | 0 [0.0%] | 0 [0.0%] | 1 [8.4%] | 0 [0.0%] | 1 [6.3%] | 3 [37.5%] |
| 3[0] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] |
| Growth activity [MIB1 index, mean ± SD %] [12.4 ± 15.4 (0 to 66.1)] (Student's t test, p < 0.0010: [A1,A2] vs [C2,D]) | ||||||
| 7.2 ± 13.4 | 4.4 ± 9.7 | 9.1 ±14.3 | 13.0 ± 12.1 | 26.3 ± 14.8 | 27.5 ± 13.6 | |
| Stage IB [58] | [4] | [9] | [11] | [12] | [9] | [12] |
| Size [mean ± SD mm] [43.2 ± 18.5 (10.0 to 120.0)] (Student's t test, no significant difference) | ||||||
| 30.0 ± 6.8 | 40.5 ± 13.7 | 35.1 ± 15.0 | 47.0 ±27.0 | 44.4 ± 18.5 | 48.9 ± 20.5 | |
| Pleural invasion (Fisher's exact test, p = 0.1715) | ||||||
| 0[34] | 4 [100.0%] | 5 [55.6%] | 8 [72.7%] | 5 [41.7%] | 5 [55.6%] | 6 [50.0%] |
| 1[15] | 0 [0.0%] | 4 [44.4%] | 1 [9.1%] | 4 [33.3%] | 4 [44.4%] | 2 [16.7%] |
| 2 [9] | 0 [0.0%] | 0 [0.0%] | 2 [18.2%] | 3 [25.0%] | 0 [0.0%] | 4 [33.3%] |
| Lymphatic canal involvement (Fisher's exact test, p = 0.5434) | ||||||
| 0[11] | 1 [25.0%] | 4 [44.4%] | 3 [27.3%] | 1 [8.3%] | 0 [0.0%] | 1 [8.3%] |
| 1[35] | 2 [50.0%] | 5 [55.6%] | 6 [54.5%] | 8 [66.7%] | 7 [77.8%] | 7 [58.4%] |
| 2 [11] | 1 [25.0%] | 0 [0.0%] | 2 [18.2%] | 3 [25.0%] | 2 [22.2%] | 3 [25.0%] |
| 3[1] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 1 [8.3%] |
| Vascular involvement (Fisher's exact test, p = 0.0547) | ||||||
| 0[31] | 3 [75.0%] | 9 [100.0%] | 6 [54.5%] | 6 [50.0%] | 3 [33.3%] | 3 [25.0%] |
| 1[17] | 0 [0.0%] | 0 [0.0%] | 4 [36.4%] | 5 [41.7%] | 3 [33.3%] | 5 [41.6%] |
| 2 [8] | 1 [25.0%] | 0 [0.0%] | 1 [9.1%] | 1 [8.3%] | 3 [33.3%] | 2 [16.7%] |
| 3 [2] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 0 [0.0%] | 2 [16.7%] |
| Growth activity [MIB1 index, mean ± SD %] [20.1 ± 26.0 (0.1 to 80.7)] (Student's t test, p < 0.0100: [A1,A2] vs [D]) | ||||||
| 10.8 ± 5.1 | 4.5 ±4.2 | 14.3 ± 11.7 | 20.0 ± 14.8 | 39.7 ± 28.2 | 37.5 ± 16.4 | |
ARC-subCLST, sub-clusters based on architectural element; SD, standard deviation; NA, not available.
Personal features among the sub-clusters
No difference in age of onset was found among the sub-clusters (Table 5). Tumors of sub-clusters A1 and A2 predominantly affected females/non-smokers. In contrast, tumors of sub-clusters C2, and D mostly affected males/ heavy smokers with a higher Brinkmann index (Table 5). Tumors of sub-cluster B almost evenly affected females/non-smokers and males/ smokers (Table 5). These findings are consistent with notion that the postoperative performance of males/smokers is generally poorer [3,12] (Figure 6 and Table 4).
