Abstract
Signaling through G protein-coupled receptors (GPCRs) promotes breast cancer metastasis. G proteins convey GPCR signals by dissociating into Gα and Gβγ subunits. The aim of the present study was to determine whether blockade of Gβγ signaling suppresses breast cancer cell migration and invasion, which are critical components of metastasis. Conditioned media (CM) of NIH-3T3 fibroblasts are widely used as chemoattractants in in vitro cancer metastasis studies. Expression of a Gβγ scavenger peptide attenuated NIH-3T3 CM-induced migration and invasion of both metastatic breast cancer MDA-MB-231 and MDA-MB-436 cells by 40 to 50% without effects on cell viability. Migration and invasion of cells in response to NIH-3T3 CM were also blocked by 8-(4,5,6-trihydroxy-3-oxo-3H-xanthen-9-yl)-1-naph-thalene-carboxylic acid) (M119K), a Gβγ inhibitor, with maximum inhibition exceeding 80% and half-maximal inhibitory concentration (IC50) values of 1 to 2 μM. M119K also attenuated Rac-dependent formation of lamellipodia, a key structure required for metastasis. Constitutively active Rac1 rescued Gβγ blockade-mediated inhibition of breast cancer cell migration, whereas dominant negative Rac1 inhibited cell migration similar to Gβγ blockade. Furthermore, M119K suppressed Gi protein-coupled CXC chemokine receptor 4 (CXCR4)-dependent MDA-MB-231 cell migration by 80% with an IC50 value of 1 μM, whereas tyrosine kinase receptor-dependent cell migration was significantly less inhibited. However, CXCR4-dependent inhibition of adenylyl cyclase, a Giα-mediated response in MDA-MB-231 cells, was not blocked by M119K but was blocked by pertussis toxin, which selectively inactivates Giα. This report is the first to directly demonstrate the role of Gβγ in cancer cell migration and invasion and suggests that targeting Gβγ signaling pathways may provide a novel strategy for suppressing breast cancer metastasis.
Breast cancer is the second largest killer of women by cancer with approximately 40,000 deaths in the United States each year (Jemal et al., 2008). Patients who cannot be cured are primarily those in whom breast cancer has metastasized. Metastasis is a complex pathophysiological process, beginning with the migration and invasion of cancer cells into the surrounding tissues and lymphatics. From these sites, cancer cells can gain access to the circulation and proliferate to form new colonies at metastatic sites in vital organs such as the lung, bone, liver, and brain (Stetler-Stevenson and Kleiner, 2001). This directed cell migration and invasion is triggered by chemoattractants such as chemokines, growth factors, and matrix metalloproteases, which are sensed by cancer cell surface receptors including integrins, receptor tyrosine kinases, and G protein-coupled receptors (GPCRs). Evidence from preclinical and clinical studies shows excessive activation of GPCRs in breast cancer caused by overexpression of receptors, abnormally elevated ligands for GPCRs, and/or down-regulation of RGS proteins (Dorsam and Gutkind, 2007; Xie et al., 2009). Extensive studies using leukocytes and Dictyostelium have demonstrated that GPCRs are important regulators of directed migration of cells in response to chemotactic gradients (Van Haastert and Devreotes, 2004). Similar to leukocytes and Dictyostelium responding to chemotactic agents, signaling initiated by various GPCRs on breast cancer cells, including CXC chemokine receptor 4 (CXCR4) and protease-activated receptors, drive cancer cells to migrate and invade through surrounding tissues and spread to distant organs (Boire et al., 2005; Burger and Kipps, 2006).
GPCRs convey signals via heterotrimeric G proteins in the form of Gα-GTP and Gβγ subunits (Gutkind, 2000). The G proteins are classified into Gs, Gi, Gq, and G12/13 subfamilies. Chemoattractant-responsive GPCRs typically activate Gi proteins, resulting in dissociation of the Giα from the Gβγ subunits (Neptune and Bourne, 1997). The signaling function of G proteins was once attributed only to the Gα subunit. However, it is now clear that Gβγ also plays a prominent role in signal transduction through its numerous downstream effectors (Sternweis, 1994; Smrcka, 2008). For instance, overexpression of the Gβγ effectors p21-activated kinase 1 (PAK1) and P-Rex1 (Welch et al., 2002; Li et al., 2003) enhances metastasis of hepatocellular carcinoma and prostate cancer (Ching et al., 2007; Qin et al., 2009). However, both PAK1 and P-Rex1 can also be activated by growth factors acting through receptor tyrosine kinases (He et al., 2001; Yoshizawa et al., 2005), which are well known to stimulate metastasis. Thus, whether or not Gβγ activates PAK1 and P-Rex1 to promote metastasis is unclear. In addition, blocking Gβγ function by a peptide derived from the carboxyl terminus of β-adrenergic receptor kinase-1 (βARK1ct) or BIM-46174, an inhibitor of the Gα/Gβγ complex, suppressed tumor formation and growth (Bookout et al., 2003; Prévost et al., 2006); however, whether blockade of Gβγ suppresses cancer metastasis is unknown. Thus, it remains uncertain whether Gβγ itself directly participates in cancer metastasis and, if it does, how Gβγ signaling controls chemotactic cancer cell movement.
Breast cancer cells migrate toward chemoattractants by restructuring their cytoskeleton, a process controlled by Rho family proteins including Rac, Rho, and Cdc42 (Yamazaki et al., 2005). Aberrant Rac signaling is found in breast cancer and mediates metastasis (Baugher et al., 2005). We reported recently that Gi-coupled receptors promote breast cancer cell migration and invasion, in part by stimulating Rac-dependent lamellipodia formation, which can be inhibited by RGS4 protein (Xie et al., 2009), an inhibitor of both Giα and Gβγ signaling (Doupnik et al., 1997; Huang et al., 1997). Because Gβγ can directly stimulate the Rac-specific activator P-Rex1, resulting in Rac activation and migration of neutrophils (Welch et al., 2002), it is possible that Gβγ plays a similar role during breast cancer metastasis, and Gβγ signaling pathways may represent new therapeutic targets for suppressing breast cancer metastasis.
The goal of the present study was to determine whether selective blockade of Gβγ signaling pathways suppresses breast cancer cell migration and invasion. The Gβγ inhibitor βARK1ct has been used to determine the biological significance of Gβγ-dependent signaling pathways (Bookout et al., 2003). Recently, Smrcka and colleagues identified a family of small-molecule inhibitors of Gβγ (M119 family) (Bonacci et al., 2006) that suppressed neutrophil migration and infiltration (Lehmann et al., 2008). However, whether or not βARK1ct and M119 compounds affect cancer metastasis has not been examined. In the present study, we found that βARK1ct or the Gβγ inhibitor M119K suppressed metastatic abilities of breast cancer cells. In addition, our results demonstrate that Gβγ promotes breast cancer cell migration and invasion via Rac-dependent lamellipodia formation.
Materials and Methods
Cells and Reagents.
