Abstract
Halothane (2-bromo-2-chloro-1,1,1-trifluoro-ethane) is an inhaled anesthetic that induces severe, idiosyncratic liver injury, i.e., “halothane hepatitis,” in approximately 1 in 20,000 human patients. We used known human risk factors (female sex, adult age, and genetics) as well as probable risk factors (fasting and inflammatory stress) to develop a murine model with characteristics of human halothane hepatitis. Female and male BALB/cJ mice treated with halothane developed dose-dependent liver injury within 24 h; however, the liver injury was severe only in females. Livers had extensive centrilobular necrosis, inflammatory cell infiltrate, and steatosis. Fasting rendered mice more sensitive to halothane hepatotoxicity, and 8-week-old female mice were more sensitive than males of the same age or than younger (4-week-old) females. C57BL/6 mice were insensitive to halothane, suggesting a strong genetic predisposition. In halothane-treated females, plasma concentration of tumor necrosis factor-α was greater than in males, and neutrophils were recruited to liver more rapidly and to a greater extent. Anti-CD18 serum attenuated halothane-induced liver injury in female mice, suggesting that neutrophil migration, activation, or both are required for injury. Coexposure of halothane-treated male mice to lipopolysaccharide to induce modest inflammatory stress converted their mild hepatotoxic response to a pronounced, female-like response. This is the first animal model of an idiosyncratic adverse drug reaction that is based on human risk factors and produces reproducible, severe hepatitis from halothane exposure with lesions characteristic of human halothane hepatitis. Moreover, these results suggest that a more robust innate immune response underlies the predisposition of female mice to halothane hepatitis.
Idiosyncratic adverse drug reactions (IADRs) occur in a minority of patients during drug therapy. They pose a unique public health problem because they cause severe illness and also often result in the withdrawal of otherwise useful drugs from the market. There are currently no predictive preclinical tests to identify drugs that have idiosyncratic potential, and the mechanisms of IADRs are poorly understood. Animal models that share the same sensitivity factors as humans and reproduce the liver lesions seen in people would be useful to study mechanisms of pathogenesis of IADRs and to develop strategies for therapy and prevention. The first step toward this effort must occur with drugs that are known to cause IADRs in people.
Halothane (2-bromo-2-chloro-1,1,1-trifluoro-ethane) is an inhaled anesthetic that produces a mild and reversible liver injury in one in five patients (Trowell et al., 1975). A more severe IADR, or “halothane hepatitis,” is observed in one in 6000 to 22,000 patients who receive the drug (Mushin et al., 1971). Although increasing numbers of reports of halothane-associated liver failure curtailed the use of halothane in the United States, it is still used in other countries, and cases of liver failure from halothane continue to be reported (Eghtesadi-Araghi et al., 2008). The most prominent histological feature seen in liver biopsies from patients who had halothane hepatitis is centrilobular necrosis (Blackburn et al., 1964). Other findings include fatty degeneration, vacuolation, and inflammatory infiltrate. Risk factors for severe halothane hepatotoxicity are female sex, genetics, age, and multiple halothane exposures (Inman and Mushin, 1974; Cousins et al., 1989). Other susceptibility factors, such as fasting before anesthesia and exposure to inflammagens that accompanies surgery, are possible. For example, it has been reported that the plasma concentration of lipopolysaccharide (LPS) increases in patients at the initiation of several types of surgery (Berger et al., 1997).
Halothane is metabolized by cytochrome P450 2E1 in hepatocytes to form trifluoroacetyl-(TFA-) chloride, which binds covalently to proteins and lipids making TFA-adducts. Direct toxicity from TFA adducts is thought to cause the mild form of injury (Bourdi et al., 2001). The severe form of halothane hepatotoxicity is widely thought to result from an adaptive immune response to TFA-adducted or halothane-modified macromolecules. This is supported by the finding that repeated exposure is a risk factor and by the appearance of antibodies and immune complexes in the sera of some halothane-treated patients (Bird and Williams, 1989).
Despite its popularity, there exists clinical evidence that is incongruent with a strictly adaptive immune-mediated hypothesis. For example, a recent retrospective study demonstrated that 39% of halothane hepatitis patients had no previous history of halothane exposure (Eghtesadi-Araghi et al., 2008). In addition, some people with antibodies in their serum did not develop liver injury (Walton et al., 1976b; Njoku et al., 2002). Attempts at developing animal models of liver damage using a halothane sensitization and challenge paradigm have resulted in a humoral immune response without associated liver pathology (Hastings et al., 1995). Pohl and Gillette noted in 1982 that after years of intensive investigation the adaptive immunity theory for halothane hepatitis was unproven (Pohl and Gillette, 1982). This remains true today.
It seems possible that halothane hepatitis occurs from a confluence of susceptibility factors, such as proposed by the “multiple determinant hypothesis,” in which an individual's chance of developing an IADR is a product of several discreet probabilities of specific risk factors particular to the individual and the drug (Li, 2002). This product would be expected to be small, which would explain the rare frequency of IADRs. If the major risk factors could be incorporated into an animal model, it follows that liver injury would occur with high frequency. Animal models of halothane hepatotoxicity have been developed, but most reproduce the mild type of injury (Lind et al., 1990; Bourdi et al., 2001; You et al., 2006). Attempts to develop animal models of severe, halothane-induced hepatotoxicity have focused on using repeated exposures (Hastings et al., 1995), drug-metabolizing enzyme inducers, glutathione depletion techniques, and/or hypoxic conditions (McLain et al., 1979; Lind et al., 1992). However, low incidence of responders, lack of severe hepatotoxicity, or both limit the usefulness of these models. High incidence of severe halothane-induced liver injury (N = 4) was reported in studies using female strain 2 guinea pigs (Lind et al., 1987), but few studies have followed the original report. Starting with knowledge gained from a mouse model of mild halothane-induced liver injury (You et al., 2006), we explored several known and likely human risk factors in developing a model of halothane hepatitis. The result is the first animal model in which severe halothane-induced liver injury occurs reproducibly without extensive chemical manipulations and that demonstrates histopathologic findings consistent with those observed in humans. The results raise the possibility that human risk factors might be useful in developing models of hepatotoxic IADRs and suggest that inflammation is a critical factor in the pathogenesis of halothane hepatitis.
Materials and Methods
Materials.
