Abstract
Background/Purpose
This study examined a stress-coping model to assess whether baseline antecedent variables predicted subsequent appraisal, and how that appraisal predicted coping and quality of life for prostate cancer patients and their spouses.
Methods
In a sample of 121 prostate cancer patient/spouse dyads, we assessed baseline antecedent variables (self-efficacy, current concerns, age, socioeconomic status, social support, communication, symptoms, phase of illness), 4-month follow-up appraisal (negative appraisal, hopelessness, uncertainty), and 8-month follow-up coping and mental and physical quality of life. Patients and spouses were assessed in a single integrated path model using structural equation modeling.
Results
The stress-coping model accounted for a significant amount of variance in mental and physical quality of life at 8 months for patients (40% and 34%, respectively) and spouses (43% and 24%, respectively). Appraisal mediated the effect of several antecedent variables on quality of life. In addition, several partner effects (e.g., spouse variables influencing patient outcomes) were found.
Conclusions
Prostate cancer patients need interventions that assist them to manage the effects of their disease. The stress-coping model suggests skills in several areas that could be improved. Programs need to include spouses because they also are negatively affected by the disease and can influence patient outcomes.
INTRODUCTION
Prostate cancer is the most common cancer affecting men in the United States; it is estimated that 218,890 men were diagnosed with prostate cancer and approximately 27,000 died of prostate cancer in 2007 (1). In light of this incidence, there is a need for more theory-driven research that prospectively examines the mechanisms that influence the mental and physical quality of life of prostate cancer patients and their spouses. This research could improve programs aimed at increasing the mental and physical functioning of families enduring prostate cancer.
Stress-coping theory, drawn from the theoretical work of Lazarus and others (2, 3) provides a conceptual framework for determining which antecedent factors may be associated with higher quality of life. According to the theory, individuals who face a potentially threatening event, such as cancer, assess the degree of threat associated with it (primary appraisal) as well as their resources to cope with the event (secondary appraisal). Based on secondary appraisal, individuals reappraise their situation as either more or less threatening. Stress-coping theorists contend that this process extends over time and can affect health outcomes such as quality of life (2).
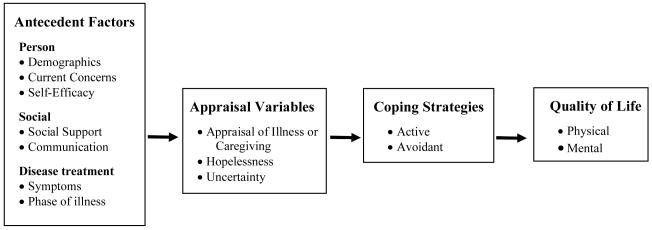
The purpose of this study was to determine if a stress-coping model (see Figure 1) could predict quality of life of men with prostate cancer and their spouses over time. To examine longitudinal effects of the model, we hypothesized that a set of antecedent variables (personal characteristics, social factors, and illness-related factors) assessed at baseline would predict appraisal variables (appraisal of illness/caregiving, hopelessness, uncertainty) measured four months later. Furthermore, these appraisal variables were hypothesized to influence coping strategies assessed at 8 months. The combination of variables (i.e., antecedent, appraisal, and coping variables) were hypothesized to predict patients’ and spouses’ mental and physical quality of life outcomes at 8 months.
Figure 1.
Stress- Coping Conceptual Model
Several studies have supported the role of the influence of the variables posited in the stress-coping model on the quality of life of patients with cancer. Findings of cross-sectional studies indicate prostate cancer patients with more co-morbid medical conditions (4), greater disease progression (5, 6), more symptoms (7), as well as less self-efficacy to manage symptoms (8) report poorer quality of life. Few longitudinal quality of life studies have been conducted with prostate cancer patients. However, several longitudinal studies of breast cancer patients found that a greater use of avoidant coping early in the illness was consistently related to poorer long-term outcomes such as emotional distress (9, 10), fear of recurrence (11), and poorer psychological adjustment three years later (12). Other personal factors such as younger age (9, 11, 13), higher perceived stress (14), more pessimism (15), and greater symptom distress (13) have also been associated with poorer adjustment outcomes of breast cancer patients at long-term follow-up. Conversely, greater use of coping through active acceptance (10, 11) have been associated with better long-term outcomes (13).
Investigators also have examined factors associated with the quality of life of spouses/partners of men with prostate cancer, who often report more emotional distress than their husbands (7). Dysfunctional problem solving, which includes avoidance, negative problem orientation, and use of less positive coping strategies have been associated with more distress in spouses (16-18). In addition, emotional distress in spouses is related to less education, greater uncertainty, worse marital quality, more negative interactions with the patient, and less positive meaning associated with the illness (16).
A few studies indicate that there is a significant relationship between the level of distress reported by prostate cancer patients and their spouses, with each person affecting the other (17, 19). Higher symptom distress in prostate cancer patientshas been associated with poorer quality of life in their spouses (7). Furthermore, greater use of constructive problem-solving coping by spouses and less social constraint in discussing the illness with their spouses have been associated with lower distress in prostate cancer patients (17, 20). These findings suggest that patients and spouses affect one another’s adjustment to the illness.
In prior research, we examined a modified version of the stress-coping model and found it predicted patient and spouse adjustment one year after diagnosis for both colon cancer (accounting for 54% of patient adjustment and 64% of spouse adjustment) (21) and breast cancer (accounting for 65% of patient adjustment and 59% of spouse adjustment) (22). In a cross-sectional study, the stress-coping model accounted for 81% of the variance in mental quality of life of patients and caregivers facing advanced breast cancer, and 72% of the variance in their physical quality of life (23). Consistent with stress-coping theory, appraisal was a key factor and it partially mediated the relationship between several antecedent and dependent variables (i.e., adjustment, QOL). While these studies provide support for theoretical underpinnings of the stress-coping model, coping variables were not included. Furthermore, one study was cross-sectional, limiting the ability to examine the mechanisms of adjustment over time.
