Abstract
Evidence suggests that the long-term adaptations in the hippocampus after repeated drug treatment may parallel its role during memory formation. The neuroplasticity that subserves learning and memory is also believed to underlie addictive processes. We have reported previously that repeated morphine administration alters local distribution of endocytic proteins at hippocampal synapses, which could in turn affect expression of glutamate receptors. Glutamatergic systems, including α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs), are believed to be involved in opiate-induced neuronal and behavioral plasticity, although the mechanisms underlying these effects are only beginning to be understood. The present study further examines the effects of repeated morphine administration on the expression and composition of AMPARs and the functional ramifications. Twelve hours after the last morphine injection, we observed an increased expression of AMPARs lacking glutamate receptor (GluR) 2 in hippocampal synaptic fractions. Immunoblotting studies show that 12 h after morphine treatment, GluR1 subunits are increased at the postsynaptic density (PSD) and at extrasynaptic sites, whereas GluR3 subunits are only increased at the PSD, and they show how this alters receptor subunit composition. In addition, we provide electrophysiological evidence that AMPARs are switched to Ca2+-permeable (GluR2-lacking) at the synapse 12 h after repeated morphine treatment, affecting the magnitude of long-term depression at hippocampal neurons. We propose that morphine-induced changes in glutamatergic synaptic transmission in the hippocampus may play an important role in the neuroadaptations induced by repeated morphine administration.
Addictive processes involve neuronal plasticity (Kauer and Malenka, 2007), thus, repeated exposure to drugs of abuse, such as morphine, produces long-term molecular and neurochemical changes that may underlie drug-induced behavioral responses. Considerable advances have been made toward understanding the anatomical substrates involved in these drug-induced neuroadaptations that may drive addictive behaviors in response to repeated morphine administration (Vanderschuren and Kalivas, 2000). Although it is accepted that the reward pathway (i.e., nucleus accumbens, VTA) is involved, recent reports highlight the role of the hippocampus in the regulation of these neuroadaptations that may underlie opiate-induced behavioral modifications (Shen et al., 2006). The hippocampus is one of the most important subcortical brain regions implicated in memory and learning; it is also involved in the rewarding effects of abused drugs (Vorel et al., 2001). Long-lasting drug-induced alterations in the hippocampus have the potential to influence the brain reward pathway and are likely to contribute to the intense memories associated with drug use and the propensity for drug relapse (Weiss, 2005). Therefore, the investigation of hippocampal neuroadaptations induced by repeated morphine treatment has great potential to reveal mechanisms contributing to the development of opiate addiction.
We have reported previously that a paradigm of repeated administration with escalating doses of morphine causes a redistribution of endocytic proteins at hippocampal synapses, which in turn may modulate glutamatergic synaptic transmission and plasticity in the hippocampus by altering clathrin-dependent endocytosis of AMPARs (Morón et al., 2007). AMPARs mediate the majority of rapid excitatory neurotransmission in the mammalian central nervous system, and their regulation directly controls synaptic efficacy (Palmer et al., 2004). Subunit composition is critical in determining the functional and trafficking properties of resulting channels (Malinow and Malenka, 2002). The majority of AMPARs in hippocampal neurons are GluR2-containing (GluR1/GluR2 and GluR2/GluR3) heteromeric complexes, although there is evidence for GluR1 homomers and GluR2-lacking heteromers (Sprengel, 2006). GluR2-containing AMPARs have low Ca2+ permeability and consequently display linear current-voltage (I-V) relationships, whereas GluR2-lacking AMPARs have high Ca2+ permeability and exhibit inwardly rectifying postsynaptic currents (Cull-Candy et al., 2006). Disruption of AMPAR composition causes critical perturbations in subunit assembly and efficiency in trafficking of the receptor to the membrane (Passafaro et al., 2001). Alterations in these cellular processes have a direct effect on synaptic transmission.
In this study we further explore the effects of repeated morphine administration on the expression and subunit composition of AMPARs at hippocampal synapses and how these effects affect AMPAR-mediated synaptic transmission. We use multiple approaches to analyze the changes in AMPARs that were due to morphine treatment, including subcellular fractionation coupled with immunoblotting, and electrophysiology. Biochemical data indicate that there are alterations in both expression and synaptic localization of GluR1 and GluR3 AMPARs subunits 12 h after morphine administration, which consequently leads to changes in AMPAR subunit composition. In addition, we provide electrophysiological evidence that AMPAR subunits are switched to Ca2+ permeable AMPARs (GluR2-lacking) at the synapse resulting in functional changes. These results provide evidence of the synaptic, molecular, and membrane mechanisms of dynamic trafficking of AMPA receptors resulting from repeated morphine administration.
Materials and Methods
Animals and Morphine Treatment.
Experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch, according to the U.S. Department of Agriculture Animal Welfare Act (Public Law 89-544) and the National Institutes of Health's Guide for the Care and Use of the Laboratory animals (Department of Health, Education, & Welfare publication no. 85-23, revised 1985). Four-week old male wild-type C57BL6 mice (15–20 g) (Harlan, Indianapolis, IN) were maintained on a 12-h light/dark cycle and allowed to acclimatize to their environment for 1 week before drug administration. Morphine sulfate (Sigma-Aldrich, St. Louis, MO) was prepared in 0.9% isotonic saline. Mice (n = 6–10 per group) were injected intraperitoneally with saline or morphine; in the latter case, four escalating doses of morphine (5, 8, 10, and 15 mg/kg) every 12 h for 48 h as described previously (Morón et al., 2007) were used. Animals were sacrificed 12 h after the last drug injection. The hippocampi from morphine or saline-treated mice were dissected and stored at −80°C until use.
