Abstract
Herpes simplex virus type-1 (HSV-1) infection of human brain cells induces changes in gene expression favorable to the propagation of the infecting agent and detrimental to the function of the host cells. We report that infection of human primary neural cells with a high phenotypic reactivator HSV-1 (17syn +) induces upregulation of a brain-enriched microRNA (miRNA)-146a that is associated with proinflammatory signaling in stressed brain cells and Alzheimer’s disease. Expression of cytoplasmic phospholipase A2, the inducible prostaglandin synthase cyclooxygenase-2, and the neuroinflammatory cytokine interleukin-1β were each upregulated. A known miRNA-146a target in the brain, complement factor H, was downregulated. These data suggest a role for HSV-1-induced miRNA-146a in the evasion of HSV-1 from the complement system, and the activation of key elements of the arachidonic acid cascade known to contribute to Alzheimer-type neuropathological change.
Keywords: Alzheimer’s disease, arachidonic acid, complement factor H, cyclooxygenase-2, cytosolic phospholipase A2, herpes simplex virus type-1, immune response, inflammation, interleukin-1β, microRNA-146a
Introduction
The double-stranded herpes simplex virus-1 (HSV-1), a neurotrophic, neuroinvasive group 1 member of the herpes virus family Herpesviridae containing at least 74 genes, is known to establish lifelong latency in nervous tissues [1]. Although a link between HSV-1 infection and Alzheimer’s disease was suggested 30 years ago, the molecular genetic mechanism of this pathogenic association is yet to be fully determined [2–4]. A generalized upregulation of inflammatory signaling has been associated with both HSV-1 infection of stressed brain cells and Alzheimer’s disease, where there are increased expression of specific members of the arachidonic acid (AA) cascade [5–7].
MicroRNAs (miRNAs) are small noncoding RNAs that through base-pair complementarity with their target mRNAs, typically located in the 3′-trailer regions, regulate gene expression posttranscriptionally [8–15]. Brain cells and tissues seem to use a specific subset of all currently known miRNAs in homeostatic brain functions [8,9]. Recently, several brain-enriched miRNAs, including miRNA-146a, have been implicated in the regulation of the host immune and inflammatory response [7–11].
In this study, we used miRNA arrays, Northern dot blot, and Western analysis to examine the effects of HSV-1 (17syn+) infection on miRNA speciation and immune and inflammatory signaling in human neural (HN) cells in primary culture. This is the first report of a coordinated upregulation of the proinflammatory markers cytosolic phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2), interleukin-1β (IL-1β), and miRNA-146a, coupled to downregulation of the immune system repressor complement factor H (CFH). cPLA2 and COX-2 are key players in the AA cascade. The recruitment of two key AA cascade members and a miRNA-146a-mediated downregulation of CFH underscore a successful strategy used by HSV-1 to subvert cellular machinery to ensure the success of infection and viral propagation.
Materials and methods
Antibodies and reagents
All reagents were purchased from commercial biomedical suppliers and were used without further purification. RNA isolation reagents such as isopropanol, nucleic acid grade ethanol, diethyl pyrocarbonate water, RNAse-free plastic reaction vials, and disposable minihomogenizers were purchased from Invitrogen (Carlsbad, California, USA) or Ambion (Austin, Texas, USA). Western immunoblots were performed using human-specific primary antibodies directed against the control marker β-actin (3598-100; Sigma-Aldrich Chemical Company, St. Louis, Missouri, USA), COX-2 (C-20; sc-1745), cPLA2 (N-216; sc-438), IL-1β (C-20; sc-1250), and CFH (H-5; sc-166608) (Santa Cruz Biotechnologies, Santa Cruz, California, USA) as described earlier [11,12].