The preceding review indicates that: a) variables assessed within a stress-coping model can account for a significant amount of variance in psychosocial outcomes of cancer patients and their caregivers; b) some variables, measured at baseline, can predict adjustment at later points in time; and c) the distress and coping style of one partner can have an affect on the well-being of the other partner. However, few studies with prostate cancer patients have used longitudinal designs, and, for the most part, studies have not included spouses or partners. Therefore, this study utilized a stress-coping model to determine if baseline antecedent variables and intermediary appraisal variables could predict coping strategies and quality of life outcomes in prostate cancer patients and their spouses at 8 months follow-up. It is expected that more baseline current concerns, lower efficacy, less social support, less communication, more symptoms, and more advanced disease would relate to more negative appraisal, hopelessness and uncertainty. These increased negative appraisals would subsequently relate to more avoidant coping and less active coping which would lead to lower mental and physical quality of life.
MATERIALS AND METHODS
Design
This study was part of a larger randomized clinical trial that examined the effects of a family-based intervention on study outcomes of prostate cancer patients and their spouse-caregivers. Detailed information pertaining to accrual and retention (24), baseline characteristics of participants (25), and outcomes of the intervention (26) have been described previously. For this longitudinal study based on a stress-coping model, only the control group was used to limit the effect of the intervention on study outcomes. Assessments were conducted at three time points: baseline, 4 month follow-up, and 8 month follow-up.
Sample
Patients were eligible if they were in one of three phases of prostate cancer (i.e., newly diagnosed, biochemical recurrence, or advanced). Newly diagnosed patients had a new diagnosis of localized or locoregional prostate cancer and received either a prostatectomy or external-beam radiation as their primary treatment. Biochemical recurrence patients completed primary treatment, had two successive rises in their PSA based on established lab values (>0.1), but had no clinical evidence of disease on radiologic exams or bone scans. They were under observation or receiving treatment. Advanced patients had clinical evidence of metastatic disease at diagnosis or a progression of the disease as indicated on diagnostic scans. They were receiving either hormonal treatments for hormone-naive disease (i.e., androgen dependent, hormone sensitive) or chemotherapy with or without additional treatments for hormone refractory disease (i.e., androgen independent, hormone resistant). In each phase, patients had a 2-month window of eligibility: (a) after completion of primary treatment (newly diagnosed), (b) after two consecutive rises in PSA post–primary treatment (>0.1), or (c) after diagnosis of metastatic disease or disease progression (advanced). This narrow window of eligibility was used to obtain dyads within each phase who were dealing with similar phase-related issues.
Other patient criteria included: ≥30 years old, a life expectancy of ≥12 months, a spouse or live-in partner, and residing within 75 miles of participating cancer centers. Patients with second primary cancers were excluded. Spouses/partners were eligible if they were: ≥21 years old and identified by patients as their primary caregiver (i.e., provider of emotional and/or physical care). Couples were excluded if spouses had been diagnosed with cancer within the prior year or were receiving cancer treatment.
During a three year period of recruitment, 429 patient-spouse dyads were referred to the study from clinic staff. Of these referrals, 46 dyads did not meet eligibility criteria, 120 refused participation, and 263 enrolled and completed baseline assessments (enrollment rate 68.7%) with 129 assigned to the intervention group and 134 assigned to the control group. Of the 134 participants from the control group, 121 completed their 8 month assessment (90% retention). Reasons for not completing the 8 month assessment included not being able to contact participants or participants being no longer interested (n=8, 62%), death of the patient (n=4, 31%), and not accepting the group assignment (n=1, 7%).
The majority of the final sample of patients (n=121) were newly diagnosed (67%), some were advanced (20%) and fewer had a biochemical recurrence (13%). The average length of time since diagnosis was 8 months for patients in the newly diagnosed phase and 67 months for patients in the later phase (biochemical recurrent/ advanced phases combined). The majority of couples were White (86%), some were African-American (13%), and a small percent were mixed-race (1%). The average length of marriage was 31.8 years (SD = 14). The mean years of education was 16.1 (SD = 3.7) for patients and 14.9 (SD = 2.8) for spouses. The majority of couples had a family income of more than $75,000 (54%), and only 6.3% had an income of $30,000 or less. Table 1 lists descriptive statistics for the variables included in the proposed stress-coping model.
Table 1.
Descriptive Characteristics for Primary Study Variables for Patients and Spouses
| Variables | Time Assessed |
Patients | Spouses | ||
|---|---|---|---|---|---|
| Alpha | M (SD) | Alpha | M (SD) | ||
| Quality of Life | 8 months | ||||
| SF-Mental | .86 | 53.5 (9.6) | .84 | 51.7 (9.9) | |
| SF-Physical | .86 | 43.4 (7.3) | .88 | 43.2 (8.6) | |
| Coping | 8 months | ||||
| Active Coping | .82 | 31.6 (7.6) | .83 | 28.4 (7.2) | |
| Avoidant Coping | .64 | 14.2 (3.4) | .65 | 15.0 (3.9) | |
| Appraisal | 4 months | ||||
| Negative Appraisal of Illness/Caregiving |
.95 | 2.29 (.77) | .87 | 2.45 (.56) | |
| Hopelessness | .85 | 2.81 (3.5) | .77 | 2.98 (3.1) | |
| Uncertainty | .94 | 60.3 (17.3) | .91 | 62.6 (17.0) | |
| Person Predictors | Baseline | ||||
| Self-efficacy | .97 | 143.8 (23.5) | .96 | 138.7 (25.6) | |
| Current Concerns | .94 | 15.8 (13.3) | .92 | 16.8 (13.6) | |
| Age (years) | 62.6 (9.3) | 59.0 (9.6) | |||
| SES* | 106.5 (58.9) | ||||
|
Social/Family
Predictors |
Baseline | ||||
| Social Support | .88 | 88.1 (12.2) | .91 | 85.0 (14.5) | |
| Communication | .91 | 3.75 (.59) | .90 | 3.77 (.54) | |
|
Illness/Treatment
Predictors (Baseline) |
Baseline | ||||
| Symptoms | .76 | 6.8 (4.0) | .77 | 5.6 (3.9) | |
| Phase of Illness Newly Diagnosed Later Phase |
67 % 33 % |
||||
Based on Hollingshead’s 4-factor weighted scaling method, using both patients’ and spouses’ occupation and education to determine the dyad’s score (47).
