Abstract
Background
In recent years, a number of studies have shown that clinical drug trials financed by pharmaceutical companies yield favorable results for company products more often than independent trials do. Moreover, pharmaceutical companies have been found to influence drug trials in various ways. This overview of current, systematic studies on this topic is intended to identify and characterize the particular aspects of the performance of a drug trial that can be affected by financial support from a pharmaceutical company.
Methods
Publications retrieved from a systematic Medline search on this topic from 1 November 2002 to 16 December 2009 were independently evaluated and selected by two of the authors. These publications were supplemented by further ones found in their references sections.
Results
57 publications were included for evaluation in Parts 1 and 2 of this article. A number of studies revealed that many trials financed by pharmaceutical companies—in some cases, as many as half of all such trials—are never published. Moreover, multiple publications of the same findings were found, and some reports were found to include selectively published data. Further studies revealed evidence of other problems including incomplete trial registration, constraints on publishing rights, withheld knowledge of adverse drug reactions, and the use of ghostwriters who were supplied by the pharmaceutical companies.
Conclusion
Financial support from a pharmaceutical company influences multiple aspects of the performance of drug trials and often leads to a favorable result for the corporate sponsor of the trial. Public access to trial protocols and results must be ensured. Moreover, more effort should be made to carry out drug trials independently, without the financial support of pharmaceutical companies.
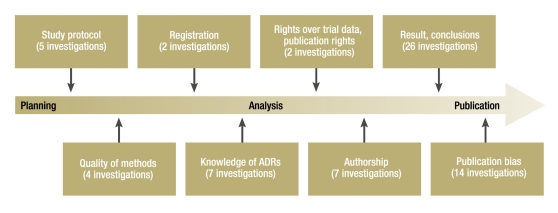
A number of studies in recent years have shown that clinical drug trials financed by pharmaceutical companies yield favorable results for the company’s product more often than independent trials do (1, 2). The authors’ own systematic review of investigations published between 1 November 2002 and 16 December 2009 comes to the same conclusion (Part 1 of this article [3]). The results of a drug trial can be influenced at many different stages of the study (Figure). For some of these areas there is clear evidence of influence exerted by pharmaceutical companies (1, 2, 4). In Part 1 the authors presented, for example, findings showing that pharmaceutical companies influence results to their advantage through the design of the study protocol, e.g., the selection of dosage, control groups, or endpoints (e1– e3).
Figure.
Topics of investigations into the influence of pharmaceutical companies (ADRs, adverse drug reactions)
Execution of the study according to plan and objective depiction of the results can also be influenced, e.g., by contractual stipulations that grant the pharmaceutical company access to the trial data or give it the power to prevent the publication of results. Moreover, the presentation of results can be manipulated by ghostwriters and guest authors. The word “ghostwriter” is used to describe a person who is not mentioned in the publication despite an essential role in performing the study or writing the manuscript. This includes statisticians who analyze the results. The term “guest author” describes someone who is listed as an author of a publication although he or she does not fulfill the recognized criteria for authorship (as outlined for example in [5]) (e4, e5). Typically, a well-known opinion former is invited to be guest author in order to underline the importance of the study results. The withholding of negative and statistically non-significant findings can result in so-called publication bias, leading to a distorted perception of the therapeutic value of the drug concerned (6).
The present investigation sets out to spotlight the different stages and aspects of drug trials that are—as shown by recent systematic reviews—influenced by funding from the pharmaceutical industry.
Methods
The methods are presented in detail in Part 1 (3). Suitable studies identified by a Medline search (1 November 2002 to 16 December 2009) were independently evaluated and selected by two of the authors (G. Schott, U. Limbach), who also added relevant publications from the reference lists.
Results
Types of influence
Investigations into the types of influence that pharmaceutical companies exert on drug trials are summarized in the Table and the eTable.
Table. Investigations into the influence on disclosure of adverse drug reactions.
