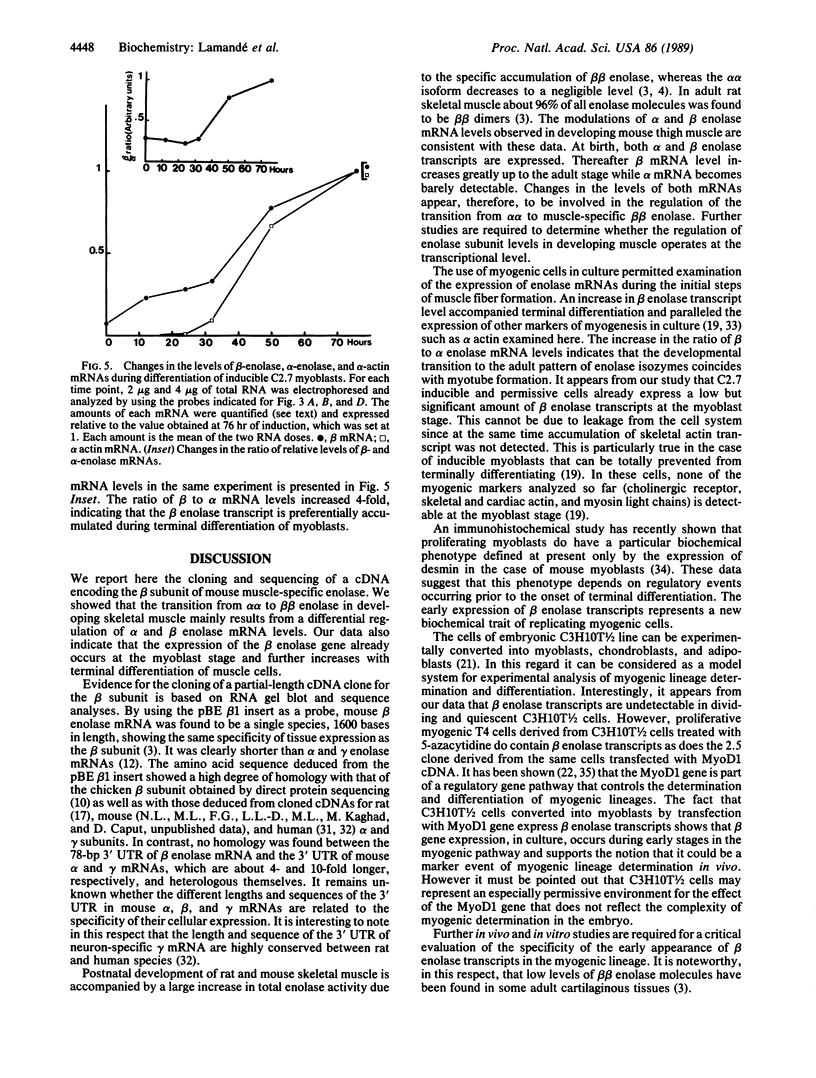
Abstract
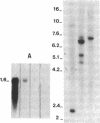
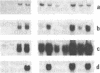
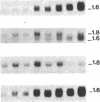
In vertebrates, the glycolytic enzyme enolase (EC 4.2.1.11) is present as homodimers and heterodimers formed from three distinct subunits of identical molecular weight, alpha, beta, and gamma. We report the cloning and sequencing of a cDNA encoding the beta subunit of murine muscle-specific enolase. The corresponding amino acid sequence shows greater than 80% homology with the beta subunit from chicken obtained by protein sequencing and with alpha and gamma subunits from rat and mouse deduced from cloned cDNAs. In contrast, there is no homology between the 3' untranslated regions of mouse alpha, beta, and gamma enolase mRNAs, which also differ greatly in length. The short 3' untranslated region of beta enolase mRNA accounts for its distinct length, 1600 bases. It is known that a progressive transition from alpha alpha to beta beta enolase occurs in developing skeletal muscle. We show that this transition mainly results from a differential regulation of alpha and beta mRNA levels. Analysis of myogenic cell lines shows that beta enolase gene is expressed at the myoblast stage. Moreover, transfection of premyogenic C3H10T1/2 cells with MyoD1 cDNA shows that the initial expression of beta transcripts occurs during the very first steps of the myogenic pathway, suggesting that it could be a marker event of myogenic lineage determination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Chin C. C., Brewer J. M., Wold F. The amino acid sequence of yeast enolase. J Biol Chem. 1981 Feb 10;256(3):1377–1384. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Day I. N., Allsopp M. T., Moore D. C., Thompson R. J. Sequence conservation in the 3'-untranslated regions of neurone-specific enolase, lymphokine and protooncogene mRNAs. FEBS Lett. 1987 Sep 28;222(1):139–143. doi: 10.1016/0014-5793(87)80207-7. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Tipler T. D., Morgan-Hughes J. A., Neerunjun J. S., Hopkinson D. A. Isozyme patterns and protein profiles in neuromuscular disorders. J Med Genet. 1982 Jun;19(3):175–183. doi: 10.1136/jmg.19.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher L., Rider C. C., Taylor C. B., Adamson E. D., Luke B. M., Graham C. F. Enolase isoenzymes as markers of differentiation in teratocarcinoma cells and normal tissues of mouse. Dev Biol. 1978 Aug;65(2):462–475. doi: 10.1016/0012-1606(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Giallongo A., Feo S., Moore R., Croce C. M., Showe L. C. Molecular cloning and nucleotide sequence of a full-length cDNA for human alpha enolase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6741–6745. doi: 10.1073/pnas.83.18.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Ibi T., Sahashi K., Kato K., Takahashi A., Sobue I. Immunohistochemical demonstration of beta-enolase in human skeletal muscle. Muscle Nerve. 1983 Nov-Dec;6(9):661–663. doi: 10.1002/mus.880060907. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kato K., Shimizu A., Semba R., Satoh T. Tissue distribution, developmental profiles and effect of denervation of enolase isozymes in rat muscles. Biochim Biophys Acta. 1985 Jul 26;841(1):50–58. [PubMed] [Google Scholar]
- Kaufman S. J., Foster R. F. Replicating myoblasts express a muscle-specific phenotype. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9606–9610. doi: 10.1073/pnas.85.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr 5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: evidence for regulatory genes controlling determination. Cell. 1984 Oct;38(3):791–800. doi: 10.1016/0092-8674(84)90274-5. [DOI] [PubMed] [Google Scholar]