Table 5.
Association between ARC-subCLST and personal characteristics
| Cluster | A1 | A2 | B | C1 | C2 | D |
|---|---|---|---|---|---|---|
| Stage I [191] | [36] | [42] | [23] | [41] | [25] | [20] |
| Age [mean ± SD Y/O] [67.8 ± 8.3 (45 to 85)] (Student's t test, no significant difference) | ||||||
| 69.2 ± 7.6 | 66.0 ± 8.0 | 66.7 ± 10.3 | 68.2 ± 8.4 | 69.9 ± 8.4 | 66.8 ± 8.1 | |
| Sex (Fisher's exact test, p = 0.0967) | ||||||
| Female [91] | 20 [55.6%] | 25 [59.5%] | 11 [47.8%] | 20 [48.8%] | 6 [24.0%] | 8 [40.0%] |
| Male [100] | 16 [44.4%] | 17 [40.5%] | 12 [52.2%] | 21 [51.2%] | 19 [76.0%] | 12 [60.0%] |
| Smoking History | ||||||
| Status (Fisher's exact test, p = 0.0012) | ||||||
| None [96] | 27 [75.0%] | 28 [66.7%] | 12 [52.2%] | 16 [40.0%] | 7 [29.2%] | 6 [33.3%] |
| Smoker [91] | 9 [25.0%] | 14 [33.3%] | 11 [47.8%] | 24 [60.0%] | 17 [70.8%] | 12 [66.7%] |
| N.A. [4] | 0 [–] | 0 [–] | 0 [–] | 1 [–] | 1 [–] | 2 [–] |
| Brinkmann index [mean ± SD] [454 ± 593 (0 to 3200)] (Student's t test, P < 0.0500: [A1,A2] vs [C2,D]) | ||||||
| 184 ± 333 | 316 ± 564 | 560 ± 708 | 479 ± 604 | 743 ± 586 | 682 ± 705 | |
| Stage IA [133] | [32] | [33] | [12] | [29] | [16] | [8] |
| Age [mean ± SD Y/O] [67.8 ± 7.7 (51 to 85)] (Student's t test, no significant difference) | ||||||
| 69.3 ± 7.1 | 66.5 ± 7.3 | 66.5 ± 8.8 | 68.2 ± 8.4 | 69.3 ± 7.6 | 70.0 ± 6.1 | |
| Sex (Fisher's exact test, p = 0.0356) | ||||||
| Female [66] | 18 [56.2%] | 21 [65.6%] | 6 [45.5%] | 16 [55.2%] | 4 [25.0%] | 1 [12.5%] |
| Male [67] | 14 [43.8%] | 12 [34.4%] | 6 [54.5%] | 13 [44.8%] | 12 [75.0%] | 7 [87.5%] |
| Smoking History | ||||||
| Status (Fisher's exact test, p = 0.0067) | ||||||
| None [71] | 24 [75.0%] | 23 [69.6%] | 5 [41.7%] | 12 [41.4%] | 5 [33.3%] | 2 [28.6%] |
| Smoker [60] | 8 [25.0%] | 10 [30.4%] | 7 [58.3%] | 17 [58.6%] | 10 [66.7%] | 5 [71.4%] |
| N.A. [2] | 0 [–] | 0 [–] | 0 [–] | 0 [–] | 1 [–] | 1 [–] |
| Brinkmann index [mean ± SD] [413 ± 574 (0 to 3200)] (Student's t test, P < 0.0500: [A1,A2] vs [C2,D]) | ||||||
| 186 ± 339 | 247 ± 436 | 670 ± 708 | 473 ± 665 | 732 ± 650 | 758 ± 754 | |
| Stage IB [58] | [4] | [9] | [11] | [12] | [9] | [12] |
| Age [mean ± SD Y/O] [67.9 ± 9.8 (45 to 84)] (Student's t test, no significant difference) | ||||||
| 68.0 ± 12.4 | 64.4 ± 10.1 | 66.8 ± 11.2 | 72.4 ± 6.8 | 71.3 ± 10.1 | 64.7 ± 8.7 | |
| Sex (Fisher's exact test, p = 0.8977) | ||||||
| Female [21] | 2 [50.0%] | 3 [33.3%] | 5 [54.5%] | 4 [33.3%] | 2 [22.2%] | 4 [33.3%] |
| Male [37] | 2 [50.0%] | 6 [66.7%] | 6 [45.5%] | 8 [66.7%] | 7 [77.8%] | 8 [66.7%] |