| Author | Purpose | Result |
| Aursnes et al. 2008 (e12) | Assessment of extent to which ADRs of the SSRI paroxetine were known to the pharmaceutical company before application for licensing but not evaluated, by analysis of trial data from 17 RCTs (1495 patients), published in 1989, that the pharmaceutical company submitted to Norwegian health authorities with licens‧ing applications | Meta-analyses show that 19 ADRs were statistically significantly more frequent during treatment with paroxetine; however, five of these ADRs (e.g., headache) are still not mentioned in the latest product information. |
| Halpern et al. 2004 (e13) | Determination of difference in average statistical power of pharmacoepidemio‧logical studies on ADRs of antiretroviral drugs funded by profit-oriented versus non-profit organizations, by analysis of all published studies on the 15 such drugs licensed up to 1999 | Only 3 of 41 studies in which funding was specified (7%) were supported by profit-oriented organizations; therefore no comparison possible. |
| Jüni et al. 2004 (e10) | Investigation whether there was evidence for cardiovascular ADRs of rofecoxib before drug was taken off market in 2004, by meta-analysis of clinical studies | At end of year 2000, RR for myocardial infarction was 2.30 (95% CI 1.22–4.33, p = 0.01); a year later, 2.24 (95% CI 1.24–4.02, p = 0.007). |
| Nieto et al. 2007 (e3) | Evaluation of differences with regard to findings on ADRs and their interpretation between 275 studies funded by a pharmaceutical manufacturer and 229 studies not funded by a pharmaceutical manufacturer; studies published between 1993 and 2002 | Statistically significant differences for ADRs were statistically significantly less frequent in studies funded by a pharmaceutical manufacturer than in studies not funded by a pharmaceutical manufacturer (34.5% vs. 65.1%; prevalence ratio 0.53, 95% CI 0.44–0.64). After adjustment for aspects of study ‧design associated with fewer ADRs, e.g., dosage, connection was no longer significant. Authors of studies funded by a pharmaceutical manufacturer were more likely to conclude that a drug is safe than authors of studies not funded by a pharmaceutical manufacturer (prevalence ratio 3.68, 95% CI 2.14–6.33). |
| Psaty et al. 2004 (e8) | Review of connection between cerivastatin intake and risk of rhabdomyolysis, by inspection of published data and pharmaceutical company’s internal documents provided in context of legal proceedings | Pharmaceutical manufacturer aware of data indicating interaction between cerivastatin and gemfibrozil by 100 days after market launch, but only after 18 months was this included in product information. Data from clinical trials with high-dose cerivastatin and from analysis of the FDA’s ADR notification system were not publicized. |
| Psaty et al. 2008 (e9) | Presentation of data on mortality in patients with Alzheimer’s dementia or cognitive disorders being treated with rofecoxib, by inspection of published data and pharmaceutical company’s internal documents provided in context of legal proceedings | Increased mortality during rofecoxib treatment was mentioned in two publications, but data were not subjected to statistical analysis. Safety stated as “well tolerated” although internal evaluation had already shown a distinct significant increase in mortality with rofecoxib (overall mortality in intention-to-treat analysis: RR 2.56; p = 0.001). This analysis was communicated neither to FDA nor to general public in timely fashion. FDA was given an analysis with shorter observation time and thus lower increase in mortality. Company answered FDA enquiries evasively. |
| Ross et al. 2009 (e11) | Investigation of whether and when analysis of published and unpublished studies would have revealed the cardiovascular risk associated with intake of rofecoxib, by person-related analysis of all the manufacturer’s trial data before September 2004 | By December 2000, 21 of a total of 30 studies had been concluded and risk of cardiovascular thromboembolic ADRs or death was higher in persons who took rofecoxib than in those who did not take rofecoxib (RR 2.18, 95% CI 0.93–5.81; p = 0.07). Statistically significantly (p = 0.05) increased risk discernible from June 2001. |
ADRs, adverse drug reactions; SSRIs, selective serotonin reuptake inhibitors; RCTs, randomized controlled trials; RR, relative risk; 95% CI, 95% confidence interval; FDA, US Food and Drug Administration
E-Table.
| Investigations into various ways in which drug trials are influenced by pharmaceutical companies |
| Authors | Subject | Result |
| Registration of drug trials | ||
| Zarin et al. 2005 (e6) | Description of registration of studies before and after implementation of ICMJE regulations by inspection of register entries at ClinicalTrials.gov on 20 May 2005 and again on 11 October 2005 | Considerable increase in registrations of clinical studies (from 13 153 to 22 714, also non-drug studies). Increase in registrations of interventional new drug studies from 2010 to 3516. Non-specific entries still found under “Name of intervention” on 11.10.2005, frequently (376/1247; 30%) no entry under “Primary outcome measure”. |
| Lott et al. 2006 (e7) | Investigation of behavior of 31 pharmaceutical companies that had placed advertisements in two major dermatology journals with regard to registration of their clinical trials after implementation of ICMJE regulations and guidelines of pharmaceutical ‧associations, by means of questionnaires, monitoring of company websites, and inspection of registers at ClinicalTrials.gov and isrctn.org in year 2005/2006 | Only 5 of 31 companies responded and one refused permission for publication of its answers. Proportion of trials registered varied from 0% to 100% at the remaining four companies. One company’s response: decision on registration made individually for each project, no formal guideline in force. Twenty of 31 companies had registered at least one study at ClinicalTrials.gov, 16 of 31 at isrctn.org. Nine pharmaceutical companies had entered no trial in either register. Twenty-three of 31 companies (74%) gave no information about registration of their clinical trials on their websites. |
| Publication of drug trials | ||
| Conen et al. 2008 (e25) | Influence of study funding source on subsequent distribution of data in 303 consecutively published studies on cardiovascular ‧research topics, published between 2000 and 2005 | Results of studies funded by pharmaceutical companies are cited more often. Among drug trials (n = 194), median number of citations was 43 for studies funded by pharmaceutical companies, 42 in the case of mixed funding, and 33 for studies financed by sources other than pharmaceutical companies (differences not statistically significant). Analysis of all studies shows a particularly clear difference for advantage of new treatment over existing standard, otherwise opposite finding. |
