Abstract
Many molecular aberrations occur in pancreatic cancer. Although aberrant epidermal growth factor receptor (EGFR), Src, and signal transducer and activator of transcription 3 (Stat3) are implicated in pancreatic cancer, therapies that target only one of these entities are undermined by signaling cross-talk. In the human pancreatic cancer lines, Panc-1 and Colo-357, pY845EGFR, pY1068EGFR, pY1086EGFR, and pY1173EGFR levels and pY416c-Src are concurrently elevated with aberrantly active Stat3 in a complex signaling cross-talk. Thus, understanding the signaling integration would facilitate the design of effective multiple-targeted therapeutic modalities. In Panc-1 and Colo-357 lines, pY845EGFR, pY1068EGFR, and pY1086EGFR levels are responsive to c-Src inhibition in contrast to pY1173EGFR, which is EGFR kinase-dependent. Constitutively active Stat3 is sensitive to both EGFR and Src inhibition, but the early suppression of aberrantly active Stat3 in response to the inhibition of EGFR and Src is countered by a Janus kinase (Jaks)-dependent reactivation, suggesting that Jaks activity is a compensatory mechanism for Stat3 induction. The inhibition of EGFR, Src, or Stat3 alone induced weak biological responses. By contrast, the concurrent inhibition of Stat3 and EGFR or Src induced greater viability loss and apoptosis and decreased the migration/invasion of pancreatic cancer cells in vitro. Significantly, the concurrent inhibition, compared with monotargeting modality, induced stronger human pancreatic tumor growth inhibition in xenografts. We infer that the tumor growth inhibition in vivo is caused by the simultaneous suppression of the abnormal functions of Stat3 and EGFR or Src. These studies strongly suggest that the concurrent targeting of Stat3 and EGFR or Src could be a beneficial therapeutic approach for pancreatic cancer.
Pancreatic cancer is a very lethal disease, with poor prognosis and mortality nearly identical to the rate of incidence. The disease also remains poorly understood. There are several genetic mutations and activated signal transduction proteins that occur during pancreatic ductal cell carcinogenesis. Understanding the critical molecular events that promote this disease and how they contribute to its maintenance and progression would facilitate the development of effective targeted therapeutic modalities. One of the major molecular abnormalities is the overexpression and/or activation of the epidermal growth factor receptor (EGFR) protein, with an incidence of 30 to 50% of pancreatic cancer cases (Tzeng et al., 2007). Evidence indicates that the hyperactive epidermal growth factor (EGF)/EGFR pathway is important in the disease maintenance and progression (Korc et al., 1986). Similarly, overexpression of the c-Src tyrosine kinase occurs in a large percentage of pancreatic adenocarcinoma and is observed to increase EGFR activities during tumorigenesis (Lutz et al., 1998; Tice et al., 1999; Trevino et al., 2006). The overactivity of the Src family kinases leads to deregulation of tumor cell growth and survival, disruption of cell-to-cell contacts, promotion of migration and invasiveness, and induction of tumor angiogenesis (Trevino et al., 2006).
Another molecular abnormality prevalent in pancreatic cancer and implicated in the disease is aberrant signal transducer and activator of transcription 3 (Stat3) (DeArmond et al., 2003; Scholz et al., 2003; Toyonaga et al., 2003; Trevino et al., 2006). Stat3 is a member of the STAT family of cytoplasmic transcription factors. As with the other STATs, Stat3 requires extrinsic tyrosine phosphorylation to become activated, and this event is induced by growth factor receptors and cytoplasmic tyrosine kinases, such as Src and Janus kinase (Jaks) families (Darnell, 2005). In contrast to normal STAT signaling that is transient in accordance with the requirements for normal biological processes, tumor cells harbor aberrant Stat3 activation, which compelling evidence indicates dysregulates cell growth and survival, promotes tumor angiogenesis and tumor cell migration and invasion, and induces tumor immune tolerance (Turkson, 2004; Yue and Turkson, 2009).
The concurrence of the hyperactive EGFR and Src tyrosine kinases, together with aberrant Stat3, in pancreatic cancer raises key questions about the contributory role of each entity to the disease. Deregulated signal transduction provides the framework for signaling cross-talk and functional cooperation that would not only support the malignant phenotype and the disease progression but also would influence drug responsiveness. Thus, a potential collaboration among hyperactive EGFR, Src, and Stat3 in support of the malignant phenotype and in regulating the response to monotargeted therapy is a reasonable model to propose. It is also a concept that would support the recent approval of the combined gemcitabine and EGFR inhibitor erlotinib for the treatment of pancreatic cancer patients (Saif, 2008). An increased understanding of the integration, functional relationship, and the collective roles of the EGFR, Src, and Stat3 in supporting pancreatic cancer is needed to derive effective, multiple-targeted therapeutic modalities for this disease. We provide evidence that the concurrent inhibition of aberrant Stat3 and EGFR or Src is effective in inducing antitumor cell responses in vitro and in inhibiting human pancreatic tumor growth in xenograft models.
Materials and Methods
Cells and Reagents.
v-Src-transformed mouse fibroblasts (NIH3T3/v-Src), human pancreatic cancer (Panc-1), and leukemic (K562) lines have been described (Turkson et al., 1998; Garcia et al., 2001; Huang et al., 2002). The human pancreatic cancer lines Colo-357 and Mia-PaCa-2 were gifts from Drs. J. M. Lancaster and M. P. Malafa (Moffitt Cancer Center, Tampa, FL). The immortalized human pancreatic duct epithelial cell (HPDEC) line was obtained from Dr. M. S. Tsao (Ontario Cancer Institute, University Health Network/Princess Margaret Hospital, Toronto, ON, Canada) (Ouyang et al., 2000). Except for the HPDEC line that was grown in keratinocytes/serum-free medium supplemented with 0.2 ng of EGF, 30 μg/ml bovine pituitary extract and containing antimycol, and the K562 line cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum and 100 units/ml penicillin/streptomycin, all other cell lines were grown in Dulbecco's modified Eagle's medium containing 5% iron-supplemented bovine calf serum and 100 units/ml penicillin/streptomycin. Recombinant human EGF is from Creative Biolabs (Port Jefferson Station, NY), and gemcitabine is from Ely Lilly & Co. (Indianapolis, IN). Erlotinib, ZD1839 (ZD/Iressa), and dasatinib (Das) were purchased from ChemieTek (Indianapolis, IN).
Nuclear Extract Preparation and Gel-Shift Assays.
Nuclear extract preparation and DNA binding with electrophoretic mobility-shift assay (EMSA) were carried out as reported previously (Turkson et al., 1998; Garcia et al., 2001). The 32P-labeled oligonucleotide probes used were high affinity sis-inducible element from the c-fos gene (m67 variant, 5′-AGCTTCATTTCCCGTAAATCCCTA) that binds Stat1 and Stat3 (Wagner et al., 1990) and the mammary gland factor element (from the bovine β-casein gene promoter, 5′-AGATTTCTAGGAATTCAA) that binds Stat1 and Stat5 (Gouilleux et al., 1995; Seidel et al., 1995).
SDS-Polyacrylamide Gel Electrophoresis/Western Blot Analysis.
