Abstract
Pulse pressure is a well established marker of vascular stiffness and is associated with increased mortality in hemodialysis patients. Here we sought to determine if a decrease in pulse pressure during hemodialysis was associated with improved outcomes using data from 438 hemodialysis patients enrolled in the 6-month Crit-Line Intradialytic Monitoring Benefit Study. The relationship between changes in pulse pressure during dialysis (2-week average) and the primary end point of non-access-related hospitalization and death were adjusted for demographics, comorbidities, medications, and laboratory variables. In the analyses that included both pre- and post-dialysis pulse pressure, higher pre-dialysis and lower post-dialysis pulse pressure were associated with a decreased hazard of the primary end point. Further, every 10 mm Hg decrease in pulse pressure during dialysis was associated with a 20% lower hazard of the primary end point. In separate models that included pulse pressure and the change in pulse pressure during dialysis, neither pre- nor post-dialysis pulse pressure were associated with the primary end point, but each 10 mm Hg decrease in pulse pressure during dialysis was associated with about a 20% lower hazard of the primary end point. Our study found that in prevalent dialysis subjects, a decrease in pulse pressure during dialysis was associated with improved outcomes. Further study is needed to identify how to control pulse pressure to improve outcomes.
Keywords: end-stage renal disease, hemodialysis, intradialytic blood pressure, morbidity and mortality, outcomes, pulse pressure
Despite significantly elevated cardiovascular morbidity and mortality in hemodialysis patients,1 the appropriate blood pressure target to optimize clinical outcomes in dialysis patients remains to be determined.2 In the general population, elevated pulse pressure, as a marker of conduit vessel stiffness, is clearly associated with adverse cardiovascular outcomes.3-5 Further, lowering pulse pressure in hypertensive patients is associated with improved cardiovascular outcomes.6 In hemodialysis patients, prior analyses demonstrate elevated pre- and post-dialysis pulse pressure to be important predictors of all-cause mortality.7,8 However, the optimal control of pulse pressure to improve outcomes in hemodialysis patients remains to be determined.
Abnormalities of the arterial system are common in dialysis patients and are associated with increased cardiovascular morbidity and mortality.9 The increased mortality associated with increased arterial stiffness (caused by increasing age, hypertension, uremia, and abnormalities in mineral metabolism) is likely the result of the increased systolic stress, which increases left ventricular afterload, decreases coronary perfusion, and leads to left ventricular hypertrophy. Non-invasive measurements of arterial stiffness include pulse wave velocity and pulse pressure (the difference between systolic and diastolic blood pressure). Whereas both increased pulse wave velocity and increased pulse pressure are associated with higher mortality in hemodialysis patients,7,8,10 only reductions in pulse wave velocity have been demonstrated to be associated with regression of left ventricular hypertrophy and improved survival.11,12 This raises the possibility that a decrease in pulse pressure during hemodialysis sessions may be a marker of improved vascular health. We hypothesized that a decrease in pulse pressure during hemodialysis is associated with improved clinical outcomes. To test this hypothesis, we analyzed data from prevalent hemodialysis subjects enrolled in a randomized controlled trial of blood volume monitoring.
RESULTS
Baseline characteristics
In the entire cohort, the mean pre- and post-dialysis pulse pressures were 70 and 64 mm Hg, respectively. Subjects with greater reductions in pulse pressure during hemodialysis had higher dry weights, larger interdialytic weight gains, and trends toward higher prevalence of left ventricular hypertrophy (Table 1). Subjects with greater reductions in pulse pressure during hemodialysis also exhibited higher predialysis systolic blood pressure and lower post-dialysis systolic blood pressure but no significant difference in pre- or post-dialysis diastolic blood pressure (Figure 1). While there were no significant differences in most laboratory variables or antihypertensive class agent use between subjects categorized by quartiles of pulse pressure change with hemodialysis, subjects with greater reductions in pulse pressure with hemodialysis exhibited higher serum calcium.
Table 1. Baseline clinical and demographic characteristics of the study population across quartiles of pulse pressure change during hemodialysis.
| Quartile 1 (Δpulse pressure <−13.8 mm Hg, n=111) |
Quartile 2 (Δpulse pressure ≥−13.8 mm Hg, <−5.4 mm Hg, n=108) |
