Abstract
Cellular mechanotransduction, the process of converting mechanical signals into biochemical responses within cells, is a critical aspect of bone health. While the effects of mechanical loading on bone are well recognized, elucidating the specific molecular pathways involved in the processing of mechanical signals by bone cells represents a challenge and an opportunity to identify therapeutic strategies to combat bone loss. In this study we have for the first time examined the relationship between the nucleocytoplasmic shuttling transcription factor nuclear matrix protein-4/cas interacting zinc finger protein (Nmp4/CIZ) and β-catenin signaling in response to a physiologic mechanical stimulation (oscillatory fluid shear stress, OFSS) in osteoblasts. Using calvaria-derived osteoblasts from Nmp4-deficient and wild-type mice, we found that the normal translocation of β-catenin to the nucleus in osteoblasts that is induced by OFSS is enhanced when Nmp4/CIZ is absent. Furthermore, we found that other aspects of OFSS-induced mechanotransduction generally associated with the β-catenin signaling pathway, including ERK, Akt and GSK3β activity, as well as expression of the β-catenin-responsive protein cyclin D1 are also enhanced in cells lacking Nmp4/CIZ. Finally, we found that in the absence of Nmp4/CIZ, OFSS-induced cytoskeletal reorganization and the formation of focal adhesions between osteoblasts and the extracellular substrate is qualitatively enhanced, suggesting that Nmp4/CIZ may reduce the sensitivity of bone cells to mechanical stimuli. Together these results provide experimental support for the concept that Nmp4/CIZ plays an inhibitory role in the response of bone cells to mechanical stimulation induced by OFSS.
Keywords: Akt, bone, ERK, fluid shear mechanosome, mechanotransduction
Introduction
Mechanical loading of the skeleton plays a vital role in bone health. Mechanical stimulation is necessary to properly coordinate the activity of bone osteoclasts and osteoblasts that is critical for normal bone turnover and remodeling. Cells within bone, including osteocytes, bone lining cells, and osteoblasts are capable of detecting and responding to the mechanical strain-induced movement of fluid through the spaces surrounding bone cells (Rubin et al., 2006; Santos et al., 2009a; You et al., 2008). Significant progress has been made in recent years in elucidating the molecular pathways involved in the cellular processing of mechanical signals induced by fluid shear stress that ultimately result in vital biochemical changes inside cells, a process referred to as cellular mechanotransduction (Papachristou et al., 2009; Wang et al., 2009). For example, mechanotransduction at sites of physical interaction between bone cells and the extracellular matrix that are mediated by integrin cell adhesion molecules are generally viewed as necessary for proper processing of mechanical signaling (Pavalko et al., 1998; Ponik and Pavalko, 2004; Young et al., 2009). Because integrins lack intrinsic catalytic activity, signaling molecules like focal adhesion kinase (FAK) that associate with the cytoplasmic domains of integrin β subunits, appear to play a key role in coupling mechanical signals detected via integrins into biochemical responses in bone cells (Young et al., 2009). Despite good progress, important aspects of mechanotransduction in bone remain poorly understood. We have suggested previously that mechanical signaling in bone involves the activity of mechanically sensitive “mechanosomes” which are nucleocytoplasmic shuttling protein complexes that transduce mechanical signals detected by integrins at the cell surface into conformational changes in the promoters of target genes in the nucleus (Pavalko et al., 2003b). Among the proteins proposed to comprise these mechanosome signaling complexes are integrins, focal adhesion kinase (FAK), vinculin, α-actinin, p130Cas, zyxin, β-catenin, and the transcription factors Lef1 (lymphoid enhancer binding factor 1) and Nmp4/CIZ (nuclear matrix protein 4/cas interacting zinc finger protein; reviewed in (Childress et al., 2009; Pavalko et al., 2003b).
Fluid shear stress and parathyroid hormone (PTH) both promote anabolic bone formation. Interestingly, both fluid shear and PTH also promote translocation of β-catenin from the cytoplasm to the nucleus, a critical aspect of mechanosome activity (Kulkarni et al., 2005; Norvell et al., 2004a; Robinson et al., 2006; Santos et al., 2009b; Tobimatsu et al., 2006). Because β-catenin plays a role in some load-induced changes in osteoblast gene expression (Case et al., 2008) and is sensitive to mechanical load and PTH (Wang et al., 2008), it may play a key role in mechanically-induced bone formation, reviewed in (Williams and Insogna, 2009) and, along with Nmp4CIZ, is a focus of the current study.
