Abstract
During ischemia ATP and phosphocreatine (PCr) decline, while intracellular hydrogen ion, Na+, Ca2+ and Mg2+ concentrations all rise. If the ischemia is relatively short and there is little irreversible injury (cell death), PCr, pH, [Na+]i, [Mg2+]i and [Ca2+]i all recovery quickly on reperfusion. ATP recovery can take up to 24 hours because of loss of adenine base from the cell and the need for de novo synthesis. There are correlative data showing that a sustained rise in [Ca2+]i during ischemia and/or lack of recovery during reperfusion is associated with irreversible cell injury. Interventions that reduce the rise in [Ca2+]i during ischemia and reperfusion have been shown to reduce cell death. Therefore a better understanding of the mechanisms responsible for the rise in [Ca2+]i during ischemia and early reperfusion could have important therapeutic implications. This review will discuss mechanisms involved in alterations in ions and high energy phosphate metabolites in perfused or intact heart during ischemia and reperfusion.
High Energy Phosphates
ATP levels have been measured in snap frozen glucose perfused hearts by enzymatic methods or by luciferase (46). Under aerobic conditions ATP levels in heart are normally about 20–25 µmol/g of tissue dry weight (6, 46, 68); this can be converted to mM by dividing by 2.5, based on 2.5 µl of intracellular water per gram dry weight of tissue. ATP content can be continuously measured in the same heart without freezing and extraction using nuclear magnetic resonance (NMR) spectroscopy. Content can be converted to concentration using standards or by measuring ATP content by biochemical methods at the end of the study. These different methods generally agree and report ATP concentrations in heart of 8–10 mM. Values for phosphate creatine (PCr) concentrations measured using similar methods are 10–25 mM (6, 46), and are more variable because this parameter is more sensitive to work state and substrate than ATP. PCr is easily degraded during extraction and tissue grinding and this may account for some of the lower levels. Inorganic phosphate is measured in the range of 2.5 mM (6). ADP measured in snap frozen hearts is typically ~2.5 to 3 µmol/g dry weight, corresponding to ~1.2 mM ADP (46). However, using NMR spectroscopy, ADP levels were undetectable. ADP calculated using the creatine kinase equilibrium is typically in the range of 0.05 to 0.08 mM, considerably lower than the values measured in extracts from frozen hearts (6, 18, 68). This difference is usually attributed to a bound ADP pool that is measure in extracts but is not measured by NMR (because the line-width of bound ADP is broadened making it undetectable) and this bound ADP does not contribute to the ATP phosphorylation potential. Table 1 shows baseline levels of high energy phosphates measured in the glucose perfused rat heart. Values are similar in other species.
Table 1.
Metabolite and Ion Concentrations During Normoxia and Ischemia
| Normoxic | Ischemic | ||
|---|---|---|---|
| ATP | 10.0 mM | 2.0 mM | |
| PCr | 20.0 mM | >1.0 mM | |
| ADP | 0.08 mM | ? | |
| Pi | 2.5 mM | 25.0 mM | |
| Mg | 0.8 mM | 2.5 mM | |
| Ca | 0.1 to 1.0 uM | 3.0 uM | |
| Na | 10.0 mM | 40.0 mM | |
| pH | 7.1 | 6.0 | |
| Mito Ca | 0.1 to 0.50 uM* | 0.3 to 0.7 uM* | |
| Mito pH | 7.8 | ? | |
| Mito Na | 1.5 mM | ? | |
| SR Ca | 1.0 mM | 1.0 mM |
depends on calibration and most studies only give relative changes.
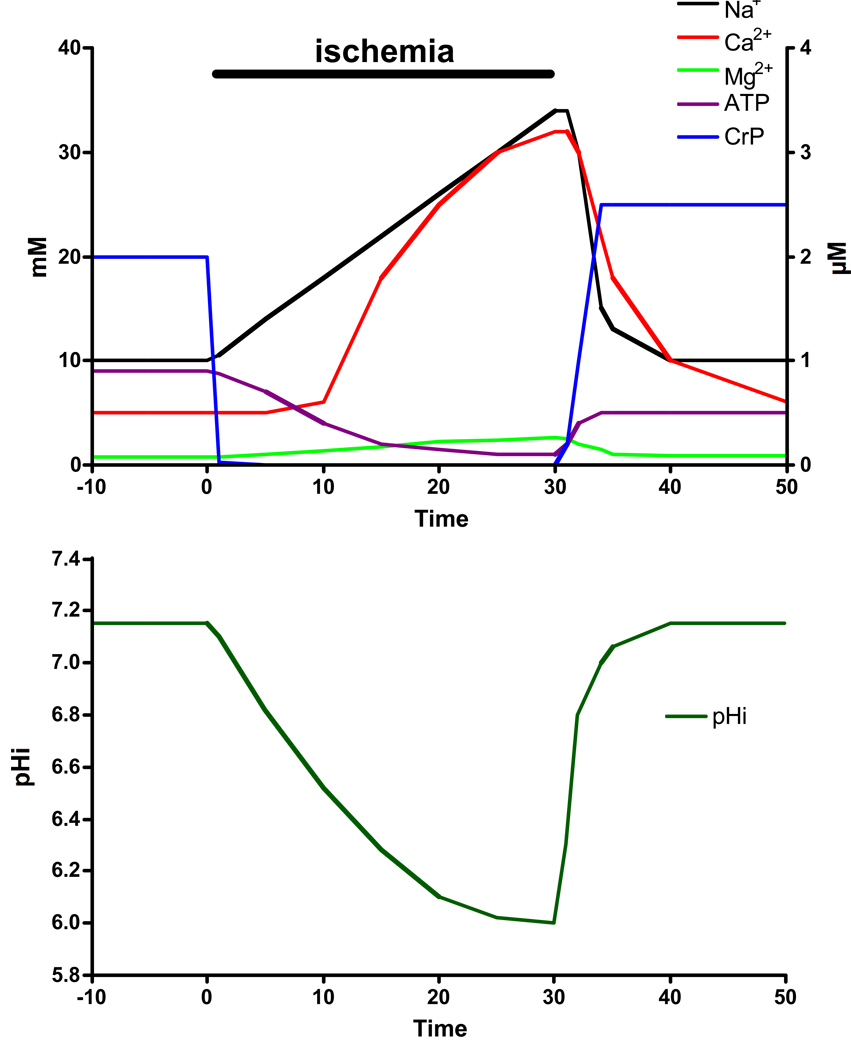
During myocardial ischemia, high energy phosphates fall rapidly (42, 46, 58) in heart (see figure 1). Creatine phosphate falls within 5 minutes to values less than 5% of initial creatine phosphate (values less than 1 mM). ATP levels are buffered by creatine phosphate and fall more slowly. By 20 minutes of ischemia ATP levels fall to 20 to 30% of initial ATP levels. High energy phosphate levels measured at 20 minutes of global ischemia in a perfused rat heart are illustrated in Table 1.
