Abstract
Dendritic spines are small actin-rich protrusions from neuronal dendrites that form the postsynaptic part of most excitatory synapses and are major sites of information processing and storage in the brain. Changes in the shape and size of dendritic spines are correlated with the strength of excitatory synaptic connections and heavily depend on remodeling of its underlying actin cytoskeleton. Emerging evidence suggests that most signaling pathways linking synaptic activity to spine morphology influence local actin dynamics. Therefore, specific mechanisms of actin regulation are integral to the formation, maturation, and plasticity of dendritic spines and to learning and memory.
Introduction
The human brain consists of a hundred billion neurons interconnected into functional neuronal circuits that underlie all our behaviors, thoughts, emotions, dreams, and memories. The capacity of neurons to function within neuronal circuits is mediated via specialized cell junctions called synapses. Chemical synapses regulate the electric communication within neural networks and pass information directly from presynaptic axon terminals to postsynaptic dendritic regions. Precise control of the development and connectivity of synapses is critical for accurate neural network activity and normal brain function. Most excitatory synapses in the mammalian brain are formed at tiny dendritic protrusions, named dendritic spines (Bourne and Harris, 2008). Experimental evidence has shown that changes in spine morphology account for functional differences at the synaptic level (Yuste and Bonhoeffer, 2001; Kasai et al., 2003). It is now widely believed that information in the brain can be stored by strengthening or weakening existing synapses, as well as appearance or disappearance of dendritic spines, which subsequently leads to the formation or elimination of synapses. These functional and structural changes at spines and synapses are believed to be the basis of learning and memory in the brain (Holtmaat and Svoboda, 2009; Kasai et al., 2010).
The primary function of dendritic spines is to compartmentalize local synaptic signaling pathways and restrict the diffusion of postsynaptic molecules (Nimchinsky et al., 2002; Newpher and Ehlers, 2009). Because the actin cytoskeleton is central to numerous cellular processes involving membrane dynamics such as cell motility and morphogenesis (Pollard and Borisy, 2003; Carlier and Pantaloni, 2007), it is not surprising that dendritic spine formation and dynamics are determined by the actin cytoskeleton. During the last decade, numerous studies on postsynaptic signaling pathways demonstrated that the actin cytoskeleton plays a pivotal role in the formation and elimination, motility and stability, and size and shape of dendritic spines (Halpain, 2000; Luo, 2002; Ethell and Pasquale, 2005; Tada and Sheng, 2006; Schubert and Dotti, 2007). In addition, modulation of actin dynamics drives the morphological changes in dendritic spines that are associated with alteration in synaptic strength (Matus, 2000; Cingolani and Goda, 2008). At synapses, the actin cytoskeleton does not only contribute to overall structure of synapses but also plays important roles in synaptic activities that range from organizing the postsynaptic density (Sheng and Hoogenraad, 2007) and anchoring postsynaptic receptors (Renner et al., 2008) to facilitating the trafficking of synaptic cargos (Schlager and Hoogenraad, 2009) and localizing the translation machinery (Bramham, 2008). It has also been shown that various memory disorders involve defects in the regulation of the actin cytoskeleton (Newey et al., 2005).
In this review, we discuss evidence for regulatory mechanisms of actin dynamics in dendritic spines. We will describe our current understanding of the organization of actin structures in spines and propose that specific actin signaling pathways regulate filopodia initiation, elongation, and spine head formation.
Dendritic spine structure and function
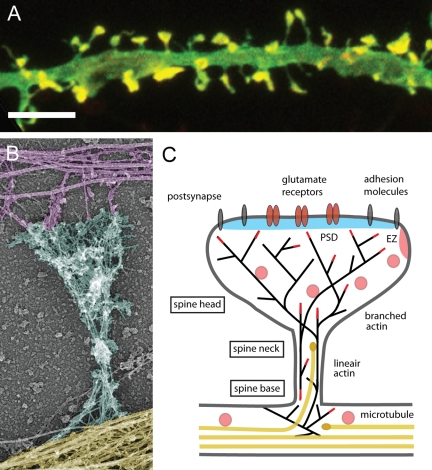
Dendritic spines are small protrusions that receive input from a single excitatory presynaptic terminal, allowing regulation of synaptic strength on a synapse-by-synapse basis. Spines occur at a density of 1–10 spines per micrometer of dendrite length, and some neurons, such as hippocampal neurons, contain thousands of spines throughout the dendritic arbors (Sorra and Harris, 2000) (Fig. 1 A). Spines consist of three distinct basic compartments: (1) a delta-shaped base at the junction with the dendritic shaft, (2) a constricted neck in the middle, and (3) a bulbous head contacting the axon (Fig. 1 B). They come in a wide range of sizes and shapes, their lengths varying from 0.2 to 2 µm and volumes from 0.001 to 1 µm3. Electron microscopy studies have identified roughly three categories of spines based on their morphology; thin, filopodia-like protrusions (“thin spines”), short spines without a well-defined spine neck (“stubby spines”) and spines with a large bulbous head (“mushroom spines”) (Bourne and Harris, 2008). The interesting feature of these spine structures is that they are not static, but change morphology continuously, even throughout adulthood, reflecting the plastic nature of synaptic connections (Grutzendler et al., 2002; Trachtenberg et al., 2002). Live imaging studies of spine dynamics reveal that the morphology of spines can be altered by neuronal activity in vitro and experience in vivo (Matsuzaki et al., 2004; Holtmaat et al., 2006; Roberts et al., 2010). Activity patterns that induce long-term potentiation (LTP), one of the major cellular mechanisms underlying learning and memory, causes enlargement of spine heads, suggesting that changes in dendritic spine morphology play an important role in memory formation (Yuste and Bonhoeffer, 2001; Kasai et al., 2003). Although de novo formation of dendritic spines in adult mice has been described, most spines are thought to arise from dendritic filopodia during early postnatal life (Holtmaat and Svoboda, 2009; Yoshihara et al., 2009).
Figure 1.
Cytoskeletal organization of dendritic spines. (A) Dendritic spine morphology (green) and localization of F-actin (red) in cultured hippocampal neurons. Merged red and green are shown in yellow. Bar, 5 µm. (B) Actin and microtubule cytoskeleton organization in a mature dendritic spine from cultured hippocampal neurons visualized by platinum replica electron microscopy (EM). Axonal cytoskeleton, purple; dendritic shaft, yellow; dendritic spine, cyan. The spine head typically contains a dense network of short cross-linked branched actin filaments, whereas the spine neck contains loosely arranged longitudinal actin filaments, both branched and linear. The base of the spine also contains branched filaments, which frequently reside directly on the microtubule network in the dendritic shaft. Image courtesy of Drs. Farida Korobova and Tatyana Svitkina (University of Pennsylvania, Philadelphia, PA). (C) Schematic diagram of a mature mushroom-shaped spine showing the postsynaptic membrane containing the postsynaptic density (PSD; blue), adhesion molecules (gray) and glutamate receptors (reddish brown), the actin (black lines) and microtubule (yellow) cytoskeleton, and organelles. The endocytic zone (EZ) is located lateral of the PSD in extrasynaptic regions of the spine and recycling endosomes (pink) are found in the shaft and spines. Dendritic spines exhibit a continuous network of both straight and branched actin filaments (black lines). The actin network is spread in the spine base, gets constricted in the neck, undergoes extensive branching at the neck–head junction, and stays highly branched in the spine head. The actin-polymerizing barbed ends are indicated as red lines. Stable microtubule arrays are predominantly present in the dendritic shaft. A small fraction of the microtubules in mature dendrites are dynamic and depart from the dendritic shaft, curve, and transiently enter dendritic spines. The microtubule plus-ends are symbolized as yellow ovals.
Dendritic spines contain the postsynaptic machinery, including glutamate receptors, postsynaptic density (PSD), and actin cytoskeleton, as well as a wide variety of membrane-bound organelles, such as smooth endoplasmic reticulum, mitochondria, and endosomes (Sheng and Hoogenraad, 2007; Fig. 1 C). The PSD is usually found at the tip of the dendritic spine head directly opposed to the presynaptic active zone. The PSD functions as a postsynaptic organizing structure where it clusters receptors, adhesion molecules, and channels and assembles a variety of signaling molecules at the postsynaptic membrane (Kennedy et al., 2005; Renner et al., 2008). Adjacent to the PSD is the endocytic zone, a stable membrane “hot spot” for clathrin-dependent endocytosis of postsynaptic receptors (Blanpied et al., 2002; Rácz et al., 2004). The main function of the endocytic zone is to capture and recycle the synaptic pool of mobile AMPA-type glutamate receptors required for synaptic potentiation (Lu et al., 2007; Petrini et al., 2009). One attractive mechanism for regulating changes in spine morphology is the local addition or removal of synaptic membrane and turnover of postsynaptic receptors. Indeed, synaptic stimulation mobilizes AMPA receptors in the recycling endosomal compartment into spines and leads to subsequent spine enlargement (Park et al., 2004, 2006). However, most other signaling pathways controlling spine shape seem to converge more directly on the actin cytoskeleton.