Nmp4 was initially characterized as a PTH-responsive nuclear matrix architectural transcription factor, i.e. a protein that alters gene activity by bending DNA (Alvarez et al., 1997; Alvarez et al., 1998; Thunyakitpisal et al., 2001). We hypothesized that the PTH- or load-induced changes in osteoblast adhesion and shape were transduced by Nmp4 into alterations in target gene DNA conformation and ultimately activity (Bidwell et al., 1998; Pavalko et al., 2003b). CIZ was independently identified as a nucleocytoplasmic shuttling Cys2His2 zinc finger transcription factor that interacts with the focal adhesion protein p130Cas (Nakamoto et al., 2000). The association of NMP4/CIZ with p130Cas, a force sensor/transducer of the cell (Sawada et al., 2006), supports the hypothesis that this association mediates communication between integrins and the nucleus (Nakamoto et al., 2000) and may be particularly relevant to changes in adhesion signaling that occur during bone cell response to PTH or load (Childress et al., 2009; Pavalko et al., 2003b). Nmp4/CIZ has been shown to regulate the expression of Mmp-13 in rat bone cells in response to fluid shear stress (Charoonpatrapong-Panyayong et al., 2007). In fact, the expression of Nmp4/CIZ itself is sensitive to fluid shear stress, consistent with a role for this protein in bone mechanotransduction. Nmp4/CIZ is a general repressor of the anabolic bone response. The independently prepared Nmp4 knockout (KO) and CIZ-KO mice both exhibit a modestly enhanced skeletal phenotype including elevated bone mineral density and content compared to wild-type (WT) mice (Morinobu et al., 2005; Robling et al., 2009). The CIZ-KO mice show a greater increase in bone formation in response to BMP2 as compared to WT mice (Morinobu et al., 2005) and the Nmp4-KO mice exhibit an enhanced increase in bone formation in response to intermittent PTH as compared to their WT counterparts (Robling et al., 2009). Remarkably Nmp4/CIZ mediates disuse-induced bone loss in mice since the CIZ-nulls are impervious to hind limb suspension (Hino et al., 2007). Therefore, Nmp4/CIZ may suppress pathways common to both PTH- and mechanical load-induced anabolic response (Childress et al., 2009).
The p130Cas/Nmp4/CIZ and β-catenin/Lef1 signaling pathways share several similar features (reviewed in, (Childress et al., 2009). Both Nmp4/CIZ and Lef1 are nucleocytoplasmic shuttling high mobility group (HMG) architectural transcription factors that associate with adhesion complex proteins (p130Cas and β-catenin, respectively) and appear to translocate between adhesion sites at the membrane and the nucleus. In this study we have for the first time examined the relationship between Nmp4/CIZ and β-catenin signaling in response to mechanical stimulation. We found that translocation of β-catenin to the nucleus in osteoblasts that is normally induced by oscillatory fluid shear stress (OFSS) is attenuated in the presence of Nmp4/CIZ. Furthermore, we show that other aspects of OFSS-induced mechanotransduction that are associated with the β-catenin pathway, including ERK, Akt and GSK3β activity, as well as expression of the β-catenin-responsive protein cyclin D1 (Tetsu and McCormick, 1999) are enhanced in cells lacking Nmp4/CIZ. Finally, we found that in the absence of Nmp4/CIZ, OFSS-induced reorganization of the actin cytoskeleton and formation of focal adhesions, as has been described previously (Ponik and Pavalko, 2004) is qualitatively enhanced, further suggesting that Nmp4/CIZ may reduce the sensitivity of bone cells to mechanical stimuli. Together, these results support the concept that Nmp4 normally plays an inhibitory role in bone cell mechanotransduction.
Materials and Methods
Isolation, culture and verification of primary mouse calvarial osteoblasts
Calvarial osteoblasts were isolated from WT and Nmp4-KO mice in a 129SvEv and C57BL/6J F2 (Nmp4-KO-F2) background and have been described previously (Robling et al., 2009). In addition, the Nmp4-KO-N6 background mice, which were derived via backcrossing the Nmp4-KO-F2 onto a C57BL/6J background through six generations, were also used for isolation of primary calvarial osteoblasts and compared to C57BL/6J mice osteoblasts (Robling et al., 2009). No differences based on background (F2 vs. N6) were observed except as noted below for β-catenin immunofluorescence nuclear:cytoplasmic ratio measurements. The isolation of primary mouse calvarial osteoblasts was carried out as described previously (Pavalko et al., 2003a). Briefly, calvaria from 10 to 12 WT or Nmp4-KO neonatal mice (day 3–5 after birth) were isolated aseptically and minced, and then digested consecutively for 5, 15 and 25 minutes at 37°C in digestion media including 0.2% collagenase P and 0.25% trypsin with shaking. Cells from the second and third digests (15 and 25 minutes digest) were pooled and cultured in minimal essential media alpha (α-MEM, Gibco) supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin (Gibco). Cells were passed at approximately 90% confluence with 0.25% trypsin and the media were changed every other day. Primary osteoblasts were confirmed by alkaline phosphatase staining with NBT/BCIP method (images not shown) and comparing to the osteoblastic cell line MC3T3-E1.
Oscillatory fluid flow
Primary calvarial osteoblasts were used between passages 2–4 for flow experiments. Prior to OFFS, WT and Nmp4-KO cells were passed onto sterile glass slides at a density of 2×105 cells per slide, grown for 24 hours and serum-starved in α-MEM media supplemented with 0.5% FCS and 1% penicillin/streptomycin for 24 hours. α-MEM media supplemented with 0.5% FCS and 1% penicillin/streptomycin was used for flow experiments. The slides were placed in specially designed chambers (described previously, (Pavalko et al., 2003a), which then were maintained in a 37°C incubator with 5% CO2 and connected to a pump with PVC tubing (Nalgene 180). The cells were subjected to the oscillatory fluid flow at a frequency of 1Hz using a pump apparatus described previously (Jacobs et al., 1998). Static controls were held in dish cultures at 37°C with 5% CO2.
