Abstract
When given challenging episodic memory tasks, young adults demonstrate notable individual differences in performance. Recent evidence suggests that individual differences in human behavior may be related to the strength of functional connectivity of large-scale functional networks as measured by spontaneous fluctuations in regional brain activity during quiet wakefulness (the “resting state”), in the absence of task performance. In this study, we sought to determine whether individual differences in memory performance could be predicted by the interhemispheric functional connectivity of the two hippocampi, hypothesized to reflect the intrinsic connectivity within the large-scale medial temporal lobe memory system. Results demonstrated that interhemispheric hippocampal functional connectivity during quiet wakefulness was predictive of the capacity to freely recall recently learned information (r = 0.47, p < 0.05). In contrast, functional connectivity of bilateral motor cortices had no relationship to free recall, supporting the specificity of the hippocampal data. Thus, individual differences in the capacity to perform episodic memory tasks, which may be persistent behavioral traits or transient states, may be at least partly subserved by individual differences in the functional connectivity of large-scale functional-anatomic memory networks.
Keywords: functional magnetic resonance imaging, hippocampus, episodic memory
Introduction
Episodic memory is a human ability that varies substantially within and between individuals depending on a variety of factors, including the type of material being learned and the conditions under which learning and retrieval are performed (Hultsch et al., 1990; Kirchhoff, 2009). One general class of factors influencing episodic memory performance is genetics, but there has been relatively little investigation of the brain mechanisms of individual differences in episodic memory (Egan et al., 2003). Although functional neuroimaging tools are commonly employed to identify similarities between individuals performing a given memory task, efforts have also been applied toward the investigation of individual differences in memory task performance (Tulving et al., 1999). Typically, this has involved analyses of individual differences in regional brain activity during encoding or retrieval processing (Alkire et al., 1998; Dickerson et al., 2007; Kirchhoff and Buckner, 2006; Nyberg et al., 1996). For example, cerebral blood flow or glucose metabolic rate in the left medial temporal lobe (MTL) during the encoding or retrieval of verbal information correlates with individual differences in performance (Alkire et al., 1998; Nyberg et al., 1996). Prefrontal or occipitotemporal activity relates to individual differences in the use of encoding strategies contributing to memory performance (Kirchhoff and Buckner, 2006). Finally, within-subject differences in memory performance on multiple lists of items relates to prefrontal, hippocampal, and fusiform activity during encoding (Dickerson et al., 2007).
In addition to these studies of individual differences in brain activity during the performance of memory tasks, there is some evidence of brain activity measures that may subserve individual differences in memory performance that are present at times other than during task performance (Desgranges et al., 1998; Eustache et al., 1995). For example, left hippocampal resting oxygen consumption correlates with individual differences in the performance of recall previously learned words (Eustache et al., 1995), suggesting the possibility that physiologic properties of memory circuits may underlie behavioral memory traits, although the stability of such measures over time has yet to be investigated.
Another emerging technique for measuring brain activity during quiet wakefulness is resting-state functional MRI (rs-fMRI), which measures the large-scale covariance of slow spontaneous oscillations of regional brain activity (Biswal et al., 1995; Fox and Raichle, 2007). From these imaging data, the degree of covariance in spontaneous fluctuation of fMRI blood oxygen level-dependent (BOLD) signal, measured as the strength of intrinsic connectivity, between two or more brain regions has been shown to be associated with individual differences in behavior (Di Martino et al., 2009; Fox et al., 2007; Hampson et al., 2006; Seeley et al., 2007). For example, spontaneous fluctuation of the BOLD signal accounts for a significant fraction of the intertrial variability in the force of a button press (Fox et al., 2007). In addition, the degree of functional connectivity (strength of correlation) within specific brain networks has been found to be related to performance on tasks of working memory and executive control (Hampson et al., 2006; Seeley et al., 2007). Collectively, these data suggest that individual differences in human behavior may be subserved at least in part by the strength of functional connectivity between two or more brain regions during quiet wakefulness. These types of relationships are only beginning to be investigated with respect to episodic memory.