Procedures
Eligible participants were identified by staff in surgery, radiation, and medical oncology clinics at three research sites. Potential participants were contacted by research staff and, if they agreed to be enrolled, were scheduled for a home visit to complete consent forms approved by institutional review boards, and to collect baseline data. Patients were stratified by treatment centers (three sites), phase of illness (three phases), and type of treatment, and then randomized with spouses into control or experimental treatment arms. Data collection nurses were blinded to group assignment.
Instruments
Established instruments were used to measure all study variables. Patients and spouses completed all measures separately, with reference to themselves. Internal consistency reliabilities for each measure were assessed and are listed in Table 1.
Quality of Life
A general QOL measure, MOS SF-12 (version 2), was used to assess patients’ and spouses’ quality of life at the 8 month follow-up (27). The MOS SF-12 yields summary scores for physical and mental QOL. The SF-12 is scored with a T-score transformation such that 50 represents general population norms. Scores above 50 indicate better quality of life than the population and scores below represent worse quality of life. (27)
Coping
Fourteen coping strategies were assessed using 2 items each at the 8 month follow-up with the 28-item Brief COPE (28). Higher-order exploratory factor analyses were conducted to determine the underlying factor structure of the 14 coping strategies. Results supported a two-factor solution that was consistent with past factor analyses using breast cancer patients (29). Humor coping, alcohol/drugs, and religion did not load (i.e., factor loading less than .40) or double loaded (i.e., factor loading greater than .40 on both factors) for either patients or spouses and therefore were not included in either the active or avoidant factor scale scores. Five scales loaded on the avoidant factor for both patients and family caregivers: denial, self-distraction, behavioral disengagement, venting, and blame. Six scales loaded on active coping: use of emotional support, positive reframing, active coping, planning, acceptance, and support seeking.
It should be noted that coping was assessed at the 8 month follow-up because Lazarus and Folkman state that coping strategies are constantly changing as a function of appraisal (2,3). Given that the appraisal variables were assessed using a “past week” time frame and coping was assessed using a “past month” time frame, it is more consistent with our model to conceptualize coping as occurring after appraisal (i.e., appraisal that occurs at 4 months should influence the coping strategies at 8 months). Furthermore, even though coping and quality of life are being assessed at 8 months, quality of life was assessed using a “past week” time frame. If coping does result from appraisal, leading to new attempts to cope, then the most recent coping efforts in the last month would be expected to have the most effect on well-being in the last week.
Appraisal Variables
Appraisal variables were assessed at the 4 month follow-up. Appraisals of illness or caregiving were assessed with separate 27-item Appraisal of Illness or Appraisal of Caregiving Scales (30, 31). These scales measure patients’ level of threat and assessment of problems associated with the illness, and spouses’ perception of caregiving threat and problems. Scores range from 27-135 with higher scores indicating more negative appraisal. Adequate construct validity and internal consistency have been reported (23, 25). Hopelessness was assessed with the 20-item true/false Beck Hopelessness Scale which measures negative expectations. Evidence of concurrent and construct validity and internal consistency have been reported (32). Uncertainty was assessed with the 28-item community version of the Mishel Uncertainty in Illness Scale. The scale ranges from 28-140 and has shown adequate construct validity and internal consistency for both patients and caregivers (33).
Person Predictors
Age and socioeconomic status (SES) were assessed using the Omega Screening Questionnaire (OSQ) developed by Mood (34). SES was computed using based on individual education and occupation codes adapted from Hollingshead (34). Current concerns was measured at baseline with a 40-item scale that asks respondents to rate the extent to which they are experiencing concerns related to self, health, family, friends, religion, existential issues, and work/finances on a scale from 1 “not true” to 3 “true” with higher scores representing more concerns. Test-retest reliability, internal consistency, predictive validity, and content validity have been reported (23, 25, 34). Self-efficacy was assessed at baseline with a 17-item modified version of the Lewis Cancer Self-Efficacy Scale that measures confidence in managing stress and changes associated with cancer or treatments (e.g., “I have the ability to handle the challenges from the cancer and its treatments”). We adapted the wording to create a separate 17-item scale that measured spouses’ confidence in their abilities as caregivers to manage the cancer. The scores ranged from 0-170 with higher scores indicating higher levels of confidence. Evidence of content and criterion validity and internal consistency have been reported (35).
Family/Social Predictors
Social support was measured at baseline with the 15-item Personal Resource Questionnaire with higher scores indicating more perceived support. Evidence about the internal consistency and predictive validity of the scale have been reported in the literature (36). Communication about illness was assessed at baseline with the 32-item Lewis Mutuality and Interpersonal Sensitivity Scale that assessed the amount and degree of communication between couples about factors related to their cancer (e.g., “We spend a lot of time talking about how things are going with the cancer”; “We understand how each of us is feeling about the prostate cancer”). Scores range from 1 to 5 with higher scores indicating better communication (35).
Illness/Treatment Predictors
General symptom distress was measured at baseline with the 16-item Symptom Scale of the OSQ, a screening measure used in another aspect of the study (37). Patients and spouses each rated the severity of their own symptoms such as fatigue, pain, urinary incontinence, and sexual difficulties. The symptom scale has had good internal consistency and content and predictive validity (23, 25). Phase of illness was originally categorized into three groups: newly diagnosed, biochemical recurrence, and advanced. Because of the low number of patients in the biochemical recurrence and advanced phases, the phase of illness variable was dichotomized: newly diagnosed vs. later phase (biochemical recurrence and advanced combined).
Data analysis
To assess the proposed stress-coping model for the patient-spouse dyads, path analyses were conducted using structural equation modeling (SEM) software. The analysis of dyadic data is often problematic because of nonindependence among their responses (e.g., quality of life of the patient often relates to quality of life of the spouse because of shared characteristics and experiences). Analyzing members of the dyad separately is one way to avoid statistical problems due to nonindependence. However, this approach fails to incorporate the interdependence of dyad members that usually is of primary interest to family-focused researchers. One approach to incorporating this interdependence is the Actor-Partner Interdependence Model (APIM)(38-40). The APIM incorporates responses from both members of a dyad into a single analysis. APIM allows assessment of whether spouses influence patients and whether patients influence spouses. This was done using path analysis with SEM software as outlined in Kenny, Kashy, and Cook (40). To incorporate the interdependence of dyadic data, correlations of all pairs of variables (e.g., age of patient is correlated with age of spouse) and error disturbances (e.g., error term for patient hopelessness is correlated with the error term for spouse hopelessness), were used in the model.