Halothane (2-bromo-2-chloro-1,1,1-trifluoro-ethane), highly refined, low acidity olive oil, sodium citrate, Oil Red O, and LPS were purchased from Sigma-Aldrich (St. Louis, MO). Isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane) was purchased from Abbott Laboratories (Abbott Park, IL). LPS from Escherichia coli O55:B5 (lot 075K4038) with an activity of 3.3 × 106 endotoxin units (EU)/mg was used in these studies. Rabbit anti-CD18 antiserum against amino acids 89 to 100 was purchased from New England Peptide (Gardnew, MA). Alanine aminotransferase (ALT) reagent was purchased from Thermo Electron Corp. (Louisville, CO). Anti-TFA-adduct rabbit serum was generously donated by Dr. Lance Pohl (the National Heart, Lung and Blood Institute, National Institutes of Health). Fluorescein isothiocyanate, goat anti-rabbit IgG, and horseradish peroxidase goat anti-rabbit IgG secondary antibodies were purchased from Invitrogen (Carlsbad, CA) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), respectively. Radioimmunoprecipitation assay buffer and halt protease inhibitor were purchased from Thermo Fisher Scientific (Waltham, MA).
Animals.
C57BL/6 or BALB/cJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used at an age of 4, 8, or 10 to 12 weeks, with weights ranging from 10 to 27 g. They were housed under conditions of controlled temperature and humidity and a 12-h light/dark cycle. They were given continuous access to bottled spring water and fed a standard chow (Rodent Chow/Tek 2018; Harlan Teklad, Madison, WI) ad libitum. The mice were allowed to acclimate for 1 week before use. Unless otherwise stated, mice were fasted for 15 h and injected with halothane between 10:00 AM and 12:00 PM. Food was returned 30 min after halothane administration. All procedures were carried out according to the humane guidelines of the American Association for Laboratory Animal Science and the University Laboratory Animal Research Unit at Michigan State University.
Experimental Protocol.
Unless otherwise stated, experiments were performed using BALB/cJ mice. Halothane was mixed with olive oil in a septum-covered glass vial at concentrations of 0.047, 0.063, 0.094, 0.187, 0.375, and 0.562 M to deliver the respective doses of 3.75, 5, 7.5, 15, 30, and 45 mmol/kg. Isoflurane mixtures were prepared similarly. Mice were fasted overnight to mimic the duration humans are typically fasted before surgery. Mice were given halothane or olive oil intraperitoneally. For polymorphonuclear leukocyte (PMN) functional inhibition, mice were treated with 100 μl of anti-CD18 serum (1:1 in saline), normal rabbit serum (1:1 in saline), or saline intravenously 6 h after halothane administration. They were anesthetized with isoflurane and euthanized at various times after the administration of halothane. Blood was drawn from the vena cava into a syringe containing sodium citrate (final concentration, 0.76%) for preparation of plasma. The left lateral lobe of the liver was fixed in 10% neutral buffered formalin (Thermo Fisher Scientific, Waltham, MA) and then blocked in paraffin. The median lobe was snap frozen, and the caudal lobe was fixed in formalin, immersed in 20% sucrose, snap-frozen in Tissue-Tek OCT embedding media (VWR Labshop, Batavia, IL), and cryosectioned.
Male BALB/cJ mice were treated with 5.0 × 106 EU/kg LPS i.v. 5.5 h after halothane administration. The dose chosen for LPS was nonhepatotoxic but consistently produces increased plasma cytokine concentrations and hepatic PMN accumulation.
Histopathology.
Paraffin-embedded left lateral liver lobes were sectioned, stained with hematoxylin and eosin, and examined by light microscopy. Snap-frozen sections were sectioned and stained with Oil Red O (0.5% Oil Red O in isopropanol) according to a previously described method (Lillie and Ashburn, 1943).
Immunohistochemistry and Microscopy.
For PMN identification, formalin-fixed liver sections were probed with anti-mouse PMN antibodies using the protocol described by Yee et al. (2003). Stained PMNs were counted in four to five random 400× fields for each animal with an Eclipse E400 light microscope (Nikon, Tokyo, Japan). For evaluation of TFA-adducted proteins, 8-μm frozen liver sections from treated animals were probed with TFA-adduct antiserum. Using a fluorescent microscope, optical fields were captured as computer images. Five centrilobular and periportal regions were analyzed, and the percentage of positive pixels in the centrilobular area was calculated and divided by the percentage in the periportal region, in which fluorescent staining was minimal in all sections examined.
Western Analysis.
Frozen liver samples were processed for whole cell protein isolation with radioimmunoprecipitation assay buffer supplemented with halt protease inhibitor according to the manufacturer's directions. Twenty micrograms of protein were loaded onto NuPAGE 12%-Tris gels (Invitrogen) and electrophoresed in Invitrogen NuPAGE MOPS SDS running buffer at 200 V for 1 h. Protein was transferred onto immuno-blot polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA) using Invitrogen NuPAGE transfer buffer at 150 mAmp for 2 h. The blots were blocked in 5% bovine serum albumin (BSA) for 1 h and hybridized with 1:10,000 TFA antiserum in 5% BSA overnight at 4°C. They were washed in Tris-buffered saline with 0.01% Tween 20 three times and hybridized with 1:5000 goat anti-rabbit horseradish peroxidase in 5% BSA for 2 h at room temperature. After the Tris-buffered saline with 0.01% Tween 20 wash, the proteins were detected using ECL Western Blotting Analysis system and Hyperfilm MP (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Tumor Necrosis Factor-α Analysis.
The plasma concentration of TNF-α was measured using an OpEIA mouse TNF ELISA kit (BD Biosciences, San Jose, CA).
Statistical Analysis.
Results are presented as mean ± S.E.M. A Student's t test was performed on comparisons of two groups. For comparison of more than two groups, a one- or two-way analysis of variance was used as appropriate after data normalization. A Student-Newman-Keuls test was performed to compare means in studies in which the analysis of variance indicated statistical significance. The criterion for significance was p < 0.05 for all experiments.
Results
Dose- and Sex-Dependent Liver Injury in Halothane-Treated Mice.
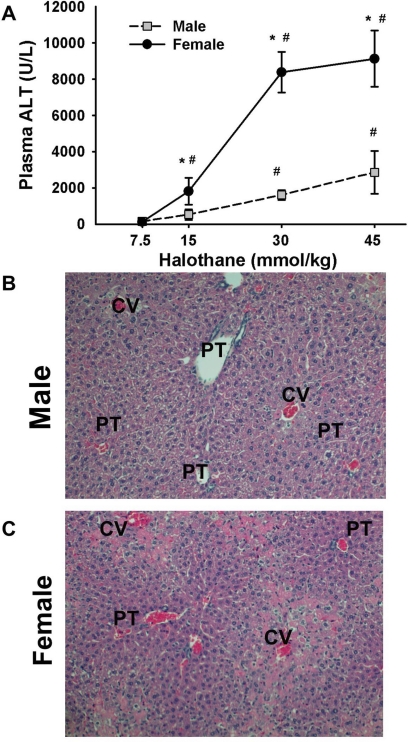
Male and female BALB/cJ mice were treated with halothane, and hepatocellular injury was assessed 24 h later from the activity of ALT in plasma and liver histopathology. Doses up to 15 mmol/kg halothane proved to be subanesthetic, with mild ataxia evident at the 15-mmol/kg dose. The 30-mmol/kg dose induced a transient anesthesia for less than 30 min. Resulting liver injury was dose-dependent in males and females (Fig. 1A). Male mice developed relatively mild liver damage at doses up to 45 mmol/kg. The response in females was greater than that in males and corresponded to severe hepatocellular injury, as plasma ALT activities approached 10,000 U/l (see Supplemental Figs. A and B for comparison of mild and severe liver injury at two doses in female mice). Livers from female mice treated with 30 mmol/kg halothane had severe lesions located primarily in centrilobular regions. Lesions were characterized by hepatocellular necrosis and inflammatory cell infiltrate (Fig. 1C). Similarly treated males had milder liver lesions than females (Fig. 1B).