Western blot analysis was performed as described previously (Turkson et al., 1998; Zhang et al., 2000). Primary antibodies used were anti-Stat3 (C20) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-pY845EGFR (Millipore Corporation, Billerica, MA), and antibodies against pY705Stat3, Stat3, pY1068EGFR, pY1086EGFR, pY1173EGFR, EGFR, pY416Src, Src, c-Myc, and β-actin (Cell Signaling Technology Inc., Danvers, MA).
Small-Interfering RNA Transfection.
Small-interfering RNA (siRNA) sequences for EGFR and Src were ordered from Dharmacon RNAi Technologies, Thermo Scientific (Lafayette, CO). Sequences used were: EGFR sense strand, 5′-GAAGGAAACUGAAUUCAAAUU-3′; EGFR antisense strand, 5′-pUUUGAAUUCAGUUUCCUUCUU-3′; control siRNA sense strand, 5′-AGUAAUACAACGGUAAAGAUU-3′; and control siRNA antisense strand, 5′-pUCUUUACCGUUGUAUUACUUU-3′. The c-Src SMARTpool siRNA reagent (NM-005417; Millipore Corporation) was used for Src. Transfection into cells was performed using 20 nM EGFR siRNA or 25 nM Src siRNA and 8 μl of Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) in OPTI-MEM culture medium (Invitrogen).
Cell Proliferation Assay and Propidium Iodide Staining or Annexin V Binding with Flow Cytometry.
Proliferating cells in 6- or 96-well plates were treated once with 0.1 to 1 μM Iressa, 100 nM Das, 50 to 100 μM S3I-201, 1 μM gemcitabine, or combinations of inhibitors for up to 96 h. Viable cells were counted by trypan blue exclusion/phase-contrast microscopy or assessed by the CyQuant cell proliferation assay according to the manufacturer's instructions (Invitrogen), or cells were stained with propidium iodide for 30 min or subjected to annexin V binding (BD Biosciences, San Jose, CA) and analyzed by flow cytometry for cell cycle distribution or apoptosis, respectively.
Colony Survival Assay.
Single-cell suspension of Panc-1 and Colo-357 cells were seeded in 6-cm dishes (500 cells/dish) and assayed as reported previously (Zhao et al., 2008). Briefly, single-cell suspension of Panc-1 and Colo-357 cells were seeded in 6-cm dishes (500 cells/well), treated the next day with inhibitors for 48 h, and allowed to grow until large colonies were visible. Colonies were stained with crystal violet for 4 h and counted under a phase-contrast microscope.
Cell Migration and Matrigel Invasion Assays.
Cell migration and invasion experiments were carried out and quantified as described previously (Siddiquee et al., 2007b) using Bio-Coat migration chambers (BD Biosciences, Franklin Lakes, NJ) of 24-well companion plates with cell culture inserts containing 8-μm pore size filters, according to the manufacturer's protocol.
Mice and in Vivo Tumor Studies.
Studies with mice were performed under approved Institutional Animal Care and Use Committee procedures and guidelines. Six-week-old female athymic nude mice were purchased from Harlan (Indianapolis, IN) and maintained in the institutional animal facilities approved by the American Association of Accreditation of Laboratory Animal Care. Athymic mice were inoculated (subcutaneously) in the left flank area with Colo-357 cells (3 × 106) in 100 μl of 1× phosphate-buffered saline or Panc-1 cells (8 × 106) in 100 μl of 1:1 (v/v) DMEM/Matrigel (Basement Membrane Matrix; BD Biosciences) suspension. Animals were monitored daily after inoculation. For Colo-357 cells, tumors of 60 mm3 were established after 4 to 6 days, whereas tumors developed in Panc-1 cells took 10 to 15 days to reach 100 mm3. Animals were grouped so that mean tumor sizes in all the groups were nearly identical. For the xenografts developed with the Colo-357 line, tumor-bearing mice were given ZD (75 mg/kg i.v.), Das (15 mg/kg i.v.), or S3I-201 (5 mg/kg i.v.) alone or in combination every 2 or 3 days for the first 2 weeks and daily for 5 days each week for the next 3 weeks. Mice bearing tumors developed using Panc-1 cells were given ZD (75 mg/kg i.v.), Das (15 mg/kg i.v.), or S3I-201 (5 mg/kg i.v.) alone or in combination every 3 to 5 days, or erlotinib (5 mg/kg i.v., every day) in combination with gemcitabine (100 mg/kg i.v., every 3 days) for 48 days. The doses and the treatment schedules used were inferred from literature reports. Tumor sizes were measured by calipers every 2 or 3 days and converted to tumor volumes by the formula V = 0.52 × a2 × b, where a is the smallest superficial diameter and b is the largest superficial diameter. In addition, at the conclusion of the studies, weights of mice were recorded. For each treatment group, the tumor volumes for each set of measurements were statistically analyzed and compared with the control (nontreated) group.
Statistical and Data Analyses.
Statistical analysis was performed on mean values using Prism software (GraphPad Software, Inc., San Diego, CA). Cell proliferation data and effect of drug combinations were analyzed with CalcuSyn software (Biosoft, Cambridge, UK) to determine additive or synergistic effects. The significance of differences between groups was determined by the paired t test at *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
Results
Aberrant EGFR, Src, and Stat3 in Pancreatic Cancer Lines.
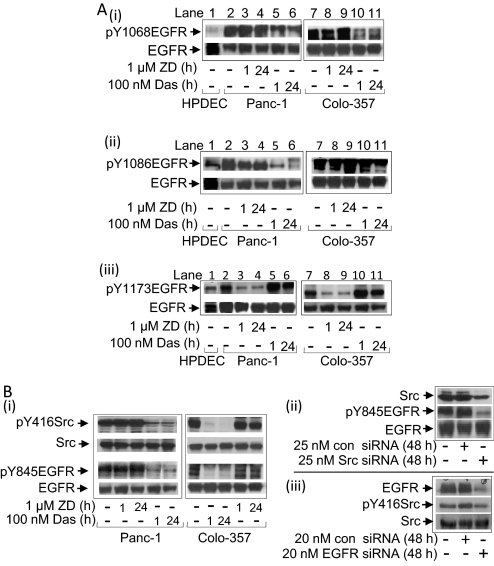
Consistent with published reports (DeArmond et al., 2003; Scholz et al., 2003), Stat3 activity, per DNA binding with EMSA analysis in nuclear extract preparations, is constitutive in Panc-1 and Colo-357, low in Mia-Paca-2, and undetectable in the normal HPDEC compared with aberrant levels in NIH3T3/v-Src (Turkson et al., 1998) (Supplemental Fig. 1Ai). Per supershift analysis, the DNA–protein complex contains Stat3 (Supplemental Fig. 1Ai, lane 3). No Stat5 activity is detectable in pancreatic cancer cells (Supplemental Fig. 1Aii) compared with aberrant levels in the K562 leukemic cells (Huang et al., 2002). Similarly, immunoblotting analysis showed pY416c-Src levels are moderate in Mia-Paca-2 but elevated in Panc-1 and Colo-357 cells as reported previously (Korc et al., 1986; Trevino et al., 2006) and similar to the levels in NIH3T3/v-Src compared with low levels in HPDEC (Supplemental Fig. 1B, top). The elevated pY416Src levels parallel the enhanced levels of the Src-sensitive pY845EGFR motif (Tice et al., 1999) in Panc-1 and Colo-357 cells compared with low levels of the same in HPDEC (Supplemental Fig. 1B, bottom). The total Src or EGFR protein remained unchanged. Immunoblotting analysis further showed enhanced EGFR autophosphorylation motifs (Downward et al., 1984), pY1068EGFR (Fig. 1Ai, lanes 2 and 7), pY1086EGFR (Fig. 1Aii, lanes 2 and 7), and pY1173EGFR (Fig. 1Aiii, lanes 2 and 7) in Panc-1 and Colo-357 compared with basal levels of the same in HPDEC (Fig. 1Ai–iii, lane 1).
