Abstract
Drug abuse-induced plasticity of putative dopaminergic (pDAergic) ventral tegmental area (VTA) neurons may play an important role in changes in the mesocorticolimbic system that lead to the development of addiction. In the present study, extracellular recordings were used to examine time-dependent effects of dopamine (DA) on pDAergic VTA neurons in rat brain slices. Administration of DA (2.5–10 μM) for 40 min resulted in inhibition followed by partial or full reversal of that inhibition. The reduced sensitivity to DA inhibition lasted 30 to 90 min after washout of the long-term dopamine administration. The inhibition reversal was not observed with 40-min administration of the D2 agonist quinpirole (25–200 nM), so this phenomenon was not the result of desensitization induced solely by stimulation of D2 DA receptors. Inhibition reversal could be observed with the coapplication of quinpirole and the D1/D5 agonist SKF38393 [(±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide], suggesting a D1/D5 mechanism for the reversal. Furthermore, D1/D5 antagonists, given in the presence of prolonged DA exposure, prevented the inhibition reversal. Application of 3 μM quinpirole caused desensitization to low quinpirole concentrations that was blocked by a D1/D5 antagonist. These data suggest that coactivation of D1/D5 receptors and D2 receptors in the VTA results in desensitization of autoinhibitory D2 receptors. Prolonged increases in pDAergic tone in the VTA that may occur in vivo with drugs of abuse could reduce the regulation of firing by D2 dopamine receptor activation, producing long-term alteration in information processing related to reward and reinforcement.
Putative dopaminergic (pDAergic) neurons of the ventral tegmental area (VTA) are important for the rewarding and reinforcing properties of numerous drugs of abuse (Wise, 1996). Drugs of abuse increase dopaminergic neurotransmission (Imperato and Di Chiara, 1986; Imperato et al., 1986; Di Chiara and Imperato, 1988). Most studies have examined dopamine (DA) release in the terminal target regions of the DA VTA neurons, the nucleus accumbens and the prefrontal cortex, and have found that drugs of abuse increase the DA concentrations in these regions (Di Chiara and Imperato, 1988; Di Chiara et al., 2004). Because there is dendritic DA release in response to increased activity of mesencephalic DA neurons (Cragg et al., 1997), it is likely that the DA concentrations in the VTA are also increased in response to most drugs of abuse. The effect of these elevated dopamine concentrations in the VTA is not known, but it is known that elevated dopamine can produce long-term changes in neurotransmission; for example, elevated dopamine can increase glutamatergic receptor expression in prefrontal cortex (Gao and Wolf, 2008; Sun et al., 2008).
There are five classes of DA receptors: two “D1-like” receptors (D1 and D5) and three “D2-like” receptors (D2, D3, and D4). D1-like (D5) receptor immunoreactivity on perikarya of mesencephalic DA neurons has been demonstrated (Ciliax et al., 2000; Khan et al., 2000), and mRNA for D5 receptors has been observed in the substantia nigra by some groups (Choi et al., 1995) but not by all groups (Meador-Woodruff et al., 1992). The D2-like mesolimbic DA receptors are predominantly D2 receptors (Sesack et al., 1994). The D1 receptors located in dopaminergic brain areas seem to be on terminals projecting to the region, not on the DA neurons themselves (Caillé et al., 1996). Dopamine D5 receptors are present on the cell bodies of dopaminergic VTA neurons (Ciliax et al., 2000).
pDAergic VTA neurons fire action potentials spontaneously in vivo (Bunney et al., 1973) and in vitro (Pinnock et al., 1979; Brodie and Dunwiddie, 1987). This spontaneous firing is inhibited by the action of DA at D2 autoreceptors on the cell bodies and dendrites of these neurons (Lacey et al., 1987). Stimulation of D2 autoreceptors activates G protein-linked potassium channels, which seem to be activated directly by G proteins without the involvement of cAMP or adenylate cyclase (Kim et al., 1995).
Drugs of abuse produce increases in dopaminergic neurotransmission, either by increasing firing rate of DA VTA neurons or by blocking reuptake or reversing DA transporter activity in terminal regions (Mueller et al., 2004), and it is likely that the DA concentration in the VTA, released in the somatodendritic area (Cragg et al., 1997; Rice et al., 1997), can remain elevated during drug abuse episodes. For this reason, we tested the effects of sustained administration of exogenous DA on DA inhibition of DA VTA neurons. In vitro electrophysiological experiments using whole-cell patch clamping cannot reliably maintain healthy neuronal recordings for periods longer than 1 h. We used extracellular recording from DA VTA neurons in brain slices, a technique that avoids disrupting the intracellular milieu and that makes it possible to monitor spontaneous firing of these neurons for long continuous time periods (1–4 h).
Materials and Methods
Animals.
Fischer 344 (F344; 90–150 g) used in these studies were obtained from Harlan Sprague-Dawley (Indianapolis, IN). All rats were treated in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all experimental methods were approved by the Animal Care Committee of the University of Illinois at Chicago.
Preparation of Brain Slices.
Brain slices containing the VTA were prepared from the subject animals as described previously (Brodie et al., 1999a). In brief, after rapid removal of the brain, the tissue was blocked coronally to contain the VTA and substantia nigra; the cerebral cortices and a portion of the dorsal mesencephalon were removed. The tissue block was mounted in the Vibratome system and submerged in chilled cutting solution. Coronal sections (400 μm thick) were cut, and the slice was placed onto a mesh platform in the recording chamber. The slice was totally submerged in aCSF maintained at a flow rate of 2 ml/min; the temperature in the recording chamber was kept at 35°C. The composition of the aCSF in these experiments was 126 mM NaCl, 2.5 mM KCl, 1.24 mM NaH2PO4, 2.4 mM CaCl2, 1.3 mM MgSO4, 26 mM NaHCO3, and 11 mM glucose. In some experiments, a HEPES-aCSF hybrid solution was used; composition of this solution was 106 mM NaCl, 2.5 mM KCl, 1.24 mM NaH2PO4, 2.4 mM CaCl2, 1.3 mM MgSO4, 20 mM HEPES, 26 mM NaHCO3, 11 and mM glucose. The composition of the cutting solution was 2.5 mM KCl, 2.4 mM CaCl2, 1.3 mM MgSO4, 26 mM NaHCO3, 11 mM glucose, and 220 mM sucrose. Both solutions were saturated with 95% O2/5% CO2 (pH 7.4). Equilibration time of at least 1 h was allowed after placement of tissue in the recording chamber before electrodes were placed in the tissue.
Cell Identification.
