A microtubule arm regulates cell–cell repulsion, pointing hemocytes in opposite directions when they contact each other in Drosophila embryos.
Abstract
Drosophila melanogaster macrophages are highly migratory cells that lend themselves beautifully to high resolution in vivo imaging experiments. By expressing fluorescent probes to reveal actin and microtubules, we can observe the dynamic interplay of these two cytoskeletal networks as macrophages migrate and interact with one another within a living organism. We show that before an episode of persistent motility, whether responding to developmental guidance or wound cues, macrophages assemble a polarized array of microtubules that bundle into a compass-like arm that appears to anticipate the direction of migration. Whenever cells collide with one another, their microtubule arms transiently align just before cell–cell repulsion, and we show that forcing depolymerization of microtubules by expression of Spastin leads to their defective polarity and failure to contact inhibit from one another. The same is true in orbit/clasp mutants, indicating a pivotal role for this microtubule-binding protein in the assembly and/or functioning of the microtubule arm during polarized migration and contact repulsion.
Introduction
It is well established that the microtubule cytoskeleton in numerous cell types plays a role in generating and maintaining polarity (Siegrist and Doe, 2007). During cell migration for example, the directed polymerization of microtubules into the leading edge is required to either establish and/or maintain the front and back organization necessary for directed movement (Small et al., 2002); this central polarity determinant must also be able to rapidly reorganize whenever a cell repolarizes in response to guidance cues. Many extracellular cues that guide a cell’s movement are soluble factors, but another important cue, particularly in vivo as cells move through tissues, will be collisions with other cells. Contact repulsion was first described more than 50 yr ago when fibroblasts were observed in vitro to rapidly repolarize upon cell–cell contact (Abercrombie and Heaysman, 1953, 1954). Since this initial observation, we have gleaned little molecular understanding of how cell–cell repulsion is regulated and only recently have begun to observe this phenomenon during migratory events in vivo (Carmona-Fontaine et al., 2008). In this study, we show that Drosophila melanogaster macrophages undergo contact repulsion during developmental dispersal in vivo and that this process is important in maintaining an even distribution of these cells within the animal. Using fluorescent probes specific to actin and microtubules, we observe the interplay of these cytoskeletal networks within hemocytes and reveal that the rapid cellular repolarization observed upon cell collisions is preceded by alignment of stable arm-like microtubule bundles in the colliding cells. We demonstrate that these microtubule arms are critical for contact repulsion and that their formation is regulated by the plus end microtubule–interacting protein Orbit.
Results and discussion
Colabeling of microtubules and actin in Drosophila macrophages reveals the dynamic interplay of these two cytoskeletal networks
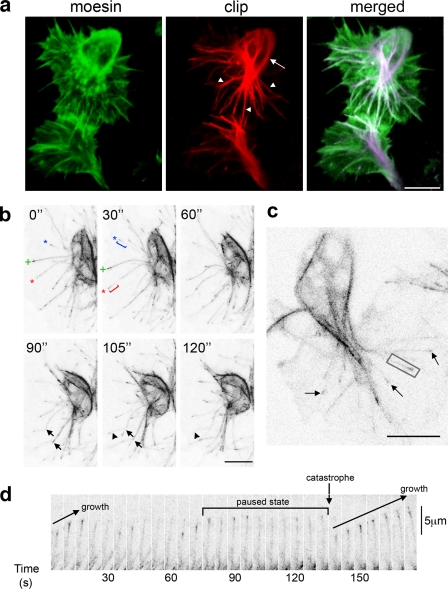
During development, Drosophila embryonic macrophages (hemocytes) disperse from their origin in the head and migrate throughout the embryo, such that by the end of embryogenesis, they are evenly distributed within the organism (Wood and Jacinto, 2007). Much of this dispersal occurs within a space between the superficial epithelium and subjacent tissues (ventrally, the ventral nerve cord), which is otherwise devoid of other cell types (Fig. S1, a–c), obliging hemocytes to interact only with one another. The organization and dynamics of the actin cytoskeleton in embryonic hemocytes has been well studied during their developmental migrations and their response to tissue damage (Stramer et al., 2005; Wood et al., 2006). In contrast, nothing is known about how microtubules are distributed in these cells. To visualize microtubule dynamics, we generated a fusion protein consisting of the microtubule-binding domain of human CLIP170 (Diamantopoulos et al., 1999; Perez et al., 1999) fused to mCherry or GFP. Coexpression of mCherry-CLIP170 and a fluorescent filamentous actin–binding construct (GFP-Moesin; Dutta et al., 2002) specifically within hemocytes allowed colabeling of both actin and microtubules in individual hemocytes within living embryos. Confocal imaging of these cells in situ as they underwent their developmental migrations or in vitro when plated out on a coverslip revealed that microtubules arranged themselves into a basket surrounding the cell body, with some extending into the lamellae (Fig. 1 a and Fig. S1 d). The lamellar microtubules of hemocytes in vivo are highly dynamic and display similar cycles of growth, pausing, catastrophe, and regrowth to those observed in cells in vitro. (Fig. 1, b and d). Expression of two copies of the fluorescent CLIP170 construct in hemocytes in vivo revealed the entire length of microtubules (Fig. 1, a and b), whereas reduced expression (only a single copy) highlighted the plus end of each filament as they grew toward the cell periphery, much as described in vitro (Fig. 1, c and d; Diamantopoulos et al., 1999). Interestingly, the microtubules extending into the lamellae colocalized with actin (Fig. 1 a and Video 1), and live imaging frequently revealed microtubules polymerizing along a fixed track within the lamellae, suggesting their extension along preexisting actin filaments (Fig. 1 d).