Quartile 3 (Δpulse pressure ≥−5.4 mm Hg, <2.6 mm Hg, n=108) |
Quartile 4 (Δpulse pressure ≥2.6 mm Hg, n=111) |
P-value* | |
|---|---|---|---|---|---|
| Age (years) | 59.7 (±13.8) | 57.9 (±15.4) | 56.2 (±17.1) | 62.9 (±15.5) | 0.01 |
| Gender (% male) | 54.1% | 52.8% | 52.8% | 45.1% | 0.5 |
| Black race (%) | 41.4% | 34.3% | 32.4% | 31.5% | 0.5 |
| Dialysis vintage (years)a | 2.2 (0.42–7.7) | 2.3 (0.32–13.5) | 1.64 (0.29–10.3) | 2.0 (0.34–8.0) | 0.13 |
| Dry weight (kg) | 82.3 (±25.1) | 76.7 (±18.2) | 76.0 (±21.7) | 73.4 (±17.0) | 0.009 |
| % IDWL | 4.09 (±1.16) | 3.83 (±1.21) | 4.04 (±1.39) | 3.60 (±1.29) | 0.01 |
| Tobacco use | 31.5% | 23.2% | 38.9% | 27.9% | 0.08 |
| Diabetes (cause of ESRD) | 37.8% | 23.2% | 27.8% | 33.3% | 0.10 |
| Hypertension | 92.8% | 86.1% | 86.1% | 87.4% | 0.4 |
| Arrhythmia | 21.6% | 16.7% | 13.0% | 19.8% | 0.4 |
| Coronary artery disease | 27.0% | 32.4% | 24.6% | 34.2% | 0.4 |
| Congestive heart failure | 21.6% | 25.9% | 19.4% | 16.2% | 0.4 |
| Cerebrovascular disease | 25.2% | 14.8% | 16.7% | 16.2% | 0.2 |
| Left ventricular hypertrophy | 46.9% | 48.2% | 38.0% | 31.5% | 0.04 |
| Peripheral vascular disease | 21.1% | 19.4% | 21.8% | 16.2% | 0.7 |
| Pre-dialysis BP (mm Hg) | |||||
| Pulse pressure | 79.5 (±13.4) | 68.8 (±12.1) | 65.1 (±13.1) | 66.5 (±15.5) | <0.0001 |
| Systolic | 160.9 (±16.0) | 148.5 (±18.1) | 144.6 (±18.8) | 144.4 (22.9) | <0.0001 |
| Diastolic | 81.3 (±11.0) | 79.6 (±11.7) | 79.5 (±13.1) | 77.9 (±13.7) | 0.2 |
| Post-dialysis BP (mm Hg) | |||||
| Pulse pressure | 59.0 (±11.3) | 59.0 (±12.2) | 63.5 (±13.5) | 75.2 (±16.0) | <0.0001 |
| Systolic | 130.7 (±14.8) | 132.1 (±17.7) | 137.8 (±18.9) | 150.7 (±21.1) | <0.0001 |
| Diastolic | 71.8 (±10.1) | 73.1 (±11.7) | 74.3 (±11.7) | 75.5 (±11.5) | 0.09 |
| ΔPulse pressure (mm Hg) | −20.5 (±7.3) | −9.9 (±2.1) | −1.6 (±2.2) | 8.8 (±5.9) | <0.0001 |
| Baseline laboratory a | |||||
| Albumin (g/dl) | 3.71 (±0.34) | 3.79 (±0.62) | 3.73 (±0.56) | 3.74 (±0.40) | 0.7 |
| Creatinine (mg/dl) | 9.6 (±3.0) | 9.6 (±2.9) | 9.1 (±3.5) | 8.7 (±2.9) | 0.08 |
| Calcium (mg/dl) | 9.4 (±0.98) | 9.3 (±0.98) | 9.0 (±0.88) | 9.1 (±0.78) | 0.006 |
| Phosphorus (mg/dl) | 5.6 (±1.8) | 6.0 (±1.9) | 5.8 (±2.0) | 5.4 (±1.7) | 0.1 |
| Hemoglobin (g/dl) | 11.3 (±1.2) | 11.6 (±1.4) | 11.6 (±1.4) | 11.2 (±1.3) | 0.05 |
| Urea reduction ratio | 0.71 (±0.07) | 0.72 (±0.07) | 0.71 (±0.09) | 0.70 (±0.31) | 0.9 |
| Number of antihypertensive medications |
1.44 (±1.14) | 1.46 (±1.10) | 1.29 (±1.17) | 1.67 (±1.08) | 0.09 |
| Antihypertensive class | |||||
| ACE-I | 34.2% | 28.7% | 23.2% | 33.3% | 0.3 |
| α-Blocker | 5.4% | 5.6% | 5.6% | 2.7% | 0.7 |
| β-Blocker | 30.6% | 38.9% | 31.5% | 36.9% | 0.5 |
| Calcium channel blk | 42.3% | 37.0% | 32.4% | 44.1% | 0.3 |
| Nitrate | 10.8% | 15.7% | 13.0% | 21.6% | 0.1 |
| Vasodilator | 17.4% | 12.0% | 19.1% | 25.2% | 0.09 |
| Epoetin use | 88.3% | 88.0% | 89.8% | 91.9% | 0.8 |
| Treatment group (versus usual care) |
50.5% | 53.7% | 48.2% | 41.4% | 0.3 |
ACE-I, angiotensin-converting enzyme inhibitor; channel blk, channel blocker; BP, blood pressure; ESRD, end-stage renal disease; IDWL, intradialytic weight loss.
Data presented as mean (s.d.) or median (interquartile range).
χ2 analysis was used for categorical P-values and analysis of variance or Kruskal–Wallis was used for continuous P-values.
IDWL=(pre-dialysis weight−post-dialysis weight/target dry weight) × 100.
Dialysis vintage is missing for 9 subjects and urea reduction ratio for 44 subjects.
Figure 1. Average pre- and post-dialysis systolic blood pressure (SBP) and diastolic blood pressure (DBP) among subjects grouped by quartiles of pulse pressure (PP) change during dialysis.
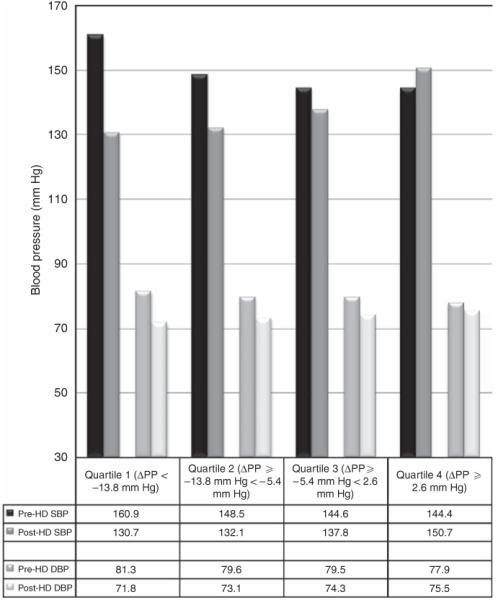
HD, hemodialysis.
Event rates across quartiles of pulse pressure change during hemodialysis
Overall, there were 24 deaths during the 6-month follow-up and 127 subjects were hospitalized for a non-access-related complication (Table 2). Subjects whose pulse pressure decreased >13.8 mm Hg during hemodialysis had the lowest event rates during follow-up, including the combined end point of time to non-access-related hospitalization or death, annual non-access related hospitalization rates, as well as all-cause mortality (Table 2).
Table 2. Outcomes among subjects grouped by quartiles of pulse pressure change during hemodialysis.
| Quartile 1 (Δpulse pressure <−13.8 mm Hg, n=111) |
Quartile 2 (Δpulse pressure ≥−13.8, <−5.4 mm Hg, n=108) |
Quartile 3 (Δpulse pressure ≥−5.4, <2.6 mm Hg, n=108) |
Quartile 4 (Δpulse pressure ≥2.6 mm Hg, n=111) |
|
|---|---|---|---|---|
| Hazard ratio of the primary end point of non-access-related hospitalization or death |
1.00 (ref) | 2.01 (1.14–3.54) | 2.46 (1.42–4.23) | 2.35 (1.36–4.06) |
| Annual non-access-related hospitalization ratesa | 0.50 | 0.93 | 1.30 | 1.16 |
| Six-month all-cause mortality (n) (%) | 3 (2.7%) | 7 (6.5%) | 8 (7.4%) | 6 (5.4%) |
ref, reference.