The VTA was clearly visible in the fresh tissue as a gray area medial to the darker substantia nigra and separated from the nigra by white matter. Recording electrodes were placed in the VTA under visual control. pDAergic neurons have been shown to have distinctive electrophysiological characteristics (Grace and Bunney, 1984; Lacey et al., 1989). Only those neurons that were anatomically located within the VTA and that conformed to the criteria for pDAergic neurons established in the literature and in this laboratory (Lacey et al., 1989; Mueller and Brodie, 1989) were studied. These criteria include broad action potentials (2.5 ms or greater, measured as the width of the biphasic or triphasic waveform at the baseline), slow spontaneous firing rate (0.5–5 Hz), and a regular interspike interval. Cells were not tested with opiate agonists as has been done by other groups to further characterize and categorize VTA neurons (Margolis et al., 2006). It should be noted that some neurons with the characteristics that we used to identify DA VTA neurons may not, in fact, be DA-containing (Margolis et al., 2006).
Drug Administration.
Drugs were added to the aCSF by means of a calibrated infusion pump from stock solutions 100 to 1000 times the desired final concentrations. The addition of drug solutions to the aCSF was performed in such a way to permit the drug solution to mix completely with aCSF before this mixture reached the recording chamber. Final concentrations were calculated from aCSF flow rate, pump infusion rate, and concentration of drug stock solution. The small volume chamber (approximately 300 μl) used in these studies permitted the rapid application and washout of drug solutions. Typically drugs reach equilibrium in the tissue after 2 to 3 min of application.
DA hydrochloride, quinpirole, and sulpiride and most of the salts used to prepare the extracellular media were purchased from Sigma-Aldrich (St. Louis, MO). SKF38393, SCH39166, and SCH23390 were purchased from Tocris (Ellisville, MO).
Extracellular Recording.
Extracellular recording was chosen for these studies because this method permits the recordings to be of long duration (routinely >4 h) and allows us to assess the effects of extended exposure (>30 min) to drugs. The limitation of only measuring spontaneous action potential frequency (rather than membrane potential or other electrophysiological parameters) is counterbalanced by the advantage of being able to determine the time course of drug actions and interactions. Extracellular recording electrodes were made from 1.5-mm diameter glass tubing with filament and were filled with 0.9% NaCl. Tip resistance of the microelectrodes ranged from 2 to 4 MΩ. A Fintronics amplifier was used in conjunction with an IBM personal computer-based data acquisition system (ADInstruments Ltd., Chalgrove, Oxfordshire, UK). Offline analysis was used to calculate, display, and store the frequency of firing 1-min intervals. Additional software was used to calculate the firing rate over 5-s intervals. Firing rate was determined before and during drug application. Firing rate was calculated over 1-min intervals before administration of drugs and during the drug effect; peak drug-induced changes in firing rate were expressed as the percentage change from the control firing rate according to the formula [(FRD − FRC)/FRC] × 100, where FRD is the firing rate during the peak drug effect and FRC is the control firing rate. Thus, the change in firing rate is expressed as a percentage of the initial firing rate, which controls for small changes in firing rate, which may occur over time. This formula was used to calculate both excitatory and inhibitory drug effects. Peak excitation was defined as the peak increase in firing rate produced by the drug (e.g., DA) greater than the predrug baseline. Inhibition was defined as the lowest firing rate below the predrug baseline. Inhibition reversal was observed as a statistically significant reduction in the inhibition.
Data Collection.
For comparison of the time course of effects on firing rate, the data were normalized and averaged. Firing rates over 1-min intervals were calculated and normalized to the 1-min interval immediately before the DA administration. These normalized data were averaged by synchronizing the data to the DA administration period, and graphs of the averaged data were made.
Statistical Analysis.
Averaged numerical values were expressed as the mean ± S.E.M. The differences among firing rates during the long drug administration intervals in these studies were assessed with repeated measures analysis of variance (ANOVA). For effects of multiple drug concentrations or more than one drug, an appropriate one- or two-way ANOVA was used, followed by Student-Newman-Keuls or Tukey post-hoc comparisons when needed (Kenakin, 1987). Statistical analyses were performed with SigmaStat (Systat, San Jose, CA).
Results
VTA Neuron Characteristics.
A total of 233 neurons were used in this study. The firing rate of VTA neurons in this study ranged from 0.6118 to 4.3833 Hz, with a mean firing rate of 1.88 ± 0.09 Hz. All of the neurons tested had regular firing rates and were inhibited by DA. DA sensitivity was initially assessed by administering dopamine (0.5–5 μM) for 5 min, and then the DA washout was performed for at least 10 min. Note that blockers of the dopamine transporter were not used because there is normally sufficient dopamine release in brain slices to cause complete cessation of spontaneous firing in the presence of dopamine transporter blockers, such as cocaine (Brodie and Dunwiddie, 1990). In the absence of DA transporter blockers, dopamine has been shown to produce inhibitory effects in the concentration range from 0.5 to 100 μM, although in dissociated DA VTA neurons, concentrations as low as 50 nM can completely inhibit spontaneous action potential firing (Brodie et al., 1999b). Cells that did not return to the pre-DA firing rate during this washout were not used. One benefit of the extracellular recording method used in these studies is that long-duration recordings can be made reliably; the average recording duration was 96 ± 18 min, with a range of 28 to 280 min.
Concentration-Response Curve: Stepwise Increases.
