Abstract
The main receptors for amyloid-beta peptide (Aβ) transport across the blood-brain barrier (BBB) from brain to blood and blood to brain are low-density lipoprotein receptor related protein-1 (LRP1) and receptor for advanced glycation end products (RAGE), respectively. In normal human plasma a soluble form of LRP1 (sLRP1) is a major endogenous brain Aβ ‘sinker’ that sequesters some 70 to 90 % of plasma Aβ peptides. In Alzheimer’s disease (AD), the levels of sLRP1 and its capacity to bind Aβ are reduced which increases free Aβ fraction in plasma. This in turn may increase brain Aβ burden through decreased Aβ efflux and/or increased Aβ influx across the BBB. In Aβ immunotherapy, anti-Aβ antibody sequestration of plasma Aβ enhances the peripheral Aβ ‘sink action’. However, in contrast to endogenous sLRP1 which does not penetrate the BBB, some anti-Aβ antibodies may slowly enter the brain which reduces the effectiveness of their sink action and may contribute to neuroinflammation and intracerebral hemorrhage. Anti-Aβ antibody/Aβ immune complexes are rapidly cleared from brain to blood via FcRn (neonatal Fc receptor) across the BBB. In a mouse model of AD, restoring plasma sLRP1 with recombinant LRP-IV cluster reduces brain Aβ burden and improves functional changes in cerebral blood flow (CBF) and behavioral responses, without causing neuroinflammation and/or hemorrhage. The C-terminal sequence of Aβ is required for its direct interaction with sLRP and LRP-IV cluster which is completely blocked by the receptor-associated protein (RAP) that does not directly bind Aβ. Therapies to increase LRP1 expression or reduce RAGE activity at the BBB and/or restore the peripheral Aβ ‘sink’ action, hold potential to reduce brain Aβ and inflammation, and improve CBF and functional recovery in AD models, and by extension in AD patients.
Keywords: low-density lipoprotein receptor related protein-1, receptor for advanced glycation end products, Fc neonatal receptor, blood-brain barrier, cerebrovascular, Alzheimer’s disease
Introduction
Alois Alzheimer, over 100 years ago, first described the symptoms, the presence of tangles in brain and extracellular deposits of a substance in the brain and blood vessels of his patient Auguste D, for the disease that is now associated with his name, Alzheimer’s disease (AD) [1]. This is a debilitating disease that affects about 5.2 million people in the US [2]. Aging is a major risk factor, and with increasing longevity by 2050 the incidence of AD will increase by about 3 fold [2]. Despite extensive research there is no treatment that alters the biological progression of the disease. However, we now understand that the brain deposits in AD are caused by progressive oligomerization of amyloid β-peptides (Aβ) to form oligomers, protofibrils and fibrils, and that these Aβ species contribute to neurotoxicity [3-5].
The relative levels and distribution of Aβ species in brain may influence the disease progression. This led to the ‘amyloid hypothesis’, as a possible explanation for the development of AD, in which Aβ is central to AD pathology [6-13]. A small number (<1%) of AD cases, familial AD (early-onset), is linked to genetic mutations which are associated with increased Aβ production [7, 14]. The cause of the majority of AD cases, sporadic (late-onset), may be due to faulty clearance of Aβ from brain [11, 13, 15, 16]. In this new concept, dementia in AD is associated with cerebrovascular disorder [13, 17-20], which leads to accumulation of Aβ on blood vessels (cerebral amyloid angiapothy, CAA) and in the brain parenchyma, extracellular deposits [9, 13, 21, 22], and intraneuronal lesions - neurofibrillar tangles [23].
In the interstitial fluid (ISF) of normal brain, Aβ concentration is rigorously regulated by its rate of production from the Aβ-precursor protein (APP), influx into the brain across the blood-brain barrier (BBB) mainly via receptor for advanced glycation end products (RAGE) [24] and by its rapid clearance across the BBB via low-density lipoprotein receptor related protein-1 (LRP1) [25-27] (Figure 1), and enzymatic degradation within brain [6]. Brain endothelial expression of RAGE is increased in AD mouse models and in AD patients [24, 28-30] whereas LRP expression at the BBB is reduced [25, 26, 29], thus making it unfavorable for Aβ clearance from brain. This in turn may lead to Aβ accumulation in brain and its gradual oligomerization and greater levels of neurotoxic Aβ oligomers [3-5]. Thus, continuous removal of Aß species from the brain by transport across the BBB and/or metabolism is essential to prevent their potentially neurotoxic accumulations in brain [31].
Figure 1. Schematic diagram showing the blood and brain compartments, and the roles of the cell surface receptors LRP1 and RAGE, and FcRn and soluble LRP (sLRP) in the regulation of Aβ transport across the blood-brain barrier (BBB).
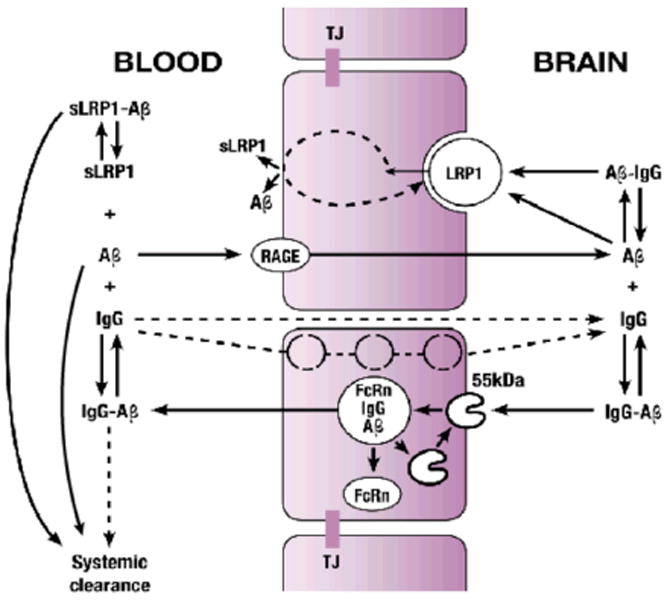
See text for details. RAGE (receptor for advanced glycation end products), LRP1 (low-density lipoprotein receptor related protein 1), FcRn (neonatal fragment crystalline (Fc) receptor) and TJ (tight junctions between cerebrovascular endothelial cells).