Figure 1.
Representative changes in Na, Ca, Mg, ATP, CrP and pH during ischemia.
With relatively short periods of ischemia and little cell death, PCr recovers rapidly on reperfusion to preischemic levels and may even exceed baseline (see figure 1). ATP levels recover somewhat, but do not reach pre-ischemic levels because adenine base is lost from the cell as adenosine, inosine and hypoxanthine during ischemia. Recovery of ATP to preischemic levels requires de novo synthesis of adenine base which typically takes about 24 hours. Inorganic phosphate also decreases quickly towards preischemic levels. With longer periods of ischemia and resulting cell death, recovery of cell high energy phosphates is incomplete. Based on NMR measurement of recovery of inorganic phosphate, it appears that this incomplete recovery is due to heterogeneous recovery between live and dead cells, particularly dead cells in areas of no-reflow, as opposed to partial recovery in all cells. Dead and dying cells do not recovery their PCr (or quickly lose any they recover) and cells that survive return PCr and inorganic phosphate to near normal levels.
Intracellular pH
Intracellular pH has been measured, at baseline and during ischemia, by 31P NMR spectroscopy using the shift difference between creatine phosphate and inorganic phosphate (see equation 1). Creatine phosphate is pH insensitive in the physiological range whereas inorganic phosphate has a pKa of ~6.9. The position of inorganic phosphate shifts depending on the pH (the degree of protonation). Therefore using a re-arrangement of the Henderson-Hasselbach equation (eqn 1), intracellular pH can be calculated from the shift difference between creatine phosphate and inorganic phosphate. Basal pHi in heart is in the range of 7.05 to 7.20 (15, 19, 42). These values agree with older literature in which intracellular pH was measured using the equilibrium of a weak acid usually with a radioactive label such as 14C(55). During total ischemia in the rodent heart, pHi rapidly declines reaching about 6.0 after 15 minutes of ischemia and remains at this level during ischemia (see figure 1 )(42).
Equation 1: pHi = log [δmin−δx]/[δx−δmax] + pK where dmax, dmin and dx are the maximum, minimum and measured shift difference between PCr and inorganic phosphate and pK is the pK for inorganic phosphate.
Intracellular Na
Intracellular Na has been measured in beating perfused hearts by 23Na NMR, using a shift agent to shift the extracellular Na+ so that it can be separated from the resonance peak for the intracellular Na+ (54). By measuring the area under the intracellular Na+ resonance peak, 23Na NMR measures the amount of intracellular Na+. The amount of Na+ can be converted to an intracellular Na+ concentration with information about intracellular volumes. Investigators using 23Na NMR have reported intracellular [Na+]i values in the range of 7–15 mM (5, 42, 48, 63). These values agree well with those measured in isolated cardiac myocytes using ion selective electrodes or fluorescent indicators (4). As illustrated in figure 1, 23Na NMR data indicate that intracellular [Na+]i rises about 3 to 4 fold during ischemia to a level in the range of 25 to 40 mM (1, 42, 48).
Cytosolic ionized Ca2+
Intracellular [Ca2+]i has been measured in cardiac myocytes using fluorescent calcium indicators by many investigators (3, 14, 66). Diastolic [Ca2+]i levels have been reported to be ~100 nM with systolic levels reported in the range of 0.6 to 2 µM (3, 14, 66). It has been more difficult to measure Ca with fluorescent indicators in a beating heart because of motion artifacts. Some measurements of surface fluorescence have been made, and they support the measurements obtained in isolated myocytes (37). Ca sensitive indicators based on BAPTA have also been labeled with fluorine to allow measurement of [Ca2+]i via 19F NMR spectroscopy (36, 42). A limitation of this NMR method is that the low sensitivity of NMR (relative to fluorescence) requires loading with high levels of the indicator which results in buffering of Ca; however gated Ca measurements agree well with measurements obtained in isolated myocytes (35). Ischemia is defined as the lack of blood flow and can therefore only be studied in an intact organ although measurements have been made of [Ca2+]i during metabolic inhibition in isolated myocytes. Studies have been done in perfused hearts using fluorescent Ca indicators (37) or NMR measurements using F-BAPTA (36, 37, 56). Surface fluorescent measurements of Indo-1 loaded hearts show a rise in [Ca2+]i during ischemia (37). 19F-NMR measurements of Ca2+ using FBAPTA have reported a rise in Ca to 3 µM by 20 minutes of global ischemia (36, 56).
Cytosolic ionized Mg2+
Cytosolic free [Mg2+] has also been measured using fluorescent and fluorine labeled NMR sensitive indicators (41, 44). Using a 19F labeled Mg2+ chelators APTRA, which undergoes an NMR chemical shift on binding Mg2+, [Mg2+]i was determined to be 0.8 mM in a beating perfused heart (44). Similar levels for free [Mg2+]i were measured using fluorescent Mg indicators loaded into cardiomyocytes (41). During ischemia, 19F-NMR was used to measure [Mg2+]i and it was reported that the rise in [Mg2+]i during ischemia tracked the decline in ATP during ischemia. At 20 minutes of ischemia, [Mg2+]i was measured at 2.1 mM in the rat heart (44).
Mechanisms Responsible for Ionic Changes During Ischemia and Reperfusion
pHi during ischemia
pHi during ischemia is determined by the cellular production of acid and the acid extrusion tranporters. The initial decline in pH during ischemia is usually attributed to anaerobic glycolysis and release of protons from ATP breakdown (53). In a global Langendorff rat heart model, glycolysis slows markedly after about 15 minutes of ischemia. The virtual cessation of production of protons during ischemia has usually been attributed to inhibition of glycolysis (due to increased NADH and low pH mediated inhibition of GAPDH) prior to complete glycogen depletion (51), whereas the rate of fall and the final extent of intracellular acidification can be modulated by interventions that reduce ATP breakdown during ischemia (reduce metabolic demand). Interestingly, the fall in pHi during ischemia is reduced (less acidification) with many cardioprotective interventions (28, 43, 58). It is also interesting that oligomycin, an inhibitor of the F1F0-ATPase, which has been shown to consume ATP during ischemia by running in reverse, also reduced ischemic acidification (11). These data suggest that the rate of fall and final pHi reached during ischemia is also influenced by factors other than direct inhibition of glycolysis.
