Abstract
The effect of the plant-derived nonpsychotropic cannabinoid, cannabidiol (CBD), on the function of hydroxytryptamine (5-HT)3A receptors expressed in Xenopus laevis oocytes was investigated using two-electrode voltage-clamp techniques. CBD reversibly inhibited 5-HT (1 μM)-evoked currents in a concentration-dependent manner (IC50 = 0.6 μM). CBD (1 μM) did not alter specific binding of the 5-HT3A antagonist [3H]3-(5-methyl-1H-imidazol-4-yl)-1-(1-methylindol-3-yl)propan-1-one (GR65630), in oocytes expressing 5-HT3A receptors. In the presence of 1 μM CBD, the maximal 5-HT-induced currents were also inhibited. The EC50 values were 1.2 and 1.4 μM, in the absence and presence of CBD, indicating that CBD acts as a noncompetitive antagonist of 5-HT3 receptors. Neither intracellular BAPTA injection nor pertussis toxin pretreatment (5 μg/ml) altered the CBD-evoked inhibition of 5-HT-induced currents. CBD inhibition was inversely correlated with 5-HT3A expression levels and mean 5-HT3 receptor current density. Pretreatment with actinomycin D, which inhibits protein transcription, decreased the mean 5-HT3 receptor current density and increased the magnitude of CBD inhibition. These data demonstrate that CBD is an allosteric inhibitor of 5-HT3 receptors expressed in X. laevis oocytes. They further suggest that allosteric inhibition of 5-HT3 receptors by CBD may contribute to its physiological roles in the modulation of nociception and emesis.
The serotonin (5-HT)3 receptor, a member of the ligand-gated ion channel family, mediates rapid and transient membrane-depolarizing effect of 5-HT in the central and peripheral nervous system (Yakel and Jackson, 1988). An involvement of 5-HT3 receptors in pain transmission, mood disorders, and drug abuse has been reported (for reviews, see Riering et al., 2004; Faerber et al., 2007; Engleman et al., 2008). Furthermore, 5-HT3 receptor antagonists are effective therapeutic agents for the treatment of chemotherapy-induced nausea and vomiting (Slatkin 2007; Thompson and Lummis, 2007).
Previous studies showed that 5-HT3 receptor antagonists and cannabinoids (CBs) produce similar pharmacological effects, such as nonopioid receptor-mediated analgesia and antiemesis (for reviews, see Tramèr et al., 2001; Martin and Wiley, 2004; Riering et al., 2004). In fact, synthetic Δ9-tetrahydrocannabinol (THC), dronabinol, (Marinol), and THC analogs such as nabilone (Cesamet) are approved by the United States Food and Drug Administration for use in chemotherapy-induced nausea and vomiting refractory to conventional antiemetic therapy (for reviews, see Tramèr et al., 2001; Martin and Wiley, 2004; Slatkin, 2007).
The limitation of the therapeutic utility of THC and its above-mentioned chemical analogs is the potential development of psychoactive effects through central nervous system CB1 receptor. Cannabidiol (CBD) is one of the most abundant cannabinoids of Cannabis sativa, with reported antioxidant, anti-inflammatory, and antiemetic effects. It is well tolerated and is without side effects when chronically administered to humans (for reviews, see Mechoulam et al., 2007; Izzo et al., 2009; Scuderi et al., 2009). Furthermore, CBD is devoid of psychoactive properties due to a low affinity for the CB1 and CB2 receptors (Mechoulam et al., 2007; Izzo et al., 2009; Pertwee, 2009). Thus, pharmaceutical interest in this compound has risen significantly in recent years (Izzo et al., 2009; Pertwee, 2009; for review, see Scuderi et al., 2009).
The effects of THC; synthetic cannabinoid receptor agonists such as WIN55,212-2 [4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1-i,j]quinolin-6-one], CP55,940 [(1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol], and JWH-015 [1-propyl-2-methyl-3-(1-naphthoyl)indole] (Fan, 1995; Barann et al., 2002); and the endocannabinoid anandamide (Oz et al., 1995, 2002a; Barann et al., 2002; Xiong, 2008) on the functional properties of 5-HT3 receptors have been shown in previous in vitro studies. However, whether nonpsychotropic cannabinoids such as cannabidiol affect 5-HT3A receptor function is unknown. In the present study, we have tested the hypothesis that CBD may produce its pharmacological effects, at least in part, via 5-HT3 receptors. For this purpose, the complementary RNA (cRNA) encoding the mouse 5-HT3 subunit A of the receptor was expressed in Xenopus laevis oocytes, and the effect of CBD on receptor function was investigated.
Materials and Methods
Mature female X. laevis frogs were purchased from Xenopus I (Ann Arbor, MI). They were housed in dechlorinated tap water at 18°C under a 12:12-h light/dark cycle and fed beef liver at least twice a week. Clusters of oocytes were removed surgically under tricaine (Sigma-Aldrich, St. Louis, MO) anesthesia (0.15%), and individual oocytes were manually dissected away in a solution containing 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.8 mM MgSO4, and 10 mM HEPES, pH 7.5. Dissected oocytes were stored 2 to 7 days in modified Barth's solution containing 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.3 mM Ca(NO3)2, 0.9 mM CaCl2, 0.8 mM MgSO4, and 10 mM HEPES, pH 7.5, supplemented with 2 mM sodium pyruvate, 10,000 IU/l penicillin, 10 mg/l streptomycin, 50 mg/l gentamicin, and 0.5 mM theophylline. Oocytes were placed in a 0.2-ml recording chamber and superfused at a constant rate of 3 to 5 ml/min. The bathing solution consisted of 95 mM NaCl, 2 mM KCl, 2 mM CaCl2, and 5 mM HEPES, pH 7.5. The amount of 5-HT3A receptor cRNA injected into oocytes varied from 1 to 30 ng, as indicated. However, the injection volume of diethylpyrocarbonate-treated distilled water was kept at 30 nl throughout the experiments. The cells were impaled with two standard glass microelectrodes filled with 3 M KCl (1–3 MΩ). The oocytes were routinely voltage-clamped at a holding potential of −70 mV using an GeneClamp-500B amplifier (Molecular Devices, Sunnyvale, CA). Current responses were digitized by A/D converter and analyzed using pClamp 8 (Molecular Devices) run on an IBM PC or directly recorded on a 2400 rectilinear pen recorder (Gould Instrument Systems Inc., Cleveland, OH). Current-voltage curves were generated by holding each membrane potential in a series for 50 to 60 s, followed by a return to −70 mV for 5 min. Oocyte capacitance was measured by a paired ramp method described previously (Oz et al., 2004a). In brief, voltage ramps were used to elicit constant capacitive current, Icap, and the charge associated with this current was calculated by the integration of Icap. Ramps had slopes of 2 V/s and durations of 20 ms and started at a holding potential of −90 mV. A series of 10 paired ramps was delivered at 1-s intervals and averaged traces were used for charge calculations. In each oocyte, the averages of five to six measurements were used to obtain values for membrane capacitance (Cm). Currents for Icap recordings were filtered at 20 kHz and sampled at 50 kHz. Current density was calculated by normalizing the average of three consecutive control currents to the oocyte capacitance.