Transport of Aβ across the BBB
The mammalian brain is separated from blood by the BBB localized to the brain capillaries and pia-subarachnoid membranes and the blood-cerebrospinal fluid (CSF) barrier localized to the choriod plexi. The physical sites of these barriers are tight junctions between brain endothelial cells (Figure 1) and epithelial cells, respectively [13, 32, 33]. There are no effective barriers to diffusion of molecules between brain ISF and CSF. While the vascular barriers restrict the transport of polar solutes, rapid transport of essential hydrophobic nutrients, such as glucose and amino acids, and peptides and proteins involves specific transporter systems and/or receptor-mediated transport, respectively [13]. Using two-photon microscopy it was recently shown that mouse parenchymal neuronal dendrites are within about 13 μm of a capillary [34], while these cells are considerably further away from CSF, especially in adult animals with large brains [32]. Therefore, the BBB plays a key role in controlling the composition of brain ISF.
Aß are small peptides (~ 4.5 kDa) and the most common isoforms are Aβ40 and Aβ42. Aβ peptides are produced by many cells, and circulate in plasma, CSF and brain ISF mainly bound to chaperone molecules in equilibrium with a small free unbound Aβ fraction [13]. In normal human CSF and plasma, Aβ40 levels are greater than that of Aβ42 by about 10- and 1.5-fold, respectively [35]. In vitro studies have shown that a number of transport proteins, such as albumin, apolipoprotein E (apoE), apolipoprotein J (apoJ), transthyretin (TTR), and α2-macroglobulin (α2M) bind Aβ [36-41]. However, in human plasma, a soluble form of LRP1, sLRP1, is a major binding protein for circulating Aβ [42]. Human sLRP1 sequesters some 70 to 90 % of plasma Aβ [42]. Using ELISA, we have shown that human sLRP1 binds the C-terminal end of Aβ, and that the interaction between sLRP1 and Aβ is completely blocked by RAP (Figure 2A).
Figure 2. Aβ binds to human plasma derived sLRP and human recombinant LRP-IV cluster but not to RAP using ELISA.
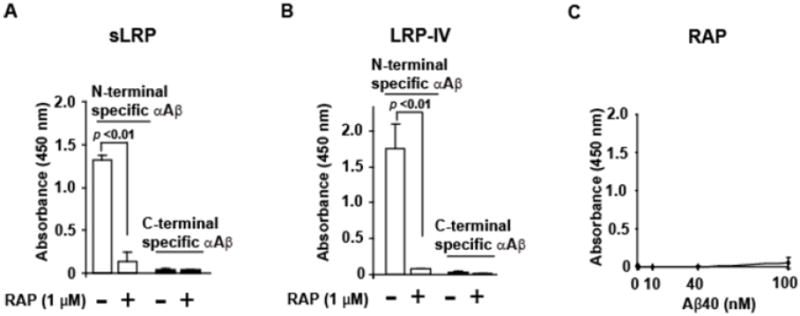
Briefly, 10 μg/ml human sLRP, recombinant LRP-IV or RAP were coated on microtiter plates and blocked with protein-free blocking buffer (Pierce 37570). For sLRP and LRP-IV, 100 nM Aβ40 was added and incubated in HBSC, pH 7.4, for 2 hours at room temperature. For RAP, various Aβ40 concentrations (0, 1, 40, 1 and 100 nM) were used. After washing with HBSC containing 0.05% Tween-20, the N-terminal specific (Cell Signaling Cat # 2454, 1μg/ml) or C-terminal specific (BA27, WAKO ELISA kit) primary antibodies were added and incubated overnight. The secondary antibody for the N-terminal anti-Aβ antibody was goat anti-rabbit (Dako; 1:2000), while the C-terminal specific primary antibody was already HRP-conjugated. The reaction was developed with 3,3′5,5′ tetramethlbenzidine (TMB; KPL,Gaithersburg, MD) and stopped with 1M HCl. Absorbance was read at 450 nm. Aβ bound to immobilized human plasma derived sLRP (A) or immobilized recombinant human LRP-IV cluster (B) in the absence or presence of RAP (1 μM) was detected using an N-terminal specific (white bars) or a C-terminal specific (black bar) anti-Aβ antibody (αAβ). C, No significant binding of monomeric Aβ40 to RAP was detected using the N-terminal specific anti-Aβ antibody. In A-C, values are mean + standard error of the mean, n=3 for each group. Pairs of groups were statistically analyzed using students t-test. These are previously unpublished data from the Zlokovic laboratory.
In CSF, apoJ, apoE, TTR and α2M can bind Aβ, and influence its clearance, metabolism and aggregation [11, 21, 43-46]. In mice, apoJ increases the BBB clearance of Aβ42, the most toxic Aβ species [44]. On the other hand, apoE disrupts the rapid LRP1-dependent clearance of free monomeric Aβ across the mouse BBB, in an isoform specific manner (apoE4>apoE3 or apoE2), by redirecting Aβ transport from LRP1 to very low density lipoprotein receptor (VLDLR) which internalizes Aβ-apoE complexes at a slower rate than LRP1 [21]. TTR increases total brain and vascular Aβ in Tg2576 mice, a model of AD [45]. In human CSF, lipocalin-type prostaclandin D synthase/β-trace appears to be another Aβ binding agent [47].
The major clearance transport mechanism of free monomeric Aβ is transcytosis across the BBB which is mediated mainly by the cell surface LRP1 localized predominantly on the abluminal side of the cerebral endothelium [25, 26]. A relatively minor transport pathway under physiological conditions is by a bulk flow of the ISF into CSF through the perivascular Virchow-Robin arterial spaces, which is followed by drainage into the blood across the arachnoid villi. In normal mice, this pathway is responsible for about 10-15% of total Aβ clearance [25,48]. Degradation of free Aβ in brain ISF has been reported to be insignificant [21, 25, 26].
i. RAGE: Transport of Aβ into brain across the BBB
Circulating Aβ enters brain in a variety of species including guinea-pigs, mice and monkeys mainly by a specific receptor-mediated transport mechanism that is dependent on RAGE expression on the luminal surface of brain vessels [24, 49-56]. Similar specific receptor-mediated transport mechanisms exist for other peptides and proteins, including arginine vasopressin [57], leu-enkephalin [58, 59], apoE [37], apoJ [39], activated protein C [60] and immunoglobulin G (IgG) [61]. Aβ transport into brain is about 5-fold lower than that of tyrosine, an essential amino acid, that is transported rapidly across the BBB or the choroid plexus [62-64].