Structural equation modeling with AMOS 6.0, using covariances and maximum likelihood estimation, was used to assess this single model that included both patients and spouses. The proposed model (see Figure 1) had baseline predictors (age, SES, current concerns, self-efficacy, social support, communication, symptoms, and phase of illness), three observed mediators assessed at 4 month follow-up for both patients and spouses (appraisal of illness/caregiving, hopelessness, and uncertainty), two coping variables assessed at 8 month follow-up (active and avoidant), and two quality of life variables assessed at 8 month follow-up (physical and mental health).
We assessed a full APIM model hypothesizing that: 1) patient predictors influenced patient appraisal, coping, and quality of life (patient actor effects), 2) spouse predictors influenced spouse appraisal, coping, and quality of life (spouse actor effects), 3) patient predictors influenced spouse appraisal, coping, and quality of life (patient partner effects), and 4) spouse predictors influenced patient appraisal, coping, and quality of life (spouse partner effects).
The model in Figure 1 represents the most parsimonious representation of stress-coping theory. However, past studies of cancer patients have also shown several direct effects of the antecedent factors on quality of life in addition to the indirect effects through appraisal (23, 25). In a sample of cancer patients, these additional direct effects are not counter to the proposed theory, but represent a slight refinement of the nature of the relationships of the antecedent variables to quality of life. We first tested the most parsimonious model shown in Figure 1. Modification indices were examined to determine if any additional direct effects from the person, social/family, or illness-related predictors to coping and quality of life would significantly improve the model fit. We did not add any parameters that improved model fit but did not make conceptual sense (e.g., correlate the error terms of patient appraisal with caregiver self-efficacy).
The model was evaluated in three ways. First, the model was evaluated for goodness of fit using three indices of fit: 1) the chi-square, 2) the root mean square error of approximation (RMSEA), and 3) the comparative fit index (CFI). Jaccard and Wan have recommended that multiple fit indices be used when evaluating the fit of a model (41). The chi-square represents how well the data fits the proposed model. Nonsignificant chi-square values show that the model fits the data. However, with relatively large sample sizes and complex models, it is common for the chi-square to be significant even with relatively good overall fit. Therefore, it is recommended that other indices of fit are used. The RMSEA is a highly recommended index (41, 42) that tests the poorness of fit (i.e., high scores indicate worse fit, low scores indicate good fit). Values range from 0 to 1 with values of 0 indicating perfect fit, and values less than .08 indicating good fit. The CFI assesses goodness of fit, with values greater than .9 indicating good fit (41, 42). Second, the model was evaluated by testing the direct and indirect effects specified in the model. This determined which variables had direct and indirect influence on the outcomes. Finally, R-square values were calculated for all mediator and outcome variables to determine the amount of variance that the predictors accounted for in the outcomes.
RESULTS
Results showed that this sample of prostate cancer patients and their spouses had slightly higher mental quality of life (although neither were significantly different using one-sample t-tests, p>.05), and significantly lower physical quality of life compared to general population norms (both p<.05).
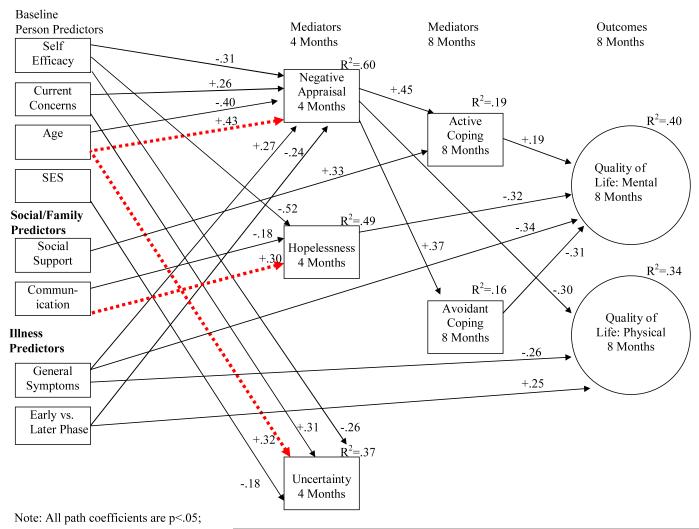
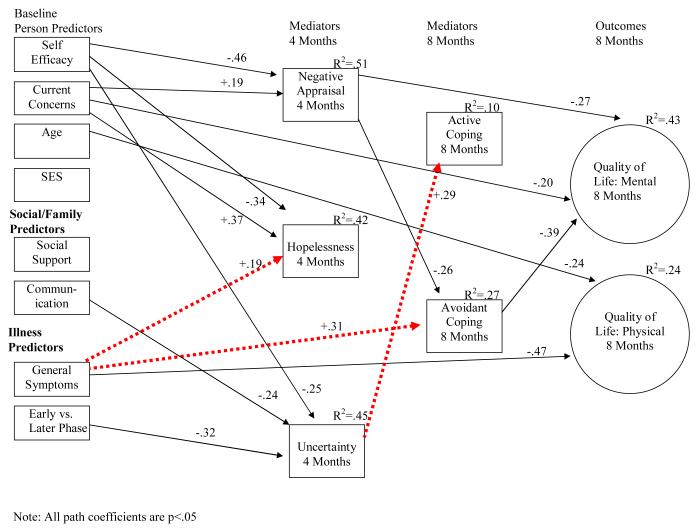
Although a single model was tested including both patient and spouse data, results are presented separately in Figure 2a for patients and Figure 2b for spouses to facilitate interpretation. Actor effects (i.e., effect of individuals’ predictor variables on their own outcomes) are shown with solid lines, and partner effects (i.e., effect of each person’s predictor variables on his/her partner’s outcomes) are shown with dashed lines.
Figure 2a.
Model Depicting Patient Relationships for Mental and Physical Quality of Life at 8 Months
Figure 2b.