Fig. 1.
Halothane-induced hepatotoxicity in male and female mice. A, plasma ALT activity evaluated 24 h after halothane treatment (intraperitoneal) of mice fed ad libitum (n = 3–6/group). *, significantly different from males given the same dose. #, significantly different from 7.5 mmol/kg-treated sex-matched animals. B and C, hematoxylin and eosin (H&E)-stained liver sections from 30 mmol/kg halothane-treated male and female mice, respectively. Liver section from the male mouse shows minimal necrosis, whereas the lesion is more severe in the female mouse. Labeled in picture are central vein (CV) and portal triad (PT).
Sensitivity to Halothane-Induced Liver Injury in Fasted Mice.
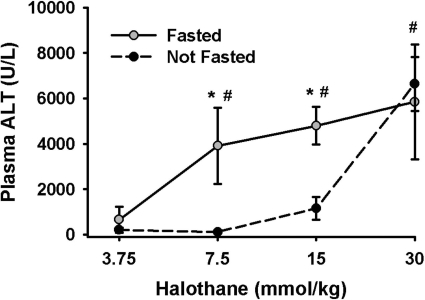
To test whether fasting increases sensitivity to halothane-induced liver injury, female BALB/cJ mice were fasted for 15 h before halothane administration, and plasma ALT activity was evaluated 24 h later. In mice fed ad libitum, there was little or no hepatic injury at doses up to 15 mmol/kg. In contrast, in fasted mice treated with 7.5 or 15 mmol/kg, plasma ALT activity reached 4000 U/l. Fasting shifted the dose-response curve to the left so that doses of 7.5 mmol/kg or greater were hepatotoxic (Fig. 2).
Fig. 2.
Fasting enhances sensitivity to halothane-induced liver injury. Female mice were either fasted overnight or not and then given halothane at the doses indicated (n = 3–5/group). Blood was collected 24 h later for the determination serum ALT activity. *, significantly different from fed mice given the same dose. #, significantly different from 3.75-mmol/kg group.
Genetic Background as a Sensitivity Factor for Halothane-Induced Liver Injury.
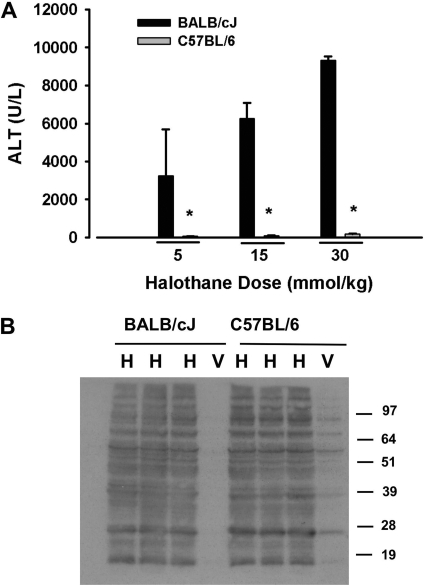
Responses to halothane were compared in two inbred mouse strains, BALB/cJ and C57BL/6. As demonstrated in Fig. 2, halothane caused dose-dependent hepatotoxicity in the BALB/cJ mice. In contrast, there were no significant increases in plasma ALT activity at any halothane dose up to 30 mmol/kg in the C57BL/6 mice (Fig. 3A). The extent of TFA-adduct formation in liver homogenates from halothane-treated C57BL/6 and BALB/cJ female mice was similar (Fig. 3B).
Fig. 3.
Genetic background is a sensitivity factor for severe halothane hepatotoxicity in mice. Fasted female C57BL/6 and BALB/cJ mice were given halothane at the doses indicated (n = 3–5/group), and blood was collected 24 h later. A, ALT activity in plasma. B, immunoblot detection of TFA-protein adducts in liver homogenates from mice treated with 15 mmol/kg halothane. *, significantly different from BALB/cJ mice.
Insensitivity to Isoflurane-Induced Liver Injury.
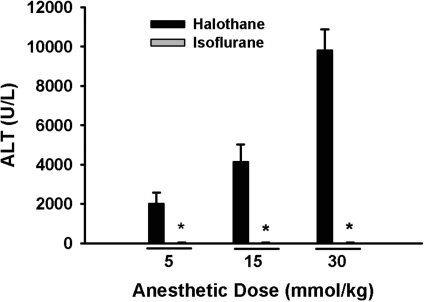
Isoflurane is an inhaled anesthetic that is structurally similar to halothane but has less propensity than halothane to cause hepatotoxic IADRs in humans (Hussey et al., 1988). In female BALB/cJ mice, halothane exposure caused dose-dependent hepatotoxicity, whereas ALT activity was not increased at doses of isoflurane up to 30 mmol/kg (Fig. 4).
Fig. 4.
Lack of isoflurane-induced hepatotoxicity in female mice. Fasted female BALB/cJ mice were given halothane or isoflurane at the doses indicated (n = 3–5/group), and blood was collected at 12 h for plasma ALT activity. All isoflurane-treated mice had plasma ALT activities <50 U/l. *, significantly different from the respective halothane-treated group.
Age as a Sensitivity Factor for Halothane-Induced Liver Injury in BALB/cJ Mice.
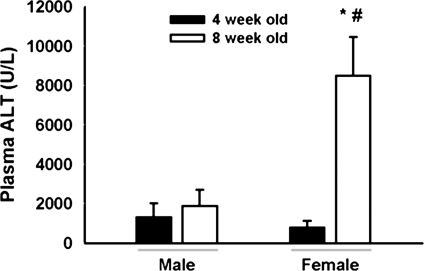
Responses to halothane were compared in 4- and 8-week-old male and female BALB/cJ mice. After 15 mmol/kg halothane exposure, plasma ALT activity was <2000 U/l in 8-week-old males and 4-week-old mice of either sex (Fig. 5). Eight-week-old female mice had plasma ALT activity of ∼8000 U/l.
Fig. 5.
Age is a sensitivity factor for severe halothane hepatotoxicity in BALB/cJ mice. Fasted 4- and 8-week-old male and female BALBc/J mice (n = 5/group) were given 15 mmol/kg halothane i.p., and blood was collected 24 h later. *, significantly different from age-matched males. #, significantly different from 4-week-old females.