Figure 1.
The N-terminal domain of human CLIP170 reveals both stable and dynamic microtubules in Drosophila hemocytes. (a) GFP-Moesin (actin) and two copies of mCherry-CLIP170 (microtubules) were expressed in stage 15 hemocytes and live-imaged by confocal microscopy. Microtubules surrounded the cell body (arrow), with some penetrating into the lamellae (arrowheads). (b) Time-lapse imaging of a hemocyte expressing two copies of GFP-CLIP170 revealed microtubules extending (asterisks), pausing (plus signs), buckling (arrows), and reextending (arrowheads) within the lamellae. Brackets indicate the length of extension from 0 to 30 s. (c) A single copy of GFP-CLIP170 labeled the tips of growing microtubules (arrows). (d) Time-lapse series of the microtubule filament highlighted in c (boxed area) revealed cycles of microtubule growth, pausing, catastrophe, and regrowth. Bars, 10 µm.
Macrophage microtubules are bundled into a microtubule arm during in vivo directed motility
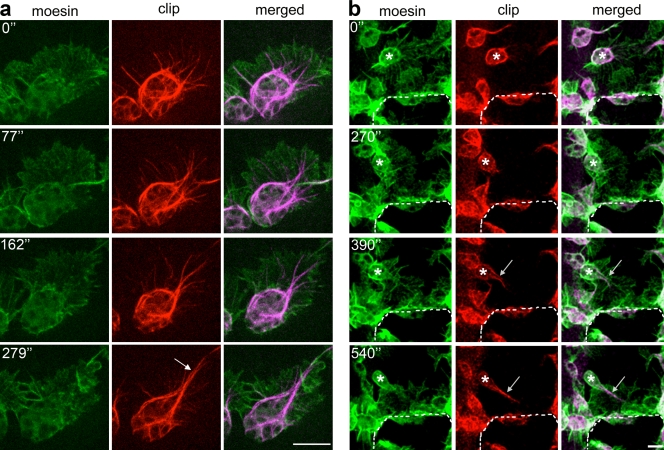
Live imaging of hemocytes as they migrate within the embryo showed that as microtubules extend into the lamellae, they are driven back apparently by actin retrograde flow and consequently converge on one another to form a stable population of centrally located microtubule bundles that coalesce to form what we termed a microtubule arm (Fig. 2 a and Video 2). This structure was not an artifact of CLIP170 overexpression and is absolutely dependent on a cell being polarized because we never observed arms in hemocytes plated out from the embryo onto a coverslip, where they failed to exhibit a polarized morphology (Fig. S1 d). We repeated the experiments using Tau-GFP, a construct widely used to label microtubules in Drosophila (Brand, 1995), and found similar microtubule dynamics in hemocytes as they migrated within the embryo (Fig. S1 e). Within a stage 15 embryo, the majority of hemocytes (81%; n = 37) displayed a clearly defined microtubule arm, which appeared to assign the cell front such that migration was always in the direction of the microtubule bundle. To assess whether assembly of this structure affects the migratory capacity of the cell, we measured directional persistence in migrating hemocytes with and without a well-defined microtubule arm and found that hemocytes with an arm have a directional persistence of 89.2 ± 1.5% (mean ± SEM; n = 18) as opposed to 18.5 ± 1.6% (n = 18) for those that lack this structure. We also noted that upon turning in response to developmental guidance cues, a hemocyte will maintain and reorient the same microtubule arm if the turn angle is <40° (92.3% of cells analyzed; n = 26); however, if the angle is >40°, the cell generally dismantles its microtubule bundle and forms a new one in the future direction of travel (88.8% of cells analyzed; n = 27). To determine whether extension of a microtubule arm directs lamellar polarization or is simply its consequence, we examined lamellar and microtubule dynamics within hemocytes responding to a polarizing chemotactic cue. We made laser wounds to embryos, which rapidly induced hemocyte migration toward the site of damage (Stramer et al., 2005, 2008), and imaged the microtubule architecture in responding cells. Within minutes of injury, hemocytes in the vicinity of the wound reorganized their microtubule cytoskeleton and extended a microtubule arm toward the wound site (Fig. 2 b). Quantitative analysis revealed that, on average, it took 4.6 ± 0.3 min (mean ± SEM; n = 13) for a hemocyte to assemble a microtubule arm, which preceded lamellar polarization and subsequent migration (Fig. 2 b and Video 3), suggesting that the arm is actively playing a role in polarizing the responding hemocyte rather than simply being a consequence of lamellar reorganization.
Figure 2.
Microtubules are transiently bundled within the lamellae of migrating hemocytes. (a) Live imaging of a hemocyte expressing GFP-Moesin (actin) and mCherry-CLIP170 (microtubules) revealed dynamic microtubules rapidly bundling into an arm (arrow) to polarize the cell’s morphology. (b) After laser ablation, a hemocyte (asterisks) in the vicinity of the wound extends a microtubule arm (arrows) before acquisition of a polarized lamellar morphology. The dashed lines indicate the wound edge. Time is shown in seconds. Bars, 10 µm.
