Abstract
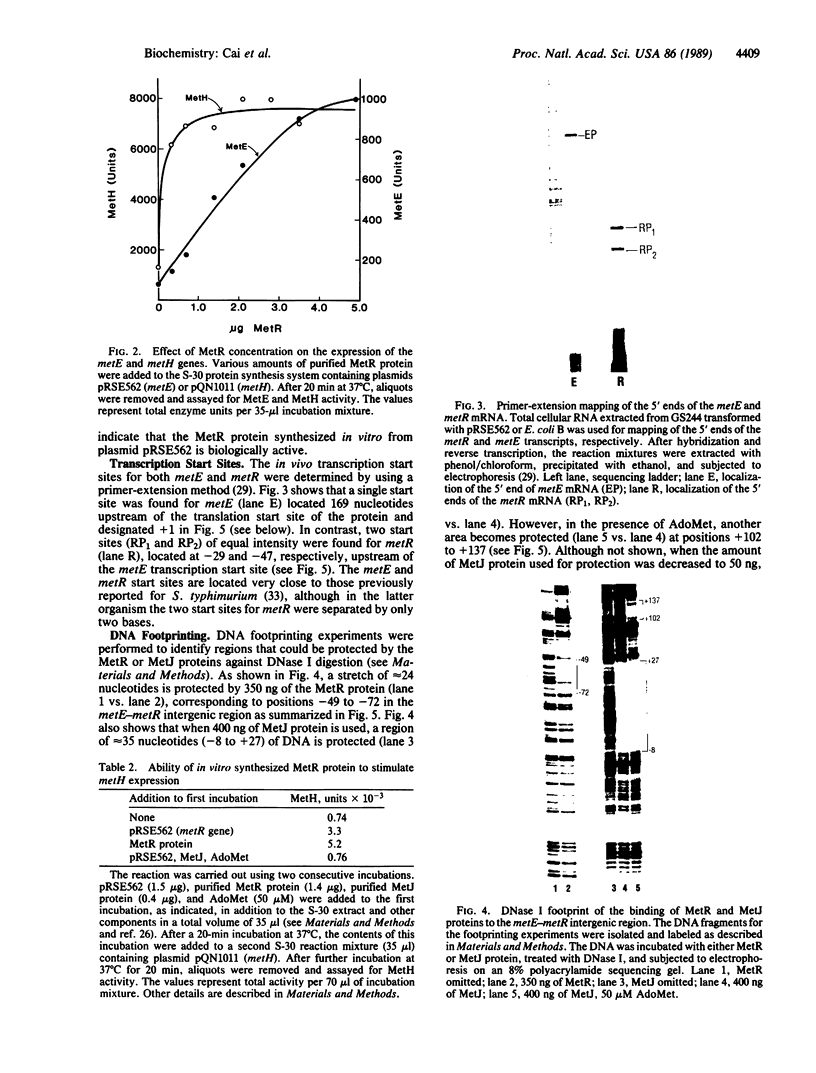
Studies by Urbanowski et al. [Urbanowski, M. L., Stauffer, L. T., Plamann, L. S. & Stauffer, G. V. (1987) J. Bacteriol. 169, 1391-1397] have identified a regulatory locus, called metR, required for the expression of the metE and metH genes. We recently purified the MetR protein from Escherichia coli and showed that it could stimulate the in vitro expression of the metE gene and autoregulate its own synthesis. In the present study, the purified MetR protein has been shown to stimulate the in vitro expression of the metH gene. Also, the in vitro synthesized MetE, MetH, and MetR proteins were shown to be biologically active. The transcription start sites for the metE and metR genes have been determined, and DNA footprinting experiments have identified regions in the metE-metR intergenic sequence that are protected by either the MetR or MetJ proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. Mechanism of repression of methionine biosynthesis in Escherichia coli. I. The role of methionine, s-adenosylmethionine, and methionyl-transfer ribonucleic acid in repression. Mol Gen Genet. 1973 Jul 16;123(4):299–324. doi: 10.1007/BF00433648. [DOI] [PubMed] [Google Scholar]
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet C., Van Meir E., ten Heggeler-Bordier B., Wittek R. Vaccinia virus produces late mRNAs by discontinuous synthesis. Cell. 1987 Jul 17;50(2):153–162. doi: 10.1016/0092-8674(87)90211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chu J., Shoeman R., Hart J., Coleman T., Mazaitis A., Kelker N., Brot N., Weissbach H. Cloning and expression of the metE gene in Escherichia coli. Arch Biochem Biophys. 1985 Jun;239(2):467–474. doi: 10.1016/0003-9861(85)90713-1. [DOI] [PubMed] [Google Scholar]
- Dawes J., Foster M. A. Vitamin B 12 and methionine synthesis in Escherichia coli. Biochim Biophys Acta. 1971 Jun 22;237(3):455–464. doi: 10.1016/0304-4165(71)90263-7. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Williams R. D., Kung H. F., Spears C., Weissbach H. Effect of methionine and vitamin B-12 on the activities of methionine biosynthetic enzymes in metJ mutants of Escherichia coli K12. Arch Biochem Biophys. 1973 Sep;158(1):249–256. doi: 10.1016/0003-9861(73)90619-x. [DOI] [PubMed] [Google Scholar]
- KERESZTESY J. C., DONALDSON K. P. Synthetic prefolic A. Biochem Biophys Res Commun. 1961 Jul 26;5:286–288. doi: 10.1016/0006-291x(61)90164-4. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Spears C., Greene R. C., Weissbach H. Regulation of the terminal reactions in methionine biosynthesis by vitamin B 12 and methionine. Arch Biochem Biophys. 1972 May;150(1):23–31. doi: 10.1016/0003-9861(72)90005-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Leustek T., Hartwig R., Weissbach H., Brot N. Regulation of ribulose bisphosphate carboxylase expression in Rhodospirillum rubrum: characteristics of mRNA synthesized in vivo and in vitro. J Bacteriol. 1988 Sep;170(9):4065–4071. doi: 10.1128/jb.170.9.4065-4071.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxon M. E., Redfield B., Cai X. Y., Shoeman R., Fujita K., Fisher W., Stauffer G., Weissbach H., Brot N. Regulation of methionine synthesis in Escherichia coli: effect of the MetR protein on the expression of the metE and metR genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):85–89. doi: 10.1073/pnas.86.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner L., Whitfield C., Weissbach H. Effect of L-methionine and vitamin B 12 on methionine biosynthesis in Escherichia coli. Arch Biochem Biophys. 1969 Sep;133(2):413–419. doi: 10.1016/0003-9861(69)90470-6. [DOI] [PubMed] [Google Scholar]
- Mulligan J. T., Margolin W., Krueger J. H., Walker G. C. Mutations affecting regulation of methionine biosynthetic genes isolated by use of met-lac fusions. J Bacteriol. 1982 Aug;151(2):609–619. doi: 10.1128/jb.151.2.609-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old I. G., Hunter M. G., Wilson D. T., Knight S. M., Weatherston C. A., Glass R. E. Cloning and characterization of the genes for the two homocysteine transmethylases of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):78–87. doi: 10.1007/BF00338396. [DOI] [PubMed] [Google Scholar]
