Abstract
The present study examined the involvement of the GABAA, N-methy-d-aspartate (NMDA), nicotinic acetylcholine, and μ-opioid receptor systems in the transduction of the discriminative stimulus effects of the abused inhalant 1,1,1-trichloroethane (TCE). Sixteen B6SJLF1/J mice were trained to discriminate 10 min of exposure to 12,000-ppm inhaled TCE vapor from air. Substitution and antagonism tests and TCE blood concentration analysis were subsequently conducted. TCE blood concentrations decreased rapidly after cessation of exposure, falling by 66% within 5 min. TCE vapor concentration-dependently substituted for the 12,000-ppm training stimulus. The volatile anesthetic halothane concentration-dependently and fully substituted for TCE. The benzodiazepine midazolam partially substituted for TCE, producing a maximum of 68% TCE-lever selection. The benzodiazepine antagonist flumazenil attenuated midazolam substitution for TCE, but not the discriminative stimulus effects of TCE itself. The noncompetitive NDMA receptor antagonists phencyclidine and dizocilpine failed to substitute for TCE. Nicotine and the central nicotinic receptor antagonist mecamylamine also failed to produce any TCE-lever selection, nor did they antagonize the discriminative stimulus of TCE. The μ-opioid receptor agonist morphine did not substitute for TCE. The opioid antagonist naltrexone failed to antagonize the discriminative stimulus of TCE. Overall, the present results, combined with previous studies, suggest that the discriminative stimulus effects of TCE are mediated primarily by positive GABAA receptor modulatory effects though a mechanism distinct from the benzodiazepine binding site.
Abused inhalants generally have been grouped into a single category based on their method of administration, leading to the misconception that abused inhalants are far more homogeneous in their neurochemical and behavioral effects than is probably warranted (Balster et al., 2009). Unfortunately, the ability to differentiate abused inhalants based on more meaningful criteria, such as abuse-related mechanisms of action, has been elusive. One classification scheme, based on common pharmacological actions, hypothesizes that the volatile hydrocarbons represent a subgroup of abused inhalants (Balster et al., 2009). However, even among volatile hydrocarbons it is likely that individual compounds or classes of compounds may possess distinct neurochemical and behavioral effects. One subgroup of volatile hydrocarbons, chlorinated hydrocarbons, are used as degreasers, spot removers, and dry cleaning fluids. The most extensively studied member of this class, in terms of abuse-related effects, is 1,1,1-trichloroethane (TCE). TCE once served as the drying agent in typewriter correction fluid, and numerous deaths have been associated with TCE abuse (King et al., 1985). The manufacture and use of TCE has been sharply curtailed for environmental reasons, but it still serves as an important research tool given that a number of other chlorinated hydrocarbons like perchloroethylene and trichloroethylene remain widely used and have also been linked to abuse and abuse-related deaths (Michaux and Delevay-Le Gueut, 1980).
Prolonged TCE vapor exposure can produce physical dependence (Evans and Balster, 1993), and acute inhalation produces a biphasic effect on locomotor activity (Bowen and Balster, 1998), locomotor sensitization (Bowen and Balster, 2006), and alterations in rates of operant responding in mice (Bowen and Balster, 1998). These studies indicate that TCE has gross behavioral affects not unlike classic central nervous system-depressant drugs (Bowen and Balster, 1996; Bowen et al., 2006). In vitro assays have shown that chlorinated hydrocarbons interact with a host of neurotransmitter receptor systems. For example, TCE can positively modulate GABAA and glycine receptors expressed in Xenopus oocytes (Beckstead et al., 2000). TCE has also been shown to potently attenuate the function of recombinant NMDA receptors (Cruz et al., 2000). Perchloroethylene inhibits nicotinic acetylcholine receptors expressed in oocytes (Bale et al., 2005) and voltage-sensitive Ca2+ channels in pheochromocytoma cells (Shafer et al., 2005). Chlorinated hydrocarbons have also been shown to have effects that may result from actions at opioid receptors (Nelson and Zenick, 1986; Paez-Martinez et al., 2008). Which, if any, of these receptor systems are responsible for the abuse-related behavioral effects of these compounds is unclear. Given the toxicity of the compounds, human studies are not possible, leaving animals models as the only means of exploring the compounds' abuse-related effects.
One behavioral procedure that may be useful for examining the neurotransmitter systems underlying the behavioral effects of TCE and related compounds is drug discrimination. Drug discrimination is an animal model of the abuse-related intoxicating effects of drugs in humans and has been used extensively to examine other classes of abused drugs. However, few studies have examined the discriminative stimulus effects of inhalants, in any fashion, and only one has used TCE as a training drug (Shelton, 2009). In that experiment, the volatile anesthetic vapors enflurane and sevoflurane produced full substitution in mice trained to discriminate TCE vapor from air. Both of these volatile anesthetics are positive modulators of GABAA receptors in in vitro preparations but also have effects on other neurotransmitter systems (Nishikawa and Harrison, 2003). In mice trained to discriminate diazepam (Bowen et al., 1999), pentobarbital (Rees et al., 1987a), or ethanol (Rees et al., 1987b) from vehicle, TCE produces partial substitution. These results support the hypothesis that the discriminative stimulus effects of TCE result, at least in part, from positive allosteric modulation of GABAA receptors. However, as noted previously, TCE and related chlorinated hydrocarbons have in vitro effects on other neurotransmitter receptor systems as well.
The primary goal of the present study was to examine whether these additional receptors systems, which have been shown to be affected by TCE or related compounds in vitro, are also involved in transducing the discriminative stimulus of TCE. Mice were trained to discriminate 10 min of exposure to a relatively high concentration of 12,000 ppm of TCE vapor from air (Bowen and Balster, 1996, 1998). Subsequently, both substitution and antagonism tests with prototypic reference drugs were conducted to more clearly delineate the in vivo pharmacological effects of TCE.
Materials and Methods
Subjects.
Sixteen adult male B6SJLF1/J mice (The Jackson Laboratory, Bar Harbor, Maine) trained in two groups of eight mice served as subjects for the drug discrimination studies and a portion of the TCE blood concentration analysis. One group of eight mice was used for the studies depicted in Figs. 1, 2, 3, 4, and 5 and Table 1. A second group of eight mice was used for the studies described in Tables 2 and 3. These mice were supplemented with an additional 12 adult male B6SJLF1/J mice used only for TCE blood concentration analysis. The B6SJLF1/J strain has been used extensively in my laboratory for drug discrimination studies with TCE and toluene vapor providing knowledge of appropriate test conditions (Shelton, 2007, 2009; Shelton and Slavova-Hernandez, 2009). The mice were individually housed on a 12-h light/dark cycle (lights on 7:00 AM). Feeding was adjusted to maintain a healthy, stable weight of between 27 and 35 g for the duration of the study. This study was conducted in accordance with the Institute of Laboratory Animal Research Guide for the Care and Use of Laboratory Animals. It was reviewed and approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
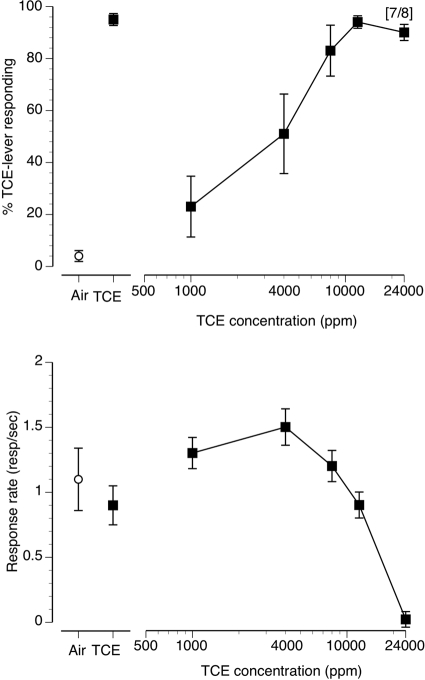
Fig. 1.