Compounds were applied by addition to the superfusate. All chemicals used in preparing the solutions were from Sigma-Aldrich. Pertussis toxin (PTX), BAPTA, actinomycin D (ActD), 5-HT, and MDL72222 [tropanyl 3,5-dichlorobenzoate] were purchased from Tocris Bioscience (Ellisville, MO). Cannabidiol was generously provided by National Institute on Drug Abuse Drug Supply System, National Institutes of Health (Rockville, MD). Procedures for the injections of PTX (50 nl; 50 μg/ml) or BAPTA (50 nl; 200 mM) were performed as described previously (Oz et al., 1998). Injections were performed 1 h before recordings using oil-driven ultramicrosyringe pumps (Micro4; WPI, Sarasota, FL). Stock solutions of CBD were prepared in dimethyl sulfoxide at a concentration of 30 mM. Dimethyl sulfoxide alone did not affect 5-HT3A receptor function when added at concentrations as high as 0.2% (v/v), a concentration 2 times greater than the most concentrated application of the agents used. Electrophysiological recordings from oocytes were conducted 3 to 4 days after cRNA injections, and both control and treatment (PTX and BAPTA) groups were recorded on the same days.
Synthesis of cRNA.
The cDNA clone of the mouse and human 5-HT3A subunits were provided by Dr. David Julius (University of California, San Francisco, San Francisco, CA) and OriGen Technologies, Inc. (Rockville, MD), respectively. cRNAs were synthesized in vitro using a mMessage mMachine RNA transcription kit (Ambion, Austin, TX). The quality and sizes of synthesized cRNAs were confirmed by denatured RNA agarose gels.
Radioligand Binding Studies.
Oocytes were injected with 10 ng of mouse 5-HT3A cRNA, and functional expression of the receptors was tested by electrophysiology on day 3. Isolation of oocyte membranes were carried out by modification of a method described previously (Oz et al., 2004b). In brief, oocytes were suspended (20 μl/oocyte) in a homogenization buffer (HB) containing 10 mM HEPES, 1 mM EDTA, 0.02% NaN3, 50 μg/ml bacitracin, and 0.1 mM phenylmethylsulfonyl fluoride, pH 7.4, at 4°C on ice and homogenized using a motorized Teflon homogenizer (six strokes, 15 s each at high speed). The homogenate was centrifuged for 10 min at 800g. The supernatant was collected and the pellet was resuspended in HB and recentrifuged at 800g for 10 min. Supernatants were then combined and centrifuged for 1 h at 36,000g. The membrane pellet was resuspended in HB at the final protein concentration of 0.5 to 0.7 mg/ml and used for the binding studies.
Binding assays were performed in 500 μl of 10 mM HEPES, pH 7.4, containing 50 μl of oocyte preparation and 1 nM [3H]3-(5-methyl-1H-imidazol-4-yl)-1-(1-methylindol-3-yl)propan-1-one (GR65630; 58.7 Ci/mmol; PerkinElmer Life and Analytical Sciences, Boston, MA). Nonspecific binding was determined using 100 μM MDL72222. Oocyte membranes were incubated with [3H]GR65630 in the absence and presence of CBD at 4°C for 1 h before bound radioligand was separated by rapid filtration onto GF/B filters (Whatman Inc. Piscataway, NJ) presoaked in 0.3% polyethylenimine. Filters were then washed with two 5-ml washes of ice-cold HEPES buffer and left in 3 ml of scintillation fluid for at least 4 h before scintillation counting was conducted to determine amounts of membrane-bound radioligand.
Data Analysis.
For the nonlinear curve fitting and regression fits of the radioligand binding data, the computer software Origin (OriginLab Corp., Northampton, MA) was used. In functional assays, average values were calculated as mean ± S.E.M.. Statistical significance was analyzed using ANOVA or Student's t test. Concentration-response curves were obtained by fitting the data to the logistic equation y = {(Emax − Emin)/(1 + [x/EC50]n)} + Emin, where x and y are concentration and response, respectively, Emax is the maximal response, Emin is the minimal response, EC50 is the half-maximal concentration, and n is the slope factor.
Results
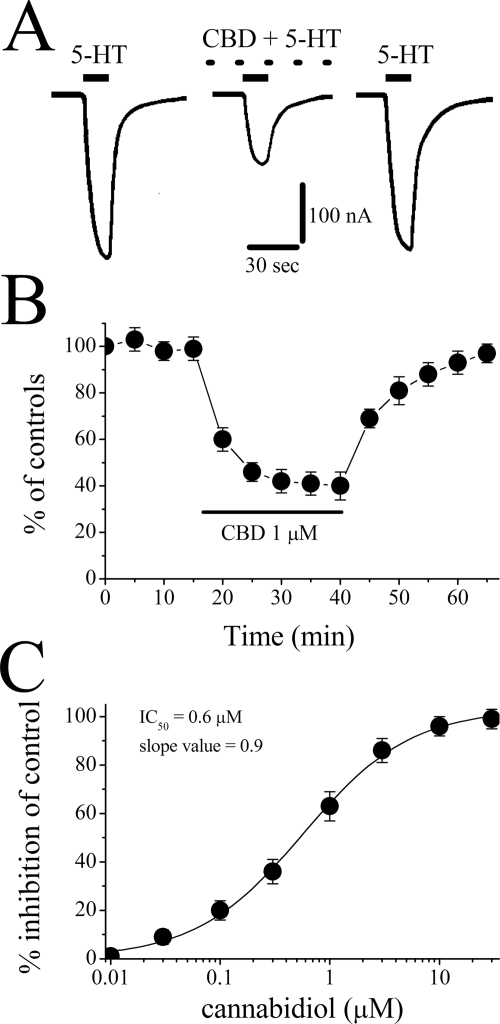
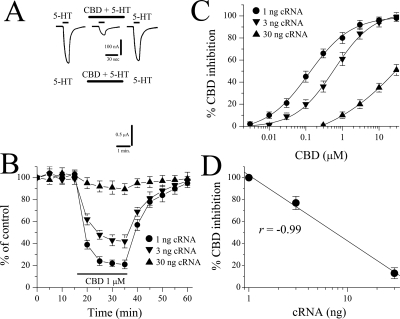
Bath application of neither 5-HT (50 μM) nor CBD (10 μM) produced detectable currents in oocytes injected with diethylpyrocarbonate-treated distilled water (30 nl/oocyte; n = 6). Application of CBD (10 μM) for 20 min did not affect membrane resistance, Cm, or resting membrane potential in oocytes injected with 3 ng of cDNA encoding the 5-HT3A receptor (Table 1). Currents evoked by 5-HT (1 μM) were maximally inhibited by CBD within 10 to 15 min after the initiation of CBD perfusion. After CBD washout, recovery was slow (e.g., 20–30 min; Fig. 1A). Time course studies assessing the effects of 25-min CBD application on the mean amplitude of 5-HT-induced currents from six oocytes are presented in Fig. 1B.