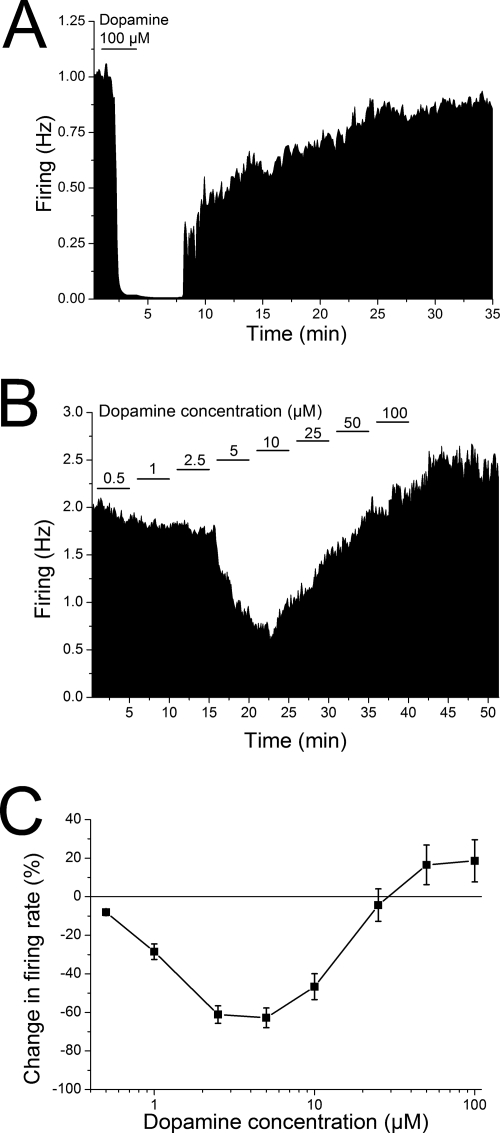
It is well known that acute application of dopamine inhibits spontaneous firing of pDAergic neurons of the VTA. Figure 1A illustrates the effect of acute application of a single high concentration of dopamine to the spontaneous firing frequency of a typical pDAergic VTA neuron. In this case, a 4-min application of 100 μM dopamine produced complete cessation of firing, which subsided upon washout of the dopamine. In the first set of experiments that formed the basis for the present study, concentration-response curves for dopamine were generated by adding DA to the superfusate in a stepwise manner; that is, the lowest concentration of DA (0.5 μM) was given for 5 min, and then the concentration was increased immediately to the next highest concentration (1 μM) and so on. An illustration of the firing rate of a typical pDAergic neuron of the VTA is shown in Fig. 1B. As shown in this rate-meter graph, the firing rate was inhibited by the lower concentrations of DA tested; however, as the concentration of dopamine was increased, the inhibition of the firing rate by dopamine was slowly reversed until, at the highest concentrations, the firing rate was higher than it was before dopamine administration. The effect of 100 μM dopamine administered in this protocol as the last of several dopamine concentrations can easily be contrasted with the effect of the acute application of dopamine shown in Fig. 1A. Due to the number of concentrations of DA tested in this protocol, some of the slices were treated with some concentration of DA for approximately 40 min. Not all cells received all DA concentrations; only cells tested with at least two concentrations were included (n = 63). The pooled results of this experiment are shown in Fig. 1C. Low concentrations of DA produced characteristic concentration-dependent inhibition of firing, but as the concentration was increased above 10 μM, the inhibitory effect of DA decreased until, at the highest concentrations in this protocol, the firing rate in the presence of DA was higher than before exogenous DA was added. There was a significant difference between the firing rate in the presence of 50 and 100 μM DA compared with the firing rate at 1 to 10 μM DA, and in addition, the DA effects at 2.5 to 10 μM (inhibition) and 100 μM (excitation) were significantly different from the baseline pre-DA firing rate (one-way repeated measures ANOVA P < 0.001, Student-Newman-Keuls post-hoc test, p < 0.05 for significant differences; n = 63). This suggests a second mechanism emerging in the presence of DA that counteracted the DA-induced inhibition.
Fig. 1.
Dopamine concentration-response curves using stepwise application of a range of dopamine concentrations. A, rate-meter graph of the effect of acute application of a high DA concentration. Vertical bars indicate the firing rate over 5-s intervals. Horizontal bars indicate the duration of application of DA (100 μM). Administration of DA produced complete cessation of firing, and the firing resumed after washout of the dopamine from the superfusate. B, rate-meter graph of the effect of stepwise application of a series of DA concentrations (0.5–100 μM). Vertical bars indicate the firing rate over 5-s intervals. Horizontal bars indicate the duration of application of DA (concentration in micromolar indicated above bar). Note that even though the concentration of dopamine was increased every 5 min, inhibition only increased up to 10 μM dopamine, and as concentrations were increased above 10 μM, the firing rate was less inhibited, and eventually firing increased above the predopamine baseline rate. C, change in spontaneous firing rate (mean ± S.E.M.) in the presence of dopamine is plotted as a function of dopamine concentration (log scale). Dopamine was applied in 5-min steps over a range of concentrations (0.5–100 μM, n = 63). There was a significant effect of concentration on inhibition with concentrations of 2.5 to 10 and 100 μM significantly different from control firing rate.
Long-Term Administration of Single Dopamine Concentrations.
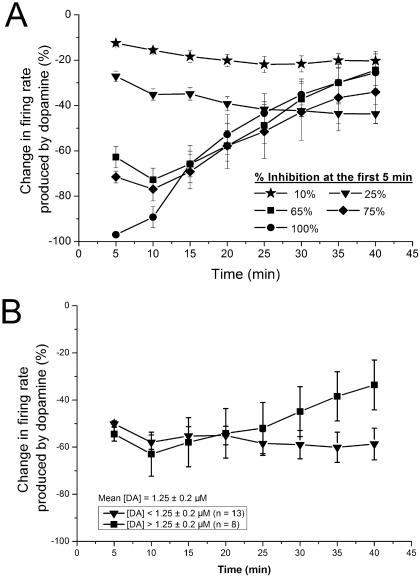
As the reversal of DA inhibition could have been the result of either the high and increasing DA concentrations or the long-duration application of DA, we tested whether the inhibitory effect of a single concentration of DA would change over time. Short (5 min) applications of DA were used at the beginning of each recording to assess the magnitude of DA inhibition; concentrations were selected that would produce inhibition of 10, 25, 65 75, or 100% during a 5-min test application. Selecting DA concentrations according to their inhibitory potency rather than the exact DA concentration controlled for cell-to-cell variability in sensitivity to DA inhibition. The results of these studies are shown in Fig. 2A. Concentrations of DA that produced 10 (mean DA concentration = 0.29 ± 0.05 μM; range = 0.25–0.5 μM; n = 7) or 25% (mean DA concentration = 0.61 ± 0.11 μM; range = 0.25–1 μM; n = 7) inhibition did not exhibit reversal over the 40-min time period during which they were applied. In contrast, concentrations of DA producing 65 (mean DA concentration = 2.54 ± 0.7 μM; range = 0.5–10 μM; n = 12), 75 (mean DA concentration = 2.82 ± 1.25 μM; range = 0.5–10 μM; n = 7), or 100% (mean DA concentration = 8.09 ± 1.02 μM; range = 2.5–10 μM; n = 11) inhibition showed clear decreases in inhibitory potency over the 40-min time course of application, with the inhibition reduced to approximately 25 to 30% at the end of the 40-min time period. When DA inhibition was approximately 50%, the responses were more variable; cells more sensitive to DA (DA concentration <1.25 μM, mean DA concentration = 0.56 ± 0.08 μM, range = 0.25–1 μM; n = 13) did not exhibit inhibition reversal, whereas cells less sensitive to DA (DA concentration >1.25 μM, mean DA concentration = 2.25 ± 0.13 μM; range = 1.5–2.5 μM; n = 8) demonstrated inhibition reversal (Fig. 2B). Inhibition reversal was indicated by a statistically significant difference between the firing rate in the presence of DA at 5 and 40 min (one-way repeated measures ANOVAs, P < 0.05; Student-Newman-Keuls post-hoc test, P < 0.05). Again, the inhibition reversal observed with the long application of a single concentration of DA suggests that a second mechanism emerges with long-term DA administration and that this mechanism requires higher DA concentrations (> 2 μM in this set of experiments).
Fig. 2.