RAGE, a multiligand receptor in the immunoglobulin superfamily, binds a number of ligands including Aβ [28, 65-67]. RAGE expression is determined by the levels of its ligands. When pathogenic Aβ species accumulate in AD brain, RAGE expression increases in affected cerebral vessels, neurons or microglia [28], or in transgenic models of β-amyloidosis and in human brain [24, 28-30]. This mechanism provides the potential for exacerbating cellular dysfunction due to RAGE-Aβ interactions. Soluble Aβ binds RAGE in the nanomolar range, and mediates its pathophysiologic cellular responses [24, 28]. RAGE/Aβ interaction is implicated in the development of Alzheimer’s neurovascular disorder by mediating transcytosis of circulating Aβ across the BBB, inflammatory responses in endothelium, brain endothelial NF-κB-dependent apoptosis and suppression of cerebral blood flow (CBF) [24, 28]. In addition, RAGE mediates Aβ-induced migration of monocytes across the human brain endothelial cell monolayers [68].
While Aß/apoJ complex is transported into brain via LRP2 [43], this process is normally saturated by the high levels of plasma apoJ and precludes significant influx of Aβ into the CNS via this route [69].
Thus RAGE, a major Aβ influx receptor at the BBB, is a potential target for therapies to lower brain Aβ burden, reduce neuroinflammation, and improve CBF and behavioral performance. Currently, a compound, PF04494700 (TTP488), which blocks RAGE/Aβ interaction, is in a Phase 2 Clinical Trial in patients with mild to moderate AD [70]. We have shown that some tertiary amides, selected by a drug screening process, block Aβ interaction with RAGE on RAGE-transfected Chinese Hamster Ovary cells which prevents oxidative stress [71]. In a mouse model of AD, these tertiary amides block Aβ transport into brain across the BBB, reduce brain Aβ burden and oxidative stress, and improve functional changes in CBF and performance in behavioral tests [71, 72]. By extension, these new compounds could be developed as potential new therapies for AD and other RAGE-related disorders.
ii. LRP1: Transport of Aβ from brain across the BBB
LRP1, a multifunctional scavenger and signaling receptor, is a member of the LDL receptor family [73, 74]. It plays a major role in the transport and metabolism of cholesterol associated with apoE-containing lipoproteins. LRP1 is synthesized as a single polypeptide precursor (600 kDa) that is processed into α and β chains [75]. The heavy α-chain of LRP1 (515 kDa), the extracellular domain, is noncovalently coupled to the 85 kDa transmembrane and cytoplasmic light β-chain domain. The α-chain contains four ligand-binding domains (clusters I-IV), consisting of 2, 8, 10, and 11 cysteine-rich complement-type repeats, respectively [76, 77]. LRP1 binds a diverse array (~ 40) of unrelated ligands, such as, apoE, α2M, tissue plasminogen activator (tPA), proteinase-inhibitors, blood coagulation factors (factor VIII) and Aβ [26, 73]. Clusters II and IV are the main ligand binding regions [44, 49]. LRP1 mediates transcytosis of Aβ and tPA across the BBB [25, 26, 78].
The cytoplasmic tail contains two NPXY, one YXXL motifs and two di-leucine motifs [79]. The YXXL motif and distal di-leucine repeat may be associated with the rapid LRP1 endocytotic rate (<0.5 seconds) [22, 26,79]. In addition, the cytoplasmic domain interacts with adaptor proteins, such as disabled-1, FE65 and PSD-95, associated with cell signaling [80,81]. The cytoplasmic tail can be phosphorylated on serine and tyrosine [82, 83]. Thus, LRP1 has dual roles as a rapid cargo transporter and transmembrane cell signaling receptor.
LRP1 has been linked to AD and CAA [23, 84-87], although some studies did not confirm the genetic link [88]. LRP1 and its ligands have been detected in senile plaque [89, 90]. LRP1 influences the processing of APP via interaction of their cytoplasmic C-terminal domains, which requires FE67, an adaptor protein [91]. Recently, it was shown that intracellular interaction between LRP1 and APP also occurs early in the secretory process and that this requires the C-terminal of LRP1 [92]. LRP1 expressed in neurons may regulate Aβ cellular uptake within brain via LRP1 ligands α2M and apoE [41, 93, 94]. However, LRP1 expression on neurons might not mediate Aβ clearance in vivo since soluble brain Aβ levels were increased in APP mice overexpressing functional LRP1 mini-receptors on neurons [95,96].
LRP1 expression in brain capillary endothelium is reduced during normal aging in rodents, non-human primates, and in AD [25, 26, 29, 97]. Since LRP1 is the main receptor for Aβ transport across the BBB in the direction of brain to blood, it’s down regulation in brain endothelium in AD and in patients with the Dutch-type of cerebrovascular β-amyloidosis will reduce Aβ clearance and promote Aβ cerebrovasccular and brain focal accumulations. Binding of Aβ to LRP1 at the abluminal side of the BBB in vivo initiates a rapid Aβ clearance via transcytosis across the BBB into blood in mice and rats [21, 25, 26, 42, 44, 98]. Human Aβ injected into different brain regions was found intact in murine plasma, confirming its vascular clearance [44, 99]. This demonstrates rapid transcytosis of intact monomeric Aβ across the BBB, from brain ISF into blood.
The affinity of Aβ40 for LRP1 is less than that of Aβ peptides with a greater β-sheet content, such as Aβ42 and the vasculotropic mutant Dutch/Iowa (DI) Aβ40 [26]. Consequently, compared to Aβ40, the Aβ peptides with higher β-sheet content are cleared less efficiently from brain. Similarly, the mutant Dutch Aβ, containing a Glu to Gln substitution on Aβ, is also cleared less efficiently from CSF to blood [100]. This may explain why the transgenic Tg DI/Swe mice (Dutch, Iowa and Swedish mutations, Thy-1 APP DI/Swe mice) develop robust Aβ brain accumulation much earlier than Tg2576 Aβ-overproducing mice despite extremely low levels of human APP in brain and low Aβ production from neurons [26, 101]. Thus, faulty vascular Aβ clearance may significantly contribute to Aβ accumulation in brain and to AD pathogenesis.