Further Characterization of Sex-Related Differences in Sensitivity to Halothane-Induced Hepatotoxicity: Development of Halothane-Induced Liver Injury in Male and Female BALB/cJ Mice.
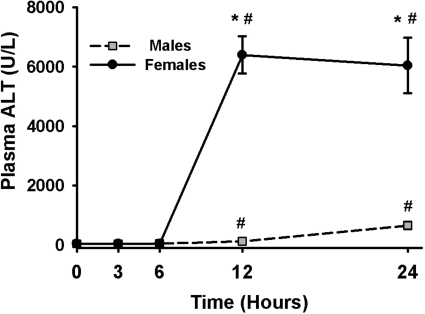
Having evaluated the ability of sex, fasting, genetic background, and age to modulate the response to halothane, we investigated further the factors involved in the sex-related difference in sensitivity. Ten- to 12-week-old male and female mice demonstrated signs of transient (<30 min), mild coordination loss after administration of 15 mmol/kg halothane. In male mice, halothane treatment caused an increase in plasma ALT activity at 12 and 24 h after administration, at which times the ALT activity was 124 and 600 U/l, respectively (Fig. 6). The injury in female mice followed a similar time course but was much more severe, reaching an ALT activity of nearly 7000 U/l at these times.
Fig. 6.
Development of halothane-induced liver injury. Fasted female and male BALB/cJ mice were given vehicle or 15 mmol/kg halothane i.p. (n = 4–6/group). Blood was collected at various times for the determination of plasma ALT activity. There was no time- or sex-related difference in ALT activity in the vehicle-treated mice, so the results were combined and represented as zero time. *, significantly different from male mice at the same time. #, significantly different from vehicle-treated animals (zero time).
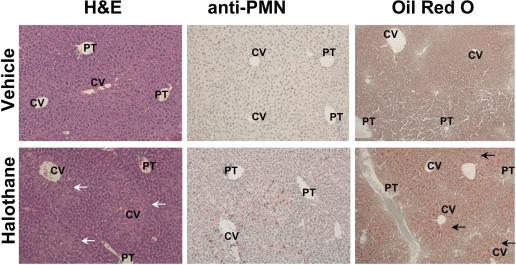
There were no significant lesions in untreated female mice, whereas halothane-treated females developed pronounced centrilobular necrosis within 12 h (Fig. 7). Immunohistochemical staining for PMNs revealed a greater number in livers of halothane-treated mice compared with control mice. Livers from halothane-treated mice also had greater Oil Red O staining at 6 h compared with livers from untreated mice at the same time (Fig. 7), indicating that halothane administration caused steatosis.
Fig. 7.
Histopathology of livers from halothane-treated mice. female BALB/cJ mice were fasted overnight and then given vehicle or halothane (15 mmol/kg i.p.). Representative liver sections from mice treated 12 h earlier were examined after hematoxylin and eosin (H&E) staining or after immunohistochemical staining for PMNs, in which PMNs appear as pink cells. Liver sections taken from mice 6 h after halothane administration were stained with Oil Red O, in which lipid appears as red dots. Labeled in the picture are central vein (CV) and portal triad (PT). Photomicrographs were taken at 200× magnification.
Halothane Bioactivation in Male and Female BALB/cJ Mice.
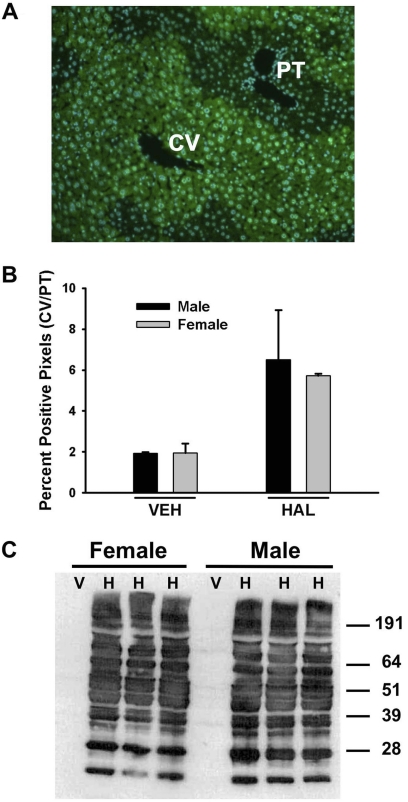
A positive correlation has been reported between the severity of liver injury and the formation of TFA-adducts in livers of halothane-treated guinea pigs (Bourdi et al., 2001). As shown in Fig. 8A, TFA-adducted proteins in the livers from halothane-treated male mice had a centrilobular distribution. A similar distribution was observed in halothane-treated female mice (data not shown). There was a similar degree of TFA-adduct formation in livers from halothane-treated male and female mice given the same dose of halothane as determined by immunohistochemistry (Fig. 8B) or by Western analysis of liver homogenates (Fig. 8C). There were no TFA-adducts in the livers of vehicle-treated animals of either sex (Fig. 8C).
Fig. 8.
Halothane metabolism is similar in male and female mice. Fasted male and female BALB/cJ mice were treated with vehicle or 15 mmol/kg halothane, and liver samples were collected 12 h later. A, representative liver section from a halothane-treated, male mouse stained immunohistochemically for TFA-adducts and visualized with green color. 4,6-Diamidino-2-phenylindole nuclear stain appears blue. B, ratio of positive pixels in the centrilobular and periportal regions [central vein (CV)/portal triad (PT)] (n = 3/group). C, immunoblot detection of TFA-protein adducts in liver homogenates.
Plasma TNF-α Concentration in Halothane-Treated Mice.
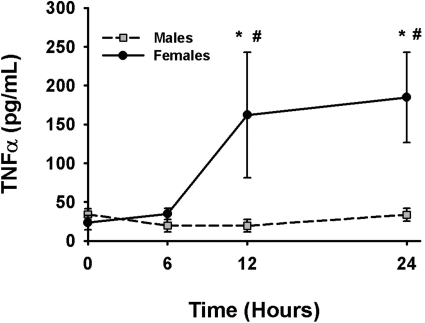
In vehicle-treated mice or in halothane-treated (15 mmol/kg) males, there was no change in plasma TNF-α concentration at any time investigated. In contrast, plasma TNF-α concentration was significantly increased 12 and 24 h after halothane exposure in female mice (Fig. 9).
Fig. 9.
Sex-specific difference in plasma TNF-α concentration after halothane exposure. Female and male BALB/cJ mice were given vehicle or 15 mmol/kg halothane (n = 4–6/group), and blood was collected at various times. Plasma TNF-α concentration was determined using an OptEIA mouse enzyme-linked immunosorbent assay kit (BD Biosciences). The average plasma concentration of TNF-α in vehicle-treated animals was less than 40 pg/ml. *, significantly different from males at the same time. #, significantly different from vehicle controls (zero time).