The human metastatic breast cancer MDA-MB-231 cell line was a gift from Dr. Zhaoyi Wang (Cancer Center, Creighton University), and the MDA-MB-436 cell line was purchased from the American Type Culture Collection (Manassas, VA). Cells were maintained in Dulbecco's modified Eagle medium (DMEM) (Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT). M119K and M119H were obtained from the Developmental Therapeutics Program, National Cancer Institute (Bethesda, MD). M119K is a xanthene derivative with high affinity for Gβγ. M119H is an analog of M119K without hydroxyl groups at the 4 and 5 positions of the xanthene moiety with 1000-fold reduced affinity for Gβγ (Bonacci et al., 2006). The adenovirus encoding βARK1ct was a gift from Dr. Terry D. Hexum (University of Nebraska Medical Center, Omaha, NE) (Li et al., 2004) and was prepared by using a ViraBind adenovirus miniprep kit (Cell Biolabs, San Diego, CA) according to the manufacturer's protocol. The βARK1ct peptide is the carboxyl-terminal 195 amino acids of βARK1 that contains a pleckstrin homology domain. The βARK1ct binds Gβγ via the pleckstrin homology domain and effectively prevents Gβγ-dependent signaling (Hawes et al., 1995). The adenovirus also expresses a green fluorescent protein (GFP), which serves as an index of transfection efficiency. pADneo2 C6-BGL, a gift from Dr. A. Himmler (Boehringer Ingelheim Research and Development, Vienna, Austria), contains six copies of the cAMP response element upstream from the firefly luciferase reporter gene. The Renilla reniformis luciferase expression plasmid, pRL-tk, was provided by Dr. Zhaoyi Wang. Constitutively active Rac1 (V12Rac1) and dominant negative Rac1 (N17Rac1) plasmids were purchased from Missouri S&T cDNA Resource Center (Rolla, MO). Unless indicated otherwise, other reagents were purchased from either Sigma-Aldrich (St. Louis, MO) or Thermo Fisher Scientific (Waltham, MA).
Adenoviral Infection.
An aliquot of frozen adenovirus was thawed and diluted in DMEM as needed. The culture medium of breast cancer cells (70% confluence) was replaced with growth medium (GM) containing recombinant adenovirus encoding GFP alone or GFP plus βARK1ct at a multiplicity of infection of 50 plaque-forming units. The cells were incubated for 36 h at 37°C. Infection efficiency was estimated based on the percentage of cells expressing GFP. Under these conditions, infection efficiency routinely was approximately 80%.
In Vitro Cell Growth Assays.
MDA-MB-231 cells were infected with adenovirus encoding GFP alone or GFP plus βARK1ct for 36 h. Cell viability was examined by a trypan blue exclusion assay. Cells were then seeded into 24-well plates at a density of 1.5 × 104 cells/well in GM (DMEM plus 10% fetal bovine serum) for 5 days and counted every 24 h with a Coulter Counter ZM (Beckman Coulter Inc., Fullerton, CA). Results shown are representative of three independent experiments performed in triplicate.
Electroporated Transfection of MDA-MB-231 Cells with Rac1 Mutants.
Four micrograms of pcDNA3.1 empty vector or vector encoding constitutively active V12Rac1 or dominant negative N17Rac1 was transfected into MDA-MB-231 cells (1.5 × 106) with a Cell Line Nucleofector Kit V using the Amaxa Nucleofector System (Lonza Walkersville Inc., Walkersville, MD), and transfected MDA-MB-231 cells were seeded on six-well plates. Transfection efficiency with the control GFP vector system was approximately 80%, as determined in three independent transfection experiments. After 24 h of incubation at 37°C, cells were infected without or with adenovirus encoding GFP alone or GFP plus βARK1ct for 36 h. Cells were harvested and subjected to transwell invasion and migration assays.
Transwell Invasion and Migration Assays.
Transwell invasion and migration assays were performed as described previously (Albini and Benelli, 2007; Xie et al., 2009). Invasion assays were carried out at 37°C for 12 h by using a 24-well Transwell apparatus (8-μm pore size with polycarbonate membrane; Corning Costar, Lowell, MA) coated with 30 μg of Matrigel (BD Biosciences, San Jose, CA). Cell suspensions were incubated with the appropriate concentrations of inhibitors or vehicle for 15 min and then added to the transwell chambers. Cells that invaded the Matrigel and migrated through the membrane, in response to chemoattractants with or without inhibitors, were quantified by staining with a Diff-Quik kit (Andwin Scientific, Woodland Hills, CA). Stained cells were counted and normalized relative to 10,000 seeded cells. Cell migration assays were performed similarly, but only for 5 h and without Matrigel coating. Conditioned media (CM) from NIH-3T3 mouse embryonic fibroblasts (American Type Culture Collection) was collected and used as a chemoattractant as described previously (Xie et al., 2009). To study the effect of inhibitors on CXCR4-dependent or EGF receptor-dependent cell migration, the insert membrane was precoated with 2 μg/ml fibronectin (BD Biosciences), and the CXCR4 agonist CXCL12 or EGF (R&D Systems, Minneapolis, MN) was used as the chemoattractant. The CXCR4 antagonist AMD3100 (20 μM, Sigma-Aldrich) (Hatse et al., 2002) was used to block CXCR4 function in MDA-MB-231 cells.
In Vitro Wound Healing Assay.
Directional cell migration was also studied by using an in vitro wound healing assay. MDA-MB-231 cells were seeded on 12-well tissue culture plates and grown to 100% confluence. Wounds were created by scraping the monolayer of cells with a sterile pipette tip followed by culture for 24 h in NIH-3T3 CM with or without inhibitors. Chemoattractants in NIH-3T3 CM triggered cell migration into the space created by the wound. The images of wounds were captured at 20× magnification with a Nikon TE200 microscope and CoolSNAP HQ2 camera (Photometrics, Tucson, AZ) immediately after wounding and 24 h later. The images were compared to estimate the effects of inhibitors on cell migration.
Immunofluorescence Microscopy.
Cortactin and F-actin double staining was used to visualize lamellipodia. MDA-MB-231 cells seeded on coverslips were serum-starved for 24 h and then cultured in NIH-3T3 CM for 5 h at 37°C in the presence or absence of 10 μM M119K or M119H. To investigate the effects of different inhibitors on CXCL12-induced lamellipodia formation, serum-starved MDA-MB-231 cells seeded on coverslips were pretreated with inhibitors or AMD3100 (20 μM) for 1 h and then stimulated without or with CXCL12 (100 ng/ml) for 30 min at 37°C. F-actin was visualized with rhodamine-labeled phalloidin (Cytoskeleton, Denver, CO). Cortactin was visualized by using anti-cortactin antibody (Cell Signaling Technology Inc., Danvers, MA) followed by fluorescein isothiocyanate-conjugated secondary antibody. Images were captured with a CoolSNAP CF camera attached to a Nikon (Tokyo, Japan) Eclipse 80i microscope. Background fluorescence was subtracted from the image, and individual cells were then digitally outlined with Image-Pro Plus software (Media Cybernetics, Inc., Bethesda, MD) to obtain a measure of total cell fluorescence for cortactin staining. The rim of cortactin staining at the leading edge was then digitally outlined, and its integrated fluorescent intensity was expressed as a percentage of the total cell fluorescence. Lamellipodia were identified as a smooth convex stretch of perpendicular actin stain at the peripheral edge of the cell as visualized by the rhodamine-labeled phalloidin stain. The summed length of lamellipodia was expressed as a percentage of total cell circumferences (Xie et al., 2009).
Rac Activation Assay.
Active Rac was assayed by using a Rac Activation Assay Kit (Millipore Corporation, Billerica, MA) according to the manufacturer's protocol. In brief, MDA-MB-231 cells were serum-starved for 24 h and then cultured in NIH-3T3 CM for 5 h at 37°C in the presence or absence of 10 μM of M119K or M119H. To investigate the effects of different inhibitors on CXCL12-induced Rac activation, serum-starved MDA-MB-231 cells were pretreated with inhibitors for 1 h and then stimulated without or with CXCL12 (100 ng/ml) for 30 min at 37°C. Cells (3 × 106/100-mm dish) were harvested in 0.6 ml of lysis buffer [25 mM HEPES (pH 7.5), 300 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, 2% glycerol, and 10 μg/ml leupeptin and aprotinin]. Cell lysates were centrifuged at 500g at 4°C for 10 min to remove nuclei and cell debris and then incubated with 10 μg of GST-PAK1-binding protein coupled to agarose beads at 4°C for 60 min with gentle agitation. Agarose beads were then washed three times with lysis buffer and collected by pulsing for 5 s in a microcentrifuge at 14,000g. The agarose beads were resuspended in 40 μl of 2× Laemmli reducing sample buffer [126 mM Tris-HCl (pH 6.8), 20% glycerol, 2% SDS, and 0.02% bromphenol blue] and boiled for 5 min. The samples were subjected to 12% SDS-polyacrylamide gel electrophoresis followed by immunoblotting with anti-Rac1 primary antibody (clone 23A8; Millipore Corporation). IRDye800-labeled anti-mouse secondary antibody was used for band detection with an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE) at a wavelength of 800 nm.
cAMP Luciferase Reporter Assays.