| Hole et al. 2009 (e23) | Determination of number of completed investigations and of ‧published results among the 245 studies registered in archive of Norwegian National Committee for Medical Research Ethics in the year 2000 | Investigation completed: 178/245 (73%); results published in journal: 131/245 (54%); published as abstract or as report to sponsor: 34/245 (14%); not published at all: 80/245 (33%). Pharmaceutical companies as sponsors seem to be conducive to both study completion and publication of results in specialist journals. |
| Jefferson et al. 2009 (e26) | Analysis of agreement between study results and conclusions (concordance), funding, and distribution of 259 studies on the ‧effect of influenza vaccinations | Studies completely or partly funded by pharmaceutical companies are more frequently published in high-ranking journals and cited more often than studies with state or private funding; this cannot be explained by quality or size of study. |
| Kuriya et al. 2008 (e27) | Quality of press releases on results of original research by the 10 leading pharmaceutical companies worldwide; published on their website in 2005 | Key trial data are reported in press releases, but limitations are rarely mentioned (6%). In 29% of cases no quantification of study results. In 47% an author is quoted, often stressing benefit. |
| Kho et al. 2009 (e22) | Determination of rate of full publication of 109 abstracts on 86 trials of rituximab in non-Hodgkin’s lymphoma, presented at annual congress of a hematology society between 1997 and 2001; also determination of time to publication and of predictors for these parameters | Publication rate of abstracts was 52.3%, median time to full publication 1.4 years. Affiliation of authors to pharmaceutical companies (OR 4.60, 95% CI 1.32–16.08) and type of presentation were independent predictors of full publication. Predictors for time to full publication were not identified. |
| Krzyzanowska et al. 2003 (e21) | Determination of factors that influence time to publication in 510 abstracts of large RCTs (≥200 patients) in oncology; presented at annual meeting of a society between 1989 and 1998 | Studies funded by pharmaceutical companies (74/510; 15%) were published more rapidly than those financed by study group (294/510; 58%, including 17 supported by pharmaceutical companies) or those with no information on funding (142/510; 28%). After 5 years 17% of the studies funded by pharmaceutical companies, 27% of those financed by the study group, and 29% of those with no reported sponsor remained unpublished (p = 0.02). |
| Lee et al. 2008 (e14) | Determination of publication status and factors associated with publication in trials of drugs licensed by FDA between 1998 and 2000 | Of 909 licensing studies for 90 newly licensed drugs (89 from pharmaceutical companies), 43% (394/909) had been published after 5 years, including 76% (257/340) of pivotal trials. Likelihood of being published was twice as high for studies with statistically significant results than for those with non-significant results. Size of sample was also positively associated with likelihood of publication. |
| Melander et al. 2003 (e17) | Importance of selective and multiple publication in 42 RCTs on five SSRIs that had been submitted to the Swedish licensing authority for commercial licensing between 1983 and 1999 | Multiple publication: 21 trials resulted in at least two publications, three trials to five publications each. Selective publication: independent publication more frequent in trials with significant results. Many studies reported more favorable per-protocol analysis, rather than intention-to-treat analysis. |
| Rising et al. 2008 (e15) | Determination of publication rate of efficacy studies presented to FDA in 2001–2002 (n = 164) and comparison with corresponding publications | By 2007, 78% (128/164) of studies had been published, with a significantly higher likelihood for those with favorable results and active controls. Publications featured more positive results than FDA documents, e.g., by addition or deletion of findings or alteration of statistical significance or conclusions. |
| Ross et al. 2009 (e24) | To check completeness of registration and extent of selective publication, data from register at ClinicalTrials.gov were compared with corresponding publications (registration after 31 December 1999, study ended by 08 June 2007) | Overall, fewer than half of studies investigated were published (311/677; 46%). Clinical trials funded by pharmaceutical companies (144/357; 40%) were less likely to be published than those funded neither by pharmaceutical companies nor by state support (110/198; 56%; p<0.001), but there was no difference from state-funded trials (57/122; 47%; p = 0,22). |
| Turner et al. 2008 (e16) | Selective publication in 74 studies on 12 antidepressives licensed between 1987 and 2004; submitted to American ‧licensing authority | Thirty-seven of 38 studies with positive result published, 3/36 studies with negative result adequately published, 11 studies published with negative result presented as positive. Separate meta-analyses of data provided to FDA and published data showed greater strength of effect in published data (range 11% to 69%, overall mean 32%). |
| Vedula et al. 2009 (e18) | Analysis of presentation of study results by comparison of pharmaceutical manufacturers’ internal documents with corresponding publications of manufacturer-funded trials of gabapentin for non-licensed (“off-label”) indications | In 8 of 12 published studies, primary endpoint in publication differed from that described in protocol. Studies with non-significant results (p≥0.05) for primary endpoint defined in protocol were published either incompletely or with altered primary endpoint. Of 21 endpoints described in protocols as primary, four were published as secondary endpoints and six not at all. Of 28 primary endpoints published, 12 were newly introduced. |
| Von Elm et al. 2008 (e20) | Factors associated with publication in 451 study protocols and 233 corresponding articles on RCTs; submitted to Swiss national ethics committee between 1988 and 1998 | Funding independent of pharmaceutical companies is associated with publication (OR 2.42, 95% CI 1.14–5.17), as are multicenter status, international cooperation, and case number >236. |
| Whittington et al. 2004 (e19) | Comparison of published (five RCTs) and unpublished data on SSRIs in children and adolescents; survey of literature up to 2003 | Published data suggest a positive benefit/harm balance for some SSRIs (paroxetine, sertraline), whereas risk predominates when unpublished data are taken into account (exception: fluoxetine). |
| Publication rights and control of trial data | ||
| Gotzsche et al. 2006 (e28) | Investigation of publication rights of all 44 RCTs initiated and published by pharmaceutical companies in a region of Denmark in 1994 and 1995 | Sponsors had right of access to data in 16/44 protocols, and 16 empowered them to end the study for any reason at any time. Limitations on publication rights in 40/44 protocols; sponsors granted rights over data, manuscript release, or both in 22. With one exception, sponsors’ rights not noted in publication. |