Fig. 1.
Immunoblotting analyses of Stat3, Src, and EGFR activities for effects of inhibitors. A and B, immunoblotting analysis of whole-cell lysates from cells untreated or treated with ZD or Das (Ai) or transfected with or without c-Src siRNA (Aii), EGFR siRNA (Aiii), or scrambled siRNA control (con) and probing for pY416c-Src (pY416Src), Src, pY845EGFR, and EGFR; and untreated or treated with ZD or Das and probing for pY1068EGFR (Bi), pY1086EGFR (Bii), and pY1173EGFR (Biii), and EGFR. Data are consistent with those obtained from four independent experiments.
Functional Integration of EGFR and Src in Pancreatic Cancer Cells.
We examined the functional relationship between the activated EGFR and Src. Immunoblotting analysis showed treatment of cells with Das inhibited Src activity (pY416Src) (Nam et al., 2005) and induced an early (1 h) and a sustained (24 h) decrease in pY845EGFR levels (Fig. 1Bi). By contrast, no detectable changes in pY416Src and pY845EGFR levels were induced by treatment with the pan-ErbB inhibitor, PD169540 (PD169) (Mahtouk et al., 2005) (data not shown) or the selective EGFR inhibitor, ZD (Wakeling et al., 2002) (Fig. 1Bi). In confirmation, the siRNA knockdown of c-Src abrogated pY845EGFR levels (Fig. 1Bii, Src siRNA), whereas the EGFR knockdown by siRNA had minimal effect on pY416Src levels (Fig. 1Biii, EGFR siRNA). Scrambled siRNA (con siRNA) has no effect (Fig. 1B, ii and iii, con siRNA). Thus, elevated pY845EGFR levels in pancreatic cancer cells are sensitive to Src activity.
Immunoblot analysis further showed that the treatment of Panc-1 and Colo-357 cells with ZD decreased pY1173EGFR levels (Fig. 1Aiii, lanes 3, 4, 8, and 9) by as early as 1 h and up to 24 h, with no effect on pY1068EGFR (Fig. 1Ai, lanes 3, 4, 8, and 9) or pY1086EGFR levels (Fig. 1Aii, lanes 3, 4, 8, and 9), suggesting that the EGFR kinase is essential for the induction of pY1173EGFR but not of pY1068EGFR or pY1086EGFR. By contrast, Das treatment decreased pY1068EGFR and pY1086EGFR levels (Fig. 1A, i and ii, lanes 5, 6, 10, and 11) with minimal effect on pYEGFR1173 levels (Fig. 1Aiii, lanes 5, 6, 10, and 11). Thus, Src activity additionally promotes pY1068EGFR and pY1086EGFR in pancreatic cancer cells.
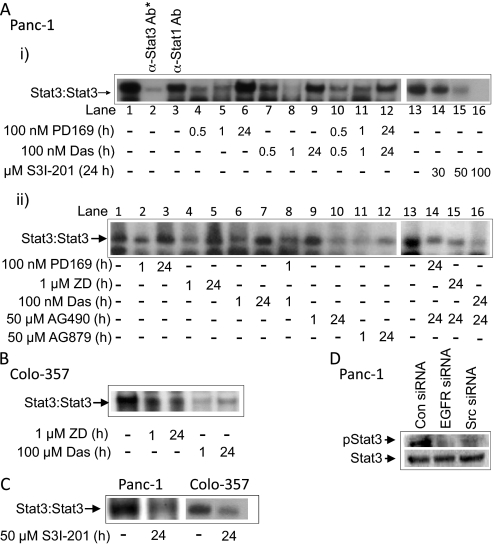
EGFR and Src Promote Aberrant Stat3 Activation but Do Not Induce ErkMAPK or Akt Activity.
Both pY1068EGFR and pY1086EGFR levels are binding sites for Stat3 (Shao et al., 2003). Given the concurrent EGFR and Src activation in Panc-1 and Colo-357 cells, we sought to define the regulation of aberrant Stat3 activation. By in vitro DNA-binding assay with EMSA analysis of nuclear extract preparations, we observed an early repression (in the first 30 min to 1 h of treatment) of constitutively active Stat3 by the pan-ErbB inhibitor, PD169, the ErbB2-selective inhibitor, AG879 (DeArmond et al., 2003), ZD, or Das (Fig. 2 Ai, lanes 4, 5, 7, and 8, Aii, lanes 2, 4, 6, and 11, and B, 1 h), or by a combined PD169 and Das (Fig. 2Ai, lanes 10 and 11 and Aii, lane 8). However, the Stat3 activity in Panc-1 cells consistently rebounded after 24-h treatments with Das, ZD, or PD169 (Fig. 2A, i and ii, 24 h), even though EGFR or Src activity remained inhibited (Fig. 1, 24 h). Twenty-four-hour treatment with the AG879 moderately inhibited Stat3 activity (Fig. 2Aii, lane 12), which we speculate may be because of its widespread activity as a pan-ErbB inhibitor. By contrast, treatment with the Jak inhibitor AG490 for 1 h had no effect on constitutive Stat3 activity but abolished Stat3 activity at 24-h treatment (Fig. 2Aii, lanes 9 and 10). Moreover, the combined treatment with AG490 and ZD, Das, or PD169 for 24 h abolished constitutively active Stat3 (Fig. 2Aii, lanes 14–16) and prevented the previously observed rebound of Stat3 activity. In Colo-357, Stat3 activity was inhibited by ZD and Das, with the effect more striking for Das (Fig. 2B). These findings together reveal a constitutive Stat3 activation in pancreatic cancer cells that is mediated by both EGFR and Src, with a compensatory, long-term Jak-dependent Stat3 activation, a pattern similarly observed in head and neck squamous carcinoma, mesothelioma, squamous cell skin carcinoma, and non–small cell lung cancer cell lines after the inhibition of Src (Johnson et al., 2007). Data also show that the concentration of S3I-201 (50 μM) used in the studies sufficiently inhibits aberrant Stat3 activation in Panc-1 and Colo-357 cells (Fig. 2C). In further support, the siRNA knockdown of EGFR (EGFR siRNA) or Src (Src siRNA) led to pStat3 suppression, as assayed by immunoblotting analysis (Fig. 2D), with no effect of scrambled siRNA (con). We confirm that treatment of Panc-1 cells with S3I-201 for 24 h inhibits aberrant Stat3 activity in a dose-dependent manner (Fig. 2Ai, lanes 13–16).