The microtubule cytoskeleton is also required for cell–cell repulsion
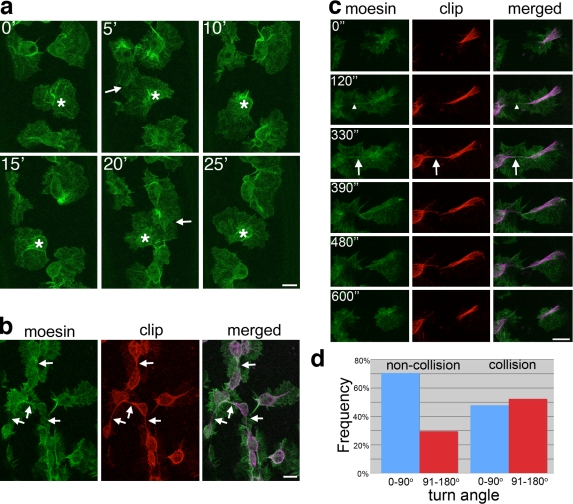
Analysis of stage 15 embryos showed that upon contact with one another, hemocytes rapidly stopped migrating and repolarized before moving away from each other (Fig. 3 a and Video 4) in a process reminiscent of the contact inhibition first observed in cultured fibroblasts more than 50 yr ago (Abercrombie and Heaysman, 1953). To understand how this repolarization occurs, we live-imaged cytoskeletal dynamics during hemocyte contact inhibition. Confocal imaging showed that when two hemocytes came into contact, their microtubule arms rapidly aligned (Fig. 3, b and c; and Video 5). Higher magnification time-lapse videos revealed that there was initial contact between lamellae and alignment of actin filaments, followed by a transient alignment of microtubule arms (Fig. 3 c and Video 6). This interaction lasted for ∼3 min before arms collapsed, and the cells subsequently repolarized and migrated away from one another (Fig. 3 c and Video 6). We then examined the turn angles 3 min after microtubule arms contacted and compared this with the turn angles of hemocytes that did not collide. Freely moving cells tended to maintain their course of direction, whereas cells that had collided showed a much greater change in direction (noncolliding, 30% turn >90°; n = 122; colliding, 52% turn >90°; n = 44; Fig. 3 d).
Figure 3.
During hemocyte contact repulsion, microtubule arms between colliding cells transiently interact. (a) Time-lapse imaging of stage 15 hemocytes expressing GFP-Moesin revealed how a single cell (asterisks) persistently collides (arrows) with neighbors and is immediately repelled from them. Time is shown in minutes. (b) Hemocytes expressing both GFP-Moesin (actin) and mCherry-CLIP170 (microtubules) indicate how the microtubule arms between contacting hemocytes align before cells retract from one another (arrows). (c) Time-lapse imaging of colliding hemocytes revealed that the lamellar interaction (arrowheads) precedes microtubule alignment (arrows). Microtubules were pseudocolored purple in the merged images. Time is shown in seconds. (d) Turn angles of colliding and noncolliding hemocytes after a 3-min time period revealed a greater change of direction after cell collision (P < 0.01; χ2 test). Bars, 10 µm.
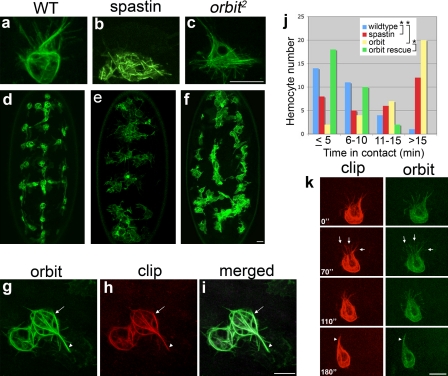
To directly test the requirement of microtubules during cell–cell repulsion, we expressed a microtubule-severing protein, Spastin, specifically in hemocytes (Trotta et al., 2004). Coexpression of GFP-CLIP170 within these hemocytes revealed that Spastin prevents assembly of a normal microtubule cytoskeleton (Fig. 4, a vs. b). Early developmental dispersal of hemocytes along the ventral midline in these embryos was delayed (Fig. S2, g and h); nonetheless, most hemocytes found their way to the ventral midline by stage 14, unlike hemocytes in mutants such as Rac that show a more catastrophic defect in migration and generally fail even to leave the head (Paladi and Tepass, 2004; Stramer et al., 2005). Interestingly, despite not being able to form a microtubule arm, these cells were still able to respond to wound stimuli, although with less efficiency than wild type; tracking experiments revealed individual cells taking a more tortuous route to the wound and displaying a mean directional persistence of 47.4 ± 3.8% (mean ± SEM) as opposed to 70.4 ± 3.7% for wild-type cells (Fig. S2, b and c). Interestingly, this failure to maintain directional persistence is countered by an increase in speed with mutant cells migrating at a mean of 4.02 ± 0.3 µm/min (mean ± SEM) when compared with wild types (2.36 ± 0.2 µm/min) such that the number of Spastin-expressing cells present at a wound 1 h after ablation is only slightly reduced relative to wild-type controls (Fig. S2 a). A more dramatic migration phenotype was seen from stage 15 onwards when Spastin-expressing hemocytes failed to disperse from the ventral midline (Fig. 4, d vs. e). Live imaging revealed that these hemocytes were unable to polarize and remained in close contact with one another at stages when they would ordinarily be exhibiting contact repulsion from one another (Video 7). To quantify these hemocyte dispersal defects, we performed a nearest neighbor analysis whereby the mean distance between each hemocyte and its nearest neighbor (dn) was measured. Spastin-expressing cells showed a significant reduction in dn when compared with wild type (11.68 µm vs. 15.69 µm, respectively; P < 0.001; Fig. S3, a, b, and e). To further quantify this deficiency in contact repulsion, we measured the length of time that wild-type versus Spastin-expressing hemocytes remained in contact with one another. We found that although wild-type hemocytes were rarely in contact with their neighbors for >10 min, Spastin-expressing hemocytes frequently retained contacts for >15 min (Fig. 4 j). Collectively, these results demonstrate that microtubules and, specifically, the microtubule arm we observe in hemocytes in vivo are essential to maintain a polarized morphology in hemocytes and, furthermore, are necessary for efficient cell–cell repulsion.