The protons generated during ischemia are removed from the cell by extrusion of weak acids such as lactic acid and other proton extrusion mechanisms (NHE), resulting in extracellular acidification. This eventually inhibits further acid efflux from the cell during ischemia in intact myocardium where the extracellular volume is limited. Concomitant measurements of intra- and extracellular pH during ischemia demonstrated final values of 5.9 and 5.5 respectively (17). Lactate efflux and proton extrusion via NHE or Na-bicarbonate cotransporters have been suggested to be the primary transporters responsible for proton extrusion during ischemia. However, the role of NHE in extruding protons during ischemia is debated because NHE is inhibited by low extracellular pH that occurs during ischemia. However, the fall in extracellular pH does not occur immediately so it is likely that inhibition of proton extrusion mechanisms does not occur immediately upon ischemia, and extracellular pH is lower than intracellular pH and this cannot be achieved by efflux of weak acids alone. Also the fall in pH can be accounted for based on metabolism without invoking inhibition of proton extrusion mechanisms (53).
pH during reperfusion
On reperfusion extracellular pH returns to normal (pH ~7.4) allowing extrusion of intracellular protons via NHE and Na-dependent bicarbonate exchange. These acid extruding mechanism, such as NHE return the intracellular pH returns to normal within a few minutes of reperfusion (26, 27, 49).
Na during ischemia
Na has been shown to rise during ischemia (34, 42, 48). This increase in Na could be due to an increase in Na influx, a decrease in Na extrusion or a combination of both. The Na-KATPase extrudes Na from the cell and thereby sets the inwardly directed Na gradient that provides the driving force for many other exchangers. Generally, an increase in a Na influx pathway does not increase intracellular Na, because of an increase in activity of the Na-K ATPase. Because intracellular Na rises during ischemia, it is generally assumed that the activity of the Na-KATPase is reduced during ischemia (10). However data suggest that the pump is active during the first few minutes of ischemia (1, 23). The reasons for the eventual inhibition of the Na-KATPase are not completely clear. Clearly a fall in ATP will result in inhibition of the Na-K ATPase; however it is not clear whether the pump becomes inhibited before ATP levels decline to concentrations that would result in inhibition of the Na pump (23). Once the pump is active, it is reported that it remains active even with ATP levels < 0.2 mM and ADP levels as high as 2 mM (23). There are also data suggesting that the Na-pump might be inhibited by post translational modifications that occur during ischemia (16). With the Na pump inhibited, Na will rise because of Na entry via Na influx pathways. Regarding the Na influx mechanism, the relative role of Na-H exchange (NHE) versus persistent (non-inactivating) Na channels has been debated (40, 67), and it is likely that both contribute. If Na-K ATPase activity is reduced during ischemia but not completely eliminated, reducing Na influx through either NHE or non-inactivating sodium channels could be sufficient to prevent sodium accumulation. Studies have shown that addition of NHE inhibitors significantly attenuates the rise in Na during ischemia, suggesting a role for NHE in the rise in Na during ischemia (8, 12, 22, 42, 49). This mechanism has been questioned for several reasons. Firstly, NHE inhibitors do not increase the fall in pHi during ischemia as might be expected. However there are other pathways that regulate pHi and if one is inhibited, other pathways can regulate pHi. Inhibition of NHE will alter the kinetics of pHi regulation. Also reducing Ca overload by reducing sodium influx can reduce ATP utilization and slow ischemic metabolism and thereby reduce the generation of protons. Secondly, it has been shown that NHE is inhibited by low extracellular pH that occurs during ischemia (64). However, as discussed above, the fall in extracellular pH does not occur immediately so it is likely that inhibition of proton extrusion mechanisms does not occur immediately during ischemia. Furthermore, although the rate of NHE activity is reduced by low extracellular pH, some low level of activity can still occur (64), which may be sufficient to extrude the intracellular acid that is being generated slowly after the first minutes of ischemia. Also, the fall in pH can be accounted for without inhibition of proton extrusion mechanisms (53). Thirdly, the early NHE inhibitors were shown to also inhibit the persistent Na channels (67). It is clear that the marked inhibition of the rise in Na during ischemia that occurs with amiloride and other non-selective NHE inhibitors is due in part to inhibition of persistent Na channels. However a role for Na channels in Na entry does not preclude a role for NHE. Indeed, recent studies with newer more specific NHE inhibitors find that these more specific inhibitors also reduce the rise in Na during ischemia (8, 22, 62), although the attenuation of the rise in [Na+]i appears to be less than with non-specific inhibitors such as amiloride. Further support for a role for NHE comes from studies using mice lacking NHE. NHE null mice were shown to be resistant to ischemia/reperfusion injury compared to wild-type, with better preserved ATP during ischemia and a reduction in the degree of contracture during ischemia (65).
A role for the persistent Na+ channels is suggested because inhibitors of these channels such as TTX and lidocaine have been shown to reduce the rise in [Na+]i during ischemia (5). Butwell et al using 23Na NMR have shown that lidocaine reduces, but does not block the rise in [Na+]i during ischemia (5, 67). Studies in cardiac myocytes using TTX or lidocaine have reported a more complete block in the rise in [Na+]i during anoxia (21). However, the metabolic activity of the cardiomyocytes is less than that in an intact heart; thus there is likely to be less metabolic generation of protons. In fact, the rise in [Na+]i during ischemia was shown to be altered by altering pacing rate(12). However, a recent study by Williams et al used the Na+ sensitive fluorescent indicator (SBFI) and monitored surface fluorescence during ischemia and reperfusion in a rat heart (67). In contrast to the study by Butwell et al (5), Williams et al reported that 300 nM TTX completely blocked the rise in [Na+]i during ischemia (67). Williams et al further show that zoniporide, an NHE inhibitor that they found to have no effect on Na+ channels, did not attenuate the rise in [Na+]i during ischemia. Williams et al also find that during ischemia [Na+]i rises to less than 20 mM, a level slightly lower (but in the same range) compared to that typically observed using 23Na NMR. This difference could possibly reflect differences between the subepicardium myocardium where some oxygen diffusion from the environment can occur and midmyocardial myocardium which is likely to be more completely anoxic during ischemia, and which is the primary source of the NMR signal but is not visible using surface fluorescence.