Human neural cells in primary culture and HSV-1 infection
Starting as primary spheroids, HN cells (CC-2599; Lonza Corporation, Walkersville, Maryland, USA) were grown in an HN maintenance medium (Lonza Corporation, Walkersville, Maryland, USA) for 2 weeks as described earlier [11,12] (Fig. 1a). HN cells displayed approximately equal populations of neurons and glia after 2 weeks; modified HN maintenance medium was changed every 3.5 days, and the total RNA and protein were extracted at 0, 24, and 48 h after HSV-1 infection. The HSV-1 strain 17syn+, a low passage isolate 3X plaque purified, was added at time ‘0’ at a multiplicity of infection (MOI) ratio of 5: 1 and 10: 1 (HSV-1 virus particle-to-HN cell ratio).
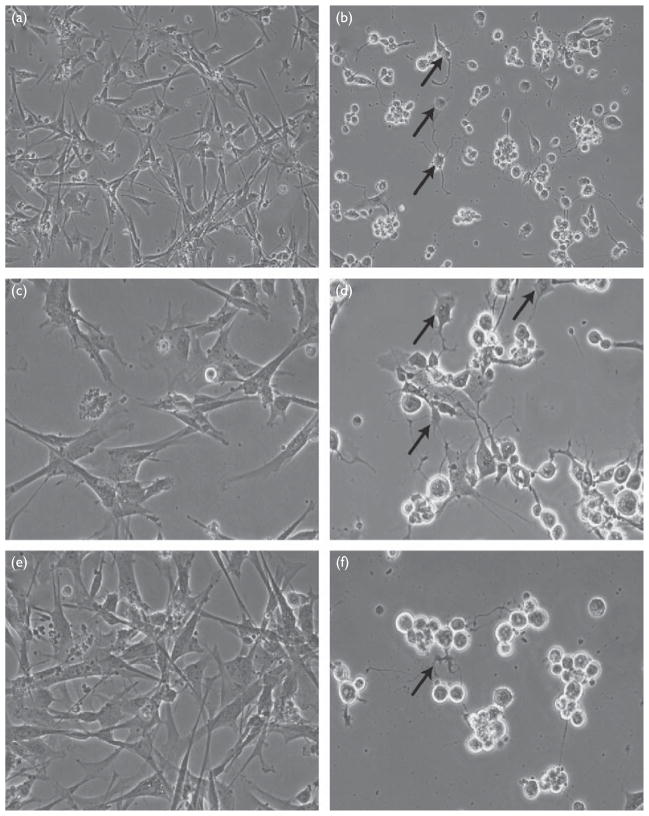
Fig. 1.
Morphology of human neural (HN) cells before (a, c, and e) and after (b, d, and f) infection with herpes simplex virus type-1 (HSV-1). (a) Control, 24 h ×10 magnification and (b) HSV-1 infected, 24 h ×10 magnification; note rounding up of cell bodies and loss of normal HN cell morphology (arrows). (c) Control, 24 h ×20 magnification, (d) HSV-1 infected, 24 h ×20 magnification, (e) control, 48 h ×20 magnification, and (f) HSV-1 infected, 48 h ×20 magnification; as indicated by arrows, note progressive atrophy of cell bodies and neurite extensions, cellular blebbing, and irregular morphology of HN cell bodies after 48 h HSV-1 infection.
RNA extraction, quality control, and Northern analysis
After 0, 24, or 48 h of HSV-1 treatment 2-week-old cultured HN cells were rapidly transferred into 1-ml ice-cold TRIzol reagent (Invitrogen). Tissues were homogenized for 2 min, following the manufacturer’s standard protocol, 0.2 ml chloroform was added, vortexed at full speed for 15 s and then centrifuged (15 min; 12 000g; 4°C). The upper aqueous phase was collected; 0.5 ml isopropanol was then added and vortexed for an additional 15 s; samples were incubated at room temperature for 10 min and then centrifuged (10 min; 12 000g; 4°C). Pellets containing total RNA were washed twice with 75% ethanol and air-dried; resulting total RNA pellets were dissolved in 35 μl diethyl pyrocarbonate water. RNA quality was assessed using an Agilent Bioanalyzer 2100 (Lucent Technologies/Caliper Technologies, Palo Alto, California, USA) [11,12]. Total RNA sample (1 μl) was loaded onto an RNA Nano Labchip (Caliper Technologies) and analyzed [11]. An electropherogram was generated for each total RNA sample and if the ratio of 28S/18S was larger than 1.4 (indicating high total RNA spectral quality), the samples were used for Northern analysis as described earlier [11–15]. Samples were analyzed individually or as pooled samples. There were no significant differences in the total RNA yield or RNA spectral quality between the control and HSV-1-infected HN cells.