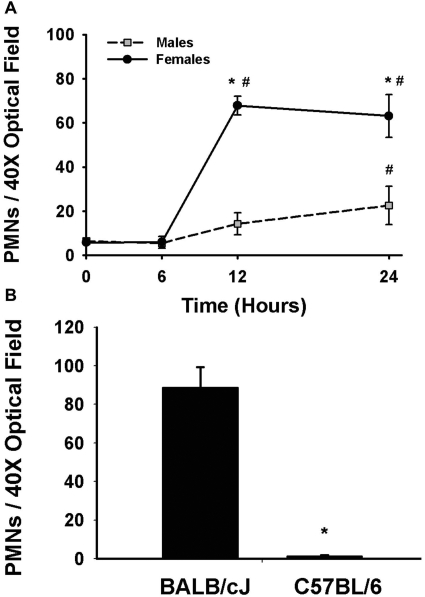
Hepatic PMN Recruitment in Halothane-Treated Mice.
There was no change in the number of hepatic PMNs in vehicle-treated mice over time. PMN number was slightly elevated by 24 h in livers of male mice given 15 mmol/kg halothane. In contrast, there was a marked increase in hepatic PMNs in female mice at 12 and 24 h after halothane administration. Hepatic PMN accumulation was significantly greater in female mice compared with male mice at 12 and 24 h (Fig. 10A). It is interesting to note that PMNs failed to accumulate in halothane-treated C57BL/6 mice (Fig. 10B), which were insensitive to halothane hepatotoxicity (see above).
Fig. 10.
Hepatic PMN recruitment is sex- and strain-dependent. Female and male BALB/cJ mice were given vehicle or 15 mmol/kg halothane (n = 4–5/group), and female BALB/cJ and C57BL/6 mice were given 30 mmol/kg halothane (n = 4). The number of PMNs in liver sections was determined by counting PMNs in immunostained tissue. A, hepatic PMNs in male and female BALB/cJ mice. The number of PMNs was less than eight for vehicle-treated mice and did not change with time, so those values were combined and represented as zero time. *, significantly different from halothane-treated males. #, significantly different from vehicle controls (zero time). B, hepatic PMNs in female, BALB/cJ and C57BL/6 mice. *, significantly different from halothane-treated BALB/cJ mice.
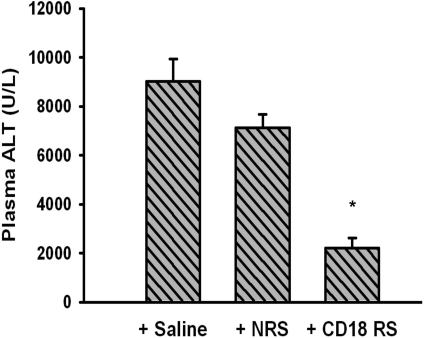
Halothane-Induced Severe Liver Injury in Female Mice Given Anti-CD18 Serum.
In a mouse model of mild halothane-induced hepatotoxicity, rabbit antiserum depleted PMNs and attenuated the increase in plasma ALT activity (You et al., 2006). CD18 is an integrin on leukocyte plasma membranes and is needed for adhesion to vascular endothelium and transmigration of PMNs into the liver parenchyma. Mice given CD18 antiserum in our model of severe halothane hepatitis had less hepatocellular injury compared with those treated with control serum or saline (Fig. 11).
Fig. 11.
CD18 neutralization attenuates severe halothane hepatotoxicity. Fasted female BALB/cJ mice were treated with 15 mmol/kg halothane i.p. and either saline, control rabbit serum (NRS), or anti-CD18 rabbit serum (CD18 RS intravenous) as described under Materials and Methods. Plasma was collected 12 h after halothane administration and evaluated for ALT activity (n = 6/group). *, significantly different from all other groups.
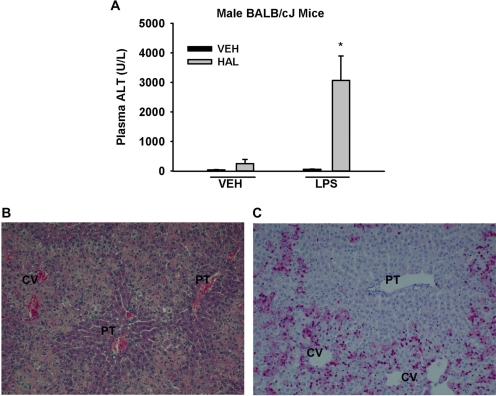
Enhanced Sensitivity of Male Mice to Halothane upon LPS Coexposure.
The results described above suggest that the greater sensitivity of female mice might be due to a more robust inflammatory response accompanying halothane exposure. Accordingly, we determined whether LPS-induced inflammation could increase the sensitivity of male mice to halothane hepatotoxicity. Treatment with vehicle or LPS alone was not hepatotoxic (Fig. 12A). The animals treated with halothane alone had a small increase in ALT activity, whereas the LPS-cotreated animals had a much larger increase (Fig. 12A). Livers from mice treated with LPS and halothane demonstrated severe centrilobular hepatocellular necrosis with marked PMN accumulation (Fig. 12, B and C).
Fig. 12.
Inflammation enhances sensitivity to halothane hepatotoxicity in male BALB/cJ mice. Mice were treated with vehicle or halothane and then either 5 × 106 EU/kg LPS or saline vehicle 6 h later (n = 3–8/group). Blood and liver samples were collected 24 h after halothane administration. A, plasma ALT activity. *, significantly different from halothane-treated and from LPS-treated animals. B, hematoxylin and eosin (H&E) section of liver from a representative halothane/LPS-cotreated mouse. C, immunohistochemical staining for PMNs in a cotreated mouse; PMNs stain bright pink and nuclei stain blue. Both photomicrographs were taken at 200× magnification. Labeled in the pictures are central vein (CV) and portal triad (PT).
Discussion
Few animal models reproduce severe liver injury caused by drugs with human idiosyncratic potential. Most of the models have involved administration of an inflammagen that prompts the appearance of liver injury from drugs that cause hepatotoxic IADRs in humans (Deng et al., 2009). In other attempts at developing animal models, liver injury has been either modest (You et al., 2006) or nonexistent (Ong et al., 2007). For example, nevirapine produces idiosyncratic dermal and liver toxicity in humans; a rat model reproduced the skin lesions but failed to reproduce the liver toxicity (Shenton et al., 2003). Accordingly, it is noteworthy that the present model in mice based on human risk factors resulted in severe liver injury without the coadministration of other agents.