The 23Na NMR and SBFI surface fluorescence studies both suggest a role for persistent Na+ channels in the rise in [Na+]i during ischemia; although they differ somewhat as to whether this is the sole Na+ entry mechanism. The preponderance of data obtained using inhibitors as well as NHE knockout mice, suggest that NHE is at least partially responsible for the rise in [Na+]i during ischemia. Taken together the data support a role for both NHE and persistent Na+ channels in the rise in [Na+]i during ischemia and the relative contribution of each may depend on the metabolic activity of the cell.
Na during reperfusion
In contrast to the debate over the mechanism responsible for the rise in [Na+]i during ischemia, there appears to be agreement that NHE is primarily responsible for the rise in [Na+]i at the start of reperfusion. On reperfusion when extracellular pH is restored the protons accumulated in the cytosol are extruded via NHE in exchange for Na+. A number of studies have shown that NHE inhibition cause a slight slowing of the rate of recovery of pHi on reperfusion (49, 59). Interestingly, even with NHE inhibition, pHi still recovers rapidly during reperfusion, demonstrating that other acid extrusion mechanisms can regulate pHi if NHE is inhibited. There is some disagreement regarding whether the Na+ that enters on reperfusion results in a measurable increase in [Na+]i or whether the Na+ entering is rapidly extruded via the Na-pump and reverse mode NCX resulting in only a slight and very transient spike in [Na+]i. Most of the 23Na NMR studies find little or no measurable rise in [Na+]i during reperfusion, unless the Na-K ATPase is inhibited (25, 63). These data suggest that on reperfusion, the Na- KATPase is reactivated and can extrude the increased Na+ that enters via NHE. Therefore on reperfusion inhibitors of NHE appear to slightly delay the recovery of pHi and slightly reduce the very transient rise in [Na+]i. In contrast to the 23Na NMR studies, Williams et al find a large rise in Na, to ~40 mM on reperfusion (67), which remains at this high level for ~10 minutes. The reason for this difference is unclear. The larger and more persistent rise in [Na+]i on reperfusion, which was measured in hearts loaded with SBFI, suggest that either ATP recovery and/or NaK ATPase recovery is slower in this study in SBFI loaded hearts or that the rise in [Na+]i is larger in this model and overwhelms the pump. pH dependent changes in the binding of Na+ to SBFI or leakage of the indicator should also be considered.
Ca during ischemia
[Ca2+]i is normally maintained several orders of magnitude below extracellular Ca by sarcoplasmic/endoplasmic reticulum CaATPase (SERCA), the sarcolemmal Ca ATPase, and the sarcolemmal Na-Ca exchanger (NCX), which uses the energy of the Na+ gradient to extrude Ca2+ from the cell. As discussed, during ischemia, intracellular [Na+]i rises and the inwardly directed Na+ gradient is reduced allowing [Ca2+]i to rise via NCX. The decline in ATP or post-translational modification of the Ca ATPase results in inhibition of the Ca ATPase. However, for a rise in [Ca2+]i to occur, Ca2+ must enter via some mechanism. The mechanism responsible for the rise in [Ca2+]i during ischemia is debated (47). There are data showing that the rise in [Ca2+]i is linked to the rise in [Na+]i. Blocking the rise in [Na+]i during ischemia has been shown to delay and attenuate the rise in [Ca2+]i. However, it is not fully agreed whether the Na+ dependent rise in [Ca2+]i is because of Ca2+ entry via reverse mode of the Na-Ca exchanger (NCX) or whether Ca2+ enters via another mechanism, but [Ca2+]i rises because Ca2+ extrusion via NCX is inhibited because of the reduced Na+ gradient. This issue could have clinical implications because inhibitors of NCX have been suggested to reduce the rise in [Ca2+]i during ischemia, and these inhibitors would only be beneficial if Ca2+ rises because of Ca2+ entry via reverse mode NCX. NCX exchanges 3 Na for 1 Ca and is therefore electrogenic. NCX is generally close to equilibrium and as indicated from the equation 2 it depends on membrane potential and the Na+ and Ca2+ gradients. Noble had questioned the level of Ca measured during ischemia, based on modeling of the equilibrium using assumptions about the Na gradient and the membrane potential (47). However, the discrepancy between the model and the data appear to be due to the value chosen for the extracellular [Na+]; in a global ischemia model, the extracellular [Na+] falls to levels approaching 120 mM, due to sodium influx and the relatively smaller extracellular volume compared to intracellular volume during ischemia in intact myocardium. If an extracellular [Na+] of 120 is assumed then the mathematic model used by Noble shows that NCX is in equilibrium with a cytosolic [Ca2+] of ~3uM (47).
| Eqn 2 |
Because NCX is inhibited by low pH, it has been suggested that NCX will not be active during ischemia when pH falls to 6.0 (32). Although NCX activity will be reduced by the low pH, it may not be totally inhibited. Furthermore, the increase in [Ca2+]i during ischemia is modulated by the level of [Na+]i consistent with NCX activity (42). Also consistent with the concept that Ca2+ entry via reverse mode of NCX has a role in Ca2+ entry during ischemia and enhances ischemic injury, studies have shown that mice lacking cardiac NCX (27) or inhibitors of NCX (24, 29, 33) reduce ischemic injury. Imahashi et al (27) found that mice with cardiac specific ablation of the plasma membrane NCX had a slower decline in ATP during ischemia, a slower onset of ischemic contracture, a reduced maximum contracture, and less of a rise in [Na+]i during ischemia. It is interesting that NCX-KO hearts had a reduced rise in [Na+]i during ischemia (27). If NCX runs in reverse mode during ischemia, it might be expected that inhibition of NCX would increase the rise in [Na+]i during ischemia. However, the reduced rise in [Ca2+]i during ischemia, which would occur due to inhibition of reverse mode NCX, would result in better preservation of ATP, which in turn might reduce metabolic generation of protons and reduce Na+ entry via NHE. Taken together these data suggest that NCX is active during ischemia, although the level of activity might be reduced. Thus there appears to be reasonable agreement that NCX is a major mechanism responsible for the rise in [Ca2+]i during ischemia and that attenuation of NCX during ischemia would be beneficial.