We have shown that both recombinant LRP-II and LRP-IV avidly bind free Aβ40 and Aβ42 in vitro by surface plasmon resonance analysis [26] and by ELISA using the N-terminal specific anti-Aβ antibody [42, 44]. However, the C-terminal specific antibodies, (BA27, Takeda Pharmaceutical Co., Ltd.) did not detect Aβ bound to immobilized LRP-IV (Figure 2B), as reported [102]. RAP completely displaces Aβ40 binding to immobilized LRP-IV cluster (Figure 2B), as reported [102], but does not interact with Aβ (Figure 2C). RAP, an intracellular ER (endoplasmic reticulum) molecular chaperone for the LDL receptor family, binds to LRP1 with high affinity, and not only prevents premature ligand binding to intracellular LRP1 but also is required for proper LRP1 folding [103]. Since the N-terminal specific, but not C-terminal specific, anti-Aβ antibody detected Aβ bound to LRP-IV it is conceivable that the C-terminal sequence of Aβ binds to LRP-IV.
In vitro studies, using an epithelial monolyear transport model, Madin-Darby Canine Kidney (MDCK) cells, transfected with LRP1 mini-receptor containing cluster IV (mLRP4) on the basolateral membrane, confirms LRP1 Aβ endocytosis at the abluminal side of the BBB which is then followed by Aβ degradation [104]. In contrast to in vivo data showing a role of P-glycoprotein (Pg-P) in the clearance of Aβ from brain [97], Pg-P was not involved in Aβ transport across the MDCK monolayer [104]. It is possible that the MDCK monolayers may lack the unique properties of brain endothelial monolayers which might account for lack of Aβ transcytosis. It is also possible that the Aβ degradation observed in MDCK monolayers over longer periods of time is compatible with systemic clearance of Aβ via kidneys [42 ]. Recently, it has been confirmed that a conditional immortalized endothelial cell line from rat brain (TR-BBB cell) binds and rapidly internalizes free Aβ via LRP1 [102].
Other lipoprotein receptors, such as low-density lipoprotein receptor and VLDLR appear to have no major role in transport of free monomeric Aβ across the BBB, into blood [26], but their role in transporting Aβ bound to its chaperone proteins, apoE and apoJ, are becoming clearer [21, 44]. Aβ clearance may be influenced by apoE , apoJ and α2M, known ligands for LRP, but formation of Aβ complexes with either of those ligands has not been shown in the CNS in vivo during relatively rapid clearance studies [25,26]. ApoE and Aβ/apoE complexes are slowly cleared from brain compared to Aβ [21, 46].
Vascular-restricted genes in the control of LRP1 expression in cerebral vessels
Transcription profiling of human brain endothelial cells indicated that a subset of age-independent genes is altered in AD compared to age-matched controls [105]. Expression of mesenchyme homebox gene 2 (MEOX2) also known as growth arrest-specific homeobox (GAX), which is restricted to the cardiovascular system in adults, is greatly reduced at the BBB in AD compared to age-matched controls, and this is associated with downregulation of LRP1 and Aβ brain accumulation, cerebral hypoperfusion and aberrant angiogenesis and vascular remodeling [105]. In vascular smooth muscle cells (VSMC) from small cerebral arteries serum response factor (SRF) and myocardin (MYOCD), two interrelated transcription factors that control VSMC differentiation, are upregulated in AD. This produces a hypercontractile phenotype in small cerebral arteries through SRF/MYOCD-directed expression of genes regulating several contractile proteins and genes that regulate calcium homeostasis and are involved in the regulation of smooth muscles contraction that reduces CBF [106]. Recently, it was shown that SRF/MYOCD overexpression in VSMC transcriptionally downregulates LRP1 through SRF/MYOCD-directed expression of sterol regulatory element binding protein-2 (SREBP2), which in turn reduces the cell surface LRP1 expression and Aβ clearance by VSMC in AD, as well as the degree of CAA and focal parenchymal brain Aβ accumulations in mouse models of AD and AD patients [22]. These two genes, MEOX2 and SRF/MYOCD and/or their protein products are potential targets for therapeutic development for AD and CAA.
Systemic Aβ clearance
Aβ systemic clearance is reduced with age in squirrel monkeys, and this is associated with enhanced Aβ deposition in brain [52,54]. Age-dependent reduction in systemic Aβ clearance may reduce the ‘sink action’ for Aβ clearance from brain and/or increase RAGE-dependent free Aβ transport across the BBB into the brain. The rapid peripheral clearance of Aβ is mediated mainly by hepatic LRP1, and this is blocked by RAP [107]. Reduced hepatic LRP1 levels are associated with decreased peripheral Aβ clearance in aged rats [107]. Insulin increases LRP1 levels in hepatic plasma membrane, and this in turn enhances peripheral Aβ clearance, which is completely blocked by RAP [108]. While the role of insulin in type II diabetes mellitus related vascular dementia is still unclear, it is possible that faulty LRP1-mediated hepatic Aβ clearance may contribute to Aβ accumulation in brain.
Transport of Aβ across the BBB during Aβ immunotherapy
The role of immunotherapy in AD has been extensively reviewed recently [109,110]. Since the accumulation of Aβ is generally believed to play a causative role in AD, a number of therapies are being developed to reduce formation or increase clearance of Aβ from brain [111]. Immunotherapy, active and/or passive, has been shown to be effective in reducing brain Aβ burden in mouse models of AD [112-120], normal aged dogs [121] and monkeys [122,123]. In contrast to mouse models of AD, in the aged dogs there was no significant improvement in cognitive function after Aβ immunization [121]. While Aβ immunization has been shown to have a number of beneficial effects in models of AD, there may be some unwanted side effects, such as increased CAA, increased T lymphocytes infiltration in brain and leucoencephalopathy [110]. In addition, some anti-Aβ antibodies may exacerbate CAA-associated microhemorrhage [124, 125]. In AD patients, even though clinical trials with Aβ immunotherapy were terminated due to neuroinflammation in about 6% of subjects, there were encouraging data regarding possible reductions in brain Aβ levels [126].