Figure 4.
Disruption of the hemocyte microtubule cytoskeleton leads to altered cell polarity and a failure in contact repulsion. (a–c) A hemocyte expressing GFP-CLIP170 revealed bundles of microtubules (a), whereas Spastin-expressing cells contained only small fragments (b), and orbit2 mutant hemocytes showed a complete loss of microtubule bundling and a loss of the microtubule arm (c). (d–f) Wild-type (WT) hemocytes expressing GFP-Moesin (d) dispersed evenly within the embryo, whereas hemocytes expressing Spastin (e) and orbit2 mutant hemocytes (f) remained clumped together. (g–i) Orbit-GFP and mCherry-CLIP170 expressed in orbit2 mutant hemocytes rescued microtubule organization with a well-defined basket of microtubules around the cell body (arrows) and a microtubule arm now clearly visible (arrowheads). (j) Graph showing contact time between neighboring hemocytes in wild-type, Spastin-expressing, orbit2 mutant, and rescued orbit2 mutant hemocytes (*, P < 0.01; χ2 test). (k) Time-lapse imaging of the rescued cells revealed that Orbit localized to the tips of microtubules (arrows) and along the entire length of microtubule bundles as the microtubule arm forms (arrowheads). Time is shown in seconds. Bars, 10 µm.
Disruption of the microtubule-stabilizing protein Orbit leads to a disorganized microtubule cytoskeleton and contact repulsion defects
A previous study implicated the microtubule plus end–binding and –stabilizing protein Orbit/Clasp in mediating growth cone repulsion from the chemorepellent Slit in Drosophila embryos (Lee et al., 2004). Given the similarity between the microtubule architecture in hemocytes and that seen in neuronal growth cones, we wondered whether Orbit might also mediate cell–cell repulsion in hemocytes. To address this, we analyzed hemocyte migration in orbit2 mutant embryos. Similar to those expressing Spastin, orbit2 mutant hemocytes exhibited a delay in migration along the ventral midline (Fig. S2, i and j), as well as a more severe defect whereby individual hemocytes failed to distribute themselves evenly at stage 15 (Fig. 4, d vs. f). Consistent with a defect in contact repulsion, quantitative analysis showed that orbit2 cells had a reduction in dn indistinguishable from Spastin-expressing cells (11.70 µm vs. 11.68 µm, respectively; P > 0.1; Fig. S3, b, c, and e). Furthermore, similar to Spastin-expressing cells, orbit2 hemocytes were frequently seen in contact with their neighbors for >15 min (Fig. 4 j). However, orbit2 hemocytes, like Spastin-expressing cells, were still capable of migrating to both epithelial wounds and in response to developmental guidance cues (Fig. S2, a, i, and j), demonstrating that this contact repulsion defect is not caused by a more general defect in motility. Furthermore, just as observed for Spastin-expressing cells, Orbit mutant hemocytes migrating to wounds exhibited reduced directional persistence (49.4% ± 3.7) and an increase in migration speed (3.19 ± 0.12 µm/min) when compared with wild-type cells (Fig. S2 d). To understand how a loss of Orbit affects the microtubule cytoskeleton, we expressed a single copy of GFP-CLIP170 to reveal microtubule dynamics in orbit2 hemocytes. Microtubules in mutant cells were highly dynamic and able to both polymerize and undergo catastrophe. However, unlike in wild-type cells, microtubule polymerization was unpolarized (Fig. S3 f): no microtubules became stabilized or bundled, and we saw no sign of a microtubule arm (Fig. 4 c and Video 8). Expression of a functional GFP-Orbit fusion protein (Lee et al., 2004), specifically in orbit2 hemocytes, rescued the formation of this structure (Fig. 4, g–i), and time-lapse analysis of these cells revealed GFP-Orbit localizing to the ends of growing microtubules as well as to the microtubule arm and the basket surrounding the cell body (Fig. 4 k). In addition to rescuing the microtubule architecture, hemocyte-specific expression of Orbit was able to restore contact repulsion such that dn and contact time between cells both returned to wild-type levels (Fig. 4 j and Fig. S3, d and e). These data demonstrate that Orbit is required for the stabilization and bundling of microtubules and that this architecture is important for both polarity and contact repulsion in hemocytes.