| Smoking History | ||||||
| Status (Fisher's exact test, p = 0.3097) | ||||||
| None [27] | 3 [75.0%] | 6 [66.7%] | 8 [72.7%] | 4 [36.4%] | 2 [22.2%] | 4 [36.4%] |
| Smoker [29] | 1 [25.0%] | 3 [33.3%] | 3 [27.3%] | 7 [63.6%] | 7 [77.8%] | 7 [63.6%] |
| N.A. [2] | 0 [–] | 0 [–] | 0 [–] | 1 [–] | 0 [–] | 1 [–] |
| Brinkmann index [mean ± SD] [544 ± 629 (0 to 2500)] (Student's t test, no significant difference) | ||||||
| 165 ± 330 | 531 ± 845 | 459 ± 723 | 496 ± 426 | 763 ± 496 | 734 ± 706 | |
ARC-subCLST, sub-clusters based on architectural element; SD, standard deviation; Y/O, year-old; None, non-smoker; N.A., not available.
Concise criteria suitable for practical use
As described above, element of histological architecture was most significantly associated with a disease recurrence. However, its prognostic value was found only in cases of stage IA, but not stage IB disease (Figure 6, Table 2 and Table 3). Thus, we thereafter focused on stage IA disease and tried to establish concise criteria suitable for practical use to subdivide cases into sub-groups with better and worse outcome. Reviewing proportions of architectural elements among the sub-clusters (Figure 7 and Table 6), those with a worse outcome (C2, and D) were found to mainly consist of ACN and/or SOL components, while those with a better outcome (A1, A2, B and C1) mainly consisted of BAC, PAP and/or TUB components (Figure 6, Figure 7, and Table 6). The findings led us to consider that a balance of low-grade (BAC, PAP and TUB) and high-grade (ACN and SOL) components could be important in determining postoperative outcome. A cut-off value, 71.5% of the high-grade component comprised by a tumor, was calculated according to the area under the curve (AUC, 0.708) using a relative operating characteristic (ROC) curve (the proportion comprising the low-grade component was found not to be suitable for setting a threshold according to mathematical logic) (Figure 8A and 8B). According to this cut-off, tumors (stage IA) were categorized into a high-grade group (tumors comprising more than or equal to 71.5% of the high component and a low-grade group (tumors comprising less than 71.5% of the high component). The groups exhibited a significant difference in postoperative outcome (Figure 8C and Table 7) [specificity 91.9%, sensitivity 42.9%]. Optionally, a cut-off value of 9.5% was set to obtain 80% sensitivity. The level of significance was decreased (Figure 8D and Table 7) and the specificity was inevitably affected [specificity 58.9%, sensitivity 80.0%].
Figure 7.