May include more than one hospitalization per subject.
Unadjusted analysis of hospitalization or death at 6 months
In unadjusted analyses, a decrease in pulse pressure during hemodialysis (per 10 mm Hg) was associated with a 24% decreased hazard ratio (HR) of hospitalization or death (HR 0.76, confidence interval (CI) 0.65–0.88, P=0.0002; Table 3). In unadjusted univariate analyses, higher pre-dialysis pulse pressure was not associated with increased HR of the primary end point, whereas higher post-dialysis pulse pressure (per 10 mm Hg) was associated with a 13% increased hazard of death or hospitalization (Table 3). However, in models including both pre- and post-dialysis pulse pressure, lower pre-dialysis pulse pressure and higher post-dialysis pulse pressure were associated with increased hazard of hospitalization or death. Interestingly, in models including either pre- or post-dialysis pulse pressure and change in pulse pressure during dialysis, neither pre- nor post-dialysis pulse pressure remained associated with the primary end point, whereas a decrease in pulse pressure during hemodialysis was associated with an ~24% reduced hazard of death or hospitalization (Table 3).
Table 3. Unadjusted and adjusted hazard of hospitalization or death associated with pre-dialysis pulse pressure (PP), post-dialysis PP, change in pulse pressure during hemodialysis, or a combination of pulse pressure measurements.
| Model | Blood pressure variable (per 10-mm Hg) | Unadjusted HR (95% CI) |
P-value | Adjusteda HR (95% CI) |
P-value |
|---|---|---|---|---|---|
| 1 | Pre-dialysis pulse pressure (per 10-mm Hg increase) | 0.94 (0.84–1.06) | 0.3 | 0.91 (0.79–1.04) | 0.2 |
| 2 | Post-dialysis pulse pressure (per 10-mm Hg increase) | 1.13 (1.01–1.27) | 0.04 | 1.06 (0.93–1.21) | 0.4 |
| 3 | Change in pulse pressure during hemodialysis (per 10-mm Hg decrease) | 0.76 (0.65–0.88) | 0.0002 | 0.80 (0.69–0.94) | 0.007 |
| 4 | Pre-dialysis pulse pressure (per 10-mm Hg increase) | 0.77 (0.65–0.91) | 0.002 | 0.79 (0.66–0.95) | 0.01 |
| Post-dialysis pulse pressure (per 10-mm Hg increase) | 1.33 (1.14–1.55) | 0.0003 | 1.22 (1.03–1.45) | 0.02 | |
| 5 | Pre-dialysis pulse pressure (per 10-mm Hg increase) | 1.03 (0.90–1.17) | 0.69 | 0.97 (0.83–1.12) | 0.6 |
| Change in pulse pressure during hemodialysis (per 10-mm Hg decrease) | 0.75 (0.64–0.87) | 0.0002 | 0.81 (0.69–0.97) | 0.02 | |
| 6 | Post-dialysis pulse pressure (per 10-mm Hg increase) | 1.03 (0.90–1.17) | 0.71 | 0.96 (0.83–1.12) | 0.6 |
| Change in pulse pressure during hemodialysis (per 10-mm Hg decrease) | 0.77 (0.65–0.91) | 0.002 | 0.79 (0.65–0.94) | 0.01 |
CI, confidence interval; HR, hazard ratio; PP, pulse pressure.
Each model is adjusted for age, race, gender, dry weight, intradialytic weight loss, tobacco use, diabetes, cerebrovascular disease, coronary artery disease, congestive heart disease, presence of left ventricular hypertrophy, hypoalbuminemia, serum calcium, phosphorus, hemoglobin, nitrate, and treatment arm.
Adjusted analyses of hospitalization or death at 6 months
In fully adjusted models, neither pre- nor post-dialysis pulse pressure tested individually was significantly associated with the primary end point (Table 3 and Figure 2). However, when tested together, lower pre-dialysis pulse pressure and higher post-dialysis pulse pressure were associated with increased hazard of the primary end point. In models including either pre- or post-dialysis pulse pressure and change in pulse pressure during hemodialysis, neither pre- nor post-dialysis pulse pressure were associated with the primary end point, whereas a decrease of pulse pressure during hemodialysis was associated with an ~20% reduction in the primary end point of death or hospitalization at 6 months (adjusted HR 0.81, CI 0.69–0.97 for pulse pressure decreasing with hemodialysis adjusted for pre-dialysis pulse pressure; HR 0.79, CI 0.65–0.94 for decreasing pulse pressure with hemodialysis adjusted for post-dialysis pulse pressure).
Figure 2. Adjusted* hazard ratio for hospitalization or death associated with the following blood pressure parameters analyzed in six separate models.
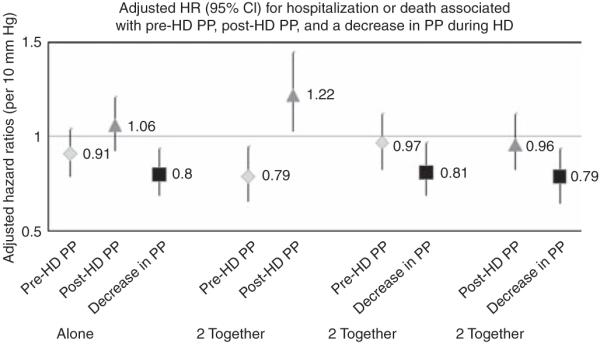
(1) Pre-dialysis pulse pressure modeled individually. (2) Post-dialysis pulse pressure modeled individually. (3) Change in pulse pressure during hemodialysis modeled individually. (4) Pre-dialysis pulse pressure and post-dialysis pulse pressure modeled together. (5) Pre-dialysis pulse pressure and change in pulse pressure during hemodialysis modeled together. (6) Post-dialysis pulse pressure and change in pulse pressure during hemodialysis modeled together. *In addition to the listed blood pressure parameter(s), each model is adjusted for age, race, gender, dry weight, intradialytic weight loss, tobacco use, diabetes, cerebrovascular disease, coronary artery disease, congestive heart failure, presence of left ventricular hypertrophy, hypoalbuminemia, serum calcium, phosphorus, hemoglobin, nitrate, and treatment arm. CI, confidence interval; HD, hemodialysis; HR, hazard ratio; PP, pulse pressure.