Although an adaptive immune response is commonly thought to be the mode of action of halothane hepatitis, not all human cases support a strictly adaptive immune mechanism. For example, four of five lymphocyte transformation tests and a leukocyte migration inhibition test failed to demonstrate evidence of cellular hypersensitivity for halothane hepatitis patients (Walton et al., 1976a). Antibodies to halothane-modified proteins form in humans (Bird and Williams, 1989); however, evidence linking antibodies to the pathogenesis of hepatitis is lacking (Pohl et al., 1988). Several retrospective clinical studies indicate that halothane hepatitis can occur on the first exposure to halothane (Walton et al., 1976b; Weber et al., 1994), with the most recent study reporting that 39% of affected patients had no previous exposure (Eghtesadi-Araghi et al., 2008). Furthermore, multiple exposures were only a risk factor for halothane hepatitis when exposures occurred within 28 days (Inman and Mushin, 1974; Inman and Mushin, 1978). This time frame supports a paradigm in which the first exposure renders the liver more sensitive to a second exposure; however, an adaptive immune response is not the only explanation for the results. For example, TFA-adducted proteins persisted in guinea pigs for at least 21 days after halothane exposure (Chen and Gandolfi, 1997). If the adducts formed in people have similar longevity, then repeated exposure at intervals that overwhelm the regenerative capacity of the liver could produce cumulative damage. Moreover, the observations that patients who developed clinical signs of liver failure upon first exposure to halothane but not upon subsequent exposure cast doubt on a strictly adaptive immune-mediated mode that explains all instances of idiosyncratic halothane hepatitis (Walton et al., 1976b).
Data presented in Fig. 1 demonstrate severe hepatotoxicity resulting from a single exposure of mice to halothane. All of halothane-treated mice developed liver injury at the largest dose administered. The lack of requirement for a priming exposure followed by re-exposure indicates a mode of action other than adaptive immune-mediated pathogenesis in this model. It is important to note that the histopathological findings in livers from halothane-treated female mice were consistent with the changes in post-mortem specimens from patients who died from halothane-induced liver failure (Blackburn et al., 1964; Kumar et al., 2005). Centrilobular necrosis, inflammatory cell infiltrate and steatosis, all hallmarks of human halothane hepatitis, were observed in livers of halothane-treated mice. Thus, this mouse model mimics to a substantial degree the histopathological features associated with idiosyncratic halothane hepatitis in human patients.
The present study extends the work of Ju and co-workers (You et al., 2006) in which exposure to 30 mmol/kg halothane produced mild liver injury in female mice and no injury in male mice at 24 h. Use of mice of adult age, female sex and a susceptible strain and imposed fasting led to severe liver injury, even at a smaller dose (i.e., 15 mmol/kg). The magnitude of the response at 30 mmol/kg was also greater, i.e., 6000 to 10,000 U/l versus 1200 U/l ALT, with more severe lesions histologically (Fig 1).
The rarity and heterogeneous clinical presentation of many IADRs and the unknown mechanisms of pathogenesis are barriers to the development of adequate animal models. We considered the influence of several risk factors known to be important in people when developing an animal model of severe halothane hepatotoxicity in mice. Female sex is a risk factor for halothane hepatotoxicity with a 2:1 preponderance in women (Cousins et al., 1989). It was recently reported that females were the most at-risk group, representing 81.4% of patients who developed halothane hepatitis (Eghtesadi-Araghi et al., 2008). Female mice developed severe hepatitis at doses of halothane that produced only a mild response in male mice (Fig. 1). The disparity in toxicity between males and females was not due to differences in bioactivation of halothane (Fig. 8). The greater sensitivity of females has been reported previously for mice and guinea pigs (Lind et al., 1987; You et al., 2006).
Children are thought to be less susceptible to halothane hepatotoxicity, with an estimated incidence of 1 in 80,000 to 200,000 (Carney and Van Dyke, 1972; Warner et al., 1984). Four-week-old female mice had a milder response to halothane than 8-week-old female mice (Fig. 5). These results indicate that the human risk factors of female sex and mature age can be recapitulated in a mouse model of severe halothane hepatitis.
The occurrence of rare halothane hepatitis among closely related family members and an increased frequency of the HLA-DR2 haplotype in patients with severe halothane hepatitis suggest a genetic predisposition (Hoft et al., 1981; Otsuka et al., 1985). The findings that guinea pigs sensitive to halothane produce offspring that are also sensitive to halothane (Lunam et al., 1986) and that there are strain-dependent variations in halothane metabolism in rats (Gourlay et al., 1981) suggest genetic determinants of halothane sensitivity in animals as well. C57BL/6 mice were insensitive to severe halothane hepatitis at all doses tested (Fig. 4A), consistent with reports in which sensitivity to more modest liver injury was strain-dependent (Lind et al., 1987; You et al., 2006). The difference in sensitivity between C57BL/6 and BALB/cJ mice was not due to a difference in halothane bioactivation (Fig. 4B). We are not aware of specific genetic differences between BALBc/J and C57BL/6 that may account for the difference in sensitivity; however, the results indicate that, as in humans, genetic differences are important in the hepatotoxic response to halothane in animals. Whether the genetic determinants that render BALB/cJ mice sensitive are the same as in susceptible humans is unknown.
Fasting has not been studied as a risk factor for human halothane hepatitis; yet, patients are uniformly fasted before general anesthesia. Fasting mice before exposure to halothane increased their sensitivity to liver injury (Fig. 2). Accordingly, consideration should be given to fasting as a potential contributor to risk for halothane hepatitis in humans. Fasting increases hepatic CYP2E1 expression and decreases hepatic glutathione stores in rodents (Qin et al., 2007), and this may reduce protection against reactive metabolites generated during oxidative metabolism of halothane. Treatment of guinea pigs with an inhibitor of glutathione biosynthesis increased covalent binding of reactive halothane intermediates and enhanced liver injury (Lind et al., 1992).
Halothane treatment resulted in elevated plasma TNF-α concentration in female, but not male, mice (Fig. 9). Moreover, in the halothane-treated, female mice hepatic PMN accumulation occurred to a greater extent than in males (Fig. 10), and liver damage was attenuated by neutralization of CD18, an adhesion molecule required for transmigration of PMNs into the hepatic parenchyma. In contrast to our results, in a model of mild injury from halothane, depletion of PMNs completely abolished liver damage (You et al., 2006). These results suggest that the magnitude or mechanism of contribution of PMNs to liver damage is different for the mild and severe forms of injury. Our findings also point to a sexually dimorphic inflammatory response to halothane, inasmuch as male mice responded to halothane with only modest liver injury, minimal hepatic accumulation of PMNs, and no increase in serum TNF-α. Sexually dimorphic inflammatory responses have been reported in both humans and experimental animals (Wichmann et al., 1996; Marriott and Huet-Hudson, 2006). For example, female sex is a risk factor in alcohol-induced liver injury in humans (Becker et al., 1997) as well as animals (Nanji et al., 2001), and alcohol consumption produces greater concentration of inflammatory mediators in female rats compared with male rats.