| Henry et al. 2005 (e29) | Characterization of relationship between pharmaceutical companies and medical specialists in Australia with regard to accomplishment of studies by means of questionnaires in 2002 and 2003 | Response rate 823/2120 (39%). Most frequent negative experience: premature discontinuation of study (114/823; 14%), in some cases on commercial grounds; also drafting of manuscript by pharmaceutical company staff (100/823; 12%). Study results delayed, distorted, or not published at all. Some 21% of physi‧cians participating in research projects report grave deficiencies. |
| Authorship—ghostwriters and guest authors | ||
| Gotzsche et al. 2007 (e4) | Investigation of prevalence and nature of ghostwriting by comparing protocol and publication of all studies initiated by pharmaceutical companies in a region of Denmark in 1994 and 1995 | Signs of involvement of ghostwriters in 33 of a total of 44 studies (75%; 95% CI 60–87%). In 31/44 publications (70%) statistical analysis was carried out by pharmaceutical company staff who were not named as authors. |
| Ross et al. 2008 (e5) | Description of type and extent of ghostwriting and guest authorship in the case of rofecoxib, by inspection of publications and pharmaceutical companies’ internal documents that were provided in the context of legal proceedings | Manuscripts for publications of clinical studies and reviews were written by company staff or contractors, then paid guest authors were recruited and frequently credited as first or second author. Support by a pharmaceutical company was mentioned in 22 (92%) of 24 publications of clinical studies but in only 36 (50%) of 72 reviews. |
ICMJE, International Committee of Medical Journal Editors; RCTs, randomized controlled trials; ADRs, adverse drug reactions; OR, odds ratio; 95% CI, 95% confidence interval; FDA, US Food and Drug Administration; SSRIs, selective serotonin reuptake inhibitors
Incomplete registration
With the aim of facilitating public access to clinical trial data and preventing pharmaceutical companies from influencing the publication of results, in 2004 the International Committee of Medical Journal Editors (ICMJE) made registration a condition for publication in any of the 11 leading medical journals (7): new trials had to be registered by 1 July 2005; those already under way, by 13 September 2005. Meanwhile several registers fulfill the ICMJE standards.
In January 2005 major pharmaceutical organizations, among them the Pharmaceutical Research and Manufacturers of America (PhRMA) and the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), implemented guidelines that obliged their members to enter trials prospectively in publicly accessible registers (8, 9).
Despite this, two of the 57 studies included in the present investigation contain data suggesting that pharmaceutical companies are still not registering important information on clinical drug trials.
One study in 2005 showed that although the introduction of the ICMJE rules was followed by an overall sharp increase in registrations at ClinicalTrials.gov, the companies’ information regarding particular aspects of their trials, e.g., primary endpoints, remained imprecise or was absent altogether (e6).
The results of the other study, published in 2006, indicate that manufacturers of pharmaceuticals used in dermatology were not registering all their studies. For several companies it remained unclear what guidelines were being applied to the registration of drug trials (e7).
Concealment of adverse drug reactions
Seven of the studies investigated concerned themselves with adverse drug reactions (ADRs) in industrially supported drug trials (Table).
Severe ADRs led to cerivastatin and rofecoxib being taken off the market 4 and 5 years, respectively, after licensing (10). In the course of subsequent legal proceedings, internal documents of the manufacturers were made public. Analysis of these documents revealed that relevant data on ADRs had not been provided to the public or to the American licensing body (Food and Drug Administration, FDA) at the appropriate time (e8, e9). In the case of cerivastatin, the manufacturer was aware of data indicating an interaction with gemfibrozil, leading to increased occurrence of rhabdomyolysis, around 100 days after their product was launched on the market; it was 18 months, however, before this was added to the contraindications in the product information (e8). As for rofecoxib, data pointing to increased mortality after intake in patients with Alzheimer’s dementia were communicated neither to the FDA nor to the general public in good time (e9); trial data on the occurrence of cardiovascular ADRs were inadequately evaluated (e10, e11).
In the case of the selective serotonin reuptake inhibitor (SSRI) paroxetine, deficient analysis of available data is the reason why well-known ADRs, e.g., paresthesia and nervousness, are still not mentioned in the product information (e12).
Exertion of influence by pharmaceutical manufacturers was also evident in studies on inhaled corticosteroids (e3). In trials financed by pharmaceutical companies, statistically significant differences in ADR occurrence between drug and control groups were significantly less common and the authors of these publications (e3) designated the drug safe for use more frequently than in trials with other sources of funding (see Part 1).
One planned analysis of the statistical power of pharmacoepidemiological studies on ADRs of antiretroviral drugs depending on the sponsor could not be carried out because too few studies were funded by pharmaceutical companies (e13).
Publication bias
Of the 57 studies included in this investigation, 14 analyzed the connection between the type of funding of a trial and publication bias.
Licensing studies, which are particularly important for the assessment of new drugs, are carried out almost exclusively by pharmaceutical companies. Comparisons of data provided to the FDA with articles published in medical journals have shown that around 25% to 50% of these studies remain unpublished (e14– e16). Positive or significant results are published more often than negative or non-significant findings (e14– e17). The authors of two studies tested and confirmed the statistical significance of this statement (e14, e15). Furthermore, negative results were portrayed as positive (e15, e16).
A study on trials of SSRIs revealed that those with significant results were more likely to be published, sometimes more than once, whereas trials with non-significant results or findings unfavorable to the drug under investigation (intention-to-treat analyses versus per-protocol analyses) were not published (e17). Another study showed how efficacy of gabapentin for unlicensed (“off-label”) indications was feigned by alteration of the primary endpoint and non-publication of unfavorable data (e18).