Western blotting and quantification
Cells subjected to static and OFSS conditions were harvested in SDS sample buffer, and protein concentrations were determined using the amido black method. Equal amounts of protein (20μg) were loaded onto SDS-PAGE gels for separation and transferred to nitrocellulose. The following primary and secondary antibodies were used: ph-Erk1/2/Erk1/2 (Santa Cruz), β-catenin (BD, #610153), ph-Akt/Akt (Cell Signaling, #9271 and #9272), ph-GSK3β/GSK3β (Cell Signaling #9336, BD #610201), COX-2 (Santa Cruz) and Cyclin D1 (Zymed #13–4500), Vinculin (Sigma), Actin (Sigma). Secondary antibodies: horseradish peroxidase (HRP) conjugated goat anti-mouse, HRP conjugated goat anti-rabbit and HRP conjugated donkey anti-goat (Santa Cruz). Densitometry was quantified using the software Multi Gauge V2.3 (Fuji).
Immunofluorescence and quantification
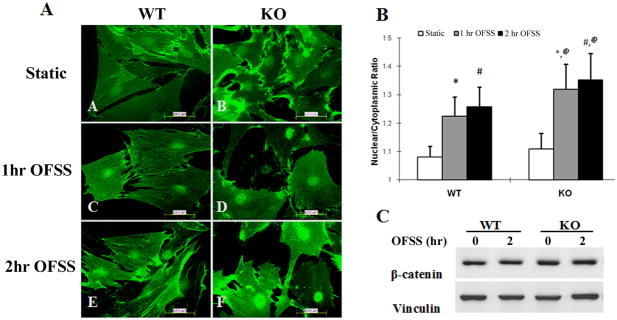
Following 24 hr of serum starvation (0.5% FCS), cells subjected to static culture conditions were fixed immediately (static) or fixed after 1 hr or 2 hr of OFSS with 4% paraformaldehyde solution and processed for immunofluorescence by permeabilization with 0.2% triton followed by rinsing in Tris-buffered saline (TBS). Slides were then treated with 1% BSA solution in TBS for 30 minutes at 37°C to block non-specific antibody binding. The following primary and secondary antibodies were used: vinculin (Sigma) or β-catenin (BD), followed by FITC-conjugated goat anti-mouse (Santa Cruz). Hoechst 33342 and Texas-Red phalloidin were used for visualizing the nucleus and F-actin, respectively. Images were captured using a Nikon inverted immunofluorescence microscope equipped with a CCD camera. Random image fields (10–15 fields/slide) were captured from 3 separate slides per experimental trial. No distinction was made between cells on the 3 separate slides within each treatment group. Fluorescence intensities of β-catenin labeling were measured in nuclei that were outlined manually based on the Hoechst 33342 stained image. Nuclear outlines were then duplicated and used to measure fluorescence intensities in the cytoplasm adjacent to the nucleus. A minimum of n=100 cells were analyzed per treatment condition (static, 1 hr or 2 hr OFSS) in each experimental trial. Results shown in Figure 1 are for cells isolated from mice with the N6 genetic background. Comparable results were obtained when the experiment was repeated using cells from the F2 genetic background with the exception that all treatment groups of the F2-KO cells exhibited a significantly greater nuclear:cytoplasmic ratio of β-catenin than that observed in the F2-WT cells. Image J software (NIH) was used for quantification.
Figure 1.
Nuclear translocation of β-catenin in response to OFSS is enhanced in Nmp4 KO primary calvarial osteoblasts compared to osteoblasts isolated from WT mice. (A) Immunofluorescence microscopy of primary calvarial osteoblasts subjected to static culture conditions or OFSS for 1 hour or 2 hour (12 dynes/cm2). Slides were fixed immediatelyand processed for immunofluorescence using an antibody against β-catenin followed by FITC-conjugated secondary antibody. Results shown are using cells isolated from N6 mice. Hoechst 33342 was used to label and identify the outline of nuclei (not shown). (B) Quantification of the nuclear/cytoplasmic ratio of β-catenin in cells isolated from N6 mice. A minimum of 100 cells in each treatment condition were selected randomly and the fluorescent grey value was calculated in the nucleus and in an identically sized area near the nucleus in the cytoplasm. Hoechst labeling was used to define the nuclear outline. The 2-way ANOVA indicated a strong genotype effect (p<0.0001), a strong treatment effect (p<0.0001) and a genotype × treatment interaction (p<0.0001). The Tukey’s HSD post hoc analysis determined that WT STATIC=KO STATIC<WT 1HR FLOW=WT 2HR FLOW<KO 1HR FLOW=KO 2HR FLOW. *p<0.0001 1h OFSS vs. static; #p<0.0001 2h OFSS vs. static; @ p<0.0001 1 hr OFSS KO vs. 1hr OFSS WT and 2 hr OFSS KO vs. 2 hr OFSS WT. NOTE: all treatment groups of the F2-KO cells exhibited a significantly greater nuclear:cytoplasmic ratio of β-catenin than that observed in the F2-WT cells (not shown). (C) Immunoblot analysis for total cellular β-catenin (normalized to total cellular vinculin) from cells maintained in static culture or subjected to OFSS for 1 hr (not shown) or 2 hr. No change in total β-catenin levels were detected as a consequence of OFSS.
Statistical evaluation
Statistical significance of immunoblots was assessed by one-tailed t-tests with a p value of <0.05 interpreted as statistically significant. A two-factor ANOVA was employed to determine if genotype (WT vs KO) or treatment (STATIC vs. 1HR OFSS vs 2HR OFSS) had an effect on the β-catenin nuclear:cytoplasmic ratio and to evaluate whether an interaction occurred between genotype × treatment. If the two-factor ANOVA indicated a significant interaction the data were then analyzed using a Tukey’s HSD post hoc test to determine significant differences between the experimental groups.