Concentration-effect curve for inhaled TCE vapor in mice (n = 8) trained to discriminate 10 min of exposure to 12,000 ppm inhaled TCE from air. The data presented are the first minute of each 5-min test session. Points above Air and TCE represent the results of air (○) and 12,000 ppm inhaled TCE (■) exposure control sessions. Top, mean (± S.E.M.) percentages TCE-lever responding are shown. Bottom, mean (± S.E.M.) response rates in responses/s are shown.
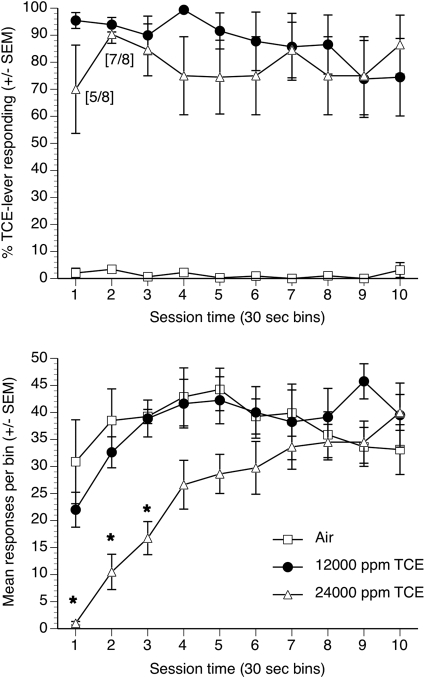
Fig. 2.
Percentages TCE lever selection (top) and operant responses (bottom) after exposure to air, 12,000 ppm TCE, or 24,000 ppm TCE in eight mice. Each point represents mean data (± S.E.M.) from each of 10 successive 30-s bins over the entire 5-min discrimination test session. *, significant suppression of operant responding (p < 0.05) compared with air exposure.
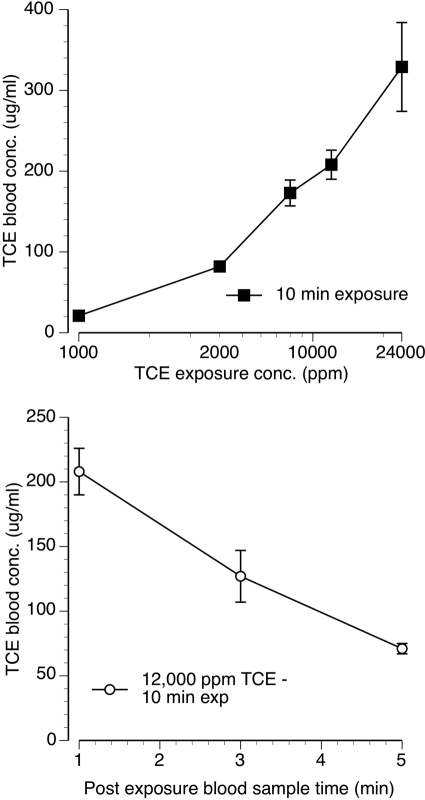
Fig. 3.
Top, mean (± S.E.M.) TCE blood concentrations measured immediately after termination of 10 min of exposure to 4000, 8000, 12,000, and 24,000 ppm TCE vapor. Bottom, mean (± S.E.M.) TCE blood concentrations measured at 1, 3, and 5 min after the termination of 10 min of exposure to 12,000 ppm TCE vapor. Each point represent data from three to four mice.
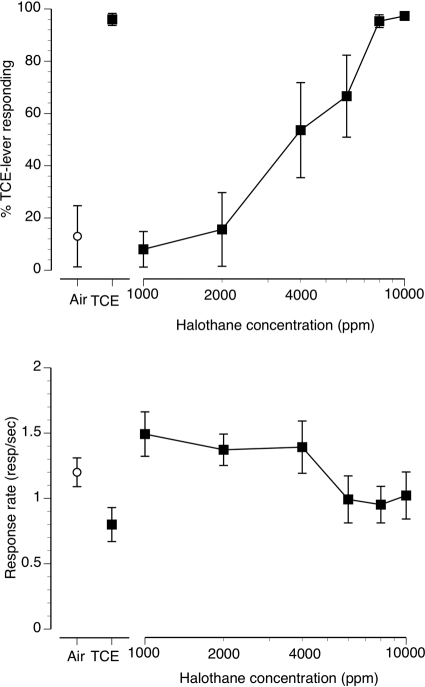
Fig. 4.
Concentration-effect curve for inhaled halothane vapor in mice (n = 8) trained to discriminate 10 min of exposure to 12,000 ppm inhaled TCE from air. The data presented are the first minute of each 5-min test session. Points above Air and TCE represent the results of air (○) and 12,000 ppm inhaled TCE (■) exposure control sessions. Top, mean (± S.E.M.) percentages of TCE-lever responding resulting from halothane exposure are shown. Bottom, mean (± S.E.M.) response rates in responses/s are shown.
Fig. 5.
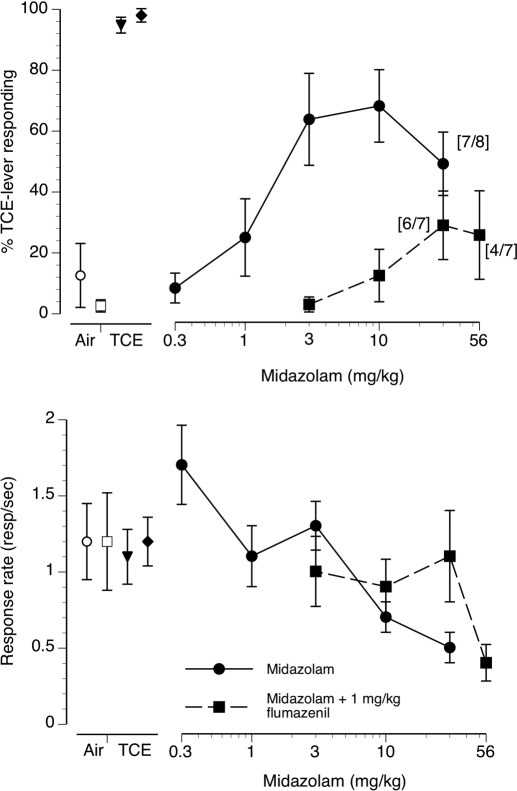
Concentration-effect curve for intraperitoneally injected midazolam alone (●) and midazolam preceded by pretreatment with 1 mg/kg flumazenil (■) in mice (n = 8) trained to discriminate 10 min of exposure to 12,000 ppm inhaled TCE from air. The data presented are the first minute of each 5-min test session. Points above Air and TCE represent the results of air (open symbols) and 12,000 ppm inhaled TCE (filled symbols) exposure control sessions. Top, mean (± S.E.M.) percentages TCE-lever responding resulting from midazolam and midazolam + flumazenil exposure are shown. Bottom, mean (± S.E.M.) response rates in responses/s are shown.