Actin organization in dendritic spines
Early electron microscopy studies have shown that actin is the major cytoskeletal component of dendritic spines (Landis and Reese, 1983). A network of long and short branching filaments is present in the spine neck and short branched actin filaments are localized in the spine head just underneath the PSD (Fig. 1 B). The most likely role of actin in mature spines is to stabilize postsynaptic proteins (Allison et al., 1998; Kuriu et al., 2006; Renner et al., 2009) and modulate spine head structure in response to postsynaptic signaling (Fischer et al., 2000; Star et al., 2002; Okamoto et al., 2004). Mass spectrometry analysis of PSD fractions has uncovered large numbers of actin-binding and actin cross-linking proteins, including Ca2+-calmodulin–dependent protein kinase IIβ (CaMKIIβ), cortactin, drebrin A, and neurabin I (Cheng et al., 2006). Down-regulation of these proteins reduces the formation and maturation of dendritic spines (Hering and Sheng, 2003; Terry-Lorenzo et al., 2005; Okamoto et al., 2007; Ivanov et al., 2009), which apparently makes them crucial for synaptic plasticity and memory formation (Wu et al., 2008; van Woerden et al., 2009; Kojima et al., 2010).
Several genetic and pharmacological experiments indicate that actin rearrangements drive the formation and loss of dendritic spines as well as their morphological plasticity, including shape, number, and motility (Halpain, 2000; Matus, 2000). Both the monomeric form of actin (G-actin) and filamentous polymers (F-actin) are present in spines and the degree of actin polymerization (and hence the G-actin/F-actin ratio) affects the various aspects of dendritic spine morphology (Cingolani and Goda, 2008). Measurements of fluorescence resonance energy transfer (FRET) between actin monomers revealed that synaptic stimulation rapidly changes the equilibrium between F-actin and G-actin (Okamoto et al., 2004). Long-term potentiation (LTP) induction shifts the G-actin/F-actin ratio toward F-actin (rise in spine actin filaments) and increases spine volume, whereas long-term depression (LTD) induction shifts the ratio toward G-actin (decrease in spine actin filaments) and results in spine shrinkage (Okamoto et al., 2004). To better understand the actin regulatory mechanisms that account for spine plasticity, we first need to consider what is known about the cytoskeleton organization in dendritic spines. Several recent studies present new insights in the organization and molecular composition of actin cytoskeleton in dendritic spines (Honkura et al., 2008; Hotulainen et al., 2009; Korobova and Svitkina, 2010). Using two-photon photoactivation of PAGFP (photoactivatable form of green fluorescent protein) fused to G-actin, the Kasai laboratory visualized the actin cytoskeleton within single dendritic spines at rest and during enlargement and found that three different actin pools dynamically regulate the structure and plasticity of the spines (Honkura et al., 2008). They also demonstrated that the actin pool at the tip of the spine treadmills to generate an expansive force in the spine head and is most likely the major determinant of spine volume. An elegant study by the Svitkina laboratory investigated the cytoskeletal organization of dendritic spines by electron microscopy and showed that spines exhibit a continuous network of both branched and long, linear actin filaments (Korobova and Svitkina, 2010). Because similar actin structures have been found in other morphogenetic processes, we will compare the recent data in dendritic spines with the actin machinery in lamellipodia and conventional filopodia in fibroblasts (see Fig. 2, A and B).
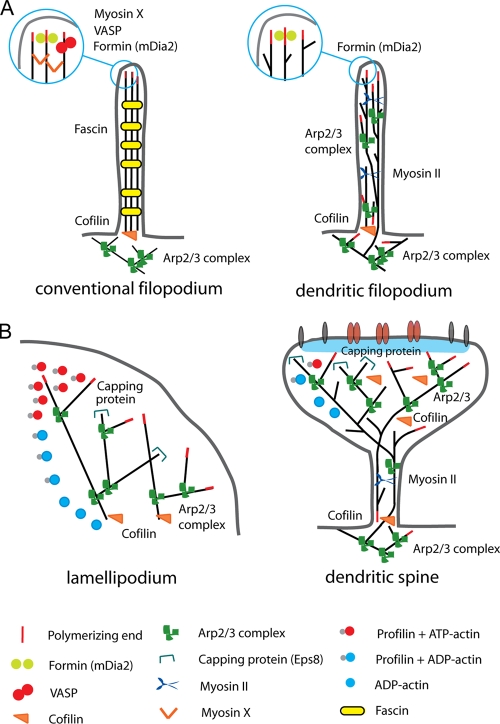
Figure 2.
Comparison of actin organization in fibroblast cells and dendritic spines. (A) In conventional filopodia from fibroblast cells, actin filaments are elongated from the tip of filopodia by mDia2, Ena/VASP, and myosin X. Polymerized actin filaments are bundled by fascin. In dendritic filopodia (neurons), mDia2 elongates actin filaments from the tip of filopodia. The functions of Ena/VASP and myosin X have not yet been studied in dendritic spines. In addition to tip polymerization, actin filaments of dendritic filopodia elongate from base. Fascin is absent from dendritic filopodia. (B) In lamellipodia from fibroblast cells, actin filaments are nucleated by Arp2/3 complex. Actin barbed ends are capped by capping protein to maintain filaments short. ADF/cofilins depolymerize pointed ends of actin filaments to replenish the actin monomer pool. Profilins change the ADP to ATP and transport ATP-actin monomers to the free barbed ends. In dendritic spine heads (neurons), functions of Arp2/3 complex and cofilin resemble those in lamellipodia. The function of capping protein has not been investigated. Profilins localize to dendritic spines in an activity-dependent manner.
The spine head contains branched actin filaments.
Actin filaments are polar structures with one end growing more rapidly (plus or “barbed” end) than the other (minus or “pointed” end). In migrating cells, the barbed ends push the plasma membrane and induce cell shape changes in the form of sheet- and rod-like extensions, termed lamellipodia and filopodia, respectively (Pollard and Borisy, 2003). Lamellipodia contain short and highly branched actin filaments, whereas filopodia consist of long, unbranched, actin filaments arranged in tight, unipolar, parallel bundles (Le Clainche and Carlier, 2008). In mature spine, the base, neck, and head all consist of a mixture of branched and linear actin filaments; the neck contains different ratios of both linear and branched filaments, whereas most branched actin filaments localize to the distal regions of the spine head (Korobova and Svitkina, 2010). Actin filaments in the spine head are very dynamic and show a high turnover by continuous treadmilling (Star et al., 2002; Honkura et al., 2008). In this way the actin cytoskeleton of mature dendritic spines, especially of the spine head, resembles actin structures found in lamellipodia (Fig. 2 B).