Dopamine concentration-response curves using long-duration application of single dopamine concentrations. Change in firing rate (mean ± S.E.M.) produced by dopamine is plotted as a function of time. A, effect of dopamine on spontaneous firing rate is plotted as a function of time. Concentrations of dopamine that produced 10, 25, 65, 75, or 100% inhibition in the first 5 min were applied for 40 min. Choice of the concentration based on initial effect of dopamine controlled for sensitivity of the neurons to the inhibitory effect of dopamine. For concentrations that initially produced 10 (n = 7) or 25% (n = 7) inhibition in the firing rate, there was no significant change in the effect of dopamine over time. For concentrations that initially produced 65 (n = 12), 75 (n = 7), or 100% (n = 11) inhibition in firing rate, there was a significant reduction in the inhibitory effect of dopamine over time, with the last three time points significantly different from the 5-min time point. B, effect of dopamine on spontaneous firing rate is plotted as a function of time for two groups of neurons in which dopamine produced 50% inhibition of firing. In more sensitive cells that were inhibited by 50% by low concentrations of dopamine ([DA] <1.25 μM; n = 13), there was no significant change in the effect of dopamine over time. In less sensitive cells that were inhibited by 50% by higher concentrations of dopamine ([DA] >1.25 μM; n = 8), there was a significant reversal of dopamine inhibition with time, with the last three time points significantly different from the 5-min time point.
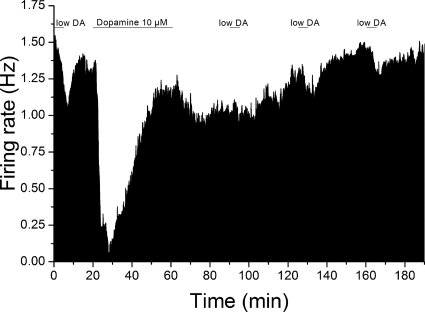
We also observed that the apparent decrease in DA potency was sustained for a relatively long time after the 40-min DA application. We observed that there was a long-lasting change in response to lower DA concentrations produced by prolonged application of 10 μM DA (Fig. 3). Before long-duration administration of 10 μM DA, low concentrations of DA (0.42 ± 0.12 μM, range = 0.25–0.5 μM; n = 6) produced 28.1 ± 3.8% decrease in the firing rate. After administration of 10 μM DA for 40 min, the inhibitory effect of the low concentration of DA was tested at 30-min intervals after washout of 10 μM DA; at 30 min, the effect was reduced significantly to 10.0 ± 4.5% (one-way ANOVA, F = 5.53, p < 0.01, Tukey test, p < 0.05; n = 7); the effects of low dopamine concentrations at 60 (17.0 ± 1.7%) and 90 min (18.2 ± 1.6%) were not significantly different from control (Fig. 3). The inhibitory effect of short applications of the low concentration of DA increased over time, suggesting a recovery from the altered DA response produced by long-term exposure to 10 μM DA.
Fig. 3.
Mean rate-meter graphs of the effect of long-duration dopamine exposure on response to shorter dopamine administration. Mean firing rate over 5-s intervals is plotted as a function of time for cells exposed to dopamine for 5-min intervals before and after a 40-min exposure to 10 μM dopamine. Administration of low concentrations of dopamine (0.42 ± 0.12 μM) for 5 min produced inhibition of firing (28.1 ± 3.8%; n = 6). After these test applications, 10 μM dopamine was applied for 40 min. Inhibition reversal was observed for this 40-min exposure to 10 μM dopamine. After washout of 10 μM dopamine, the effect of brief 5-min exposures to lower concentrations of dopamine (same concentrations for each cell as tested before long-term dopamine exposure) were significantly reduced at 30 min (10.0 ± 4.5%) but was not significantly different at 60 (17.0 ± 1.7%) or 90 min (18.2 ± 1.6%) after the long-term exposure (one-way ANOVA, F = 5.53, p < 0.01; Tukey post-hoc test, P < 0.05 for significance).
In a separate set of cells, we carefully examined the percentage of cells that exhibited dopamine-inhibition reversal. Putative dopaminergic neurons (n = 13) were tested with concentrations of dopamine that produced a mean 70% inhibition in the first 5 min (range from −43 to −97%), and dopamine was left on for 40 min. The effect of dopamine at 5 min (−70.0 ± 4.8%) was significantly different from that at 40 min (−39.7 ± 6.7%) (t test, p < 0.002; n = 13). Using the criterion of a reduction in inhibition by dopamine of at least 15%, 12 of the 13 cells exhibited reversal, whereas in the nonreversed neuron, dopamine-induced inhibition went from −82% at 5 min to −73% at the end of 40 min. Results in HEPES-aCSF hybrid buffer were similar. The effect of dopamine at 5 min (−76.0 ± 3.8%) was significantly different from that at 40 min (−44.9 ± 5.6%) (t test, p < 0.005; n = 12). Using the criterion of a reduction in inhibition by dopamine of at least 15%, 10 of the 12 cells exhibited reversal, whereas in the nonreversed neurons, one dopamine-induced inhibition went from −84% at 5 min to −70.5% at the end of 40 min, and the other went from −66% at 5 min to −100% at the end of 40 min.
Long-Term Administration of Single Quinpirole Concentrations.
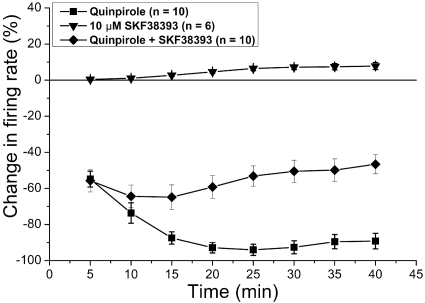
One possibility is that there is DA D2 receptor desensitization as a result of the long application of higher DA agonist concentrations. To test this possibility, we assessed the effect of a long administration of the D2 receptor agonist quinpirole (concentration causing a greater than 50% inhibition at 5 min, mean concentration of quinpirole = 43.5 ± 3.42 nM; mean change in firing rate at 10 min = −73.6%) (Fig. 4). Unlike the effect of DA, prolonged application of quinpirole produced inhibition that was not reversed with time; inhibition became significantly greater between 5 and 15 min, and then no significant change in inhibition was evident between 15 and 40 min (one-way repeated measures ANOVA P < 0.001, Student-Newman-Keuls post-hoc test, significant differences P < 0.05). This observation suggests that the reversal of DA inhibition was not induced solely by desensitization of D2 receptors.
Fig. 4.
Inhibitory effects of long-duration treatment with quinpirole and SKF38393. Long-duration applications to VTA dopamine neurons of D2 agonist quinpirole, D1/D5 agonist SKF38393, and the combination were performed. Quinpirole (43.5 ± 3.4 nM; n = 10) alone produced significant inhibition (one-way repeated measures ANOVA, P < 0.05; n = 10) that reached a peak at approximately 15 min, and the firing rate remained inhibited for the duration of quinpirole application. SKF38393 (10 μM) alone (n = 6) had a small but significant excitatory effect on the firing rate. In the presence of 10 μM SKF38393, quinpirole (102 ± 20 nM) inhibition partially reversed so that the inhibition at 40 min was significantly less than that at 5 or 10 min (n = 10).