Results
OFSS-induced nuclear translocation of β-catenin is enhanced in Nmp4 KO osteoblasts compared to WT controls
To evaluate β-catenin signaling in bone cells from Nmp4−/− (KO) mice in response to OFSS, primary calvarial osteoblasts were isolated from Nmp4 KO or WT mice and subjected to OFSS for either 1 or 2 hours. Cells were fixed immediately after cessation of OFSS and processed for immunofluorescence microscopy. As expected, both 1 hr and 2 hr of OFSS resulted in nuclear translocation of β-catenin compared to cells maintained in static culture. However, it appeared that OFSS-induced nuclear translocation of β-catenin in osteoblasts isolated from Nmp4 KO mice was consistently greater than that observed in osteoblasts isolated from WT mice (Fig. 1). This was confirmed by quantitative assessment of nuclear vs. cytoplasmic staining for β-catenin (Fig. 1). Interestingly, there was no significant difference in the basal nuclear/cytoplasmic ratio of β-catenin in Nmp4 KO and WT osteoblasts maintained under static culture conditions suggesting that the absence of Nmp4 from KO cells affected only the mechanically-induced change in β-catenin distribution and not basal β-catenin activity. Total cellular levels of β-catenin as measured by western blot analysis were not different between Nmp4 KO and WT cells (Fig. 1).
OFSS-induced activation of Erk, Akt and GSK3βin Nmp4 KO osteoblasts
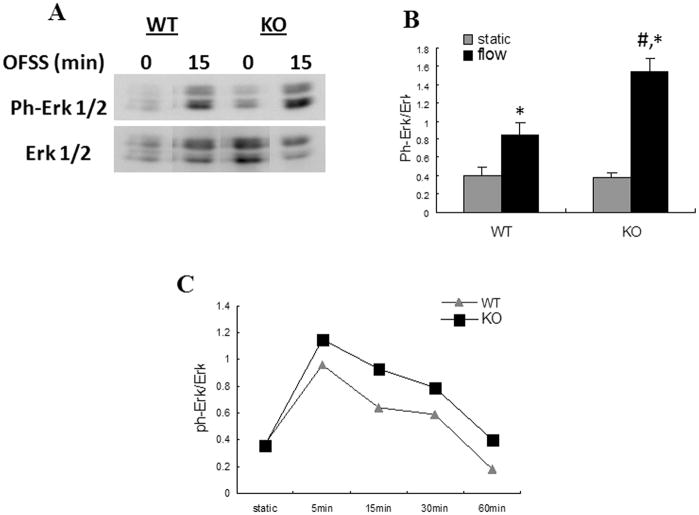
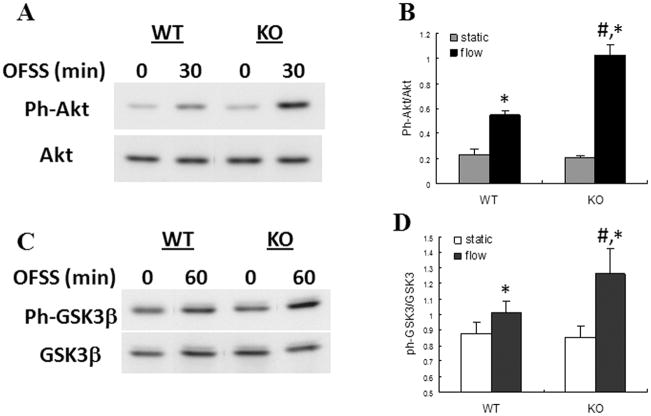
To further evaluate the role of Nmp4/CIZ in mediating the response of osteoblasts to mechanical loading, we subjected cells from WT and Nmp4 KO animals to OFSS for 5, 15, 30 or 60 min and analyzed activation of Erk1/2, Akt and GSK3β. As seen previously, activation of Erk1/2 increased and peaked within 15 min of OFSS (Fig. 2). A similar time course of Erk1/2 activation was observed in both WT and Nmp4 KO cells (Fig. 2). Erk1/2 activation in Nmp4-KO cells trended higher than levels seen in WT cells at all time points examined, but was significantly higher only at the peak time of 15 min after onset of OFSS (Fig. 2). Activation of Akt and GSK3β increased in response to OFSS in WT osteoblasts (Fig. 3). This increase was significantly greater in Nmp4 KO cells at 30 and 60 min for Akt and GSK3β, respectively (Fig. 3).
Figure 2.
Activation of ERK 1/2 in response to 15 min OFSS is enhanced in Nmp4 KO osteoblasts compared to WT controls. (A) Representative immunoblot of total cellular protein from Nmp4 and WT osteoblasts maintained in static culture or subjected to OFSS for 15 min. Blots were probed either with antibody specific for phosphorylated ERK 1/2 or for total ERK 1/2 protein. (B) Quantification of immunoblots illustrated in (A), n=3. The static vs. 15 min OFSS experiment was repeated three times with comparable results. *p<0.05 vs static; #p<0.05 vs WT flow. (C) Representative time course of ERK 1/2 phosphorylation at 5, 15, 30 and 60 min of OFSS. Although phosphorylation of ERK 1/2 in Nmp4 KO osteoblasts was consistently elevated above WT osteoblasts in response to OFSS at all time points, and was significantly higher at 15 min, statistically significant differences could not be measured at 5, 30 or 60 min (not shown).