In a previous study of the functional neuroanatomy of encoding that leads to successful free recall, we observed that bilateral hippocampal activation was present during encoding for successfully recalled items, and that stronger coupling of the hemodynamic response between bilateral hippocampi during task performance was associated with successful subsequent free recall compared with encoding that did not lead to successful recall (Dickerson et al., 2007).
In the present study, we sought to answer the following question: do individuals with more strongly correlated activity within the episodic memory network during the resting state prior to a task perform better on an episodic memory task than individuals with less strongly correlated activity within the episodic memory network? Because of our previous results demonstrating that the strength of inter-hemispheric hippocampal functional connectivity during a task is associated with better memory performance (Dickerson et al., 2007) and because the spontaneous activity of the hippocampal formation is typically functionally coupled to the contralateral hippocampal formation at rest (Buckner et al., 2008; Greicius et al., 2004; Rombouts et al., 2003), we performed a focused analysis of the strength of correlation between left and right hippocampi. We hypothesized that inter-individual (between-subject) variability in episodic memory performance would be predicted by inter-individual variability of interhemispheric hippocampal intrinsic connectivity during a period of quiet wakefulness prior to the performance of the memory task. In addition, to address the specificity of this relationship (i.e., the question of whether the strength of such correlated activity is a reflection of global factors affecting many brain networks), we investigated interhemispheric functional connectivity of the motor cortex, hypothesizing no relationship to memory performance.
Twenty-six adults (19 women, 7 men, ages 18–35, mean = 23.5) who were right-handed, native English speakers participated this study. Participants were recruited via local advertisement and were paid for their participation. All participants were screened to exclude individuals with a history of neurologic or psychiatric disorders, or those taking medication with central nervous system pharmacologic activity. Informed consent was obtained from each subject. The study was approved by the Partners Healthcare System Human Research Committee.
The resting-state scans were acquired as part of an fMRI session in which participants performed an episodic memory task (task-related fMRI data not described here). A resting-state run was scanned at the beginning of the scanning session, prior to the administration of the memory paradigm. During the resting-state run, participants were scanned for 6 minutes and 20 seconds while they were instructed to relax and remain still with their eyes open.
The encoding and free recall paradigm was modified slightly from a previous version and is described only briefly here (Dickerson et al., 2007). The paradigm consisted of ten lists of pictures of objects from the Snodgrass and Vanderwart corpus. Each list consisted of twelve pictures that were balanced for natural and man-made objects, randomly ordered. During encoding, participants were instructed to press a button to indicate whether each object was “natural” or “man-made,” and to try to learn the item for subsequent memory testing. Each encoding run was followed immediately by a 16-s distractor task during which subjects were instructed to count (out loud) backward by threes. Immediately following the distractor task, the subjects were asked to freely recall the names of as many of the items as possible from the previous list, in any order, in sixty seconds. A word was counted as a free recall “Hit” if it was a specific descriptor of one of the items viewed in the immediately preceding encoding list. The percentage of free recall Hits was calculated as a sum of recalled items from the 10 lists divided by the total number of items (120); this value was used as the primary measure of each subject’s capacity to freely recall recently learned information.
Subjects were scanned using a Siemens Trio 3.0 Tesla scanner (Siemens Medical Systems, Erlingan, Germany) with a twelve-channel head coil. Two runs of high-resolution structural images were obtained with T1-weighted magnetization prepared rapid acquisition gradient echo (MP-RAGE) sequence (repetition time (TR) = 2300ms, echo time (TE) = 2.98ms, flip angle (FA) = 9°, voxel size: 1 × 1 × 1 mm). Resting-state functional images were acquired by using a gradient-echo echo-planar sequence (TR = 5000ms, TE = 30ms, FA = 90). Fifty-five axial slices parallel to the anterior posterior commissure line with 2 × 2 × 2 mm voxel size were acquired in each of the resting-state functional volumes, with 76 whole-brain volumes acquired in the run.