Consistent with this idea, several lamellipodial actin-binding proteins described in fibroblasts are enriched in dendritic spines (Table I). (1) The Arp2/3 complex (a stable complex of seven conserved subunits including the two actin-related proteins Arp2 and Arp3 and as well as ARPC1, ARPC2, ARPC3, ARPC4, and ARPC5) is the main nucleator of actin filaments in lamellipodia and necessary for lamellipodia formation (Hotulainen and Lappalainen, 2006). The Arp2/3 complex binds to the sides of existing actin filaments to nucleate a new filament, which subsequently results in the formation of a branched network of actin filaments (Goley and Welch, 2006). Arp2/3 complex is concentrated in spines (Rácz and Weinberg, 2008) and its knockdown from hippocampal neurons revealed its importance in dendritic spine head formation (Wegner et al., 2008; Hotulainen et al., 2009). Depletion of the Arp2/3 complex activators, including cortactin, Abi2, WAVE-1 (WASP-family verprolin homology protein-1), N-WASP (neural Wiskott-Aldrich syndrome protein), and Abp1 (actin-binding protein 1) alters the morphology and number of spines (Hering and Sheng, 2003; Grove et al., 2004; Kim et al., 2006; Soderling et al., 2007; Haeckel et al., 2008; Wegner et al., 2008). Consistently, WAVE-1 and Abi2 knock-out mice show hippocampal-dependent learning and memory deficits (Grove et al., 2004; Soderling et al., 2007). (2) Profilin I and profilin II are important assistants in actin polymerization. Profilins change actin nucleotide from ADP to ATP to promote barbed-end polymerization (Pollard et al., 2000). Endothelial cells lacking profilin I exhibit defects in membrane protrusion and cell migration (Ding et al., 2006). Profilin II is the major brain isoform, although profilin I is also present (Witke et al., 2001). Interestingly, profilins are localized to only a subset of dendritic spines in resting hippocampal neurons, whereas after chemical or electrical stimulation, profilins target to many more spines (Ackermann and Matus, 2003; Neuhoff et al., 2005). Consistently, fear conditioning in rats led to the relocalization of profilin from the dendritic shaft into dendritic spines (Lamprecht et al., 2006). A peptide competitor of profilin binding to polyproline-rich proteins prevented profilin targeting and destabilized spine structures (Ackermann and Matus, 2003), suggesting a role for profilin in activity-dependent actin regulation. However, detailed genetic studies are missing to clarify the roles of profilins in dendritic spine morphogenesis. (3) Another important factor for actin assembly in lamellipodia is a barbed end–binding protein called capping protein. Capping protein keeps the filaments short and maintains the actin monomer pool by restricting the polymerization only to the areas where it is required (Pollard and Borisy, 2003). The actin monomer pool is pivotal for the fast reorganization of the actin cytoskeleton. Without capping protein, actin filaments in lamellipodia grow too long, resulting in filopodia formation and reduction of lamellipodia (Mejillano et al., 2004). Capping protein localizes throughout dendritic spines (Korobova and Svitkina, 2010) but no functional studies have been reported. Eps8 (EGF receptor pathway substrate 8), another protein with actin-capping function, has been studied in dendritic spine morphogenesis. Actin-capping activity of Eps8 was shown to be the relevant biochemical activity required to inhibit filopodia formation (Menna et al., 2009). (4) ADF (actin-depolymerizing factor)/cofilins, which induce depolymerization of actin filaments from their pointed ends, are essential for maintaining the cellular actin monomer pool (Hotulainen et al., 2005; Kiuchi et al., 2007). Cofilin depletion from motile fibroblasts induces stabilization of actin filaments, thus “freezing” cell dynamics (Hotulainen et al., 2005). In neurons, cofilin1 is required for proper actin turnover and morphology of the dendritic spines (Hotulainen et al., 2009). Consistently, inactivation of LIM kinase 1 (LIMK1), which inhibits the activity of ADF/cofilins, results in altered dendritic spine morphology and synaptic function (Meng et al., 2002). Consistent with the structural and electrophysiological deficits, LIMK-1 knock-out mice exhibited abnormalities in behavioral responses, including altered fear responses and spatial learning.
Table I.
Actin-binding proteins and signaling pathways in dendritic spine morphogenesis
| Protein | Effect on actin cytoskeletona | Function in dendritic spine morphogenesis | Signaling pathwaya |
| Nucleating | |||
| Arp2/3 complex | Nucleates branched actin filaments | Required for spine head growth (Wegner et al., 2008; Hotulainen et al., 2009) | Rac and Cdc42 signaling cascades activate Arp2/3 complex |
| DRF3/ mDia2 | Induces elongation of straight actin filaments | Required for proper dendritic filopodia and spine neck formation (Hotulainen et al., 2009) | Rif activates DRF3/mDia2 |
| Proteins regulating Arp2/3 complex | |||
| Cortactin | Activates Arp2/3 complex | Regulates spine density; binds Shank (Hering and Sheng, 2003) | Src family kinases activate cortactin |
| N-WASP | Activates Arp2/3 complex | Regulates spine density (Wegner et al., 2008) | Cdc42, Rac, and PIP2 activate N-WASP |
| WAVE-1 | Activates Arp2/3 complex | Regulates spine density (Soderling et al., 2007) | Rac and PIP3 binding activate WAVE-1 |
| Abp1 | Controls Arp2/3 via N-WASP | Abp1 expression increases mushroom spine; binds Shank (Haeckel et al., 2008) | |
| ADP/ATP exchanger | |||
| Profilin | Enhances exchange of ADP to ATP and actin treadmilling rate | Stabilizes spine morphology and moves to spines upon activity (Ackermann and Matus, 2003) | |
| Depolymerizing | |||
| ADF, Cofilin | Depolymerizes and severs actin filaments | Required for spine head morphology and stabilization during LTP formation (Chen et al., 2007; Hotulainen et al., 2009) | PAK3 phosphorylates LIM kinase, which inactivates ADF/cofilins |
| Capping | |||
| Eps8 | Caps plus-ends of actin filaments | Inhibits BDNF-induced neuronal filopodia formation (Menna et al., 2009) | MAPK phosphorylation inhibits Eps8 |
| Cross-linking | |||
| α-Actinin | Bundles actin filaments | Expression induces spine elongation and thinning (Hoe et al., 2009) | FAK reduces binding of α-actinin to actin |
| Calponin | Bundles and stabilizes actin filaments | Expression induces spine elongation and increase in density (Rami et al., 2006) | |
| CaMKIIβ | Bundles and stabilizes actin filaments | Required for spine maturation and LTP-induced stabilization (Okamoto et al., 2007) | CaMKIIβ autoinhibition is released by NMDA receptor activation |
| Neurabin I | Bundles actin filaments | Required for spine maturation (Terry-Lorenzo et al., 2005) | Cdk5 phosphorylation inhibits Neurabin I |
| Drebrin | Bundles and stabilizes actin filaments | Expression induces spine elongation (Ivanov et al., 2009) | |
| Motor proteins | |||
| Myosin II | ATP-driven, actin-based motor | Required for proper spine head and neck morphology (Ryu et al., 2006) | RhoA activates myosin II |
| Myosin VI | ATP-driven, actin-based motor | Required for spine formation (Osterweil et al., 2005) |
For review see Le Clainche and Carlier, 2008.
The spine neck contains branched and linear actin filaments with mixed polarity.
In both immature dendritic filopodia and the spine neck of mature spines, the actin filaments are arranged roughly longitudinally along the structure (Korobova and Svitkina, 2010). The straight actin filaments do not span the entire neck, but begin or end with unbound ends or branch off of the side of another filament. Surprisingly, the actin filaments in dendritic filopodia (and the mature spine neck) differ from conventional filopodia in many aspects (Fig. 2 A). (1) Conventional filopodia consist of stiff bundles of straight actin filaments. Dendritic filopodia are made of a mixture of branched and straight actin filaments, but not to the extent of forming a tight bundle (Korobova and Svitkina, 2010). Consistently, the conventional actin filament bundling protein fascin is absent from the dendritic filopodia, whereas the Arp2/3 complex and capping protein are present (Korobova and Svitkina, 2010). (2) Actin filaments of conventional filopodia are polymerized on the tip of filopodia, whereas actin filaments in dendritic filopodia are polymerized from both the tip and the base (Hotulainen et al., 2009). (3) Conventional filopodia consist of parallel, unipolar arrays of actin filaments. Actin filaments of dendritic filopodia exhibit mixed polarity, although the predominant orientation is with the barbed end pointing toward the tip of filopodia (Hotulainen et al., 2009; Korobova and Svitkina, 2010). (4) The actin-dependent motor myosin II that binds and contracts actin filaments is absent from conventional filopodia but present in the dendritic filopodia (Korobova and Svitkina, 2010), consistent with the presence of anti-parallel actin filaments in dendritic filopodia. Despite these major differences, some similarities can be found between conventional filopodia and dendritic filopodia. The formin DRF3/mDia2 (human Diaphanous-related formin 3/mouse Diaphanous 2) is one of the main factors inducing elongation of actin filaments in conventional filopodia. The formin family proteins are known to polymerize straight actin filaments (Paul and Pollard, 2009). Depletion of DRF3/mDia2 from neurons resulted in reduced number of filopodia and abnormal spine neck morphology (Hotulainen et al., 2009). Moreover, Enabled/vasodilator-stimulated phosphoprotein (Ena/VASP) family proteins play an important role in filopodia formation in motile cells. Ena/VASP proteins anti-cap the polymerizing plus-ends of branched actin filaments, resulting in elongation of straight actin filaments. In the developing cortex, Ena/VASP proteins are required for filopodia formation and neuritogenesis (Dent et al., 2007; Kwiatkowski et al., 2007). Although recent evidence suggests that the transmembrane heparan sulfate proteoglycan Syndecan-2 promotes the formation of filopodia via the Ena/VASP pathway (Lin et al., 2007), the role of Ena/VASP family proteins in dendritic spines has been poorly investigated. In addition to DRF3/mDia2 and Ena/VASP proteins, myosin X has a key role in formation of filopodia in fibroblasts (Bohil et al., 2006). However, there is presently no study published on the role of myosin X in dendritic filopodia formation.