Cells were transiently transfected by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For cAMP assays, a reporter plasmid containing the luciferase gene under the transcriptional control of multiple units of the cAMP response element (pADneo2-C6-BGL) was used to measure cAMP-dependent signaling as described previously (Xie et al., 2007) with modifications. In brief, MDA-MB-231 cells were grown to 70% confluence in 24-well plates. pADneo2-C6-BGL (0.2 μg) was routinely cotransfected with R. reniformis luciferase expression pRL-tk plasmid (0.1 μg, an internal control for transfection efficiency) into cells. Cells were pretreated with or without pertussis toxin (PTx; 100 ng/ml overnight) and then stimulated with 5 μM forskolin in the presence or absence of the CXCR4 agonist CXCL12 (100 ng/ml) and various inhibitors for 8 h. Luciferase activities in cell lysates were measured with a Sirius luminometer (Berthold Technologies, Bad Wildbad, Germany) using the dual luciferase assay system (Promega, Madison, WI) and were normalized to the R. reniformis activities of the samples.
Statistical Analysis.
Statistical comparisons were made by using Student's t test. A probability (p) value < 0.05 was considered significant, with a Bonferroni correction used for multiple comparisons. Results are the mean ± S.E. of at least three determinations.
Results
Depletion of Free Gβγ Subunits by the βγ-Sequestering Peptide βARK1ct Inhibited the Migratory and Invasive Abilities of Breast Cancer Cells.
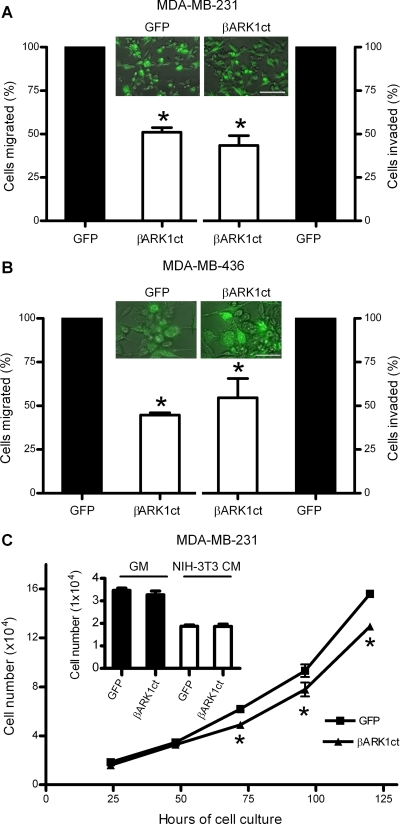
NIH-3T3 CM contains various cytokines and growth factors and is widely used as a chemoattractant in in vitro cancer metastasis assays (Boire et al., 2005; Qin et al., 2009; Xie et al., 2009). To determine whether depletion of free Gβγ would affect metastatic abilities of breast cancer cells, adenovirus encoding GFP alone or GFP and βARK1ct were used to infect metastatic breast cancer MDA-MB-231 cells and MDA-MB-436 cells. The abilities of those cells to migrate and invade through Matrigel in response to NIH-3T3 CM were measured in transwell chamber assays. As seen in Fig. 1A, the migratory and invasive abilities of MDA-MB-231 cells expressing βARK1ct were 51 ± 3 and 43 ± 6%, respectively, less than that of control cells expressing GFP alone. A similar inhibition of cell migration and invasion by βARK1ct was observed in MDA-MB-436 cells (Fig. 1B). It should be noted that infection efficiency was approximately 80% as determined by counting GFP-positive cells; 40 to 50% inhibition of cell migration and invasion by βARK1ct may be underestimated.
Fig. 1.
Depletion of free Gβγ subunits by the βγ-sequestering peptide βARK1ct inhibits the migration and invasion of breast cancer cells. A and B, metastatic breast cancer cells, MDA-MB-231 (A) and MDA-MB-436 (B), were infected with adenovirus encoding GFP alone or GFP and βARK1ct for 36 h and subjected to migration and invasion assays in response to NIH-3T3 CM. Data are the percentage of cells migrated or invaded per 10,000 loaded cells compared with control cells expressing GFP alone. A score of 100% is equal to 675 ± 78 migrated or 250 ± 43 invaded MDA-MB-231 cells (A) or 857 ± 94 migrated or 314 ± 63 invaded MDA-MB-436 cells (B). Columns, means; bar, SE (n = 4); *, p < 0.01 compared with GFP control. Overlaid images are representative of four independent experiments at 36 h postinfection under the fluorescence and bright-field channels. Scale bar, 100 μm. C, in vitro growth of MDA-MB-231 cells expressing GFP alone or GFP and βARK1ct in GM. Data shown are representative of three independent experiments performed in triplicate with *, p < 0.05 compared with cells expressing GFP alone. Inset, effects of βARK1ct on MDA-MB-231 cell growth after 48 h cultured in GM or NIH-3T3 CM.
Because overexpression of βARK1ct regulates prostate cancer cell growth and proliferation (Bookout et al., 2003), we investigated whether the effects of βARK1ct on breast cancer cell migration and invasion are caused by inhibition of cell growth and/or survival. Trypan blue exclusion assays showed that viability of MDA-MB-231 cells expressing GFP alone or GFP and βARK1ct were 93 ± 5 and 91 ± 6%, respectively, suggesting that expression of βARK1ct did not reduce cell viability. We then compared the growth characteristics of those MDA-MB-231 cells in culture. As shown in Fig. 1C, overexpression of βARK1ct inhibited MDA-MB-231 cell growth in GM by approximately 15%. However, this growth difference became statistically significant only after 48-h cell culture. Furthermore, MDA-MB-231 cells grew much slower in NIH-3T3 CM than in GM. After 48-h cell culture, there was no difference in total cell numbers with or without expression of βARK1ct (Fig. 1C, inset). Because the duration of our migration and invasion experiments were 5 and 12 h in NIH-3T3 CM, respectively, the impact of a difference in cell growth on cell migration and invasion should be negligible. These data suggest that free Gβγ is required for chemoattractant-stimulated migration and invasion of breast cancer cells.
The Gβγ Inhibitor M119K Reduced the Migratory and Invasive Abilities of Breast Cancer Cells.
