NMDA receptor antibody encephalitis typically begins as a fulminant encephalopathy, with prominent neuropsychiatric manifestations, seizures, dyskinesias, and autonomic instability. After this often dramatic presentation, 1-3 relapses may occur. Most patients either die or recover from the disease.1 We describe a 15-year-old girl who initially presented with encephalopathy, hypoventilation, dyskinesias, and seizures. Her subsequent course was atypical, with more than 10 relapses during the next year, with longitudinally extensive transverse myelitis (LETM) and optic neuritis (ON) in addition to multifocal, contrast-enhancing gray and white matter lesions. These findings have not been previously reported in anti-NMDA receptor encephalitis. Her disease was ultimately controlled on an aggressive combined regimen of monthly plasmapheresis, pulse methylprednisolone and cyclophosphamide, and rituximab.
Case report.
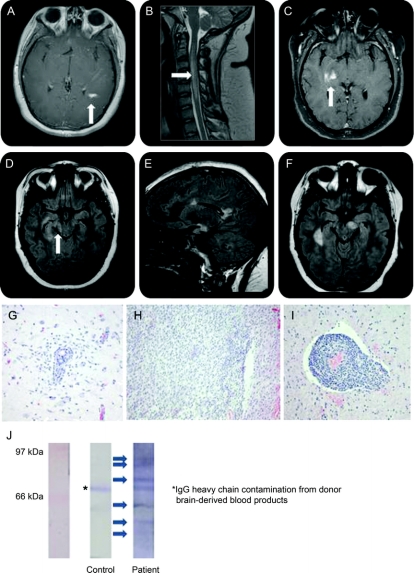
A 15-year-old girl presented with headaches, photophobia, complex partial seizures, and encephalopathy dominated by hyporesponsiveness. Orofacial dyskinesias were noted. She required intubation for hypoventilatory failure. Her CSF demonstrated 420 leukocytes/mm3 (13% neutrophils, 79% lymphocytes, 8% monocytes). Protein was 103 mg/dL; glucose 38 mg/dL. MRI demonstrated a contrast-enhancing periatrial lesion (figure, A). After a 2-week hospitalization, she recovered without residual symptoms.
Figure Features of atypical anti-NMDA receptor encephalitis
MRI: (A) Initial contrast-enhancing lesion; (B) longitudinally extensive transverse myelitis; (C) continued development of contrast-enhancing lesions; (D) retrochiasmatic optic neuritis; (E, F) continued accumulation of T2/fluid-attenuated inversion recovery (FLAIR) hyperintense lesion burden, with sagittal FLAIR hyperintensities reminiscent of Dawson's fingers (E). Brain biopsy (from contrast-enhancing frontal lobe lesion): (G) perivascular infiltrate with associated reactive microgliosis; (H) widespread parenchymal destruction mediated by infiltrative lymphocytes and macrophages without selective demyelination; (I) prominent mixed perivascular infiltrate (macrophages, T- and B-lymphocytes with uncommon neutrophils and rare eosinophils). Western blot: (J) Western blot depicting the presence of several additional serum-derived autoantibodies reactive against cerebellar protein extract from control human brain.
Profound headaches recurred within 1 month, and she developed right-sided weakness, ataxia, and dysarthria. MRI again demonstrated contrast enhancement, now multifocal. LETM was also noted (figure, B). She began treatment with prednisone, 60 mg/day, for presumed acute disseminated encephalomyelitis with significant improvement.
She remained clinically stable on oral steroids for several months before being weaned, without overt clinical symptoms despite the appearance of new enhancing lesions (figure, C). Six months into her course, she developed retrochiasmatic ON (figure, D). LP was repeated, demonstrating 4 leukocytes/mm3 (94% lymphocytes, 6% monocytes). CSF analysis demonstrated 6 oligoclonal bands absent from serum, consistent with intrathecal immunoglobulin G synthesis. All other CSF indices were normal, as were CSF cytology and flow cytometry. Magnetic resonance angiography was unremarkable. Nutritional studies and testing for chronic meningoencephalitis were unrevealing. Extensive autoantibody testing (including NMO antibody testing; table e-1 on the Neurology® Web site at www.neurology.org) was negative.
The patient then developed recurrences at least monthly for the next 6 months (figure, E and F), with ataxia, weakness, sensory loss, internuclear ophthalmoplegia progressing to one-and-a-half syndrome, dysarthria, dysphagia, gait impairment, urinary incontinence, and later, cognitive decline (impaired problem solving, memory, and executive function). Labile emotionality, depression, and panic attacks were prominent later features. New symptoms occurred despite the use of interferon-β therapy for several consecutive months, followed by pulse cyclophosphamide for 2 months. Pulse IV steroid therapy was moderately effective in treating acute attacks. IV immunoglobulin was ineffective, while her response to plasmapheresis was excellent (supporting a role for peripherally produced autoantibodies in her disease), but not sustained for more than a few weeks.