Signaling to the actin cytoskeleton in dendritic spines
The actin-signaling pathways in spines are regulated by many synaptic receptors such as the excitatory NMDA and AMPA-type glutamate receptors (Fischer et al., 2000). NMDA receptor regulates actin cytoskeleton in at least two ways: (1) by mediating the influx of Ca2+ ions into postsynaptic neurons, which modulate the activity of many actin binding proteins, e.g., CaMKIIβ (Lisman et al., 2002) and gelsolin (Nag et al., 2009); and (2) by binding directly to actin-binding or -regulating proteins, e.g., CaMKII (Raveendran et al., 2009), α-actinin (Wyszynski et al., 1997), and myosin regulatory light chain (Bajaj et al., 2009). Also, various receptor tyrosine kinases, such as members of the Trk (BDNF receptor; Menna et al., 2009) and Eph/ephrin families (Schubert and Dotti, 2007), as well as synaptic adhesion molecules (Yoshihara et al., 2009), have been shown to be important in regulating actin in spines. The major signaling hot spots in actin cytoskeleton regulation in fibroblasts are small Rho and Ras GTPases (Ethell and Pasquale, 2005; Tada and Sheng, 2006). Rho GTPases, including RhoA, Rac, and Cdc42 have been extensively studied in neurons and have profound influence on dendritic spine morphogenesis. In dendritic spines, RhoA activation has been shown to be necessary for expression of LTP via cofilin inactivation (Rex et al., 2009). In fibroblasts, Cdc42 induces filopodia formation, but in dendritic spines it induced spine head enlargement (Irie and Yamaguchi, 2002; Wegner et al., 2008; Hotulainen et al., 2009). In a simplified view, RhoA is important to inhibit cofilin activity, which will result in actin filament and spine stabilization. Rac and Cdc42 regulate spine head formation, mainly by activating Arp2/3 complex–induced nucleation and inhibiting actin depolymerization via cofilin. The Rho family GTPase Rif (Rho in filopodia) is an important regulator of filopodia formation (Hotulainen et al., 2009).
The Ras family of GTPases and their downstream signaling pathways as well as many of the Rho and Ras GTPase activators (GTP exchange factors [GEFs]) and inhibitors (GTPase-activating proteins [GAPs]) have been shown to be fundamental for dendritic spine morphology and neuronal functioning (Kennedy et al., 2005). A major postsynaptic inhibitor of Ras signaling is synaptic GAP (SynGAP), which is abundantly enriched in the PSD. Interestingly, heterozygous deletion of SynGAP in mice has been shown to cause an increase in the number of mushroom spines on hippocampal neurons (Vazquez et al., 2004). The spine phenotype is most likely explained by the cross talk of multiple signaling pathways where increased activation of Ras due to the absence of SynGAP leads to the downstream activation of Rac, which subsequently decreases cofilin function (Carlisle et al., 2008). Interestingly, mutations in the several Rho and Ras family GEFs and GAPs have been shown to cause mental retardation (Newey et al., 2005).
Actin dynamics modulate dendritic spine formation during development
During neuronal development upon synaptic contact with the axon, thin and highly motile dendritic filopodia can transform into more stable mushroom spines (Craig et al., 2006; Arikkath and Reichardt, 2008; Yoshihara et al., 2009). We will now discuss specific mechanisms of actin regulation controlling filopodia initiation, elongation, and spine head formation (Fig. 3).
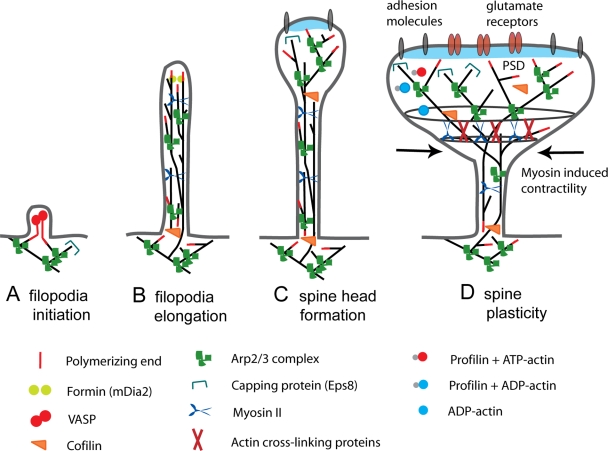
Figure 3.
Actin regulatory mechanisms during spine development and plasticity. (A) Spine development starts with the initiation of the dendritic filopodium and its elongation. Eps8 inhibits filopodia initiation by its capping activity. We propose that Ena/VASP proteins could induce filopodia elongation from Arp2/3 complex–generated branched filaments by anti-capping the actin barbed ends. (B) mDia2 promotes actin filament polymerization in the filopodium tip. We propose that Ena/VASP and myosin X take part in filopodia elongation. At this stage, the elongation of dendritic filopodia protrusions is mechanistically more similar to the promotion of lamellipodia protrusions. The factors driving actin filament polymerization in the base of filopodia remain to be identified. (C) Extensive actin branching occurs at the filopodium tip and the spine head begins to form. The mechanism of actin assembly is now increased and the large Arp2/3-nucleated branched actin filament network leads to enlargement of the spine head. The function of ADF/cofilins, in addition to replenishing the cytoplasmic actin monomer pool in neurons, is to control the proper length of actin filaments and thus to prevent formation of abnormal protrusions from spine heads. (D) Mature spines are still dynamic but maintain their overall morphology. Dynamics occur as small Arp2/3 complex–induced protrusions on the surface of the spine head (morphing). Myosin II–dependent contractility and cross-linking of actin filaments further modulate the shape of the spine head. We propose that during LTP, the activities of Arp2/3, profilin, actin cross-linking proteins, myosin II, and actin filament capping proteins are increased whereas activity of cofilin is reduced. The actin-ring structure is oversimplified to highlight the possible dynamic changes in the spine head morphology.
Initiation and elongation of dendritic filopodia.
The molecular mechanisms involved in the initiation of dendritic filopodia are currently unknown. Dendritic filopodia initiate directly from the dendritic shaft, often from preexisting patches of branched actin or from small lamellipodia (Andersen et al., 2005; Korobova and Svitkina, 2010). The actin-rich sites of initiation subsequently become the base of the filopodia. Current literature supports a model of both random initiation and signal-induced initiation. It has been shown that glutamate released from presynaptic sites influences filopodia initiation and elongation (Tashiro et al., 2003; Andersen et al., 2005). Here, we list some possible mechanisms involved in the initiation and elongation process (see Fig. 3, A and B). (1) Ena/VASP-induced anti-capping of filaments generated by the Arp2/3 complex could initiate filament elongation. (2) DRF3/mDia2 formin is important for proper filopodia formation (Hotulainen et al., 2009). It is plausible that also other proteins of the formin family are involved in polymerization of straight actin filaments for filopodia initiation and elongation. (3) Myosin X could initiate filopodia formation. It has been proposed that myosin X moves barbed ends laterally along the leading edge. This lateral movement converges the barbed ends of the actin filaments, thus producing the base of filopodia (Tokuo et al., 2007). (4) Actin filaments frequently reside on and branch off microtubules in the dendritic shaft (Korobova and Svitkina, 2010). In this way, microtubule-associated actin filament nucleators or actin–microtubule cross-linking factors could initiate filopodia formation. (5) Microtubules and actin filament–binding protein septin 7 localizes to the base of dendritic protrusions (Tada et al., 2007). Septins polymerize into heterotrimeric filaments and form small ring structures, which could initiate filopodia formation and work as a “diffusion barrier” in dendritic spine maturation. (6) Filopodia initiation could occur through membrane-deforming proteins that contain I-BAR (inverse Bin/Amphiphysin/Rvs) domains (Saarikangas et al., 2009; Yang et al., 2009). Electrostatic interactions between the positively charged poles of I-BAR domains and the negatively charged PI(4,5)P2 head groups induce clustering of PI(4,5)P2 and generation of membrane curvature due to the convex geometry of the lipid-binding interface of the domain (Saarikangas et al., 2009). In cultured neurons, acute depletion of I-BAR–containing protein IRSp53 has been shown to affect dendritic spine morphogenesis (Choi et al., 2005).
Formation of the spine head.
Once a dendritic filopodia is formed and axonal contact is made, spine motility gradually decreases and the spine structure is stabilized, which requires the assembly of pre- and postsynaptic components (Craig et al., 2006; Arikkath and Reichardt, 2008; Yoshihara et al., 2009). Newly formed spines are usually thin and elongated and in general have a small spine head. The Arp2/3 complex and its proper regulation are necessary for spine head growth (Grove et al., 2004; Kim et al., 2006; Soderling et al., 2007; Wegner et al., 2008; Hotulainen et al., 2009) (Fig. 3 C). A recent study showed that a complex with WAVE and Arp2/3 inhibits DRF3/mDia2-induced filopodia formation in fibroblast cells (Beli et al., 2008). This is an attractive mechanism to induce a switch from DRF3/mDia2-based actin polymerization to Arp2/3 complex–based actin polymerization, leading to the initiation of spine head growth (Hotulainen et al., 2009). Alternatively, transient microtubule entry into dendritic spines may induce Arp2/3 complex activation. We recently demonstrated that microtubule invasion into spines is associated with transient morphological changes, such as the formation of spine head protrusions and spine growth (Jaworski et al., 2009). Dynamic microtubules may induce a signaling cascade affecting actin dynamics through the microtubule plus-end tracking protein EB3 (Jaworski et al., 2009; Fig. 1 C). A proteomics screen for EB-binding partners in hippocampal neurons uncovered p140Cap (also known as SNAP-25 interacting protein) as an abundant PSD protein in spines (Jaworski et al., 2009) and regulator of Src tyrosine kinase (Di Stefano et al., 2007). p140Cap also interacts with cortactin (Jaworski et al., 2009), a Src substrate that can activate the Arp2/3 complex, stabilize branched actin filament network in spines, and promote formation of mushroom-like spines (Hering and Sheng, 2003). It is likely that the association between EB3-bound microtubule ends and p140Cap controls Src kinase activity and regulates cortactin function, which could lead to Arp2/3 complex activation and spine head growth. In this way, dynamic microtubules in mature neurons can serve as signaling devices to locally reorganize the actin cytoskeleton and regulate spine head size.