- Lazar M., Lucas M., Lamandé N., Bishop J. G., Gros F., Legault-Demare L. Isolation of murine neuron-specific and non-neuronal enolase cDNA clones. Biochem Biophys Res Commun. 1986 Nov 26;141(1):271–277. doi: 10.1016/s0006-291x(86)80364-3. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Gall I., Campbell P., Frischauf A. M. Isolation and characterization of cDNA clones from mouse skeletal muscle actin mRNA. DNA. 1986 Jun;5(3):235–238. doi: 10.1089/dna.1986.5.235. [DOI] [PubMed] [Google Scholar]
- Lucas M., Lamande N., Lazar M., Gros F., Legault-Demare L. Developmental expression of alpha- and gamma-enolase subunits and mRNA sequences in the mouse brain. Dev Neurosci. 1988;10(2):91–98. doi: 10.1159/000111960. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Ohshima Y., Mitsui H., Takayama Y., Kushiya E., Sakimura K., Takahashi Y. cDNA cloning and nucleotide sequence of rat muscle-specific enolase (beta beta enolase). FEBS Lett. 1989 Jan 2;242(2):425–430. doi: 10.1016/0014-5793(89)80515-0. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Pinset C., Montarras D., Chenevert J., Minty A., Barton P., Laurent C., Gros F. Control of myogenesis in the mouse myogenic C2 cell line by medium composition and by insulin: characterization of permissive and inducible C2 myoblasts. Differentiation. 1988 Jun;38(1):28–34. doi: 10.1111/j.1432-0436.1988.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Prewitt M. A., Salafsky B. Enzymic and histochemical changes in fast and slow muscles after cross innervation. Am J Physiol. 1970 Jan;218(1):69–74. doi: 10.1152/ajplegacy.1970.218.1.69. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Rider C. C., Taylor C. B. Enolase isoenzymes in rat tissues. Electrophoretic, chromatographic, immunological and kinetic properties. Biochim Biophys Acta. 1974 Sep 13;365(1):285–300. doi: 10.1016/0005-2795(74)90273-6. [DOI] [PubMed] [Google Scholar]
- Russell G. A., Dunbar B., Fothergill-Gilmore L. A. The complete amino acid sequence of chicken skeletal-muscle enolase. Biochem J. 1986 May 15;236(1):115–126. doi: 10.1042/bj2360115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K., Kushiya E., Obinata M., Takahashi Y. Molecular cloning and the nucleotide sequence of cDNA to mRNA for non-neuronal enolase (alpha alpha enolase) of rat brain and liver. Nucleic Acids Res. 1985 Jun 25;13(12):4365–4378. doi: 10.1093/nar/13.12.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford J. E., Lebherz H. G. Effect of denervation on the levels and rates of synthesis of specific enzymes in "fast-twitch" (breast) muscle fibers of the chicken. J Biol Chem. 1981 Jun 25;256(12):6423–6429. [PubMed] [Google Scholar]
- Shimizu A., Suzuki F., Kato K. Characterization of alpha alpha, beta beta, gamma gamma and alpha gamma human enolase isozymes, and preparation of hybrid enolases (alpha gamma, beta gamma and alpha beta) from homodimeric forms. Biochim Biophys Acta. 1983 Oct 28;748(2):278–284. doi: 10.1016/0167-4838(83)90305-9. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Sugisaki K., Nakashima K. Purification, characterization, and distribution of enolase isozymes in chicken. J Biochem. 1985 Dec;98(6):1527–1534. doi: 10.1093/oxfordjournals.jbchem.a135421. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Sugisaki K., Nakashima K. Switching in levels of translatable mRNAs for enolase isozymes during development of chicken skeletal muscle. Biochem Biophys Res Commun. 1985 Dec 31;133(3):868–872. doi: 10.1016/0006-291x(85)91215-x. [DOI] [PubMed] [Google Scholar]