Comparison of published and unpublished data from pharmaceutical companies showed that published data on SSRIs suggested a positive benefit/risk balance, but risk predominated when unpublished data were taken into account (e19). The strengths of effect of 12 different antidepressives as stated in published data were frequently greater than those derived from information supplied to the licensing authorities (e16).
There are varying data on whether the type of funding of a trial influences the likelihood of publication. While an investigation of protocols provided to an ethics committee showed that funding independent of pharmaceutical companies is associated with a greater likelihood of publication (e20), two studies in oncology (e21, e22) and an investigation of the protocols supplied to a different ethics committee (e23) revealed that trials funded by pharmaceutical companies were published more often (e22, e23) or more quickly (e21). A further investigation of publications of registered trials showed that low publication rates are not limited to particular sponsors of a trial; rather, they are a problem with both industry-funded and state-financed trials (e24).
Two investigations, one of cardiovascular research studies (e25) and the other of studies on the efficacy of influenza vaccines (e26) found evidence that results of clinical trials funded by pharmaceutical companies are cited more often. Pharmaceutically sponsored studies on the efficacy of influenza vaccines were also published in higher ranking journals. An analysis of pharmaceutical companies’ press releases on clinical trials showed that key data were provided but there was often no mention of study limitations and no quantification of the results (e27).
Rights over trial data and restricted publication rights
Two investigations on this topic were identified. Both an analysis of the protocols of all studies initiated and published by pharmaceutical companies in a particular region of Denmark in 1994 and 1995 (e28) and a questionnaire survey of medical specialists in Australia (e29) indicated that in some trials pharmaceutical companies secure the rights over the data and place constraints on publication rights.
Ghostwriters and guest authors
A case study on rofecoxib (e5) and an investigation into the above-mentioned studies in Denmark both found evidence of frequent resort to ghostwriters and guest authors in industry-funded publications. The Denmark investigation showed that statisticians employed by pharmaceutical companies are frequently not mentioned in the published articles (e4).
Discussion
Drug trials financed by the pharmaceutical industry yield results favorable to the funding company much more often than studies with other sources of support. This has been shown by the present systematic review (Part 1 [3]) and in other comparable investigations (1, 2).
This finding may be explained by various factors, some of them supported by systematic investigations. Publication bias, from selective publication of positive results or withholding of negative findings (e15– e20), probably goes a long way to explaining the predominance of positive results in studies funded by pharmaceutical companies. Non-publication of results constitutes scientific malpractice and can ultimately lead to patients receiving inadequate treatment. Besides the companies and organizations that fund studies, scientists, ethics committees, and journal editors must also take responsibility for publication of all study results, whether they be positive or negative (11).
Publication bias may be favored by the fact that some pharmaceutical companies still do not give complete information when registering their trials, despite their commitment to do so (8, 9, 12). This is indicated by the results of two studies, albeit published as early as 2005 and 2006 (e6, e7). Given that study results are often published incompletely, in distorted form, or not in accordance with the study protocol (e15– e19, e30), all planned drug trials should be registered and their protocols be made publicly available before conclusion of the investigations.
Furthermore, there were indications that pharmaceutical companies use ghostwriters (frequently staff statisticians) (e4, e5) and that knowledge of ADRs is withheld (e8– e10, e12, e13). The gravity of the potential consequences of this concealment is illustrated by the withholding of mortality data on rofecoxib (e9).
Some aspects of pharmaceutical companies’ influence on the results and publication of drug trials have not been systematically investigated. For instance, Richard Smith, long-serving editor of the British Medical Journal, drew attention to the fact that many medical journals derive a substantial income from the pharmaceutical industry, e.g., from advertisements and reprints. He discerned therein a risk to the independence of journals and postulated that they often serve as extensions of the marketing departments of pharmaceutical companies (e31, 13). Medical journals should therefore publish their income on a regular basis, e.g., annually. This demand is a logical consequence of an investigation which showed that industry-funded studies on the efficacy of influenza vaccines were published in higher-ranking journals than studies with other sources of financing (e26).
The present investigation has various limitations. One of them—also described by Lexchin et al. (2)—is the difficulty in identifying the relevant literature. The authors’ PubMed search yielded only 38 of the 57 studies covered; the remaining 19 were found on inspection of the reference lists or were the subject of personal communications. Moreover, there was no assessment of the quality of the studies included, one of the preconditions for quantitative analysis. Furthermore, publications were included in which the terms “conflict of interest” and “funding by the pharmaceutical industry” were defined in different ways. Both served the goal of this qualitative study, namely comprehensive portrayal of the various ways in which influence can be exerted.
Conclusion
This systematic review clearly shows that clinical trials with the involvement of pharmaceutical companies often present the therapeutic benefit of a drug in too positive a light and also fail to mention risks. Clinical studies are increasingly being funded by pharmaceutical companies (e32– e35). Professional medical bodies construct evidence-based guidelines on the basis of published trial results, so their recommendations may be flawed. This contributes to excessive prescription of expensive new drugs whose efficacy is overestimated and risks underestimated. Moreover, because the evidence is distorted patients do not receive adequate information (14).