In addition to actin filament polymerization, three-dimensional organization of actin filaments is required in spine head formation. Both activation and inhibition of myosin II activity modifies the size and shape of spines, indicating that myosin II–induced contractility is an important process in dendritic spine morphogenesis (Zhang et al., 2005; Ryu et al., 2006; Fig. 3 C). Moreover, the actin cross-linking proteins CaMKIIβ, neurabin I, and drebrin A could also play roles in spine head modification and stabilization (Terry-Lorenzo et al., 2005; Okamoto et al., 2007; Ivanov et al., 2009). Regulation of cofilin activity is important for the proper morphology and stabilization of spines (Hotulainen et al., 2009) (Fig. 3 C). It is plausible that capping activity is required to establish the normal shape of the spine head and inhibit the formation of extra protrusions.
Synaptic plasticity is associated with a rapid and persistent reorganization of the spine actin cytoskeleton (Cingolani and Goda, 2008). Sequestering free actin monomers by latrunculin A blocks LTP expression, suggesting that actin polymerization is required for LTP (Fukazawa et al., 2003; Okamoto et al., 2004; Chen et al., 2007; Ramachandran and Frey, 2009). It seems evident that the Arp2/3 complex, helped by relocalized profilin, induces spine head enlargement shortly after LTP induction. During LTP expression, the actin cytoskeleton is stabilized and is resistant to latrunculin A (Ramachandran and Frey, 2009), which has been shown to occur through cofilin inactivation (Fukazawa et al., 2003; Chen et al., 2007; Fedulov et al., 2007). In addition, actin cross-linking by CaMKII and other actin cross-linking proteins may assist to stabilize actin filaments and spine heads during LTP formation (Okamoto et al., 2007). Myosin II–induced contractility could further regulate spine morphology and synaptic strength by controlling the geometry of the spine neck (Fig. 3 D), which is important for the diffusional coupling between the spine head and dendritic shaft (Bloodgood and Sabatini, 2005). Additional plus- and minus-end actin-capping proteins could further stabilize the actin filaments (Fig. 3 D).
Future perspectives: role of actin dynamics in dendritic spines in learning and memory
Over the past decades the function of the cytoskeleton has been extensively studied in developing and mature neurons in vitro and in vivo (Cingolani and Goda, 2008; Conde and Cáceres, 2009; Hoogenraad and Bradke, 2009), and there has been tremendous progress in understanding the molecular mechanisms that underlie dendritic spine changes and synaptic plasticity (Sheng and Hoogenraad, 2007; Bourne and Harris, 2008; Holtmaat and Svoboda, 2009; Newpher and Ehlers, 2009). Future studies will continue to visualize the localization and trafficking of synaptic and spine components and test the influence of synaptic activity on these processes (Fischer et al., 1998; Blanpied et al., 2002; Wang et al., 2008; Jaworski et al., 2009; Kapitein et al., 2010). Monitoring the spatiotemporal dynamics of postsynaptic signals (Lee et al., 2009) and photo-activation localization microscopy (PALM)–based single-molecule tracking to analyze cytoskeletal structures in single dendritic spines (Tatavarty et al., 2009) will be of great additional value.
There is substantial evidence that structural changes in spines, driven by remodeling of the actin cytoskeleton, underlie the stabilization of memories after learning (Lamprecht and LeDoux, 2004). For instance, drugs that block actin polymerization suppress LTP (Krucker et al., 2000; Fukazawa et al., 2003; Okamoto et al., 2004; Chen et al., 2007; Ramachandran and Frey, 2009); LIMK1 knock-out mice, which are unable to regulate cofilin activity, have enhanced hippocampal LTP (Meng et al., 2002); and WAVE-1 and Abi2 knock-out mice exhibit deficits in learning and memory (Grove et al., 2004; Soderling et al., 2007). The precise biological functions of many other actin regulatory proteins, however, remain largely unknown and are subjects for future studies. Recent data from Drosophila provide a strong basis to genetically dissect the role of the actin cytoskeleton in learning and memory formation in flies. Dendrites of visual system interneurons have recently been demonstrated to exhibit protrusions closely resembling vertebrate spines and consist of actin filaments and Rac1 (Leiss et al., 2009). Moreover, a recent report showed that Rac in mushroom body neurons contributes to both passive memory decay and interference-induced forgetting, suggesting that actin reorganization may contribute to memory erasure in Drosophila (Shuai et al., 2010). It has become clear that the actin cytoskeleton is engaged in multiple aspects of spine morphology and synaptic function and that local actin rearrangements and synaptic activity have a close cooperative feedback relationship. Synaptic activity regulates the integrity of the spine actin cytoskeleton; in its turn, actin dynamics affect spine morphology and thereby synaptic plasticity. However, it should be noted that actin polymerization in spines is required but not sufficient for functional LTP. Actin depolymerization by pharmacological approaches prevents spine enlargement and LTP, but actin-polymerizing agents do not potentiate synaptic transmission or induce LTP on their own (Okamoto et al., 2004).
In recent years it has become evident that many psychiatric and neurological disorders ranging from mental retardation and autism to Alzheimer’s disease and addiction are accompanied by alterations in spine morphology and synapse number (Blanpied and Ehlers, 2004). For instance, mutations in synaptic scaffolding protein Shank3 and adhesion molecules neuroligin-3 and neuroligin-4 have been linked to autism spectrum disorder (Südhof, 2008). Further evidence suggests that various memory disorders involve defects in the regulation of actin cytoskeleton (Newey et al., 2005). For example, the Schizophrenia risk factor DISC1 regulates dendritic spine morphology via Rac1 (Hayashi-Takagi et al., 2010) and mutations in the cofilin kinase PAK3 (p21-activated kinase) gene lead to X-linked mental retardation (Allen et al., 1998). Moreover, decreased levels of PAK3 relate to synaptic dysfunction in Alzheimer’s disease (Kreis and Barnier, 2009). PAK3 inhibition in mice causes cofilin pathology and memory impairment, consistent with a potential causal role of PAK defects in cognitive deficits in Alzheimer’s disease (Zhao et al., 2006). Future genetic studies will most likely identify new mutations that impact spine actin regulation in association with synapse dysfunction. Moreover, investigating basic cell biological mechanisms underlying spine development and plasticity will lead to a better understanding of synaptic plasticity, brain function, and neurological diseases.
Acknowledgments
The authors wish to thank Drs. Farida Korobova and Tatyana Svitkina for providing the electron microscopy picture of actin cytoskeleton in dendritic spine (Fig. 1 B), and Dr. Tamás Balla (National Institutes of Health, Bethesda, MD) and Dr. Roland Wedlich-Soldner (Max Planck Institute of Biochemistry, Martinsried, Germany) for the generous gift of the GFP-PLCγ1 PH domain and LifeAct-RFP construct, respectively (Fig. 1 A). Drs. Tatyana Svitkina, Enni Bertling, Lukas Kapitein, Esther de Graaff, Dick Jaarsma, and Robert van den Berg are acknowledged for critical reading of the manuscript.
P. Hotulainen is supported by the Finish Academy (SA 1125867). C.C. Hoogenraad is supported by the Netherlands Organization for Scientific Research (NWO-ALW/ECHO), the Netherlands Organization for Health Research and Development (ZonMw-VIDI/TOP), European Science Foundation (European Young Investigators [EURYI] Award), EMBO Young investigators program (YIP), and Human Frontier Science Program Career Development Award (HFSP-CDA).