TABLE 1.
Effects of cannabidiol (10 μ M) on the passive membrane properties of the X. laevis oocytes expressing 5-HT3 receptors
| Rm | Cm | Vm | |
|---|---|---|---|
| MΩ | nF | mV | |
| Control (n = 11) | 1.1 ± 0.3 | 193 ± 17 | −35.2 ± 3.4 |
| 20-min CBD (n = 9) | 1.4 ± 0.3 | 197 ± 14 | −36.9 ± 3.8 |
Rm, membrane resistance; Vm resting membrane potential.
Fig. 1.
Effect of cannabidiol on 5-HT3 receptor-mediated ion currents. A, records of currents activated by 1 μM 5-HT in control (left), coapplication of 1 μM CBD and 5-HT after 20-min CBD application (middle), and 30-min recovery (right). B, time course of the effect of CBD application (25 min) on the maximal amplitudes of the currents induced by 1 μM 5-HT at 5-min intervals. Data points represent means ± S.E.M. of six cells. C, concentration-response curve for cannabidiol inhibition of 5-HT3 receptor-mediated ion currents. For all concentrations used, CBD was applied for 25 min. Data points are the mean ± S.E.M. (n = 6–7); error bars not visible are smaller than the size of the symbols. The curve is the best fit of the data to the logistic equation described under Materials and Methods. The IC50 value for CBD was 0.6 ± 0.1 μM, with a slope value of 0.9.
In the next series of experiments, we examined the concentration-response relationship of the CBD effects on the function of 5-HT3 receptors (Fig. 1C). The threshold concentration for inhibition by CBD was 0.1 μM, and maximal inhibition was achieved in concentrations ranging between 10 and 30 μM. The inhibition of 5-HT (1 μM)-induced current by 25-min CBD application was concentration-dependent, with an IC50 value of 0.6 ± 0.1 μM and a slope value of 0.9 (Fig. 1C).
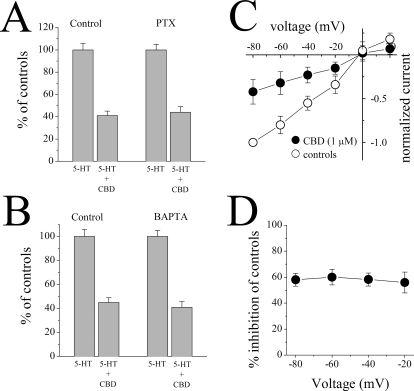
Because the participation of Gi/o proteins in the signaling of the receptors activated by the cannabinoids and certain CBD analogs have been reported previously (Járai et al., 1999), we tested the effect of CBD in control (distilled water-injected) and PTX-injected oocytes expressing 5-HT3 receptors. There was no significant difference in CBD inhibition of 5-HT responses between controls and PTX-injected cells (ANOVA: F3,18 = 130.9, P < 0.001, n = 5–6 for the effect of CBD compared with controls in distilled water-injected and PTX-injected groups; Bonferroni test: P > 0.05 for the significance of CBD inhibition between controls and PTX group; Fig. 2A).
Fig. 2.
Effects of pertussis toxin treatment, calcium chelator, BAPTA injection, and membrane potential changes on cannabidiol inhibition of 5-HT3 receptor-mediated ion currents. A, bar presentation of the effects of 1 μM CBD application (20 min) on the maximal amplitudes of 5-HT-induced currents in oocytes injected with 50 nl of distilled water (controls; n = 6) or 50 nl of PTX (50 μg/ml; n = 5). Bars represent the means ± S.E.M. B, bar presentation of the effects of 1 μM CBD application (20 min) on the maximal amplitudes of 5-HT-induced currents in oocytes injected with 50 nl of distilled water, controls (n = 5) or BAPTA (50 nl; 200 mM; n = 7). Bars represent the means ± S.E.M. C, current-voltage relationship of 5-HT-activated current in the absence (open circles) and presence (closed circles) of 1 μM CBD. Currents were activated by 1 μM 5-HT in the same oocyte. D, percentage inhibition of 5-HT-activated current by 1 μM CBD at different membrane potentials; there are no significant differences among these values at different membrane potentials (ANOVA: P > 0.05; n = 5).
Because CBD has been shown to increase intracellular Ca2+ levels in neurons and glia (Drysdale et al., 2006; Ryan et al., 2006), we investigated the effect of the Ca2+ chelator BAPTA on CBD inhibition of 5-HT responses. In oocytes injected with BAPTA, the inhibition of 5-HT responses by 20 min CBD application was not significantly different from controls (ANOVA: F3,20 = 110.7, P < 0.001, n = 5–7 for the effect of CBD compared with controls in distilled water-injected and PTX-injected groups; Bonferroni test: P > 0.05 for the significance of CBD inhibition between distilled water-injected and PTX-injected group; Fig. 2B).
Examination of the voltage dependence of the CBD inhibition indicated that the degree of inhibition of the 5-HT (1 μM)-induced currents did not vary with membrane potential (Fig. 2C). In addition, there was no change of the reversal potential of the 5-HT-activated ion currents [control: 2 ± 2 mV in controls; CBD (1 μM): 4 ± 3 mV], indicating that neither the ion selectivity of the channel nor the driving force on Na+ and Ca2+ was affected by CBD. Moreover, quantitative evaluation of data for the inhibitory effect of CBD at different membrane potentials (Fig. 2D) showed no statistically significant differences on the effect of CBD at different holding potentials (among −20, −40, −60, and −80 mV groups; ANOVA: F3,16 = 0.11, P = 0.953, n = 5 for each group).
