Abstract
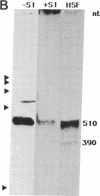
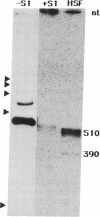
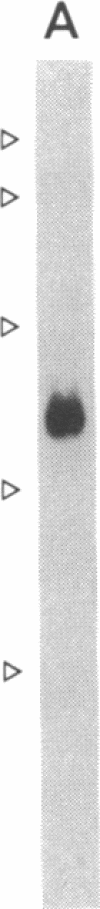
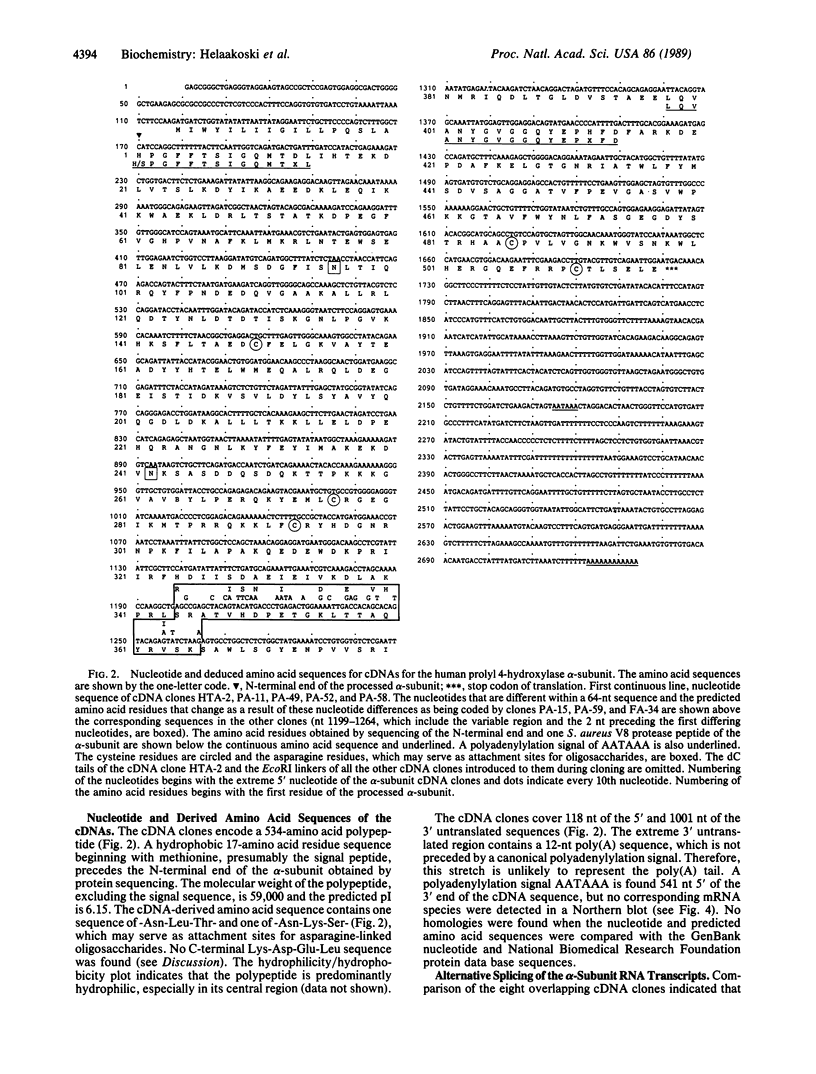
Prolyl 4-hydroxylase [procollagen-proline, 2-oxyglutarate 4-dioxygenase; procollagen-L-proline, 2-oxoglutarate:oxygen oxidoreductase (4-hydroxylating), EC 1.14.11.2], an alpha 2 beta 2 tetramer, catalyzes the formation of 4-hydroxyproline in collagens by the hydroxylation of proline residues in peptide linkages. We report here on the isolation of cDNA clones encoding the alpha-subunit of the enzyme from human tumor HT-1080, placenta, and fibroblast cDNA libraries. Eight overlapping clones covering almost all of the corresponding 3000-nucleotide mRNA, including all the coding sequences, were characterized. These clones encode a polypeptide of 517 amino acid residues and a signal peptide of 17 amino acids. Previous characterization of cDNA clones for the beta-subunit of prolyl 4-hydroxylase has indicated that its C terminus has the amino acid sequence Lys-Asp-Glu-Leu, which, it has been suggested, is necessary for the retention of a polypeptide within the lumen of the endoplasmic reticulum. The alpha-subunit does not have this C-terminal sequence, and thus one function of the beta-subunit in the prolyl 4-hydroxylase tetramer appears to be to retain the enzyme within this cell organelle. Interestingly, three of the cDNA clones for the alpha-subunit contained a 64-nucleotide sequence homologous but not identical to the corresponding 64-nucleotide sequence found in four other cDNA clones. Nuclease S1 mapping experiments demonstrated that this difference was due to the existence of two types of mRNA present in approximately equal amounts. Southern blot analyses of human genomic DNA with a cDNA probe for the alpha-subunit suggested the presence of only one gene encoding the two types of mRNA, which appear to result from mutually exclusive alternative splicing of primary transcripts of one gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Berg R. A., Kedersha N. L., Guzman N. A. Purification and partial characterization of the two nonidentical subunits of prolyl hydroxylase. J Biol Chem. 1979 Apr 25;254(8):3111–3118. [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Cardinale G. J., Udenfriend S. Homology between a prolyl hydroxylase subunit and a tissue protein that crossreacts immunologically with the enzyme. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4420–4424. doi: 10.1073/pnas.74.10.