Our observation of microtubules in Drosophila hemocytes in vivo revealed that although these cells have some similarities with isolated cells in vitro, they also exhibit significant and interesting differences. Hemocytes in vivo, like many cultured cells (e.g. S2 cells), do possess a dynamically unstable population of microtubules in the lamellae. However, hemocytes in vivo also assemble a stable basket of microtubules surrounding the cell body and a microtubule arm that protrudes into the lamellae and polarizes the cell. Although it is possible that this architecture is unique to hemocytes, it appears more likely that the differences are a result of the 3D environment in which the hemocyte migrates in vivo because Drosophila hemocyte cell lines (Rogers et al., 2004; Sousa et al., 2007), and more revealingly primary isolated hemocytes plated onto a 2D substrate in vitro (Fig. S1 d), do not show a bundled microtubule architecture. Furthermore, none of these cells in vitro have a polarized morphology. Interestingly, it was recently reported that fibroblasts migrating in vitro on 1D lines of matrix move with an anterior microtubule bundle mimicking the movement we observe for hemocytes in vivo (Doyle et al., 2009). Hemocyte cell lines are increasingly being used as screening tools to elucidate genes controlling several processes such as cytoskeletal regulation (Kiger et al., 2003; Rogers et al., 2003). However, to date, no in vitro screen has highlighted orbit or actin regulatory genes such as fascin as important for cellular morphology (Kiger et al., 2003; Rogers et al., 2003; Baum, B., personal communication), but we know that they play a role within hemocytes in vivo (Zanet et al., 2009; this study). Our findings indicate that microtubule organization is very different in hemocytes in the embryo and highlight the importance of complementing in vitro screens with in vivo analysis.
Obvious similarities exist between the cytoskeletal architecture within hemocytes in vivo and that seen in migrating neuronal growth cones: both possess a central bundle of microtubules and actin microspikes radiating from the cell body toward the cortex. In both cell types, the local modification of microtubule dynamics appears capable of regulating directed migration. However, in this study, we also show that microtubules are critical for mediating cell–cell repulsive events in hemocytes (Fig. 5). That microtubules may have a role in both directed migration and contact repulsion initially seems paradoxical but, in fact, has a simple explanation that may parallel microtubule roles in growth cone guidance. Stabilization of growth cone microtubules in the direction of a chemotactic cue leads to the cell turning toward this signal (Zhou et al., 2004; Zumbrunn et al., 2001), whereas local depolymerization of microtubules results in the opposite event, cell repulsion (Buck and Zheng, 2002). Indeed, when microtubule dynamics are altered in neurons, growth cones fail to turn in response to many different cues (Williamson et al., 1996; Lee et al., 2004; Rajnicek et al., 2006). Our data suggest that cell collision is simply another guidance cue requiring a dynamic microtubule cytoskeleton that rapidly collapses upon cell–cell contact to enable cell–cell repulsion and turning.
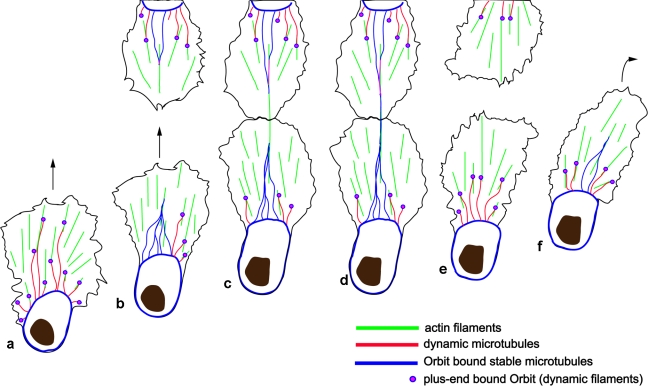
Figure 5.
Model for how microtubule bundles regulate both polarized migration and contact repulsion in hemocytes. (a) Stabilized, Orbit-bound microtubules surround the cell cortex, whereas dynamic microtubules with Orbit-decorated plus ends probe the lamella by extending along actin filaments. (b) Dynamic microtubules coalesce during directed migration to form the bundled microtubule arm that becomes Orbit bound over the entire filament length. (c and d) Upon collision with another cell, there is initial alignment of the actin cytoskeletons (c), followed by microtubule arms colliding at the site of cell–cell contact (d). (e and f) Subsequently, microtubules are depolymerized in the vicinity of the cell–cell contact site (e), leading to cellular repolarization and contact repulsion (f).
How might microtubules be directed to disassemble upon cell–cell contact, and how does this lead to cell repulsion? Previous studies showed that catastrophe of microtubules is induced by their growth against an immovable cellular object (Janson et al., 2003; Laan et al., 2008). The collision of two growing microtubules in colliding hemocytes may generate sufficient force for their depolymerization, which would lead to stochastic cellular repolarization. Another, nonmutually exclusive possibility is that microtubules play an active signaling role to break cell contacts. Fibroblasts undergoing contact inhibition in vitro make transient cell–cell adhesions (Gloushankova et al., 1998; Omelchenko et al., 2001), and the alignment of cytoskeletal filaments between two colliding hemocytes suggests similar transient contacts. Microtubules have also been shown to target focal adhesions in fibroblasts to induce their disassembly (Kaverina et al., 1999), leading to the intriguing possibility that cell–cell contacts might be another form of adhesion regulated by microtubules.