In addition to the role of microglia in Aβ clearance, the BBB mechanisms by which anti-Aβ clears brain Aβ fall into two categories: peripheral Aβ ‘sink action’ and central action. Sequestration of plasma Aβ by some anti-Aβ antibodies may enhance the endogenous ‘sink action’ of sLRP1 which in turn could alters Aβ transport dynamics across the BBB [127-129]. Also, the formation of anti-Aβ antibody/Aβ immune complexes in plasma reduces free Aβ transport into brain via RAGE [24,129]. The peripheral ‘sink action’ can also be enhanced by other Aβ-binding agents, such as gelsolin, MG1 (a ganglioside) [130] or recombinant LRP1 clusters [42], all of which have been shown to reduce brain Aβ burden in a mouse model of AD. Recombinant LRP-IV cluster can effectively sequester plasma Aβ40 and Aβ42 present in human AD patient plasma and in plasma of a mouse model of AD [42].
Clearance of Aβ may also depend on the entry of some anti-Aβ antibodies (IgG) into the brain. IgGs with a pK close to 7.4 are transported into the brain from the cerebral circulation by a saturable mechanism across the BBB [61]. In addition, they can enter the brain by passive diffusion at sites deficient in BBB [131-133]. Within brain, anti-Aβ antibodies may disrupt the formation of Aβ aggregates and solubilize Aβ deposits. The anti-Aβ antibody/Aβ immune complexes may be cleared by Fc-dependent activation of microglia followed by phagocytosis of Aβ deposits [120, 134, 135] and Fc-independent mechanisms [115, 136]. In addition, anti-Aβ antibody/Aβ immune complexes are rapidly transported across the BBB via neonatal Fc receptor for IgG (FcRn) that is present in the adult brain endothelium [129, 137,138] (Figure 1). Although, the mechanism of FcRn-dependent transcytosis of IgG is unclear, it has been suggested that FcRn mediates Aβ transcytosis across the BBB after anti-Aβ antibody/Aβ immune complexes are internalized at the abluminal surface of the brain endothelium [129, 138] (Figure 1).
In Tg-SwDI mice, a AD mouse model that exhibits early and robust cerebrovascular deposits due to reduced cerebrovascular LRP1 levels related to the low affinity of the DI-Aβ for LRP1 [26, 101], Aβ immunization had no effect on parenchymal or vascular Aβ deposits [139]. However, injection of purified anti-Aβ antibodies from plasma of immunized mice into the hippocampus rapidly cleared diffused Aβ, as reported [140], but not vascular amyloid deposits [139]. These results may suggest that while some anti-Aβ antibodies clear brain Aβ, their effecacy is determined by the levels of anti-Aβ antibody in brain ISF at the site of Aβ deposits, and by the levels of cell surface LRP1 at the BBB.
Conclusion
RAGE and LRP1 on the BBB and sLRP1 in plasma play a key role in controlling brain ISF Aβ concentration. In plasma, sLRP1 sequesters Aβ and maintains the peripheral ‘sink’ action which maintains continuous Aβ clearance from brain. The C-terminal sequence of Aβ interacts with sLRP1 and LRP-IV cluster. In AD, RAGE levels at the BBB are increased and LRP1 levels at the BBB and the capacity of sLRP1 binding of peripheral Aβ are reduced, favoring Aβ accumulation in the brain. Thus, therapies focused on upregulation of the cell surface LRP1 levels, reducing RAGE activity on brain endothelial cells and/or restoring the peripheral Aβ ‘sink’ action by replacement of sLRP1 in plasma, as for example with recombinant high affinity Aβ binding LRP1 clusters or some anti-Aβ antibodies, represent promising approaches to control brain Aβ levels and the associated pathology by targeting transport processes at the BBB.
Acknowledgments
This study was supported by R37AG023084 and R37NS34467 to BVZ, and 5R01 AG029481-02 and IIRG-036-06118 to RD.
References
- 1.Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8(6):429–31. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. Alzheimer’s disease Facts and Figures. [Jan 16, 2009];2008 doi: 10.1016/j.jalz.2008.02.005. http://www.alz.org/national/documents/report_alzfactsfigures2008.pdf. [DOI] [PubMed]
- 3.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 4.Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. The role of cell-derived oligomers of Abeta in Alzheimer’s disease and avenues for therapeutic intervention. Biochem Soc Trans. 2005;33(Pt 5):1087–90. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- 5.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32(2):177–80. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- 7.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–8. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 8.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–55. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Hardy J. Amyloid double trouble. Nat Genet. 2006;38(1):11–2. doi: 10.1038/ng0106-11. [DOI] [PubMed] [Google Scholar]
- 10.Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Gen. 2006;38(1):24–6. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 11.Holtzman DM, Zlokovic BV. In: Alzheimer’s disease: Advances in genetics, molecules and cellular biology. Sisodia S, Tanzi R, editors. Springer; New York: 2007. pp. 151–54. [Google Scholar]
- 12.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 13.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 1992;360(6405):672–4. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 15.Zlokovic BV, Shinya Y, Holyzman D, Ghiso J, Frangione B. Clearance of amyloid β-peptide from brain: transport or metaboloism. Nat Med. 2000;6:718–719. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 16.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43(5):605–8. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28(4):202–8. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5(5):347–60. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 19.Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem. 2004;89(4):807–11. doi: 10.1111/j.1471-4159.2004.02385.x. [DOI] [PubMed] [Google Scholar]
- 20.Han BH, Zhou ML, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, Cirrito JR, Milner E, Holtzman DM, Zipfel GJ. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J Neurosci. 2008;28(50):13542–50. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118(12):4002–13. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, Miano JM, Zlokovic BV. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2008 Dec 21; doi: 10.1038/ncb1819. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8(9):663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 24.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9(7):907–13. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 25.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106(12):1489–99. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43(3):333–44. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35(Suppl 1):2628–31. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 28.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 29.Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112(4):405–15. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 30.Miles MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG. Hippocampus RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res. 2008;1230:273–80. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med. 2000;6(7):718–9. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 32.Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56(12):1765–91. doi: 10.1016/j.addr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Redzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev. 2004;56(12):1695–716. doi: 10.1016/j.addr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol. 2007;5(5):e119. doi: 10.1371/journal.pbio.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 36.Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem. 1996;271(51):32916–22. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- 37.Martel CL, Mackic JB, Matsubara E, Governale S, Miguel C, Miao W, McComb JG, Frangione B, Ghiso J, Zlokovic BV. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood-brain barrier transport of circulating Alzheimer’s amyloid beta. J Neurochem. 1997;69(5):1995–2004. doi: 10.1046/j.1471-4159.1997.69051995.x. [DOI] [PubMed] [Google Scholar]