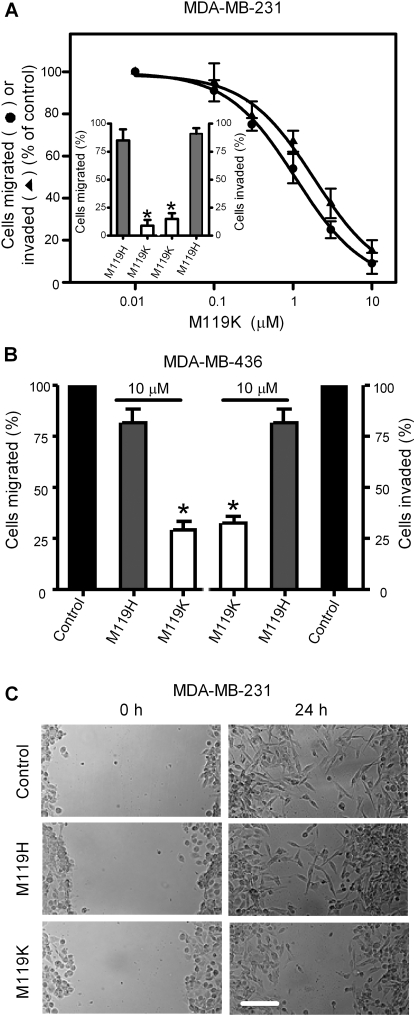
The Gβγ inhibitor M119K suppressed migration and invasion of MDA-MB-231 cells in a dose-dependent manner. The maximum inhibition of migration and invasion by M119K was over 85% with IC50 values of 1.0 ± 0.2 and 1.8 ± 0.5 μM, respectively (Fig. 2A). M119K (10 μM) also inhibited the migration and invasion of another metastatic breast cancer cell line, MDA-MB-436, by 70 ± 8% (Fig. 2B). In addition, we used M119H, an analog of M119K with much lower affinity for Gβγ (Bonacci et al., 2006), to identify nonspecific effects of this drug class. As expected, 10 μM M119H reduced migration and invasion of MDA-MB-231 and MDA-MB-436 cells by only approximately 20% (Fig. 2, A Inset, and B). Wound healing assays showed that Gβγ is required for enhanced cell motility in metastatic breast cancer cells, because 10 μM M119K, but not M119H, significantly suppressed NIH-3T3 CM-induced migration of MDA-MB-231 cells (Fig. 2C). It should be noted that cell viability was not affected by 10 μM M119K during experiments because more than 95% of treated cells were trypan blue negative.
Fig. 2.
The Gβγ inhibitor M119K reduces migration and invasion abilities of breast cancer cells. A and B, MDA-MB-231 cells (A) or MDA-MB-436 cells (B) were preincubated with dimethyl sulfoxide (control) and various concentrations of M119K or its less active analog M119H for 15 min, and then subjected to transwell migration and invasion assays in response to NIH-3T3 CM. Data are the percentage of cells migrated (●) or invaded (▲) compared with control cells. Inset, effects of 10 μM M119K or M119H on MDA-MB-231 cell migration and invasion. Points or columns, means; bar, SE (n = 3). *, p < 0.01 compared with control. C, movement of MDA-MB-231 cells into cleared space within a cell monolayer (wound) was monitored in a wound healing assay. Cell monolayers with wounds were incubated with NIH-3T3 CM in the absence (control) and presence of 10 μM M119K or M119H. Representative phase-contrast images of the wounded cell monolayers were taken immediately after wounding and 24 h later. Scale bar, 100 μm.
The Gβγ Inhibitor M119K Blocked Rac-Dependent Lamellipodia Formation in Breast Cancer Cells.
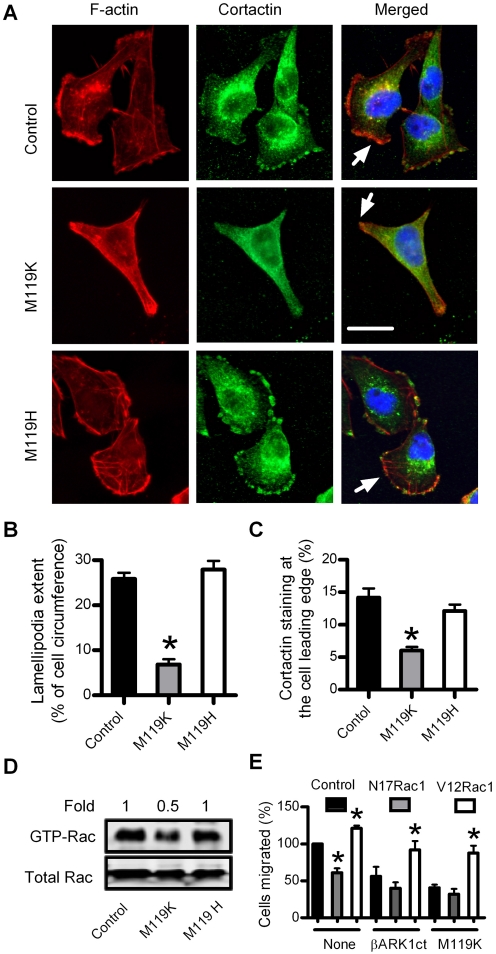
NIH-3T3 CM contains various cytokines and growth factors that can induce breast cancer cell migration and invasion through Rac-dependent lamellipodia formation (Xie et al., 2009). Lamellipodia is the flattened F-actin-rich leading edge of migrating cells, which is a key structure for cancer cell migration and invasion during metastatic progression (Condeelis et al., 2001). Because blocking Gβγ reduces NIH-3T3 CM-induced breast cancer cell migration and invasion, we investigated the effects of the Gβγ inhibitor M119K on lamellipodia formation of MDA-MB-231 cells cultured in NIH-3T3 CM. M119K (10 μM), but not M119H, caused less cell spreading and reduced the extent of lamellipodia in MDA-MB-231 cells by 68 ± 9% (Fig. 3, A and B). Because activated Rac recruits cortactin from the cytosol to the plasma membrane where cortactin stimulates F-actin polymerization to form the lamellipodia (Weed and Parsons, 2001), we further examined effects of Gβγ inhibition by M119K on subcellular localization of cortactin and the levels of active Rac in breast cancer cells. As shown in Fig. 3A, most cortactin in MDA-MB-231 control cells is cytosolic, with a small fraction found near or on the plasma membrane. A quantitative analysis of changes of cortactin distribution was attempted by measuring the percentage of total cortactin staining associated with cell leading edges. As shown in Fig. 3C, 10 μM M119K significantly reduced cortactin staining at the cell leading edge from 14.2 ± 1.4 to 6.0 ± 0.6% (p < 0.001). In contrast, 10 μM M119H reduced cortactin staining at the cell leading edge to only 12.1 ± 1.0% (p = 0.18). Interestingly, 10 μM M119K, but not M119H, also decreased the levels of activated GTP-bound Rac in MDA-MB-231 cells by approximately 50% (Fig. 3D). These data show that blockade of Gβγ suppresses lamellipodia formation by reducing Rac activity in breast cancer cells.
Fig. 3.
The Gβγ inhibitor M119K inhibits Rac-activated lamellipodia formation in breast cancer cells. Serum-starved MDA-MB-231 cells were incubated with NIH3T3 CM for 5 h in the absence (control) and presence of 10 μM M119K or M119H. A, cells were stained with rhodamine-conjugated phalloidin (red) for detection of F-actin or anticortactin antibody for detection of cortactin followed by fluorescein isothiocyanate-conjugated secondary antibody (green). Arrows indicate lamellipodia at cell edges. Scale bar, 25 μm. B, lamellipodia extent at cell edges was quantified as a percentage of the cell circumference on 20 randomly selected cells in each group. Columns, means; bar, SE (n = 3). *, p < 0.001 compared with control. C, quantification of the cortactin staining at cell leading edges. The cortactin staining of the whole cell and its leading edge was determined in 20 randomly selected cells from each group by using Image-Pro Plus software. Data shown represent the percentage of cortactin staining at the cell leading edge. Columns, means; bar, SE (n = 20). *, p < 0.001 by paired t test, referring to the difference between M119K-treated and control cells. D, GST-PAK1-binding protein pull-down assays for active GTP-bound Rac in MDA-MB-231 cell lysates. Ten percent of cell lysates used in Rac activation assays were also immunoblotted for total amounts of Rac. Rac protein was quantified by densitometry, and optical density units in each group are expressed as fold of optical density units of control cells. Data are representative of two separate experiments. E, effects of transient expression of a constitutively active Rac1 (V12Rac1) or a dominant negative Rac1 (N17Rac1) by electroporated transfection on NIH-3T3 CM-induced migration of MDA-MB-231 cells in the absence (none) or presence of βARK1ct or 3 μM M119K. *, p < 0.01 compared with control cells transfected with empty vector (n = 3).