Figure 3.
Activation of AKT and GSK3β in response to 30 and 60 min of OFSS, respectively, was enhanced in Nmp4 KO osteoblasts compared to WT controls. (A) Representative immunoblot of total cellular protein from Nmp4 and WT osteoblasts maintained in static culture or subjected to OFSS for 30 min and probed either with antibody specific for phosphorylated Akt or for total Akt. (B) Quantification of immunoblots illustrated in (A), n=3. The static vs. 30 min OFSS experiment was repeated three times with comparable results. *p<0.05 vs. static; #p<0.05 vs. WT flow. (C) Representative immunoblot of total cellular protein from Nmp4 and WT osteoblasts maintained in static culture or subjected to OFSS for 60 min and probed either with antibody specific for phosphorylated GSK3β or for total GSK3β. (D) Quantification of immunoblots illustrated in (C), n=3. The static vs. 60 min OFSS experiment was repeated three times with comparable results. *p<0.05 vs. static; #p<0.05 vs. WT flow.
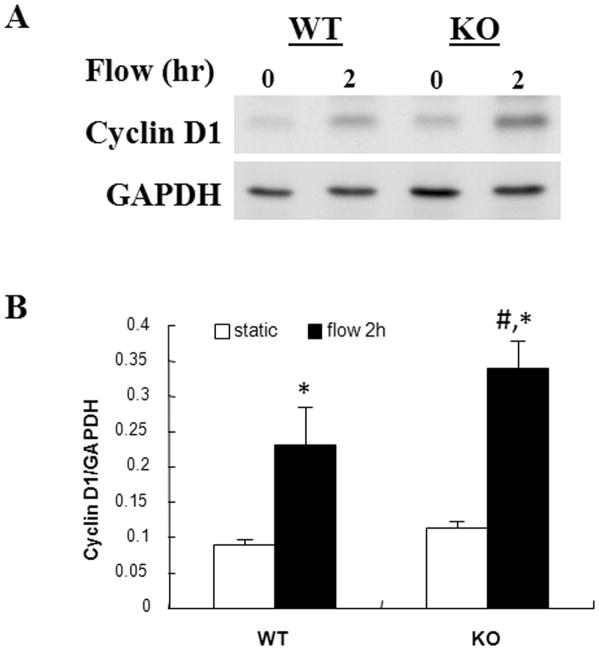
OFSS-induced expression of the β-catenin responsive gene cyclin D1 is increased in Nmp4 KO osteoblasts compared to WT controls
To evaluate the functional significance of enhanced OFSS-induced nuclear translocation of β-catenin, we measured expression of the β-catenin responsive gene cyclin D1 (Fig. 4). As expected, OFSS for 2 hr resulted in increased expression of cyclin D1, consistent with previously published reports. We observed no significant difference in the basal levels of cyclin D1 protein between Nmp4 KO and WT osteoblasts maintained in static culture (Fig. 4). However, following 2 hr of OFSS, the protein levels of cyclin D1 was significantly higher in Nmp4 KO cells compared to WT cells suggesting that Nmp4 may normally function to inhibit OFSS-induced expression of these proteins in osteoblasts. Although cyclin D1 was elevated in response to OFSS and was higher in Nmp4 KO cells, there was no measureable difference in the rate of proliferation between WT and KO cells (not shown). We also examined the expression of another mechanically responsive protein, cyclooxygenase-2 (Cox-2), in response to OFSS. No significant difference in the expression of Cox-2 was detected in response to 1 or 2 hr of OFSS between WT and KO cells (not shown).
Figure 4.
Induction of the β-catenin responsive protein cyclin D1 is enhanced in Nmp4 KO osteoblasts following 2 h r of OFSS compared to WT controls. (A) Representative immunoblot for cyclin D1 in osteoblasts from Nmp4 KO or WT osteoblasts maintained in static culture or subjected to OFSS for 2 hr. (B) Quantification of immunoblots illustrated in (A), n=3. The static vs. 2 hr OFSS experiment was repeated twice times with comparable results. *p<0.05 vs. static; #p<0.05 vs. WT flow.
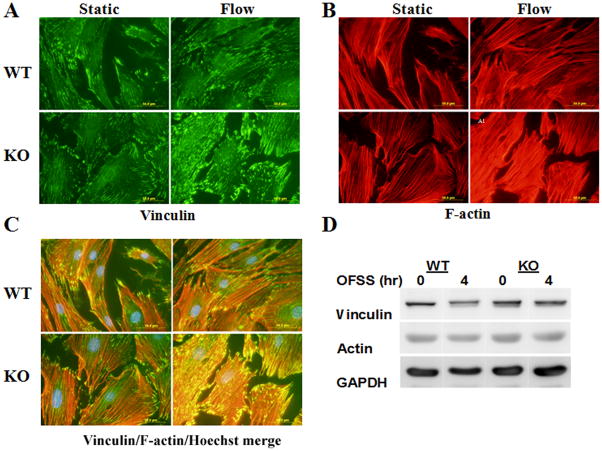
Nmp4 deficiency enhances the OFSS-induced formation of stress fiber and focal adhesions
We and others have previously reported a dramatic reorganization of actin filaments (F-actin) into large bundles (stress fibers) and an increase in recruitment of integrins to focal adhesions in response to both unidirectional and oscillatory fluid shear (Pavalko et al., 1998; Ponik et al., 2007). Therefore, we also evaluated the effect of OFSS on the formation of actin stress fibers and focal adhesions in WT and Nmp4 KO osteoblasts. Immunofluorescence microscopy for F-actin and for the focal adhesion protein vinculin showed that 4 hours of OFSS enhanced the formation of stress fibers and promoted the formation of focal adhesions as observed previously (Fig. 5). However, the extent of both stress fiber and focal adhesion formation appeared to be more robust in Nmp4 KO cells than in the WT cells (Fig. 5). Although the absence of Nmp4/CIZ appeared to enhance OFSS-induced reorganization of F-actin and formation of focal adhesions, western blot analysis showed that the total levels of actin and vinculin did not differ between WT and Nmp4 KO cells (Fig. 5).