Fig. 2.
EMSA and immunoblotting analyses for effects of inhibitors on Stat3 activation. EMSA analysis of Stat3 DNA-binding activity in Panc-1 (A) or Colo-357 (B) cells treated or untreated with the pan ErbB inhibitor PD169, ZD, Das, the Jak inhibitor AG490, the ErbB2-selective inhibitor AG879, or inhibitor combinations for the indicated times, or Panc-1 and Colo-357 cells treated for 24 h with S3I-201 (C), or immunoblotting analysis of whole-cell lysates from Panc-1 cells transfected with EGFR siRNA, Src siRNA, or scrambled siRNA (control) and probing for pStat3 or Stat3 (D). *, supershift analysis. Data are consistent with those obtained from three independent experiments.
In contrast to the effects on Stat3 activity, immunoblotting analysis showed elevated pErk1/pErk2MAPK and pAkt activities in Panc-1 and Colo-357 cells, which were not responsive to ZD or Das treatment (Supplemental Fig. 2), suggesting that aberrant EGFR and Src kinases do not promote the baseline elevated ErkMAPK and Akt activities in pancreatic cancer cells. Thus, Erk and Akt are not key mediators of EGFR and Src functions in pancreatic cancer.
Inhibition of Stat3 Sensitizes Pancreatic Cancer Cells to Effects of EGFR and Src Inhibitors.
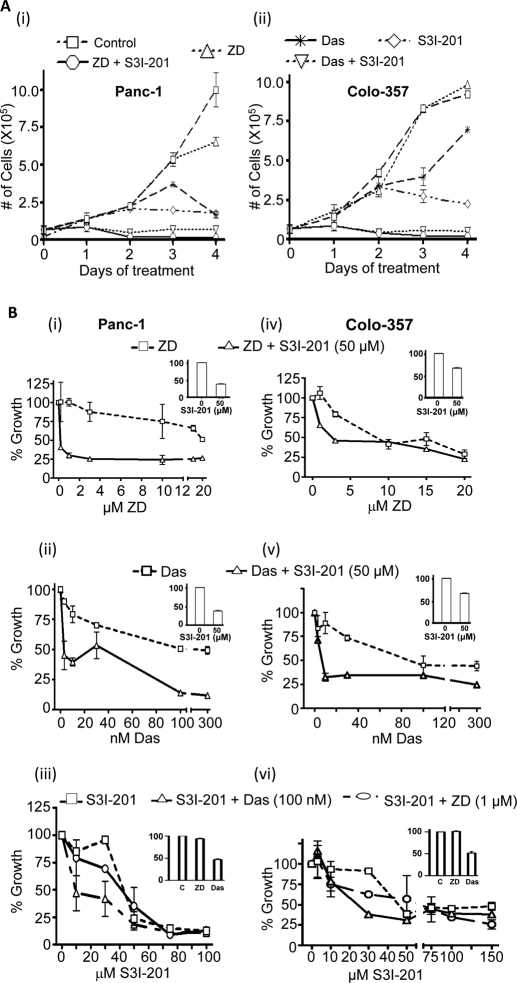
We investigated the biological and therapeutic implications of the unique inter-relationship between EGFR, Src, and Stat3 activation. Das and ZD were used at 100 nM and 0.1 to 1 μM, respectively, as in the literature reports (Mahtouk et al., 2005; Nam et al., 2005), whereas the Stat3 inhibitor S3I-201 was used at the suboptimum, 50 μM, or at the 100 μM required to inhibit Stat3 activation (Siddiquee et al., 2007a). Cell growth was measured as viable cell count by trypan blue exclusion/phase-contrast microscopy. Cells treated with 1 μM ZD, 100 nM Das, or 50 μM S3I-201 alone for 24 h showed minimal effect on growth (Fig. 3A, day 1). Treatment for 48 to 96 h with Das or S3I-201 alone appeared to slow down cell growth, whereas treatment for the same period with ZD showed minimal effect (Fig. 3A, i and ii, days 2–4). The concurrent treatment with both S3I-201 and ZD or S3I-201 and Das for 24 h seemed to decrease growth (Fig. 3A, day 1). By contrast, the concurrent treatment with both S3I-201 and ZD or S3I-201 and Das for 48 to 96 h induced larger decreases in the number of viable cells (Fig. 3A, days 2–4). These data were confirmed by the CyQuant cell proliferation assay (Invitrogen). The CyQuant assay showed that, to some extent, the 48-h treatment with each inhibitor alone decreased cell growth in a dose-dependent manner (Fig. 3B, ZD, Das, and S3I-201). However, the observed effects of any single agent were significantly weaker than the effects of the concurrent treatment with a Stat3 inhibitor and an inhibitor of EGFR or Src. Thus, treatment with 50 μM S3I-201 increased the sensitivity of Panc-1 and Colo-357 cells to ZD and Das, shifting the dose-response curves to the left (Fig. 3B, ZD + S3I-201 and Das + S3I-201; insert shows the effect of 50 μM S3I-201 alone on growth). Cotreatment with S3I-201 significantly decreased the IC50 values from 17 to 0.4 μM and 100 to 6 nM, respectively, for ZD and Das against Panc-1 growth (Fig. 3B, i and ii), and from 6.5 to 2.4 μM and 90 to 8 nM, respectively, for ZD and Das against Colo-357 growth (Fig. 3B, iv and v). Analysis, per Chou and Talalay equation, using CalcuSyn (Biosoft) indicated synergism for the combination of S3I-201 and ZD and S3I-201 and Das, with combination indexes of <1. The CyQuant cell proliferation assay also showed that Das but not ZD increased the sensitivity of both cell lines to S3I-201, decreasing the IC50 value from 40 to 15 μM and 45 to 20 μM, respectively, for effects on Panc-1 and Colo-357 cells (Fig. 3B, iii and iv). Thus, treatment with S3I-201 sensitized pancreatic cancer cells to ZD and Das.
Fig. 3.
Cell proliferation and viability studies for the effects of inhibitors. A, trypan blue exclusion/phase-contrast microscopy for viable Panc-1 or Colo-357 cells after treatment for 0 to 96 h with 1 μM ZD, 100 nM Das, or 50 μM S3I-201, or combinations. B, CyQuant cell proliferation assay for the viability of Panc-1 (left) or Colo-357 (right) cells in response to 48-h treatments with the designated concentrations of ZD, Das, and S3I-201, alone and in combinations. Values, mean and S.D. n = 4 experiments, each in triplicate.