In the past few years measures have been taken worldwide to deal with the problems described here. Laws have been enacted, for example, with the intention of securing public access to research data (15– 18). In the USA, for instance, a law of 27 September 2008 prescribes the registration and publication of the results of clinical trials in a register accessible on the internet (15, 19). In the European Union, directive 2001/20/EC requires registration of all clinical studies (16). A guideline implemented in 2008 lays down what classes of information from the EudraCT database—accessible only to governmental authorities—should be made available in the publicly accessible EudraPharm drug database, which thus remains incomplete (17, 18).
Pharmaceutical organizations have implemented recommendations that are intended to ensure comprehensive publication of research findings, whether positive or negative (9, 12, 20). This initiative on the part of the pharmaceutical industry is welcome; however, the present investigation shows that negative results are still not being published in timely fashion and control mechanisms have failed.
Official regulatory measures to guarantee public access to study protocols and results and prevent the withholding of information about dangerous ADRs are urgently required. This would also give independent drug bulletins and bodies representing physicians, e.g., the Drug Commission of the German Medical Association, the opportunity to obtain detailed, unbiased information about new drugs. Furthermore, it should be obligatory to prove that a new drug provides additional benefit compared with existing pharmacological and non-pharmacological forms of treatment. More public funding should be made available for independent studies (21, 22).
Measures must be taken at many levels to ensure that commercial interests do not undermine the knowledge of scientifically correct study planning, study execution, and publication (4, 5, e15, 15, 23– 25, e36, e37). A large number of physicians are involved in the planning and conduct of drug trials. For the benefit of their patients, they should assume greater responsibility and work to counteract the economic self-interest of pharmaceutical companies in research and clinical practice.
Acknowledgments
This work was supported by funds from the Initiative for Health Services Research (Förderinitiative Versorgungsforschung) of the German Medical Association (Bundesärztekammer, BÄK). The 110th German Medical Assembly tasked the BÄK with investigating, in the framework of the Initiative for Health Services Research, the influence of sponsors on the scientific results of clinical drug trials.
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289:454–465. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- 2.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326:1167–1170. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schott G, Pachl H, Limbach U, Gundert-Remy U, Ludwig WD, Lieb K. The financing of drug trials by pharmaceutical companies and its consequences: part 1. A qualitative, systematic review of the literature on possible influences on the findings, protocols, and quality of drug trials [Finanzierung von Arzneimittelstudien durch pharmazeutische Unternehmen und die Folgen - Teil 1: Qualitative systematische Literaturübersicht zu Einfluss auf Studienergebnisse, -protokoll und -qualität] Dtsch Arztebl Int. 2010;107(16):279–285. doi: 10.3238/arztebl.2010.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bero LA, Rennie D. Influences on the quality of published drug studies. Int J Technol Assess Health Care. 1996;12:209–237. doi: 10.1017/s0266462300009582. [DOI] [PubMed] [Google Scholar]
- 5.International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to biomedical journals: writing and editing for biomedical publication. www.icmje.org. Updated: Oktober 2007. [PubMed]
- 6.Arbeitsgruppe Glossar im DNEbM e.V. EbM-Glossar: Publikationsbias: www.ebm-netzwerk.de/grundlagen/glossar#publik. Deutsches Netzwerk Evidenzbasierte Medizin e.V. Stand: 16. März 2008. Zuletzt geprüft: 22. Oktober 2009.
- 7.DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA. 2004;292:1363–1364. doi: 10.1001/jama.292.11.1363. [DOI] [PubMed] [Google Scholar]
- 8.Pharmaceutical Research and Manufacturers of America. PhRMA Clinical Trial Registry Proposal: www.phrma.org/publications/policy_papers/phrma_clinical_trial_registry_proposal/. Zuletzt geprüft: 22. Oktober 2008.
- 9.International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), European Federation of Pharmaceutical Industries and Associations (EFPIA), Japan Pharmaceutical Manufacturers Association (JPMA), Pharmaceutical Research and Manufacturers of America (PhRMA) Gemeinsame Position zur Offenlegung von Informationen zu klinischen Studien über Register und Datenbanken: clinicaltrials.ifpma.org. Bekannt gegeben: 06. Januar 2005.
- 10.Giacomini KM, Krauss RM, Roden DM, Eichelbaum M, Hayden MR, Nakamura Y. When good drugs go bad. Nature. 2007;446:975–977. doi: 10.1038/446975a. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers I. Underreporting research is scientific misconduct. JAMA. 1990;263:1405–1408. [PubMed] [Google Scholar]
- 12.International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), European Federation of Pharmaceutical Industries and Associations (EFPIA), Japan Pharmaceutical Manufacturers Association (JPMA), Pharmaceutical Research and Manufacturers of America (PhRMA) Gemeinsame Position zur Offenlegung sensibler Daten in Studienregistern: http://clinicaltrials.ifpma.org. Bekannt gegeben: 5. September 2005.
- 13.Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunge M, Muhlhauser I, Steckelberg A. What constitutes evidence-based patient information? Overview of discussed criteria. Patient Educ Couns. 2009 doi: 10.1016/j.pec.2009.10.029. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.PLoS Medicine Editors. Next stop, don’t block the doors: opening up access to clinical trials results. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Directive 2001/20/EEC of the european parliament and of the council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official Journal of the European Communities. 2001;L 121:34–44. [PubMed] [Google Scholar]
- 17.European Commission—Enterprise and Industry Directorate-General, Consumer Goods, Pharmaceuticals. Draft list of fields contained in the ’EudraCT’ clinical trials database to be included in the ’EudraPharm’ database on medicinal products and made public, in accordance with Article 57(2) of Regulation (EC) No 726/2004. http://ec.europa.eu. Public Consultation Paper. Version: 15 July 2008.