Figure 5.
OFSS-induced reorganization of actin filaments into stress fibers, and formation of focal adhesions is qualitatively more extensive in Nmp4 KO osteoblasts compared to osteoblasts from WT mice. (A) Vinculin immunofluorescence to detect focal adhesions. (B) Texas-red-phalloidin labeling of F-actin. (C) Merged image of staining in (A) and (B). Cells were subjected to OFSS for 4 hr. (D) Immunoblot analysis to measure total levels of vinculin and actin in Nmp4 KO and WT osteoblasts maintained in static culture or subjected to 4 hr OFSS.
Discussion
Our results suggest a potentially important regulatory mechanism to explain the high bone mass phenotype of mice that lack expression of the nucleocytoplasmic shuttling transcription factor Nmp4/CIZ. Previous work has suggested that Nmp4/CIZ functions as a transcriptional repressor of genes that are crucial to normal regulation of osteoblast differentiation and of genes that are activated by anabolic stimuli such as PTH and BMP-2 (Morinobu et al., 2005; Robling et al., 2009). However, the molecular mechanisms that mediate this effect remain to be determined. Our identification of the β-catenin signaling pathway as a target of Nmp4/CIZ-mediated action in primary osteoblasts from Nmp4 KO mice represents a potential point of convergence between mechanical load- and PTH-induced regulation of bone formation (Childress et al., 2009).
To investigate the role of Nmp4/CIZ in mechanotransduction, we used oscillatory fluid shear stress (OFSS), arguably the most physiologically relevant type of mechanical stimulus for bone cells. We found that OFSS-induced signals in osteoblasts that are triggered both upstream (ERK, Akt and GSK-3β activation) and downstream (cyclin D1 expression) of β-catenin are more robust when Nmp4/CIZ is absent. Our findings do not yet allow us to identify a specific molecular role for Nmp4/CIZ in load-induced β-catenin signaling. However, our data build on several existing lines of experimental support in favor of a role for Nmp4/CIZ in regulating the anabolic effects of mechanical loading in bone cells. First, Nmp4/CIZ interacts with two important focal adhesion-associated proteins: p130Cas, via the p130Cas SH3 domain (Nakamoto et al., 2000), or indirectly via an association with zyxin, which also interacts directly with ZNF384, the human ortholog of Nmp4/CIZ (Janssen and Marynen, 2006). Nmp4/CIZ has also been shown to regulate the fluid shear-induced expression of Mmp-13 in bone cells and is itself regulated by fluid shear stress (Charoonpatrapong-Panyayong et al., 2007).
CIZ-KO mice do not lose bone during hind limb unloading providing strong evidence of a role for Nmp4/CIZ on load-regulated bone formation in vivo (Hino et al., 2007). Thus, at both the cellular and tissue level there is evidence that Nmp4/CIZ may suppress load-induced mechanical signals that promote new bone formation. The present data suggest that while in the cytoplasm, Nmp4/CIZ inhibits β-catenin nuclear translocation as well as other signaling pathways thus reinforcing its repressive action on the transcription of target genes (Charoonpatrapong-Panyayong et al., 2007; Shah et al., 2004; Shen et al., 2002; Thunyakitpisal et al., 2001).
Reorganization of the actin cytoskeleton into contractile bundles (stress fibers) and enhanced formation of focal adhesions in osteoblasts in response to both unidirectional and oscillatory fluid shear stress has been previously reported (Pavalko et al., 1998; Ponik et al., 2007). Although our group has shown that cytoskeletal reorganization is not required for all mechanically-induced gene expression (Norvell et al., 2004b), other studies suggest that inhibiting actin assembly inhibits osteoblast gene expression in response to pulsating fluid flow (Ajubi et al., 1996; Toma et al., 1997). Less controversial is the requirement for focal adhesion formation in mechanotransduction. When the ability of cells to form focal adhesions in response to mechanical load (Ponik and Pavalko, 2004; Wozniak et al., 2000) or the activity of the focal adhesion-associated tyrosine kinase FAK (Young et al., 2009) are blocked, load-induced signaling, including prostaglandin production and gene expression are severely inhibited. We found that in the absence of Nmp4/CIZ OFSS-induced stress fiber formation and focal adhesion formation are qualitatively enhanced. These results provides additional support for the notion that Nmp4CIZ functions to render cells less responsive to mechanical stimulation by fluid shear stress. Future studies aimed at evaluating the effect of Nmp4/CIZ directly on integrin-mediated (focal adhesion) signaling activity should be able to more directly evaluate the mechanistic role of this protein in mechanotransduction.