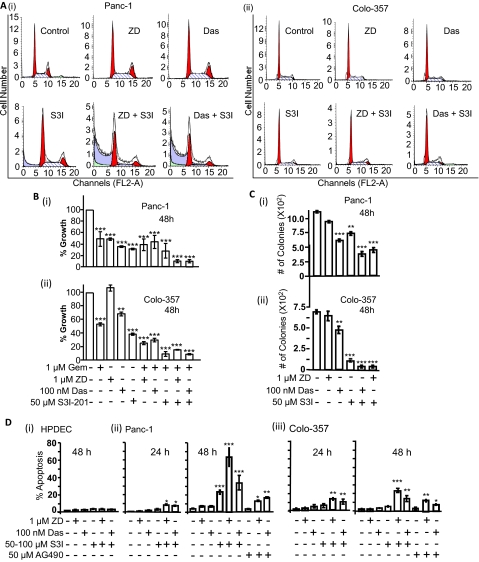
Given the potential that the concurrent treatment with agents slowed down growth of pancreatic cancer cells, propidium iodide staining with flow cytometry was performed for cell cycle profile analysis. Treatment of Panc-1 or Colo-357 cells with ZD, Das, or S3I-201 alone for 24 h had no significant impact on the cell cycle distribution, with nearly identical distribution at G0/G1, S, and G2/M phases as in the control, dimethyl sulfoxide-treated cells (Fig. 4A, ZD, Das, and S3I-201). Similarly, the combined treatment with S3I-201 and ZD or S3I-201 and Das for 24 h appeared to have no dramatic effect on the cell cycle distribution of Colo-357 cells (Fig. 4Aii). For Panc-1, a noticeable decrease in the population in the S phase, with no dramatic increase in G0/G1, and an increased sub-G0 population occur in response to the combined treatment with ZD and S3I-201 or Das and S3I-201 (Fig. 4Ai, ZD + S3I and Das + S3I). We deduce that the combined treatment with the inhibitors of Stat3 and Src or EGFR induces some degree of apoptosis in Panc-1 cells at 24 h, with no dramatic change in the cell cycle profile in Colo-357 cells.
Fig. 4.
Cell cycle distribution, cell growth, and colony survival and apoptosis studies for the effects of inhibitors. A, cells were untreated (control) or treated with ZD (1 μM), Das (100 nM), S3I-201 (50 μM), ZD + S3I-201, or Das + S3I-201 for 24 h, stained with propidium iodide, and analyzed by flow cytometry for cell cycle distribution. B, CyQuant cell proliferation assay for the viability of Panc-1 (i) and Colo-357 (ii) cells after treatments for 48 h with the designated concentrations of ZD, Das, S3I-201, and gemcitabine (Gem), alone and in combinations. C, number of colonies emerging from cells in culture (500/6-cm dish) untreated or treated once with ZD, Das, S3I-201 (S3I), or combinations and allowed to culture. D, annexin V binding/flow cytometry analysis of normal HPDEC, Panc-1, or Colo-357 cells treated or untreated with inhibitors or combinations. Values, mean and S.D. n = 4 experiments each in triplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Given the clinical implications of these findings, we extended these studies to examine the effect of inhibitors of the EGFR, Src, and Stat3 pathway on the sensitivity of pancreatic cancer lines to gemcitabine, the antimetabolite agent used for treating pancreatic cancer. The rationale for this study is the approval of erlotinib and gemcitabine combination therapy for locally advanced metastatic pancreatic cancer. CyQuant cell proliferation studies showed that inhibition of EGFR, Src, or Stat3 alone did induce some sensitization of Panc-1 and Colo-357 cells to gemcitabine (Fig. 4B). More importantly, the combined inhibition of Stat3 and EGFR or Src induced a higher sensitization of cells to gemcitabine (Fig. 4B), further supporting the ability to substantially suppress the malignant phenotype by the combined inhibition of Stat3 and EGFR or Src.
To further explore the sensitization potential of Stat3 inhibitors, we performed colony survival assay (Zhao et al., 2008). Generally, colonies were formed by day 21 after the single, 48-h treatment, and there were no differences between control (untreated) and treated samples in the length of time to form colonies and the sizes of colonies. Panc-1 cells showed decreased sensitivity to inhibitors, and the treatment with single inhibitors had minimal effect on colony numbers compared with the combined treatment with S3I-201 (S3I) and Das or S3I-201 and ZD (Fig. 4Ci). Colo-357 cells were less sensitive to Das or ZD but showed a high sensitivity to S3I-201 and the combined S3I-201 and Das or ZD (Fig. 4Cii). Except for the Colo-357 response to S3I-201, overall the results indicate that Panc-1 and Colo-357 cells are more sensitive to the concurrent treatment with S3I-201 and Das or S3I-201 and ZD. Furthermore, annexin V binding/flow cytometric analysis showed higher percentages of Panc-1 and Colo-357 cells undergo apoptosis in response to the concurrent inhibition of Stat3 and EGFR or Src than to the treatment with any single agent alone. Treatment of Panc-1 and Colo-357 cells with S3I-201, ZD, or Das alone induced lower percentage of apoptosis (Fig. 4D, ii and iii). By contrast, combined treatment with S3I-201 and Das or S3I-201 and ZD induced apoptosis in both Panc-1 and Colo-357 cells at 24 h and a higher percentage of apoptosis at 48 h (Fig. 4D, ii and iii). Panc-1 cells showed higher sensitivity to the combined treatment with S3I-201 and ZD or Das, with up to 60% apoptosis, compared with 25% apoptosis in Colo-357 cells (Fig. 4D, ii and iii). Similar results were obtained for the concurrent treatments with AG490 and ZD or Das (Fig. 4D, ii and iii), whereas similar treatments of normal HPDEC cells showed no significant apoptosis in response to combination treatments (Fig. 4Di). Altogether, our findings indicate that pancreatic cancer cells have a higher sensitivity to the combined inhibition of Stat3 and EGFR or Stat3 and Src than to the inhibition of a single entity.
EGFR, Src, and Stat3 Together Promote Pancreatic Cancer Cell Migration and Invasion.
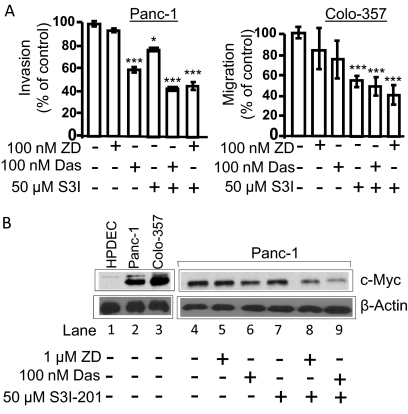
Consistent with roles for Src and Stat3 in tumor cell motility, migration, invasion, and metastasis (Nam et al., 2005; Trevino et al., 2006), in vitro Matrigel assay showed that Das or S3I-201 alone suppresses migration and invasion (Fig. 5A). However, the concurrent inhibition for 24 h of Stat3 and EGFR or Src induced stronger effects on Colo-357 migration and Panc-1 invasion, except for the similar effect of Src inhibition on Panc-1 migration (Fig. 5A). At the 24-h treatment when these studies were done, there is no significant effect on the growth of Panc-1 and Colo-357 cells (Fig. 3), although for Panc-1 cells, cell cycle analysis revealed some evidence of increased sub-G0 population after 24-h treatment with ZD and S3I-201 or Das and S3I-201 (Fig. 4Ai), which may contribute to the reduced number of migrating Panc-1 cells.
Fig. 5.
Concurrent inhibition of Stat3 and EGFR or Src inhibits migration and invasion and suppresses c-Myc expression. A, effects of 24-h treatment with ZD, Das, and/or S3I-201 (S3I) on migration and invasion. B, immunoblotting analysis of whole-cell lysates for c-Myc and β-actin expression in Panc-1 cells after treatment with inhibitors for 24 h. Values, mean and S.D. n = 3 or 4 experiments each in triplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
EGFR, Src, and Stat3 Module Regulates c-Myc Overexpression in Pancreatic Cancer Cells.