Model Depicting Spouse Relationships for Mental and Physical Quality of Life at 8 Months
The initial model showed less than adequate fit, (χ2(198) = 443.19, p = <.01, RMSEA=.10, RMSEA 90% CI=.09-.11, CFI=.81), and modification indices suggested adding several direct paths, not indicated in the more parsimonious initial model. Therefore, paths were added among patient variables between the following: from symptoms and hopelessness to mental quality of life; from phase of illness, symptoms, and negative appraisal of illness to physical quality of life; and from social support to active coping. Paths among spouse variables were also added: from current concerns and negative appraisal of caregiving to mental quality of life; from age and symptoms to physical quality of life. One new partner effect was added for spouses: from patient symptoms to avoidant coping. The addition of these direct paths significantly improved the model fit and resulted in an overall excellent fitting model (χ2(189) = 283.42, p<.01, RMSEA=.06, RMSEA 90% CI=.04-.08, CFI=.93).
Results showed that the majority of the significant effects were actor effects (e.g., patient variables influencing patient outcomes; spouse variables influencing spouse outcomes). There were three partner effects of spouse predictors directly influencing patient variables, and two partner effects of patient predictors directly influencing spouse variables (see dashed lines in Figures 2a and 2b).
Patient Outcomes
Quality of Life
The model accounted for 40% of the variance in patients’ mental quality of life at the 8 month follow-up, and 34% of their physical quality of life. Overall, patients had better mental quality of life at the 8-month follow-up if they had more active coping at 8 months (β= +.19), lower avoidant coping at 8 months (β= −.31), less hopelessness at 4 months (β= −.32), and fewer baseline symptoms (β= −.34). Patients had better physical quality of life at the 8 month follow-up if they had less negative appraisal of illness at 4 months (β= −.30), fewer baseline symptoms (β= −.26), and were in the newly diagnosed phase of disease (β= +.25).
Coping resources
In addition, the model accounted for 19% of the variance of patient active coping and 16% of patient avoidant coping at 8 months. Patients with more negative appraisal of illness at 4 months used more active (β= +.45) and avoidant coping (β= +.37) at 8 months. In addition, patients with more baseline social support (β= +.33) used more active coping at 8 months.
Appraisal variables
The model accounted for 60% of the variance of negative appraisal of illness, 49% of hopelessness, and 37% of uncertainty at 4 months. Patients with less self-efficacy (β= −.31), more current concerns (β= +.26), more symptoms (β= +.27), younger (β= −.40), and in a later phase of illness (e.g., biochemical recurrent or advanced) (β= −.24), had more negative appraisal of illness at 4 months. Furthermore, a spouse partner effect (dotted line) was found such that older spouses (β= +.43) were related to more patient negative appraisal of illness at 4 months. Patients with less self-efficacy (β= −.52), and less communication (β= −.18) had more subsequent hopelessness. A spouse partner effect also was found for hopelessness, indicating that patients had higher hopelessness if their spouses reported more communication (β= +.30). Patients with less self-efficacy (β= −.52), more current concerns (β= +.31), and lower SES (β= −.18) had more uncertainty. Finally, another spouse partner effect was found, indicating that patients who have older spouses had more uncertainty (β= +.32).
In addition, several of these predictors showed indirect effects on coping and quality of life through the appraisal variables. Symptoms had indirect effects on active coping (β= +.11, p<.05) and avoidant coping (β= +.11, p<.05) through negative appraisal. Phase of illness (β= −.12, p<.05) and current concerns (β= +.11, p<.05) had indirect effects on avoidant coping through negative appraisal. Spouse communication (β= −.09 p<.05) had indirect effects on patient mental quality of life through hopelessness. Patient efficacy (β= +.18 p<.05) had indirect effects on patient mental quality of life through hopelessness and negative appraisal of illness. Social support (β= +.07, p<.05) had indirect effects on patient mental quality of life through active coping. Patient age (β= +.10, p<.05), spouse age (β= −.11, p<.05), patient symptoms (β= −.07, p<.05), and patient efficacy (β= +.09 p<.05) had indirect effects on patient physical quality of life through negative appraisal of illness.
Spouse Outcomes
Quality of life
For spouses, the model accounted for 43% of the variance of mental quality of life, and 24% in their physical quality of life. Spouses had better mental quality of life at the 8 month follow-up if they used less avoidant coping at 8 months (β= −.39), had less negative appraisal of caregiving at 4 months (β= −.27), and less baseline current concerns (β= −.20). There were no significant effects for spouse active coping and either mental or physical quality of life. Spouses had better physical quality of life at the 8 month follow-up if they had fewer baseline symptoms of their own (β= −.47), and were younger (β= −.24).
Coping resources
In addition, the model accounted for 10% of spouse active coping and 27% of avoidant coping at 8 months. Spouses with less negative appraisal of illness at 4 months (β= −.26) had more avoidant coping at 8 months. In addition, there was a partner effect showing that more baseline symptoms in patients (β= +.31) was related to more spouse avoidant coping at 8 months. Another partner effect indicated that more patient uncertainty at 4 months (β= +.29) related to more spouse active coping.
Appraisal variables
The model accounted for 51% of the variance of negative appraisal of caregiving, 42% of hopelessness, and 45% of uncertainty at 4 months. Spouses with less self-efficacy (β= −.46), and more current concerns (β= +.19) at baseline had more negative appraisal of caregiving at 4 months. Spouses with less self-efficacy (β= −.34), and more current concerns at baseline (β= +.37) had more subsequent hopelessness. A partner effect was found for hopelessness, indicating that, when patients reported more symptoms (β= +.19), spouses reported more hopelessness. Furthermore, spouses with less self-efficacy (β= −.25), less communication (β= −.24), and those who have husbands in later phase of illness (i.e., recurrent or advanced) (β= −.32) had more uncertainty.
In addition, several of these predictors showed indirect effects on coping and quality of life through the appraisal variables. Self-efficacy had indirect effects on avoidant coping (β= −.12, p<.05) through negative appraisal of caregiving. Spouses’ own symptoms (β= −.17, p<.05), self-efficacy (β= +.18, p<.05), and current concerns (β= −.08, p<.05) had indirect effects on spouses’ mental quality of life through negative appraisal of caregiving. Negative appraisal of caregiving (β= −.10, p<.05) had indirect effects on spouses’ mental quality of life through avoidant coping.