In the final multivariable model adjusted for age, gender, race, comorbidity, and relevant laboratory variables, a decrease of pulse pressure during hemodialysis (per 10 mm Hg) was significantly associated with a 20% reduced hazard of the primary end point (HR 0.80, CI 0.69–0.94, P=0.007; Table 4). Variables associated with increased hazard of the primary end point included history of tobacco use and presence of cerebrovascular disease, coronary artery disease, and congestive heart failure. Higher hemoglobin and higher dry weights were associated with decreased hazard of the primary end point. When Δpulse pressure was analyzed as a time-varying covariate in fully adjusted models, there was a 13% reduced hazard of the primary end point associated with every 10 mm Hg decrease in pulse pressure during hemodialysis (HR 0.87, CI 0.80–0.95, P=0.002).
Table 4. Cox proportional hazards model of time to hospitalization or death among end-stage renal disease subjects.
| Variable | HR (95% CI) | P-value |
|---|---|---|
| Change in pulse pressure during hemodialysis (per 10-mm Hg decrease) |
0.80 (0.69–0.94) | 0.007 |
| Dry weight (per 1-kg increase) | 0.98 (0.98–1.00) | 0.02 |
| Tobacco use | 1.51 (1.01–2.26) | 0.04 |
| Cerebrovascular disease | 1.51 (1.13–1.71) | 0.01 |
| Coronary artery disease | 1.85 (1.23–2.77) | 0.003 |
| Congestive heart failure | 1.47 (0.97–2.21) | 0.07 |
| Hemoglobin (per 1-g/dl increase) | 0.82 (0.72–0.94) | 0.006 |
CI, confidence interval; HR, hazard ratio.
Model also adjusted for age; gender; race; intradialytic weight loss; diabetes, presence of left ventricular hypertrophy, serum calcium, serum phosphorus, hypoalbuminemia, nitrates, and treatment group. Variables removed from the final model with P-value of >0.15 included history of arrhythmia, hypertension, Hispanic ethnicity, peripheral vascular disease, serum creatinine, erythropoietin stimulating agent use, and the use of angiotensin-converting enzyme inhibitor, β-blocker, and calcium channel blocker.
Dialysis-related events and pulse pressure changes during follow-up
Considering we identified differences in outcomes across groups of subjects based on pulse pressure responses to hemodialysis, we explored the subsequent occurrence of dialysis-related complications among subjects during the 6-month follow-up (Table 5). In general, subjects whose pulse pressure decreased during dialysis had greater frequency of intradialytic hypotension. There was also a trend toward greater use of intradialytic medications among subjects whose pulse pressure decreased with hemodialysis. Interestingly, there was no difference across subjects with regards to symptomatic complaints of cramping, dizziness, or nausea/vomiting. While rates of treatment sessions using hypertonic saline or sodium modeling was higher among subjects whose pulse pressure decreased during hemodialysis, use of cool dialysate was lowest among these subjects.
Table 5. Rates of treatment-related complications or use of specific therapies during hemodialysis occurring in the 6-month follow-up among patients categorized by baseline pulse pressure changes during hemodialysis.
| Complication or therapy | ΔPulse pressure (<−13.8 mm Hg, n=111) |
ΔPulse pressure (≥−13.8 mm Hg, <−5.4 mm Hg, n=108) |
ΔPulse pressure (≥−5.4 mm Hg, <2.6 mm Hg, n=108) |
ΔPulse pressure (≥2.6 mm Hg, n=111) |
P-value |
|---|---|---|---|---|---|
| Intradialytic hypotension | 0.087 (0.13) | 0.069 (0.14) | 0.049 (0.10) | 0.058 (0.14) | 0.008 |
| Treated or symptomatic intradialytic hypotension |
0.047 (0.07) | 0.049 (0.12) | 0.029 (0.05) | 0.027 (0.05) | 0.006 |
| Intradialytic use of medication | 0.11 (0.12) | 0.10 (0.10) | 0.084 (0.08) | 0.069 (0.07) | 0.06 |
| Dialysis session complicated by cramping, dizziness, or nausea/ vomiting |
0.12 (0.13) | 0.12 (0.13) | 0.10 (0.09) | 0.11 (0.11) | 0.8 |
| Administration of hypertonic saline or use of sodium modeling |
0.19 (0.34) | 0.23 (0.37) | 0.15 (0.32) | 0.095 (0.27) | 0.08 |
| Use of cool dialysate (temp.<36°C) | 0.019 (0.09) | 0.009 (0.03) | 0.033 (0.10) | 0.022 (0.05) | 0.009 |
Rates are defined as the number of treatments during which the event occurred divided by the total number of dialysis sessions. Event rates are reported as means (s.d.); comparisons of events were performed with the non-parametric Kruskal–Wallis test.
During the 6-month follow-up, regression to the mean occurred for the monthly mean change in pulse pressure during hemodialysis among subjects whose pulse pressure increased during hemodialysis (quartile 4; Figure 3) or whose pulse pressure decreased during hemodialysis (quartile 1; Figure 3). Despite this, subjects generally demonstrated similar pulse pressure responses to hemodialysis at month 6 as they did at baseline. For example, subjects in quartile 1 consistently demonstrated >10-mm Hg decreases in pulse pressure during hemodialysis throughout the 6-month follow-up period.
Figure 3. Monthly average change in pulse pressure during dialysis across quartiles of subjects categorized by baseline pulse pressure change during hemodialysis.
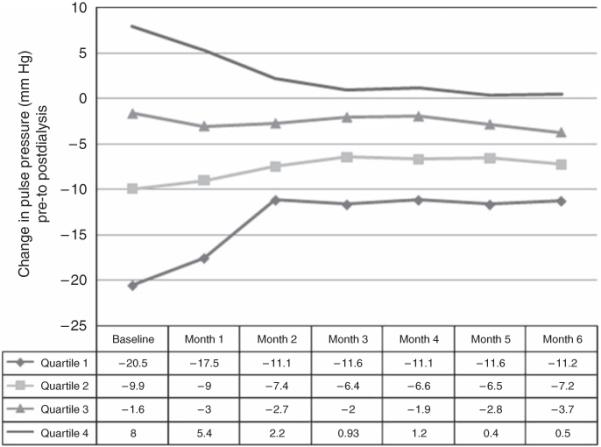
Sensitivity analyses
Since pre-dialysis systolic blood pressure is an important determinant of clinical outcomes, pre-dialysis systolic blood pressure was added to the final model to determine if it modified the relationship between decrease in pulse pressure during hemodialysis and clinical outcomes. In models adjusted for systolic blood pressure, there continued to be lower hazard of death or hospitalization associated with decrease of pulse pressure during hemodialysis (HR 0.81, CI 0.68–0.96, P=0.01)
A separate model analyzing the association between decrease in pulse pressure during hemodialysis and the primary combined end point across strata of pre-dialysis systolic blood pressure was designed. Figure 4 demonstrates that the association between decrease in pulse pressure during hemodialysis and lower hazard of the primary end point persisted across subjects whose pre-dialysis systolic blood pressure values were ≥120 mm Hg, which represents 93% of the cohort. As expected, subjects whose pre-dialysis systolic blood pressure was low (<120 mm Hg) exhibited a trend toward worse outcomes associated with a decrease in pulse pressure during hemodialysis.