- 38.Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, Ladu MJ, Rostagno A, Frangione B, Ghiso J. Lipidation of apolipoprotein E influences it isoform-specific interaction with Alzheimer’s amyloid beta peptides. Biochem J. 2000;348(Pt 2):359–65. [PMC free article] [PubMed] [Google Scholar]
- 39.Calero M, Rostagno A, Matsubara E, Zlokovic B, Frangione B, Ghiso J. Apolipoprotein J (clusterin) and Alzheimer’s disease. Microsc Res Tech. 2000;50(4):305–15. doi: 10.1002/1097-0029(20000815)50:4<305::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Sousa JC, Cardoso I, Marques F, Saraiva MJ, Palha JA. Transthyretin and Alzheimer’s disease: where in the brain? Neurobiol Aging. 2007;28(5):713–8. doi: 10.1016/j.neurobiolaging.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Narita M, Holtzman DM, Schwartz AL, Bu G. Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem. 1997;69(5):1904–11. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- 42.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13(9):1029–31. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, Ghiso J. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci U S A. 1996;93(9):4229–34. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27(5):909–18. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wati H, Kawarabayashi T, Matsubara E, Kasai A, Hirasawa T, Kubota T, Harigaya Y, Shoji M, Maeda S. Transthyretin accelerates vascular Abeta deposition in a mouse model of Alzheimer’s disease. Brain Pathol. 2009;19(1):48–57. doi: 10.1111/j.1750-3639.2008.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zlokovic BV. Cerebrovascular transport of Alzheimer’s amyloid beta and apolipoproteins J and E: Possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 1996;59(18):1483–97. doi: 10.1016/0024-3205(96)00310-4. [DOI] [PubMed] [Google Scholar]
- 47.Kanekiyo T, Ban T, Aritake K, Huang ZL, Qu WM, Okazaki I, Mohri I, Murayama S, Ozono K, Taniike M, Goto Y, Urade Y. Lipocalin-type prostaglandin D synthase/beta-trace is a major amyloid beta-chaperone in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 2007;104(15):6412–7. doi: 10.1073/pnas.0701585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D. Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2003;2(8):506–11. doi: 10.1016/s1474-4422(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 49.Zlokovic BV, Ghiso J, Mackic JB, McComb JG, Weiss MH, Frangione B. Blood-brain barrier transport of circulating Alzheimer’s amyloid beta. Biochem Biophys Res Commun. 1993;197(3):1034–40. doi: 10.1006/bbrc.1993.2582. [DOI] [PubMed] [Google Scholar]
- 50.Maness LM, Banks WA, Podlisny MB, Selkoe DJ, Kastin AJ. Passage of human amyloid beta-protein 1-40 across the murine blood-brain barrier. Life Sci. 1994;55(21):1643–50. doi: 10.1016/0024-3205(94)00331-9. [DOI] [PubMed] [Google Scholar]
- 51.Martel CL, Mackic JB, McComb JG, Ghiso J, Zlokovic BV. Blood-brain barrier uptake of the 40 and 42 amino acid sequences of circulating Alzheimer’s amyloid beta in guinea pigs. Neurosci Lett. 1996;206(2-3):157–60. doi: 10.1016/s0304-3940(96)12462-9. [DOI] [PubMed] [Google Scholar]
- 52.Mackic JB, Weiss MH, Miao W, Kirkman E, Ghiso J, Calero M, Bading J, Frangione B, Zlokovic BV. Cerebrovascular accumulation and increased blood-brain barrier permeability to circulating Alzheimer’s amyloid beta peptide in aged squirrel monkey with cerebral amyloid angiopathy. J Neurochem. 1998;70(1):210–5. doi: 10.1046/j.1471-4159.1998.70010210.x. [DOI] [PubMed] [Google Scholar]
- 53.Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D, Schmidt AM, Frangione B, Zlokovic BV. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1-40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102(4):734–43. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackic JB, Bading J, Ghiso J, Walker L, Wisniewski T, Frangione B, Zlokovic BV. Circulating amyloid-beta peptide crosses the blood-brain barrier in aged monkeys and contributes to Alzheimer’s disease lesions. Vascul Pharmacol. 2002;38(6):303–13. doi: 10.1016/s1537-1891(02)00198-2. [DOI] [PubMed] [Google Scholar]
- 55.LaRue B, Hogg E, Sagare A, Jovanovic S, Maness L, Maurer C, Deane R, Zlokovic BV. Method for measurement of the blood-brain barrier permeability in the perfused mouse brain: application to amyloid-beta peptide in wild type and Alzheimer’s Tg2576 mice. J Neurosci Methods. 2004;138(1-2):233–42. doi: 10.1016/j.jneumeth.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 56.Clifford PM, Zarrabi S, Siu G, Kinsler KJ, Kosciuk MC, Venkataraman V, D’Andrea MR, Dinsmore S, Nagele RG. Abeta peptides can enter the brain through a defective blood-brain barrier and bind selectively to neurons. Brain Res. 2007;1142:223–36. doi: 10.1016/j.brainres.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 57.Zlokovic BV, Banks WA, el Kadi H, Erchegyi J, Mackic JB, McComb JG, Kastin AJ. Transport, uptake, and metabolism of blood-borne vasopressin by the blood-brain barrier. Brain Res. 1992;590(1-2):213–8. doi: 10.1016/0006-8993(92)91098-y. [DOI] [PubMed] [Google Scholar]
- 58.Zlokovic BV, Mackic JB, Djuricic B, Davson H. Kinetic analysis of leucine-enkephalin cellular uptake at the luminal side of the blood-brain barrier of an in situ perfused guinea-pig brain. J Neurochem. 1989;53(5):1333–40. doi: 10.1111/j.1471-4159.1989.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 59.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336(1):125–32. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 60.Deane R, LaRue B, Sagare AP, Castellino FJ, Zhong Z, Zlokovic BV. Endothelial protein C receptor-assisted transport of activated protein C across the mouse blood-brain barrier. J Cereb Blood Flow Metab. 2009;29(1):25–33. doi: 10.1038/jcbfm.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zlokovic BV, Skundric DS, Segal MB, Lipovac MN, Mackic JB, Davson H. A saturable mechanism for transport of immunoglobulin G across the blood-brain barrier of the guinea pig. Exp Neurol. 1990;107(3):263–70. doi: 10.1016/0014-4886(90)90144-h. [DOI] [PubMed] [Google Scholar]
- 62.Segal MB, Preston JE, Collis CS, Zlokovic BV. Kinetics and Na independence of amino acid uptake by blood side of perfused sheep choroid plexus. Am J Physiol. 1990;258(5 Pt 2):F1288–94. doi: 10.1152/ajprenal.1990.258.5.F1288. [DOI] [PubMed] [Google Scholar]
- 63.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2007;4(2):191–7. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 64.Deane R, Sagare A, Zlokovic BV. The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer’s disease. Curr Pharm Des. 2008;14(16):1601–5. doi: 10.2174/138161208784705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 66.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274(44):31740–9. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 67.Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002;54(12):1615–25. doi: 10.1016/s0169-409x(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 68.Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VK. beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279(6):C1772–81. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- 69.Shayo M, McLay RN, Kastin AJ, Banks WA. The putative blood-brain barrier transporter for the beta-amyloid binding protein apolipoprotein j is saturated at physiological concentrations. Life Sci. 1997;60(7):PL115–8. doi: 10.1016/s0024-3205(96)00685-6. [DOI] [PubMed] [Google Scholar]
- 70.Pfizer, ClinicalTrials.gov: A Phase 2 Study Evaluating The Efficacy And Safety Of PF 04494700 In Mild To Moderate Alzheimer’s Disease. [Jan 16, 2009]; http://clinicaltrials.gov/ct2/show/NCT00566397?term=RAGE&rank=2.