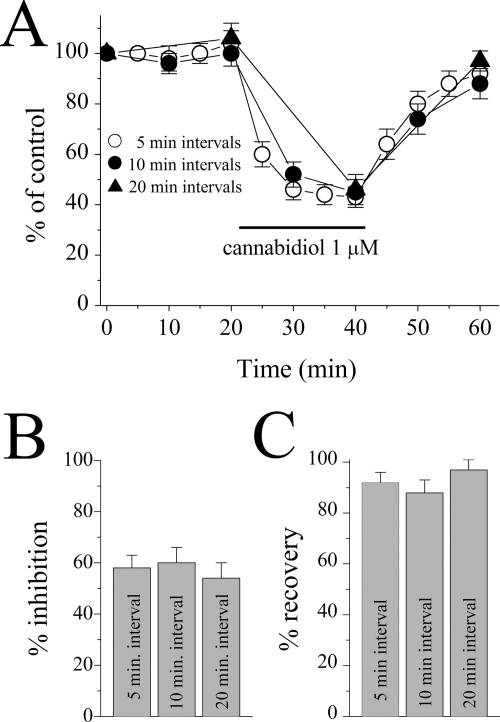
By definition, an open channel blockade requires the opening of the channel by the binding of the agonist to the receptor. Thus, in the absence of an agonist, the degree of blockade should be related to the frequency of channel activation. Therefore, the extent of CBD inhibition of the 5-HT3A receptors was compared in cells exposed to 5-HT at 5-min intervals with those exposed at 10- and 20-min intervals (Fig. 3A). During application of 1 μM CBD for 20 min, CBD was equally effective in inhibiting currents activated at 5-, 10-, and 20-min intervals (between 5-, 10-, and 20-min interval groups, ANOVA: F2,14 = 0.29, P = 0.746, n = 5–7; Fig. 3B), indicating that the frequency of channel opening does not alter the extent of CBD inhibition and that the channel does not need to be opened by the agonist for CBD to be effective. Recovery from an open channel blocker would be facilitated by the increases in opening frequencies. Therefore, we analyzed the extent of recovery from CBD inhibition at different 5-HT stimulation intervals (Fig. 3C). The recovery from CBD inhibition was not altered by 5-HT stimulation intervals, suggesting that CBD is not trapped in the channel when the channel closes, as can occur with open channel blocking drugs (between 5-, 10-, and 20-min interval groups, ANOVA: F2,14 = 1.06, P = 0.379, n = 5–7).
Fig. 3.
Effect of 5-HT stimulation-interval alterations on the inhibition of 5-HT3A receptor-mediated responses by cannabidiol. A, time course of the effect of CBD on the maximal amplitudes of the currents induced by 1 μM 5-HT at 5- (open circles), 10- (closed circles), and 20 (closed triangles)-min intervals. Data points represent means ± S.E.M of five to seven cells. B, percentage of CBD inhibition on the 5-HT3A receptor-mediated currents recorded at the end of a 20-min application period was not different among oocytes stimulated with 5-HT application at 5-, 10-, and 20-min intervals (ANOVA: P > 0.05; n = 5–7). C, percentage of controls (recovery) from CBD inhibition of the 5-HT3A receptor-mediated currents recorded at the end of a 20-min recovery period was not different between oocytes stimulated and not stimulated with 5-HT application every 10 min (ANOVA: P > 0.05; n = 5–7). Duration of CBD application (20 min) is indicated by the horizontal bar in the figure.
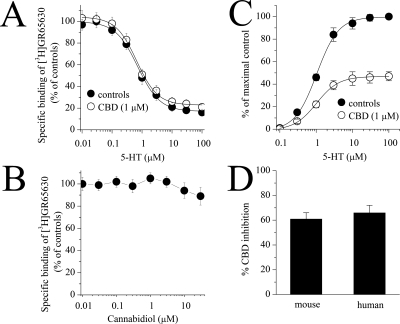
CBD may alter 5-HT3 receptor function via competitive inhibition of 5-HT binding to the receptor. To examine this issue, we performed radioligand binding assays (Fig. 4, A and B). In competition experiments, 5-HT concentration-dependently inhibited the specific binding of 1 nM [3H]GR65630 (Fig. 4A). The concentration-dependent inhibition of [3H]GR65630 binding by 5-HT was not altered by 1 μM CBD (Fig. 4A). The IC50 value in the absence and presence of CBD was 0.7 ± 0.3 and 0.6 ± 0.2 μM, respectively (Student's t test: t = 1.2, df = 17, P = 0.24, n = 8–11). Likewise, increasing CBD concentrations did not reduce specific [3H]GR65630 binding to membranes of oocytes expressing 5-HT3A receptor cDNA (Fig. 4B).
Fig. 4.
Effects of cannabidiol and 5-HT on the specific binding of [3H]GR65630 and the effect of cannabidiol on 5-HT concentration-response curves from X. laevis oocytes expressing 5-HT3A receptors. A, inhibition of specific [3H]GR65630 binding by nonlabeled 5-HT in membranes after 1-h preincubation with 1 μM cannabidiol. The concentration of [3H]GR65630 was 1 nM. The inhibition curve shows pooled data from 8 to 11 measurements from three experiments. B, effects of increasing concentration of cannabidiol on the specific binding of [3H]GR65630. Experiments were conducted in the presence of 1 nM [3H]GR65630. The results present data from 9 to 11 measurements. Data points indicate mean ± S.E.M. C, concentration-response curves for 5-HT-activated current in the absence (open circles) and presence (closed circles) of 1 μM CBD. Currents were activated by 5-HT concentrations ranging from 0.1 to 100 μM. Cannabidiol was applied for 20 min, and 5-HT and CBD were then coapplied for 10 to 15 s. Data points are the mean ± S.E.M. (n = 4–5); error bars are not visible are smaller than the size of the symbols. The curve is the best fit of the data to the logistic equation described in the methods. The control concentration-response curve is normalized to the maximal response. The CBD concentration-response curve is the percentage of the maximal control. D, comparison of the 1 μM CBD effect on the mouse and human 5-HT3A receptors expressed in oocytes (3 ng/oocyte, recorded on postinjection day 3). Bar graph shows average inhibition (mean ± S.E.M.) of 5-HT (1 μM)-induced currents by 20-min CBD application in five oocytes expressing mouse 5-HT3A receptors and seven oocytes expressing 5-HT3A receptors.