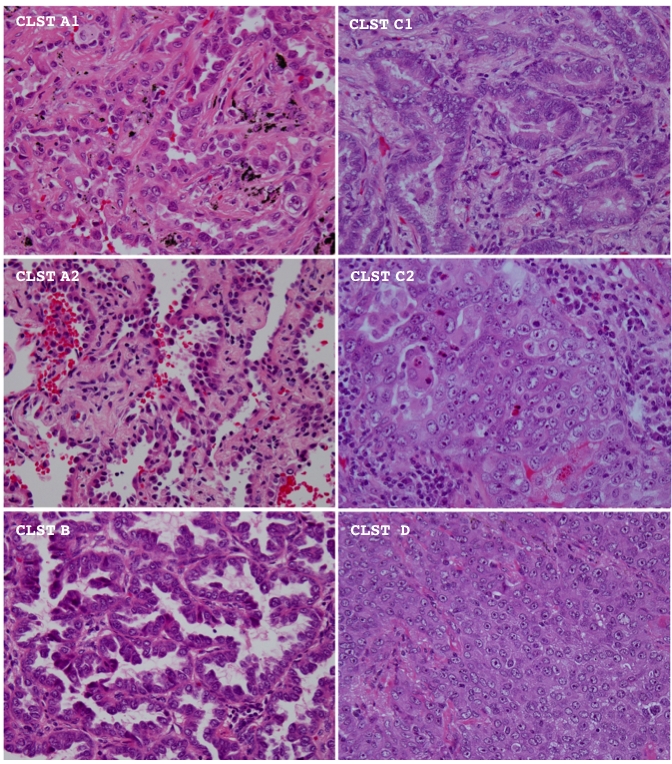
The representative histological appearance of tumors among different architectural sub-clusters is shown. Hematoxylin and eosin stain. CLST means cluster. Magnification is ×400.
Table 6.
Proportions of different elements among ARC-subCLSTs in stage IA disease
| Cluster | A1 [32] | A2 [33] | B[12] | C1 [29] | C2 [16] | D[8] |
|---|---|---|---|---|---|---|
| BAC | 64.9 ± 9.8 | 90.9 ± 7.1 | 22.3 ± 16.1 | 22.4 ± 13.1 | 4.7 ± 6.2 | 2.8 ± 7.0 |
| TUB | 27.7 ± 9.8 | 6.0 ± 4.5 | 16.8 ± 12.5 | 49.1 ± 17.9 | 11.3 ± 7.9 | 3.7 ± 5.2 |
| ACI | 4.7 ± 4.2 | 0.7 ± 1.7 | 8.6 ± 13.6 | 19.1 ± 11.0 | 55.0 ± 19.9 | 6.2 ± 10.6 |
| DCT | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.3 ± 3.5 |
| PAP | 1.3 ± 2.8 | 2.0 ± 3.9 | 50.9 ± 22.1 | 5.2 ± 4.5 | 5.3 ± 7.2 | 2.5 ± 7.0 |
| SOL | 0.5 ± 2.0 | 0.0 ± 0.0 | 2.3 ± 3.4 | 1.8 ± 3.8 | 17.2 ± 14.0 | 70.3 ± 14.8 |
| TRB | 0.7 ± 1.5 | 0.3 ± 0.8 | 0.3 ± 0.9 | 1.6 ± 2.5 | 0.9 ± 1.6 | 6.6 ± 8.5 |
| SGL | 0.3 ± 0.7 | 0.2 ± 0.6 | 0.2 ± 0.6 | 0.6 ± 1.2 | 0.4 ± 0.8 | 4.6 ± 8.4 |
| STR | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.9 | 1.6 ± 6.3 | 1.9 ± 3.7 |
| NER | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.8 ± 15.0 | 1.2 ± 5.6 |
ARC-subCLST, sub-clusters based on architectural element; mean ± standard deviation is shown; BAC, bronchioloalveolar; TUB, tubular; ACI, acinar; DCT, ductal; PAP, papillary; SOL, solid; TRB, trabecular; SGL, single; STR, striated; NER, neuroendocrinal.
Figure 8.