- 71.Deane R, Perry S, Sagare A, Cheng T, LaRue B, Miller B, Zlokovic B. Tertiary amides block RAGE-mediated Aβ transport into brain in a mouse model of AD. Program No. 412.3. Society for Neuroscience Meeting Planner; Alanta, GA. 2006. [Google Scholar]
- 72.Deane R, Sagare A, Paquette N, Bell R, LaRue B, Ross N, Miller B, Zlokovic B. Tertiary amides improves cerebral blood flow and behavioral performance in a mouse model of AD by blocking RAGE/Aβ interaction. Program No. 704.3. Society for Neuroscience Meeting Planner; Washington, DC. 2008. [Google Scholar]
- 73.Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29(3):571–81. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 74.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108(6):779–84. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willnow TE, Rohlmann A, Horton J, Otani H, Braun JR, Hammer RE, Herz J. RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J. 1996;15(11):2632–9. [PMC free article] [PubMed] [Google Scholar]
- 76.Meijer AB, Rohlena J, van der Zwaan C, van Zonneveld AJ, Boertjes RC, Lenting PJ, Mertens K. Functional duplication of ligand-binding domains within low-density lipoprotein receptor-related protein for interaction with receptor associated protein, alpha2-macroglobulin, factor IXa and factor VIII. Biochim Biophys Acta. 2007;1774(6):714–22. doi: 10.1016/j.bbapap.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Obermoeller-McCormick LM, Li Y, Osaka H, FitzGerald DJ, Schwartz AL, Bu G. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J Cell Sci. 2001;114(Pt 5):899–908. doi: 10.1242/jcs.114.5.899. [DOI] [PubMed] [Google Scholar]
- 78.Benchenane K, Berezowski V, Fernandez-Monreal M, Brillault J, Valable S, Dehouck MP, Cecchelli R, Vivien D, Touzani O, Ali C. Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process. Stroke. 2005;36(5):1065–70. doi: 10.1161/01.STR.0000163050.39122.4f. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Lu W, Marzolo MP, Bu G. Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J Biol Chem. 2001;276(21):18000–6. doi: 10.1074/jbc.M101589200. [DOI] [PubMed] [Google Scholar]
- 80.Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273(50):33556–60. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 81.Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275(33):25616–24. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 82.van der Geer P. Phosphorylation of LRP1: regulation of transport and signal transduction. Trends Cardiovasc Med. 2002;12(4):160–5. doi: 10.1016/s1050-1738(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 83.Bu G, Sun Y, Schwartz AL, Holtzman DM. Nerve growth factor induces rapid increases in functional cell surface low density lipoprotein receptor-related protein. J Biol Chem. 1998;273(21):13359–65. doi: 10.1074/jbc.273.21.13359. [DOI] [PubMed] [Google Scholar]
- 84.Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 1997;49(1):56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- 85.Lambert JC, Wavrant-De Vrieze F, Amouyel P, Chartier-Harlin MC. Association at LRP gene locus with sporadic late-onset Alzheimer’s disease. Lancet. 1998;351(9118):1787–8. doi: 10.1016/s0140-6736(05)78749-3. [DOI] [PubMed] [Google Scholar]
- 86.Wavrant-DeVrieze F, Lambert JC, Stas L, Crook R, Cottel D, Pasquier F, Frigard B, Lambrechts M, Thiry E, Amouyel P, Tur JP, Chartier-Harlin MC, Hardy J, Van Leuven F. Association between coding variability in the LRP gene and the risk of late-onset Alzheimer’s disease. Hum Genet. 1999;104(5):432–4. doi: 10.1007/s004390050980. [DOI] [PubMed] [Google Scholar]
- 87.Christoforidis M, Schober R, Krohn K. Genetic-morphologic association study: association between the low density lipoprotein-receptor related protein (LRP) and cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2005;31(1):11–9. doi: 10.1111/j.1365-2990.2004.00614.x. [DOI] [PubMed] [Google Scholar]
- 88.Bertram L, Blacker D, Crystal A, Mullin K, Keeney D, Jones J, Basu S, Yhu S, Guenette S, McInnis M, Go R, Tanzi R. Candidate genes showing no evidence for association or linkage with Alzheimer’s disease using family-based methodologies. Exp Gerontol. 2000;35:1353–61. doi: 10.1016/s0531-5565(00)00193-5. [DOI] [PubMed] [Google Scholar]
- 89.Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the alpha 2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann Neurol. 1995;37(2):211–7. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- 90.Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT. LRP and senile plaques in Alzheimer’s disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104(1):38–46. doi: 10.1016/s0169-328x(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 91.Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci. 2004;24:4259–65. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waldron E, Heilig C, Schweitzer A, Nadella N, Jaeger S, Martin AM, Weggen S, Brix K, Pietrzik CU. LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiol Dis. 2008;31:188–97. doi: 10.1016/j.nbd.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Qiu Z, Strickland DK, Hyman BT, Rebeck GW. Alpha2-macroglobulin enhances the clearance of endogenous soluble beta-amyloid peptide via low-density lipoprotein receptor-related protein in cortical neurons. J Neurochem. 1999;73(4):1393–8. doi: 10.1046/j.1471-4159.1999.0731393.x. [DOI] [PubMed] [Google Scholar]
- 94.DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41(2):193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- 95.Zerbinatti CV, Bu G. LRP and Alzheimer’s disease. Rev Neurosci. 2005;16(2):123–35. doi: 10.1515/revneuro.2005.16.2.123. [DOI] [PubMed] [Google Scholar]
- 96.Zerbinatti CV, Wozniak DF, Cirrito J, Cam JA, Osaka H, Bales KR, Zhuo M, Paul SM, Holtzman DM, Bu G. Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A. 2004;101(4):1075–80. doi: 10.1073/pnas.0305803101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bading JR, Yamada S, Mackic JB, Kirkman L, Miller C, Calero M, Ghiso J, Frangione B, Zlokovic BV. Brain clearance of Alzheimer’s amyloid-beta40 in the squirrel monkey: a SPECT study in a primate model of cerebral amyloid angiopathy. J Drug Target. 2002;10(4):359–68. doi: 10.1080/10611860290031831. [DOI] [PubMed] [Google Scholar]