Protein isolation and Western analysis
Total proteins were simultaneously isolated using TRIzol reagent and concentrations were determined using dotMETRIC microassay (sensitivity 0.3 ng protein/ml; Chemicon, Temecula, California, USA) [11]. To ascertain whether HSV-1 infection was associated with an increase in the expression of inflammatory or pathogenic proteins that correlated to mRNA levels in the Northern assays, Western immunoblots were performed for cPLA2, COX-2, IL-1β, and CFH proteins using β-actin as a control and the antibodies described above. Signals were detected with an anti-IgG fluor-linked secondary antibody/an ECL+Western immune blotting system (RPN2132/PA45007; Amersham Bioscience, Piscataway, New Jersey, USA) [11,12].
Brain-enriched miRNA array analysis
As a preliminary screen, miRNA arrays (LC Sciences, Houston, Texas, USA) were probed with total small miRNAs isolated at 0, 24, and 48 h from HSV-1-infected HN cells or control HN cells cultured in parallel. Specific miRNAs showing strong hybridization signals were studied further and subjected to Northern dot blot analysis [9,11,14]. Total small RNA extracts (25 μg) containing total miRNA were spotted onto GeneScreen membranes (PerkinElmer, Waltham, Massachusetts, USA), transferred using a vacuum manifold, cross-linked, baked, hybridized, and probed with specific DNA oligomers corresponding to specific small RNAs and miRNAs, as described earlier [11–14].
Statistical analysis and data interpretation
All statistical procedures for cPLA2, COX-2, IL-1β, and CFH Northern [messenger RNA (mRNA)] and Western (protein) abundance were analyzed using a two-way factorial analysis of variance (P value) using programs and procedures in the SAS language (Statistical Analysis Institute, Cary, North Carolina, USA), and as described earlier by our group [11–14]. Only P values of less than 0.05 (analysis of variance) were considered to be statistically significant. Figures were generated using Microsoft Office Excel 2007 (Microsoft Corporation, Redmond, Washington, USA) and Adobe Designer Version 6.0 (Adobe, San Jose, California, USA).
Results
Morphological changes in human brain cells after HSV-1 infection
The culture of control HN cells exhibited a progressive increase in cell number from their original plating as neurospheres, reaching about 40% confluency, and made up of about equal populations of neurons and glia, after 2 weeks of culture [11,12]. In contrast, HSV-1-treated cells cultured in parallel in tissue culture showed progressively heterogeneous morphologies as HSV-1 infection progressed from 0 to 48 h (Fig. 1). HSV-1-infected brain cells typically showed a ‘rounding up’ of cell bodies and atrophy of neurite processes (Fig. 1). Preliminary data showed that an MOI of 10: 1 yielded more significant miRNA and mRNA changes and was used in all subsequent experiments.