Finally, there was a significant correlation between patients’ and spouses’ mental quality of life (r =.25, p<.05), but no relationship between patients’ and spouses’ physical quality of life (r =.07, p =.45). There was no relationship between their own physical and mental quality of life for either patients (r = .09, p = .35) or for spouses (r = −.14, p =.13).
DISCUSSION
One of the important contributions of this study was that it provided a theory-based, longitudinal analysis of factors associated with the quality of life of both prostate cancer patients and their spouses. This analysis of factors enabled us to examine the process of adjustment over time. Findings indicated that selected antecedent variables measured at baseline had a significant effect on patients’ and spouses’ appraisal at 4 months and on their coping and quality of life outcomes at 8 months.
We tested a modified version of Lazarus’s theoretical model and found that the model accounted for a significant amount of variance in both prostate cancer patients’ and their spouses’ quality of life. Consistent with Lazarus’s theory, appraisal mediated the effect of the antecedents on quality of life. Although Lazarus’s theory suggests a fully mediated model as shown in Figure 1, we found that appraisal had both direct and indirect effects on quality of life. Illness-related factors, such as symptom distress (both patients’ and spouses’) had a direct negative effect on quality of life outcomes, consistent with findings in previous studies (23).
Using the APIM analysis to examine patients’ and spouses’ self-assessments in a single analysis, we found more actor effects than partner effects. Thus, patients’ personal, social, and illness-related variables had the most significant effects on their own negative appraisal at 4 months, and ultimately on their own quality of life. Similarly, spouses’ own antecedent factors were more predictive of their own appraisal measures and thus their quality of life.
However, the few observed partner effects were quite interesting. One partner effect that emerged pertained to the age of the spouse. The older the spouse, the more negative appraisal of illness, and uncertainty, that was reported by the patient. Patients may perceive older spouses as more vulnerable to the effects of illness and less able to meet patients’ support needs. Another partner effect that emerged indicated that, when patients reported more baseline symptoms, their spouses reported more hopelessness at 4 months follow-up. This finding is consistent with reports of others who found that, as patients’ symptoms increased, spouses’ mental health decreased (7, 43). On the other hand, if patients reported more uncertainty, their spouses reported using more active coping strategies, perhaps to create a greater sense of predictability in their lives. These partner effects, as well as the significant relationship found between patients’ and spouses’ mental quality of life, underscore the importance of assessing both patients’ and spouses’ quality of life because each partner affects the well-being of the other.
Of the mediators examined, appraisal served as an important mediator between antecedent variables and quality of life for both patients and spouses. Hopelessness was a mediator for patients’ but not for spouses’ quality of life. Uncertainty did not mediate either patients’ or spouses’ quality of life outcomes, a finding supported in an earlier study of breast cancer patients and their partners (23). It may be more accurate to view uncertainty as an outcome (endpoint) rather than as a mediator of quality of life.
Among the baseline predictors, self-efficacy was a key variable in the model for both patients and spouses. The longitudinal analyses illustrated the paths through which self-efficacy influenced outcomes. Less self-efficacy at baseline led to more negative appraisals of illness/caregiving, more hopelessness, and greater uncertainty for both patients and spouses at the 4 month follow-up and, as predicted by the theoretical model, some of these variables led to poorer quality of life outcomes at the 8 month follow-up. Because of its far-reaching effects on patient and spouse outcomes over time, self-efficacy should be included in patients’ and spouses’ clinical evaluations.
The number of current concerns reported by patients and spouses also was an important predictor in the model. Patients and spouses with more initial concerns had more negative appraisal of their illness and caregiving. A pile-up of concerns may occur (i.e., both illness- and nonillness-related) which overloads patients’ and spouses’ resources and leads to a more negative view of the illness or of caregiving. Note that higher baseline concerns had both a direct and indirect negative effect on spouses’ mental quality of life eight months later, but not on patient’s quality of life. Perhaps spouses with many baseline current concerns, who take on the caregiving role and assume roles that patients can no longer fulfill, deplete their resources and this negatively affects their long-term mental quality of life. Patients, on the other hand, appear able to reverse some of the effects of these early concerns as they proceed through the illness experience, by making better use of active coping, which leads to more positive long-term mental quality of life.
Interesting findings emerged for patient-spouse communication. Spouses benefited from more communication with their husbands because it helped to reduce their uncertainty about the illness. In addition, patients who reported more communication with their spouses had less hopelessness. Researchers have found that supportive relationships help prostate cancer patients to cognitively process the cancer experience, which is associated with better mental health (44). However, there may be a limit to how much patients benefit from communicating about the illness. We found a spouse partner effect that, when spouse-caregivers reported communicating more about the illness, the patients subsequently reported more hopelessness. While this finding warrants further investigation, it may suggest that some communication is helpful, but too much communication may prevent men from putting more of their attention on other matters in their day-to-day life. Another possible explanation is that not all communication about cancer is helpful. Helgeson and colleagues found that certain types of communication (e.g., urging patients to perform restorative health behaviors) led to poorer physical and mental health (45). Therefore, it may be important to intervene with spouses to provide them skills and knowledge about the proper amount and type of communication strategies to use to best help patients cope with their illness.
The proposed model examined factors that promote the use of active and avoidant coping strategies as well as the effect of these coping strategies on quality of life. Patients with more negative appraisal of illness used both active and avoidant coping strategies, with greater use of active coping leading to better mental quality of life while avoidant coping led to poorer mental quality of life, consistent with reports by others (9, 10, 12). However, a different picture emerged for spouses. There were no direct paths to active coping or from active coping to quality of life for spouses (with the exception of one partner effect). In essence, active coping exists in isolation from the other variables in the model, suggesting that it was not a factor used by spouses to maintain or enhance their quality of life. For the most part, spouses used avoidant coping rather than active coping to manage their own symptom distress. While spouses may cope with their physical symptoms by denying or minimizing their own needs, our findings indicate that this has serious consequences for them and is associated with poorer mental quality of life, and also may lead to poorer physical health for spouses (46). Furthermore, it should be noted that neither active nor avoidant coping related to physical quality of life. This relates to previous studies that showed that coping related to mental functioning and not physical functioning (23). Despite the use of coping strategies, it may be difficult to change actual physical functioning. Coping strategies seem to relate more to how individuals emotionally deal with their physical health than their actual physical health.