It is interesting to note that although male BALB/cJ mice responded to halothane with only modest liver injury and inflammation, cotreatment with a nontoxic dose of LPS led to pronounced hepatotoxicity. Similar results have been observed by others in a hypoxia-halothane model in rats (Lind et al., 1984). LPS effects biological responses through Toll-like receptor 4. A viral mimetic (PoIyI:C) that stimulates Toll-like receptor 3 potentiated halothane hepatotoxicity in female mice (Cheng et al., 2009). These results suggest that inflammation might contribute to halothane hepatotoxicity and that the contribution of inflammatory stimuli might be mediated through at least Toll-like receptors 3 or 4. It is tempting to speculate that the modest hepatotoxic response to halothane alone in male mice is tantamount to the mild liver injury seen in 20% of patients, whereas the pronounced response seen in combination with LPS relates to the rarer severe halothane hepatitis seen in humans.
The report by You et al. (2006) provided a useful starting point for our study, because it found female mice to be more sensitive than males to the mild form of halothane hepatotoxicity and BALB/cJ mice to be a sensitive strain. The thrust of our study was to determine whether a murine model of severe halothane hepatitis could be developed by incorporating known and probable human risk factors (without additional cotreatments). By use of adult, female, BALB/cJ mice that were fasted, we obtained pronounced liver injury as evidenced by serum ALT activity of approximately 10,000 U/l and histopathologic changes that included pronounced, bridging, centrilobular necrosis with inflammatory infiltrate and steatosis, hallmarks of halothane hepatitis in humans. As You et al. (2006) had found in their study of mild halothane toxicity, genetic predisposition and female sex were important sensitivity determinants for the development of severe liver injury. In addition, we identified age and fasting as other sensitivity factors and elucidated the dose-response relationship for halothane toxicity. Furthermore, we found that isoflurane, a structurally related halogenated anesthetic with far less capacity to cause hepatitis in humans, did not produce hepatotoxicity in mice. This observation is an important step toward validating the animal model. Finally, we explored why females are more sensitive than males. You et al. (2006) state that the “basis for this sex difference in both humans and this mouse model remains to be elucidated.” We addressed this issue by providing evidence that a more robust inflammatory response to halothane underlies the greater susceptibility of female mice to halothane hepatitis.
In summary, the animal model of severe halothane hepatitis described herein shares the human risk factors of female sex, mature age, and genetic predisposition and a contribution of fasting, which is imposed on all human patients before general anesthesia. It is noteworthy that the results suggest that an adaptive immune response requiring sensitization and challenge exposures to halothane is not needed to precipitate severe halothane hepatitis, because injury was produced in mice upon a single exposure to the drug. The greater sensitivity of female mice was associated with a more robust inflammatory response than occurred in males, and the importance of inflammatory stress in the hepatotoxicity was suggested by the ameliorative effect of CD18 neutralization. The ability of a nontoxic dose of LPS to convert a modestly hepatotoxic response in males to a more robust, female-like response further testifies to the importance of an inflammatory response in the expression of halothane hepatitis in this model. This animal model should expand our thinking about the mode(s) of action of human halothane hepatitis and may prove to be useful in the future study of mechanisms underlying IADRs from halothane and other drugs.
Supplementary Material
This work was supported by National Institutes of Health [Grant DK061315]. C.M.D. was supported by National Institutes of Health National Institute of Environmental Health Sciences [Grant T32-ES07255].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.164541.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- IADR
- idiosyncratic adverse drug reaction
- LPS
- lipopolysaccharide
- TFA
- trifluoroacetyl-
- EU
- endotoxin unit(s)
- ALT
- alanine aminotransferase
- PMN
- polymorphonuclear leukocyte
- MOPS
- 4-morpholinepropanesulfonic acid
- BSA
- bovine serum albumin
- TNF
- tumor necrosis factor.
References
- Becker PU, Deis A, Sørensen TI, Grønbaek MN, Borch-Johnsen K, Müller CF, Schnohr P, Jensen GB. (1997) [Alcohol intake and risk of liver disease–significance of gender. A population study]. Ugeskr Laeger 159:3782–3786 [PubMed] [Google Scholar]
- Berger D, Bölke E, Seidelmann M, Beger HG. (1997) Time-scale of interleukin-6, myeloid related proteins (MRP), C reactive protein (CRP), and endotoxin plasma levels during the postoperative acute phase reaction. Shock 7:422–426 [DOI] [PubMed] [Google Scholar]
- Bird GL, Williams R. (1989) Detection of antibodies to a halothane metabolite hepten in sera from patients with halothane-associated hepatitis. J Hepatol 9:366–373 [DOI] [PubMed] [Google Scholar]
- Blackburn WR, Ngai SH, Lindenbaum J. (1964) Morphologic changes in hepatic necrosis following halothane anesthesia in man. Anesthesiology 25:270–283 [DOI] [PubMed] [Google Scholar]
- Bourdi M, Amouzadeh HR, Rushmore TH, Martin JL, Pohl LR. (2001) Halothane-induced liver injury in outbred guinea pigs: role of trifluoroacetylated protein adducts in animal susceptibility. Chem Res Toxicol 14:362–370 [DOI] [PubMed] [Google Scholar]
- Carney FM, Van Dyke RA. (1972) Halothane hepatitis: a critical review. Anesth Analg 51:135–160 [PubMed] [Google Scholar]
- Chen M, Gandolfi J. (1997) Characterization of the humoral immune response and hepatotoxicity after multiple halothane exposures in guinea pigs. Drug Metab Rev 29:103–122 [DOI] [PubMed] [Google Scholar]
- Cheng L, You Q, Yin H, Holt M, Franklin C, Ju C. (2009) Effect of polyI:C cotreatment on halothane-induced liver injury in mice. Hepatology 49:215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MJ, Plummer JL, Hall PD. (1989) Risk factors for halothane hepatitis. Aust N Z J Surg 59:5–14 [DOI] [PubMed] [Google Scholar]