However, blocking the rise in [Na+]i during ischemia does not completely block the rise in [Ca2+]i (42), so there are also likely to be Na+ independent mechanisms for Ca2+ entry. Ca2+ entry via the L-type Ca channel appears to play a role in the rise in [Ca2+]i during ischemia (7, 61). Inhibition of the L-type Ca channel via S-nitrosylation has been shown to reduce ischemic injury (60, 61). A rise in cytosolic [Ca2+]i could also occur due to release of Ca2+ from intracellular organelles such as the SR or the mitochondria. Ca2+ uptake into the SR is mediated by the SERCA which uses the energy from ATP hydrolysis to transport Ca2+ into the SR against a concentration gradient. Under normoxic conditions, [Ca2+] in the SR is reported to be near 1 mM (6); thus the Ca2+ gradient across the SR is ~ 4 orders of magnitude, a value close to the thermodynamic potential based on the ΔG for ATP. During ischemia, when ATP falls, it might be expected that Ca2+ would be released from the SR. However, measurements of SR [Ca2+] during ischemia, show no measurable change in SR [Ca2+]. The lack of decline in SR [Ca2+] is consistent with the rise in cytosolic [Ca2+]; calculations show that during ischemia even with the reduced ΔG for ATP (calculations show it falls from −60 to −49 kJ/mol), there is still sufficient energy to maintain an SR [Ca2+] of ~1 mM with a cytosolic [Ca2+] of 3 uM (see (6) for details). Because SR [Ca2+] does not change during ischemia, it does not appear that release of SR Ca2+ during ischemia is a significant source of the rise in [Ca2+]i.
The role of mitochondria in regulating cytosolic [Ca2+]i has long been debated (). Currently there is controversy as to whether mitochondrial matrix [Ca2+] follows cytosolic Ca2+ transients or whether the change in matrix [Ca2+] reflects a more time averaged change in cytosolic [Ca2+]. Several studies (2, 50) report that mitochondrial [Ca2+] cycles on a beat to beat basis from ~0.2 uM to 0.9 uM. Others such as Miyata et al (37) suggest that mitochondrial Ca is in the range of 0.1 to 0.2 uM, and increases to a higher steady state as cytosolic [Ca2+] is increased by increasing beating frequency or by addition of isoproterenol. One key issue is whether a mitochondrial release mechanism exists with sufficient time resolution to extrude Ca from the mitochondria on a beat to beat basis. The contribution of mitochondrial Ca2+ to changes in cytosolic [Ca2+] during ischemia is even less clear. Ca2+ is taken up into the mitochondria by the Ca uniporter, which uses the energy of the mitochondrial Δp (protomotive force) as a driving force. The mitochondrial Δp and thus the driving force for Ca2+ uptake into the mitochondrial falls during ischemia. Ca2+ can exit cardiac mitochondria via a Na-Ca exchanger. The matrix Na+ level has been reported to be regulated by mitochondrial NHE and thus by the inwardly directed pH gradient across the inner mitochondrial membrane. In energized mitochondria, the Na+ gradient is therefore outwardly directed and [Na+] is reported to be as much as 8-fold lower in the matrix (13, 30).
There are very few studies measuring mitochondrial [Ca2+] in a perfused heart during true ischemia. Miyamae et al (37) measured mitochondrial [Ca2+] in perfused hearts with indo-1 and surface fluorescence, using Mn2+ to quench cytosolic Ca2+. They found a rise in mitochondrial [Ca2+] during ischemia and an inverse correlation between mitochondrial [Ca2+] during ischemia and recovery of LVDP on reperfusion. A number of studies have measured mitochondrial [Ca2+] during simulated ischemia, and most studies suggest that there is a small rise in mitochondrial [Ca2+] (20, 38, 39, 45, 52). Interestingly, Griffiths et al observed that the rise in mitochondrial [Ca2+] during ischemia was inhibited by clonazepam (an inhibitor of mitochondrial NCX), thus suggesting a role for mitochondrial NCX operating in the reverse mode to increase mitochondrial matrix [Ca2+] (20). It is suggested that during ischemia the mitochondrial Na+ gradient decreases as a result of the decrease in mitochondrial pH gradient, and this will reduce the inwardly directed Na+ gradient (20). The decrease in Na+ gradient along with the rise in [Ca2+]i, may contribute to Ca2+ entry into the mitochondria by Na-Ca2+ exchange. Taken together, the data suggest that mitochondrial Ca2+ efflux does not contribute to the rise in cytosolic [Ca2+] during ischemia. In fact the data suggest the contrary, that the rise in cytosolic [Ca2+] during ischemia contributes to the rise in mitochondrial [Ca2+]. In summary, Ca2+ entry via NCX and the L-type Ca channel appear to be the primary mechanisms responsible for the rise in Ca2+ during ischemia.
Ca during reperfusion
During the first few minutes of reperfusion, before the Na+ gradient is restored to normal and during the time of increased Na+ entry by NHE (stimulated by the pH gradient that occurs with the return of the normal extracellular pH), a rise in cytosolic Ca2+ can occur due to reverse mode NCX. Within a few minutes of reperfusion after 20–30 minutes of global ischemia, the Na+ gradient is restored (by the Na-K ATPase and the return of normal the normal pH gradient which reduces intracellular Na+ loading) to its normal inwardly directed gradient allowing Ca2+ extrusion via NCX. ATP is also restored allowing full operation of Ca-ATPases. [Ca2+]i typically returns to preischemic levels within a few minutes of reperfusion after 20– 30 minutes of global ischemia, resulting in more or less normal Ca2+ transients. On reperfusion the mitochondrial Δp is also restored providing a large driving force for Ca2+ uptake into the mitochondria. If the Na-KATPase returns to normal function, it will extrude Na+, sparing the cell from a large Ca2+ overload. If a rise in [Ca2+]i on reperfusion is sustained, this can lead to mitochondrial Ca+2 uptake, which dissipates Δψ, thus reducing mitochondrial ATP generation (see (43)). Accumulation of mitochondrial Ca is also reported to activate a poorly define mitochondrial permeability transition pore, which will totally dissipate the mitochondrial Δp and is suggested to result in immediate cell death.
Consequence of ionic alterations
The rise in cytosolic [Ca2+] during ischemia may lead to activation of enzymes such as calpains that are involved in initiating apoptosis and necrosis. Data seems to suggest that the rise in [Ca2+]i at the start of reperfusion is a major factor in the development of irreversible injury. This rise in [Ca2+]i can lead to increased mitochondrial Ca2+ uptake, which will use the energy from electron transport for Ca2+ influx rather than to make ATP and if mitochondrial [Ca2+] increases above a threshold amount, it can activate the mitochondrial permeability transition pore that triggers cell death (43). It should be noted however that there are some data suggesting that a rise in [Ca2+]i on reperfusion is not an important trigger for the MPT (31).