In oocytes expressing 5-HT3A receptor, the concentration-response curve of 5-HT was examined in the absence and presence of 1 μM CBD. The EC50 value (mean ± S.E.M.) in the absence and presence of CBD was 1.2 ± 0.2 and 1.4. ± 0.1 μM, respectively (Student's t test: t = −0.96, df = 7, P = 0.36, n = 4–5). As shown in Fig. 4C, CBD did not significantly alter EC50 values and inhibited the maximal 5-HT-responses to the same percentage of control values (n = 5), suggesting that CBD inhibits 5-HT-activated ion currents in a noncompetitive manner.
Because our experiments were conducted using mouse 5-HT3A receptor cRNA, we compared the effect of 1 μM CBD on the function of mouse and human 5-HT3A receptors expressed in X. laevis oocytes (3 ng of cRNA/oocyte). Application of CBD for 20 min caused a significant inhibition of currents induced by 1 μM 5-HT. The magnitude of CBD inhibition did not differ between mouse (61 ± 4% inhibition; n = 5) and human 5-HT3A receptors (65 ± 5% inhibition; n = 7; Student's t test: t = −0.63, df = 10, P = 0.54, n = 5–7; Fig. 4D).
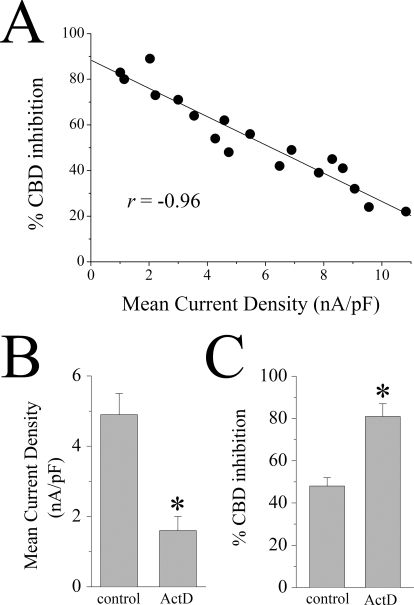
In a recent study, it was demonstrated that the magnitude of inhibition induced by the endocannabinoid anandamide is inversely correlated with the amount of 5-HT3 receptor cRNA injected into X. laevis oocytes (Xiong et al., 2008). For this reason, we compared the effects of CBD on 5-HT3 receptors in X. laevis oocytes injected with increasing concentrations of cRNA encoding for this receptor. Increasing the concentration of 5-HT3 receptor cRNA reversed the inhibitory effect of CBD at this receptor (Fig. 5A). For example, the maximal inhibition induced by 1 μM CBD was 81 ± 5% (n = 6) in oocytes injected with 1 ng of 5-HT3A receptor cRNA, whereas the maximal inhibition was only 11 ± 3% (n = 5) in oocytes injected with 30 ng of 5-HT3A receptor cRNA. These values were significantly different (Student's t test: t = −12.8, df = 9, P < 0.001, n = 5–6; Fig. 5B). The IC50 values of CBD inhibition differed by nearly 230-fold between oocytes previously injected with 1 and 30 ng of cRNAs (Fig. 5C); the IC50 values for CBD inhibition were 121 ± 11 nM, 587 ± 62 nM, and 29 ± 4 μM (means ± S.E.M.) in cells injected with 1, 3, and 30 ng of cRNA, respectively (Student's t test: t = −7.1, df = 10, P < 0.001, n = 5–7; Fig. 5C). Likewise, the magnitude of inhibition produced by 1 μM CBD was highly correlated with the amount of the cRNA injected into the oocytes (r = −0.99; Fig. 5D). As would be expected, the amplitude of current activated by 1 μM 5-HT also increased with the amount of cRNA injected (Figs. 5 and 6A), indicating that the levels of functional receptor expression correlate with the amount of cRNA expressed in the oocytes. The magnitude of inhibition produced by 1 μM CBD was also highly correlated with the mean current density of the receptor (r = −0.96; Fig. 6A). To further confirm the relationship between receptor expression and CBD inhibition, we pretreated oocytes for 24 h before recordings with 15 μg/ml ActD, which inhibits RNA transcription. Pretreatment with ActD significantly reduced mean current density (activated by 30 μM 5-HT) from 4.9 ± 0.6 to 1.6 ± 0.4 nA/pF (ANOVA: F1,12 = 25.9, P < 0.001, n = 6–8; Fig. 6B, right), suggesting that ActD reduces the functional expression of 5-HT3A receptors. In contrast, ActD significantly increased the magnitude of CBD inhibition from 48 ± 4 to 81 ± 6% (ANOVA: F1,12 = 18.2, P < 0.002, n = 6–8; Fig. 6B, left).
Fig. 5.
Cannabidiol inhibition is inversely correlated with the amount of 5-HT3A receptor cRNA injected into X. laevis oocytes. A, current traces demonstrating CBD inhibition of 5-HT-activated currents in cells previously injected with 1 ng (top) and 30 ng (bottom) of 5-HT3A receptor cRNAs. B, time course of CBD inhibition of maximal currents induced by 1 μM 5-HT in oocytes previously injected with 1, 3, and 30 ng of the 5-HT3A receptor cRNAs. The solid bar indicates the duration (20 min) of 1 μM CBD application. Each data point represents mean ± S.E.M. from the average of five to six cells. C, concentration-response curves of CBD inhibition of 5-HT-activated current in oocytes injected with 1, 3, and 30 ng of 5-HT3A receptor cRNAs. The curves were best fit to the logistic equation as described under Materials and Methods. Each data point represents mean ± S.E. from five to seven oocytes. D, correlation between the magnitude of inhibitory effect induced by 1 μM CBD and increasing concentrations of 5-HT3A cRNAs injected into oocytes (linear regression, r = −0.99; n = 5–7).
Fig. 6.
Cannabidiol inhibition of 5-HT3A receptors is inversely correlated with the mean current density and ActD treatment-induced decreases in 5-HT-induced currents. A, correlation between the percentage of CBD inhibition of mean current density (MCD; linear regression, r = −0.96). B, bar graphs of average amplitude of current induced by maximal concentration of 5-HT (30 μM) with and without ActD treatment of X. laevis oocytes expressing mouse 5-HT3A receptors. Each bar represents mean ± S.E.M. from six to eight cells. C, bar graphs represents average percentage of CBD inhibition of 5-HT3A receptors without and with ActD treatment. Each bar represents mean ± S.E.M. from six to eight cells. *, P < 0.05, significant difference compared with control (ANOVA).