Given that there is evidence for D1/D5 receptors in the VTA and that activation of these receptors results in depolarization of DA VTA neurons (Schilström et al., 2006), we tested whether concomitant stimulation of D1/D5 receptors is involved in the reversal of DA inhibition. A D1/D5 agonist, SKF38393 (10 μM), was added to the superfusate with quinpirole (102 ± 20 nM; n = 10). We do not know the reason for the need for higher quinpirole concentrations in the presence of SKF38393 (102 nM) compared with the quinpirole concentration needed in the absence of SKF38393 (43.5 nM); it is possible that this is due to partial blockade of D2 receptors by SKF38393, because the affinity of SKF38393 for the D1 receptor is only 150 times greater than its affinity for the D2 receptor (Seeman and Van Tol, 1994). This concomitant stimulation of D2 receptors (by quinpirole) and D1/D5 receptors (by SKF38393) should mimic the stimulation of these receptors by dopamine. With the treatment with SKF38393, some reversal of the quinpirole inhibition was observed; there was a significant difference between the firing rate at 10, 15 and 20 min compared with the firing rate at 40 min (Friedman repeated measures ANOVA on a Ranks test P < 0.001, Tukey post-hoc test, significant differences P < 0.05). Treatment with SKF38393 alone produced a small but significant increase in the firing rate at 40 min compared with that at 5, 10, and 15 min (one-way repeated measures ANOVA P < 0.001, Student-Newman-Keuls post-hoc test, significant differences P < 0.05) (Fig. 4). These results suggest that stimulation of D1/D5 receptors is required for dopamine-inhibition reversal.
We also tested whether long-term treatment with dopamine itself can produce excitation in the absence of D2-mediated inhibition. We assessed the effect of a 40-min application of dopamine in the presence of the specific D2 receptor antagonist sulpiride. With coapplication of sulpiride (35 ± 5.67 μM) and a single concentration of DA (9.4 ± 5.61 μM), the DA inhibition was blocked, and dopamine over 40 min produced no statistically significant increase in the firing rate (one-way repeated measures ANOVA, P > 0.05; n = 4) (data not shown).
Effect of Quinpirole after Induction of Dopamine-Inhibition Reversal.
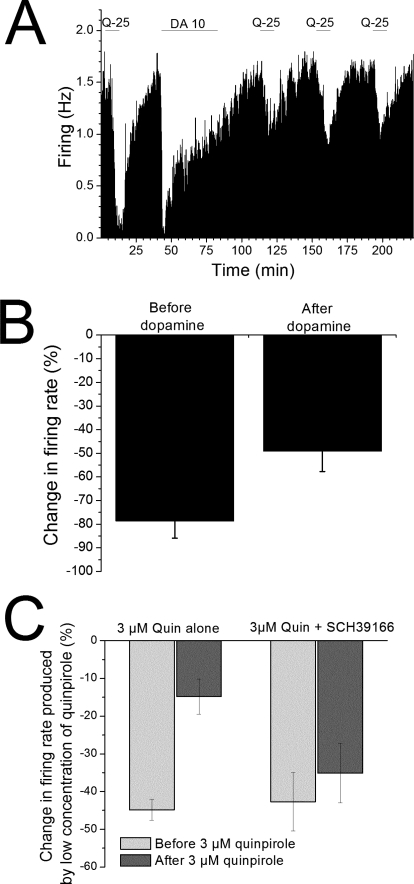
It is clear from the results described above that quinpirole alone does not induce inhibition reversal. We also tested whether there was alteration in sensitivity to D2 receptor stimulation after induction of dopamine-inhibition reversal by dopamine itself. Quinpirole (25 nM) was applied for 10 min to a population of pDA VTA neurons and reduced the spontaneous firing rate (range 46–99%, mean 78.6 ± 7.3%; n = 9). Quinpirole was washed out for at least 30 min, and the firing rate was restored to the prequinpirole baseline. Dopamine (10 μM) then was applied for 40 min, and dopamine-inhibition reversal was induced (Fig. 5A). Dopamine was washed out for 30 min, and then quinpirole was tested again to assess any change in the effect of this D2 agonist after the dopamine treatment. After dopamine-inhibition reversal was induced by dopamine, the effect of short-term administration of quinpirole was significantly reduced compared with the effect of quinpirole before dopamine treatment (Fig. 5B). The mean inhibition produced by quinpirole before dopamine was 78.6 ± 7.3%; however, 30 min after washout of dopamine, the quinpirole-induced inhibition was 49.1 ± 8.6% (t test, P < 0.02; n = 9). This indicates that there is desensitization of D2 receptors after dopamine-inhibition reversal. To compare our studies with those of Bartlett et al. (2005), we tested the effect of low concentrations of quinpirole before and after a 25-min exposure to 3 μM quinpirole. After exposure to this high quinpirole concentration, there was significant desensitization to the inhibitory effect of low quinpirole. We repeated the experiment in the presence of D1/D5 antagonist SCH39166 and found that the D1/D5 antagonist blocked the desensitization induced by high quinpirole exposure (Fig. 5C). It is noteworthy that the lower concentration of quinpirole needed to produce approximately 45% inhibition was almost eight times higher in the presence of SCH39166 (285 ± 77 nM) than in its absence (36 ± 13 nM).
Fig. 5.
Effect of quinpirole after induction of dopamine-inhibition reversal. A, rate-meter graph from a single putative dopaminergic neuron. Vertical bars indicate the firing rate averaged over a 5-s interval; horizontal bars indicate the duration of application of either 25 nM quinpirole (Q-25) or 10 μM dopamine (DA-10). Note that the inhibitory effect of quinpirole after the 40-min dopamine treatment is much less than that observed before the dopamine treatment. Initially, quinpirole produced inhibition of approximately 90%; after dopamine treatment, the quinpirole-induced inhibition (tested at 30-min intervals) was 25, 45, and 44%, respectively. B, effect of quinpirole before and after dopamine in the population of cells tested. Nine cells were tested in a protocol similar to that illustrated in A, and comparison was made between responses to quinpirole before and after dopamine treatment. The mean inhibition produced by quinpirole before dopamine was 78.6 ± 7.3%; 30 min after washout of dopamine, the quinpirole-induced inhibition was 49.1 ± 8.6% (t test, P < 0.02; n = 9). C, effect of quinpirole before and after 3 μM quinpirole. Thirteen cells were tested in a protocol similar to that illustrated in A, with the exception that 3 μM quinpirole was applied for 25 min instead of the 40-min dopamine application. Comparison was made between responses to low concentrations of quinpirole before (light bar) and after (dark bar) 3 μM quinpirole treatment. The mean inhibition produced by low quinpirole (mean concentration = 36 ± 13 nM) before the 3 μM application was 44.8 ± 2.8%; within 1 h of the washout of 3 μM quinpirole, the same concentration of quinpirole induced an inhibition of only 14.8 ± 4.7% (t test, P < 0.005; n = 6). In the presence of 10 μM SCH39166, the mean inhibition produced by low quinpirole (mean concentration = 285 ± 77 nM) before the 3 μM application was 42.7 ± 7.8%; within 1 h of the washout of 3 μM quinpirole, the same concentration of quinpirole induced an inhibition of 35.1 ± 7.9% that was not significantly different from the value before 3 μM quinpirole treatment (t test, P > 0.05; n = 7).