The findings of this longitudinal study illustrated the mechanism through which antecedent, appraisal and coping variables affected quality of life outcomes in prostate cancer patients and their spouses. The analysis of this model supported many of the hypotheses derived from Lazarus’s Stress-Coping Theory: i.e., antecedent personal, resource, and illness variables, assessed at baseline, predicted appraisal variables 4 months later, which in turn led to secondary appraisal, evidenced by the use of coping strategies that affected quality of life at eight months. However, this theory does not explain all of the significant paths that emerged in testing this model. Rather it appears that the explanation of the mechanisms and outcomes are more complex than suggested by the basic model.
There are some limitations of this study that need to be considered. First, although the analysis was conducted with participants in the control group of a randomized control trial, the study may have had some effects on the responses of participants who were in the control group; they met with a nurse data collector who asked them questions about themselves. Furthermore, participants who agree to be part of a randomized control trial dealing with family involvement may not be generalizable to the population of prostate cancer patients. These results do not represent patients that do not have spouses or caregivers that are involved enough to agree to participation in an intervention. In addition, the relationship needs to be functioning enough to allow for recruitment into the study. Also, individuals who agree to be part of an intervention that allows nurses to visit their home regularly, may not be representative of prostate cancer patients as a whole. Second, the sample size was relatively small for an SEM analysis, though within the guidelines to yield stable parameter estimates (41). Future studies using larger samples should replicate and expand this model by assessing additional complex relationships (e.g., whether factors such as stage of disease or baseline self-efficacy moderate model paths). Third, the small samples of participants in later phases of illness may have limited power to test the effects of illness phase on outcomes. Fourth, some findings may be related to being male or female rather than to being a patient or a spouse, and these could not be separated out in this study of male patients and primarily female spouses. Fifth, the selected mediators focus on only one aspect of appraisal (e.g., negative appraisal). There is less attention to positive appraisal (except active coping) variables that may also effect quality of life. Sixth, the sample was primarily white, wealthy, and well-educated. Therefore, it is important to assess whether the model fits well in lower or middle class settings. Furthermore, some of the associations may be different (e.g., the non-significant association of SES) among a more heterogeneous sample.
The results of this study suggest a number of implications that are clinically relevant to the healthcare providers of prostate cancer patients and their spouses. The study identified specific predictors of quality of life that are within the domain of healthcare providers to identify and intervene. For example, self-efficacy was shown to be a strong indirect predicator of both patient and spouse quality of life. Healthcare providers can screen individuals to identify those with low levels of self-confidence, and then provide or refer those individuals to appropriate interventions to increase their levels of self-efficacy. Furthermore, study results suggest that spouses need more help in using effective coping strategies when in their caregiving role.
Other quality of life predictors that are identifiable in the healthcare setting include age, phase of illness, general symptoms, and current concerns. Older spouses of prostate cancer patients, younger patients, patients with more symptom distress and current concerns, and patients experiencing biochemical recurrence or advanced disease are at greater risk for decreased quality of life. Armed with knowledge of these predictors, healthcare providers can assess these risk factors and intervene if needed, and thus assure that patients and their spouse-caregivers achieve their highest level of quality of life along their cancer trajectory.
Acknowledgments
Grant support: R01CA90739 from NCI (L. Northouse PI)
REFERENCES
- 1.Control CfD . Estimated New Cancer Cases and Deaths By Sex for All Sites, US, 2006. National Center for Health Statistics, Center for Disease Control and Prevention; Atlanta: 2006. [Google Scholar]
- 2.Lazarus RS. Stress and emotion: A new synthesis. Springer; New York: 1999. [Google Scholar]
- 3.Lazarus RS, Folkman S, editors. Stress, appraisal, and coping. Springer; New York: 1984. [Google Scholar]
- 4.Schag CA, Ganz PA, Wing DS, Sim MS, Lee JJ. Quality of life in adult survivors of lung, colon and prostate cancer. Qual Life Res. 1994;3:127–141. doi: 10.1007/BF00435256. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfield B, Roth AJ, Gandhi S, Penson D. Differences in health-related quality of life of prostate cancer patients based on stage of cancer. Psychooncology. 2004;13:800–807. doi: 10.1002/pon.797. [DOI] [PubMed] [Google Scholar]
- 6.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–556. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 7.Kornblith AB, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73:2791–2802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Campbell LC, Keefe FJ, McKee DC, et al. Prostate cancer in African Americans: Relationship of patient and partner self-efficacy to quality of life. J Pain Symptom Manage. 2004;28:433–444. doi: 10.1016/j.jpainsymman.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Low CA, Stanton AL, Thompson MA, Kwan L, Ganz PA. Contextual life stress and coping strategies as predictors of adjustment to breast cancer survivorship. Ann Behav Med. 2006;32:235–244. doi: 10.1207/s15324796abm3203_10. [DOI] [PubMed] [Google Scholar]
- 10.Stanton AL, Danoff-Burg S, Cameron CL, et al. Emotionally expressive coping predicts psychological and physical adjustment to breast cancer. J Consult Clin Psychol. 2000;68:875–882. [PubMed] [Google Scholar]