Subcellular Fractionation.
Subcellular fractionation was performed as described previously (Morón et al., 2007). In brief, hippocampi (3–5 animals/group) were homogenized in 1.5 ml of 0.32 M sucrose, 0.1 mM CaCl2 containing protease and phosphatase inhibitors (Sigma-Aldrich). The homogenate was brought to a final concentration of 1.25 M sucrose by adding 2 M sucrose and 0.1 mM CaCl2. The homogenate was then placed in an ultracentrifuge tube and overlaid with 1 M sucrose and subjected to centrifugation at 100,000g for 3 h at 4°C. The synaptosomal fraction was collected at the 1.25 M/1 M sucrose interface. To obtain synaptic junctions, the synaptosomal fraction was diluted with 20 mM Tris-Cl, pH 6, 0.1 mM CaCl2, containing 1% Triton X-100 (TX-100), mixed for 20 min at 4°C, and centrifuged at 40,000g for 20 min at 4°C. The pellet containing the isolated synaptic junctions was collected. To separate presynaptic proteins from the PSD, the pellet was resuspended in 20 mM Tris-Cl, pH 8, 1% TX-100, and 0.1 mM CaCl2. The mixture was again mixed for 20 min at 4°C, and centrifuged at 40,000g for 20 min at 4°C. The insoluble pellet containing the PSD fraction was collected and stored at −80°C until use.
Immunoblotting.
For immunoblotting, equal amounts of total proteins from fractions obtained from morphine- and saline-treated animals were separated by 7.5% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes by electroblotting. Membranes were then incubated with selective antibodies to GluR1 (1:1000) (C terminus), phosphoGluR1 Ser845 (1:1000), GluR2 (1:1000) (N terminus), or GluR3 (1:500) (N terminus; all from Millipore Bioscience Research Reagents, Temecula, CA). After incubation with appropriate fluorescent secondary antibody (1:10,000–1:20,000) (LI-COR Biosciences, Lincoln, NE), membranes were scanned directly by the Odyssey infrared fluorescent imaging system (LI-COR Biosciences). Blots were reprobed with antibody to tubulin (Sigma-Aldrich) to ensure equal loading and transfer. Band densities were analyzed using Odyssey software. Quantification was performed by measuring the intensity of the band with protein-specific antibodies and comparing with that of tubulin to control for variation in loading and transfer. These values were normalized to saline control values. Differences between groups were determined by one-way ANOVA followed by Tukey test for multiple comparisons among groups.
Quantitative Coimmunoprecipitation.
Synaptosomal membranes from mice hippocampi were prepared as described previously (Morón et al., 2007) with minor modifications. In brief, hippocampi from individual animals were homogenized using a glass homogenizer in ice-cold 10 mM Tris-HCl, pH 7.4, 5 mM NaF, 1 mM EDTA buffer (TE) containing 320 mM sucrose, and protease and phosphatase inhibitors. The homogenate was then centrifuged at 1000g for 10 min at 4°C to pellet cellular debris and nuclei. The supernatant (S1) was collected and then centrifuged at 9200g for 15 min at 4°C. The resulting pellet (P2) containing the crude synaptosomal fraction was then resuspended in TE buffer containing 35.6 mM sucrose and incubated on ice for 30 min. The samples were then centrifuged for 20 min at 25,000g at 4°C. The supernatant (LS1) was removed and the pellet (LP1) containing synaptosomal membranes was then resuspended in TE buffer with 1% Triton X-100. Samples were kept at −80°C until use.
Coimmunoprecipitation was carried out as described by Conrad et al. (2008) with minor modifications. In brief, 4 to 5 μg of antibody (GluR1, GluR2) or an equal amount of control IgG was incubated with 20 μl of 50% protein A agarose slurry (Thermo Fisher Scientific, Waltham, MA) for 4 h at 4°C. The beads bound with antibody were pelleted by centrifugation at 1000g for 30 s and washed three times with 0.1% TBS/Triton X-100. Synaptosomal membranes (100 μl) were then added to the beads and incubated overnight with gentle rocking at 4°C. The samples were then centrifuged at 1000g for 30 s to pellet the agarose-bound antibody. We obtained two fractions, the bound (pellet) and the unbound (supernatant). The unbound fraction was then subjected to a second round of immunoprecipitation. After the final round of immunoprecipitation, the unbound sample was quantified using the BCA assay kit from Pierce. Equal amounts of unbound proteins (12 μg) were run on 7.5% gels, transferred to nitrocellulose membranes and immunoblotted as described above. The percentage of total AMPA receptor remaining in the unbound fraction was calculated based on the standard curve [i.e., 14 μg (100%), 7 μg (50%), and 0.7 μg (5%)] created from control IgG immunoprecipitated sample.
Electrophysiology.
Mice were sacrificed 12 h after receiving their last injection of saline or morphine as described above. Hippocampal slices were prepared by following standard procedures. In brief, brains were removed rapidly and sliced to 400 μm thickness in an ice-cold cutting solution containing 240 mM sucrose, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 0.5 mM CaCl2, and 7 mM MgCl2, and bubbled continuously with 95% O2/5% CO2. After incubation for at least 30 min at room temperature, the slices were transferred and submerged in the recording chamber. Both incubating and recording chambers contained artificial cerebral spinal fluid (ACSF): 119 mM NaCl, 3.0 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 25 mM NaHCO3, and 11.5 mM glucose. The temperature of the recording chamber was kept at 32 ± 1°C.