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. Y., Gong Q. H., Parkison C., Robinson E. A., Appella E., Merlino G. T., Pastan I. The nucleotide sequence of a human cellular thyroid hormone binding protein present in endoplasmic reticulum. J Biol Chem. 1987 Aug 15;262(23):11221–11227. [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Geetha-Habib M., Noiva R., Kaplan H. A., Lennarz W. J. Glycosylation site binding protein, a component of oligosaccharyl transferase, is highly similar to three other 57 kd luminal proteins of the ER. Cell. 1988 Sep 23;54(7):1053–1060. doi: 10.1016/0092-8674(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Nakazawa M., Aida T., Everson W. V., Kao C. W., Seyer J. M., Hughes S. H. Isolation of cDNA clones and genomic DNA clones of beta-subunit of chicken prolyl 4-hydroxylase. Connect Tissue Res. 1988;18(3):157–174. doi: 10.3109/03008208809016805. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Tkacz J. S., Berg R. A. Biosynthesis of prolyl hydroxylase: evidence for two separate dolichol-media pathways of glycosylation. Biochemistry. 1985 Oct 8;24(21):5960–5967. doi: 10.1021/bi00342a041. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R., Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989 Mar;3(5):1609–1617. [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Recent developments in posttranslational modification: intracellular processing. Methods Enzymol. 1987;144:96–114. doi: 10.1016/0076-6879(87)44175-x. [DOI] [PubMed] [Google Scholar]
- Koivu J., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K. I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem. 1987 May 15;262(14):6447–6449. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Parkkonen T., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase beta-subunit, protein disulphide-isomerase and a cellular thyroid-hormone-binding protein. Comparison of cDNA-deduced amino acid sequences with those in other species. Biochem J. 1988 Dec 15;256(3):1005–1011. doi: 10.1042/bj2561005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Myers J. C. Characterization of a pro-alpha 2(I) collagen gene mutation by nuclease S1 mapping. Methods Enzymol. 1987;145:213–222. doi: 10.1016/0076-6879(87)45011-8. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Tryggvason K., Myers J. C., Kurkinen M., Lebo R., Cheung M. C., Prockop D. J., Boyd C. D. cDNA clones coding for the pro-alpha1(IV) chain of human type IV procollagen reveal an unusual homology of amino acid sequences in two halves of the carboxyl-terminal domain. J Biol Chem. 1985 Jun 25;260(12):7681–7687. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K., Yamamoto T., Hayashi H., Koya S., Takikawa H., Toyoshima K., Horiuchi R. Sequence of membrane-associated thyroid hormone binding protein from bovine liver: its identity with protein disulphide isomerase. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1485–1492. doi: 10.1016/0006-291x(87)90817-5. [DOI] [PubMed] [Google Scholar]