A brain biopsy demonstrated a mixed inflammatory infiltrate primarily affecting gray matter (figure, G–I). Significant demyelination was conspicuously absent. Anti-NR1/NR2 heteromer (NMDA receptor) antibody was detected in the CSF (diluted 1:10), but was not detected in any serum samples (diluted 1:10). Western blot of human brain extract probed with patient serum demonstrated several additional antineuronal autoantibodies (figure, J). One of these was identified as anti-myelin basic protein immunoglobulin G, although citrullinated forms (seen in some cases of multiple sclerosis and acute disseminated encephalomyelitis)2 were conspicuously absent. Chest/abdomen/pelvis CT scan and pelvic MRI failed to demonstrate an occult teratoma.3 Despite accumulating significant disability early in her course, with a combined regimen of monthly plasmapheresis, pulse methylprednisolone, rituximab, and pulse cyclophosphamide, she became asymptomatic.
Discussion.
Our patient was positive for NR1/NR2 antibodies, a highly specific finding that until now has only been identified in patients with a characteristic set of symptoms.1,4 Although our patient initially exhibited most of the symptoms associated with this disorder, the clinical course was highly unusual. Moreover, her MRI findings of LETM and ON in addition to irregular contrast-enhancing supratentorial and infratentorial lesions were atypical. Her biopsy findings and autoantibody testing did not support a diagnosis of multiple sclerosis, but could be considered consistent with seronegative NMO.5 Her case thus appears to represent a previously unrecognized overlap syndrome, potentially involving other neuronal autoantibodies, as suggested by her Western blot results. We suggest that her early course was typical for anti-NMDA receptor encephalitis, and that epitope-spreading may have occurred6 and led to evolution into the syndrome described. The contribution of multiple autoantibodies acting concurrently may be important in the course and evolution of autoimmune encephalitides. CSF autoantibody testing may be crucial in identifying pathogenic autoantibodies. Characterization of these disorders is important because, as shown in our patient, findings may guide treatment strategies, and warrant intensifying immunotherapy. Despite initial accumulation of disability, with aggressive immunotherapy, good outcomes may be possible (table e-2).
Supplementary Material
Supplemental data at www.neurology.org
Disclosure: Dr. Kruer has received funding for travel from UCB; and receives research support from the NIH (NCRR UWXY3099 [PI] and via NCRR grant UL1 RR024140). Dr. Koch serves on the editorial advisory board of Pediatric Neurology; and has received honoraria for lectures or educational activities not funded by commercial entities. Dr. Bourdette serves on the editorial board of the Journal of Medicinal Medicine and as a Section Editor for Current Neurology and Neuroscience Reports; and serves on speakers' bureaus for Teva Pharmaceutical Industries Ltd., Biogen Idec, and EMD Serono Inc. Dr. Chabas has received honoraria from Teva Pharmaceutical Industries Ltd., EMD Serono Inc., and Pfizer Inc; has received license fee payments and may accrue revenue on office of technology licensing Stanford Docket #501-085 (issued 5/6/2003): osteopontin related compositions and methods; and receives research support from the National Multiple Sclerosis Society and the Nancy Davis Foundation. Dr. Waubant has received travel expenses and/or honoraria for lectures or educational activities not funded by industry; and receives research support from Biogen Idec, Pfizer Inc, sanofi-aventis, the National MS Society, The Immune Tolerance Network, and the Nancy Davis Foundation. Dr. Moscarello receives research support from the Multiple Sclerosis Society of Canada. Dr. Dalmau has received honoraria for lectures not funded by industry; receives research support from EUROIMMUN and the NIH/NCI [RO1CA107192 (PI) and RO1CA89054-06A2 (PI)]; has received license fee payments from EUROIMMUN for an NMDA receptor autoantibody test (patent pending PCT/US07/18092, filed: August 15, 2007); and has received royalty payments and may accrue revenue for US Patent 6,387,639, issued: May 14th, 2002: Patent for Ma2 autoantibody test. Dr. Woltjer receives research support from the NIH (NIA P30 AG08017 [Director of neuropathology core of Alzheimer's Disease Center] and NIA 5R01AG026916 [Consultant]), and from the Medical Research Foundation of Oregon, OHSU Dean's Fund for Research Collaboration, and Oregon Tax Checkoff. Dr. Adamus and Dr. Mueller report no disclosures.
Received September 4, 2009. Accepted in final form February 24, 2010.
Address correspondence and reprint requests to Dr. Michael Kruer, Divisions of Pediatric Neurology and Developmental Pediatrics, CDRC-P, 707 SW Gaines St., Oregon Health & Science University, Portland, OR 97239; kruerm@ohsu.edu
&NA;
- 1.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res 2007;32:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 2009;66:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485– 1489. [DOI] [PubMed] [Google Scholar]
- 6.Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol 2009;65:239–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.