What relationship (if any) is there between altered ion gradients during ischemia and the rise in [Ca2+]i at the start of reperfusion. Another way of phrasing this question is – is the rise in [Ca2+]i during ischemia a predictor of the rise in [Ca2+]i on reperfusion? Correlative data suggest that irreversible injury correlates with the rise in cytosolic and/or mitochondrial [Ca2+] during ischemia (37, 57). However this increase in [Ca2+] may just indicate an increase in severity of injury in general and it may not be the increase in [Ca2+] per se that is injurious. In support of a causal role for Ca2+, interventions that reduce the rise in [Ca2+]i during ischemia also reduce the amount of injury (57). Furthermore, in mice with cardiac specific ablation of plasma membrane NCX or NHE, there was a reduced rate of fall in ATP during ischemia, suggesting a beneficial effect of reducing [Ca2+]i during ischemia (27, 65). It is also possible that the rise in [Ca2+]i during ischemia primes the mitochondria for opening of the MPT when pH is restored to normal on reperfusion thus removing the inhibition of the MPT by low pH. The increase in cytosolic Ca is also a factor in maintaining SR Ca2+ during ischemia and this might contribute to SR Ca oscillations on reperfusion.
The fall in pHi during ischemia has a number of effects. The low pHi inhibits contractility which will help to conserve ATP. The low pH during ischemia also contributes to Na+ and Ca2+ loading of the cell and organelles. Furthermore the low pHi during ischemia inhibits the MPT. On reperfusion, intracellular pH is rapidly restored allowing contractility to resume, but also allowing activation of MPT. Indeed it has been suggested that postconditioning protects in part by slowing the recovery of intracellular pH (9).
In summary, during ischemia, ATP and PCr decline, while intracellular hydrogen ion concentration, [Na+]i, [Ca2+]i, and [Mg2+]i all rise. If the ischemia is relatively short and there is little irreversible injury (cell death), PCr, pHi, [Na+]i, [Ca2+]i, and [Mg2+]i all recover quickly on reperfusion. ATP recovery can take up to 24 hours because of loss of adenine base and the need for de novo synthesis. Interventions that reduce the rise in [Ca2+]i during ischemia and reperfusion have been shown to reduce cell death. Therefore a better understanding of the mechanisms responsible for the rise in [Ca2+]i during ischemia and early reperfusion could have important therapeutic implications. A rise in [Na+]i occurs during ischemia due to NHE and persistent Na channel activity; this rise in [Na+]i leads to an increase in [Ca2+]i via NCX. Ca2+ entry via the L-type Ca channel may also contribute to the rise in [Ca2+]i during ischemia. On reperfusion, there is additional Na+ entry via NHE, but if PCr recovers and ATP phosphorylation potential is restored, there is little or no rise in bulk Na+ because of extrusion via the Na-KATPase and NCX. However, Na+ extrusion via NCX results in increased Ca2+ entry into the cell which can increase mitochondrial Ca2+ loading, activating the mitochondrial permeability pore, resulting in cell death. The increase in [Ca2+]i can also lead to Ca2+ cycling across the SR which impairs contractility and leads to futile cycling. The relative role of the rise in [Ca2+]i during ischemia versus reperfusion has been debated. It appears the Ca2+ entry during ischemia and reperfusion both contribute to irreversible cell death, and reducing Ca2+ entry during both phases would be worthwhile therapeutic targets. Interventions initiated at the start of reperfusion are more clinically relevant; however it is important that the interventions be applied during the first few seconds of reperfusion. Thus strategies to reduce [Ca2+]i during ischemia and reperfusion are worth developing and testing.
References
- 1.Anderson SE, Dickinson CZ, Liu H, Cala PM. Effects of Na-K-2Cl cotransport inhibition on myocardial Na and Ca during ischemia and reperfusion. The American journal of physiology. 1996;270:C608–C618. doi: 10.1152/ajpcell.1996.270.2.C608. [DOI] [PubMed] [Google Scholar]
- 2.Bell CJ, Bright NA, Rutter GA, Griffiths EJ. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. The Journal of biological chemistry. 2006;281:28058–28067. doi: 10.1074/jbc.M604540200. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovascular research. 2003;57:897–912. doi: 10.1016/s0008-6363(02)00656-9. [DOI] [PubMed] [Google Scholar]
- 5.Butwell NB, Ramasamy R, Lazar I, Sherry AD, Malloy CR. Effect of lidocaine on contracture, intracellular sodium, and pH in ischemic rat hearts. The American journal of physiology. 1993;264:H1884–H1889. doi: 10.1152/ajpheart.1993.264.6.H1884. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, London R, Murphy E, Steenbergen C. Regulation of the Ca2+ gradient across the sarcoplasmic reticulum in perfused rabbit heart. A 19F nuclear magnetic resonance study. Circulation research. 1998;83:898–907. doi: 10.1161/01.res.83.9.898. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circulation research. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 8.Choy IO, Schepkin VD, Budinger TF, Obayashi DY, Young JN, DeCampli WM. Effects of specific sodium/hydrogen exchange inhibitor during cardioplegic arrest. The Annals of thoracic surgery. 1997;64:94–99. doi: 10.1016/s0003-4975(97)00245-2. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 10.Cross HR, Radda GK, Clarke K. The role of Na+/K+ ATPase activity during low flow ischemia in preventing myocardial injury: a 31P, 23Na and 87Rb NMR spectroscopic study. Magn Reson Med. 1995;34:673–685. doi: 10.1002/mrm.1910340505. [DOI] [PubMed] [Google Scholar]
- 11.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. The Journal of physiology. 1995;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dizon J, Burkhoff D, Tauskela J, Whang J, Cannon p, Katz J. Metabolic inhibition in the perfused rat heart: evidence for glycolytic requirement for normal sodium homeostasis. The American journal of physiology. 1998;274:H1082–H1089. doi: 10.1152/ajpheart.1998.274.4.H1082. [DOI] [PubMed] [Google Scholar]
- 13.Donoso P, Mill JG, O'Neill SC, Eisner DA. Fluorescence measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. The Journal of physiology. 1992;448:493–509. doi: 10.1113/jphysiol.1992.sp019053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisner DA, Choi HS, Diaz ME, O'Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circulation research. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty JT, Weisfeldt ML, Bulkley BH, Gardner TJ, Gott VL, Jacobus WE. Mechanisms of ischemic myocardial cell damage assessed by phosphorus-31 nuclear magnetic resonance. Circulation. 1982;65:561–570. doi: 10.1161/01.cir.65.3.561. [DOI] [PubMed] [Google Scholar]