Synaptic responses were evoked through a concentric bipolar electrode placed in the Schaffer collateral fiber bundles. Extracellular field excitatory postsynaptic potential (fEPSP) measurements were recorded using a glass Ag/AgCl electrode filled with ACSF in the presence of 100 μM picrotoxin (PTX), 2 μM (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl] amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride (CGP55845), and 50 μM d-APV. Stimulation parameters consisted of a square-wave current pulse of 150-μs duration. Stimulus-response curves were performed at the beginning of each experiment, and pulses at an intensity eliciting 50% of a maximum slope were used. In the paired-pulse paradigm, the first conditioning response (fEPSP1) and the second, or test response (fEPSP2), were elicited at intervals of 30 to 140 ms. Peak amplitude of the paired fEPSP responses was measured. The magnitude of facilitation was calculated as [fEPSP2/fEPSP1]. The paired-pulse facilitation (PPF) was plotted as a function of the interstimulus interval for saline- and morphine-treated mice. Long-term potentiation (LTP) was produced by two 1-s long trains of high-frequency stimulation (HFS, 100 Hz at 10-s intertrain interval) after baseline synaptic responses had been stable for at least 10 min. LTD was induced by applying a low-frequency stimulus (LFS, 1 Hz for 15 min) in the presence of 50 μM PTX and 2 μM CGP55845; concentrations of CaCl2 and MgSO4 in ACSF were 4 mM. Whole-cell patch-clamp recordings were obtained using the blind-patch technique. excitatory postsynaptic currents (EPSCs) were recorded in CA1 pyramidal cells while stimulating the Schaffer collateral-commissural afferents. Evoked currents were measured using an Axoclamp 2A amplifier and analyzed with pClamp 9.1 software (Molecular Devices, Sunnyvale, CA). The composition of the internal solution of the patch electrode was 115 mM Cs-MeSO4, 5 mM NaCl, 1 mM EGTA, 0.3 mM CaCl2, 2 mM MgCl2, 5 mM Na-ATP, 0.4 mM Na-GTP, 10 mM HEPES, and 5 mM N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium (QX314). The I-V curve of the AMPAR-mediated EPSCs was obtained by including spermine 0.1 mM in the internal solution. The rectification index (RI) was calculated as the ratio of AMPAR excitatory postsynaptic currents EPSCs at −60 mV to AMPAR EPSC at +40 mV (−60/+40 mV). In the experiments in which we studied involvement of GluR2-lacking (Ca2+-permeable) receptors, slices were perfused with 10 μM philanthotoxin (Phtx) or 500 nM Joro spider toxin (JST) as described previously (Bellone and Lüscher, 2006; Frade et al., 2009).
The AMPAR/NMDAR ratio was measured from the dual-component EPSCs evoked while holding cells at +40 mV. The pure AMPAR-mediated EPSC was isolated by application of 100 μM d-APV and 100 μM PTX. The NMDAR-mediated EPSC was obtained by digital subtraction of the AMPAR EPSC from the dual EPSC. AMPA-evoked whole-cell currents were recorded by bath application of S-AMPA in the presence of 1 μM tetrodotoxin.
Data Analysis.
Results are expressed as the mean ± S.E.M. Statistical comparisons were made using one-way ANOVA with Tukey's test for multiple comparisons, two-way ANOVA, or unpaired t test where appropriate. Significance was set at p < 0.05. Statistical analyses of data were generated by using Prism software (ver. 5.0; GraphPad Software Inc., San Diego, CA).
Results
Morphine Administration Results in Increased Levels and Phosphorylation of GluR2-Lacking Receptors in Synaptic Fractions.
Exposure to drugs of abuse, such as morphine, has been shown to alter the levels and composition of AMPARs at the synapse (Zhong et al., 2006). Regulation of the number of postsynaptic AMPARs at a given synapse is recognized as an integral feature of rapid long-term changes in synaptic strength (Kauer and Malenka, 2007) that may be responsible for the long-term behavioral modifications elicited by drug administration. Using the morphine administration paradigm described above, we specifically examined the effects of morphine on the expression and phosphorylation of AMPAR subunits in synaptic fractions 12 h after morphine treatment to elucidate neuroadaptations in response to morphine. By coupling subcellular fractionation with immunoblotting, we were able to assess changes in both expression and location of the receptors within the synapse, with focus on the PSD (Morón et al., 2007; Billa et al., 2009).