Gβγ Stimulated Breast Cancer Cell Migration Involves Rac1 Signaling.
We next determined whether specific activation of Rac1 would rescue impaired migration of MDA-MB-231 cells caused by blockade of Gβγ. To accomplish this, we transiently expressed a constitutively active Rac1 (V12Rac1) or a dominant negative Rac1 (N17Rac1) in MDA-MB-231 cells by electroporated transfection and studied the effects on cell migration in the absence and presence of βARK1ct or M119K. As shown in Fig. 3E, expression of βARK1ct or 3 μM M119K treatment resulted in 50 to 60% inhibition of NIH-3T3 CM-induced cell migration. Expression of constitutively active V12Rac1 only enhanced NIH-3T3 CM-induced cell migration by approximately 20%, but significantly rescued defective migration of MDA-MB-231 cells with the expression of βARK1ct or M119K treatment. These data demonstrate that Gβγ stimulated breast cancer cell migration involves Rac1 signaling. Interestingly, expression of N17Rac1 alone reduced NIH-3T3 CM-induced cell migration by 40% but only slightly increased βARK1ct or M119K-mediated inhibition of cell migration from 50 to 60% to approximately 70%.
Gβγ Signaling Is Required for the Gi-Coupled Receptor CXCR4-Dependent Migration of Breast Cancer Cells.
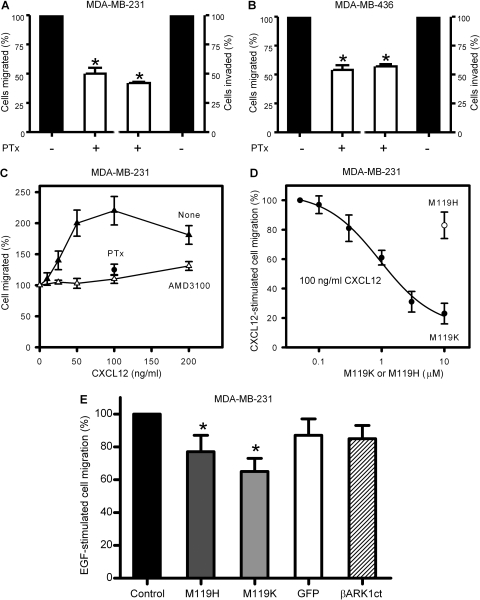
Heterotrimeric Gi proteins are the major donors of Gβγ after activation of GPCRs during chemotaxis (Neptune and Bourne, 1997). Gi-protein signaling can be selectively inhibited by PTx-dependent ADP ribosylation of the Giα subunit. Pretreatment of MDA-MB-231 and MDA-MB-436 cells with PTx (100 ng/ml overnight) blocked NIH-3T3 CM-stimulated cell migration and invasion by approximately 50% (Fig. 4, A and B), suggesting that activation of Gi proteins is required for breast cancer cell migration and invasion. Previous studies have demonstrated that the Gi protein-coupled receptor CXCR4 promotes breast cancer metastasis (Burger and Kipps, 2006; Xie et al., 2009). Indeed, the CXCR4 agonist CXCL12 caused an increase in MDA-MB-231 cell migration in a dose-dependent manner with maximum stimulation of 2.3-fold at 100 ng/ml (Fig. 4C). Pretreatment of cells with PTx (100 ng/ml overnight) or the CXCR4 antagonist AMD3100 (20 μM) (Hatse et al., 2002) blocked CXCL12-stimulated MDA-MB-231 cell migration (Fig. 4C). Interestingly, the Gβγ inhibitor M119K also suppressed CXCL12 (100 ng/ml)-stimulated migration of MDA-MB-231 cells in a dose-dependent manner. The maximum inhibition of migration by M119K was 80 ± 7% with an IC50 value of 0.9 ± 0.2 μM (Fig. 4D). In contrast, 10 μM M119H reduced CXCL12-stimulated migration of MDA-MB-231 cells by only 18 ± 10% (Fig. 4D). These data suggest that Gβγ signaling is required for the Gi-coupled receptor CXCR4-stimulated migration of breast cancer cells.
Fig. 4.
Blockade of Gβγ selectively inhibits Gi-coupled receptor CXCR4-dependent migration of breast cancer cells. A and B, metastatic breast cancer cells, MDA-MB-231(A) and MDA-MB-436 (B), were pretreated with or without PTx (100 ng/ml overnight) and subjected to transwell migration and invasion assays in response to NIH-3T3 CM. Data are the percentage of cells migrated and invaded compared with untreated cells. Points or columns, means; bar, SE (n = 3). *, p < 0.01 compared with no treatment (−). C, transwell migration assays using MDA-MB-231 cells stimulated with various concentrations of the CXCR4 agonist CXCL12 in the presence or absence of the CXCR4 antagonist AMD3100 (20 μM). Pretreatment of cells with PTx (100 ng/ml overnight) blocked 100 ng/ml CXCL12-stimulated cell migration by 81 ± 6%. Data are the percentage of cells migrated compared with untreated cells with 100% equal to 116 ± 27 cells migrated per 10,000 loaded cells (n = 3). D, effects of the Gβγ inhibitors M119K and M119H on the CXCL12-stimulated migration of MDA-MB-231 cells. Data are the percentage of increase in migrated cells in response to 100 ng/ml CXCL12 in the absence of M119K and M119H (n = 3). E, effects of Gβγ blockade on EGF (5 ng/ml)-stimulated migration of MDA-MB-231 cells. Cells were infected without (control) or with adenovirus encoding GFP alone or GFP and βARK1ct for 36 h. Cells were then stimulated with 5 ng/ml EGF in the absence or presence of 10 μM M119K or M119H in transwell migration assays. EGF (5 ng/ml) stimulated migration of control MDA-MB-231 cells by 1.9-fold. Data shown are the percentage of increase in migration compared with control cells. *, p < 0.05 compared with control (n = 4).
As a control, we also examined migration of breast cancer cells in response to a process dependent on tyrosine kinase receptors but not G proteins. EGF (5 ng/ml) stimulated MDA-MB-231 cell migration by 1.9-fold. The potent Gβγ inhibitor M119K (10 μM) reduced EGF stimulated cell migration by 35 ± 8% compared with 23 ± 10% with 10 μM of the less potent analog M119H (Fig. 4E). We also examined the effect of overexpression of βARK1ct on EGF-stimulated MDA-MB-231 cell migration. As shown in Fig. 4E, there was no significant difference in 5 ng/ml EGF-stimulated cell migration between MDA-MB-231 cells expressing GFP alone and cells expressing GFP and βARK1ct. Thus, the effects of stimulating migration pathways known to rely on tyrosine kinase receptors were little inhibited by blockade of Gβγ.
Gβγ Subunits Are Required for CXCR4-Stimulated, Rac-Dependent Lamellipodia Formation in Breast Cancer Cells.