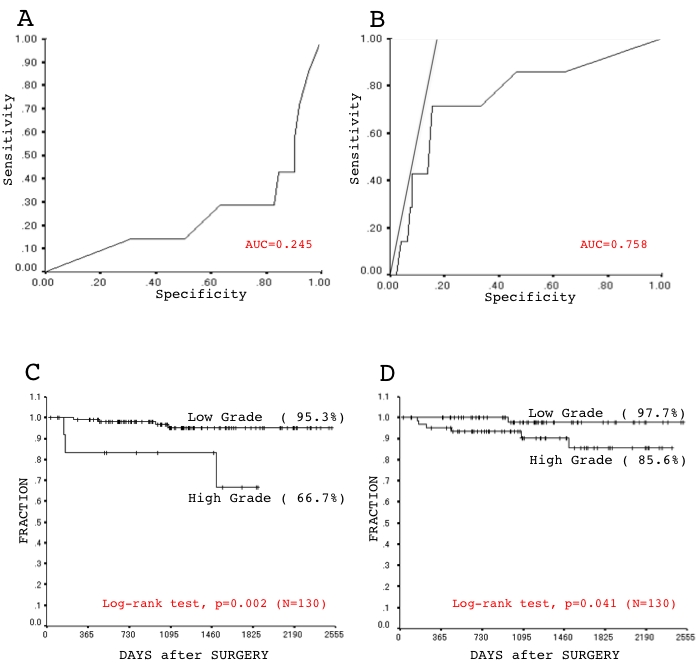
The relative operating characteristic (ROC) curve was described based on the proportion of either low-grade (A) or high-grade (B) components among tumors of stage IA ADC. AUC is the area under the curve (AUC). Five-year disease-free survival curves was evaluated based on a Kaplan-Meier analysis of the sub-groups according to a cut-off value of 72.0% (C) and of 9.0% (D) in a proportion of the high-grade component among stage IA disease. Values in parentheses are estimated five-year disease-free survival rates.
Table 7.
Postoperative recurrence rates and hazard ratios in high- and low-grade groups at stage IA
| Crude recurrence rate % [No.] | Hazard ratio [95% CI] | |
|---|---|---|
| Cut-off 71.5% | P = 0.022 | P = 0.002 |
| Low -grade group [116] | 3.4 [4] | 1.00 [ref.] |
| High-grade group [13] | 23.1 [3] | 7.35 [1.64- 33.0] |
| Cut-off 9.5% | P = 0.058 | P = 0.041 |
| Low -grade group [66] | 1.5 [1] | 1.00 [ref.] |
| High-grade group [63] | 9.5 [6] | 6.72 [0.81- 55.79] |
ARC-CLSTs, clusters based on architectural elements; No., number of cases; P, P value for crude reccyrrence rate and harad ratio are calculated by Fisher's exact test and and Log-rank test, respectively; CI, confidence interval; ref., reference; N.A., not available
Discussion
The main aim of the present study was to establish a practical sub-typing system for predicting postoperative outcome in cases of stage I ADC. We used the morphometric profiling. Possible differences in postoperative outcome among clusters based on multiple morphometric elements were analyzed. Histological architecture had the most prognostic value, especially in stage IA disease (Figure 6, Table 2 and Table 3). The elements of histological architecture could be roughly categorized into low-grade (BAC, PAP and TUB) and high-grade (ACN and SOL) components. A cut-off value was determined as 71.5% of the high-grade component (Figure 8 and Table 7). The sub-grouping system according to the cut-off is concise enough for practical use, however its sensitivity seems not satisfactory [specificity 91.9%, sensitivity 42.9%]. Eighty percent sensitivity requires a cut-off value of 9.0%, which affects specificity [specificity 58.9%, sensitivity 80.0%] (Figure 8C). We consider this could be the limit of the prognostic accuracy provided by morphological features of tumor parenchyma alone. Studies have demonstrated the excellent prognostic potential of the proportion of fibrotic area or of the status of fibrotic reactions in collapsed lesions in ADC with a BAC component [4,7,14]. Moreover, certain molecular genetic alterations in tumor cells were reported to closely associate with postoperative outcome in cases of stage I ADC [10,15]. Therefore, we are interested in combining such factors with our sub-grouping system to further improve its accuracy.