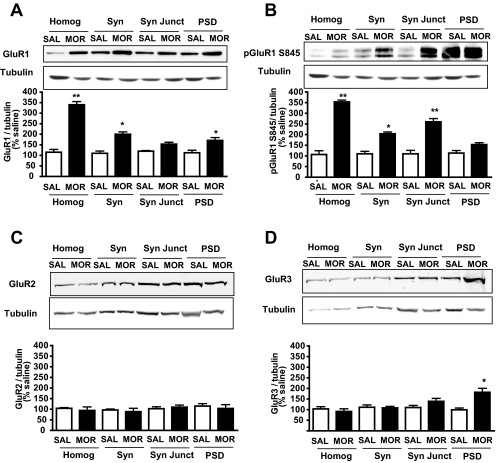
Hippocampi were dissected, and subcellular fractionation was performed as reported previously (Morón et al., 2007; Billa et al., 2009). Quantification of phosphoGluR1, GluR1, GluR2, and GluR3 subunits was performed by Western blot analysis in synaptic fractions from saline- and morphine-treated animals, and results obtained are shown in Fig. 1. Data show that morphine treatment produced a robust increase in the total levels of GluR1 in the hippocampal homogenate and synaptosomes (*, p < 0.05; **, p < 0.01; one-way ANOVA with Tukey's test). In addition, morphine treatment also increased GluR1 levels at the PSD, albeit to a lesser extent (*, p < 0.05; one-way ANOVA with Tukey's test) (see Fig. 1A). Because expression of GluR1 at the cell surface is regulated by phosphorylation (Ehlers, 2000), we next examined the effect of morphine treatment on the phosphorylation of GluR1 at serine 845, a site known to be phosphorylated by protein kinase A (Colledge et al., 2000). Repeated morphine treatment led to a significant increase in GluR1 phosphorylation in the total homogenate, synaptosomes, and synaptic junctions (*, p < 0.05; **, p < 0.01; one-way ANOVA with Tukey's test) (Fig. 1B). Although we also observed an increase in GluR1 phosphorylation at the PSD after morphine treatment, this was not significant. To determine whether the increase in pGluR1 was due to an elevation in total GluR1 levels, we analyzed whether the ratio of pGluR1/total GluR1 was altered. This ratio was calculated using the normalized band intensities for pGluR1 and GluR1, respectively, for each fraction. A significant increase in the ratio of pGluR1/total GluR1 (180 ± 15%) was found at the synaptic junctions (p < 0.01; one-way ANOVA with Tukey's test) relative to saline-treated animals. In addition, these data showed that repeated morphine administration did not alter the expression of GluR2 at hippocampal synapses (Fig. 1C), but levels of GluR3 were significantly increased at the PSD (*, p < 0.05; one-way ANOVA with Tukey's test) (Fig. 1D). These data indicate an increase in GluR2-lacking receptor levels at 12 h upon repeated morphine exposure.
Fig. 1.
Morphine-induced expression changes of AMPA subunits in hippocampal synaptic fractions 12 h after treatment. Fractions representing homogenate (Homog), synaptosomes (Syn), synaptic junctions (Syn Jct), and PSD were subjected to Western blot analyses. Morphine treatment increased levels of GluR1 at the homogenate, the synaptosomal fraction, and the PSD (A). Phosphorylation of GluR1 at Ser845 was also increased at the homogenate, synaptosomes, and synaptic junctions (B). In contrast, GluR2 levels were not altered upon morphine treatment (C). Morphine treatment induced an increase of GluR3 levels at the PSD (D). Blots were standardized with tubulin. Quantification was performed relative to tubulin levels (*, p < 0.05, **, p < 0.01 relative to saline-treated animals, one-way ANOVA with Tukey's multiple comparison test, n = 6). SAL, saline; MOR, morphine.
Morphine Administration Alters AMPAR Subunit Composition.
Given the observation that repeated morphine administration resulted in an increase in GluR1 and GluR3 expression without affecting levels of the GluR2 subunit, we wanted to directly examine whether morphine administration had an effect on AMPAR subunit composition. To this end, we performed quantitative coimmunoprecipitation studies in hippocampal synaptosomal membranes from morphine- and saline-treated mice; results are shown in Fig. 2. Two independent experiments showed that repeated morphine administration decreased both the association between GluR1 and GluR2 and between GluR1 and GluR3; in contrast, morphine increased the association between GluR2 and GluR3: 1) after GluR1 immunoprecipitation (IP), morphine-treated mice showed an increase in unbound GluR2 (24% in saline- versus 42% in morphine-treated mice) and GluR3 (56% in saline- versus 75% in morphine-treated mice), indicating an increase in GluR2 or GluR3 not associated with GluR1; and 2) after GluR2 IP, morphine-treated mice showed a decrease in GluR3 remaining in the unbound fraction (82% in saline- versus 72% in morphine-treated mice) and an increase in unbound GluR1 (26% in saline- versus 43% in morphine-treated mice), indicating an increase in GluR3 associated with GluR2 and a decrease in GluR1 associated with GluR2, respectively. In addition, the increase in the absolute amount of GluR1 in both the synaptosomal fraction and the PSD shown above (see Fig. 1) that does not seem to be associated with GluR2 or GluR3 may indicate an increase in homomeric GluR1.
Fig. 2.
Quantitative coimmunoprecipitation of AMPA subunits in hippocampal synaptosomal membranes 12 h after repeated morphine administration. AMPAR subunit composition was compared in hippocampal synaptosomal membranes from saline- and morphine-treated mice 12 h after discontinuation of morphine treatment. Immunoblots (IB) show the percentage of AMPAR subunits remaining (unbound fraction) after IP of synaptosomal membranes. Data shown represent the average of two independent experiments. The antibodies used for IB are indicated at the left. The left three lanes in each row show immunoblotting of IgG control immunoprecipitated sample. After IP with the respective antibody, the percentage remaining in the unbound fraction was calculated from the standard curve generated by controls of 100 (14 μg), 50 (7 μg), and 5% (0.7 μg) run on each blot. Results obtained indicate that morphine administration decreases the association between GluR1 and GluR2 and GluR3 and increases the association between GluR2 and GluR3.
Repeated Morphine Increases Basal Synaptic Transmission.