TABLE 1.
TCE lever selection and operant response rates produced by nicotine, mecamylamine, morphine, dizocipline (MK-801), and (PCP)
Data represent the first minute of each 5-min test session. Numerals in brackets indicate the number of animals in the group that emitted sufficient responses to be included in this determination.
| Drug | Drug Dose or Vapor Concentration | % TCE-Lever Responding (± S.E.M.) | Response Rate in Responses/s (± S.E.M.) |
|---|---|---|---|
| Nicotine | Air control | 1 (0.2) | 1.3 (0.2) |
| 12,000 ppm TCE | 99 (0.1) | 1.2 (0.1) | |
| 0.1 mg/kg | 1 (0.1) | 1.3 (0.2) | |
| 0.3 mg/kg | 13 (12.5) | 1.2 (0.1) | |
| 1 mg/kg | 22 (14.3) | 0.8 (0.1) | |
| 1.7 mg/kg | 1 (0.6) [6/8] | 0.7 (0.2) | |
| 2.5 mg/kg | — | 0.0 (0.0) | |
| Mecamylamine | Air control | 2 (0.2) | 1.7 (0.2) |
| 12,000 ppm TCE | 99 (1.0) | 1.2 (0.1) | |
| 0.3 mg/kg | 1 (0.2) | 1.8 (0.2) | |
| 1 mg/kg | 1 (0.5) | 1.6 (0.2) | |
| 3 mg/kg | 1 (0.7) | 1.2 (0.2) | |
| 5.6 mg/kg | 0 (0) | 1.3 (0.2) | |
| Morphine | Air control | 0 (0) | 1.4 (0.3) |
| 12,000 ppm TCE | 98 (0.3) | 1.4 (0.3) | |
| 1 mg/kg | 0 (0) | 1.2 (0.4) | |
| 1.7 mg/kg | 4 (3.9) | 1.3 (0.3) | |
| 3 mg/kg | 1 (0.7) | 1.2 (0.1) | |
| 10 mg/kg | 3 (2.2) [3/6] | 0.5 (0.2) | |
| Dizocilpine (MK-801) | Air control | 17 (12.5) | 1.3 (0.2) |
| 12,000 ppm TCE | 97 (2.0) | 1.4 (0.2) | |
| 0.03 mg/kg | 9 (7.0) | 1.2 (0.2) | |
| 0.1 mg/kg | 2 (1.2) | 1.4 (0.2) | |
| 0.17 mg/kg | 13 (12.5) | 1.2 (0.3) | |
| 0.3 mg/kg | — | 0.2 (0.1) | |
| PCP | Air control | 1 (0.3) | 1.1 (0.2) |
| 12,000 ppm TCE | 98 (1.0) | 1.2 (0.2) | |
| 0.5 mg/kg | 2 (1.1) | 1.5 (0.3) | |
| 1 mg/kg | 4 (1.8) | 1.3 (0.3) | |
| 2 mg/kg | 14 (11.9) | 1.5 (0.3) | |
| 4 mg/kg | 6 (2.7) | 1.1 (0.2) | |
| 6 mg/kg | 9 (7.1) [6/7] | 0.7 (0.2) | |
| 8 mg/kg | — | 0.4 (0.3) |
TABLE 2.
TCE lever selection and operant response rates: TCE vapor alone and in combination with naltrexone, flumazenil or mecamylamine (n = 8)
Data represent the first minute of each 5-min test session.
| TCE Concentration | Percentage TCE-Lever Responding (±S.E.M.) |
Response Rate in Responses/s (±S.E.M.) |
||||||
|---|---|---|---|---|---|---|---|---|
| TCE Alone | TCE + 10 mg/kg Naltrexone | TCE + 1 mg/kg Flumazenil | TCE + 10 mg/kg Flumazenil | TCE Alone | TCE + 10 mg/kg Naltrexone | TCE + 1 mg/kg Flumazenil | TCE + 10 mg/kg Flumazenil | |
| Air only | 1 (0.2) | 1 (0.4) | 4.7 (2.9) | 1 (0.2) | 1.5 (0.2) | 1.2 (0.1) | 1.1 (0.1) | |
| Air + antagonist | — | 6.9 (5.4) | 2 (0.8) | 1 (0.4) | — | 1.4 (0.2) | 1.4 (0.1) | 1.5 (0.2) |
| 12000 ppm TCE control | 100 (0) | 99 (0.1) | 99 (0.2) | 99 (0.9) | 100 (0) | 1.6 (0.2) | 0.9 (0.2) | 1.4 (0.1) |
| 1000 ppm TCE | 14 (11.3) | 13 (12.4) | 3.5 (2.2) | 2 (1.9) | 1.8 (0.2) | 1.3 (0.2) | 1.4 (0.2) | 1.3 (0.4) |
| 2000 ppm TCE | 37 (15.5) | 1 (0.6) | 51 (18.7) | 19 (14.3)) | 1.6 (0.2) | 1.4 (0.1) | 1.4 (0.1) | 1.5 (0.3) |
| 4000 ppm TCE | 71 (15.6) | 73 (15.6) | 80 (13.3) | 51 (22.2) | 1.8 (0.2) | 1.2 (0.2) | 1.3 (0.2) | 1.3 (0.3) |
| 8000 ppm TCE | 85 (10.0) | 86 (11.4) | 99 (1.1) | 94 (4.3) | 1.6 (0.3) | 1.2 (0.1) | 1.5 (0.2) | 1.4 (0.3) |
| 12000 ppm TCE | 99 (0.2) | 99 (0.1) | 99 (0.4) | 98 (1.1) | 1.5 (0.2) | 1.3 (0.1) | 1.4 (0.1) | 1.1 (0.3) |
TABLE 3.