It is unclear how Nmp4/CIZ inhibits nuclear translocation of β-catenin. One potential convergence point between the p130Cas/Nmp4/CIZ and Lef1/β-catenin pathways may be the SMAD proteins (Childress et al., 2009). R-SMAD activity is upregulated by both PTH and load and these proteins are important components of the anabolic pathways in bone (Li, 2008; Ogita et al., 2008; Sowa et al., 2003; Tobimatsu et al., 2006). The R-SMADs physically interact with the Lef1/β-catenin proteins and together synergistically enhance osteoblast gene expression (Guo et al., 2008; Labbe et al., 2000; Sato et al., 2009). Nmp4/CIZ attenuates R-SMAD activity (Shen et al., 2002) but it is not yet clear whether this occurs in the cytoplasm, the nucleus or both. Additionally, p130Cas, a major binding partner of Nmp4/CIZ, suppresses R-SMAD activation (Kim et al., 2008). Elucidating the components and interactions comprising this putative mechanosome network is necessary for understanding the cellular and molecular basis of the skeleton’s response to PTH and loading.
Acknowledgments
This work was supported by grants R01AR052682 to FMP and DK053796 to JPB.
LITERATURE CITED
- Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes--a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225(1):62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Long H, Onyia J, Hock J, Xu W, Bidwell J. Rat osteoblast and osteosarcoma nuclear matrix proteins bind with sequence specificity to the rat type I collagen promoter. Endocrinology. 1997;138(1):482–489. doi: 10.1210/endo.138.1.4852. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Thunyakitpisal P, Morrison P, Onyia J, Hock J, Bidwell JP. PTH-responsive osteoblast nuclear matrix architectural transcription factor binds to the rat type I collagen promoter. J Cell Biochem. 1998;69(3):336–352. doi: 10.1002/(sici)1097-4644(19980601)69:3<336::aid-jcb11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Bidwell JP, Alvarez M, Feister H, Onyia J, Hock J. Nuclear matrix proteins and osteoblast gene expression. J Bone Miner Res. 1998;13(2):155–167. doi: 10.1359/jbmr.1998.13.2.155. [DOI] [PubMed] [Google Scholar]
- Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283(43):29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoonpatrapong-Panyayong K, Shah R, Yang J, Alvarez M, Pavalko FM, Gerard-O’Riley R, Robling AG, Templeton E, Bidwell JP. Nmp4/CIZ contributes to fluid shear stress induced MMP-13 gene induction in osteoblasts. J Cell Biochem. 2007;102(5):1202–1213. doi: 10.1002/jcb.21349. [DOI] [PubMed] [Google Scholar]
- Childress P, Robling AG, Bidwell JP. Nmp4/CIZ: Road block at the intersection of PTH and load. Bone. 2009 doi: 10.1016/j.bone.2009.09.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Flanagan J, Jasuja R, Kirkland J, Jiang L, Bhasin S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/beta-catenin signaling pathways. J Biol Chem. 2008;283(14):9136–9145. doi: 10.1074/jbc.M708968200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino K, Nakamoto T, Nifuji A, Morinobu M, Yamamoto H, Ezura Y, Noda M. Deficiency of CIZ, a nucleocytoplasmic shuttling protein, prevents unloading-induced bone loss through the enhancement of osteoblastic bone formation in vivo. Bone. 2007;40(4):852–860. doi: 10.1016/j.bone.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31(11):969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen H, Marynen P. Interaction partners for human ZNF384/CIZ/NMP4--zyxin as a mediator for p130CAS signaling? Exp Cell Res. 2006;312(7):1194–1204. doi: 10.1016/j.yexcr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kim W, Seok Kang Y, Soo Kim J, Shin NY, Hanks SK, Song WK. The integrin-coupled signaling adaptor p130Cas suppresses Smad3 function in transforming growth factor-beta signaling. Mol Biol Cell. 2008;19(5):2135–2146. doi: 10.1091/mbc.E07-10-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95(6):1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci U S A. 2000;97(15):8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. Bone morphogenetic protein-Smad pathway as drug targets for osteoporosis and cancer therapy. Endocr Metab Immune Disord Drug Targets. 2008;8(3):208–219. doi: 10.2174/187153008785700127. [DOI] [PubMed] [Google Scholar]
- Morinobu M, Nakamoto T, Hino K, Tsuji K, Shen ZJ, Nakashima K, Nifuji A, Yamamoto H, Hirai H, Noda M. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med. 2005;201(6):961–970. doi: 10.1084/jem.20041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto T, Yamagata T, Sakai R, Ogawa S, Honda H, Ueno H, Hirano N, Yazaki Y, Hirai H. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol Cell Biol. 2000;20(5):1649–1658. doi: 10.1128/mcb.20.5.1649-1658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int. 2004a;75(5):396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- Norvell SM, Ponik SM, Bowen DK, Gerard R, Pavalko FM. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol. 2004b;96(3):957–966. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]