- 11.Stanton A, Danoff-Burg S, Huggins ME. The first year after breast cancer diagnosis: Hope and coping strategies as predictors of adjustment. Psychooncology. 2002;11:93–102. doi: 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- 12.Hack TF, Degner LF. Coping responses following breast cancer diagnosis predict psychological adjustment three years later. Psychooncology. 2004;13:235–247. doi: 10.1002/pon.739. [DOI] [PubMed] [Google Scholar]
- 13.Bower JE, Meyerowitz BE, Desmond KA, Bernaards CA, Rowland JH, Ganz PA. Perceptions of positive meaning and vulnerability following breast cancer: Predictors and outcomes among long-term breast cancer survivors. Ann Behav Med. 2005;29:236–245. doi: 10.1207/s15324796abm2903_10. [DOI] [PubMed] [Google Scholar]
- 14.Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio S, et al. Traumatic Stress, perceived global stress, and life events: Prospectively predicting quality of life in breast cancer patients. Health Psychol. 2005;24:288–296. doi: 10.1037/0278-6133.24.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schou I, Ekeberg O, Ruland CM, Sandvik RL, Karesen R. Pessimism as a predictor of emotional morbidity one year following breast cancer surgery. Psychooncology. 2004;13:309–320. doi: 10.1002/pon.747. [DOI] [PubMed] [Google Scholar]
- 16.Eton DT, Lepore SJ, Helgeson VS. Psychological distress in spouses of men treated for early-stage prostate carcinoma. Cancer. 2005;103:2412–2418. doi: 10.1002/cncr.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko CM, Malcarne VL, Varni JW, et al. Problem-solving and distress in prostate cancer patients and their spousal caregivers. Support Care Cancer. 2005;13:367–374. doi: 10.1007/s00520-004-0748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malcarne VL, Banthia R, Varni JW, Sadler GR, Greenbergs HJ, Ko CM. Problem-solving skills and emotional distress in spouses of men with prostate cancer. J Cancer Educ. 2002;17:150–154. doi: 10.1080/08858190209528823. [DOI] [PubMed] [Google Scholar]
- 19.Cliff AM, MacDonagh RP. Psychosocial morbidity in prostate cancer: II. A comparison of patients and partners. BJU Intl. 2000;86:834–839. doi: 10.1046/j.1464-410x.2000.00914.x. [DOI] [PubMed] [Google Scholar]
- 20.Lepore SJ, Helgeson V. Social constraints, intrusive thoughts, and mental health after prostate cancer. Journal of Social and Clinical Psychology. 1998;17:89–106. [Google Scholar]
- 21.Northouse LL, Mood D, Templin T, Mellon S, George T. Couples’ patterns of adjustment to colon cancer. Soc Sci Med. 2000;50:271–284. doi: 10.1016/s0277-9536(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 22.Northouse LL, Templin T, Mood D. Couples’ adjustment to breast disease during the first year following diagnosis. J Behav Med. 2001;24:115–136. doi: 10.1023/a:1010772913717. [DOI] [PubMed] [Google Scholar]
- 23.Northouse LL, Mood D, Kershaw T, et al. Quality of life of women with recurrent breast cancer and their family members. J Clin Oncol. 2002;20:4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- 24.Northouse LL, Rossett T, Phillips L, Mood D, Schafenacker A, Kershaw T. Research with families facing cancer: The challenge of accrual and retention. Res Nurs Health. 2006;29:199–211. doi: 10.1002/nur.20128. [DOI] [PubMed] [Google Scholar]
- 25.Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: Patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25:4171–4177. doi: 10.1200/JCO.2006.09.6503. [DOI] [PubMed] [Google Scholar]
- 26.Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 110:2809–2818. doi: 10.1002/cncr.23114. [DOI] [PubMed] [Google Scholar]
- 27.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Carver CS. You want to measure coping but your protocol’s too long: Consider the Brief COPE. International Journal of Behavioral Medicine. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 29.Kershaw T, Northouse L, Kritpracha C, Schafenacker A, Mood D. Coping strategies and quality of life in women with advanced breast cancer and their family caregivers. Psychology and Health. 2004;19:139–155. [Google Scholar]
- 30.Oberst MT. Appraisal of Illness Scale: Manual for Use. Wayne State University; Detroit: 1991. [Google Scholar]
- 31.Oberst MT. Appraisal of Caregiving Scale: Manual for Use. Wayne State University; Detroit: 1991. [Google Scholar]
- 32.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- 33.Mishel M, Epstein D. Uncertainty in Illness Scales: Manual. University of Arizona; Tucson, AZ: 1990. [Google Scholar]
- 34.Mood DW, Streater A. Assessing cancer patients’ risk for distress. Midwest Nursing Research Conference; Milwaukee, WI.. 1994.p. 129. [Google Scholar]
- 35.Lewis FM. Family Home Visitation Study Final Report. National Cancer Institute, National Intitutes of Health; 1996. [Google Scholar]
- 36.Weinert C, editor. Measuring social support:PRQ 2000. Springer Publishing Company; New York: [Google Scholar]
- 37.Mood D, Bickes J. Strategies to enhance self-care in radiation therapy. Oncol Nurs Forum. 1989;16:143. [Google Scholar]
- 38.Campbell L, Kashy DA. Estimating actor, partner, and interaction effects for dyadic data using PROC MIXED and HLM: A user-friendly guide. Personal Relationships. 2002;9:327–342. [Google Scholar]
- 39.Kashy DA, Kenny DA. The analysis of data from dyads and groups. Cambridge University Press; New York, NY: 2000. [Google Scholar]
- 40.Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. Guilford; New York, NY: 2006. [Google Scholar]
- 41.Jaccard J, Wan CK. Lisrel Approaches to Interaction Effects in multiple Regression. sage; Newbury Park, CA: 1996. [Google Scholar]
- 42.Loehlin JC. Latent variable models: An introduction to factor, path, and structural analysis. 3rd ed Lawrence Erlbaum; Hilldale, NJ: 1998. [Google Scholar]
- 43.Kornblith AB, Herndon JE, Zuckerman E, et al. The impact of docetaxel, estramustine, and low dose hydrocortisone on the quality of life of men with hormone refractory prostate cancer and their partners: A feasibility study. Ann Oncol. 2001;12:633–641. doi: 10.1023/a:1011102619058. [DOI] [PubMed] [Google Scholar]
- 44.Roberts KJ, Lepore SJ, Helgeson V. Social-cognitive correlates of adjustment to prostate cancer. Psychooncology. 2006;15:183–192. doi: 10.1002/pon.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helgeson V, Novak SA, Lepore SJ, Eton DT. Spouse social control efforts: Relations to health behavior and well-being among men with prostate cancer. Journal of Social and Personal Relationships. 2004;21:53–68. [Google Scholar]
- 46.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 47.Hollingshead AB, Redlich FC. Social class and mental illness: A community study. John C. Wiley; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]