TCE lever selection and operant response rates: TCE vapor alone and in combination with mecamylamine or nicotine (n = 8)
Data represent the first minute of each 5-min test session.
| TCE Concentration | Percentage TCE-Lever Responding (±S.E.M.) |
Response Rate in Responses/s (±S.E.M.) |
||||||
|---|---|---|---|---|---|---|---|---|
| TCE Alone | TCE + 1 mg/kg Mecamylamine | TCE + 5.6 mg/kg Mecamylamine | TCE + 1 mg/kg Nicotine | TCE Alone | TCE + 1 mg/kg Mecamylamine | TCE + 5.6 mg/kg Mecamylamine | TCE + 1 mg/kg Nicotine | |
| Air | 1 (0.2) | 3 (1.8) | 3 (1.8) | 2 (1.1) | 1.5 (0.2) | 1.3 (0.1) | 1.3 (0.1) | 1.4 (0.2) |
| Air + combination drug | — | 2 (0.7) | 2 (0.7) | 6 (5.5) | — | 1.3 (0.1) | 1.3 (0.1) | 1.1 (0.3) |
| 12,000 ppm TCE control | 100 (0) | 99 (0.9) | 99 (0.9) | 94 (5.5) | 1.6 (0.2) | 1.3 (0.2) | 1.3 (0.2) | 1.0 (0.1) |
| 1000 ppm TCE | 14 (11.3) | 1 (0.4) | 1 (0.4) | 5 (4.1) | 1.8 (0.2) | 1.5 (0.1) | 1.3 (0.2) | 1.4 (0.2) |
| 2000 ppm TCE | 37 (15.5) | 28 (17.1) | 0 (0) | 15 (14.2) | 1.6 (0.2) | 1.5 (0.2) | 1.3 (0.1) | 1.8 (0.1) |
| 4000 ppm TCE | 71 (15.6) | 70 (17.5) | 57 (18.1) | 47 (14.9) | 1.8 (0.2) | 1.2 (0.2) | 1.0 (0.1) | 1.6 (0.1) |
| 8000 ppm TCE | 85 (10.0) | 99 (0.7) | 97 (1.9) | 97 (3.3) | 1.6 (0.3) | 1.3 (0.2) | 1.0 (0.1) | 1.5 (0.1) |
| 12,000 ppm TCE | 99 (0.2) | 99 (1.1) | 99 (0.8) | 98 (1.5) | 1.5 (0.2) | 1.2 (0.1) | 0.9 (0.1) | 1.1 (0.1) |
Compounds.
TCE (99.5% anhydrous), nicotine bitartrate, flumazenil, naltrexone hydrochloride, dizocilpine maleate (MK-801), and mecamylamine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Midazolam maleate was a gift of Roche Pharmaceuticals (Nutley, NJ). Morphine sulfate and phencyclidine hydrochloride (PCP) were obtained from the National Institute on Drug Abuse (Bethesda, MD). Halothane was purchased from Webster Veterinary Supply (Charlotte, NC). Injected doses of mecamylamine, morphine, dizocipline, naltrexone, and PCP were based on the salt weight and dissolved in 0.9% physiological saline. Flumazenil was prepared in a 45% β-cyclodextrin/sterile water vehicle. Nicotine doses were calculated by using the base weight, dissolved in 0.9% saline, and the pH was adjusted to between 6 and 7 with 0.1 N NaOH. All injected drugs were administered in a volume of 10 ml/kg. Midazolam, dizocilpine, PCP, naltrexone, mecamylamine, and flumazenil were administered intraperitoneally. Nicotine was administered subcutaneously. All drugs were prepared on a biweekly basis and stored refrigerated between uses at 3°C.
Apparatus.
Drug discrimination sessions were conducted in standard two-lever mouse operant conditioning chambers equipped with 0.01-ml liquid dippers and housed in sound-attenuating cubicles (model ENV-307AW; MED Associates, St. Albans, VT). A single 5-W incandescent house light was located at the top center of the chamber rear wall. Drug discrimination schedule conditions and data recording were controlled by a MED Associates interface and MED-PC version 4 software (MED Associates). The milk solution reinforcer consisted of 25% sugar, 25% nonfat powdered milk, and 50% tap water (by volume).
The static vapor chambers and general procedures used to expose the mice to TCE vapor before drug discrimination testing have been described previously (Shelton, 2007). Closed-loop recirculation of chamber atmosphere through a Miran 1A single-wavelength infrared spectrophotometer (Foxboro Co., Sharon, MA) indicated that TCE vapor concentration in the chambers reached equilibrium in less than 1 min for all tested concentrations (see Shelton, 2007 for more details of this procedure).
Discrimination Training.
Fifteen-minute training sessions were conducted 5 days per week (Monday through Friday). Both lever lights and the house light were illuminated for the duration of the session. The mice were first reinforced for responding on only one of the two levers on a fixed ratio 1 (FR1) response schedule for several daily sessions. Upon completion of the FR requirement the dipper cup was available for 3 s. Responses occurring while the dipper was elevated did not count toward completion of the next FR. Once the animals were reliably responding at FR1 on either lever, the operant session length was decreased to 5 min and discrimination training was initiated. During each 5-min TCE or air-discrimination training session the correct lever was determined by whether the subject received a 10-min exposure to 12,000 ppm TCE vapor or air immediately before the discrimination training session. Training exposures were presented according to a double alternation schedule (i.e., 2 TCE vapor days followed by 2 air days). Over the course of 10 to 20 sessions, the response requirement was increased to FR12. Responding on the inactive lever reset the FR requirement on the correct lever. These training conditions were in effect for the remainder of the study. Animals were determined to have acquired the 12,000 ppm TCE vapor versus air discrimination when the first FR was completed on the correct lever, before the completion of a FR on the incorrect lever, in 8 of 10 consecutive training sessions. In addition, the mice were required to emit more than 80% of responses on the correct lever during all 10 of these sessions.
Substitution and Antagonism Test Procedure.
After acquisition, substitution and antagonism tests were conducted on Tuesday and Friday, providing that the mice continued to exhibit accurate stimulus control on the Monday, Wednesday, and Thursday training sessions. Test sessions were temporarily suspended if an animal did not emit the first FR on the correct lever and produce more than 80% correct-lever responding during all training sessions since the last test session. Test sessions were not resumed until the animal emitted the first FR on the correct lever and produced more than 80% correct-lever responding in three consecutive training sessions. Substitution tests with TCE and halothane vapors were preceded by a 10-min exposure to a single concentration of vapor in the exposure chamber. In substitution test sessions with a single injected drug, the injection was given and the animals were then exposed to air for 10 min in the vapor exposure chamber before being removed and tested for substitution. In the antagonism tests, naltrexone, flumazenil, or mecamylamine was administered 10 min before exposure to 10 min of TCE vapor. In the midazolam + flumazenil combination tests, flumazenil pretreatment was administered immediately before midazolam, which was again followed by a 10-min exposure to air only in the exposure chamber. Naltrexone, flumazenil, and mecamylamine doses were chosen to be well in excess of those reported in the literature to fully antagonize the discriminative stimulus effects of effective training doses of morphine, midazolam, or nicotine, respectively (Shannon and Holtzman, 1976; Sannerud and Ator, 1995; Varvel et al., 1999).
Drug discrimination test sessions were 5 min in duration, and completion of the FR requirement on either lever resulted in dipper presentation. Vapor concentrations and drug doses were generally administered in an ascending order. Before each vapor concentration-effect curve, two control substitution test sessions were conducted, one with the training concentration of 12,000 ppm TCE vapor and a second with air. Control sessions before the dose-effect curves using injected drugs were conducted in a similar manner with the addition of injection of the test drugs vehicle before TCE or air exposure. Control test sessions were also conducted on Tuesday and Friday in place of other substitution tests.
Blood TCE Level Analysis.