The data were preprocessed using SPM2 (Wellcome Department of Imaging Neuroscience, London, UK). The first three volumes in resting-state data were discarded to allow for T1 equilibration effects. For the remaining functional images, timing differences between slices were removed and then motion correction was applied using the first volume as reference. A 4mm full width at half maximum Gaussian smoothing kernel was applied. The preprocessing provided a record of head motion within resting run, which was later included as a set of nuisance regressors in subsequent correlation analysis. Each subject’s mean functional image was also coregistered to that subject’s structural data, which allowed for the localization of functional data to each individual’s native neuroanatomical space.
Several additional preprocessing steps were carried out to optimize the data for correlation analysis (Fox et al., 2006; Vincent et al., 2006). First, temporal filtering (0.009 Hz < f < 0.08 Hz) was applied to the time series of each voxel to remove low- and high- frequency components of resting fMRI data. Next, distinct source of spurious variance along with their temporal derivatives were further removed from the data by linear regression: (1) six parameters generated from realignment of head motion; (2) the whole brain signal averaged from a subject specific mask region; (3) signal from a ventricular region of interest (ROI) and ROIs located in bilateral deep brain white matter. Regression of each of these signals was performed in a stepwise manner and the residual time course was retained for subsequent computation of correlation strength. For computation of correlation strength between a pair of ROIs of relevance for the study, the time courses were extracted separately from each of the individual ROIs anatomically defined in each subject’s structural scan (in native space) and the Pearson correlation coefficients were computed, then converted to z values using Fisher’s transformation for subsequent statistical analyses.
The structural MRI data were processed using the fully automated standard processing stream in Freesurfer (http://surfer.nmr.mgh.harvard.edu) to generate anatomic ROIs (Dale et al., 1999; Fischl et al., 1999a; Fischl et al., 1999b). For each subject, the left and right hippocampi (including hippocampus proper, dentate gyrus, and subiculum for each hippocampus) were identified using the automated segmentation algorithm that examines variations in voxel intensities and spatial relationships to classify subcortical regions (Fischl et al., 2002), and ROIs were manually inspected to ensure accuracy. In this study, no editing of hippocampal ROIs was required. Since in some samples of subjects (usually older subjects or those with neurologic or psychiatric disorders) hippocampal volume relates to memory performance, we also examined this relationship although there was none expected. The volumes of the left and right hippocampi were also calculated and divided by intracranial volume to adjust for head size. A segmentation of the left and right hippocampi in a single subject is illustrated in Figure 1. The left and right motor cortices were manually labeled on the Freesurfer average surface on the precentral gyrus in the region of the omega-shaped convolution typically associated with hand movement (Boling et al., 2008). These two spherical atlas-space labels were converted to individual spherical space label by using a surface registration method (Fischl et al., 1999b), which were further converted to individual volume space ROIs for application to the functional data.
Figure 1.
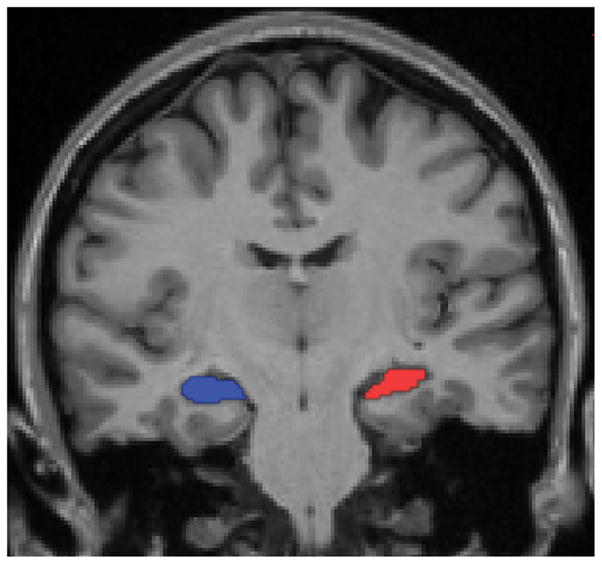
This T1-weighted coronal MRI image displays the regions of interests (ROIs) for the left (red) and right (blue) hippocampi in a representative subject. The hippocampal ROIs were automatically segmented from each individual subject’s structural MRI data and visually inspected to verify accuracy. Functional data were extracted from these ROIs at the individual subject level in native space for analysis.