- 16.Fuller W, Parmar V, Eaton P, Bell JR, Shattock MJ. Cardiac ischemia causes inhibition of the Na/K ATPase by a labile cytosolic compound whose production is linked to oxidant stress. Cardiovascular research. 2003;57:1044–1051. doi: 10.1016/s0008-6363(02)00810-6. [DOI] [PubMed] [Google Scholar]
- 17.Gabel SA, Cross HR, London RE, Steenbergen C, Murphy E. Decreased intracellular pH is not due to increased H+ extrusion in preconditioned rat hearts. The American journal of physiology. 1997;273:H2257–H2262. doi: 10.1152/ajpheart.1997.273.5.H2257. [DOI] [PubMed] [Google Scholar]
- 18.Gard JK, Kichura GM, Ackerman JJ, Eisenberg JD, Billadello JJ, Sobel BE, Gross RW. Quantitative 31P nuclear magnetic resonance analysis of metabolite concentrations in Langendorff-perfused rabbit hearts. Biophysical journal. 1985;48:803–813. doi: 10.1016/S0006-3495(85)83839-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garlick PB, Radda GK, Seeley PJ. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. The Biochemical journal. 1979;184:547–554. doi: 10.1042/bj1840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths EJ, Ocampo CJ, Savage JS, Rutter GA, Hansford RG, Stern MD, Silverman HS. Mitochondrial calcium transporting pathways during hypoxia and reoxygenation in single rat cardiomyocytes. Cardiovascular research. 1998;39:423–433. doi: 10.1016/s0008-6363(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 21.Haigney MC, Lakatta EG, Stern MD, Silverman HS. Sodium channel blockade reduces hypoxic sodium loading and sodium-dependent calcium loading. Circulation. 1994;90:391–399. doi: 10.1161/01.cir.90.1.391. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann M, Decking UK. Blocking Na(+)-H+ exchange by cariporide reduces Na(+)-overload in ischemia and is cardioprotective. Journal of molecular and cellular cardiology. 1999;31:1985–1995. doi: 10.1006/jmcc.1999.1029. [DOI] [PubMed] [Google Scholar]
- 23.Hilgemann DW, Yaradanakul A, Wang Y, Fuster D. Molecular control of cardiac sodium homeostasis in health and disease. Journal of cardiovascular electrophysiology. 2006;17 Suppl 1:S47–S56. doi: 10.1111/j.1540-8167.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 24.Hobai IA, O'Rourke B. The potential of Na+/Ca2+ exchange blockers in the treatment of cardiac disease. Expert opinion on investigational drugs. 2004;13:653–664. doi: 10.1517/13543784.13.6.653. [DOI] [PubMed] [Google Scholar]
- 25.Imahashi K, London RE, Steenbergen C, Murphy E. Male/female differences in intracellular Na+ regulation during ischemia/reperfusion in mouse heart. Journal of molecular and cellular cardiology. 2004;37:747–753. doi: 10.1016/j.yjmcc.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Imahashi K, Mraiche F, Steenbergen C, Murphy E, Fliegel L. Overexpression of the Na+/H+ exchanger and ischemia-reperfusion injury in the myocardium. American journal of physiology. 2007;292:H2237–H2247. doi: 10.1152/ajpheart.00855.2006. [DOI] [PubMed] [Google Scholar]
- 27.Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E. Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circulation research. 2005;97:916–921. doi: 10.1161/01.RES.0000187456.06162.cb. [DOI] [PubMed] [Google Scholar]
- 28.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circulation research. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 29.Inserte J, Garcia-Dorado D, Ruiz-Meana M, Padilla F, Barrabes JA, Pina P, Agullo L, Piper HM, Soler-Soler J. Effect of inhibition of Na(+)/Ca(2+) exchanger at the time of myocardial reperfusion on hypercontracture and cell death. Cardiovascular research. 2002;55:739–748. doi: 10.1016/s0008-6363(02)00461-3. [DOI] [PubMed] [Google Scholar]
- 30.Jung DW, Apel LM, Brierley GP. Transmembrane gradients of free Na+ in isolated heart mitochondria estimated using a fluorescent probe. The American journal of physiology. 1992;262:C1047–C1055. doi: 10.1152/ajpcell.1992.262.4.C1047. [DOI] [PubMed] [Google Scholar]
- 31.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. American journal of physiology. 2006;290:H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 32.Lazdunski M, Frelin C, Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. Journal of molecular and cellular cardiology. 1985;17:1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- 33.Lee C, Hryshko LV. SEA0400: a novel sodium-calcium exchange inhibitor with cardioprotective properties. Cardiovascular drug reviews. 2004;22:334–347. doi: 10.1111/j.1527-3466.2004.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 34.Malloy CR, Buster DC, Castro MM, Geraldes CF, Jeffrey FM, Sherry AD. Influence of global ischemia on intracellular sodium in the perfused rat heart. Magn Reson Med. 1990;15:33–44. doi: 10.1002/mrm.1910150105. [DOI] [PubMed] [Google Scholar]
- 35.Marban E, Kitakaze M, Chacko VP, Pike MM. Ca2+ transients in perfused hearts revealed by gated 19F NMR spectroscopy. Circulation research. 1988;63:673–678. doi: 10.1161/01.res.63.3.673. [DOI] [PubMed] [Google Scholar]
- 36.Marban E, Kitakaze M, Kusuoka H, Porterfield JK, Yue DT, Chacko VP. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:6005–6009. doi: 10.1073/pnas.84.16.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamae M, Camacho SA, Weiner MW, Figueredo VM. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. The American journal of physiology. 1996;271:H2145–H2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- 38.Miyata H, Lakatta EG, Stern MD, Silverman HS. Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circulation research. 1992;71:605–613. doi: 10.1161/01.res.71.3.605. [DOI] [PubMed] [Google Scholar]
- 39.Murata M, Akao M, O'Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca(2+) overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circulation research. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- 40.Murphy E, Cross H, Steenbergen C. Sodium regulation during ischemia versus reperfusion and its role in injury. Circulation research. 1999;84:1469–1470. doi: 10.1161/01.res.84.12.1469. [DOI] [PubMed] [Google Scholar]