MicroRNA changes
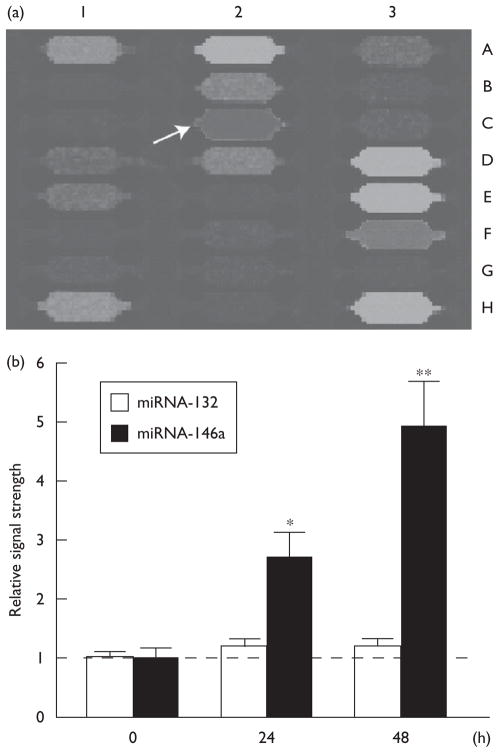
Analysis of miRNA panels that display 911 control RNAs, small RNAs, and miRNA levels showed consistently upregulated levels of miRNA-146a to 2.7-fold and 5.0-fold, 24 and 48 h after HSV-1 infection, respectively (Fig. 2). There was no change in the abundance of a closely related brain-enriched miRNA-132 analyzed in the same sample. All miRNA levels were normalized against (i) the abundance of 5S RNA in each sample, and also (ii) against eight hybridization controls on the miRNA panels whose expression levels remained unchanged either before or after HSV-1 infection (Fig. 2).
Fig. 2.
(a) Specific upregulation of miRNA-146a (coordinate 2C) on miRNA array panels and (b) signal quantitation of miRNA Northern dot blots. Individual miRNA panels were probed with total miRNA obtained from control and herpes simplex virus type-1 (HSV-1)-infected human neural (HN) cells (48 h) (a) and the results were compared; by convention, blue fluorescence indicates no expression detected and green-brown fluorescence indicates nonsignificant changes compared with controls. Column 1A–1H represents eight hybridization controls whose identity can be found at www.lcsciences.com; other miRNA signals are for miRNA-144 (2A), miRNA-145 (2B) miRNA-146b (2D), miRNA-147 (2E), miRNA-148a (2F), miRNA-148b (2G), miRNA-149 (2H). Those miRNAs that were detected in HN cells, but whose relative signal strength neither increased nor decreased significantly after HSV-1 infection were miRNA-200b (3D), miRNA-200c (3E), miRNA-202 (3F) and miRNA-203 (3H). (b) N=4; significance over ‘0’ time controls; *P<0.05; **P<0.01 (analysis of variance). miRNA, microRNA.
RNA and protein extraction and quality control
Controls and HSV-1-treated cell samples each yielded total RNA samples with 28S/18S >1.45 and single, sharp protein bands for cPLA2, COX-2, IL-1β, and CFH with no evidence of protein degradation.
Changes in cPLA2, COX-2, IL-1β, and CFH
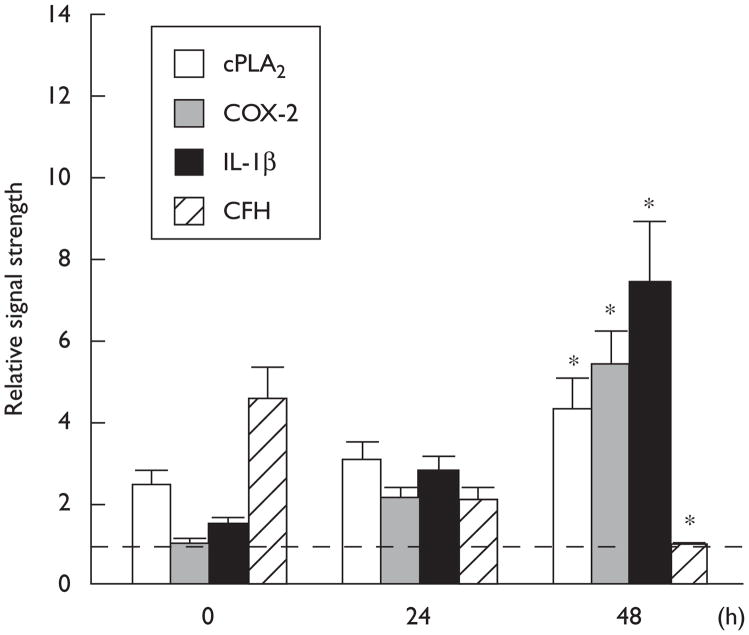
Western analysis showed upregulation of the inflammatory markers cPLA2, COX-2, and IL-1β, and downregulation of CFH, when compared with control β-actin levels in the same HN cell sample. Forty-eight hours after HSV-1 infection, cPLA2, COX-2, and IL-1β were found to be upregulated over 0 h controls 4.5-fold, 5.1-fold, and 7.2-fold, respectively, and CFH was found to be reduced 4.5-fold when compared with 0 h controls (Fig. 3).