To test the a priori hypothesis of this study, simple linear correlations were performed to investigate relationships between the percentage of free recall Hits and the strength of interhemispheric hippocampal intrinsic functional connectivity, as well as the strength of intrinsic connectivity between left and right motor cortices. To determine statistically whether the hypothesized hippocampal-memory correlation is stronger than the motor-memory correlation, we used a procedure comparing two correlations sharing one variable, described by Steiger (1980) as:
where rjk denotes the hippocampal-memory correlation, and rjh denotes the motor-memory correlation, and rkh denotes the correlation between interhemispheric functional connectivity of bilateral hippocampi and that of bilateral motor cortices, and |R| = (1 − rjk2 − rjh2 − rkh2) + rjk (rh2rkh), and , and . The T2 has a t distribution with df = N − 3. The statistical analyses were performed using SPSS 11.0 (Chicago, IL), and results were considered statistically significant if p < 0.05.
The percentage of items freely recalled during the task was 62% ± 10% (mean ±SD), ranging from 42% to 80%, demonstrating notable inter-individual variability in these young adults. The strength (correlation coefficient “r”) of intrinsic connectivity between the hippocampi in the two hemispheres was 0.77 ± 0.09, ranging from 0.49 to 0.89, again illustrating the substantial inter-individual variability that can be found in these measures. Most importantly, the strength of interhemispheric hippocampal intrinsic connectivity predicted the capacity to freely recall recently learned information (r = 0.47, p < 0.05) (Figure 2). Time courses of spontaneous activity within the two hippocampi for two subjects are shown in Figure 3, showing that the best performer has higher interhemispheric intrinsic hippocampal coupling than the worst performer.
Figure 2.

Group data demonstrating the relationship between individual differences in episodic memory performance and individual differences in correlated spontaneous hippocampal activity. The z-transformed correlation coefficients of bilateral hippocampal spontaneous activity (x axis) are plotted against free recall performance on this episodic memory task (percentage of free recall Hits, y axis). The strength of intrinsic hippocampal connectivity prior to the performance of the task predicts episodic memory performance on the task, r = 0.47, p < 0.05. The subjects with the best and the poorest performance are highlighted with a thick and a thin arrow, respectively; BOLD time course data for these two individuals is shown in Fig. 3.
Figure 3.
Illustration of time courses of spontaneous fluctuations in hippocampal activity in two individual participants, one with high memory performance and one with low memory performance. The time courses of spontaneous blood oxygen level dependent (BOLD) signal were extracted from left (red) and right (blue) hippocampal ROIs in the subject who had the best free recall performance on this episodic memory task (A, marked with thick arrow in Fig. 2), and in the subject who had the poorest performance on this memory task (B, marked with thin arrow in Fig. 2). The best performer has spontaneous physiologic fluctuations that are highly correlated between bilateral hippocampi (r value of 0.76) while the worst performer has a somewhat lower correlation of activity between the two hippocampi (r value of 0.66).