- 41.Murphy E, Freudenrich CC, Levy LA, London RE, Lieberman M. Monitoring cytosolic free magnesium in cultured chicken heart cells by use of the fluorescent indicator Furaptra. Proceedings of the National Academy of Sciences of the United States of America. 1889;86:2981–2984. doi: 10.1073/pnas.86.8.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy E, Perlman M, London RE, Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circulation research. 1991;68:1250–1258. doi: 10.1161/01.res.68.5.1250. [DOI] [PubMed] [Google Scholar]
- 43.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annual review of physiology. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 44.Murphy E, Steenbergen C, Levy LA, Raju B, London RE. Cytosolic free magnesium levels in ischemic rat heart. The Journal of biological chemistry. 1989;264:5622–5627. [PubMed] [Google Scholar]
- 45.Namekata I, Shimada H, Kawanishi T, Tanaka H, Shigenobu K. Reduction by SEA0400 of myocardial ischemia-induced cytoplasmic and mitochondrial Ca2+ overload. Eur J Pharmacol. 2006;543:108–115. doi: 10.1016/j.ejphar.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Neely JR, Rovetto MJ, Whitmer JT, Morgan HE. Effects of ischemia on function and metabolism of the isolated working rat heart. The American journal of physiology. 1973;225:651–658. doi: 10.1152/ajplegacy.1973.225.3.651. [DOI] [PubMed] [Google Scholar]
- 47.Noble D. Simulation of Na/Ca exchange activity during ischemia. Annals of the New York Academy of Sciences. 2002;976:431–437. doi: 10.1111/j.1749-6632.2002.tb04772.x. [DOI] [PubMed] [Google Scholar]
- 48.Pike MM, Kitakaze M, Marban E. 23Na-NMR measurements of intracellular sodium in intact perfused ferret hearts during ischemia and reperfusion. The American journal of physiology. 1990;259:H1767–H1773. doi: 10.1152/ajpheart.1990.259.6.H1767. [DOI] [PubMed] [Google Scholar]
- 49.Pike MM, Luo CS, Clark MD, Kirk KA, Kitakaze M, Madden MC, Cragoe EJ, Jr, Pohost GM. NMR measurements of Na+ and cellular energy in ischemic rat heart: role of Na(+)-H+ exchange. The American journal of physiology. 1993;265:H2017–H2026. doi: 10.1152/ajpheart.1993.265.6.H2017. [DOI] [PubMed] [Google Scholar]
- 50.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, Pozzan T. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. The EMBO journal. 2001;20:4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rovetto MJ, Lamberton WF, Neely JR. Mechanisms of glycolytic inhibition in ischemic rat hearts. Circulation research. 1975;37:742–751. doi: 10.1161/01.res.37.6.742. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Meana M, Garcia-Dorado D, Miro-Casas E, Abellan A, Soler-Soler J. Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovascular research. 2006;71:715–724. doi: 10.1016/j.cardiores.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Smith GL, Donoso P, Bauer CJ, Eisner DA. Relationship between intracellular pH and metabolite concentrations during metabolic inhibition in isolated ferret heart. The Journal of physiology. 1993;472:11–22. doi: 10.1113/jphysiol.1993.sp019932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Springer CS, Jr, Pike MM, Balschi JA, Chu SC, Frazier JC, Ingwall JS, Smith TW. Use of shift reagents for nuclear magnetic resonance studies of the kinetics of ion transfer in cells and perfused hearts. Circulation. 1985;72:IV89–IV93. [PubMed] [Google Scholar]
- 55.Steenbergen C, Deleeuw G, Rich T, Williamson JR. Effects of acidosis and ischemia on contractility and intracellular pH of rat heart. Circulation research. 1977;41:849–858. doi: 10.1161/01.res.41.6.849. [DOI] [PubMed] [Google Scholar]
- 56.Steenbergen C, Murphy E, Levy L, London RE. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circulation research. 1987;60:700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- 57.Steenbergen C, Murphy E, Watts JA, London RE. Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart. Circulation research. 1990;66:135–146. doi: 10.1161/01.res.66.1.135. [DOI] [PubMed] [Google Scholar]
- 58.Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circulation research. 1993;72:112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- 59.Stromer H, de Groot MC, Horn M, Faul C, Leupold A, Morgan JP, Scholz W, Neubauer S. Na(+)/H(+) exchange inhibition with HOE642 improves postischemic recovery due to attenuation of Ca(2+) overload and prolonged acidosis on reperfusion. Circulation. 2000;101:2749–2755. doi: 10.1161/01.cir.101.23.2749. [DOI] [PubMed] [Google Scholar]
- 60.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning Results in S-Nitrosylation of Proteins Involved in Regulation of Mitochondrial Energetics and Calcium Transport. Circulation research. 2007 doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 61.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circulation research. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 62.ten Hove M, van Emous JG, van Echteld CJ. Na+ overload during ischemia and reperfusion in rat hearts: comparison of the Na+/H+ exchange blockers EIPA, cariporide and eniporide. Molecular and cellular biochemistry. 2003;250:47–54. doi: 10.1023/a:1024985931797. [DOI] [PubMed] [Google Scholar]
- 63.Van Emous JG, Schreur JH, Ruigrok TJ, Van Echteld CJ. Both Na+-K+ ATPase and Na +-H+ exchanger are immediately active upon post-ischemic reperfusion in isolated rat hearts. Journal of molecular and cellular cardiology. 1998;30:337–348. doi: 10.1006/jmcc.1997.0597. [DOI] [PubMed] [Google Scholar]
- 64.Vaughan-Jones RD, Wu ML. Extracellular H+ inactivation of Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. The Journal of physiology. 1990;428:441–466. doi: 10.1113/jphysiol.1990.sp018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Meyer JW, Ashraf M, Shull GE. Mice with a null mutation in the NHE1 Na+-H+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circulation research. 2003;93:776–782. doi: 10.1161/01.RES.0000094746.24774.DC. [DOI] [PubMed] [Google Scholar]
- 66.Wier WG. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annual review of physiology. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]
- 67.Williams IA, Xiao XH, Ju YK, Allen DG. The rise of [Na(+)] (i) during ischemia and reperfusion in the rat heart-underlying mechanisms. Pflugers Arch. 2007;454:903–912. doi: 10.1007/s00424-007-0241-3. [DOI] [PubMed] [Google Scholar]
- 68.Zimmer SD, Ugurbil K, Michurski SP, Mohanakrishnan P, Ulstad VK, Foker JE, From AH. Alterations in oxidative function and respiratory regulation in the post-ischemic myocardium. The Journal of biological chemistry. 1989;264:12402–12411. [PubMed] [Google Scholar]