One final question is whether or not the repolarization event itself is actively signaled or is a passive consequence of microtubule reorganization. It was recently reported that when two neural crest cells collide, RhoA becomes transiently activated at the site of cell–cell contact (Carmona-Fontaine et al., 2008) and may therefore provide a cell repolarization signal during contact inhibition. Intriguingly, microtubules are capable of regulating Rho signaling by interactions with Rho guanine nucleotide exchange factors (Ren et al., 1998; Glaven et al., 1999; van Horck et al., 2001; Krendel et al., 2002; Rogers et al., 2004). These data are interesting in light of our previous finding that Rho mutant hemocytes clump and maintain cell–cell contacts during migration (Stramer et al., 2005). There is no doubt that our ability to examine these processes in a genetically tractable organism such as Drosophila will greatly aid in the dissection of the molecular events downstream of microtubules during persistent migration and contact repulsion in vivo.
Materials and methods
Fly stocks
For microtubule labeling, the N-terminal 350 aa of human CLIP170 (Perez et al., 1999) were tagged with GFP and mCherry at the N terminus and cloned into pUASp with 5′ KpnI–NotI and 3′ NotI–BamHI sites. To visualize actin, srp-Gal4 (on the second chromosome; Brückner et al., 2004) was recombined with UAS-GFP-Moesin (Dutta et al., 2002) or UAS-mCherry-Moesin (Millard and Martin, 2008). To colabel both actin and microtubules, a stable fly line was generated expressing srp-Gal4, UAS-GFP-Moesin; UAS-mCherry-CLIP170. To depolymerize the microtubule cytoskeleton specifically in hemocytes and visualize the actin cytoskeleton, fly lines were generated expressing srp-Gal4, UAS-GFP-Moesin; UAS-Spastin (Trotta et al., 2004) and srp-Gal4, UAS-mCherry-Moesin; UAS-Spastin-GFP (Jankovics and Brunner, 2006). To visualize the microtubule cytoskeleton in hemocytes also expressing Spastin, a fly line was generated expressing srp-Gal4, UAS-GFP-CLIP170; UAS-Spastin. To visualize the actin cytoskeleton in orbit2 mutants, a fly line was generated expressing srp-Gal4, UAS-GFP-Moesin; orbit2/TTG. TTG is a fluorescent GFP balancer (Halfon et al., 2002) that allowed us to select for homozygous orbit2 mutants by selecting nonfluorescent embryos. To visualize microtubules in orbit mutants, a fly line was generated expressing srp-Gal4, UAS-GFP-CLIP170; orbit2/TTG. To overexpress Orbit-GFP, a UAS-Orbit-GFP line (on the second chromosome; Lee et al., 2004) was recombined with srp-Gal4, which yielded viable progeny. To rescue orbit mutants, the orbit2 allele was recombined with UAS-mCherry-CLIP170 and expressed along with srp-Gal4, UAS-Orbit-GFP.
Imaging and quantification
Stage 14 or 15 embryos were dechorionated in bleach and mounted in Voltalef oil under a coverslip on a gas-permeable culture dish (Greiner Lumox; Sigma-Aldrich). For a detailed protocol, see Stramer and Wood (2009). Images were collected on a confocal microscope (SP5; Leica) or a spinning disk microscope (Ultraview; PerkinElmer) at room temperature with a 63× NA 1.4 Plan-Apochromat lens. Time-lapse images were processed using ImageJ (National Institutes of Health) or Volocity (PerkinElmer). To quantify the time hemocytes were in contact during their normal migration, 45-min time-lapse videos were acquired of stage 15 hemocytes with a z stack taken every 30 s. Videos were then processed in ImageJ, and the time hemocyte lamellae remained in contact was quantified.
Online supplemental material
Fig. S1 shows that hemocytes migrate within an acellular ventral space beneath the epithelium and that microtubule bundling is not an artifact of expression of UAS-CLIP170. Fig. S2 shows that hemocytes either expressing Spastin or mutant for Orbit are still capable of migrating within the embryo. Fig. S3 shows quantification of the hemocyte clumping defects in Spastin-expressing and orbit2 mutant embryos using nearest neighbor analysis and quantification of microtubule dynamics in wild-type and orbit2 mutant embryos. Video 1 is a 3D reconstruction of hemocytes expressing mCherry-CLIP170 and GFP-Moesin, which label microtubules and actin, respectively. Video 2 is a spinning disk confocal video of a hemocyte with fluorescently labeled actin and microtubules, revealing the colocalization of these two cytoskeletal components and the bundling of microtubules. Video 3 shows a confocal sequence of a hemocyte responding to a laser wound and reveals the time course of microtubule and lamellae dynamics upon consequent repolarization of this cell. Video 4 shows GFP-Moesin–labeled hemocytes undergoing contact repulsion during their embryonic migrations. Video 5 shows a confocal series of actin- and microtubule-labeled hemocytes colliding within the embryo and reveals the transient alignment of the cells’ microtubule bundles upon contact. Video 6 shows a collision between two hemocytes, revealing lamellar contact occurring immediately before microtubule alignment and subsequent repolarization. Video 7 shows wild-type versus Spastin-expressing hemocytes as they disperse within the embryo and reveals how cells clump without microtubules. Video 8 shows a confocal series of wild-type versus orbit2 mutant hemocytes expressing GFP-CLIP170 to reveal the altered microtubule dynamics in orbit2 mutants. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200912134/DC1.