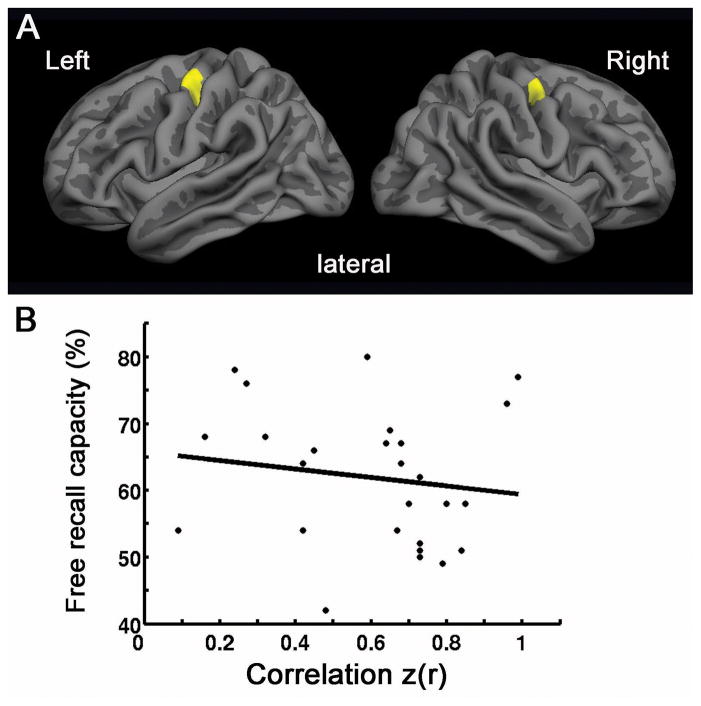
The location of the ROIs labeling bilateral motor areas on the average surface is shown in Figure 4A. The strength (r) of intrinsic connectivity between the motor cortices in the two hemispheres was 0.52 ± 0.18, ranging from 0.09 to 0.76. The variability of intrinsic connectivity between interhemispheric motor cortices did not predict free recall performance capacity (p = 0.4) (Figure 4B). The interhemispheric hippocampal functional connectivity was significantly more strongly correlated with free recall performance than interhemispheric functional connectivity between bilateral motor cortices (t = 2.53, p < 0.05).
Figure 4.
The interhemispheric functional connectivity between motor cortices chosen as control ROIs for this analysis show no relationship between spontaneous activity and free recall performance. A) Motor cortex ROIs (shown in yellow) were manually labeled on the approximate hand areas of the left and right precentral gyri. Labels are shown on the semi-inflated cortical surface of the Freesurfer average brain with light gray regions representing gyri and dark grey regions representing sulci. B) The strength of correlation of spontaneous fluctuations in motor cortical activity (z-transformed correlation coefficients, x axis) are plotted against free recall performance (y axis). Strength of correlation between bilateral motor cortical ROIs does not predict episodic memory performance (p = 0.4).
We also found no relationship between free recall performance and the adjusted left or right hippocampal volumes (both p values > 0.4).
Despite observations that healthy young adults vary substantially in their ability to perform challenging episodic memory tasks, few functional neuroimaging studies of episodic memory have investigated inter-individual differences in brain activity in memory tasks. Many of the previous functional neuroimaging studies that have investigated inter- or intra-individual differences in memory performance have done so using functional neuroimaging data collected during the performance of the task of interest (Alkire et al., 1998; Dickerson et al., 2007; Fernandez et al., 1998; Kirchhoff and Buckner, 2006; Nyberg et al., 1996). Only a few studies have investigated the relationship of functional brain activity “at rest” and episodic memory performance (Desgranges et al., 1998; Eustache et al., 1995; Wig et al., 2008). None of these studies have focused on “resting state” or intrinsic functional connectivity of nodes within the episodic memory circuit.
In this study, we found that the strength of functional connectivity between bilateral hippocampi measured during a six-minute period of quiet wakefulness prior to the performance of an episodic memory task predicts how well individuals will perform on that task. Although it is possible that the differences between individuals in hippocampal functional connectivity observed here are a reflection of global inter-individual differences across multiple large-scale brain networks in the coupling of low-frequency spontaneous fluctuations, the present finding that bilateral motor cortical connectivity does not predict memory performance mitigates this concern. That is, a region of the precentral gyrus in the vicinity of the hand motor area is strongly functionally coupled with the homologous region in the contralateral hemisphere. Yet the variation in this coupling between individuals, which is substantial, shows no relationship with memory performance. This finding supports the hypothesis that the hippocampal connectivity-memory performance relationship is specific to the episodic memory network.