Discussion
The results presented indicate that the plant-derived nonpsychoactive cannabinoid CBD inhibits the function of both mouse and human 5-HT3 receptors expressed in X. laevis oocytes. The inhibitory effect of CBD on 5-HT-induced currents was concentration-dependent and related to 5-HT3 receptor expression. The IC50 values varied from 121 nM to 29 μM in oocytes injected with 1 to 30 ng of cRNA, respectively. Increasing the concentration of 5-HT did not overcome CBD inhibition of 5-HT-induced ion currents; i.e., the maximal amplitudes of 5-HT-induced currents were also inhibited, suggesting that CBD inhibition is noncompetitive.
CBD is a major nonpsychotropic constituent of C. sativa. Unlike THC, it is virtually inactive at both CB1 and CB2 receptors (Pertwee, 2009; for review, see Izzo et al., 2009). CB1 and CB2 receptors are not expressed in X. laevis oocytes (Hejazi et al., 2006; Oz et al., 2007). Therefore, it is unlikely that the effect of CBD on 5-HT3 receptors is mediated by the activation of CB1 or CB2 receptors. CBD analogs such as abnormal-cannabidiol are reported to activate non-CB1 and non-CB2 receptor by a PTX-sensitive G protein (Járai et al., 1999). However, CBD inhibited 5-HT3 receptor function in PTX-treated oocytes, indicating that the PTX-sensitive receptors do not mediate the functional interaction of CBD with the 5-HT3 receptor.
Cannabidiol increases intracellular Ca2+ levels in neurons and glia (Drysdale et al., 2006; Ryan et al., 2006). However, the magnitude of CBD inhibition of 5-HT3A currents was not significantly altered by intracellular injection of BAPTA, a high-affinity Ca2+ chelator. Furthermore, during our experiments, application of CBD in the highest concentration of used (30 μM) in this study, did not modify baseline currents, indicating that intracellular Ca2+ concentration was not affected by CBD. Because Ca2+-activated Cl− channels are highly sensitive to intracellular levels of Ca2+ (for review, see Dascal, 1987), the release of Ca2+ from internal stores of this ion would be reflected by changes in holding current in voltage-clamp conditions. This was not seen. In addition, other passive membrane properties such as membrane capacitance and oocyte input resistance were not significantly altered (Table 1), suggesting that CBD, at the concentrations used in this study, also did not disrupt the integrity of the lipid membrane.
CBD suppresses nausea and vomiting in animal models. In shrews, pretreatments with CBD suppress lithium chloride-induced vomiting (Parker et al. 2004). In rats, CBD interferes with nausea elicited by lithium chloride and with conditioned nausea elicited by a flavor paired with lithium chloride (Parker et al., 2002). Because CBD does not activate known CB receptors (Izzo et al., 2009; Pertwee, 2009), and the effect of CBD was not reversed by the CB1 receptor antagonist SR-141617A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboximide hydrochloride], this suppression of nausea and vomiting does not appear to be linked to activity of the CB1 or CB2 receptors (Kwiatkowska et al., 2004).
Cannabinoid receptor-independent actions of various cannabinoids on the function of 5-HT3 receptors have been demonstrated in several previous investigations (for review, see Oz, 2006). In previous in vitro electrophysiological studies, direct effects of the cannabinoid receptor ligands THC, anandamide, WIN55,212-2, and CP55,940 on the function of 5-HT3 receptors have been reported in both in vitro (Fan, 1995; Barann et al., 2002; Xiong et al., 2008; Oz et al. 1995, Oz et al. 2002) and in vivo studies (Godlewski et al., 2003; Przegalinski et al., 2005; Racz et al., 2008). Our results provide the first demonstration that nonpsychotropic phytocannabinoids such as CBD also modulate the function of 5-HT3 receptors.
Commonly used doses of CBD (3–10 mg/kg) produce brain levels of 200 nM to 3 μM (Varvel et al., 2006). Therefore, functional modulation of 5-HT3A receptors demonstrated in this study (IC50 = 121 and 587 nM for 1- and 3-ng cRNA-injected oocytes, respectively) can mediate some of the cannabinoid receptor-independent actions of CBD. In previous studies, direct actions of CBD on several integral membrane proteins, including various subtypes of glycine receptors (Ahrens et al., 2009), 5-HT receptors (Russo et al., 2005), opioid receptors (Kathmann et al., 2006), transient receptor potential channels (Bisogno et al., 2001; De Petrocellis et al., 2008; Qin et al., 2008), and T-type Ca2+ channels (Ross et al., 2008), have also been demonstrated (for a recent review, see Izzo et al., 2009). In addition, anti-inflammatory, analgesic, and antiepileptic actions of CBD are mediated by mechanisms independent of known cannabinoid receptors (for review, see Izzo et al., 2009).
Open-channel blockade is a widely used model to describe the block of ligand-gated ion channels. However, this model cannot account for the results of the present study. First, for open channel blockers, the presence of the agonist is required to let the blocker enter the channel after the receptor has undergone an agonist-induced conformational change to open the channel. In contrast to open channel blockers, preincubation of CBD caused a further inhibition (Figs. 1A and 3A), indicating that CBD can interact with the closed state of the 5-HT3A receptor. Second, inhibition by CBD is not voltage-sensitive, suggesting that the CBD binding site is not charged and that the site is not within the transmembrane electric field. Likewise, there was an absence of use-dependent blockade (Fig. 3A), and CBD had little effect when coadministered with 5-HT without CBD preincubation (data not shown). Third, recovery from CBD inhibition occurred independent of agonist application intervals (Fig. 3C), indicating that CBD is not trapped in the channel when the channel closes, as can occur with open channel blocking drugs. Finally, CBD did not significantly affect the reversal potential of 5-HT-induced currents, indicating that current inhibition is not due to an alteration in the ion selectivity of the channels.