Figure 4. Adjusted* hazard ratio for hospitalization or death associated with decrease in pulse pressure during hemodialysis (per 10 mm Hg) among the following four strata of pre-dialysis systolic blood pressure.
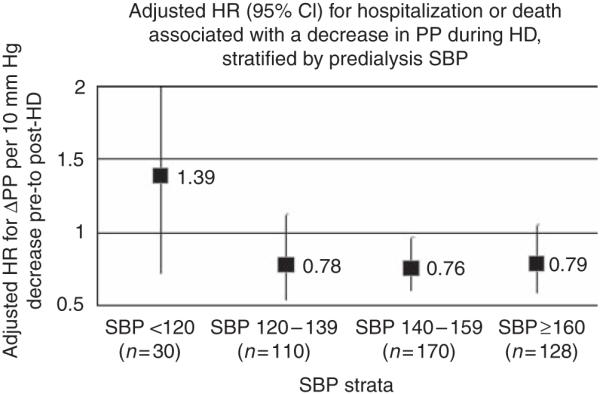
(1) Pre-dialysis systolic blood pressure <120 mm Hg (HR 1.39, CI 0.72–2.76, per 10-mm Hg decrease in pulse pressure with hemodialysis). (2) Pre-dialysis systolic blood pressure 120–139 mm Hg (HR 0.78, CI 0.54–1.13, per 10-mm Hg decrease in pulse pressure with hemodialysis). (3) Pre-dialysis systolic blood pressure 140–159 mm Hg (HR 0.76, CI 0.60–0.97, per 10-mm Hg decrease in pulse pressure with hemodialysis). (4) Pre-dialysis systolic blood pressure ≥160 mm Hg (HR 0.79, CI 0.59–1.06, per 10-mm Hg decrease in pulse pressure with hemodialysis). *Each model is adjusted for age, race, gender, dry weight, intradialytic weight loss, tobacco use, pre-dialysis systolic blood pressure, diabetes, cerebrovascular disease, coronary artery disease, congestive heart failure, presence of left ventricular hypertrophy, hypoalbuminemia, serum calcium, phosphorus, hemoglobin, nitrate, and treatment arm. CI, confidence interval; HD, hemodialysis; HR, hazard ratio; PP, pulse pressure; SBP, systolic blood pressure.
Finally, we analyzed whether the relationship between decrease in pulse pressure and lower hazard of the primary end point was modified by the amount of intradialytic weight loss or use of specific antihypertensive agents. There was no interaction between intradialytic weight loss and decrease in pulse pressure during hemodialysis (P=0.3) as a predictor of the primary end point. Further, the relationship between decreased pulse pressure during hemodialysis and lower hazard of the primary end point persisted across all strata of intradialytic weight loss (data not shown). In the initial models, including the entire cohort of subjects, no specific blood pressure medications were associated with the primary end point (data not shown). Overall, 76% (333/438) of the cohort was treated with antihypertensive medications, but none of the blood pressure medications were associated with the primary end point nor did their use modify the primary results (data not shown).
DISCUSSION
The principal new finding in this study is that a decrease in pulse pressure during hemodialysis is associated with better short-term outcomes in prevalent hemodialysis subjects. Furthermore, our investigation demonstrated that despite higher rates of intradialytic hypotension, decrease in pulse pressure during hemodialysis was more strongly associated with improved clinical outcomes than pre- or post-dialysis pulse pressure either alone or combined. Finally, while this study was not targeted toward lowering pulse pressure with hemodialysis, the observation that subjects whose pulse pressure decreased during hemodialysis had better outcomes suggests blood pressure responses to hemodialysis may be an important risk factor which warrants further investigations.
Prior investigations in hemodialysis patients have identified elevated pre- and post-dialysis pulse pressure to be associated with higher mortality.7,8 In an investigation of 37,069 prevalent hemodialysis patients, every 10-mm Hg increase in post-dialysis pulse pressure was associated with a 12% increased hazard of death at 1 year. Similarly, this study identified every 10-mm Hg increase in post-dialysis pulse pressure (when adjusted for pre-dialysis pulse pressure) to be associated with a 22% increased hazard of death or hospitalization. Interestingly, the relationship between elevated post-dialysis pulse pressure and adverse outcomes was minimized when decreasing pulse pressure during hemodialysis was added to the model; thus suggesting that decrease in pulse pressure with hemodialysis is more strongly predictive of clinical outcomes than elevated post-dialysis pulse pressure. This may be due to the known inaccuracies of dialysis-unit-obtained blood pressure measurements in estimating interdialytic hemodynamic burden,13 and blood pressure changes during hemodialysis may be better reflections of a patients underlying arterial compliance and cardiovascular risk. Alternatively, reduction in pulse pressure during hemodialysis may represent a patient with better overall blood pressure control and lower risk for subsequent cardiovascular outcomes.
While this study is the first to suggest a positive association between decrease in pulse pressure during hemodialysis and short-term outcomes in hemodialysis subjects, studies in the general population have shown that reductions in pulse pressure (by decreasing systolic blood pressure more than diastolic blood pressure) with therapeutic interventions are associated with improved cardiovascular outcomes.6 In a meta-analysis of 10 randomized controlled trials of blood pressure reduction in hypertensive patients, greater reductions in systolic blood pressure relative to diastolic blood pressure (across all age groups) were associated with decreased fatal and non-fatal cardiovascular events.6 Although there are few available randomized controlled trials of antihypertensive therapy in hemodialysis patients,14-20 the use of certain agents (such as inhibitors of the renin–angiotensin system or carvedilol) has been demonstrated to improve cardiovascular outcomes. Further, longer slower dialysis modalities, which control blood pressure better than conventional hemodialysis, have been demonstrated to improve left ventricular hypertrophy.21,22 Whether therapeutic interventions (such as certain medications or changes in dialysis procedure) targeted specifically toward lowering pulse pressure can improve cardiovascular outcomes in dialysis patients remains to be determined.