Fig. 3.
Western-based analysis showing specific upregulation relative abundance of human cytosolic phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2), and interleukin-1β (IL-1β) in 0, 24, and 48 h herpes simplex virus type-1 (HSV-1)-infected human neural cells and downregulation of complement factor H (CFH); the relative signal strength for β-actin did not significantly change after HSV-1 infection; N=4; significance over ‘0’ time controls; *P<0.01 (analysis of variance).
Discussion
The course of HSV-1 infection of nervous tissue
HSV-1 type strains are abundant in human nervous tissue and their presence is not related to either age or sex [16]. HSV-1 type strains can be separated into low-reactivation and high-reactivation phenotypes. Low-phenotypic reactivators are typified by HSV-1 strains F, KOS, and 17ΔPst, whereas high-phenotypic reactivators include HSV-1 strains 17syn+ and McKrae [1]. In animal models, HSV-1 strains F, KOS, and 17ΔPst exhibit low-reactivation frequencies when latent animals are given stress inducers. In contrast, HSV-1 strains 17syn+ and McKrae exhibit high-reactivation frequency when given inducers of physiological stress [1]. As expected, an infection of HSV-1 (17syn+) in brain cells using an MOI (HSV-1 particle-to-cell ratio) of both 5 to 10 produced a typical progressive loss of processes and cell rounding (Fig. 1) that have been characterized earlier [4,5].
Gene expression in HSV-1-infected brain cells: upregulation of cPLA2, COX-2, and IL-1β
The actions of the inducible cPLA2 (group IVA, cytosolic, calcium-dependent), the oxidoreductase COX-2, and the brain abundant inflammatory cytokine IL-1β are significantly interrelated in driving the development of brain cell degeneration. The cPLA2, an inducible membrane-associated attack enzyme hydrolyzes the sn-2 position of membrane glycerophospholipid stores to generate AA. As a primary initiator of the AA cycle, cPLA2 and subsequent actions of COX-2 generate eicosanoids and lipid mediators that through lipid–protein and trans-synapse interactions regulate vital signaling and biophysical aspects of plasma membrane biology [16,17]. Excessive cPLA2 action may destabilize plasma membranes and favor HSV-1 entry into cells (unpublished observations). The genes encoding cPLA2 and COX-2 are physically linked at human chromosome 1q25.2-q25.3, and may be under coordinate genetic control as they are often coinduced by oxidative and other physiological stressors (Fig. 3) [16–18]. Interestingly, cPLA2 activity copurifies with HSV-1-specified Fc receptor protein, and COX-2 inhibitors, including nonsteroidal anti-inflammatory drugs, also inhibit HSV-1 proliferation [19]. COX-2 and cPLA2 are found to be consistently overexpressed in Alzheimer-affected brain regions and seem to be centrally involved in the acquisition and maintenance of proinflammatory and apoptotic signaling events [13,17,18]. Levels of several brain-abundant inflammatory peptide cytokines including IL-1β are also elevated in Alzheimer brain [13]. As a potent inducer of COX-2 expression, IL-1β not only sustains, but propagates existing inflammatory and apoptotic processes by positive reinforcement mechanisms [11–13]. Indeed, various layers of regulation are used by neural cells to avoid uncontrolled immune and inflammatory responses. Chronic inflammatory signaling by COX-2 and cPLA2, driven by the excessive induction of a pathogenic host immune response, may be used as a key strategy by viruses to ensure their survival and propagation in host cells.