- Ogita M, Rached MT, Dworakowski E, Bilezikian JP, Kousteni S. Differentiation and proliferation of periosteal osteoblast progenitors are differentially regulated by estrogens and intermittent parathyroid hormone administration. Endocrinology. 2008;149(11):5713–5723. doi: 10.1210/en.2008-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou DJ, Papachroni KK, Basdra EK, Papavassiliou AG. Signaling networks and transcription factors regulating mechanotransduction in bone. Bioessays. 2009;31(7):794–804. doi: 10.1002/bies.200800223. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998;275(6 Pt 1):C1591–1601. [PubMed] [Google Scholar]
- Pavalko FM, Gerard RL, Ponik SM, Gallagher PJ, Jin Y, Norvell SM. Fluid shear stress inhibits TNF-alpha-induced apoptosis in osteoblasts: a role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J Cell Physiol. 2003a;194(2):194–205. doi: 10.1002/jcp.10221. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A Model for mechanotransduction in bone cells: The load-bearing mechanosomes. J Cell Biochem. 2003b;88(1):104–112. doi: 10.1002/jcb.10284. [DOI] [PubMed] [Google Scholar]
- Ponik SM, Pavalko FM. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. J Appl Physiol. 2004;97(1):135–142. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]
- Ponik SM, Triplett JW, Pavalko FM. Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J Cell Biochem. 2007;100(3):794–807. doi: 10.1002/jcb.21089. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281(42):31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- Robling AG, Childress P, Yu J, Cotte J, Heller A, Philip BK, Bidwell JP. Nmp4/CIZ suppresses parathyroid hormone-induced increases in trabecular bone. J Cell Physiol. 2009;219(3):734–743. doi: 10.1002/jcp.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A, Bakker AD, Klein-Nulend J. The role of osteocytes in bone mechanotransduction. Osteoporos Int. 2009a;20(6):1027–1031. doi: 10.1007/s00198-009-0858-5. [DOI] [PubMed] [Google Scholar]
- Santos A, Bakker AD, Zandieh-Doulabi B, de Blieck-Hogervorst JM, Klein-Nulend J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/akt, and focal adhesion kinase. Biochem Biophys Res Commun. 2009b doi: 10.1016/j.bbrc.2009.11.064. [DOI] [PubMed] [Google Scholar]
- Sato MM, Nakashima A, Nashimoto M, Yawaka Y, Tamura M. Bone morphogenetic protein-2 enhances Wnt/beta-catenin signaling-induced osteoprotegerin expression. Genes Cells. 2009;14(2):141–153. doi: 10.1111/j.1365-2443.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127(5):1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Alvarez M, Jones DR, Torrungruang K, Watt AJ, Selvamurugan N, Partridge NC, Quinn CO, Pavalko FM, Rhodes SJ, Bidwell JP. Nmp4/CIZ regulation of matrix metalloproteinase 13 (MMP-13) response to parathyroid hormone in osteoblasts. Am J Physiol Endocrinol Metab. 2004;287(2):E289–296. doi: 10.1152/ajpendo.00517.2003. [DOI] [PubMed] [Google Scholar]
- Shen ZJ, Nakamoto T, Tsuji K, Nifuji A, Miyazono K, Komori T, Hirai H, Noda M. Negative regulation of bone morphogenetic protein/Smad signaling by Cas-interacting zinc finger protein in osteoblasts. J Biol Chem. 2002;277(33):29840–29846. doi: 10.1074/jbc.M203157200. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Canaff L, Hendy GN, Tsukamoto T, Yamaguchi T, Miyazono K, Sugimoto T, Chihara K. Inactivation of menin, the product of the multiple endocrine neoplasia type 1 gene, inhibits the commitment of multipotential mesenchymal stem cells into the osteoblast lineage. J Biol Chem. 2003;278(23):21058–21069. doi: 10.1074/jbc.M302044200. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Thunyakitpisal P, Alvarez M, Tokunaga K, Onyia JE, Hock J, Ohashi N, Feister H, Rhodes SJ, Bidwell JP. Cloning and functional analysis of a family of nuclear matrix transcription factors (NP/NMP4) that regulate type I collagen expression in osteoblasts. J Bone Miner Res. 2001;16(1):10–23. doi: 10.1359/jbmr.2001.16.1.10. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T, Kaji H, Sowa H, Naito J, Canaff L, Hendy GN, Sugimoto T, Chihara K. Parathyroid hormone increases beta-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology. 2006;147(5):2583–2590. doi: 10.1210/en.2005-1627. [DOI] [PubMed] [Google Scholar]
- Toma CD, Ashkar S, Gray ML, Schaffer JL, Gerstenfeld LC. Signal transduction of mechanical stimuli is dependent on microfilament integrity: identification of osteopontin as a mechanically induced gene in osteoblasts. J Bone Miner Res. 1997;12(10):1626–1636. doi: 10.1359/jbmr.1997.12.10.1626. [DOI] [PubMed] [Google Scholar]
- Wang H, Li M, Lin PH, Yao Q, Chen C. Fluid shear stress regulates the expression of TGF-beta1 and its signaling molecules in mouse embryo mesenchymal progenitor cells. J Surg Res. 2008;150(2):266–270. doi: 10.1016/j.jss.2007.12.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24(2):171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak M, Fausto A, Carron CP, Meyer DM, Hruska KA. Mechanically strained cells of the osteoblast lineage organize their extracellular matrix through unique sites of alphavbeta3-integrin expression. J Bone Miner Res. 2000;15(9):1731–1745. doi: 10.1359/jbmr.2000.15.9.1731. [DOI] [PubMed] [Google Scholar]
- You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42(1):172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SR, Gerard-O’Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24(3):411–424. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]