- 98.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115(11):3285–90. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shiiki T, Ohtsuki S, Kurihara A, Naganuma H, Nishimura K, Tachikawa M, Hosoya K, Terasaki T. Brain insulin impairs amyloid-beta(1-40) clearance from the brain. J Neurosci. 2004;24(43):9632–7. doi: 10.1523/JNEUROSCI.2236-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23(3):405–12. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 101.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279(19):20296–306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 102.Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G, Basak JM, Holtzman DM, Ohtsuki S, Terasaki T, Iwatsubo T. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J Biol Chem. 2008;283(50):34554–62. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bu G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int Rev Cytol. 2001;209:79–116. doi: 10.1016/s0074-7696(01)09011-8. [DOI] [PubMed] [Google Scholar]
- 104.Nazer B, Hong S, Selkoe DJ. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol Dis. 2008;30(1):94–102. doi: 10.1016/j.nbd.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11(9):959–65. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 106.Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A. 2007;104(3):823–8. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tamaki C, Ohtsuki S, Iwatsubo T, Hashimoto T, Yamada K, Yabuki C, Terasaki T. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm Res. 2006;23(7):1407–16. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 108.Tamaki C, Ohtsuki S, Terasaki T. Insulin facilitates the hepatic clearance of plasma amyloid beta-peptide (1 40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Mol Pharmacol. 2007;72(4):850–5. doi: 10.1124/mol.107.036913. [DOI] [PubMed] [Google Scholar]
- 109.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–93. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boche D, Nicoll JA. The role of the immune system in clearance of Abeta from the brain. Brain Pathol. 2008;18(2):267–78. doi: 10.1111/j.1750-3639.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–84. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 112.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 113.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408(6815):982–5. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 114.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408(6815):979–82. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 115.Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma-/- knock-out mice. J Neurosci. 2003;23(24):8532–8. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lemere CA, Spooner ET, LaFrancois J, Malester B, Mori C, Leverone JF, Matsuoka Y, Taylor JW, DeMattos RB, Holtzman DM, Clements JD, Selkoe DJ, Duff KE. Evidence for peripheral clearance of cerebral Abeta protein following chronic, active Abeta immunization in PSAPP mice. Neurobiol Dis. 2003;14(1):10–8. doi: 10.1016/s0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 117.Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a nontoxic/nonfibrillar amyloid-beta homologous peptide reduces Alzheimer’s disease-associated pathology in transgenic mice. Am J Pathol. 2001;159(2):439–47. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat Neurosci. 2002;5(5):452–7. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 119.Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22(15):6331–5. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci. 2003;23(9):3745–51. doi: 10.1523/JNEUROSCI.23-09-03745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Head E, Pop V, Vasilevko V, Hill M, Saing T, Sarsoza F, Nistor M, Christie LA, Milton S, Glabe C, Barrett E, Cribbs D. A two-year study with fibrillar beta-amyloid (Abeta) immunization in aged canines: effects on cognitive function and brain Abeta. J Neurosci. 2008;28(14):3555–66. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165(1):283–97. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Trouche SG, Asuni A, Rouland S, Wisniewski T, Frangione B, Verdier JM, Sigurdsson EM, Mestre-Frances N. Antibody response and plasma Abeta1-40 levels in young Microcebus murinus primates immunized with Abeta1-42 and its derivatives. Vaccine. 2008 Dec 27; doi: 10.1016/j.vaccine.2008.12.012. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pfeifer M, Boncristiano L, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrage after passice anti-Abeta immunotherapy. Science. 2002;298(5597):1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 125.Racke MM, Boone LI, Hepburn DL, Pasadainian M, Bryan MT, Ness DK, Pioroozi KS, Jordon WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, Demattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci. 2005;25(3):629–36. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 127.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98(15):8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295(5563):2264–7. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 129.Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, Wu Z, Holtzman DM, Zlokovic BV. IgG-assisted age-dependent clearance of Alzheimer’s amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J Neurosci. 2005;25(50):11495–503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matsuoka Y, Saito M, LaFrancois J, Saito M, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23(1):29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol. 1986;251(2):260–80. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- 132.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 1993;120(2):245–63. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- 133.Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Passage of amyloid beta protein antibody across the blood-brain barrier in a mouse model of Alzheimer’s disease. Peptides. 2002;23(12):2223–6. doi: 10.1016/s0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 134.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 135.Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzman DM. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci. 2008;28(52):14156–64. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22(18):7873–8. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J Neurochem. 2002;81(1):203–6. doi: 10.1046/j.1471-4159.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 138.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 139.Vasilevko V, Xu F, Previti ML, Van Nostrand WE, Cribbs DH. Experimental investigation of antibody-mediated clearance mechanisms of amyloid-beta in CNS of Tg-SwDI transgenic mice. J Neurosci. 2007;27(49):13376–83. doi: 10.1523/JNEUROSCI.2788-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-beta deposits in brains of lining mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7(3):369–72. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]