Upregulation of miRNA-146a and downregulation of CFH
The role of miRNAs as downregulators of gene expression in health and disease has been investigated using miRNA-array and DNA-array analytical strategies, miRNA and mRNA pair-matching and bioinformatics, and by gain-of-function and loss-of-function approaches. The miRNA-146a was first identified as an immune regulator, responsive to induction by IL-1β and other proinflammatory cytokines in human monocytes [8,10]. Extension of these studies to human brain cells and tissues has shown that miRNA-146a is an inducible, low-to-moderately abundant small RNA in human brain, has a relatively short half-life, in the order of approximately 1.5 h, and is rapidly induced by IL-1β, by amyloid-β42 peptides, by oxidative stress, and by HSV-1 infection (Fig. 2) [9,11,14]. Interestingly, IL-1β, amyloid-β42 peptides, oxidative stress, and viral infection all upregulate pathogenic gene expression by their activation of the proinflammatory transcription factor NF-κB, and miRNA- 146a is under NF-κB transcriptional control [10,11,20]. Moreover, miRNA-146a upregulation is strongly linked to downregulation of CFH, an important repressor of the complement signaling cascade [8,21]. Conversely, blocking of NF-κB activation using pyrrolidine dithiocarbamate or the resveratrol analog CAY10512 in human brain cells displays strong anti-miRNA-146a effects, resulting in increased CFH expression and consequent rescue of proinflammatory signaling markers [11]. The known inhibition of HSV-1 replication and suppression of HSV-1- induced activation of NF-κB by the phytoalexin resveratrol could be, in part, regulated by reduced miRNA-146a signaling; however, this mechanism remains to be investigated [11,22]. More recently, Epstein–Barr virus and vesicular stomatitis virus have been shown to significantly upregulate the expression of miRNA-146a, and act as a miRNA-146a-mediated negative regulator of tumor necrosis factor receptor-associated factor 6 (TRAF6), and interleukin-1 receptor-associated kinase-1 (IRAK1), key elements of the host immune and inflammatory response [23,24]. Although this is the first reported coordinated upregulation of cPLA2, COX-2, IL-1β, and miRNA-146a in HSV-1-infected HN cells coupled to a decrease in the expression of the miRNA-146a target, CFH, specific interactions between miRNA-146a and cPLA2, COX-2, or IL-1β gene expression, if any, are not currently known. Finally, in addition to host miRNAs, HSV-1 has been reported to induce specific ‘viral miRNAs’, the functions of which are not completely understood [25].
Conclusion
We showed an elevation of cPLA2, COX-2, IL-1β, and miRNA-146a in HSV-1-infected HN cells, and a decrease in the expression of a known miRNA-146a target, CFH. These data suggest an evasion of HSV-1 from complement activation, a major first-line host defense mechanism. Anti-inflammatory, antiviral, or anti-miRNA strategies, or their combination, may be useful in retarding the success of HSV-1 infection.
Acknowledgments
The authors thank Darlene Guillot and Aileen Pogue for expert technical assistance. This study was supported, in part, through Translational Research Initiative Grants from the LSU Health Sciences Center, New Orleans (J.M.H. and W.J.L.); the Alzheimer Association (W.J.L.); NIH UIO6311; AG23085; EY 2377; EY06311 Research to prevent Blindness Senior Investigator (J.M.H.); Research to Prevent Blindness, New York, NY; The Louisiana Vaccine Center and the South Louisiana Institute for Infectious Disease Research (Louisiana Board of Regents); Louisiana Eye Lions and Lions International Foundation.