While decrease in pulse pressure with hemodialysis was associated with improved outcomes in this study, the underlying mechanisms behind individual blood pressure responses to ultrafiltration and hemodialysis remain unknown. Prior investigations have identified roughly half of hemodialysis patients exhibit a fall in systolic blood pressure during hemodialysis23 and that this relationship is short-lived with a rise in systolic blood pressure over the subsequent 12–24 h.24,25 Greater reductions in blood pressure during hemodialysis have been postulated to be mediated by greater decreases in the renin–angiotensin system and/or decreased sympathetic nervous system activity in response to decreases in blood volume.2 Alternatively, greater reductions in blood pressure with hemodialysis may be due to higher fluid or sodium solute removal in these individuals; however, 2 prior studies noted that individual blood pressure responses to hemodialysis are not related to blood volume changes or volume overload.26,27 Other potential etiologies for differential individual blood pressure responses to hemodialysis include timing of blood pressure medications, decreased removal of blood pressure medications with hemodialysis, different dialysate prescriptions, differing doses of erythropoietin stimulating agents, or some other unknown factor. While it is likely that all or some of these factors contribute to individual blood pressure responses to hemodialysis, in our investigation, subjects with greater reductions in pulse pressure trended toward greater fluid removal with hemodialysis and we found no difference in class of antihypertensive medication.
Interestingly, we identified that subjects with the greatest reduction in pulse pressure with hemodialysis exhibited higher dry weights. One prior investigation, analyzing the relationship between blood pressure and body mass index in hemodialysis patients, also noted that patients with higher body mass index exhibited lower post-dialysis systolic blood pressure compared to under-weight patients.28 The reasons for this are unclear but possible mechanisms include under-dialysis in underweight individuals, higher relative volume shifts triggering excess sympathetic response in lower weight patients, or alternatively an excess presence of proinflammatory cytokines in underweight and malnourished patients that may alter endothelial response to hemodialysis resulting in excess endothelin-1 and vasoconstriction. Under-dialysis in lower weight patients is unlikely as these patients tend to receive greater doses of dialysis relative to their body weight compared to overweight patients.29 Greater volume shifts relative to body weight resulting in excess sympathetic tone is possible, but in this study subjects with the greatest decrease in pulse pressure with hemodialysis also had the highest percent fluid removal with dialysis. Finally, higher body mass index has been associated with better nutritional status and better clinical outcomes in dialysis patients,30-33 thus the possibility of an abnormal inflammatory response during hemodialysis and resultant vasoconstriction in underweight individuals is possible.
This study also noted higher serum calcium among subjects with greater reductions in pulse pressure during hemodialysis. Prior investigations have identified higher serum calcium to be associated with decreased arterial compliance and increased pulse pressure.34 It is also well established in hemodialysis patients that an acute rise in serum calcium with the use of higher calcium dialysate concentrations increases cardiac contractility, cardiac output, and can improve hemodynamic instability during hemodialysis.35-38 If one considers dialysate calcium concentration to be a major determinant of serum calcium level, then one would expect a lower calcium level among subjects with greater reductions in pulse pressure during hemodialysis, which we did not identify. However, considering numerous other factors contribute to serum calcium levels (such as calcium containing binders and vitamin D administration) in hemodialysis patients and we lack information on dialysate calcium, it is uncertain what influence calcium dialysate concentrations had on individual blood pressure responses to hemodialysis in this study.
There are several limitations to the findings of this study. First, given the observational nature of the present study, we cannot make any conclusions regarding cause and effect. Whether pulse pressure reduction with hemodialysis is merely a marker of underlying improved vascular compliance or a marker of better overall blood pressure control remains to be determined. Second, the blood pressure parameters used for this analysis were averaged from 2 weeks of blood pressure measurements; while prior analyses have suggested home blood pressure recordings to be better estimates of ambulatory blood pressure,39 a recent study identified median hemodialysis unit blood pressure to correlate with ambulatory blood pressure40 suggesting a pre- to post-dialysis change in pulse pressure may be a useful correlate of overall hemodynamic burden. This study also lacked information on calcium and bicarbonate dialysate concentrations, thus limiting our ability to determine whether these parameters contributed mechanistically to changes in pulse pressure with dialysis. Finally, the cohort used for this analysis was part of a randomized controlled trial; given the known volunteer bias, which likely resulted in a healthier cohort with lower mortality, these findings may not be applicable to the wider range of prevalent end-stage renal disease patients included in the United States Renal Data System.
In conclusion, a greater decrease in pulse pressure during hemodialysis was associated with improved short-term clinical outcomes, including hospitalization and death. The beneficial association between decrease in pulse pressure during hemodialysis and improved outcomes was not modified by either high or low pre-dialysis or post-dialysis pulse pressure, and was most pronounced among subjects who exhibited pre-dialysis systolic blood pressure ≥120 mm Hg. Further research into the underlying etiology of individual blood pressure responses to hemodialysis is needed to determine whether this risk factor is merely a marker of underlying vascular disease or overall blood pressure burden, and whether blood pressure responses to hemodialysis are modifiable.
MATERIALS AND METHODS
Subject population
Subjects enrolled in the Crit Line Intradialytic Monitoring Benefit Study (CLIMB) were included in this analysis. The methods and results of the original CLIMB study have been reported previously.41 In summary, entry criteria to CLIMB included age between 18–85 years and thrice weekly in-center hemodialysis for >2 months. Patients were excluded if they had any of the following: blood pressure immeasurable by standard techniques, active gastrointestinal bleed, severe malnutrition (albumin <2.6 g/dl), active hematologic disease, planned move or planned living donor renal transplant, malignancy requiring chemotherapy, and inability to provide informed consent. The Institutional Review Board (IRB) at each of the six participating centers approved the original study protocol and the Duke University IRB approved this analysis.
Study measurements and definitions
In the CLIMB study, hemodialysis subjects were observed for 2 weeks and then randomized to 6 months of intradialytic blood volume monitoring using Crit-Line* (Hema Metrics Inc. (*formerly In-Line Diagnostics), Kaysville, UT, USA) or conventional clinical strategies. Following randomization, subjects were followed for 6 months and the primary end point was non-access-related hospitalization rates. For this analysis, the primary outcome is a combined end point of non-access-related hospitalization or death.