- Plamann L. S., Stauffer G. V. Nucleotide sequence of the Salmonella typhimurium metR gene and the metR-metE control region. J Bacteriol. 1987 Sep;169(9):3932–3937. doi: 10.1128/jb.169.9.3932-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann L. S., Urbanowski M. L., Stauffer G. V. Salmonella typhimurium metE operator-constitutive mutations. Gene. 1988 Dec 15;73(1):201–208. doi: 10.1016/0378-1119(88)90326-5. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Belfaiza J., Guillou Y., Perrin D., Guiso N., Bârzu O., Cohen G. N. Interactions of the Escherichia coli methionine repressor with the metF operator and with its corepressor, S-adenosylmethionine. J Biol Chem. 1986 Aug 15;261(23):10936–10940. [PubMed] [Google Scholar]
- Schulte L. L., Stauffer L. T., Stauffer G. V. Cloning and characterization of the Salmonella typhimurium metE gene. J Bacteriol. 1984 Jun;158(3):928–933. doi: 10.1128/jb.158.3.928-933.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Coleman T., Redfield B., Greene R. C., Smith A. A., Saint-Girons I., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: effect of metJ gene product and S-adenosylmethionine on the in vitro expression of the metB, metL and metJ genes. Biochem Biophys Res Commun. 1985 Dec 17;133(2):731–739. doi: 10.1016/0006-291x(85)90965-9. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Redfield B., Coleman T., Greene R. C., Smith A. A., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: Effect of metJ gene product and S-adenosylmethionine on the expression of the metF gene. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3601–3605. doi: 10.1073/pnas.82.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly S., Coleman T., Fu C. F., Brot N., Weissbach H. Correlation between the 32-kDa sigma factor levels and in vitro expression of Escherichia coli heat shock genes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8365–8369. doi: 10.1073/pnas.84.23.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. A., Greene R. C., Kirby T. W., Hindenach B. R. Isolation and characterization of the product of the methionine-regulatory gene metJ of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6104–6108. doi: 10.1073/pnas.82.18.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Weissbach H. N5-methyltetrahydrofolate-homocysteine transmethylase. Partial purification and properties. J Biol Chem. 1967 Apr 10;242(7):1502–1508. [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Regulation of the metR gene of Salmonella typhimurium. J Bacteriol. 1987 Dec;169(12):5841–5844. doi: 10.1128/jb.169.12.5841-5844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Regulation of the metR gene of Salmonella typhimurium. J Bacteriol. 1987 Dec;169(12):5841–5844. doi: 10.1128/jb.169.12.5841-5844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. The control region of the metH gene of Salmonella typhimurium LT2: an atypical met promoter. Gene. 1988 Dec 15;73(1):193–200. doi: 10.1016/0378-1119(88)90325-3. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. The metH gene from Salmonella typhimurium LT2: cloning and initial characterization. Gene. 1986;44(2-3):211–217. doi: 10.1016/0378-1119(86)90184-8. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer L. T., Plamann L. S., Stauffer G. V. A new methionine locus, metR, that encodes a trans-acting protein required for activation of metE and metH in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1987 Apr;169(4):1391–1397. doi: 10.1128/jb.169.4.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH H., PETERKOFSKY A., REDFIELD B. G., DICKERMAN H. STUDIES ON THE TERMINAL REACTION IN THE BIOSYNTHESIS OF METHIONINE. J Biol Chem. 1963 Oct;238:3318–3324. [PubMed] [Google Scholar]
- Whitfield C. D., Steers E. J., Jr, Weissbach H. Purification and properties of 5-methyltetrahydropteroyltriglutamate-homocysteine transmethylase. J Biol Chem. 1970 Jan 25;245(2):390–401. [PubMed] [Google Scholar]