Footnotes
Abbreviations used in this paper:
- CaMKIIβ
- Ca2+-calmodulin–dependent protein kinase IIβ
- Ena
- Enabled
- LIMK1
- LIM kinase 1
- LTP
- long-term potentiation
- PSD
- postsynaptic density
- VASP
- vasodilator-stimulated phosphoprotein
References
- Ackermann M., Matus A. 2003. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat. Neurosci. 6:1194–1200 10.1038/nn1135 [DOI] [PubMed] [Google Scholar]
- Allen K.M., Gleeson J.G., Bagrodia S., Partington M.W., MacMillan J.C., Cerione R.A., Mulley J.C., Walsh C.A. 1998. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat. Genet. 20:25–30 10.1038/1675 [DOI] [PubMed] [Google Scholar]
- Allison D.W., Gelfand V.I., Spector I., Craig A.M. 1998. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J. Neurosci. 18:2423–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R., Li Y., Resseguie M., Brenman J.E. 2005. Calcium/calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. J. Neurosci. 25:8878–8888 10.1523/JNEUROSCI.2005-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikkath J., Reichardt L.F. 2008. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 31:487–494 10.1016/j.tins.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj G., Zhang Y., Schimerlik M.I., Hau A.M., Yang J., Filtz T.M., Kioussi C., Ishmael J.E. 2009. N-methyl-D-aspartate receptor subunits are non-myosin targets of myosin regulatory light chain. J. Biol. Chem. 284:1252–1266 10.1074/jbc.M801861200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beli P., Mascheroni D., Xu D., Innocenti M. 2008. WAVE and Arp2/3 jointly inhibit filopodium formation by entering into a complex with mDia2. Nat. Cell Biol. 10:849–857 10.1038/ncb1745 [DOI] [PubMed] [Google Scholar]
- Blanpied T.A., Ehlers M.D. 2004. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol. Psychiatry. 55:1121–1127 10.1016/j.biopsych.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Blanpied T.A., Scott D.B., Ehlers M.D. 2002. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 36:435–449 10.1016/S0896-6273(02)00979-0 [DOI] [PubMed] [Google Scholar]
- Bloodgood B.L., Sabatini B.L. 2005. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 310:866–869 10.1126/science.1114816 [DOI] [PubMed] [Google Scholar]
- Bohil A.B., Robertson B.W., Cheney R.E. 2006. Myosin-X is a molecular motor that functions in filopodia formation. Proc. Natl. Acad. Sci. USA. 103:12411–12416 10.1073/pnas.0602443103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J.N., Harris K.M. 2008. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 31:47–67 10.1146/annurev.neuro.31.060407.125646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham C.R. 2008. Local protein synthesis, actin dynamics, and LTP consolidation. Curr. Opin. Neurobiol. 18:524–531 10.1016/j.conb.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Carlier M.F., Pantaloni D. 2007. Control of actin assembly dynamics in cell motility. J. Biol. Chem. 282:23005–23009 10.1074/jbc.R700020200 [DOI] [PubMed] [Google Scholar]
- Carlisle H.J., Manzerra P., Marcora E., Kennedy M.B. 2008. SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin. J. Neurosci. 28:13673–13683 10.1523/JNEUROSCI.4695-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.Y., Rex C.S., Casale M.S., Gall C.M., Lynch G. 2007. Changes in synaptic morphology accompany actin signaling during LTP. J. Neurosci. 27:5363–5372 10.1523/JNEUROSCI.0164-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D., Hoogenraad C.C., Rush J., Ramm E., Schlager M.A., Duong D.M., Xu P., Wijayawardana S.R., Hanfelt J., Nakagawa T., et al. 2006. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol. Cell. Proteomics. 5:1158–1170 10.1074/mcp.D500009-MCP200 [DOI] [PubMed] [Google Scholar]
- Choi J., Ko J., Racz B., Burette A., Lee J.R., Kim S., Na M., Lee H.W., Kim K., Weinberg R.J., Kim E. 2005. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J. Neurosci. 25:869–879 10.1523/JNEUROSCI.3212-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani L.A., Goda Y. 2008. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9:344–356 10.1038/nrn2373 [DOI] [PubMed] [Google Scholar]
- Conde C., Cáceres A. 2009. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10:319–332 10.1038/nrn2631 [DOI] [PubMed] [Google Scholar]
- Craig A.M., Graf E.R., Linhoff M.W. 2006. How to build a central synapse: clues from cell culture. Trends Neurosci. 29:8–20 10.1016/j.tins.2005.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E.W., Kwiatkowski A.V., Mebane L.M., Philippar U., Barzik M., Rubinson D.A., Gupton S., Van Veen J.E., Furman C., Zhang J., et al. 2007. Filopodia are required for cortical neurite initiation. Nat. Cell Biol. 9:1347–1359 10.1038/ncb1654 [DOI] [PubMed] [Google Scholar]
- Di Stefano P., Damiano L., Cabodi S., Aramu S., Tordella L., Praduroux A., Piva R., Cavallo F., Forni G., Silengo L., et al. 2007. p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity. EMBO J. 26:2843–2855 10.1038/sj.emboj.7601724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Lambrechts A., Parepally M., Roy P. 2006. Silencing profilin-1 inhibits endothelial cell proliferation, migration and cord morphogenesis. J. Cell Sci. 119:4127–4137 10.1242/jcs.03178 [DOI] [PubMed] [Google Scholar]
- Ethell I.M., Pasquale E.B. 2005. Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol. 75:161–205 10.1016/j.pneurobio.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Fedulov V., Rex C.S., Simmons D.A., Palmer L., Gall C.M., Lynch G. 2007. Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J. Neurosci. 27:8031–8039 10.1523/JNEUROSCI.2003-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Kaech S., Knutti D., Matus A. 1998. Rapid actin-based plasticity in dendritic spines. Neuron. 20:847–854 10.1016/S0896-6273(00)80467-5 [DOI] [PubMed] [Google Scholar]
- Fischer M., Kaech S., Wagner U., Brinkhaus H., Matus A. 2000. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat. Neurosci. 3:887–894 10.1038/78791 [DOI] [PubMed] [Google Scholar]
- Fukazawa Y., Saitoh Y., Ozawa F., Ohta Y., Mizuno K., Inokuchi K. 2003. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 38:447–460 10.1016/S0896-6273(03)00206-X [DOI] [PubMed] [Google Scholar]
- Goley E.D., Welch M.D. 2006. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7:713–726 10.1038/nrm2026 [DOI] [PubMed] [Google Scholar]
- Grove M., Demyanenko G., Echarri A., Zipfel P.A., Quiroz M.E., Rodriguiz R.M., Playford M., Martensen S.A., Robinson M.R., Wetsel W.C., et al. 2004. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol. Cell. Biol. 24:10905–10922 10.1128/MCB.24.24.10905-10922.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J., Kasthuri N., Gan W.B. 2002. Long-term dendritic spine stability in the adult cortex. Nature. 420:812–816 10.1038/nature01276 [DOI] [PubMed] [Google Scholar]
- Haeckel A., Ahuja R., Gundelfinger E.D., Qualmann B., Kessels M.M. 2008. The actin-binding protein Abp1 controls dendritic spine morphology and is important for spine head and synapse formation. J. Neurosci. 28:10031–10044 10.1523/JNEUROSCI.0336-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S. 2000. Actin and the agile spine: how and why do dendritic spines dance? Trends Neurosci. 23:141–146 10.1016/S0166-2236(00)01576-9 [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A., Takaki M., Graziane N., Seshadri S., Murdoch H., Dunlop A.J., Makino Y., Seshadri A.J., Ishizuka K., Srivastava D.P., et al. 2010. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat. Neurosci. 13:327–332 10.1038/nn.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H., Sheng M. 2003. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J. Neurosci. 23:11759–11769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe H.S., Lee J.Y., Pak D.T. 2009. Combinatorial morphogenesis of dendritic spines and filopodia by SPAR and alpha-actinin2. Biochem. Biophys. Res. Commun. 384:55–60 10.1016/j.bbrc.2009.04.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A., Svoboda K. 2009. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10:647–658 10.1038/nrn2699 [DOI] [PubMed] [Google Scholar]
- Holtmaat A., Wilbrecht L., Knott G.W., Welker E., Svoboda K. 2006. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 441:979–983 10.1038/nature04783 [DOI] [PubMed] [Google Scholar]
- Honkura N., Matsuzaki M., Noguchi J., Ellis-Davies G.C., Kasai H. 2008. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 57:719–729 10.1016/j.neuron.2008.01.013 [DOI] [PubMed] [Google Scholar]
- Hoogenraad C.C., Bradke F. 2009. Control of neuronal polarity and plasticity—a renaissance for microtubules? Trends Cell Biol. 19:669–676 10.1016/j.tcb.2009.08.006 [DOI] [PubMed] [Google Scholar]