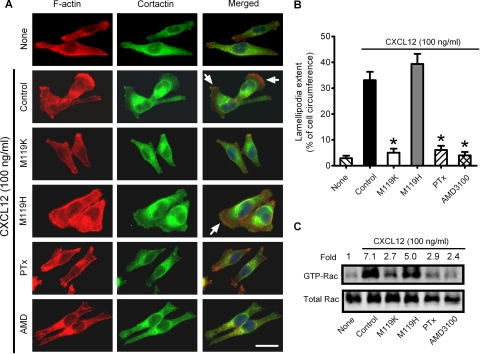
GPCR activation triggers actin cytoskeleton reorganization to form lamellipodia. Indeed, CXCL12 (100 ng/ml) induced MDA-MB-231 cells to spread out and form lamellipodia, which was blocked by pretreatment of cells with the CXCR4 antagonist AMD3100 (20 μM) or PTx (100 ng/ml overnight) (Fig. 5, A and B), indicating a Gi-coupled CXCR4-dependent response. Interestingly, 10 μM M119K, but not M119H, attenuated CXCL12-stimulated lamellipodia formation by 75 ± 4% (Fig. 5, A and B). Because Rac activation stimulates lamellipodia formation, we also examined effects of Gβγ inhibition by M119K on the levels of active Rac and subcellular localization of cortactin in CXCL12-stimulated breast cancer cells. As shown in Fig. 5C, CXCL12 treatment (100 ng/ml) increased the levels of activated GTP-bound Rac by approximately 7-fold. M119K (10 μM) attenuated CXCL12-stimulated Rac activation by 70%. In contrast, 10 μM M119H reduced CXCL12-stimulated Rac activation by only 25%. In addition, we found that 10 μM M119K, but not M119H, attenuated the translocation of cortactin from the cytosol to the lamellipodia in response to CXCL12 in MDA-MB-231 cells (Fig. 5A). As expected, Rac activation and cortactin translocation in response to CXCL12 were also blocked by pretreatment of MDA-MB-231 cells with AMD3100 (20 μM) or PTx (Fig. 5C). These data show that Gβγ signaling is required for CXCR4-stimulated Rac activation and lamellipodia formation in breast cancer cells. Thus, blockade of Gβγ would suppress breast cancer cell migration by reducing Rac-dependent lamellipodia formation.
Fig. 5.
Gβγ subunits are required for CXCR4-dependent Rac activation and lamellipodia formation in breast cancer cells. MDA-MB-231 cells were pretreated without or with PTx (100 ng/ml overnight) and then incubated without (none) or with CXCL12 (100 ng/ml) for 30 min in the absence (control) and presence of 10 μM M119K, 10 μM M119H, or 20 μM AMD3100. A, cells were stained for F-actin (red) or cortactin (green). Arrows indicate lamellipodia at cell edges. Scale bar, 25 μm. B, lamellipodia extent at cell edges was quantified as a percentage of the cell circumference on 20 randomly selected cells in each group. Columns, means; bar, SE (n = 3); *, p < 0.001 compared with cells treated with CXCL12 alone (control). C, GST-PAK1 binding protein pull-down assays for active GTP-bound Rac in MDA-MB-231 cell lysates. Ten percent of cell lysates used in Rac activation assays were also immunoblotted for total amounts of Rac. Rac protein was quantified by densitometry, and optical density units in each group are expressed as fold of optical density units of untreated cells (none). Images are representative of two separate experiments.
The Gβγ Inhibitor M119K Does Not Affect CXCR4-Dependent Inhibition of Adenylyl Cyclase Activity in Breast Cancer Cells.
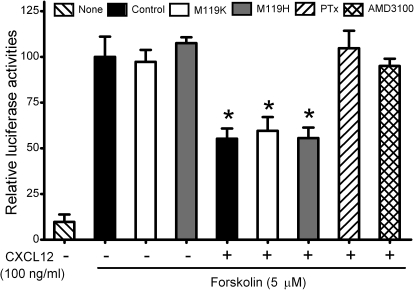
Activation of Gi protein by CXCR4 results in dissociation of Giα from Gβγ subunits. Giα inhibits adenylyl cyclase activity to reduce the level of cAMP in cells (Gilman, 1987). To differentiate Gβγ from Giα signaling pathways, we investigated the effects of M119K on CXCR4-dependent inhibition of adenylyl cyclase in MDA-MB-231 cells. Using a cAMP-dependent luciferase reporter assay (Xie et al., 2007), we found that CXCL12 (100 ng/ml) inhibited forskolin (5 μM)-stimulated cAMP production by 40 ± 7% (Fig. 6). Neither M119K nor M119H blocked the CXCL12 effects, suggesting that the CXCR4 receptor could still activate Gi proteins to release Giα subunit to inhibit adenylyl cyclase. In contrast, PTx, which inactivates the Giα subunit, completely blocked the CXCL12-mediated inhibition of cAMP generation (Fig. 6). As expected, the CXCR4 antagonist AMD3100 (20 μM) also significantly blocked the CXCL12 effect. These data show that M119K attenuates Gi-coupled receptor CXCR4 signaling by selectively suppressing Gβγ-dependent pathways without effects on Giα-dependent pathways in breast cancer cells.
Fig. 6.
PTx, but not M119K, attenuated CXCR4-dependent inhibition of adenylyl cyclase. MDA-MB-231 cells were transfected with dual luciferase reporter genes (pADneo2-C6-BGL and pRL-tk plasmids). Cells were pretreated without or with PTx (100 ng/ml) for 12 h and then stimulated without (none) or with 5 μM of the adenylyl cyclase activator forskolin in the presence or absence of CXCL12 (100 ng/ml) for 8 h. M119K (10 μM), M119H (10 μM), AMD3100 (20 μM), or the vehicle (control) was added 15 min before forskolin stimulation. Luciferase activities of cell lysates were measured by using the dual luciferase assay system. The results are presented as relative luciferase activities (ratio of reporter luciferases/R. reniformis luciferases). Columns, means; bar, SE (n = 3). *, p < 0.01 compared with cells treated with forskolin alone (Control).
Discussion
A wide range of evidence has appeared over the past several years supporting a role for GPCRs during cancer growth and metastasis (Daaka 2004; Dorsam and Gutkind, 2007). Various studies have shown that excessive activation of GPCRs in cancer is caused by abnormally elevated levels of GPCR ligands, overexpression of GPCRs, and/or alterations in GPCR regulation (Dorsam and Gutkind, 2007; Xie et al., 2009). Unfortunately, the large number of GPCR ligands and receptors that have been implicated in controlling chemotaxis precludes targeting a specific GPCR or its ligand to inhibit breast cancer metastasis. Thus, identifying the downstream components that are common to the GPCRs that are activated in cancer may provide an effective strategy for suppressing breast cancer metastasis.
GPCRs convey signals via heterotrimeric G proteins in the form of free Gα-GTP and Gβγ subunits. In this study, we found that Gβγ-sequestering peptide βARK1ct and the Gβγ inhibitor M119K inhibit the interaction between Gβγ and Rac-dependent signaling. Blocking Gβγ signaling pathways attenuates Rac-dependent processes such as lamellipodia formation and consequently inhibits cell migration and invasion, which are critical elements of breast cancer metastasis. Our study is the first to directly demonstrate that Gβγ is important for cancer chemotaxis. Because metastasis is the major cause of morbidity and mortality from breast cancer, our data suggest that identifying drugable components in Gβγ signaling cascades may be crucial for limiting the spread of cancer cells and making breast cancer more manageable by potentially curative procedures such as surgery, radiation, and immunotherapy.
G proteins have been shown to be directly linked to intracellular pathways that promote breast cancer invasion (Kelly et al., 2006). We previously showed that PTx inhibited migration and invasion of breast cancer cells, suggesting a role for Gi signaling pathways in metastasis (Xie et al., 2009). Because PTx specifically catalyzes ADP ribosylation of Giα and interrupts the receptor-dependent activation of G proteins it blocks both Gα- and Gβγ-dependent signaling (Ye, 2001). Whether Giα and/or Gβγ are responsible for chemotaxis in breast cancer cells is unknown. It has been reported that Gαi is not required for neutrophil chemotaxis mediated by Gi-coupled receptors (Neptune et al., 1999). Instead, disruption of Gβγ signaling inhibits neutrophil chemotaxis (Lehmann et al., 2008). In addition, sequestration of Gβγ subunits or using constitutively activated Giα subunit suppress cellular invasion of Madin-Darby canine kidney cells, suggesting that only Gβγ signaling is responsible for cell invasion (Faivre et al., 2001). Thus, we hypothesized that Gβγ signaling is important for breast cancer metastasis. Indeed, depletion of free Gβγ by its sequestering protein βARK1ct inhibited breast cancer migration and invasion in response to chemoattractants. In addition, we used M119K, a representative of a class of compounds that bind to Gβγ and interfere with the interaction between Gβγ and its effectors (Bonacci et al., 2006), thus suppressing Gβγ-dependent cellular response (Zhao et al., 2007; Lehmann et al., 2008). As expected, M119K also reduced NIH-3T3 CM-induced migration and invasion of metastatic breast cancer cells.