Differences between individuals in the activity of the hippocampus and adjacent medial temporal cortices during memory task performance relates to individual differences in performance on the task. For example, left MTL blood flow during retrieval correlated with performance on a recognition memory test (Nyberg et al., 1996); left hippocampual metabolic rate during encoding correlated with free verbal recall performance (Alkire et al., 1998); hippocampal BOLD signal during encoding was correlated with the number of words recalled (Fernandez et al., 1998). Intra-individual variance in memory performance also relates to hippocampal activation. When individuals encoded multiple short word lists, greater differential hippocampal activity during encoding of items that were later recalled compared with encoding of those not recalled was associated with better recall performance for a given list (Dickerson et al., 2007). The present finding extends these results by demonstrating that individual differences in memory performance can be predicted not only by differences in activity during performance of the task but also by differences in activity during quiet wakefulness prior to task performance, suggesting that a state-related (or possibly trait-related) property of the MTL memory system prior to a task influences performance on the task.
Several previous blood flow or metabolism studies identified relationships between these resting state physiologic measures and memory task performance. For example, resting oxygen consumption in the left hippocampus was correlated with individual differences in the performance of recall previously learned words (Eustache et al., 1995); resting cerebral glucose utilization in the medial temporal cortices in a group of patients with Alzheimer’s disease correlated with the performance of story recall (Desgranges et al., 1998). The present finding extends these results by showing that individual differences in the level of functional connectivity in spontaneous fluctuations in brain activity, which are thought to reflect functional-anatomic connectivity of specific cerebral networks (Vincent et al., 2007), can predict individual differences in memory task performance.
The anatomic basis of interhemispheric hippocampal connectivity is likely the dorsal hippocampal commissure, which includes fibers originating in presubiculum, entorhinal cortex, and posterior parahippocampal cortex and terminating predominantly on contralateral entorhinal cortex (Gloor et al., 1993).
Although the functional connectivity identified between brain regions using the method described here is thought to relate in part to direct or indirect synaptic connections (Vincent et al., 2007), it is also possible that ongoing mental activity during the resting state immediately prior to a task contributes to performance on the task. Previous fMRI studies have shown that not only is hippocampal activity higher during resting state than during the performance of simple cognitive task (Stark and Squire, 2001), but more importantly that the magnitude of a task-induced decrease in hippocampal activity during simple tasks predicts inter-individual differences in mnemonic ability (Wig et al., 2008). The present data may be interpreted as consistent with individual differences either in relatively static (possibly functional-anatomic) traits of the MTL memory system or in potentially dynamic states that may be present immediately prior to a task but not necessarily stable over time, an issue which deserves further study.
Previous studies have related differences within and between individuals in a variety of behaviors to the variance in functional connectivity in brain networks thought to subserve those behaviors. In the first such study, the within-subject trial-to-trial spontaneous variability in the force of a right-handed button press was predicted by similar variability in task-evoked hemodynamic response in the left motor cortex, which was strongly related to the degree to which low-frequency spontaneous fluctuations in BOLD signal in right motor cortex was correlated with such activity in the left motor cortex (Fox et al., 2007). In a commentary on this article, the point was made that there could be many artifactual factors that may account for such findings, although most of them had been adequately controlled for in the study, but more importantly that it was not yet clear whether such resting state functional connectivity measures would relate to more complex behaviors (Birn, 2007). Since then, several studies have reported clear relationships between the degree of resting-state functional connectivity of spatially-distributed networks and inter-individual differences in complex behaviors (Di Martino et al., 2009; Hampson et al., 2006; Seeley et al., 2007). For example, individual differences in the coherence of spontaneous activity within the frontoinsular-anterior cingulate “salience” network was associated with differences between subjects in prescan anxiety ratings, while connectivity of the frontoparietal executive control network was associated with set-shifting performance on the Trail Making Test (Seeley et al., 2007). The spontaneous correlation between the posterior cingulate cortex and medial prefrontal/ventral anterior cingulate cortices is relevant to individual differences in working memory performance (Hampson et al., 2006); between pregenual anterior cingulate cortex and anterior mid-insula is relevant to autistic traits in young adults (Di Martino et al., 2009). The data reported here extend these observations to episodic memory. In addition, given that interhemispheric hippocampal connectivity constitutes a subsystem in default network (Buckner et al., 2008; Vincent et al., 2006), these findings support the hypothesis that one of the functions default network subserves is episodic memory processing (Buckner et al., 2008; Greicius et al., 2003). Further investigations will be needed to determine the degree to which the functional connectivity between other nodes of the default mode network, or other networks, contribute to episodic memory performance.