- 18.Mitteilung der Kommission betreffend die Leitlinie zu den Datenfeldern der in Artikel 11 der Richtlinie 2001/20/EG vorgesehenen Datenbank über klinische Versuche, die in die gemäß Artikel 57 der Verordnung (EG) Nr. 726/2004 eingerichtete Datenbank über Arzneimittel aufzunehmen sind (2008/C 168/02). Amtsblatt der Europäischen Union 2008; C 168: 3-4 [Google Scholar]
- 19.Kaiser J. Making clinical data widely available. Science. 2008;322:217–218. doi: 10.1126/science.322.5899.217. [DOI] [PubMed] [Google Scholar]
- 20.Verband Forschender Arzneimittelhersteller e. V. (VFA) Ethik-Handbuch: Ethisches Verhalten bei der Zusammenarbeit der forschenden Arzneimittelhersteller mit Ärzten, Patientenorganisationen und den anderen Partnern im Gesundheitswesen. Ausgabe November 2007. Berlin: VFA; 2007. [Google Scholar]
- 21.Garattini S, Chalmers I. Patients and the public deserve big changes in evaluation of drugs. BMJ. 2009;338 doi: 10.1136/bmj.b1025. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig W-D, Fetscher S, Schildmann J. Teure Innovationen in der Onkologie - für alle? Der Onkologe. 2009;15:1004–1014. [Google Scholar]
- 23.Booth CM, Tannock I. Reflections on medical oncology: 25 years of clinical trials—where have we come and where are we going? J Clin Oncol. 2008;26:6–8. doi: 10.1200/JCO.2007.13.8156. [DOI] [PubMed] [Google Scholar]
- 24.DeAngelis CD, Fontanarosa PB. Impugning the integrity of medical science: the adverse effects of industry influence. JAMA. 2008;299:1833–1835. doi: 10.1001/jama.299.15.1833. [DOI] [PubMed] [Google Scholar]
- 25.Young NS, Ioannidis JP, Al-Ubaydli O. Why current publication practices may distort science. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Katz KA, Karlawish JH, Chiang DS, Bognet RA, Propert KJ, Margolis DJ. Prevalence and factors associated with use of placebo control groups in randomized controlled trials in psoriasis: a cross-sectional study. J Am Acad Dermatol. 2006;55:814–822. doi: 10.1016/j.jaad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- e2.Waldinger MD, Schweitzer DH. Premature ejaculation and pharmaceutical company-based medicine: the dapoxetine case. J Sex Med. 2008;5:966–997. doi: 10.1111/j.1743-6109.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- e3.Nieto A, Mazon A, Pamies R, Linana JJ, Lanuza A, Jimenez FO, et al. Adverse effects of inhaled corticosteroids in funded and nonfunded studies. Arch Intern Med. 2007;167:2047–2053. doi: 10.1001/archinte.167.19.2047. [DOI] [PubMed] [Google Scholar]
- e4.Gotzsche PC, Hrobjartsson A, Johansen HK, Haahr MT, Altman DG, Chan AW. Ghost authorship in industry-initiated randomised trials. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Ross JS, Hill KP, Egilman DS, Krumholz HM. Guest authorship and ghostwriting in publications related to rofecoxib: a case study of industry documents from rofecoxib litigation. JAMA. 2008;299:1800–1812. doi: 10.1001/jama.299.15.1800. [DOI] [PubMed] [Google Scholar]
- e6.Zarin DA, Tse T, Ide NC. Trial Registration at ClinicalTrials.gov between May and October 2005. N Engl J Med. 2005;353:2779–2787. doi: 10.1056/NEJMsa053234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Lott JP, Katz KA. Pharmaceutical companies’ policies and practices regarding prospective registration of dermatology-related clinical trials. Br J Dermatol. 2006;155:635–638. doi: 10.1111/j.1365-2133.2006.07386.x. [DOI] [PubMed] [Google Scholar]
- e8.Psaty BM, Furberg CD, Ray WA, Weiss NS. Potential for conflict of interest in the evaluation of suspected adverse drug reactions: use of cerivastatin and risk of rhabdomyolysis. JAMA. 2004;292:2622–2631. doi: 10.1001/jama.292.21.2622. [DOI] [PubMed] [Google Scholar]
- e9.Psaty BM, Kronmal RA. Reporting mortality findings in trials of rofecoxib for Alzheimer disease or cognitive impairment: a case study based on documents from rofecoxib litigation. JAMA. 2008;299:1813–1817. doi: 10.1001/jama.299.15.1813. [DOI] [PubMed] [Google Scholar]
- e10.Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:2021–2029. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- e11.Ross JS, Madigan D, Hill KP, Egilman DS, Wang Y, Krumholz HM. Pooled analysis of rofecoxib placebo-controlled clinical trial data: lessons for postmarket pharmaceutical safety surveillance. Arch Intern Med. 2009;169:1976–1985. doi: 10.1001/archinternmed.2009.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Aursnes I, Gjertsen MK. Common adverse events associated with an SSRI: meta-analysis of early paroxetine data. Pharmacoepidemiol Drug Saf. 2008;17:707–713. doi: 10.1002/pds.1596. [DOI] [PubMed] [Google Scholar]
- e13.Halpern SD, Barton TD, Gross R, Hennessy S, Berlin JA, Strom BL. Epidemiologic studies of adverse effects of anti-retroviral drugs: how well is statistical power reported. Pharmacoepidemiol Drug Saf. 2005;14:155–161. doi: 10.1002/pds.1059. [DOI] [PubMed] [Google Scholar]