For insight into the underlying molecular mechanisms, we studied the regulation of key cancer-relevant genes and observed that c-Myc is overexpressed in pancreatic cancer lines compared with normal HPDEC, and its expression was consistently more responsive to the 24-h treatment with both S3I-201 and ZD or S3I-201 and Das than the inhibition of Stat3, Src, or EGFR alone (Fig. 5B). These data raise the potential that Stat3, EGFR, and Src functionally cooperate to induce c-Myc overexpression in pancreatic cancer cells.
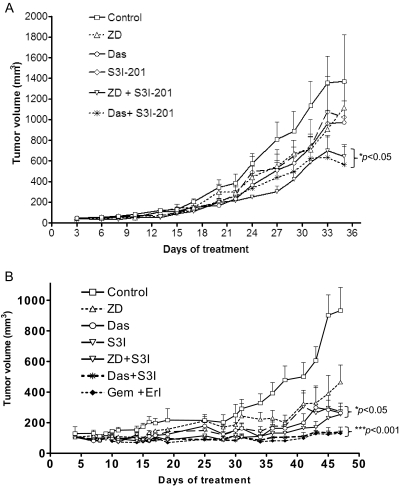
Concurrent Inhibition of Stat3 and EGFR or Src Inhibits Human Pancreatic Tumor Growth in Xenografts.
Subcutaneous xenografts of Colo-357, a metastatic pancreatic adenocarcinoma line, and the epithelioid carcinoma, Panc-1, line were used to study the therapeutic implication of the Stat3, EGFR, and Src inter-relationships and evaluate the in vivo antitumor effects of concurrent inhibition of Stat3 and EGFR or Src. Data showed that, in general, xenografts of Colo-357 cells showed low responsiveness to treatment with inhibitor of EGFR, Src, or Stat3 alone, although as the therapy progressed, tumors treated with only one inhibitor alone appeared to show reduced growth, which was statistically not significant from control, nontreated tumors (Fig. 6A). On the other hand, xenografts of Panc-1 cells showed greater sensitivity to the Src or the Stat3 inhibitor, which induced significant (p < 0.05) inhibition of tumor growth (Fig. 6B, Das and S3I-201). Importantly, for both Colo-357 and Panc-1 lines, xenograft tumors were much more sensitive to the combined inhibition of Stat3 and EGFR or Stat3 and Src than to the inhibition of either one alone, with tumors consistently showing reduced growth and smaller sizes in response to the combined treatment throughout the entire study (Fig. 6, ZD + S3I-201 and Das + S3I-201). Results also showed that xenograft tumors of Panc-1 showed increased sensitivity to the concurrent treatment with the approved combination therapy, gemcitabine and erlotinib, similarly to the treatment with Das and S3I-201 (Fig. 6B, gemcitabine + erlotinib compared with Das + S3I-201). Studies also showed that xenografts of Panc-1 were more sensitive to drug combinations than xenografts derived from Colo-357 cells (Fig. 6B, compare with Fig. 6A), which is consistent with the in vitro cell growth/viability and apoptosis data (Figs. 3 and 4D). Thus, significant differences (p < 0.05) between the tumor volumes (sizes) for Colo-357 tumors in mice treated with the combination inhibitors and the control tumors occurred at day 20 and onward posttreatment, compared with the significant differences that occurred at day 10 posttreatment between the tumor volumes for xenografts of Panc-1 that were treated with the combination of Das and S3I-201 or gemcitabine and erlotinib and the tumor volumes for control tumors (Fig. 6). The in vivo antitumor effects of the combination treatment with S3I-201 and Das or S3I-201 and ZD are consistent with the in vitro antitumor cell data and together indicate that aberrant Stat3 cooperates with hyperactive EGFR or Src to sustain human pancreatic cancer. There were no obvious signs of toxicity of any of the drugs or drug combinations, and the average weights of mice for control and treated mice were 23 to 26 mg at the end of the studies.
Fig. 6.
In vivo study of growth inhibition of human pancreatic cancer xenografts. Human pancreatic Colo-357 tumor-bearing mice were given ZD (75 mg/kg i.v.), Das (15 mg/kg i.v.), and S3I-201 (5 mg/kg i.v.) alone or in combination every 2 or 3 days for the first 2 weeks and then daily for 5 days each week for the next 3 weeks (A), or Panc-1 tumor-bearing mice were given ZD (75 mg/kg i.v.), Das (15 mg/kg i.v.), and S3I-201 (5 mg/kg i.v.) alone or in combination every 2 or 3 days, or given erlotinib (5 mg/kg i.v., every day) in combination with gemcitabine (100 mg/kg i.v., every 3 days) for 48 days (B). Tumor sizes, measured at 2- to 5-day intervals, were converted to tumor volumes and plotted against days. Values, mean and S.E.M. n = 7 or 8 mice per group. *, p < 0.05; **, p < 0.001.
Discussion
Pancreatic cancer is a lethal disease that has remained a challenge to treatments. The lack of understanding of the disease has also limited the chances of designing effective therapeutic modalities. We present evidence from in vitro and in vivo studies that the multiple targeting of Stat3 and EGFR or Stat3 and Src has the potential to induce strong antitumor responses in pancreatic cancer. Our study shows that the concurrent treatment with the Stat3 inhibitor, S3I-201, and the EGFR inhibitor, ZD, or S3I-201 and the Src inhibitor, Das, induced an enhanced tumor growth inhibition, with reduced tumor sizes in xenograft models developed from the metastatic pancreatic cancer line, Colo-357, and the epithelioid carcinoma line, Panc-1. The robust antitumor effects of the combination therapy contrast the weaker antitumor responses to the inhibition of EGFR, Src, or Stat3 alone in the xenografts of Colo-357 and Panc-1, suggesting the monotargeting of EGFR, Src, or Stat3 alone might not be sufficient to suppress the malignant phenotype and induce significant antitumor effects. These findings are generally consistent with the reports of dismal responses in molecular targeted monotherapy, such as the anti-EGFR monotherapy (Philip, 2008; Saif, 2008). Although the combined therapy of gemcitabine and the EGFR inhibitor, erlotinib, for locally advanced/metastatic pancreatic cancer (Senderowicz et al., 2007; Burris and Rocha-Lima, 2008) may be justified by the same argument as the multiple-targeted therapy in our study, it is not without toxicities (Saif, 2008). In our study, the treatment with Das and S3I-201 showed a similar antitumor efficacy. Given that the Das and S3I-201 combination therapy is based on molecular targeting and is mechanism-based, this therapeutic modality presents advantages over the approved gemcitabine and erlotinib combination. We also note from our in vitro study that the concurrent inhibition of Stat3 and EGFR or Src promotes a higher sensitization of pancreatic cancer cells to gemcitabine than the inhibition of EGFR or Src alone. The present in vitro data also provide evidence that the combined inhibition of Stat3 and Src or EGFR induces stronger cell growth inhibition and apoptosis, mechanisms that would contribute to the decreased tumor growth in vivo. Molecularly, the enhanced antitumor effects of the concurrent inhibition of Stat3 and EGFR or Src against pancreatic cancer could in part be the result of the increased potential to down-regulate critical tumor-relevant genes. Altogether, the present study shows the in vivo efficacy of the concurrent targeting of aberrant Stat3 and EGFR or Src and provides support for the use of this multiple-targeting modality as a therapeutic approach for pancreatic cancer.