It should be noted that the maximum inhibition of cell migration and invasion by M119K is 70 to 85% compared with 40 to 50% inhibition by βARK1ct. There are two possible reasons for this difference. First, infection efficiency of cells by adenovirus was approximately 80%; thus, inhibition of cell migration and invasion by βARK1ct may be underestimated. Second, M119K may also inhibit breast cancer cell migration and invasion via Gβγ-independent mechanisms. In fact, 10 μM M119H, an analog of M119K with an IC50 of 300 μM for Gβγ (Bonacci et al., 2006), still reduced migration and invasion of MDA-MB-231 and MDA-MB-436 cells by approximately 20%, suggesting some nonspecific effects of this drug class. Nevertheless, blockade of Gβγ suppressed breast cancer cell migration and invasion.
Similar to other motile cells, breast cancer cells also respond to chemotactic signals by restructuring their cytoskeleton. Rho family members including Rac, Rho, and Cdc42 are intracellular regulators of these processes. Rac activation leads to membrane ruffling and the formation of lamellipodia as cells advance. We reported previously that reduction of lamellipodia formation with NSC23766, an inhibitor of Rac1 activation, significantly reduced migration and invasion of breast cancer cells (Xie et al., 2009). In the present study, we found that expression of dominant negative Rac1 mutant suppressed breast cancer cell migration. Interestingly, blocking Gβγ with M119K reduced the level of active GTP-bound Rac in MDA-MB-231 cells cultured in NIH-3T3 CM, which correlated with suppression of lamellipodia formation and cell migration. There was no additive effect of blocking Gβγ and Rac1 on cell migration, suggesting that both Gβγ and Rac1 function in the same signaling pathway. In addition, defective migration of MDA-MB-231 cells caused by Gβγ blockade was rescued by expression of constitutively active Rac1 mutant, suggesting that Rac1 functions downstream of Gβγ. Taken together, our results indicate a substantial contribution of Gβγ signaling to the maintenance of Rac activity that is important for breast cancer cells to migrate and invade in response to chemoattractants in NIH-3T3 CM.
NIH-3T3 CM contains a range of factors capable of activating different GPCRs and tyrosine kinase receptors. We found that pretreatment of MDA-MB-231 cells and MDA-MB-436 cells with PTx partially blocked NIH-3T3 CM-induced cell migration, suggesting that Gi-coupled receptor activation may be important in breast cancer metastasis. Gi protein-coupled receptor CXCR4 expression is a prognostic marker in breast cancer. Stromal-derived CXCL12 attracts breast cancer cells via its receptor, CXCR4, and plays an important role in the spread and progression of breast cancer (Burger and Kipps, 2006). Thus the CXCL12-CXCR4 signaling pathway may be a potential new target for treatment of breast cancer (Luker and Luker, 2006). However, downstream signaling components of the CXCL12-CXCR4 involved in breast cancer metastasis have not been defined. In the present study, we used the Gβγ inhibitor M119K to determine the role of Gβγ signaling in CXCR4-dependent breast cancer cell migration. Our data indicated that 10 μM M119K attenuated the MDA-MB-231 cell migratory response to CXCL12 by 80%, similar to the inhibitory effect of PTx treatment. In contrast, effects of stimulating migration pathways known to rely on EGF tyrosine kinase receptors were significantly less inhibited by M119K or βARK1ct. Thus, blockade of Gβγ selectively affects GPCR-stimulated cell migration and invasion. Interestingly, CXCL12 also induced Rac activation and lamellipodia formation, which was blocked by the CXCR4 antagonist AMD3100, suggesting a CXCR4-dependent response. More importantly, we found that blocking Gβγ by M119K attenuated CXCL12-stimulated Rac activation, which correlated with suppression of lamellipodia formation in CXCL12-treated cells. Thus, Gβγ is important for Gi-coupled receptor-dependent Rac activation, lamellipodia formation and cell migration in breast cancer cells.
However, the requirement for Gβγ in cell migration may not be related to signaling per se, but rather the G protein heterotrimer can not reform if Gβγ is blocked by M119K; therefore, the CXCR4 receptor can no longer activate Gi proteins. In this case all downstream signaling would be via Giα, and the Gβγ requirement is solely for receptor coupling to Gi proteins. The M119 family was originally described as a selective inhibitor of Gβγ downstream signaling without interfering with the binding between Gα and Gβγ (Bonacci et al., 2006). Indeed, we found that CXCL12 acting through CXCR4 receptors to release Giα was capable of inhibiting forskolin-stimulated adenylyl cyclase activation, even in the presence of M119K. Thus, the functional GPCR–G protein complex could be formed in the presence of M119K, and the CXCR4 receptor can still activate Gi proteins to release Giα and inhibit adenylyl cyclase. In contrast, PTx, which causes ADP ribosylation of Giα and blocks both Giα- and Gβγ-dependent signaling by preventing the activation and dissociation of Gi protein into Giα and Gβγ (Ye, 2001), not only attenuated CXCR4-dependent Rac activation and cell migration but also blocked inhibition of adenylyl cyclase activity. Taken together, these data demonstrate that Gβγ signaling, but not Giα signaling, plays a crucial role in the movement of breast cancer cells in response to chemoattractants.
In summary, our data show that the Gβγ released from PTx-sensitive Gi proteins are responsible primarily for the migration and invasion of breast cancer cells in response to chemotactic factors acting through GPCRs. Our studies suggest that blockade of Gβγ could significantly attenuate breast cancer metastasis by suppressing Rac activation. However, Gβγ cannot be blocked indiscriminately, and the challenge will be to identify and target Gβγ effectors that are vital to breast cancer metastasis but inconsequential for physiologically invasive normal cells. P-Rex1, a nonessential Rac-specific guanine nucleotide exchange factor with limited expression in normal adult tissues, is directly activated by Gβγ (Welch et al., 2002). P-Rex1 interacts directly with Rac, thereby leading to increased GTP-bound active Rac, which eventually causes actin polymerization to form lamellipodia. We recently reported that P-Rex1 was selectively up-regulated in metastatic prostate cancer and plays an important role in promoting prostate cancer metastasis (Qin et al., 2009). Interestingly, P-Rex1 was also up-regulated in breast cancer cells (Y. Xie, unpublished observation). Thus, Gβγ-dependent P-Rex1 or other nonessential cancer cell-specific Gβγ effectors could be important therapeutic targets for preventing breast cancer metastasis.
Acknowledgments
We thank Drs. Thomas F. Murray, Frank Dowd, Margaret A. Scofield, and Laura A. Hansen for helpful suggestions.
This work was supported, in part, by the National Institutes of Health National Cancer Institute [Grant R011CA125661] (to Y.T.) and the State of Nebraska Research Fund [Grants LB595, LB692] (to Y.T. and Y.X.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.164814.