Blood TCE level analysis used mice undergoing drug discrimination testing and 12 naive B6JSLF1/J mice. Initial results showed that blood TCE concentrations resulting from exposure to 10 min of 12,000 ppm TCE did not differ between mice undergoing TCE discrimination training/testing and naive mice; therefore, the blood TCE concentration data from the two groups were combined. TCE exposure conditions before blood sampling were identical to drug discrimination exposure conditions, except that rather than being tested in the discrimination procedure, each mouse was briefly restrained and approximately 0.1 ml of blood was obtained from the submandibular vascular bundle by using a 5-mm lancet. Blood droplets were captured in a micro collection tube containing EDTA (Lavender Top Microtainer; Becton Dickinson and Co., Franklin Lakes, NJ). The tube was briefly agitated, and a 20-μl blood sample was then removed and placed into a 20-ml headspace vial to which 980 μl of type 1 ultrapure water had been previously added. The blood sample was then immediately tested for TCE concentration by using a Hewlett Packard (Palo Alto, CA) model 5890A gas chromatograph equipped with a flame ionization detector, 2-m 5% Carbowax 20M 80/120 mesh packed column (Restek, Bellefonte, PA), and CTC Combi-Pal headspace autosampler (LEAP Technologies, Inc., Carrboro, NC). The gas chromatograph parameters were: 5-min sample incubation at 70°C, headspace sample volume 1.25 ml, 7-min sample run time, injector temp 200°C, oven temperature isothermal 90°C, detector temperature 200°C, helium carrier gas flow rate 30 ml/min, flame ionization detector hydrogen flame flow rate 25 ml/min, and flame ionization detector air flow rate 400 ml/min. Data were collected and analyzed by using Clarity chromatography software (Apex Data Systems, Prague, CZ) using a linear regression analysis with no weighting. A seven-point calibration curve preceded the analysis of blood samples, and quality-control TCE standards were interspersed with each set of blood samples. Up to three replicates were analyzed from each animal and averaged if sufficient blood was collected. Each blood concentration data point represents a mean (± S.E.M.) TCE blood concentration (μg/ml) generated from at least three mice.
Drug Discrimination Data Analysis.
Percentage of TCE-lever responding and response rates (responses/s) were recorded in 30-s bins for each of the 5-min discrimination test sessions. Response rates in each 30-s bin for air, the training concentration of 12,000 ppm TCE, and the highest tested concentration of 24,000 ppm TCE were analyzed by two-way analysis of variance (TCE concentration × 30-s bin) and Bonferroni post hoc tests. The results from this analysis showed that the response rate-altering effects of TCE were extremely short-lived. Therefore, to insure that the discrimination and operant rate suppression data represented a time frame during which the maximal behavioral effects TCE were exhibited, with the exception of the time course experiment, only the first min of each TCE vapor discrimination test session was examined. Because of their longer durations of action, the data from the injected drugs were qualitatively similar when the first-minute data were compared with the data from the entire 5-min session (data not shown). However, to maintain consistency across the entire study, only the first-minute data were examined for these compounds as well. Group means (± S.E.M.) were calculated for percentage of first-minute TCE-lever selection and first-minute response rate. Any inhalant concentration or injected drug dose that suppressed response rates to the extent that the animal did not complete at least one FR during the first minute of the test session resulted in the exclusion of that mouse's datum from the group lever-selection analysis, although that animal's datum was included in the response rate determination. A criterion of 80% or more mean TCE vapor-appropriate responding was selected to indicate full substitution for the training concentration of 12,000 ppm TCE vapor. Mean TCE vapor-lever responding between 20 and 79% was defined as partial substitution. Mean TCE vapor-lever responding of less than 20% was considered evidence of no substitution. When possible, EC50 or ED50 values [and 95% confidence limits (CLs)] for TCE vapor-lever selection and response rate suppression were calculated. In brief, mean dose-effect curves were first plotted and visually inspected to determine the linear portion of the curve, with the restriction that it must include at least three drug doses or vapor concentrations. Individual animal data for the points in the linear portion of the mean dose-effect curve were then entered into a Microsoft (Redmond, WA) Excel spreadsheet based on SAS Pharm/PCS version 4 (SAS Institute, Cary, NC) (Tallarida and Murray, 1986) to determine group EC50 or ED50 values. EC50 or ED50 values for individual concentration- or dose-effect curves were considered significantly different from each other when their respective 95% confidence limits did not overlap.
Results
TCE vapor concentration-dependently substituted for the 12,000 ppm TCE vapor training concentration (Fig. 1, top) with an EC50 value of 2894 ppm (CL: 1984–4223 ppm). Concentrations of 8000, 12,000, and 24,000 ppm TCE all resulted in full substitution. The control tests after 10 min of exposure to air and 12,000 ppm TCE produced 4 and 95% TCE-lever responding, respectively. TCE vapor exposure also resulted in concentration-dependent suppression of operant responding during the first minute of the 5-min test session (Fig. 1, bottom) with an EC50 of 21,888 ppm (CL: 14,837–32,289 ppm). Figure 2, top, shows substitution data for entire 5-min test sessions plotted in 30-s bins during a second series of test sessions with the training concentration of 12,000 ppm TCE, air, and the highest tested concentration of 24,000 ppm TCE. Air resulted in almost exclusively air-appropriate responding across all bins. The training concentration of 12,000 ppm TCE vapor and 24,000 ppm TCE vapor produced predominantly TCE-appropriate responding across each 30-s bin. The 24,000 ppm TCE concentration significantly suppressed operant responding in a time-dependent manner (Fig. 2, bottom) compared with air exposure [F(9140) = 5.89, P < 0.001]. Specifically, operant responding was significantly suppressed in the first three 30-s bins (p < 0.05) and progressively recovered over the course of the 5-min test session to levels no different from those of air. In the first 30-s bin at the 24,000 ppm TCE concentration, only five of eight animals emitted any operant responses but by the third 30-s bin all eight mice emitted at least one response.
TCE blood concentration was directly related to exposure concentration and negatively related with postexposure blood sample time (Fig. 3). When assessed within 1 min of removal from the vapor exposure chamber, the training condition of 10 min of exposure to 12,000 ppm TCE vapor resulted in a mean TCE blood concentration of 208 μg/ml (Fig. 3, top). Exposure to 10 min of 1000 ppm TCE, a concentration that produced almost exclusively air-appropriate responding in drug discrimination testing, resulted in a mean TCE blood concentration of 21 μg/ml. The highest tested concentration of 24,000 ppm TCE produced a maximal mean blood concentration of 329 μg/ml. TCE blood concentration after cessation of exposure decreased rapidly (Fig. 3, bottom). Specifically, mean TCE blood concentration measured 5 min after cessation of exposure to 10 min of 12,000 ppm TCE fell by 66% compared with the blood concentration assessed immediately after removal from the exposure chamber.
The volatile anesthetic vapor halothane produced concentration-dependent increases in TCE-lever selection. Concentrations of 8000 and 10,000 ppm fully substituted for the 12,000 ppm TCE training condition (Fig. 4, top). The EC50 for halothane substitution for TCE was 3839 ppm (CL: 2966–4960 ppm). Concentrations of halothane up to 10,000 ppm failed to alter operant response rates sufficiently to generate an EC50 for response-rate suppression (Fig. 4, bottom).