Effect of D1/D5 Agents on Dopamine-Inhibition Reversal.
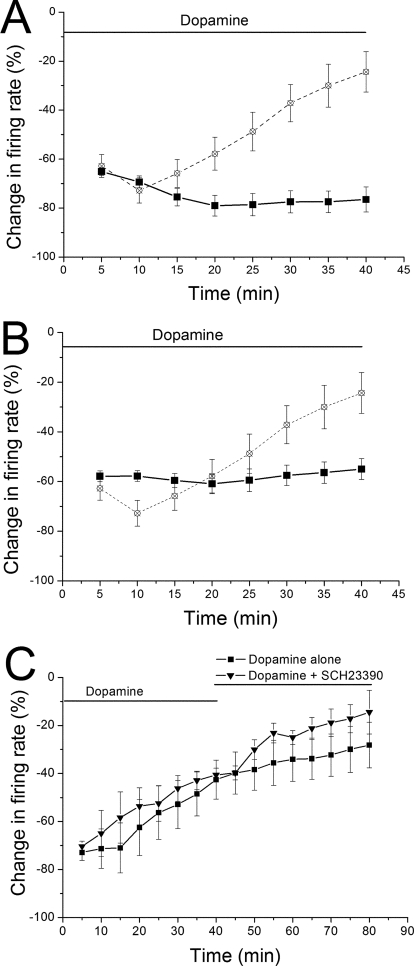
We also tested the involvement of D1/D5 receptors by applying DA in the presence of D1/D5 receptor antagonists. Both SCH39166 (10 μM; n = 10) (Fig. 6A) and SCH23390 (10 μM; n = 8) (Fig. 6B) prevented the characteristic reversal of DA inhibition, supporting the idea that pDAergic stimulation of D1/D5 receptors is necessary for inhibition reversal. With SCH39166, there was a statistically significant decrease in the firing rate with time in DA but no inhibition reversal (Friedman repeated measures ANOVA on a Ranks test, Tukey post-hoc test, P < 0.05 for comparison between 5-min time point and 20–35-min time points). With SCH23390, there was no statistical difference in the effect of DA at the 5, 10, and 40 time points, indicating a blockade of the DA inhibition reversal (Friedman repeated measures ANOVA on a Ranks test, Tukey post-hoc test, P > 0.05 for comparison between time points of 5 and 40 min).
Fig. 6.
Effects of D1/D5 antagonists on dopamine-inhibition reversal. A and B, antagonists applied 10 min before dopamine: a concentration of dopamine sufficient to cause inhibition of 60% or greater was applied for 40 min in the presence of either 10 μM SCH39166 (dopamine concentration = 2.4 ± 0.4 μM; n = 10) (A) or 10 μM SCH23390 (dopamine concentration = 3.9 ± 1.4 μM; n = 8) (B). No significant reduction in dopamine-induced inhibition was observed in the presence of either antagonist. On both A and B, the response to a concentration of dopamine that produced 65% inhibition (from Fig. 2) is shown for reference (open symbols and dashed line). C, antagonists applied after dopamine: a concentration of dopamine sufficient to cause inhibition of 60% or greater was applied for 40 min, and then dopamine was continued for an additional 40 min, either alone (n = 6, mean dopamine concentration = 5.1 ± 0.9 μM) or in the presence of 10 μM SCH23390 (n = 4, dopamine concentration = 4.0 ± 1.4 μM). There was a significant reduction in the effect of dopamine over the 80-min period in both groups (two-way ANOVA, F = 7.37, p < 0.001) and a statistically significant difference in the effect of dopamine overall between the cells treated with dopamine alone (n = 6) or with dopamine plus SCH23390 (n = 4) (two-way ANOVA, p < 0.02). Note that there was no antagonism of the dopamine-inhibition reversal with the addition of SCH23390.
We examined the possibility that a D1/D5 antagonist could antagonize dopamine-inhibition reversal once it occurs. A concentration of dopamine sufficient to cause inhibition of 60% or greater was applied for 40 min, and then dopamine was continued for an additional 40 min, either alone (n = 6, mean dopamine concentration = 5.1 ± 0.9 μM) or in the presence of 10 μM SCH23390 (n = 4, dopamine concentration = 4.0 ± 1.4 μM). There was a significant reduction in the effect of dopamine over the 80-min period in both groups (two-way ANOVA, F = 7.37, p < 0.001). In addition, there was a statistically significant difference in the effect of dopamine overall between the cells treated with dopamine alone (n = 6) or with dopamine plus SCH23390 (n = 4) (two-way ANOVA, p < 0.02), but there was no antagonism of the dopamine-inhibition reversal (Fig. 6C).
Discussion
Our results demonstrate that elevation of DA concentration in the VTA initially reduces the firing rate, as shown by numerous laboratories previously, but sustained inhibition of pDAergic neurons by DA exhibits reversal with time. This reversal is apparently due to D2 receptor desensitization induced by concurrent stimulation of D2 and D1/D5 receptors but not by stimulation of D2 receptors alone. This desensitization is long-lasting, affecting the response to dopamine for up to 90 min. Desensitization of D1 receptors has been demonstrated previously (Ng et al., 1994, 1995). Although D2 desensitization has been observed previously (Barton et al., 1991; Bartlett et al., 2005), this is the first report of a necessity of interaction of D1/D5 receptors and D2 receptors that results in a functional decrease in the effect of D2 receptor stimulation in the VTA. We have termed this phenomenon “dopamine-inhibition reversal,” because it requires stimulation of both D2 and D1/D5 receptors, rather than classic desensitization that requires stimulation of only the primary receptor (i.e., in this case, the D2 receptor).
The results of the present study suggest that reversal of dopamine inhibition depends on a balance between D2 and D1/D5 stimulation. With sufficiently large D2 stimulation [e.g., 3 μM quinpirole (Fig. 5C)], the modest stimulation of D1/D5 receptors by ambient concentrations of dopamine may be sufficient to produce desensitization of D2 receptors. The need for D1/D5 stimulation is shown by the blockade of this desensitization by a D1/D5 antagonist (Fig. 5C). In the cases of more modest D2 receptor stimulation (40–100 nM quinpirole), stimulation of D1/D5 receptors by ambient dopamine in the brain slice is not sufficient to produce inhibition reversal (Fig. 4). Some reversal of the inhibition was observed when these lower concentrations of quinpirole were used in the presence of the D1/D5 agonist (Fig. 4). More detailed concentration-response studies using different D2 and D1/D5 agonists are needed to determine the specific ratios of D2 and D1/D5 stimulation that result in inhibition reversal and long-lasting desensitization of the D2 receptor.