- Hotulainen P., Lappalainen P. 2006. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 173:383–394 10.1083/jcb.200511093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P., Paunola E., Vartiainen M.K., Lappalainen P. 2005. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol. Biol. Cell. 16:649–664 10.1091/mbc.E04-07-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P., Llano O., Smirnov S., Tanhuanpää K., Faix J., Rivera C., Lappalainen P. 2009. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J. Cell Biol. 185:323–339 10.1083/jcb.200809046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie F., Yamaguchi Y. 2002. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat. Neurosci. 5:1117–1118 10.1038/nn964 [DOI] [PubMed] [Google Scholar]
- Ivanov A., Esclapez M., Pellegrino C., Shirao T., Ferhat L. 2009. Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons. J. Cell Sci. 122:524–534 10.1242/jcs.033464 [DOI] [PubMed] [Google Scholar]
- Jaworski J., Kapitein L.C., Gouveia S.M., Dortland B.R., Wulf P.S., Grigoriev I., Camera P., Spangler S.A., Di Stefano P., Demmers J., et al. 2009. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 61:85–100 10.1016/j.neuron.2008.11.013 [DOI] [PubMed] [Google Scholar]
- Kapitein L.C., Schlager M.A., Kuijpers M., Wulf P.S., van Spronsen M., MacKintosh F.C., Hoogenraad C.C. 2010. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol. 20:290–299 10.1016/j.cub.2009.12.052 [DOI] [PubMed] [Google Scholar]
- Kasai H., Matsuzaki M., Noguchi J., Yasumatsu N., Nakahara H. 2003. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 26:360–368 10.1016/S0166-2236(03)00162-0 [DOI] [PubMed] [Google Scholar]
- Kasai H., Fukuda M., Watanabe S., Hayashi-Takagi A., Noguchi J. 2010. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 33:121–129 10.1016/j.tins.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Kennedy M.B., Beale H.C., Carlisle H.J., Washburn L.R. 2005. Integration of biochemical signalling in spines. Nat. Rev. Neurosci. 6:423–434 10.1038/nrn1685 [DOI] [PubMed] [Google Scholar]
- Kim Y., Sung J.Y., Ceglia I., Lee K.W., Ahn J.H., Halford J.M., Kim A.M., Kwak S.P., Park J.B., Ho Ryu S., et al. 2006. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 442:814–817 10.1038/nature04976 [DOI] [PubMed] [Google Scholar]
- Kiuchi T., Ohashi K., Kurita S., Mizuno K. 2007. Cofilin promotes stimulus-induced lamellipodium formation by generating an abundant supply of actin monomers. J. Cell Biol. 177:465–476 10.1083/jcb.200610005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Hanamura K., Yamazaki H., Ikeda T., Itohara S., Shirao T. 2010. Genetic disruption of the alternative splicing of drebrin gene impairs context-dependent fear learning in adulthood. Neuroscience. 165:138–150 10.1016/j.neuroscience.2009.10.016 [DOI] [PubMed] [Google Scholar]
- Korobova F., Svitkina T. 2010. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol. Biol. Cell. 21:165–176 10.1091/mbc.E09-07-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis P., Barnier J.V. 2009. PAK signalling in neuronal physiology. Cell. Signal. 21:384–393 10.1016/j.cellsig.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Krucker T., Siggins G.R., Halpain S. 2000. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc. Natl. Acad. Sci. USA. 97:6856–6861 10.1073/pnas.100139797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriu T., Inoue A., Bito H., Sobue K., Okabe S. 2006. Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms. J. Neurosci. 26:7693–7706 10.1523/JNEUROSCI.0522-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski A.V., Rubinson D.A., Dent E.W., Edward van Veen J., Leslie J.D., Zhang J., Mebane L.M., Philippar U., Pinheiro E.M., Burds A.A., et al. 2007. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron. 56:441–455 10.1016/j.neuron.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Lamprecht R., LeDoux J. 2004. Structural plasticity and memory. Nat. Rev. Neurosci. 5:45–54 10.1038/nrn1301 [DOI] [PubMed] [Google Scholar]
- Lamprecht R., Farb C.R., Rodrigues S.M., LeDoux J.E. 2006. Fear conditioning drives profilin into amygdala dendritic spines. Nat. Neurosci. 9:481–483 10.1038/nn1672 [DOI] [PubMed] [Google Scholar]
- Landis D.M., Reese T.S. 1983. Cytoplasmic organization in cerebellar dendritic spines. J. Cell Biol. 97:1169–1178 10.1083/jcb.97.4.1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clainche C., Carlier M.F. 2008. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88:489–513 10.1152/physrev.00021.2007 [DOI] [PubMed] [Google Scholar]
- Lee S.J., Escobedo-Lozoya Y., Szatmari E.M., Yasuda R. 2009. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 458:299–304 10.1038/nature07842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiss F., Koper E., Hein I., Fouquet W., Lindner J., Sigrist S., Tavosanis G. 2009. Characterization of dendritic spines in the Drosophila central nervous system. Dev. Neurobiol. 69:221–234 10.1002/dneu.20699 [DOI] [PubMed] [Google Scholar]
- Lin Y.L., Lei Y.T., Hong C.J., Hsueh Y.P. 2007. Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. J. Cell Biol. 177:829–841 10.1083/jcb.200608121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J., Schulman H., Cline H. 2002. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 3:175–190 10.1038/nrn753 [DOI] [PubMed] [Google Scholar]
- Lu J., Helton T.D., Blanpied T.A., Rácz B., Newpher T.M., Weinberg R.J., Ehlers M.D. 2007. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 55:874–889 10.1016/j.neuron.2007.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. 2002. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 18:601–635 10.1146/annurev.cellbio.18.031802.150501 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M., Honkura N., Ellis-Davies G.C., Kasai H. 2004. Structural basis of long-term potentiation in single dendritic spines. Nature. 429:761–766 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. 2000. Actin-based plasticity in dendritic spines. Science. 290:754–758 10.1126/science.290.5492.754 [DOI] [PubMed] [Google Scholar]
- Mejillano M.R., Kojima S., Applewhite D.A., Gertler F.B., Svitkina T.M., Borisy G.G. 2004. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 118:363–373 10.1016/j.cell.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Meng Y., Zhang Y., Tregoubov V., Janus C., Cruz L., Jackson M., Lu W.Y., MacDonald J.F., Wang J.Y., Falls D.L., Jia Z. 2002. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 35:121–133 10.1016/S0896-6273(02)00758-4 [DOI] [PubMed] [Google Scholar]
- Menna E., Disanza A., Cagnoli C., Schenk U., Gelsomino G., Frittoli E., Hertzog M., Offenhauser N., Sawallisch C., Kreienkamp H.J., et al. 2009. Eps8 regulates axonal filopodia in hippocampal neurons in response to brain-derived neurotrophic factor (BDNF). PLoS Biol. 7:e1000138 10.1371/journal.pbio.1000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S., Ma Q., Wang H., Chumnarnsilpa S., Lee W.L., Larsson M., Kannan B., Hernandez-Valladares M., Burtnick L.D., Robinson R.C. 2009. Ca2+ binding by domain 2 plays a critical role in the activation and stabilization of gelsolin. Proc. Natl. Acad. Sci. USA. 106:13713–13718 10.1073/pnas.0812374106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H., Sassoè-Pognetto M., Panzanelli P., Maas C., Witke W., Kneussel M. 2005. The actin-binding protein profilin I is localized at synaptic sites in an activity-regulated manner. Eur. J. Neurosci. 21:15–25 10.1111/j.1460-9568.2004.03814.x [DOI] [PubMed] [Google Scholar]
- Newey S.E., Velamoor V., Govek E.E., Van Aelst L. 2005. Rho GTPases, dendritic structure, and mental retardation. J. Neurobiol. 64:58–74 10.1002/neu.20153 [DOI] [PubMed] [Google Scholar]
- Newpher T.M., Ehlers M.D. 2009. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 19:218–227 10.1016/j.tcb.2009.02.004 [DOI] [PubMed] [Google Scholar]
- Nimchinsky E.A., Sabatini B.L., Svoboda K. 2002. Structure and function of dendritic spines. Annu. Rev. Physiol. 64:313–353 10.1146/annurev.physiol.64.081501.160008 [DOI] [PubMed] [Google Scholar]
- Okamoto K., Nagai T., Miyawaki A., Hayashi Y. 2004. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci. 7:1104–1112 10.1038/nn1311 [DOI] [PubMed] [Google Scholar]
- Okamoto K., Narayanan R., Lee S.H., Murata K., Hayashi Y. 2007. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. USA. 104:6418–6423 10.1073/pnas.0701656104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil E., Wells D.G., Mooseker M.S. 2005. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J. Cell Biol. 168:329–338 10.1083/jcb.200410091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Penick E.C., Edwards J.G., Kauer J.A., Ehlers M.D. 2004. Recycling endosomes supply AMPA receptors for LTP. Science. 305:1972–1975 10.1126/science.1102026 [DOI] [PubMed] [Google Scholar]