Allosteric modulators alter the functional properties of ligand-gated ion channels by interacting with site(s) that are topographically distinct from the ligand binding sites (for review, see Onaran and Costa, 2009). In electrophysiological studies, although the potency of the 5-HT, a natural ligand (agonist) for this receptor, was not altered, its efficacy was significantly inhibited by CBD, indicating that CBD did not compete with the 5-HT binding site on the receptor. In agreement with these findings, radioligand binding studies indicated that displacement of [3H]GR65630 by 5-HT was not significantly affected by CBD, further suggesting that CBD does not interact with 5-HT binding site on the receptor. These findings indicate that CBD acts as an allosteric modulator of 5-HT3 receptor. In previous studies, CBD has been reported to be an allosteric modulator of several structurally different ion channels (Izzo et al., 2009); i.e., CBD binds to site(s) topographically distinct from the 5-HT binding sites on the receptor-ion channel complex. The noncompetitive property of the allosteric CBD inhibition puts it in an advantageous position, because the increases in concentration of endogenous agonist (5-HT) in synaptic cleft cannot alter the efficacy of CBD.
It is likely that CBD, a highly lipophilic agent, first dissolves into the lipid membrane and then diffuses into a nonannular lipid space to inhibit the ion channel-receptor complex. Consistent with this idea, the effect of CBD on 5-HT3 receptor reached to a maximal level within 10 to 15 min of application time. Likewise, actions of several hydrophobic allosteric modulators, such as endocannabinoids (Oz et al., 2002a; Spivak et al., 2007; Xiong et al., 2008), fatty acids (Oz et al., 2004c), steroids (Oz et al., 2002b), and general anesthetics (Zhang et al., 1997), on ligand-gated ion channels require 5 to 20 min to reach their maxima (for review, see Oz, 2006), suggesting that the binding site(s) for these allosteric modifiers is located inside the lipid membrane and require a relatively slow (in minutes) time course to modulate the function of the receptor. It is likely that these hydrophobic agents act as gating modifiers (for review, see Oz, 2006), affecting the energy requirements for the gating-related conformational changes in ligand-gated ion channels (Spivak et al., 2007).
It is interesting that we found an inverse correlation between the magnitude of CBD inhibition and the amount of cRNA injected into oocytes. In a recent study, biotinylation experiments indicated that the increase in the amount of cRNA injected into X. laevis oocytes enhances the surface expression of 5-HT3A receptors and attenuates the magnitude of anandamide inhibition of 5-HT3A receptor (Xiong et al., 2008). This phenomenon has been suggested to be due to the increased tendency of 5-HT3A receptors to desensitize at low expression levels. Various conditions that decrease the desensitization of the receptor also attenuate anandamide inhibition (Xiong et al., 2008). By definition, receptors are required to be open before their transition into a desensitized state. However, as mentioned, in the majority of reports, the effects of highly lipophilic substances, such as cannabinoid receptor ligands (Barann et al., 2002; Oz et al., 2002a) and steroids (Oz et al., 2002b), require a long (several seconds)-lasting exposure time before the opening of the channel by agonist application (for review, see Oz, 2006). Thus, it appears that cannabinoids can interact with 5-HT3A receptors during the closed state and facilitate desensitization during agonist activation of the receptor.
It is plausible to predict that CBD, similar to the effect of anandamide on nicotinic acetylcholine receptors (Spivak et al., 2007), reduces current amplitude by lowering the energy barrier for receptors to enter a desensitized state. In addition, 5-HT3A receptor density can contribute to the free-energy barrier required for conformational changes during a receptor desensitization process and facilitate the effect of CBD on the desensitization of 5-HT3A receptor. Clearly, further investigations in which receptor kinetics can be studied in a more detailed and precise manner are required to delineate the mechanisms by which CBD affects 5-HT3A receptor function.
In conclusion, our results indicate that CBD inhibits the function of homomerically expressed 5-HT3A receptor by a noncompetitive (allosteric) mechanism and that the expression level of 5-HT3A receptors significantly influences the sensitivity of the receptor to the inhibitory effect of CBD. These data add to a growing body of evidence (Izzo et al., 2009), indicating that cannabinoid-receptor-independent targets can contribute to pharmacological actions of CBD.
Acknowledgments
We thank Dr. David Julius for providing 5-HT3A receptor cDNA and Dr. Mary Pfeiffer (Intramural Research Program, National Institute on Drug Abuse) for careful editing of the manuscript.
This work was supported in part by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse and from the Faculty of Medicine and Health Science, United Arab Emirates University.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.162594.
- 5-HT
- 5-hydroxytryptamine
- THC
- tetrahydrocannabinol
- CB
- cannabinoid
- CBD
- cannabidiol
- WIN55,212-2
- 4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1-i,j]quinolin-6-one
- CP55,940
- (1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol
- JWH-015
- 1-propyl-2-methyl-3-(1-naphthoyl)indole
- cRNA
- complementary RNA
- Icap
- capacitive current
- Cm
- membrane capacitance
- PTX
- pertussis toxin
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- ActD
- actinomycin D
- MDL72222
- tropanyl 3,5-dichlorobenzoate
- HB
- homogenization buffer
- GR65630
- 3-(5-methyl-1H-imidazol-4-yl)-1-(1-methylindol-3-yl)propan-1-one
- ANOVA
- analysis of variance
- SR-141716A
- N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboximide hydrochloride.