Table 1 shows the percentage of TCE-lever selection and response rates after administration of nicotine (n = 8), mecamylamine (n = 8), morphine (n = 6), dizocilpine (n = 8), and PCP (n = 7). Nicotine produced a maximum of 22% TCE-lever responding at the 1 mg/kg dose. This reflected data from two of eight animals emitting 75% and 97% of their first minute responding on the TCE-appropriate lever, respectively. The remaining six mice emitted exclusively air-appropriate responding at the 1 mg/kg nicotine dose. Nicotine dose-dependently suppressed operant responding with an ED50 of 1.5 mg/kg (CL: 0.8–2.8 mg/kg). Mecamylamine, up to a dose of 5.6 mg/kg, only produced modest reductions in response rates and no TCE-appropriate responding. Morphine doses of 1–10 mg/kg failed to produce any TCE-like discriminative stimulus effects but did dose-dependently suppress operant responding with an ED50 of 8.7 mg/kg (CL: 3.9–19.1 mg/kg). Neither dizocilpine nor PCP substituted for TCE, although both drugs dose-dependently suppressed operant responding with ED50s of 0.23 mg/kg (CL: 0.19–0.27) and 7.3 mg/kg (CL: 5.4–10.1), respectively.
Table 2 shows the percentage substitution generated by a concentration-effect curve of TCE alone in a second group of eight mice. TCE concentration-dependently and fully substituted for itself with an EC50 of 3260 ppm (CL: 2533–4195 ppm). This EC50 value was not significantly different from the EC50 of 2894 ppm (CL: 1984–4223 ppm) generated in the first group of TCE-trained mice. Also shown in Table 2 is TCE in combination with pretreatments of 10 mg/kg naltrexone, 1 mg/kg flumazenil, and 10 mg/kg flumazenil. Pretreatment with 10 mg/kg naltrexone failed to significantly alter the TCE concentration-effect curve, producing a TCE substitution EC50 of 3351 ppm (CL: 2601–4316 ppm). Likewise, neither 1 nor 10 mg/kg flumazenil had a significant effect on the TCE concentration-effect function, resulting in TCE substitution EC50s of 2357 ppm (CL: 1823–3048 ppm) and 3424 ppm (CL: 2604–4504 ppm), respectively.
For comparison purposes, Table 3 shows the same substitution curve for TCE alone that was depicted in Table 2. Also shown are concentration-effect curves for TCE in combination with pretreatments of 1 and 5.6 mg/kg mecamylamine and TCE in combination with pretreatment with 1 mg/kg nicotine. Pretreatment with 1 and 5.6 mg/kg mecamylamine did not significantly alter the TCE concentration-effect curve, producing TCE substitution EC50s of 2869 ppm (CL: 2237–3680 ppm) and 3558 ppm (CL: 2885–4388 ppm), respectively. A dose of 1 mg/kg nicotine also failed to alter the TCE concentration-effect curve, the combination of the two resulting in an EC50 of 3474 ppm (CL: 2694–4479 ppm).
Midazolam produced dose-dependent partial substitution for TCE vapor (Fig. 5, top, ●) with a maximum of 68% TCE-lever selection at the 10 mg/kg dose. The ED50 for midazolam substitution for TCE was 3.5 mg/kg (CL: 1.6–7.6 mg/kg). Preinjection of 1 mg/kg i.p. flumazenil before midazolam administration shifted the midazolam dose-effect curve downward and to the right, almost completely antagonizing the ability of midazolam doses up to 56 mg/kg to substitute for TCE (Fig. 5, top, ■). Midazolam dose-dependently suppressed operant response rates (Fig. 5, bottom, ●) with an ED50 of 26.3 mg/kg (CL: 14.0–49.6 mg/kg). Preinjection with 1 mg/kg flumazenil resulted in an apparent rightward shift in the midazolam response-rate suppression curve (Fig. 5, bottom, ■). An ED50 value for rate suppression could not be calculated because of insufficient suppression of responding at the 56 mg/kg midazolam + 1 mg/kg flumazenil dose combination.
Discussion
We have previously examined the substitution profiles of representative inhalants in animals trained to discriminate TCE from air (Shelton, 2009). My laboratory and others have also assessed cross-substitution of TCE in animals trained to discriminate other drugs from vehicle (Bowen et al., 1999; Shelton and Balster, 2004). The present results expand on these published findings by examining the substitution of the vapor anesthetic halothane and representative members of several pharmacological classes for TCE. The halogenated hydrocarbon vapor anesthetic halothane has been demonstrated to affect a number of ion channel receptors in a manner similar to that reported for TCE (Beckstead et al., 2000; Cruz et al., 2000). Halothane enhances both GABAA and glycine receptors and negatively modulating NMDA receptors in vitro (Perouansky et al., 1998; Westphalen and Hemmings, 2006). Halothane also suppresses nicotine-evoked dopamine release from rat striatal synaptosomes (Salord et al., 1997). In the present study, halothane produced concentration-dependent full substitution for TCE. This result suggests common mechanisms may underlie the discriminative stimulus effects of both compounds. The present halothane data are consistent with results from a previous experiment showing that the halogenated ether vapor anesthetics enflurane and sevoflurane also fully substitute for TCE (Shelton, 2009). Interestingly, a third halogenated ether isoflurane failed to completely substitute for TCE in the prior study, suggesting that there may be some subtle differences in the in vivo pharmacological effects of volatile anesthetics that can be detected by drug discrimination.
TCE itself has not been examined, but nicotinic acetylcholine receptors expressed in oocytes are inhibited by a closely related chlorinated hydrocarbon, percholoroethylene (Bale et al., 2005). Volatile anesthetics also inhibit the function of native (Borghese et al., 2003) and recombinant nicotinic acetylcholine receptors (Zhang et al., 1997), inhibit nicotinic receptor-evoked norepinephrine release (Rowley and Flood, 2008), and reduce agonist affinity at nicotinic receptors (Rada et al., 2003). These results suggest that TCE may be functioning as a nicotinic receptor antagonist. However, in the present experiment, neither the central nicotinic receptor antagonist mecamylamine nor for that matter nicotine itself substituted for TCE (Table 1). In addition, neither nicotine nor mecamylamine pretreatment shifted the TCE concentration-effect curve (Table 3). These data suggest that nicotinic acetylcholine receptors are unlikely to be involved in transducing the discriminative stimulus effects of TCE.
Although the evidence is limited, there are also data to suggest that chlorinated hydrocarbons affect opioid receptors. For instance, acute exposure to 12,000 ppm TCE significantly decreased μ-opioid receptor binding in mice examined 24 h after exposure (Paez-Martinez et al., 2008). Trichloroethylene suppression of sexual behavior in male rats is blocked by naltrexone (Nelson and Zenick, 1986), and trichloroethylene anesthesia reduced the need for postoperative analgesia compared with other inhaled anesthetics (Rice and Reynolds, 1987). The μ-opioid receptor agonist morphine, up to doses that suppressed responses rates by more than 50%, failed to produce TCE-like discriminative stimulus effects (Table 1). Pretreatment with naltrexone, which will not only antagonize the discriminative stimulus effects of morphine (Jarbe and Rollenhagen, 1978) but under certain conditions can alter the discriminative stimulus and reinforcing effects of drugs that indirectly modulate opioid receptors (Solinas and Goldberg, 2005), failed to shift the TCE concentration-effect curve (Table 2). These findings suggest that μ-opioid receptors are not involved in producing the discriminative stimulus effects of TCE but do not rule out the involvement of other opioid receptor subtypes.