- e14.Lee K, Bacchetti P, Sim I. Publication of clinical trials supporting successful new drug applications: a literature analysis. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Rising K, Bacchetti P, Bero L. Reporting bias in drug trials submitted to the Food and Drug Administration: review of publication and presentation. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- e17.Melander H, Ahlqvist-Rastad J, Meijer G, Beermann B. Evidence b(i)ased medicine-selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications. BMJ. 2003;326:1171–1173. doi: 10.1136/bmj.326.7400.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e18.Vedula SS, Bero L, Scherer RW, Dickersin K. Outcome reporting in industry-sponsored trials of gabapentin for off-label use. N Engl J Med. 2009;361:1963–1971. doi: 10.1056/NEJMsa0906126. [DOI] [PubMed] [Google Scholar]
- e19.Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 2004;363:1341–1345. doi: 10.1016/S0140-6736(04)16043-1. [DOI] [PubMed] [Google Scholar]
- e20.von Elm E, Rollin A, Blumle A, Huwiler K, Witschi M, Egger M. Publication and non-publication of clinical trials: longitudinal study of applications submitted to a research ethics committee. Swiss Med Wkly. 2008;138:197–203. doi: 10.4414/smw.2008.12027. [DOI] [PubMed] [Google Scholar]
- e21.Krzyzanowska MK, Pintilie M, Tannock IF. Factors associated with failure to publish large randomized trials presented at an oncology meeting. JAMA. 2003;290:495–501. doi: 10.1001/jama.290.4.495. [DOI] [PubMed] [Google Scholar]
- e22.Kho ME, Brouwers MC. Conference abstracts of a new oncology drug do not always lead to full publication: proceed with caution. J Clin Epidemiol. 2009;62:752–758. doi: 10.1016/j.jclinepi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- e23.Hole OP, Nitter-Hauge S, Cederkvist HR, Winther FO. An analysis of the clinical development of drugs in Norway for the year 2000: the completion of research and publication of results. Eur J Clin Pharmacol. 2009;65:315–318. doi: 10.1007/s00228-008-0601-8. [DOI] [PubMed] [Google Scholar]
- e24.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrialsG.gov: a cross-sectional analysis. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e25.Conen D, Torres J, Ridker PM. Differential citation rates of major cardiovascular clinical trials according to source of funding: a survey from 2000 to 2005. Circulation. 2008;118:1321–1327. doi: 10.1161/CIRCULATIONAHA.108.794016. [DOI] [PubMed] [Google Scholar]
- e26.Jefferson T, Di PC, Debalini MG, Rivetti A, Demicheli V. Relation of study quality, concordance, take home message, funding, and impact in studies of influenza vaccines: systematic review. BMJ. 2009;338 doi: 10.1136/bmj.b354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e27.Kuriya B, Schneid EC, Bell CM. Quality of pharmaceutical industry press releases based on original research. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e28.Gotzsche PC, Hrobjartsson A, Johansen HK, Haahr MT, Altman DG, Chan AW. Constraints on publication rights in industry-initiated clinical trials. JAMA. 2006;295:1645–1646. doi: 10.1001/jama.295.14.1645. [DOI] [PubMed] [Google Scholar]
- e29.Henry DA, Kerridge IH, Hill SR, McNeill PM, Doran E, Newby DA, et al. Medical specialists and pharmaceutical industry-sponsored research: a survey of the Australian experience. Med J Aust. 2005;182:557–560. doi: 10.5694/j.1326-5377.2005.tb06813.x. [DOI] [PubMed] [Google Scholar]
- e30.Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291:2457–2465. doi: 10.1001/jama.291.20.2457. [DOI] [PubMed] [Google Scholar]
- e31.Smith R. Medical journals and pharmaceutical companies: uneasy bedfellows. BMJ. 2003;326:1202–1205. doi: 10.1136/bmj.326.7400.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e32.Booth CM, Cescon DW, Wang L, Tannock IF, Krzyzanowska MK. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008;26:5458–5464. doi: 10.1200/JCO.2008.16.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e33.Peppercorn J, Blood E, Winer E, Partridge A. Association between pharmaceutical involvement and outcomes in breast cancer clinical trials. Cancer. 2007;109:1239–1246. doi: 10.1002/cncr.22528. [DOI] [PubMed] [Google Scholar]
- e34.Patsopoulos NA, Ioannidis JP, Analatos AA. Origin and funding of the most frequently cited papers in medicine: database analysis. BMJ. 2006;332:1061–1064. doi: 10.1136/bmj.38768.420139.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e35.Jagsi R, Sheets N, Jankovic A, Motomura AR, Amarnath S, Ubel PA. Frequency, nature, effects, and correlates of conflicts of interest in published clinical cancer research. Cancer. 2009 doi: 10.1002/cncr.24315. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- e36.Graf C, Battisti WP, Bridges D, Bruce-Winkler V, Conaty JM, Ellison JM, et al. Research Methods & Reporting. Good publication practice for communicating company sponsored medical research: the GPP2 guidelines. BMJ. 2009;339 doi: 10.1136/bmj.b4330. [DOI] [PubMed] [Google Scholar]
- e37.Equator network website: www.equator-network.org/. Last accessed: 8. Februar 2010.