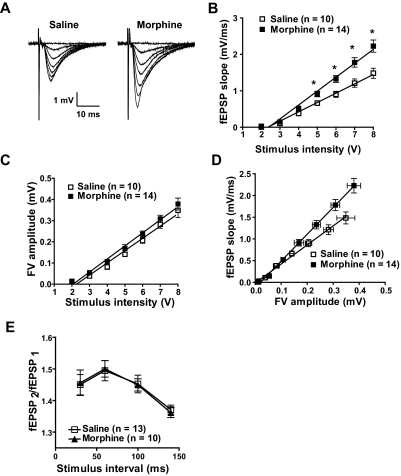
The changes in AMPAR expression and subunit composition shown above have direct consequences on synaptic function. To investigate the functional impact on glutamatergic synaptic transmission within the hippocampus, we used a series of electrophysiological approaches. First, we examined the effects of morphine treatment on glutamatergic transmission at the Schaeffer collateral to CA1 synapse. We performed these experiments in the presence of D-APV (50 μM) to block NMDA receptors. Changes in the slope of the input-output relationship provide a measure of efficacy of information transfer and synaptic gain. Input-output relationships were measured by varying stimulus input and measuring fEPSP slope as a function of the presynaptic fiber volley (FV) amplitude in slices from mice sacrificed 12 h after receiving their last injection of saline or morphine as described above. fEPSP slope magnitude significantly increased with increasing stimulus intensity and was greater in the morphine-treated population suggesting an increase in synaptic transmission (F1,126 = 9.25; *, p < 0.05, two-way ANOVA) (Fig. 3B). Furthermore, the relationship between FV amplitude and stimulus intensity was similar in the two groups, suggesting that presynaptic mechanisms may not be involved in morphine's effect (Fig. 3C). Finally, the relationship of FV versus fEPSP showed that morphine treatment significantly increased fEPSP magnitude at similar presynaptic FV amplitudes (Fig. 3D) (linear regression comparison of the fit of two curves, F3,294 = 31.8, p < 0.0001). The slope of the input-output curve in neurons from morphine-treated animals (6.18 ± 0.28 ms−1, n = 14) was significantly greater than that in neurons from saline-treated animals (4.39 ± 0.25 ms−1, n = 10, p < 0.001). These data suggest an increase in synaptic efficacy in hippocampal CA1 neurons from morphine-treated animals. In addition, we examined PPF, which is a sensitive measure of changes in the probability of neurotransmitter release. Figure 3E shows that PPF was unchanged after morphine administration, indicating that morphine-induced increase in synaptic gain within the hippocampal circuitry involves postsynaptic events.
Fig. 3.
Morphine increases fEPSP but not fiber volley magnitude. A, traces of FVs and fEPSPs were recorded 12 h after discontinuation of treatment in hippocampal slices from saline- and morphine-treated animals. B, fEPSP magnitude increased as a function of stimulus intensity and was greater in morphine-treated animals (F1,126 = 9.25, *, p < 0.05, two-way ANOVA). C, relationship between FV amplitude and stimulus intensity did not differ between saline- and morphine-treated animals. D, input (FV)-output (fEPSP) shows that morphine treatment increased fEPSP magnitude at similar presynaptic fiber volley amplitudes (linear regression comparison of fit of two curves, F3,294 = 31.8, p < 0.0001). E, PPF does not differ between saline- and morphine-treated animals.
Our biochemical data show that morphine administration, besides increasing synaptic GluR1 and GluR3 levels, also produces a significant increase in the expression of extrasynaptic GluR1 (Fig. 1). To examine whether some of this extrasynaptic GluR1 pool could be responsible for the observed effects on synaptic transmission, we recorded whole-cell responses to bath application of AMPA, which activates AMPARs through the cell, including somatic and dendritic extrasynaptic AMPARs as well as synaptic AMPARs. We found that bath application of AMPA did not affect the size of currents in morphine-treated animals compared with those of saline-treated animals (Fig. 4, A and B). These results suggest that although we observe an increase in extrasynaptic receptors in our biochemical studies, this pool of receptors is not involved in the synaptic responses obtained with our electrophysiological approaches.
Fig. 4.
Morphine-induced increased in excitatory synaptic transmission is not related to extrasynaptic receptors but is paralleled by an increase in the AMPAR/NMDA ratio. A and B, extrasynaptic AMPAR-mediated responses are not affected by morphine treatment. Whole-cell currents evoked by bath application of AMPA (1 or 10 μM) at the holding potential of −70 mV do not differ between saline- (n = 6) and morphine-treated (n = 6) groups. C and D, the ratio of AMPAR to NMDAR current is increased in morphine-treated animals (AMPAR/NMDAR ratio: saline, 0.95 ± 0.04, n = 9; morphine, 1.59 ± 0.07, n = 9; p < 0.0001).
The relative contribution of AMPAR and NMDAR to excitatory synaptic currents is believed to play an important role in synaptic plasticity (Malinow and Malenka, 2002). We next measured the ratio of AMPAR- to NMDAR-mediated EPSCs in both morphine- and saline-treated animals. Figure 4D shows that the AMPAR/NMDAR ratio increased significantly 12 h after morphine administration (saline, 0.95 ± 0.04, n = 9; morphine, 1.59 ± 0.07, n = 9; p < 0.0001), indicating that the enhancement in synaptic transmission induced by morphine treatment is specific to the AMPAR component.
Repeated Morphine Treatment In Vivo Induces Inwardly Rectifying AMPAR EPSCs at Hippocampal Synapses.