TCE prevents NMDA-induced seizures in mice (Cruz et al., 2003) and attenuates NMDA receptor function in oocytes (Cruz et al., 2000). Prior drug discrimination experiments have shown that TCE produces, at best, a low level of partial substitution in mice trained to discriminate the noncompetitive NMDA antagonists PCP (Bowen et al., 1999) or dizocilpine (Shelton and Balster, 2004) from vehicle. The present results are generally consistent with prior data in that neither dizocilpine nor PCP produced any TCE-like discriminative stimulus effects in TCE-trained animals. These findings support the conclusion that the discriminative stimulus effects of TCE are unlikely to be the result of noncompetitive antagonism of NMDA receptors. However, given data showing that competitive and noncompetitive NMDA receptor antagonists do not fully substitute for one another (France et al., 1991), the possibility that one or more of the other sites on the NMDA receptor may be involved in transducing the discriminative stimulus effects of TCE will require additional testing to resolve.
Aside from halothane, the only compound tested in the present study that showed appreciable substitution for TCE was the benzodiazepine-site GABAA receptor-positive modulator, midazolam. Midazolam produced a maximum of 68% group mean TCE-lever responding at a dose of 10 mg/kg (Fig. 4). These data are in agreement with results showing that in diazepam-trained mice 14,000 ppm TCE produced approximately 70% diazepam-lever selection (Bowen et al., 1999). Although the mean level of substitution produced by midazolam was not as great as TCE itself, the group data somewhat underestimate the degree of substitution produced in individual animals. Specifically, at least one dose of midazolam produced full substitution for TCE in seven of the eight mice tested. The maximal substitution exhibited by individual animals generally occurred at the dose just below that which resulted in a marked reduction in that animal's rate of operant responding that varied greatly between mice. This pattern of small, but notable, differences in the optimal substitution dose between subjects has been repeatedly demonstrated in inhalant discrimination experiments. For example, in mice trained to discriminate intraperitoneal toluene from vehicle, both methohexital and oxazepam showed substantial intersubject dose-effect curve variability (Knisely et al., 1990). In mice trained to discriminate ethanol from vehicle, the volatile anesthetics isoflurane and desflurane produced only partial substitution as a group, but when examined in individual subjects, at least one concentration of both compounds fully substituted for ethanol in almost all of the mice tested (Bowen and Balster, 1997). Taken together, these data suggest that partial substitution results in experiments with inhalants may benefit from both group and more detailed individual subject data analysis.
Typical of training drugs in the drug discrimination procedure, TCE vapor concentration-dependently substituted for the 12,000-ppm training concentration. The within-session time course data suggest that the discriminative stimulus effects of both 12,000 and 24,000 ppm TCE appeared to persist for the duration of the 5-min test session (Fig. 2, top), whereas the response-rate suppressing effects of TCE were more short-lived (Fig. 2, bottom). The lack of diminution of TCE-lever selection for the duration of the 5-min test session could be reflective of persistent discriminative stimulus effects. Alternatively, this result could be an artifact of reinforcer presentations during test sessions that would tend to promote continued responding on a single lever once a reinforcer had been obtained. Our blood-level assay found that TCE blood concentrations decreased quite rapidly after the animals were removed from the exposure chamber (Fig. 3, bottom), suggesting that the latter hypothesis may be correct. However, during discrimination training the mice would have also been subject to a descending range of internal TCE concentrations. This could have resulted in a discrimination based not on the discrete stimulus effects of 12,000 ppm TCE vapor, but one more broadly based on the band of internal TCE concentrations experienced by the animals during the 5-min discrimination training session. Although a similar effect probably occurs with other discrimination training drugs and routes of administration, the magnitude of the difference between the stimulus properties at the onset and the end of the discrimination test session is likely more pronounced with inhalants. Additional studies with training sessions shorter than the 5 min used in the present series of experiments would be necessary to conclusively address this question.
In summary, regardless of whether one chooses to characterize the degree of substitution produced by midazolam as full or partial, the present data are consistent with the hypothesis that the discriminative stimulus effects of TCE are at least in part mediated by positive modulation of GABAA receptors. The finding that the benzodiazepine antagonist flumazenil antagonizes the ability of midazolam to substitute for TCE, but not the ability of TCE to substitute for itself, argues against a direct interaction of TCE at the benzodiazepine binding site. This result is consistent with oocyte data showing that recombinant GABAA receptors that do not contain the γ subunit, which confers benzodiazepine sensitivity, are nonetheless positively modulated by TCE (Beckstead et al., 2000). Additional studies in which other site-selective GABAA agonists and positive modulators and ligands preferentially effecting GABAA receptors composed of certain subunit combinations will be necessary to further delineate and refine our understanding of the role of GABAA receptors in the in vivo pharmacological effects of TCE.
This work was supported by the National Institutes of Health [Grant RO1-DA020553].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.158949.
- TCE
- 1,1,1-trichloroethane
- FR
- fixed ratio
- NMDA
- N-methy-d-aspartate
- PCP
- phencyclidine
- MK-801
- (+)-5-methyl-10,11- dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
- CL
- confidence limit.