Acknowledgments
The authors thank Mary Foley and Larry White for technical assistance. This study was supported by grants from the NIA R01-AG29411, R21-AG29840, P50-AG05134, NINDS R01-NS042861, NCRR P41-RR14075, U24-RR021382, the Alzheimer’s Association, and the Mental Illness and Neuroscience Discovery (MIND) Institute.
References
- Alkire MT, Haier RJ, Fallon JH, Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proc Natl Acad Sci U S A. 1998;95(24):14506–10. doi: 10.1073/pnas.95.24.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM. The behavioral significance of spontaneous fluctuations in brain activity. Neuron. 2007;56(1):8–9. doi: 10.1016/j.neuron.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boling W, Parsons M, Kraszpulski M, Cantrell C, Puce A. Whole-hand sensorimotor area: cortical stimulation localization and correlation with functional magnetic resonance imaging. J Neurosurg. 2008;108(3):491–500. doi: 10.3171/JNS/2008/108/3/0491. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, de la Sayette V, Petit-Taboue MC, Benali K, Landeau B, Lechevalier B, Eustache F. The neural substrates of memory systems impairment in Alzheimer’s disease. A PET study of resting brain glucose utilization. Brain. 1998;121 (Pt 4):611–31. doi: 10.1093/brain/121.4.611. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166(8):891–9. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, Sperling RA. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: an event-related functional-anatomic MRI study. Hippocampus. 2007;17(11):1060–70. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eustache F, Rioux P, Desgranges B, Marchal G, Petit-Taboue MC, Dary M, Lechevalier B, Baron JC. Healthy aging, memory subsystems and regional cerebral oxygen consumption. Neuropsychologia. 1995;33(7):867–87. doi: 10.1016/0028-3932(95)00021-t. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weyerts H, Schrader-Bolsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, et al. Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci. 1998;18(5):1841–7. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8(4):272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56(1):171–84. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Gloor P, Salanova V, Olivier A, Quesney LF. The human dorsal hippocampal commissure. An anatomically identifiable and functional pathway. Brain. 1993;116 (Pt 5):1249–73. doi: 10.1093/brain/116.5.1249. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychol Aging. 1990;5(3):356–68. doi: 10.1037//0882-7974.5.3.356. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA. Individual differences in episodic memory: the role of self-initiated encoding strategies. Neuroscientist. 2009;15(2):166–79. doi: 10.1177/1073858408329507. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51(2):263–74. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Houle S, Nilsson LG, Tulving E. Activation of medial temporal structures during episodic memory retrieval. Nature. 1996;380(6576):715–7. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20(2):1236–45. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98(22):12760–6. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87(2):245–251. [Google Scholar]
- Tulving E, Habib R, Nyberg L, Lepage M, McIntosh AR. Positron emission tomography correlations in and beyond medial temporal lobes. Hippocampus. 1999;9(1):71–82. doi: 10.1002/(SICI)1098-1063(1999)9:1<71::AID-HIPO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–31. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Wolford GL, Petersen SE, Kelley WM. Medial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adults. Proc Natl Acad Sci U S A. 2008;105(47):18555–60. doi: 10.1073/pnas.0804546105. [DOI] [PMC free article] [PubMed] [Google Scholar]