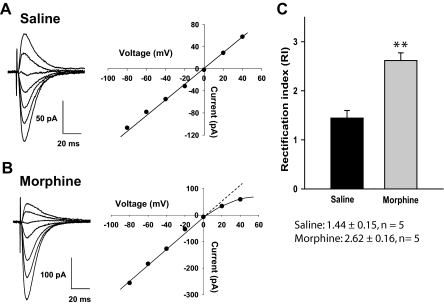
Next, we studied whether the observed increase in synaptic gain after repeated morphine administration was mediated through an alteration in RI [i.e., switching from linear Ca2+-impermeable (GluR2-containing) receptors to inwardly rectifying Ca2+-permeable (GluR2-lacking) receptors]. To this end, 12 h after the last injection, hippocampal neurons from saline- and morphine-treated animals were recorded with patch electrodes. AMPAR EPSCs were isolated by superfusing PTX (100 μM), CGP55845 (2 μM), and d-APV (50 μM) in ACSF; in addition, spermine (100 μM) and QX314 (5 mM) were included in the electrode solution. In CA1 neurons from saline-treated animals, the AMPAR EPSCs displayed an approximately linear I-V relationship with inward and outward currents of equivalent magnitude (Fig. 5A). In contrast, neurons from morphine-treated animals exhibited only limited ability to conduct outward AMPAR EPSCs and demonstrated inward rectification, a marker for the presence of GluR2-lacking receptors (Fig. 5B). Furthermore, the RI [calculated as EPSC (−60/+40 mV)] was significantly increased in morphine-treated animals (2.62 ± 0.16) compared with saline-treated animals (1.44 ± 0.15) (**, p < 0.01, unpaired t test; Fig. 5C). Because the RI is a measure of Ca2+-permeable AMPARs, these data suggested that, although homomers may be present in saline-treated control, after morphine treatment, AMPARs most likely are GluR1/3 heteromers or GluR1 or GluR3 homomers, which are Ca2+-permeable.
Fig. 5.
Morphine treatment results in the insertion of Ca2+-permeable AMPA receptors. A, left, traces of EPSCs; right, linear current-voltage relationship obtained by plotting EPSC amplitude as a function of holding potential indicates presence of GluR2 containing AMPARs. B, Same as in A but recorded in hippocampal neurons from morphine-treated animals. Note that current-voltage relationship deviates from linearity, a marker of Ca2+ passing AMPARs not containing the GluR2 subunit. C, RI calculated as EPSC (−60/+40 mV) was increased by morphine indicating the removal of GluR2 (**, p < 0.01, unpaired t test). The patch electrode contained 100 μM spermine to saturate polyamine binding sites.
Morphine Treatment Increases Effects of a Polyamine Toxin that Specifically Blocks Ca2+-Permeable AMPA Receptors.
The above data suggest increased insertion of GluR2-lacking receptors (Ca2+-permeable) at hippocampal synapses upon repeated administration of morphine. To further confirm this effect, we analyzed fEPSP in the presence of a polyamine toxin that specifically blocks Ca2+-permeable receptors (Tóth and McBain, 1998). Recording of fEPSP in the presence of 10 μM Phtx in slices from saline- and morphine-treated animals during basal synaptic transmission was performed as described above. Figure 6 shows that 12 h after discontinuation of morphine treatment, fEPSPs in hippocampal neurons showed a significant increase in the inhibitory effect of Phtx 15 min after infusion of the toxin, whereas fEPSP recorded in slices from saline-treated animals did not exhibit appreciable sensitivity to Phtx (**, p < 0.01, unpaired t test). These data provided further evidence of a greater insertion of Ca2+-permeable AMPA (GluR2-lacking) receptors after morphine treatment.
Fig. 6.
Morphine treatment increases the effects of Phtx, a polyamine toxin that blocks Ca2+-permeable AMPARs. Traces above show fEPSPs recorded before and 60 min after Phtx (10 μM) in slices from saline (left) and morphine (right)-treated animals. fEPSP recorded in slices from morphine-treated animals exhibited increased sensitivity to Phtx compared with saline-treated animals (**, p < 0.01, unpaired t test).
Repeated Morphine Treatment Has No Impact on Hippocampal LTP but Reduces LTD at CA1 Synapses.
Activity-dependent insertion of AMPA-type glutamate receptors is believed to underlie the mechanisms of LTP (Plant et al., 2006; Derkach et al., 2007). A transient increase in postsynaptic GluR2-lacking AMPA receptor ion channels is required for the stabilization of LTP (Plant et al., 2006). Because morphine-induced dynamic trafficking of AMPARs may affect both basal synaptic transmission and plasticity, we next examined the effect of repeated morphine administration on LTP at Schaffer collateral fiber synapses in CA1 region. As shown in Fig. 7A, HFS produced a robust induction of LTP in slices from both saline- and morphine-treated rats. Comparing two groups at 60 min after HFS, the slopes of fEPSP increased by 168.5 ± 8.9% of baseline in the saline group and 159.0 ± 13.9% in the morphine group, respectively (p > 0.05, unpaired t test). These results indicate that repeated morphine treatment has no significant affect on the induction and maintenance phases of HFS-induced LTP in hippocampal synapses.
Fig. 7.