References
- Ahrens J, Demir R, Leuwer M, de la Roche J, Krampfl K, Foadi N, Karst M, Haeseler G. (2009) The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-beta glycine receptor function. Pharmacology 83:217–222 [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barann M, Molderings G, Brüss M, Bönisch H, Urban BW, Göthert M. (2002) Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br J Pharmacol 137:589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. (1987) The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem 22:317–387 [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, Di Marzo V. (2008) Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther 325:1007–1015 [DOI] [PubMed] [Google Scholar]
- Drysdale AJ, Ryan D, Pertwee RG, Platt B. (2006) Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology 50:621–631 [DOI] [PubMed] [Google Scholar]
- Engleman EA, Rodd ZA, Bell RL, Murphy JM. (2008) The role of 5-HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS Neurol Disord Drug Targets 7:454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P. (1995) Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J Neurophysiol 73:907–910 [DOI] [PubMed] [Google Scholar]
- Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. (2007) The neuronal 5-HT3 receptor network after 20 years of research–evolving concepts in management of pain and inflammation. Eur J Pharmacol 560:1–8 [DOI] [PubMed] [Google Scholar]
- Godlewski G, Göthert M, Malinowska B. (2003) Cannabinoid receptor-independent inhibition by cannabinoid agonists of the peripheral 5-HT3 receptor-mediated von Bezold-Jarisch reflex. Br J Pharmacol 138:767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi N, Zhou C, Oz M, Sun H, Ye JH, Zhang L. (2006) Delta9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol Pharmacol 69:991–997 [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. (2009) Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30:515–527 [DOI] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, et al. (1999) Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA 96:14136–14141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. (2006) Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol 372:354–361 [DOI] [PubMed] [Google Scholar]
- Kwiatkowska M, Parker LA, Burton P, Mechoulam R. (2004) A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology 174:254–259 [DOI] [PubMed] [Google Scholar]
- Martin BR, Wiley JL. (2004) Mechanism of action of cannabinoids: how it may lead to treatment of cachexia, emesis, and pain. J Support Oncol 2:305–314; discussion 314–316 [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. (2007) Cannabidiol–recent advances. Chem Biodivers 4:1678–1692 [DOI] [PubMed] [Google Scholar]
- Onaran HO, Costa T. (2009) Allosteric coupling and conformational fluctuations in proteins. Curr Protein Pept Sci 10:110–115 [DOI] [PubMed] [Google Scholar]
- Oz M. (2006) Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol Ther 111:114–144 [DOI] [PubMed] [Google Scholar]
- Oz M, Brauneis U, Zhang L, Weight FF. (1995) Inhibition by the endogenous cannabinoid anandamide, of 5-HT3 receptor-mediated ion current in Xenopus oocytes. Proc Eur Symp Drug Addiction AIDS 3:64 [Google Scholar]
- Oz M, Melia MT, Soldatov NM, Abernethy DR, Morad M. (1998) Functional coupling of human L-type Ca2+ channel and angiotensin AT1A receptor coexpressed in Xenopus laevis oocytes: involvement of the carboxyl-terminal Ca2+ sensors. Mol Pharmacol 54:1106–1112 [DOI] [PubMed] [Google Scholar]
- Oz M, Spivak CE, Lupica CR. (2004a) Tween 80 is a potent inhibitor of α7-nicotinic acetylcholine receptor-mediated currents in Xenopus oocytes. J Neurosci Methods 137:167–173 [DOI] [PubMed] [Google Scholar]
- Oz M, Yang KH, Dinc M, Shippenberg TS. (2007) The endogenous cannabinoid anandamide inhibits cromakalim-activated K+ currents in follicle-enclosed Xenopus oocytes. J Pharmacol Exp Ther 323:547–554 [DOI] [PubMed] [Google Scholar]
- Oz M, Zakharova I, Dinc M, Shippenberg T. (2004b) Cocaine inhibits cromakalim-activated K+ currents in follicle-enclosed Xenopus oocytes. Naunyn Schmiedebergs Arch Pharmacol 369:252–259 [DOI] [PubMed] [Google Scholar]
- Oz M, Zhang L, Morales M. (2002a) Endogenous cannabinoid, anandamide, acts as a noncompetitive inhibitor on 5-HT3 receptor-mediated responses in Xenopus oocytes. Synapse 46:150–156 [DOI] [PubMed] [Google Scholar]
- Oz M, Zhang L, Spivak CE. (2002b) Direct noncompetitive inhibition of 5-HT(3) receptor-mediated responses by forskolin and steroids. Arch Biochem Biophys 404:293–301 [DOI] [PubMed] [Google Scholar]
- Oz M, Zhang L, Ravindran A, Morales M, Lupica CR. (2004c) Differential effects of endogenous and synthetic cannabinoids on α7-nicotinic acetylcholine receptor-mediated responses in Xenopus oocytes. J Pharmacol Exp Ther 310:1152–1160 [DOI] [PubMed] [Google Scholar]
- Parker LA, Kwiatkowska M, Burton P, Mechoulam R. (2004) Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology 171:156–161 [DOI] [PubMed] [Google Scholar]
- Parker LA, Mechoulam R, Schlievert C. (2002) Cannabidiol, a non-psychoactive component of cannabis and its synthetic dimethylheptyl homolog suppress nausea in an experimental model with rats. Neuroreport 13:567–570 [DOI] [PubMed] [Google Scholar]
- Pertwee RG. (2009) Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol 156:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegaliński E, Göthert M, Frankowska M, Filip M. (2005) WIN 55,212-2-induced reduction of cocaine hyperlocomotion: possible inhibition of 5-HT(3) receptor function. Eur J Pharmacol 517:68–73 [DOI] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. (2008) TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28:6231–6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rácz I, Bilkei-Gorzo A, Markert A, Stamer F, Göthert M, Zimmer A. (2008) Anandamide effects on 5-HT(3) receptors in vivo. Eur J Pharmacol 596:98–101 [DOI] [PubMed] [Google Scholar]
- Riering K, Rewerts C, Zieglgänsberger W. (2004) Analgesic effects of 5-HT3 receptor antagonists. Scand J Rheumatol Suppl 119:19–23 [PubMed] [Google Scholar]
- Ross HR, Napier I, Connor M. (2008) Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol. J Biol Chem 283:16124–16134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. (2005) Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 30:1037–1043 [DOI] [PubMed] [Google Scholar]
- Ryan D, Drysdale AJ, Pertwee RG, Platt B. (2006) Differential effects of cannabis extracts and pure plant cannabinoids on hippocampal neurones and glia. Neurosci Lett 408:236–241 [DOI] [PubMed] [Google Scholar]
- Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G. (2009) Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res 23:597–602 [DOI] [PubMed] [Google Scholar]
- Slatkin NE. (2007) Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting: beyond prevention of acute emesis. J Support Oncol 5:1–9 [PubMed] [Google Scholar]
- Spivak CE, Lupica CR, Oz M. (2007) The endocannabinoid anandamide inhibits the function of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 72:1024–1032 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SC. (2007) The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets 11:527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. (2001) Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 323:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR. (2006) Psychopharmacology (Berl) 186:226–234 [DOI] [PubMed] [Google Scholar]
- Xiong W, Hosoi M, Koo BN, Zhang L. (2008) Anandamide inhibition of 5-HT3A receptors varies with receptor density and desensitization. Mol Pharmacol 73:314–322 [DOI] [PubMed] [Google Scholar]
- Yakel JL, Jackson MB. (1988) 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron 1:615–621 [DOI] [PubMed] [Google Scholar]
- Zhang L, Oz M, Stewart RR, Peoples RW, Weight FF. (1997) Volatile general anaesthetic actions on recombinant nAChα7, 5-HT3 and chimeric nAChα7-5-HT3 receptors expressed in Xenopus oocytes. Br J Pharmacol 120:353–355 [DOI] [PMC free article] [PubMed] [Google Scholar]