In summary, the present study has confirmed mathematically that histological architecture is the most important morphological feature of tumor parenchyma, and has proposed a concise sub-grouping system (cut-off value, 71.5% (practically 70%) of the high grade component (ACN or SOL)) for predicting postoperative recurrences in cases of stage I, especially stage I A, ADC. Importantly, we have subdivided the acinar pattern of the traditional classification system [2] into “TUB", which is defined as a tubular or glandular structure lined with single-layered neoplastic cells, and “ACN", which is defined as a tubular or glandular structure lined with poly-layered neoplastic cells or as a fused glandular structure such as the cribriform pattern, and also demonstrated their utility. This proposal is worthy of note. We hope our efforts will aid in improving the histopathologic classification of ADC.
Acknowledgments
This work was supported by the Japanese Ministry of Education, Culture, Sports, and Science (Tokyo Japan), the Smoking Research Foundation (Tokyo, Japan), and by a grant from Yokohama Medical Facility (Yokohama, Japan). We especially thank Masaichi IKEDA (Department of Pathology, Yokohama City University Graduate School of Medicine) and Shigeko IWANADE and Emi HONDA (Division of Pathology, Kanagawa Prefectural Cardiovascular and Respiratory Center Hospital) for technical assistance.
References
- 1.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–392. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. Pathology and Genetics of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. World Health Organization Classification of Tumors. [DOI] [PubMed] [Google Scholar]
- 3.Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, et al. A Japanese lung cancer registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi M, Morikawa A, Kawasaki M, Matsuno Y, Yamada T, et al. Small adenocar-cinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I non-small cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California cancer registry from 1989 to 2003. Cancer. 2007;110:1532–1541. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 6.Tsuchiya T, Akamine S, Muraoka M, Kamohara R, Tsuji K, Urabe S, et al. Stage IA non-small cell lung cancer: Vessel invasion is a poor prognostic factor and a new target of adjuvant chemotherapy. Lung Cancer. 2007;56:341–348. doi: 10.1016/j.lungcan.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Yokose T, Suzuki K, Nagai K, Nishiwaki Y, Sasaki S, Ochiai A. Favorable and unfavorable morphological prognostic factors in peripheral adenocar-cinoma of the lung 3 cm or less in diameter. Lung Cancer. 2000;29:179–188. doi: 10.1016/s0169-5002(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 8.Hamada C, Tanaka F, Ohta M, Fujimura S, Kodama K, Imaizumi M, Wada H. Meta-analysis of postoperative adjuvant chemotherapy with tega-ful-uracil in non-small-cell lung cancer. J Clin Oncol. 2005;23:4999–5006. doi: 10.1200/JCO.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarci-noma of the lung. N Engl J Med. 2004;350:1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 10.Okudela K, Woo T, Mitsui H, Yazawa T, Shi-moyamda H, Tajiri M, et al. Morphometric Profiling of Lung Cancers - its association with clini-copathologic, biological and molecular genetic features - Am J Surg Pahtol. 2010 doi: 10.1097/PAS.0b013e3181c79a6f. in press [doi: 10.1097/PAS.0b013e3181c79a6f] [DOI] [PubMed] [Google Scholar]
- 11.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 12.Bertheau P, Cazals-Hatem D, Meignin V, Roquan-court A, Verola O, Lesourd A, et al. Variability of immunohistochemical reactivity on stored paraffin slides. J Clin Pathol. 1998;51:370–374. doi: 10.1136/jcp.51.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiVito KA, Charette LA, Rimm DL, Camp RL. Long -term preservation of antigenicity on tissue mi-croarrays. Lab Invest. 2004;84:1071–1078. doi: 10.1038/labinvest.3700131. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Yokose T, Yoshida J, Nishimura M, Takahashi K, Nagai K, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000;69:893–897. doi: 10.1016/s0003-4975(99)01331-4. [DOI] [PubMed] [Google Scholar]
- 15.Woo T, Okudela K, Yazawa T, Wada N, Ogawa N, Ishiwa N, et al. Prognostic value of KRAS mutations and Ki-67 expression in stage I lungadeno-carcinomas. Lung Cancer. 2009;65:355–62. doi: 10.1016/j.lungcan.2008.11.020. [DOI] [PubMed] [Google Scholar]