Morphine treatment does not alter the magnitude or time course of LTP in hippocampal CA1 neurons but decreases the magnitude of LTD in hippocampal CA1 neurons, an effect prevented by blocking Ca2+-permeable AMPARs. A, traces above show fEPSPs recorded before and 60 min after HFS in slices from saline- (left) and morphine (right)-treated animals. The graph depicts the lack of effect of morphine on the induction and maintenance phases of LTP in hippocampal synapse. B, repeated morphine exposure decreased the magnitude of LTD and this effect is reversed by Phtx and Joro spider toxin. Graph comparing the LTD induced by LFS (1 Hz for 15 min) of hippocampal Schaffer collateral-CA1 synapses in brain slices from saline- and morphine-treated mice (fEPSP slopes 50 min after LFS: saline, 80.4 ± 3.4% of baseline; morphine, 89.4 ± 3.8% of baseline; p < 0.05, unpaired t test). Phtx and JST reverse morphine-induced decrease in the magnitude of LTD, respectively (fEPSP slopes 50 min after LFS: morphine, 89.4 ± 3.7% of baseline versus Phtx-morphine, 78.8 ± 1.5% of baseline, unpaired t test, p < 0.05; morphine, 89.4 ± 3.7% of baseline versus JST-morphine of baseline, 80.1 ± 3.0%, unpaired t test, p < 0.05).
The data above indicated an increase in the expression and synaptic insertion of GluR2-lacking AMPA receptors (Figs. 1–3, 5, and 6), which have been suggested to be important for synaptic transmission (Chung et al., 2003). However, it was found that LTP was not altered after morphine treatment (Fig. 7A). We further tested whether LTD, another form of synaptic plasticity, had been altered. To this end, we measured the magnitude of LTD induced by LFS (1 Hz, 15 min) of the Schaffer collateral to CA1 synapses in hippocampal slices from saline- and morphine-treated mice. Figure 7B shows that LFS delivery produced a significant depression of fEPSP lasting at least 50 min in both saline- and morphine-treated animals. It is interesting that the magnitude of LTD was significantly decreased in morphine-treated mice compared with saline-treated mice (fEPSP slopes 50 min after LFS: saline, 80.5 ± 3.4% of baseline; morphine, 89.4 ± 3.8% of baseline; p < 0.05, unpaired t test). These data suggest that repeated morphine administration decreases the magnitude of LTD in the CA1 region. Finally, we further tested whether this effect was due to an increased insertion of GluR2-lacking (Ca2+-permeable receptors). To this end, we analyzed morphine-induced alteration of LTD in the presence of 10 μM Phtx or 500 nM JST, polyamine toxins that specifically block Ca2+-permeable receptors, in slices from saline- and morphine-treated animals. Figure 7B shows that infusion of Phtx or JST completely reversed the morphine-induced decrease in LTD magnitude (fEPSP slopes 50 min after LFS: morphine, 89.4 ± 3.7% of baseline; Phtx-morphine, 78.8 ± 1.5% of baseline; JST-morphine, 80.1 ± 3.0% of baseline; p < 0.05, unpaired t test). These data confirm that the alteration in LTD observed after morphine administration is associated with an increase in the insertion of GluR2-lacking receptors.
Discussion
Behavioral evidence demonstrates that drugs of abuse produce changes in brain function that can be persistent (Kauer and Malenka, 2007). In this study, our goal was to examine these neuroadaptations in response to repeated morphine administration. In the present study, our biochemical and electrophysiological data indicate that morphine administration elicits changes in glutamate receptors and synaptic neurotransmission 12 h after discontinuation of the treatment.
It is known that GluR2-containing receptors are prevalent in the hippocampus during basal conditions (Sprengel, 2006). Here, we report an increase in the expression of the GluR1 subunit of AMPARs 12 h after repeated morphine treatment both at extrasynaptic and synaptic hippocampal sites. In addition, we found that morphine treatment also increased phosphorylation of GluR1 subunit at serine 845, the protein kinase A site (Lee et al., 2000), at synaptic fractions, which is in agreement with the effects observed with total GluR1. Up-regulation in the phosphorylation state of the GluR1 subunit in response to drug treatment has been reported previously. For example, an increase in the phosphorylation state of GluR1 at serine 845 in the dorsal hippocampus has been observed during cocaine-conditioned place preference behavior (Tropea et al., 2008). This effect has also been observed during withdrawal from heroin self-administration in the amygdala and the VTA (Edwards et al., 2009). Furthermore, previous studies obtained from our laboratory have shown that the extinction of morphine-conditioned place preference behavior is associated with an increase in the phosphorylation of GluR1 at hippocampal synapses (Billa et al., 2009).
In addition, we find that repeated morphine administration led to an increase in the expression of the GluR3 subunit at the PSD. In contrast, no changes in GluR2 levels were observed in any synaptic fraction upon morphine administration. GluR2 levels are not altered by morphine treatment because insertion of this subunit does not depend on synaptic activity (Shi et al., 2001). In addition, morphine-induced increase in GluR3 receptors may compete with the recycling of GluR2-containing receptors to the cell membrane (Shi et al., 2001). Overall, these data suggest that morphine administration may lead to changes in both the expression and location of AMPARs.
Subunit composition of AMPARs is also critical in determining the functional properties of resulting channels (Malinow and Malenka, 2002). Our data show that morphine administration could replace GluR1/GluR2-containing receptors for GluR2/GluR3-containing receptors, without affecting the total levels of GluR2. These results are similar to those reported by Conrad et al. (2008) during withdrawal from cocaine self-administration. In addition, these data are in agreement with the subcellular fractionation data that show a dramatic increase in GluR1 levels with no changes in GluR2 levels. Furthermore, the coimmunoprecipitation data indicate that this increased GluR1 does not seem to be associated with any other subunit, and therefore, this could reflect an increase in homomeric GluR1, which would lead to an overall increase in GluR2-lacking receptors.