References
- Bale AS, Meacham CA, Benignus VA, Bushnell PJ, Shafer TJ. (2005) Volatile organic compounds inhibit human and rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Toxicol Appl Pharmacol 205:77–88 [DOI] [PubMed] [Google Scholar]
- Balster RL, Cruz SL, Howard MO, Dell CA, Cottler LB. (2009) Classification of abused inhalants. Addiction 104:878–882 [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. (2000) Glycine and γ-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol 57:1199–1205 [PubMed] [Google Scholar]
- Borghese CM, Henderson LA, Bleck V, Trudell JR, Harris RA. (2003) Sites of excitatory and inhibitory actions of alcohols on neuronal α2β4 nicotinic acetylcholine receptors. J Pharmacol Exp Ther 307:42–52 [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. (1996) Effects of inhaled 1,1,1-trichloroethane on locomotor activity in mice. Neurotoxicol Teratol 18:77–81 [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. (1997) Desflurane, enflurane, isoflurane, and ether produce ethanol-like discriminative stimulus effects in mice. Pharmacol Biochem Behav 57:191–198 [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. (1998) A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Exp Clin Psychopharmacol 6:235–247 [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. (2006) Tolerance and sensitization to inhaled 1,1,1-trichloroethane in mice: results from open-field behavior and a functional observational battery. Psychopharmacology (Berl) 185:405–415 [DOI] [PubMed] [Google Scholar]
- Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. (2006) The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol 28:636–647 [DOI] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Jones HE, Balster RL. (1999) Phencyclidine- and diazepam-like discriminative stimulus effects of inhalants in mice. Exp Clin Psychopharmacol 7:28–37 [DOI] [PubMed] [Google Scholar]
- Cruz SL, Balster RL, Woodward JJ. (2000) Effects of volatile solvents on recombinant N-methyl-d-aspartate receptors expressed in Xenopus oocytes. Br J Pharmacol 131:1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz SL, Gauthereau MY, Camacho-Munoz C, Lopez-Rubalcava C, Balster RL. (2003) Effects of inhaled toluene and 1,1,1-trichloroethane on seizures and death produced by N-methyl-d-aspartic acid in mice. Behav Brain Res 140:195–202 [DOI] [PubMed] [Google Scholar]
- Evans EB, Balster RL. (1993) Inhaled 1,1,1-trichloroethane-produced physical dependence in mice: effects of drugs and vapors on withdrawal. J Pharmacol Exp Ther 264:726–733 [PubMed] [Google Scholar]
- France CP, Moerschbaecher JM, Woods JH. (1991) MK-801 and related compounds in monkeys: discriminative stimulus effects and effects on a conditional discrimination. J Pharmacol Exp Ther 257:727–734 [PubMed] [Google Scholar]
- Jarbe TU, Rollenhagen C. (1978) Morphine as a discriminative cue in gerbils: drug generalization and antagonism. Psychopharmacology (Berl) 58:271–275 [DOI] [PubMed] [Google Scholar]
- King GS, Smialek JE, Troutman WG. (1985) Sudden death in adolescents resulting from the inhalation of typewriter correction fluid. J Am Med Assoc 253:1604–1606 [PubMed] [Google Scholar]
- Knisely JS, Rees DC, Balster RL. (1990) Discriminative stimulus properties of toluene in the rat. Neurotoxicol Teratol 12:129–133 [DOI] [PubMed] [Google Scholar]
- Michaux P, Delevay-Le Gueut M. (1980) Three cases of death in trichloroethylene addicts. Acta Med Leg Soc (Liege) 30:89–94 [PubMed] [Google Scholar]
- Nelson JL, Zenick H. (1986) The effect of trichloroethylene on male sexual behavior: possible opioid role. Neurobehav Toxicol Teratol 8:441–445 [PubMed] [Google Scholar]
- Nishikawa K, Harrison NL. (2003) The actions of sevoflurane and desflurane on the γ-aminobutyric acid receptor type A: effects of TM2 mutations in the α and β subunits. Anesthesiology 99:678–684 [DOI] [PubMed] [Google Scholar]
- Paez-Martinez N, Ambrosio E, Garcia-Lecumberri C, Rocha L, Montoya GL, Cruz SL. (2008) Toluene and TCE decrease binding to μ-opioid receptors, but not to benzodiazepine and NMDA receptors in mouse brain. Ann NY Acad Sci 1139:390–401 [DOI] [PubMed] [Google Scholar]
- Perouansky M, Kirson ED, Yaari Y. (1998) Mechanism of action of volatile anesthetics: effects of halothane on glutamate receptors in vitro. Toxicol Lett 100/101:65–69 [DOI] [PubMed] [Google Scholar]
- Rada EM, Tharakan EC, Flood P. (2003) Volatile anesthetics reduce agonist affinity at nicotinic acetylcholine receptors in the brain. Anesth Analg 96:108–111 [DOI] [PubMed] [Google Scholar]
- Rees DC, Knisely JS, Balster RL, Jordan S, Breen TJ. (1987a) Pentobarbital-like discriminative stimulus properties of halothane, 1,1,1-trichloroethane, isoamyl nitrite, flurothyl, and oxazepam in mice. J Pharmacol Exp Ther 241:507–515 [PubMed] [Google Scholar]
- Rees DC, Knisely JS, Breen TJ, Balster RL. (1987b) Toluene, halothane, 1,1,1-trichloroethane, and oxazepam produce ethanol-like discriminative stimulus effects in mice. J Pharmacol Exp Ther 243:931–937 [PubMed] [Google Scholar]
- Rice AS, Reynolds F. (1987) Comparison of trichloroethylene and enflurane as adjuncts to nitrous oxide and relaxant anesthesia. Anesthesia 42:1320–1323 [DOI] [PubMed] [Google Scholar]
- Rowley TJ, Flood P. (2008) Isoflurane prevents nicotine-evoked norepinephrine release from the mouse spinal cord at low clinical concentrations. Anesth Analg 107:885–889 [DOI] [PubMed] [Google Scholar]
- Salord F, Keita H, Lecharny JB, Henzel D, Desmonts JM, Mantz J. (1997) Halothane and isoflurane differentially affect the regulation of dopamine and γ-aminobutyric acid release mediated by presynaptic acetylcholine receptors in the rat striatum. Anesthesiology 86:632–641 [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Ator NA. (1995) Drug discrimination analysis of midazolam under a three-lever procedure: I. Dose-dependent differences in generalization and antagonism. J Pharmacol Exp Ther 272:100–111 [PubMed] [Google Scholar]
- Shafer TJ, Bushnell PJ, Benignus VA, Woodward JJ. (2005) Perturbation of voltage-sensitive Ca2+ channel function by volatile organic solvents. J Pharmacol Exp Ther 315:1109–1118 [DOI] [PubMed] [Google Scholar]
- Shannon HE, Holtzman SG. (1976) Blockade of the discriminative effects of morphine in the rat by naltrexone and naloxone. Psychopharmacology (Berl) 50:119–124 [DOI] [PubMed] [Google Scholar]
- Shelton KL. (2007) Inhaled toluene vapor as a discriminative stimulus. Behav Pharmacol 18:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL. (2009) Discriminative stimulus effects of inhaled 1,1,1-trichloroethane in mice: comparison to other hydrocarbon vapors and volatile anesthetics. Psychopharmacology (Berl) 203:431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL, Balster RL. (2004) Effects of abused inhalants and GABA-positive modulators in dizocilpine discriminating inbred mice. Pharmacol Biochem Behav 79:219–228 [DOI] [PubMed] [Google Scholar]
- Shelton KL, Slavova-Hernandez G. (2009) Characterization of an inhaled toluene drug discrimination in mice: effect of exposure conditions and route of administration. Pharmacol Biochem Behav 92:614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. (2005) Involvement of μ-, delta- and κ-opioid receptor subtypes in the discriminative-stimulus effects of delta-9-tetrahydrocannabinol (THC) in rats. Psychopharmacology (Berl) 179:804–812 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. (1986) Manual of Pharmacological Calculations with Computer Programs, Springer-Verlag, New York: [Google Scholar]
- Varvel SA, James JR, Bowen S, Rosecrans JA, Karan LD. (1999) Discriminative stimulus (DS) properties of nicotine in the C57BL/6 mouse. Pharmacol Biochem Behav 63:27–32 [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr (2006) Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine-evoked release. J Pharmacol Exp Ther 316:216–223 [DOI] [PubMed] [Google Scholar]
- Zhang L, Oz M, Stewart RR, Peoples RW, Weight FF. (1997) Volatile general anaesthetic actions on recombinant nACh α7, 5-HT3 and chimeric nACh α 7-5-HT3 receptors expressed in Xenopus oocytes. Br J Pharmacol 120:353–355 [DOI] [PMC free article] [PubMed] [Google Scholar]