At baseline, the following parameters were collected and included in this analysis: demographics (race, age, and sex); target dry weight; intradialytic weight loss; dialysis vintage; tobacco use (defined as current or quit within last 10 years); treatment center; and past medical history including history of diabetes mellitus, diabetes as cause of end-stage renal disease hypertension, peripheral vascular disease, coronary artery disease (defined as history of myocardial infarction, coronary artery bypass graft, or percutaneous coronary intervention), congestive heart failure (defined as a history of congestive heart disease or left ventricular dysfunction), cerebrovascular disease (history of transient ischemic attack or stroke), arrhythmia (cardiac arrest, atrial fibrillation/flutter, atrial/ventricular tachycardia, or ventricular fibrillation), antihypertensive medication class, and routine laboratory data.
Baseline blood pressure used for this analysis was averaged from six hemodialysis sessions over 2 weeks during enrollment. Blood pressure parameters were measured by trained nurses using automated devices after the subject was at rest for 5 min. For this analysis, pulse pressure was defined as: systolic–diastolic blood pressure, and Δpulse pressure was defined as: post-dialysis pulse pressure–pre-dialysis pulse pressure.
During the course of the follow-up, data from each individual dialysis sessions were recorded thrice weekly. The rate of occurrence of the following events and treatments were available for analysis: intradialytic hypotension (defined as a lowest intradialytic systolic blood pressure ≤85 mm Hg); treated or symptomatic intradialytic hypotension (defined as lowest intradialytic systolic blood pressure ≤85 mm Hg and treatment was given or the patient developed symptoms); use of intradialytic medications; subject complaints of symptoms during dialysis, including cramping, dizziness, nausea, or vomiting; use of sodium modeling or administration of hypertonic saline; and use of cool dialysate (temperature <36°C).
Primary end point
The primary end point for this analysis was time to first non-access-related hospitalization or death.
Statistical analysis
Four hundred and thirty-eight of the original 443 subjects initially enrolled in the CLIMB study were included in this analysis. Subjects were excluded if they failed to follow-up after enrollment (n=1) or if they were missing baseline pre- or post-dialysis blood pressure measurements (n=4).
For descriptive purposes, subjects were grouped into quartiles of pulse pressure changes during dialysis. Categorical variables are presented as percentages and compared with χ2-tests. Continuous variables are reported as means (±s.d.) unless otherwise noted. One-way analysis of variance was used to compare normally distributed continuous variables; otherwise non-parametric Kruskal–Wallis test was used. Categorical variables with missing data were assumed to be absent (~1%). For continuous variables with missing data (only creatinine and hemoglobin, <5%), the variable was replaced with the overall mean for the final analyses. Final models were analyzed with and without imputation and the results are similar to those presented.
In unadjusted analysis stratified by original randomization, Kaplan–Meier curves were used to separately describe the relationship between pre-dialysis pulse pressure, post-dialysis pulse pressure, Δpulse pressure, and the primary end point of time to first non-access-related hospitalization or death. Cox proportional hazards assumptions were tested by formal and graphical methods. Continuous variables with non-linear associations with the primary end point were transformed into categorical variables. In adjusted analysis, Cox proportional hazards models were used to determine the relationship between Δpulse pressure (as a continuous variable) and the primary end point while controlling for demographics, comorbid conditions, laboratory variables, and blood pressure medications. Demographic variables and variables, which have been demonstrated to be associated with adverse outcomes (age, gender, race, diabetes, treatment group, and albumin), were included in all models. Variables, which were significantly different between quartiles of patients categorized by pulse pressure changes with hemodialysis, were also included in all models. Other variables, which were not associated with the primary end point (P>0.15), were removed from the final model by backward selection. Additional adjusted models were created, which analyzed the relationship between pre-dialysis pulse pressure, post-dialysis pulse pressure, and Δpulse pressure (tested individually and in combination), and the primary composite end point. Box plots were created to graphically display the adjusted HRs and 95% CIs associated with the different blood pressure parameters. Adjusted analyses were also performed with Δpulse pressure as a time-varying covariate in which monthly average Δpulse pressure was analyzed in association with the subsequent primary end point.
Sensitivity analyses were performed by adding pre-dialysis systolic blood pressure to the final model to determine if this modified the relationship between Δpulse pressure and the primary end point. Separate stratified analyses among four strata of pre-dialysis systolic blood pressure (<120, 120–139, 140–159, and >160 mm Hg) were performed to determine whether the relationship between Δpulse pressure and the primary end point differed with the level of pre-dialysis systolic blood pressure. Separate models also tested whether interactions were present between Δpulse pressure and intradialytic weight loss and antihypertensive agent class.
All statistical analyses were performed using SAS Eguide (version 9.2; SAS Institute, Cary, NC, USA).