Acknowledgments
We would like to thank David Van Vactor for orbit lines, Pernille Rorth and Andrea Daga for Drosophila Spastin lines, Debbie Carter for assistance with transmission electron microscopy, and Kate Nobes for helpful discussions.
This work was initially funded by a Medical Research Council project grant to P. Martin. B. Stramer is currently funded by a Biotechnology and Biological Sciences Research Council project grant. W. Wood is funded by a Wellcome Trust Career Development Fellowship. T. Millard was funded by a Wellcome Trust Advanced Training Fellowship. S. Moreira is funded by a Gulbenkian PhD Program in Biomedicine/Fundação para a Ciência e Tecnologia studentship.
References
- Abercrombie M., Heaysman J.E. 1953. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp. Cell Res. 5:111–131 10.1016/0014-4827(53)90098-6 [DOI] [PubMed] [Google Scholar]
- Abercrombie M., Heaysman J.E. 1954. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp. Cell Res. 6:293–306 10.1016/0014-4827(54)90176-7 [DOI] [PubMed] [Google Scholar]
- Brand A. 1995. GFP in Drosophila. Trends Genet. 11:324–325 10.1016/S0168-9525(00)89091-5 [DOI] [PubMed] [Google Scholar]
- Brückner K., Kockel L., Duchek P., Luque C.M., Rørth P., Perrimon N. 2004. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev. Cell. 7:73–84 10.1016/j.devcel.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Buck K.B., Zheng J.Q. 2002. Growth cone turning induced by direct local modification of microtubule dynamics. J. Neurosci. 22:9358–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C., Matthews H.K., Kuriyama S., Moreno M., Dunn G.A., Parsons M., Stern C.D., Mayor R. 2008. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 456:957–961 10.1038/nature07441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulos G.S., Perez F., Goodson H.V., Batelier G., Melki R., Kreis T.E., Rickard J.E. 1999. Dynamic localization of CLIP-170 to microtubule plus ends is coupled to microtubule assembly. J. Cell Biol. 144:99–112 10.1083/jcb.144.1.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A.D., Wang F.W., Matsumoto K., Yamada K.M. 2009. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184:481–490 10.1083/jcb.200810041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D., Bloor J.W., Ruiz-Gomez M., VijayRaghavan K., Kiehart D.P. 2002. Real-time imaging of morphogenetic movements in Drosophila using Gal4-UAS-driven expression of GFP fused to the actin-binding domain of moesin. Genesis. 34:146–151 10.1002/gene.10113 [DOI] [PubMed] [Google Scholar]
- Glaven J.A., Whitehead I., Bagrodia S., Kay R., Cerione R.A. 1999. The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J. Biol. Chem. 274:2279–2285 10.1074/jbc.274.4.2279 [DOI] [PubMed] [Google Scholar]
- Gloushankova N.A., Krendel M.F., Alieva N.O., Bonder E.M., Feder H.H., Vasiliev J.M., Gelfand I.M. 1998. Dynamics of contacts between lamellae of fibroblasts: essential role of the actin cytoskeleton. Proc. Natl. Acad. Sci. USA. 95:4362–4367 10.1073/pnas.95.8.4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon M.S., Gisselbrecht S., Lu J., Estrada B., Keshishian H., Michelson A.M. 2002. New fluorescent protein reporters for use with the Drosophila Gal4 expression system and for vital detection of balancer chromosomes. Genesis. 34:135–138 10.1002/gene.10136 [DOI] [PubMed] [Google Scholar]
- Jankovics F., Brunner D. 2006. Transiently reorganized microtubules are essential for zippering during dorsal closure in Drosophila melanogaster. Dev. Cell. 11:375–385 10.1016/j.devcel.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Janson M.E., de Dood M.E., Dogterom M. 2003. Dynamic instability of microtubules is regulated by force. J. Cell Biol. 161:1029–1034 10.1083/jcb.200301147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Small J.V. 1999. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146:1033–1044 10.1083/jcb.146.5.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger A.A., Baum B., Jones S., Jones M.R., Coulson A., Echeverri C., Perrimon N. 2003. A functional genomic analysis of cell morphology using RNA interference. J. Biol. 2:27 10.1186/1475-4924-2-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M., Zenke F.T., Bokoch G.M. 2002. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4:294–301 10.1038/ncb773 [DOI] [PubMed] [Google Scholar]
- Laan L., Husson J., Munteanu E.L., Kerssemakers J.W., Dogterom M. 2008. Force-generation and dynamic instability of microtubule bundles. Proc. Natl. Acad. Sci. USA. 105:8920–8925 10.1073/pnas.0710311105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Engel U., Rusch J., Scherrer S., Sheard K., Van Vactor D. 2004. The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron. 42:913–926 10.1016/j.neuron.2004.05.020 [DOI] [PubMed] [Google Scholar]