Because the excitatory effect produced by the D1/D5 agonist SKF38393 was small in comparison to the difference between maximal DA inhibition and the inhibition after 40 min of DA exposure, a synergistic action of DA at D2 and D1/D5 receptors is possible. However, our observation that the inhibitory effect of quinpirole is reduced after 40 min of dopamine exposure (Fig. 5, A and B) indicates that there is a long-term change in D2 receptor sensitivity. This type of interaction has been observed in other preparations. The release of atrial natruretic factor is inhibited by DA at lower concentrations but the inhibition of release is reversed at higher concentrations. The concentration-dependent reversal of inhibition of atrial natruretic factor release at higher DA concentrations in that study appeared to be due to activation of adenylate cyclase and protein kinase A, possibly as a result of stimulation of D1/D5 receptors (Lee et al., 2000). Future studies will examine possible G protein interactions that might be involved in the mechanism of the reversal of DA inhibition in the VTA.
Desensitization of D2 receptors has been observed in some expression systems (Namkung and Sibley, 2004; Cho et al., 2006). Desensitization has been well studied in other G protein-coupled receptors, such as the β-adrenergic receptor. The mechanism is generally related to phosphorylation of the G protein-coupled receptors under conditions of agonist occupancy, which results in decoupling of the receptor and the G protein, decreasing the function of the receptors (Namkung and Sibley, 2004). A similar effect was observed in a study by use of DA VTA neurons in a brain slice preparation (Bartlett et al., 2005). In that study, 3 μM quinpirole was observed to induce desensitization of DA VTA neurons to subsequent quinpirole administration, an effect dependent upon G protein-coupled receptor-associated sorting protein. In the present study, quinpirole alone (<200 nM) did not produce desensitization but required concomitant stimulation of D1/D5 receptors (Fig. 4). The higher concentration of quinpirole by described by Bartlett et al. (2005) may partially account for this difference. Endogenous dopamine may provide sufficient D1/D5 stimulation in combination with very large quinpirole concentrations to produce desensitization because even 3 μM quinpirole in our study failed to produced significant desensitization in the presence of the D1/D5 antagonist SCH39166 (Fig. 5C). It is unclear why, in the presence of SCH39166, higher concentrations of quinpirole were needed to produce a similar level of inhibition, because we did not need higher DA in the presence of D1/D5 antagonists (Fig. 6). Additional studies will be necessary to determine whether G protein-coupled receptor-associated sorting protein, or other cell factors, participates in the interaction between D2 sensitivity and D1/D5 receptor activation.
In the present study, in addition to D2 receptor occupancy, D1/D5 receptor stimulation was needed to reduce the inhibitory action of the D2 agonist. Once produced, the desensitization was persistent for a long time after removal of inhibitory concentrations of dopamine. In addition, once the inhibition reversal was achieved, addition of a D1/D5 antagonist was ineffective in antagonizing the desensitization. These observations suggest that a long-duration event such as phosphorylation results from concurrent D2 and D1/D5 stimulation; stimulation of D1/D5 receptors is apparently necessary for induction but not maintenance of this long-duration event. Candidates for such possible phosphorylation are the receptor itself, proteins of the postsynaptic density (Carlsson and Carlsson, 2008), or dopamine-receptor interacting proteins (Kabbani and Levenson, 2007). The precise mechanism and the need for D1/D5 stimulation for induction but not maintenance of this phenomenon will be the subject of future studies.
The regulation of DA autoinhibition shown above could be functionally important to neurons of the VTA under conditions in which persistent and prolonged release of DA might otherwise produce sustained inhibition of DA VTA firing. Under those conditions, DA could begin to stimulate the D1/D5 receptors, resulting in desensitization not apparent with D2 stimulation alone and could serve to overcome the persistent inhibition of DA VTA neurons. Although it seems to require concentrations of DA that cause significant inhibition of firing (> 50%), it does not require high DA concentrations that cause complete cessation of firing. Furthermore, this desensitization may occur more robustly if there is a gradual increase in DA concentration (see Fig. 1).
Adaptation of DA VTA neurons to administration of cocaine has been observed after repeated cocaine administration (Chen et al., 2008). Cocaine-induced long-term potentiation has been observed in brain slices, suggesting a local mechanism of LTP induction (Argilli et al., 2008). Cocaine increases the availability of extracellular DA in the VTA (Brodie and Dunwiddie, 1990). Sustained DA in the VTA (possibly caused by cocaine administration) may increase excitability in the VTA by reducing D2 receptor-mediated autoinhibition. This increase in excitability may increase the effect of spontaneously released glutamate as an increased response to α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate and N-methyl-d-aspartate receptor stimulation, ultimately leading to a form of LTP. Additional studies will be needed to determine interactions between glutamatergic and dopaminergic systems under the conditions necessary to produce dopamine-inhibition reversal.
Numerous drugs of abuse increase DA neurotransmission from the VTA (Di Chiara et al., 2004) in a sustained manner, and sustained increase in DA concentration in the VTA neurons may be factor common to all drugs of abuse. Extended increases in dopamine levels in the VTA could lead to D2 desensitization, which would alter information processing and autoregulation in this important brain area. It has been demonstrated, for example, that intermittent morphine produces a long-lasting desensitization of D2 receptors in nucleus accumbens (Nestby et al., 1995). Although the focus of these studies is often on the target regions of the nucleus accumbens and the prefrontal cortex, given that the VTA is the source of pDAergic innervation to these regions as well as other parts of the extended amygdala, the importance of adaptive changes in neurotransmission in the VTA may be a common feature of drug abuse disorders.
One unique aspect of the present study is the duration of the continuous recordings, which demonstrated the time dependence and concentration dependence of dopamine-inhibition reversal. Although there is a vast literature on the physiology and pharmacology of dopamine systems, previous electrophysiological studies generally examined shorter time courses of responses. For example, in a study of the effects of cocaine on VTA neurons in vivo, Einhorn et al. (1988) observed the effects of systemically administered dopaminergic agents and cocaine over periods lasting from 10 to 20 min and did not report inhibition reversal. Those shorter duration recordings mostly tested doses of agents producing less than 50% inhibition. We found that longer duration application and concentrations reducing the firing rate by 50% or more are needed to observe dopamine-inhibition reversal.
There are other pathological states that are associated with an increase in dopamine in the central nervous system. For example, the DA hypothesis of schizophrenia posits that increased DA levels are an important facet of this illness (Howes and Kapur, 2009). The increased DA in this pathological state may result in reduced autoregulation of dopaminergic neurotransmission. Knowledge of the mechanisms of D2 desensitization may lead to a better understanding of the pathology and treatment of schizophrenia.
Acknowledgments
We thank Maureen McElvain for excellent technical assistance and Chayant Tantipathananandh for custom software development and support.
This study was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Public Health Service Grants AA09125, AA05846].