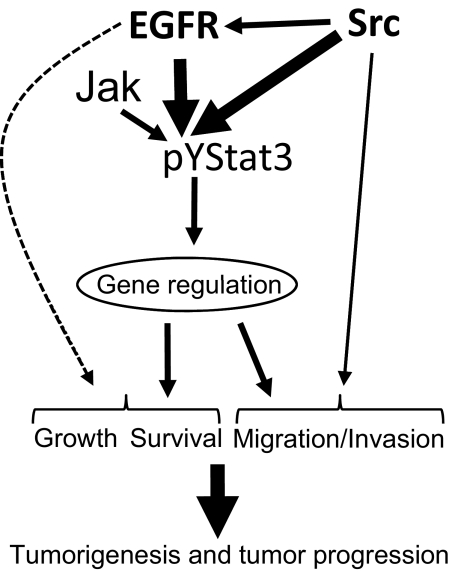
In vitro studies provide the biochemical and biological insights into the enhanced antitumor effects in vivo of the multiple targeting of Stat3 and EGFR or Src in pancreatic cancer. Our study provides strong evidence for a complex signaling cross-talk and a functional cooperation (Fig. 7), which would undercut the biological response of targeting only the EGFR, Src, or Stat3 protein. In the context of this signal integration (Fig. 7), data presented reveal a strong role for Src in increasing the aberrant EGFR activity in pancreatic cancer cells by promoting the phosphorylation of multiple tyrosine residues, including the Y1068EGFR and Y1086EGFR motifs. The induction of aberrant Stat3 activation is promoted by both hyperactive EGFR and Src activities. In this context, the induction of pY1068EGFR and pY1086EGFR by Src would serve to further promote Stat3 activation, given that those two EGFR tyrosine motifs are essential sites for the binding and the activation of Stat3 (Shao et al., 2003). We also note that apart from the baseline, constitutive Stat3 activation in pancreatic cancer cells, which is caused primarily by EGFR and Src, there is a contributory role for Jak tyrosine kinases in the induction of Stat3 activation (Fig. 7). However, Jaks are unlikely to be the predominant mediators of the baseline, constitutive Stat3 activity, given that their inhibition by AG490 did not abolish aberrant Stat3 activation at the earliest time point, whereas the EGFR or Src inhibition completely attenuated the baseline, aberrant Stat3 activity. Instead, Jaks could provide a secondary, compensatory mechanism for Stat3 activation, as a part of a two-phase model for the induction of Stat3 activity in pancreatic cancer cells. A similar secondary induction of Stat3 activation via Jak activities has previously been observed in the head and neck squamous cell carcinoma line (Johnson et al., 2007) and is suggested to be caused by the growth-stimulatory factors that are released from tumor cells (Salomon et al., 1995), which in turn induce Jaks activity and thereby promote Stat3 activation. In this complex signaling structure (Fig. 7), the inhibition of EGFR or Src alone is unlikely to abolish the functional effects of abnormal Stat3 signaling. Instead, combining the direct inhibition of aberrant Stat3 with the EGFR or Src inhibition serves to provide the framework for substantially thwarting the oncogenic events with measureable beneficial outcome. Our data would suggest that the inhibition of Stat3 enhances the sensitivity of pancreatic cancer cells to the antitumor effects of ZD and Das.
Fig. 7.
A model of collaborative function of hyperactive EGFR, Src, and Stat3 in support of human pancreatic cancer. Hyperactive c-Src contributes to promoting the aberrant EGFR activation. Both hyperactive EGFR and Src induce constitutive Stat3 activation, whereas Jak activity contributes to promoting Stat3 signaling by serving as a compensatory mechanism. Aberrantly active Stat3 dysregulates the induction of target genes and, together with other potential mechanisms induced by the hyperactive EGFR and Src, induces cell growth, survival, and migration/invasion, thereby promoting pancreatic tumorigenesis and progression.
Additional in vitro biological data further indicate a collective role of EGFR, Src, and Stat3 in supporting pancreatic cancer. Although the inhibition of the activity of each of the three proteins induced some degree of antitumor cell response in vitro, albeit minimal, data are convincing that the simultaneous targeting of Stat3 and EGFR or Src has a higher potential to be more effective in inducing diverse antitumor cell effects against pancreatic cancer cells than the inhibition of EGFR or Src alone. The hyperactivation of EGFR signaling has been deemed a prognostic indicator of low survival among pancreatic cancer patients (Dong et al., 1998; Ueda et al., 2004). In addition, there is evidence to indicate that the concurrence of hyperactive Src signaling potentiates the effects of aberrant EGFR (Tice et al., 1999). Such increase of effect or the cooperation of activities and/or functions between the critical molecular entities that supports the pancreatic cancer phenotype can undermine the efficacy of a targeted monotherapy in pancreatic cancer. Indeed, the present data that the simultaneous inhibition of Stat3 and EGFR or Src induced greater antitumor cell effects and a higher sensitization to gemcitabine strongly supports this viewpoint. We infer from our study that the more robust tumor growth inhibition in vivo by simultaneous inhibition of Stat3 and EGFR or Src is the result of the concomitant suppression of the abnormal functions of both Stat3 and EGFR or Stat3 and Src, suggesting that the concurrent targeting of Stat3 and EGFR or Stat3 and Src could be a more effective, multitargeted therapeutic modality for pancreatic cancer than the inhibition of EGFR, Src, or Stat3 alone.
Supplementary Material
Acknowledgments
We thank colleagues and members of our laboratory for stimulating discussions.
This work was supported by the National Institutes of Health National Cancer Institute [Grants CA106439, CA128865].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.162669.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- EGFR
- epidermal growth factor receptor
- EGF
- epidermal growth factor
- ErkMAPK
- extracellular signal-regulated kinase-mitogen-activated protein kinase
- STAT/Stat
- signal transducer and activator of transcription
- Jaks
- Janus kinase
- HPDEC
- human pancreatic duct epithelial cell
- ZD/Iressa
- ZD1839
- Das
- dasatinib
- EMSA
- electrophoretic mobility-shift assay
- siRNA
- small-interfering RNA
- PD169
- PD169540 {N-[4-(3-bromophenylamino)-7-[3-(4-morpholinyl)propoxy]-6-quinazolinyl]-2-propenamide}
- S3I-201
- 2-hydroxy-4-(2-{[(4-methylbenzene)sulfonyl]oxy}acetamido)benzoic acid
- AG879
- α-cyano-(3,5-di-t-butyl-4-hydroxy)thiocinnamide.