- GPCR
- G protein-coupled receptor
- βARK1ct
- carboxyl terminus of β-adrenergic receptor kinase-1
- CM
- conditioned media
- GM
- growth medium
- IC50
- half-maximal inhibitory concentration
- GFP
- green fluorescent protein
- PTx
- pertussis toxin
- CXCR4
- CXC chemokine receptor 4
- CXCL12
- CXC chemokine ligand 12
- PAK1
- p21-activated kinase 1
- DMEM
- Dulbecco's modified Eagle medium
- EGF
- epidermal growth factor
- M119K
- NSC119893 [8-(4,5,6-trihydroxy-3-oxo-3H-xanthen-9-yl)-1-naphthalenecarboxylic acid)]
- M119H
- NSC119888 [3-(6-hydroxy-3-oxo-3H-xanthen-9-yl)-benzoic acid]
- AMD3100
- 1,1′-[1,4-phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane]octohydrobromide dihydrate.
References
- Albini A, Benelli R. (2007) The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation. Nat Protoc 2:504–511 [DOI] [PubMed] [Google Scholar]
- Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF. (2005) Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res 7:965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. (2005) PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120:303–313 [DOI] [PubMed] [Google Scholar]
- Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. (2006) Differential targeting of Gβγ-subunit signaling with small molecules. Science 312:443–446 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Finney AE, Guo R, Peppel K, Koch WJ. (2003) Targeting Gβγ signaling to inhibit prostate tumor formation and growth. J Biol Chem 278:37569–37573 [DOI] [PubMed] [Google Scholar]
- Burger JA, Kipps TJ. (2006) CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 107:1761–1767 [DOI] [PubMed] [Google Scholar]
- Ching YP, Leong VY, Lee MF, Xu HT, Jin DY, Ng IO. (2007) P21-activated protein kinase is overexpressed in hepatocellular carcinoma and enhances cancer metastasis involving c-Jun NH2-terminal kinase activation and paxillin phosphorylation. Cancer Res 67:3601–3608 [DOI] [PubMed] [Google Scholar]
- Condeelis JS, Wyckoff JB, Bailly M, Pestell R, Lawrence D, Backer J, Segall JE. (2001) Lamellipodia in invasion. Semin Cancer Biol 11:119–128 [DOI] [PubMed] [Google Scholar]
- Daaka Y. (2004) G proteins in cancer: the prostate cancer paradigm. Sci STKE 2004:re2 [DOI] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. (2007) G protein-coupled receptors and cancer. Nat Rev Cancer 7:79–94 [DOI] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA, Kofuji P. (1997) RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA 94:10461–10466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S, Régnauld K, Bruyneel E, Nguyen QD, Mareel M, Emami S, Gespach C. (2001) Suppression of cellular invasion by activated G-protein subunits Gαo, Gαi1, Gαi2, and Gαi3 and sequestration of Gβγ. Mol Pharmacol 60:363–372 [DOI] [PubMed] [Google Scholar]
- Gilman AG. (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649 [DOI] [PubMed] [Google Scholar]
- Gutkind JS. (2000) Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci STKE 2000: re1 [DOI] [PubMed] [Google Scholar]
- Hatse S, Princen K, Bridger G, De Clercq E, Schols D. (2002) Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett 527:255–262 [DOI] [PubMed] [Google Scholar]
- Hawes BE, van Biesen T, Koch WJ, Luttrell LM, Lefkowitz RJ. (1995) Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J Biol Chem. 270:17148–17153 [DOI] [PubMed] [Google Scholar]
- He H, Levitzki A, Zhu HJ, Walker F, Burgess A, Maruta H. (2001) Platelet-derived growth factor requires epidermal growth factor receptor to activate p21-activated kinase family kinases. J Biol Chem 276:26741–26744 [DOI] [PubMed] [Google Scholar]
- Huang C, Hepler JR, Gilman AG, Mumby SM. (1997) Attenuation of Gi- and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc Natl Acad Sci USA 94:6159–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. (2008) Cancer statistics. CA Cancer J Clin 58:71–96 [DOI] [PubMed] [Google Scholar]
- Kelly P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, Dewhirst MW, Fields TA, Casey PJ. (2006) The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci USA 103:8173–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann DM, Seneviratne AM, Smrcka AV. (2008) Small-molecule distruption of G protein subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol 73:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ikezu T, Hexum TD. (2004) βγ Subunits mediate the NPY enhancement of ATP-stimulated inositol phosphate formation. Peptides 25:267–274 [DOI] [PubMed] [Google Scholar]
- Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, et al. (2003) Directional sensing requires Gβγ-mediated PAK1 and PIX α-dependent activation of Cdc42. Cell 114:215–227 [DOI] [PubMed] [Google Scholar]
- Luker KE, Luker GD. (2006) Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett 238:30–41 [DOI] [PubMed] [Google Scholar]
- Neptune ER, Bourne HR. (1997) Receptors induce chemotaxis by releasing the βγ subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci USA 94:14489–14494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Iiri T, Bourne HR. (1999) Gαi is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem 274:2824–2828 [DOI] [PubMed] [Google Scholar]
- Prévost GP, Lonchampt MO, Holbeck S, Attoub S, Zaharevitz D, Alley M, Wright J, Brezak MC, Coulomb H, Savola A, et al. (2006) Anticancer activity of BIM-46174, a new inhibitor of the heterotrimeric Gα/Gβγ protein complex. Cancer Res 66:9227–9234 [DOI] [PubMed] [Google Scholar]
- Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, Scofield MA, Dowd FJ, Lin MF, Tu Y. (2009) Up-regulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene 28:1853–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV. (2008) G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci 65:2191–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis PC. (1994) The active role of βγ in signal transduction. Curr Opin Cell Biol 6:198–203 [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Kleiner DE., Jr (2001) Molecular biology of cancer: invasion and metastases, in Cancer: Principles and Practice of Oncology, 6th ed (DeVita VT, Jr., Hellmann S, Rosenberg SA. eds), pp 123–136, Lippincott Williams & Wilkins, Philadelphia: [Google Scholar]
- Van Haastert PJ, Devreotes PN. (2004) Chemotaxis: signaling the way forward. Nat Rev Mol Cell Biol 5:626–634 [DOI] [PubMed] [Google Scholar]
- Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. (2002) P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell 108:809–821 [DOI] [PubMed] [Google Scholar]
- Weed SA, Parsons JT. (2001) Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20:6418–6434 [DOI] [PubMed] [Google Scholar]
- Xie Y, Wolff DW, Lin MF, Tu Y. (2007) Vasoactive intestinal peptides transactivates the androgen receptor through a protein kinase A-dependent extracellular signal-regulated kinase pathway in prostate cancer LNCaP cells. Mol Pharmacol 72:73–85 [DOI] [PubMed] [Google Scholar]
- Xie Y, Wolff DW, Wang B, Deng C, Kirui JK, Jiang H, Qin J, Abel PW, Tu Y. (2009) Breast cancer migration and invasion depends on proteasome degradation of regulator of G protein signaling 4. Cancer Res 69:5743–5751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Kurisu S, Takenawa T. (2005) Regulation of cancer cell motility through actin reorganization. Cancer Sci 96:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Kawauchi T, Sone M, Nishimura YV, Terao M, Chihama K, Nabeshima Y, Hoshino M. (2005) Involvement of a Rac activator, P-Rex1, in neurotrophin-derived signaling and neuronal migration. J Neurosci 25:4406–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye RD. (2001) Regulation of nuclear factor κB activation by G protein-coupled receptors. J Leukocyte Biol 70:839–848 [PubMed] [Google Scholar]
- Zhao T, Nalbant P, Hoshino M, Dong X, Wu D, Bokoch GM. (2007) Signaling requirements for translocation of P-Rex1, a key Rac2 exchange factor involved in chemoattractant-stimulated human neutrophil function. J Leukocyte Biol 81:1127–1136 [DOI] [PubMed] [Google Scholar]