References
- 1.Toma HS, Murina AT, Areaux RG, Neumann DM, Bhattacharjee PS, Foster TP, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23:249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- 2.Ball MJ. Limbic predilection in Alzheimer dementia: is reactivated herpes virus involved? Can J Neurol Sci. 1982;9:303–306. doi: 10.1017/s0317167100044115. [DOI] [PubMed] [Google Scholar]
- 3.Itzhaki RF, Wozniak MA. Herpes simplex virus type 1 in Alzheimer’s disease: the enemy within. J Alzheimers Dis. 2008;13:393–405. doi: 10.3233/jad-2008-13405. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson GA, Maitland NJ, Craske J, Wilcock GK, Itzhaki RF. Detection of herpes simplex virus type 1 DNA sequences in normal and Alzheimer’s disease brain using polymerase chain reaction. Biochem Soc Trans. 1991;19:122S. doi: 10.1042/bst019122s. [DOI] [PubMed] [Google Scholar]
- 5.Hill JM, Lukiw WJ, Gebhardt BM, Higaki S, Loutsch JM, Myles ME, et al. Gene expression analyzed by microarrays in HSV-1 latent mouse trigeminal ganglion following heat stress. Virus Genes. 2001;23:273–280. doi: 10.1023/a:1012517221937. [DOI] [PubMed] [Google Scholar]
- 6.Lukiw WJ. Gene expression profiling in fetal, aged, and Alzheimer hippocampus: a continuum of stress-related signaling. Neurochem Res. 2004;29:1287–1297. doi: 10.1023/b:nere.0000023615.89699.63. [DOI] [PubMed] [Google Scholar]
- 7.Lehtinen M, Koivisto V, Lahtinen P, Lehtinen T, Aaran RK, Leinikki P. Phospholipase A2 activity is copurified together with herpes simplex virus-specified Fc receptor proteins. Intervirology. 1988;29:50–56. doi: 10.1159/000150028. [DOI] [PubMed] [Google Scholar]
- 8.Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 9.Prerna S, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett. 2009;459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 10.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukiw WJ, Cui JG, Zhao Y. An NF-κB-sensitive microRNA-146a-mediated inflammatory circuit in Alzheimer’s disease and in stressed human brain cells. J Biol Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TPA, Percy ME, et al. Characterization of an NF-κB-regulated, miRNA-146a-mediated downregulation of CFH in metal-sulfate-stressed human brain cells. J Inorganic Biochem. 2009;111:156–164. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Lukiw WJ. Micro RNA speciation in fetal, aged and Alzheimer hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 14.Lukiw WJ, Pogue AI. Induction of specific miRNA species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higaki S, Deai T, Fukuda M, Shimomura Y. Microarray analysis in the HSV-1 latently infected mouse trigeminal ganglia. Cornea. 2004;23:S42–S47. doi: 10.1097/01.ico.0000136665.56247.89. [DOI] [PubMed] [Google Scholar]
- 16.Hill JM, Ball MJ, Neumann DM, Azcuy AM, Bhattacharjee PS, Bouhanik S, et al. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82:8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandrov P, Cui JG, Zhao Y, Lukiw WJ. 24S-hydroxycholesterol induces inflammatory gene expression in human neural cells. Neuroreport. 2005;16:909–913. doi: 10.1097/00001756-200506210-00007. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport SI. In vivo approaches and rationale for quantifying kinetics and imaging brain lipid metabolic pathways. Prostagland Other Lipid Mediat. 2005;77:185–196. doi: 10.1016/j.prostaglandins.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Higaki S, Watanabe K, Itahashi M, Shimomura Y. Cyclooxygenase-inhibiting drug reduces HSV-1 reactivation in a mouse eye model. Curr Eye Res. 2009;34:171–176. doi: 10.1080/02713680802650377. [DOI] [PubMed] [Google Scholar]
- 20.Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Huemer HP, Wang Y, Garred P, Koistinen V, Oppermann S. Herpes simplex virus glycoprotein C: molecular mimicry of complement regulatory proteins by a viral protein. Immunology. 1993;79:639–647. [PMC free article] [PubMed] [Google Scholar]
- 22.Faith SA, Sweet TJ, Bailey E, Booth T, Docherty JJ. Resveratrol suppresses nuclear factor-κB in herpes simplex virus infected cells. Antiviral Res. 2006;72:242–251. doi: 10.1016/j.antiviral.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Motsch N, Pfuhl T, Mrazek J, Barth S, Grässer FA. Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a. RNA Biology. 2007;4:131–137. doi: 10.4161/rna.4.3.5206. [DOI] [PubMed] [Google Scholar]
- 24.Hou J, Wang P, Lin L, Liu X, Ma F, An H, et al. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 25.Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]