References
- Burris H, 3rd, Rocha-Lima C. (2008) New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist 13:289–298 [DOI] [PubMed] [Google Scholar]
- Darnell JE. (2005) Validating Stat3 in cancer therapy. Nat Med 11:595–596 [DOI] [PubMed] [Google Scholar]
- DeArmond D, Brattain MG, Jessup JM, Kreisberg J, Malik S, Zhao S, Freeman JW. (2003) Autocrine-mediated ErbB-2 kinase activation of STAT3 is required for growth factor independence of pancreatic cancer cell lines. Oncogene 22:7781–7795 [DOI] [PubMed] [Google Scholar]
- Dong M, Nio Y, Guo KJ, Tamura K, Tian YL, Dong YT. (1998) Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res 18:4613–4619 [PubMed] [Google Scholar]
- Downward J, Parker P, Waterfield MD. (1984) Autophosphorylation sites on the epidermal growth factor receptor. Nature 311:483–485 [DOI] [PubMed] [Google Scholar]
- Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, et al. (2001) Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20:2499–2513 [DOI] [PubMed] [Google Scholar]
- Gouilleux F, Moritz D, Humar M, Moriggl R, Berchtold S, Groner B. (1995) Prolactin and interleukin-2 receptors in T lymphocytes signal through a MGF-STAT5-like transcription factor. Endocrinology 136:5700–5708 [DOI] [PubMed] [Google Scholar]
- Huang M, Dorsey JF, Epling-Burnette PK, Nimmanapalli R, Landowski TH, Mora LB, Niu G, Sinibaldi D, Bai F, Kraker A, et al. (2002) Inhibition of Bcr-Abl kinase activity by PD180970 blocks constitutive activation of Stat5 and growth of CML cells. Oncogene 21:8804–8816 [DOI] [PubMed] [Google Scholar]
- Johnson FM, Saigal B, Tran H, Donato NJ. (2007) Abrogation of signal transducer and activator of transcription 3 reactivation after Src kinase inhibition results in synergistic antitumor effects. Clin Cancer Res 13:4233–4244 [DOI] [PubMed] [Google Scholar]
- Korc M, Meltzer P, Trent J. (1986) Enhanced expression of epidermal growth factor receptor correlates with alterations of chromosome 7 in human pancreatic cancer. Proc Natl Acad Sci USA 83:5141–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Lührs H, Friess H, Büchler MW, Adler G. (1998) Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun 243:503–508 [DOI] [PubMed] [Google Scholar]
- Mahtouk K, Hose D, Rème T, De Vos J, Jourdan M, Moreaux J, Fiol G, Raab M, Jourdan E, Grau V, et al. (2005) Expression of EGF-family receptors and amphiregulin in multiple myeloma. Amphiregulin is a growth factor for myeloma cells. Oncogene 24:3512–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. (2005) Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res 65:9185–9189 [DOI] [PubMed] [Google Scholar]
- Ouyang H, Mou Lj, Luk C, Liu N, Karaskova J, Squire J, Tsao MS. (2000) Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol 157:1623–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip PA. (2008) Targeted therapies for pancreatic cancer. Gastrointest Cancer Res 2 (Suppl 2):S16–S19 [PMC free article] [PubMed] [Google Scholar]
- Saif MW. (2008) Erlotinib: the first biologic in the management of pancreatic cancer. Exp Opin Pharmacother 9:1595–1607 [DOI] [PubMed] [Google Scholar]
- Salomon DS, Brandt R, Ciardiello F, Normanno N. (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19:183–232 [DOI] [PubMed] [Google Scholar]
- Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, Schirner M, Wiedenmann B, Rosewicz S. (2003) Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology 125:891–905 [DOI] [PubMed] [Google Scholar]
- Seidel HM, Milocco LH, Lamb P, Darnell JE, Jr, Stein RB, Rosen J. (1995) Spacing of palindromic half sites as a determinant of selective STAT (signal transducers and activators of transcription) DNA binding and transcriptional activity. Proc Natl Acad Sci USA 92:3041–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderowicz AM, Johnson JR, Sridhara R, Zimmerman P, Justice R, Pazdur R. (2007) Erlotinib/gemcitabine for first-line treatment of locally advanced or metastatic adenocarcinoma of the pancreas. Oncology (Williston Park) 21:1696–1706; discussion 1706–1709, 1712, 1715 [PubMed] [Google Scholar]
- Shao H, Cheng HY, Cook RG, Tweardy DJ. (2003) Identification and characterization of signal transducer and activator of transcription 3 recruitment sites within the epidermal growth factor receptor. Cancer Res 63:3923–3930 [PubMed] [Google Scholar]
- Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, et al. (2007a) Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 104:7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiquee KA, Gunning PT, Glenn M, Katt WP, Zhang S, Schrock C, Schroeck C, Sebti SM, Jove R, Hamilton AD, et al. (2007b) An oxazole-based small-molecule Stat3 inhibitor modulates Stat3 stability and processing and induces antitumor cell effects. ACS Chem Biol 2:787–798 [DOI] [PubMed] [Google Scholar]
- Tice DA, Biscardi JS, Nickles AL, Parsons SJ. (1999) Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA 96:1415–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyonaga T, Nakano K, Nagano M, Zhao G, Yamaguchi K, Kuroki S, Eguchi T, Chijiiwa K, Tsuneyoshi M, Tanaka M. (2003) Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett 201:107–116 [DOI] [PubMed] [Google Scholar]
- Trevino JG, Gray MJ, Nawrocki ST, Summy JM, Lesslie DP, Evans DB, Sawyer TK, Shakespeare WC, Watowich SS, Chiao PJ, et al. (2006) Src activation of Stat3 is an independent requirement from NF-κB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis 9:101–110 [DOI] [PubMed] [Google Scholar]
- Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, Donato NJ, Abbruzzese JL, Baker CH, Gallick GE. (2006) Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol 168:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkson J. (2004) STAT proteins as novel targets for cancer drug discovery. Exp Opin Ther Targets 8:409–422 [DOI] [PubMed] [Google Scholar]
- Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. (1998) Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol 18:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng CW, Frolov A, Frolova N, Jhala NC, Howard JH, Vickers SM, Buchsbaum DJ, Heslin MJ, Arnoletti JP. (2007) EGFR genomic gain and aberrant pathway signaling in pancreatic cancer patients. J Surg Res 143:20–26 [DOI] [PubMed] [Google Scholar]
- Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K, Mochizuki H. (2004) The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas 29:e1–e8 [DOI] [PubMed] [Google Scholar]
- Wagner BJ, Hayes TE, Hoban CJ, Cochran BH. (1990) The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J 9:4477–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. (2002) ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 62:5749–5754 [PubMed] [Google Scholar]
- Yue P, Turkson J. (2009) Targeting STAT3 in cancer: how successful are we? Exp Opin Invest Drugs 18:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Turkson J, Carter-Su C, Smithgall T, Levitzki A, Kraker A, Krolewski JJ, Medveczky P, Jove R. (2000) Activation of Stat3 in v-Src-transformed fibroblasts requires cooperation of Jak1 kinase activity. J Biol Chem 275:24935–24944 [DOI] [PubMed] [Google Scholar]
- Zhao S, Venkatasubbarao K, Lazor JW, Sperry J, Jin C, Cao L, Freeman JW. (2008) Inhibition of STAT3 Tyr705 phosphorylation by Smad4 suppresses transforming growth factor β-mediated invasion and metastasis in pancreatic cancer cells. Cancer Res 68:4221–4228 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.