- Millard T.H., Martin P. 2008. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development. 135:621–626 10.1242/dev.014001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko T., Fetisova E., Ivanova O., Bonder E.M., Feder H., Vasiliev J.M., Gelfand I.M. 2001. Contact interactions between epitheliocytes and fibroblasts: formation of heterotypic cadherin-containing adhesion sites is accompanied by local cytoskeletal reorganization. Proc. Natl. Acad. Sci. USA. 98:8632–8637 10.1073/pnas.151247698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladi M., Tepass U. 2004. Function of Rho GTPases in embryonic blood cell migration in Drosophila. J. Cell Sci. 117:6313–6326 10.1242/jcs.01552 [DOI] [PubMed] [Google Scholar]
- Perez F., Diamantopoulos G.S., Stalder R., Kreis T.E. 1999. CLIP-170 highlights growing microtubule ends in vivo. Cell. 96:517–527 10.1016/S0092-8674(00)80656-X [DOI] [PubMed] [Google Scholar]
- Rajnicek A.M., Foubister L.E., McCaig C.D. 2006. Growth cone steering by a physiological electric field requires dynamic microtubules, microfilaments and Rac-mediated filopodial asymmetry. J. Cell Sci. 119:1736–1745 10.1242/jcs.02897 [DOI] [PubMed] [Google Scholar]
- Ren Y., Li R., Zheng Y., Busch H. 1998. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J. Biol. Chem. 273:34954–34960 10.1074/jbc.273.52.34954 [DOI] [PubMed] [Google Scholar]
- Rogers S.L., Wiedemann U., Stuurman N., Vale R.D. 2003. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J. Cell Biol. 162:1079–1088 10.1083/jcb.200303023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S.L., Wiedemann U., Häcker U., Turck C., Vale R.D. 2004. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol. 14:1827–1833 10.1016/j.cub.2004.09.078 [DOI] [PubMed] [Google Scholar]
- Siegrist S.E., Doe C.Q. 2007. Microtubule-induced cortical cell polarity. Genes Dev. 21:483–496 10.1101/gad.1511207 [DOI] [PubMed] [Google Scholar]
- Small J.V., Geiger B., Kaverina I., Bershadsky A. 2002. How do microtubules guide migrating cells? Nat. Rev. Mol. Cell Biol. 3:957–964 10.1038/nrm971 [DOI] [PubMed] [Google Scholar]
- Sousa A., Reis R., Sampaio P., Sunkel C.E. 2007. The Drosophila CLASP homologue, Mast/Orbit regulates the dynamic behaviour of interphase microtubules by promoting the pause state. Cell Motil. Cytoskeleton. 64:605–620 10.1002/cm.20208 [DOI] [PubMed] [Google Scholar]
- Stramer B., Wood W. 2009. Chemotaxis: inflammation and wound healing in Drosophila embryos. In Chemotaxis: methods and protocols. Methods in Molecular Biology, Vol. 571 Jin T., Hereld D., Humana Press Inc, Totowa, NJ: 137–149 [DOI] [PubMed] [Google Scholar]
- Stramer B., Wood W., Galko M.J., Redd M.J., Jacinto A., Parkhurst S.M., Martin P. 2005. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 168:567–573 10.1083/jcb.200405120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B., Winfield M., Shaw T., Millard T.H., Woolner S., Martin P. 2008. Gene induction following wounding of wild-type versus macrophage-deficient Drosophila embryos. EMBO Rep. 9:465–471 10.1038/embor.2008.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta N., Orso G., Rossetto M.G., Daga A., Broadie K. 2004. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr. Biol. 14:1135–1147 10.1016/j.cub.2004.06.058 [DOI] [PubMed] [Google Scholar]
- van Horck F.P., Ahmadian M.R., Haeusler L.C., Moolenaar W.H., Kranenburg O. 2001. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J. Biol. Chem. 276:4948–4956 10.1074/jbc.M003839200 [DOI] [PubMed] [Google Scholar]
- Williamson T., Gordon-Weeks P.R., Schachner M., Taylor J. 1996. Microtubule reorganization is obligatory for growth cone turning. Proc. Natl. Acad. Sci. USA. 93:15221–15226 10.1073/pnas.93.26.15221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W., Jacinto A. 2007. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat. Rev. Mol. Cell Biol. 8:542–551 10.1038/nrm2202 [DOI] [PubMed] [Google Scholar]
- Wood W., Faria C., Jacinto A. 2006. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell Biol. 173:405–416 10.1083/jcb.200508161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanet J., Stramer B., Millard T., Martin P., Payre F., Plaza S. 2009. Fascin is required for blood cell migration during Drosophila embryogenesis. Development. 136:2557–2565 10.1242/dev.036517 [DOI] [PubMed] [Google Scholar]
- Zhou F.Q., Zhou J., Dedhar S., Wu Y.H., Snider W.D. 2004. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 42:897–912 10.1016/j.neuron.2004.05.011 [DOI] [PubMed] [Google Scholar]
- Zumbrunn J., Kinoshita K., Hyman A.A., Näthke I.S. 2001. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr. Biol. 11:44–49 10.1016/S0960-9822(01)00002-1 [DOI] [PubMed] [Google Scholar]