- Park M., Salgado J.M., Ostroff L., Helton T.D., Robinson C.G., Harris K.M., Ehlers M.D. 2006. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 52:817–830 10.1016/j.neuron.2006.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.S., Pollard T.D. 2009. Review of the mechanism of processive actin filament elongation by formins. Cell Motil. Cytoskeleton. 66:606–617 10.1002/cm.20379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini E.M., Lu J., Cognet L., Lounis B., Ehlers M.D., Choquet D. 2009. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 63:92–105 10.1016/j.neuron.2009.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T.D., Borisy G.G. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465 10.1016/S0092-8674(03)00120-X [DOI] [PubMed] [Google Scholar]
- Pollard T.D., Blanchoin L., Mullins R.D. 2000. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29:545–576 10.1146/annurev.biophys.29.1.545 [DOI] [PubMed] [Google Scholar]
- Rácz B., Weinberg R.J. 2008. Organization of the Arp2/3 complex in hippocampal spines. J. Neurosci. 28:5654–5659 10.1523/JNEUROSCI.0756-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rácz B., Blanpied T.A., Ehlers M.D., Weinberg R.J. 2004. Lateral organization of endocytic machinery in dendritic spines. Nat. Neurosci. 7:917–918 10.1038/nn1303 [DOI] [PubMed] [Google Scholar]
- Ramachandran B., Frey J.U. 2009. Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro. J. Neurosci. 29:12167–12173 10.1523/JNEUROSCI.2045-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami G., Caillard O., Medina I., Pellegrino C., Fattoum A., Ben-Ari Y., Ferhat L. 2006. Change in the shape and density of dendritic spines caused by overexpression of acidic calponin in cultured hippocampal neurons. Hippocampus. 16:183–197 10.1002/hipo.20145 [DOI] [PubMed] [Google Scholar]
- Raveendran R., Devi Suma Priya S., Mayadevi M., Steephan M., Santhoshkumar T.R., Cheriyan J., Sanalkumar R., Pradeep K.K., James J., Omkumar R.V. 2009. Phosphorylation status of the NR2B subunit of NMDA receptor regulates its interaction with calcium/calmodulin-dependent protein kinase II. J. Neurochem. 110:92–105 10.1111/j.1471-4159.2009.06108.x [DOI] [PubMed] [Google Scholar]
- Renner M., Specht C.G., Triller A. 2008. Molecular dynamics of postsynaptic receptors and scaffold proteins. Curr. Opin. Neurobiol. 18:532–540 10.1016/j.conb.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Renner M., Choquet D., Triller A. 2009. Control of the postsynaptic membrane viscosity. J. Neurosci. 29:2926–2937 10.1523/JNEUROSCI.4445-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex C.S., Chen L.Y., Sharma A., Liu J., Babayan A.H., Gall C.M., Lynch G. 2009. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J. Cell Biol. 186:85–97 10.1083/jcb.200901084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T.F., Tschida K.A., Klein M.E., Mooney R. 2010. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 463:948–952 10.1038/nature08759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J., Liu L., Wong T.P., Wu D.C., Burette A., Weinberg R., Wang Y.T., Sheng M. 2006. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron. 49:175–182 10.1016/j.neuron.2005.12.017 [DOI] [PubMed] [Google Scholar]
- Saarikangas J., Zhao H., Pykäläinen A., Laurinmäki P., Mattila P.K., Kinnunen P.K., Butcher S.J., Lappalainen P. 2009. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr. Biol. 19:95–107 10.1016/j.cub.2008.12.029 [DOI] [PubMed] [Google Scholar]
- Schlager M.A., Hoogenraad C.C. 2009. Basic mechanisms for recognition and transport of synaptic cargos. Mol Brain. 2:25 10.1186/1756-6606-2-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert V., Dotti C.G. 2007. Transmitting on actin: synaptic control of dendritic architecture. J. Cell Sci. 120:205–212 10.1242/jcs.03337 [DOI] [PubMed] [Google Scholar]
- Sheng M., Hoogenraad C.C. 2007. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 76:823–847 10.1146/annurev.biochem.76.060805.160029 [DOI] [PubMed] [Google Scholar]
- Shuai Y., Lu B., Hu Y., Wang L., Sun K., Zhong Y. 2010. Forgetting is regulated through Rac activity in Drosophila. Cell. 140:579–589 10.1016/j.cell.2009.12.044 [DOI] [PubMed] [Google Scholar]
- Soderling S.H., Guire E.S., Kaech S., White J., Zhang F., Schutz K., Langeberg L.K., Banker G., Raber J., Scott J.D. 2007. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J. Neurosci. 27:355–365 10.1523/JNEUROSCI.3209-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorra K.E., Harris K.M. 2000. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 10:501–511 [DOI] [PubMed] [Google Scholar]
- Star E.N., Kwiatkowski D.J., Murthy V.N. 2002. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat. Neurosci. 5:239–246 10.1038/nn811 [DOI] [PubMed] [Google Scholar]
- Südhof T.C. 2008. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 455:903–911 10.1038/nature07456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Sheng M. 2006. Molecular mechanisms of dendritic spine morphogenesis. Curr. Opin. Neurobiol. 16:95–101 10.1016/j.conb.2005.12.001 [DOI] [PubMed] [Google Scholar]
- Tada T., Simonetta A., Batterton M., Kinoshita M., Edbauer D., Sheng M. 2007. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr. Biol. 17:1752–1758 10.1016/j.cub.2007.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A., Dunaevsky A., Blazeski R., Mason C.A., Yuste R. 2003. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors: a two-step model of synaptogenesis. Neuron. 38:773–784 10.1016/S0896-6273(03)00299-X [DOI] [PubMed] [Google Scholar]
- Tatavarty V., Kim E.J., Rodionov V., Yu J. 2009. Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging. PLoS One. 4:e7724 10.1371/journal.pone.0007724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-Lorenzo R.T., Roadcap D.W., Otsuka T., Blanpied T.A., Zamorano P.L., Garner C.C., Shenolikar S., Ehlers M.D. 2005. Neurabin/protein phosphatase-1 complex regulates dendritic spine morphogenesis and maturation. Mol. Biol. Cell. 16:2349–2362 10.1091/mbc.E04-12-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuo H., Mabuchi K., Ikebe M. 2007. The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J. Cell Biol. 179:229–238 10.1083/jcb.200703178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg J.T., Chen B.E., Knott G.W., Feng G., Sanes J.R., Welker E., Svoboda K. 2002. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 420:788–794 10.1038/nature01273 [DOI] [PubMed] [Google Scholar]
- van Woerden G.M., Hoebeek F.E., Gao Z., Nagaraja R.Y., Hoogenraad C.C., Kushner S.A., Hansel C., De Zeeuw C.I., Elgersma Y. 2009. betaCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat. Neurosci. 12:823–825 10.1038/nn.2329 [DOI] [PubMed] [Google Scholar]
- Vazquez L.E., Chen H.J., Sokolova I., Knuesel I., Kennedy M.B. 2004. SynGAP regulates spine formation. J. Neurosci. 24:8862–8872 10.1523/JNEUROSCI.3213-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Edwards J.G., Riley N., Provance D.W., Jr., Karcher R., Li X.D., Davison I.G., Ikebe M., Mercer J.A., Kauer J.A., Ehlers M.D. 2008. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 135:535–548 10.1016/j.cell.2008.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A.M., Nebhan C.A., Hu L., Majumdar D., Meier K.M., Weaver A.M., Webb D.J. 2008. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J. Biol. Chem. 283:15912–15920 10.1074/jbc.M801555200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Sutherland J.D., Sharpe A., Arai M., Kwiatkowski D.J. 2001. Profilin I is essential for cell survival and cell division in early mouse development. Proc. Natl. Acad. Sci. USA. 98:3832–3836 10.1073/pnas.051515498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.J., Ren M., Wang H., Kim S.S., Cao X., Zhuo M. 2008. Neurabin contributes to hippocampal long-term potentiation and contextual fear memory. PLoS One. 3:e1407 10.1371/journal.pone.0001407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M., Lin J., Rao A., Nigh E., Beggs A.H., Craig A.M., Sheng M. 1997. Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature. 385:439–442 10.1038/385439a0 [DOI] [PubMed] [Google Scholar]
- Yang C., Hoelzle M., Disanza A., Scita G., Svitkina T. 2009. Coordination of membrane and actin cytoskeleton dynamics during filopodia protrusion. PLoS One. 4:e5678 10.1371/journal.pone.0005678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y., De Roo M., Muller D. 2009. Dendritic spine formation and stabilization. Curr. Opin. Neurobiol. 19:146–153 10.1016/j.conb.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Yuste R., Bonhoeffer T. 2001. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 24:1071–1089 10.1146/annurev.neuro.24.1.1071 [DOI] [PubMed] [Google Scholar]
- Zhang H., Webb D.J., Asmussen H., Niu S., Horwitz A.F. 2005. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 25:3379–3388 10.1523/JNEUROSCI.3553-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Ma Q.L., Calon F., Harris-White M.E., Yang F., Lim G.P., Morihara T., Ubeda O.J., Ambegaokar S., Hansen J.E., et al. 2006. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat. Neurosci. 9:234–242 10.1038/nn1630 [DOI] [PubMed] [Google Scholar]