- Deng X, Luyendyk JP, Ganey PE, Roth RA. (2009) Inflammatory stress and idiosyncratic hepatotoxicity: hints from animal models. Pharmacol Rev 61:262–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghtesadi-Araghi P, Sohrabpour A, Vahedi H, Saberi-Firoozi M. (2008) Halothane hepatitis in Iran: a review of 59 cases. World J Gastroenterol 14:5322–5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay GK, Adams JF, Cousins MJ, Hall P. (1981) Genetic differences in reductive metabolism and hepatotoxicity of halothane in three rat strains. Anesthesiology 55:96–103 [DOI] [PubMed] [Google Scholar]
- Hastings KL, Thomas C, Brown AP, Gandolfi AJ. (1995) Trifluoroacetylation potentiates the humoral immune response to halothane in the guinea pig. Immunopharmacol Immunotoxicol 17:201–213 [DOI] [PubMed] [Google Scholar]
- Hoft RH, Bunker JP, Goodman HI, Gregory PB. (1981) Halothane hepatitis in three pairs of closely related women. N Engl J Med 304:1023–1024 [DOI] [PubMed] [Google Scholar]
- Hussey AJ, Aldridge LM, Paul D, Ray DC, Beckett GJ, Allan LG. (1988) Plasma glutathione S-transferase concentration as a measure of hepatocellular integrity following a single general anaesthetic with halothane, enflurane or isoflurane. Br J Anaesth 60:130–135 [DOI] [PubMed] [Google Scholar]
- Inman W, Mushin W. (1974) Jaundice after repeated exposure to halothane: an analysis of reports to the committee on safety of medicines. Br Med J 1:5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman W, Mushin W. (1978) Jaundice after repeated exposure to halothane: a further analysis of reports to the Committee on Safety of Medicines. Br Med J 25:1455–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GP, Bhat VJ, Sowdi V. (2005) Fulminant hepatic failure following halothane anaesthesia. J Clin Forensic Med 12:271–273 [DOI] [PubMed] [Google Scholar]
- Li AP. (2002) A review of the common properties of drugs with idiosyncratic hepatotoxicity and the “multiple determinant hypothesis” for the manifestation of idiosyncratic drug toxicity. Chem Biol Interact 142:7–23 [DOI] [PubMed] [Google Scholar]
- Lillie R, Ashburn L. (1943) Supersaturated solutions of fat stains in dilute isopropanol for demonstration of acute fatty degeneration not shown by Herxheimer's technique. Arch Pathol 36:432 [Google Scholar]
- Lind RC, Gandolfi AJ, Hall PD. (1990) Covalent binding of oxidative biotransformation intermediates is associated with halothane hepatotoxicity in guinea pigs. Anesthesiology 73:1208–1213 [DOI] [PubMed] [Google Scholar]
- Lind RC, Gandolfi AJ, Brown BR, Jr, Hall PM. (1987) Halothane hepatotoxicity in guinea pigs. Anesth Analg 66:222–228 [PubMed] [Google Scholar]
- Lind RC, Gandolfi AJ, Hall PM. (1992) Glutathione depletion enhances subanesthetic halothane hepatotoxicity in guinea pigs. Anesthesiology 77:721–727 [DOI] [PubMed] [Google Scholar]
- Lind RC, Gandolfi AJ, Sipes IG, Brown BR., Jr (1984) The involvement of endotoxin in the halothane-associated liver injury. Anesthesiology 61:544–550 [DOI] [PubMed] [Google Scholar]
- Lunam CA, Cousins MJ, Hall PM. (1986) Genetic predisposition to liver damage after halothane anesthesia in guinea pigs. Anesth Analg 65:1143–1148 [PubMed] [Google Scholar]
- Marriott I, Huet-Hudson Y. (2006) Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res 34:177–192 [DOI] [PubMed] [Google Scholar]
- McLain GE, Sipes IG, Brown BR., Jr (1979) An animal model of halothane hepatotoxicity: roles of enzyme induction and hypoxia. Anesthesiology 51:321–326 [DOI] [PubMed] [Google Scholar]
- Mushin W, Rosen M, Bowen D, Campbell H. (1971) Post-halothane jaundice in relation to previous administration of halothane. Br Med J 3:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Fotouhinia M, Rahemtulla A, Thomas P, Tipoe GL, Su GL, Dannenberg AJ. (2001) Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am J Physiol Gastrointest Liver Physiol 281:G1348–G1356 [DOI] [PubMed] [Google Scholar]
- Njoku DB, Greenberg RS, Bourdi M, Borkowf CB, Dake EM, Martin JL, Pohl LR. (2002) Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesth Analg 94:243–249 [DOI] [PubMed] [Google Scholar]
- Ong MM, Latchoumycandane C, Boelsterli UA. (2007) Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci 97:205–213 [DOI] [PubMed] [Google Scholar]
- Otsuka S, Yamamoto M, Kasuya S, Ohtomo H, Yamamoto Y, Yoshida TO, Akaza T. (1985) HLA antigens in patients with unexplained hepatitis following halothane anesthesia. Acta Anaesthesiol Scand 29:497–501 [DOI] [PubMed] [Google Scholar]
- Pohl LR, Gillette JR. (1982) A perspective on halothane-induced hepatotoxicity. Anesth Analg 61:809–811 [PubMed] [Google Scholar]
- Pohl LR, Satoh H, Christ DD, Kenna JG. (1988) The immunologic and metabolic basis of drug hypersensitivities. Annu Rev Pharmacol Toxicol 28:367–387 [DOI] [PubMed] [Google Scholar]
- Qin L, Wang Y, Xu J, Kaneko T, Sato A, Wang P. (2007) One-day dietary restriction changes hepatic metabolism and potentiates the hepatotoxicity of carbon tetrachloride and chloroform in rats. Tohoku J Exp med 212:379–387 [DOI] [PubMed] [Google Scholar]
- Shenton JM, Teranishi M, Abu-Asab MS, Yager JA, Uetrecht JP. (2003) Characterization of a potential animal model of an idiosyncratic drug reaction: nevirapine-induced skin rash in the rat. Chem Res Toxicol 16:1078–1089 [DOI] [PubMed] [Google Scholar]
- Trowell J, Peto R, Smith A. (1975) Controlled trial of repeated halothane anaesthetics in patients with carcinoma of the uterine cervix treated with radium. Lancet 1:821–824 [PubMed] [Google Scholar]
- Walton B, Hamblin A, Dumonde DC, Simpson R. (1976a) Absence of cellular hypersensitivity in patients with unexplained hepatitis following halothane. Anesthesiology 44:391–397 [DOI] [PubMed] [Google Scholar]
- Walton B, Simpson BR, Strunin L, Doniach D, Perrin J, Appleyard AJ. (1976b) Unexplained hepatitis following halothane. Br Med J 1:1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LO, Beach TP, Garvin JP, Warner EJ. (1984) Halothane and children: the first quarter century. Anesth Analg 63:838–840 [PubMed] [Google Scholar]
- Weber P, Scheurlen M, Irkin I, Viebahn R, Schareck W, Becker W, de Groot H, Lauchart W. (1994) [Liver transplantation in halothane-induced liver necrosis]. Zentralbl Chir 119:305–308 [PubMed] [Google Scholar]
- Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. (1996) Enhanced immune responses in females, as opposed to decreased responses in males following haemorrhagic shock. Cytokine 8:853–863 [DOI] [PubMed] [Google Scholar]
- Yee SB, Hanumegowda UM, Hotchkiss JA, Ganey PE, Roth RA. (2003) Role of neutrophils in the synergistic liver injury from monocrotaline and bacterial lipopolysaccharide exposure. Toxicol Sci 72:43–56 [DOI] [PubMed] [Google Scholar]
- You Q, Cheng L, Reilly TP, Wegmann D, Ju C. (2006) Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology 44:1421–1431 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.