ACKNOWLEDGMENTS
This work was presented in part at the American Society of Nephrology Annual Meeting in Philadelphia, PA, USA, on November 7, 2008, as a poster exhibit. JKI was supported by National Institutes of Health (NIH) grant K23 HL092297. UDP was supported by NIH grant K23 DK075929. RDT was supported by NIH grant K24 DK02818.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.US Renal Data Systems . USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2007. [Google Scholar]
- 2.National Kidney Foundation K/DOQI Clinical Practice Guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(Suppl 4):s49–s59. s69–s75. [PubMed] [Google Scholar]
- 3.Millar JA, Lever AF, Burke V. Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens. 1999;17:1065–1072. doi: 10.1097/00004872-199917080-00004. [DOI] [PubMed] [Google Scholar]
- 4.Benetos A, Rudnichi A, Safar M, et al. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998;32:560–564. doi: 10.1161/01.hyp.32.3.560. [DOI] [PubMed] [Google Scholar]
- 5.Domanski M, Norman J, Wolz M, et al. Cardiovascular risk assessment using pulse pressure in the first national health and nutrition examination survey (NHANES I) Hypertension. 2001;38:793–797. doi: 10.1161/hy1001.092966. [DOI] [PubMed] [Google Scholar]
- 6.Wang JG, Staessen JA, Franklin SS, et al. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension. 2005;45:907–913. doi: 10.1161/01.HYP.0000165020.14745.79. [DOI] [PubMed] [Google Scholar]
- 7.Klassen PS, Lowrie EG, Reddan DN, et al. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287:1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 8.Tozawa M, Iseki K, Iseki C, et al. Pulse pressure and risk of total mortality and cardiovascular events in patients on chronic hemodialysis. Kidney Int. 2002;61:717–726. doi: 10.1046/j.1523-1755.2002.00173.x. [DOI] [PubMed] [Google Scholar]
- 9.Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 10.London GM, Blacher J, Pannier B, et al. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 11.Guerin AP, Blacher J, Pannier B, et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 12.London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol. 2001;12:2759–2767. doi: 10.1681/ASN.V12122759. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Peixoto AJ, Santos SFF, et al. Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1:389–398. doi: 10.2215/CJN.01891105. [DOI] [PubMed] [Google Scholar]
- 14.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 15.Cice G, Ferrara L, Di Benedetto A, et al. Dilated cardiomyopathy in dialysis patients—beneficial effects of carvedilol: a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2001;37:407–411. doi: 10.1016/s0735-1097(00)01158-x. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi A, Takase H, Toriyama T, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis—a randomized study. Nephrol Dial Transplant. 2006;21:2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 17.Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70:1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Kanno Y, Sugahara S, et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52:501–506. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Ichihara A, Hayashi M, Kaneshiro Y, et al. Low doses of losartan and trandolapril improve arterial stiffness in hemodialysis patients. Am J Kidney Dis. 2005;45:866–874. doi: 10.1053/j.ajkd.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Shibasaki Y, Masaki H, Nishiue T, et al. Angiotensin II type 1 receptor antagonist, losartan, causes regression of left ventricular hypertrophy in end-stage renal disease. Nephron. 2002;90:256–261. doi: 10.1159/000049060. [DOI] [PubMed] [Google Scholar]
- 21.Chan CT, Floras JS, Miller JA, et al. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002;61:2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 22.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 23.Inrig JK, Oddone EZ, Hasselblad V, et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71:454–461. doi: 10.1038/sj.ki.5002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos SF, Mendes RB, Santos CA, et al. Profile of interdialytic blood pressure in hemodialysis patients. Am J Nephrol. 2003;23:96–105. doi: 10.1159/000068038. [DOI] [PubMed] [Google Scholar]
- 25.Cheigh JS, Milite C, Sullivan JF, et al. Hypertension is not adequately controlled in hemodialysis patients. Am J Kidney Dis. 1992;19:453–459. doi: 10.1016/s0272-6386(12)80954-1. [DOI] [PubMed] [Google Scholar]
- 26.Boon D, van Montfrans GA, Koopman MG, et al. Blood pressure response to uncomplicated hemodialysis: the importance of changes in stroke volume. Nephron Clin Pract. 2004;96:c82–c87. doi: 10.1159/000076745. [DOI] [PubMed] [Google Scholar]
- 27.Chaignon M, Chen WT, Tarazi RC, et al. Blood pressure response to hemodialysis. Hypertension. 1981;3:333–339. doi: 10.1161/01.hyp.3.3.333. [DOI] [PubMed] [Google Scholar]
- 28.Salahudeen AK, Fleischmann EH, Bower JD, et al. Underweight rather than overweight is associated with higher prevalence of hypertension: BP vs BMI in haemodialysis population. Nephrol Dial Transplant. 2004;19:427–432. doi: 10.1093/ndt/gfg523. [DOI] [PubMed] [Google Scholar]
- 29.Salahudeen AK, Fleischmann EH, Bower JD. Impact of lower delivered Kt/V on the survival of overweight patients on hemodialysis. Kidney Int. 1999;56:2254–2259. doi: 10.1046/j.1523-1755.1999.00766.x. [DOI] [PubMed] [Google Scholar]
- 30.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65:597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe RA, Ashby VB, Daugirdas JT, et al. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis. 2000;35:80–88. doi: 10.1016/S0272-6386(00)70305-2. [DOI] [PubMed] [Google Scholar]
- 32.Kopple JD, Zhu X, Lew NL, et al. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 33.Lowrie EG, Li Z, Ofsthun N, et al. Body size, dialysis dose and death risk relationships among hemodialysis patients. Kidney Int. 2002;62:1891–1897. doi: 10.1046/j.1523-1755.2002.00642.x. [DOI] [PubMed] [Google Scholar]
- 34.Kyriazis J, Stamatiadis D, Mamouna A. Intradialytic and interdialytic effects of treatment with 1.25 and 1.75 mmol/l of calcium dialysate on arterial compliance in patients on hemodialysis. Am J Kidney Dis. 2000;35:1096–1103. doi: 10.1016/s0272-6386(00)70046-1. [DOI] [PubMed] [Google Scholar]
- 35.Leunissen KM, van den Berg BW, van Hooff JP. Ionized calcium plays a pivotal role in controlling blood pressure during haemodialysis. Blood Purif. 1989;7:233–239. doi: 10.1159/000169600. [DOI] [PubMed] [Google Scholar]
- 36.Gabutti L, Bianchi G, Soldini D, et al. Haemodynamic consequences of changing bicarbonate and calcium concentrations in haemodialysis fluids. Nephrol Dial Transplant. 2009;24:973–981. doi: 10.1093/ndt/gfn541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman RA, Bialy GB, Gazinski B, et al. The effect of dialysate calcium levels on blood pressure during hemodialysis. Am J Kidney Dis. 1986;8:244–247. doi: 10.1016/s0272-6386(86)80033-6. [DOI] [PubMed] [Google Scholar]
- 38.Maynard JC, Cruz C, Kleerekoper M, et al. Blood pressure response to changes in serum ionized calcium during hemodialysis. Ann Intern Med. 1986;104:358–361. doi: 10.7326/0003-4819-104-3-358. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal R, Andersen MJ, Bishu K, et al. Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int. 2006;69:900–906. doi: 10.1038/sj.ki.5000145. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal R, Metiku T, Tegegne GG, et al. Diagnosing hypertension by intradialytic blood pressure recordings. Clin J Am Soc Nephrol. 2008;3:1364–1372. doi: 10.2215/CJN.01510308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddan DN, Szczech LA, Hasselblad V, et al. Intradialytic blood volume monitoring in ambulatory hemodialysis patients: a randomized trial. JAm Soc Nephrol. 2005;16:2162–2169. doi: 10.1681/ASN.2004121053. [DOI] [PubMed] [Google Scholar]