Some of this work was presented previously: Nimitvilai S and Brodie MS (2008) Biphasic effects of dopamine on the firing rate of dopaminergic ventral tegmental area neurons: involvement of h-current; 2009 Nov 15–19; 2008 Neuroscience Meeting Planner. Program 727.4, Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.163931.
- pDAergic
- putative dopaminergic
- DA
- dopamine
- VTA
- ventral tegmental area
- LTP
- long term potentiation
- SKF38393
- (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide
- SCH39166
- (6aS-trans)-11-chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-5H-benzo[d]naphth[2,1-b]azepin-12-ol hydrobromide
- SCH23390
- (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- ANOVA
- analysis of variance
- aCSF
- artificial cerebrospinal fluid.
References
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. (2008) Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci 28:9092–9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M, Mailliard WS, Armstrong R, Bonci A, et al. (2005) Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci U S A 102:11521–11526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton AC, Black LE, Sibley DR. (1991) Agonist-induced desensitization of D2 dopamine receptors in human Y-79 retinoblastoma cells. Mol Pharmacol 39:650–658 [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. (1987) Cholecystokinin potentiates dopamine inhibition of mesencephalic dopamine neurons in vitro. Brain Res 425:106–113 [DOI] [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. (1990) Cocaine effects in the ventral tegmental area: Evidence for an indirect dopaminergic mechanism of action. Naunyn Schmiedebergs Arch Pharmacol 342:660–665 [DOI] [PubMed] [Google Scholar]
- Brodie MS, McElvain MA, Bunney EB, Appel SB. (1999a) Pharmacological reduction of small conductance calcium-activated potassium current (SK) potentiates the excitatory effect of ethanol on ventral tegmental area dopamine neurons. J Pharmacol Exp Ther 290:325–333 [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. (1999b) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res 23:1848–1852 [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. (1973) Dopaminergic neurons: effects of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther 185:560–571 [PubMed] [Google Scholar]
- Caillé I, Dumartin B, Bloch B. (1996) Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res 730:17–31 [DOI] [PubMed] [Google Scholar]
- Carlsson A, Carlsson ML. (2008) Adaptive properties and heterogeneity of dopamine D(2) receptors - pharmacological implications. Brain Res Rev 58:374–378 [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. (2008) Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 59:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Beom S, Van Tol HH, Caron MG, Kim KM. (2006) Characterization of the desensitization properties of five dopamine receptor subtypes and alternatively spliced variants of dopamine D2 and D4 receptors. Biochem Biophys Res Commun 350:634–640 [DOI] [PubMed] [Google Scholar]
- Choi WS, Machida CA, Ronnekleiv OK. (1995) Distribution of dopamine D1, D2, and D5 receptor mRNAs in the monkey brain: ribonuclease protection assay analysis. Brain Res Mol Brain Res 31:86–94 [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. (2000) Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse 37:125–145 [DOI] [PubMed] [Google Scholar]
- Cragg S, Rice ME, Greenfield SA. (1997) Heterogeneity of electrically evoked dopamine release and reuptake in substantia nigra, ventral tegmental area, and striatum. J Neurophysiol 77:863–873 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. (2004) Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47 (Suppl 1):227–241 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic dopamine system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. (1988) Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci 8:100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Wolf ME. (2008) Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J Neurochem 106:2489–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. (1984) The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4:2866–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. (2009) The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull 35:549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. (1986) Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther 239:219–228 [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. (1986) Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol 132:337–338 [DOI] [PubMed] [Google Scholar]
- Kabbani N, Levenson R. (2007) A proteomic approach to receptor signaling: molecular mechanisms and therapeutic implications derived from discovery of the dopamine D2 receptor signalplex. Eur J Pharmacol 572:83–93 [DOI] [PubMed] [Google Scholar]
- Kenakin TP. (1987) Analysis of dose-response data, in Pharmacologic Analysis of Drug-Receptor Interaction, pp 129–162, Raven Press, New York: [Google Scholar]
- Khan ZU, Gutiérrez A, Martín R, Peñafiel A, Rivera A, de la Calle A. (2000) Dopamine D5 receptors of rat and human brain. Neuroscience 100:689–699 [DOI] [PubMed] [Google Scholar]
- Kim KM, Nakajima Y, Nakajima S. (1995) G protein-coupled inward rectifier modulated by dopamine agonists in cultured substantia nigra neurons. Neuroscience 69:1145–1158 [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. (1987) Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol 392:397–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. (1989) Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci 9:1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Huang W, Lim AT. (2000) Dopamine induces a biphasic modulation of hypothalamic ANF neurons: a ligand concentration-dependent effect involving D5 and D2 receptor interaction. Mol Psychiatry 5:39–48 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. (2006) The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577:907–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Grandy DK, Damask SP, Civelli O, Watson SJ., Jr. (1992) Distribution of D5 dopamine receptor mRNA in rat brain. Neurosci Lett 145:209–212 [DOI] [PubMed] [Google Scholar]
- Mueller AL, Brodie MS. (1989) Intracellular recording from putative dopamine-containing neurons in the ventral tegmental area of Tsai in a brain slice preparation. J Neurosci Methods 28:15–22 [DOI] [PubMed] [Google Scholar]
- Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. (2004) Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res 121:60–69 [DOI] [PubMed] [Google Scholar]
- Namkung Y, Sibley DR. (2004) Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J Biol Chem 279:49533–49541 [DOI] [PubMed] [Google Scholar]
- Nestby P, Tjon GH, Visser DT, Drukarch B, Leysen JE, Mulder AH, Schoffelmeer AN. (1995) Intermittent morphine treatment causes long-term desensitization of functional dopamine D2 receptors in rat striatum. Eur J Pharmacol 294:771–777 [DOI] [PubMed] [Google Scholar]
- Ng GY, Mouillac B, George SR, Caron M, Dennis M, Bouvier M, O'Dowd BF. (1994) Desensitization, phosphorylation and palmitoylation of the human dopamine D1 receptor. Eur J Pharmacol 267:7–19 [DOI] [PubMed] [Google Scholar]
- Ng GY, Trogadis J, Stevens J, Bouvier M, O'Dowd BF, George SR. (1995) Agonist-induced desensitization of dopamine D1 receptor-stimulated adenylyl cyclase activity is temporally and biochemically separated from D1 receptor internalization. Proc Natl Acad Sci USA 92:10157–10161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock RD, Woodruff GN, Turnbull MJ. (1979) Sulpiride blocks the action of dopamine in the rat substantia nigra. Eur J Pharmacol 56:413–414 [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. (1997) Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol 77:853–862 [DOI] [PubMed] [Google Scholar]
- Schilström B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, et al. (2006) Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci 26:8549–8558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. (1994) Dopamine receptor pharmacology. Trends Pharmacol Sci 15:264–270 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. (1994) Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci 14:88–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. (2008) Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci 28:4216–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243–251 [DOI] [PubMed] [Google Scholar]