Therefore, we hypothesize that morphine exposure may increase the insertion of GluR2-lacking AMPARs at hippocampal synapses. Studies characterizing the electrophysiological mechanisms contributing to AMPAR subunit composition at glutamatergic synapses during basal synaptic transmission after repeated morphine administration were performed. We found that morphine treatment increased the fEPSP magnitude without altering fiber volley magnitude, which would be expected with a postsynaptic increase in synaptic transmission. This was further confirmed with our assay of PPF that showed no effect on presynaptic release after morphine treatment. This would be in agreement with our hypothesis that morphine-induced changes in glutamatergic transmission are related to changes at the postsynaptic terminal. Next, we examined whether changes in the gain of synaptic transmission after repeated morphine administration were mediated through an alteration in rectification index switching from linear Ca2+-impermeable receptors (GluR2-containing) to inwardly rectifying, GluR2-lacking, Ca2+-permeable AMPA receptors. We found that repeated morphine administration significantly increased the rectification index, a measure of GluR2-containing receptors (Washburn et al., 1997), reflecting an increased rectification in the current-voltage relationship and indicating an altered AMPAR composition at the synapse. This effect has also been observed in the VTA after a single injection of cocaine (Bellone and Lüscher, 2006). In addition, we also examined fEPSP sensitivity to Phtx as a measure of insertion of Ca2+-permeable AMPA receptors under basal conditions. The data obtained indicate that repeated morphine administration led to an increased sensitivity of basal fEPSPs to Phtx, which further validates the idea that morphine treatment promotes the insertion of GluR2-lacking receptors during basal synaptic transmission by altering the dynamics of AMPAR trafficking at the synapse.
Our biochemical data showed that in addition to increased synaptic levels of GluR1, morphine administration induced a dramatic increase in extrasynaptic GluR1. However, data with whole-cell recordings and bath application of AMPA, which activates both synaptic and extrasynaptic receptors, did not show an increase in AMPA responses after morphine administration. These data do not agree with the observed increase in extrasynaptic GluR1 obtained in our biochemical studies. One possible explanation is that these increased GluR1 receptors at extrasynaptic sites are not functionally available, particularly because morphine may also affect the rate of receptor endocytosis at the synapse (Morón et al., 2007).
At first sight, one would expect that an increase in the AMPA/NMDA ratios would be associated with an increase in LTP; however, our data show that morphine administration does not affect LTP. In addition, this lack of effect on LTP does not seem to agree with the biochemical results or with the electrophysiological recordings performed at baseline. One possible explanation is that if a synapse is already potentiated, the synapse may not show further potentiation, whereas the observed increase in GluR1 or GluR3 levels after morphine administration would be reflected as an increase in AMPA/NMDA ratio during baseline recordings. On the other hand, we should consider the existing controversy regarding the involvement of GluR2-lacking receptors on expression of hippocampal LTP. In this regard, some reports show that blockers of GluR2-lacking receptors prevent LTP in hippocampal slices (Plant et al., 2006; Lu et al., 2007), although this effect seems to be age-dependent. In contrast, another report shows that insertion and activation of GluR2-lacking receptors are not involved in LTP at excitatory synapses in the hippocampal CA1 region (Gray et al., 2007). AMPAR modulation of LTP after cocaine exposure has also been studied in other brain areas such as the VTA (Argilli et al., 2008) with different results to those obtained in our study. Again, it is quite likely that AMPAR systems may respond specifically to a particular drug of abuse and administration paradigm and that this response may be different depending on the brain structure studied.
On the other hand, the present study shows that repeated morphine administration decreases the magnitude of LTD and that this effect is related to an increased insertion of GluR2-lacking (Ca2+-permeable) receptors. More specifically, this effect on LTD is quite likely to be associated with the observed increase in the phosphorylation state of the GluR1 subunit at Ser845 after morphine administration. In this regard, it has been reported that dephosphorylation of GluR1 at Ser845 is an expression mechanism of LTD (Lee et al., 2000). By increasing phosphorylation of GluR1 at Ser845, morphine could alter LTD and thus affect its magnitude. Likewise, it is known that increased phosphorylation at this site is associated with stabilization of Ca2+-permeable (GluR2-lacking) receptors (He et al., 2009). Another possible explanation is that LTD depends on clathrin-dependent endocytosis (Wang, 2008). We have previously reported that the paradigm of morphine administration used in the present study may affect endocytosis of the GluR1 subunit (Morón et al., 2007). Therefore, morphine could also affect LTD by impairing clathrin-dependent endocytosis of GluR1-containing AMPARs.
Overall, data presented in this study provides electrophysiological and biochemical evidence that AMPAR subunits are switched to Ca2+-permeable AMPARs 12 h after repeated morphine treatment. This suggests that repeated use of opiates may lead to maladaptive changes in hippocampal synaptic transmission and plasticity, such as formation of GluR2-lacking receptors. Modulation of specific AMPAR subtypes may provide a viable therapeutic intervention in the development of opiate addiction.
Acknowledgments
We thank Dr. Joanne Cousins for critical reading of this manuscript.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R03-DA023454, R01-DA025036].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.060301.
- VTA
- ventral tegmental area
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- AMPAR
- α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor
- PSD
- postsynaptic density
- LTP
- long-term potentiation
- LTD
- long-term depression
- fEPSP
- extracellular field excitatory postsynaptic potential
- EPSC
- excitatory postsynaptic currents
- HFS
- high-frequency stimulation
- LFS
- low-frequency stimulation
- d-APV
- 2-amino-5-phosphonovalerate
- RI
- rectification index
- Phtx
- philanthotoxin
- JST
- Joro spider toxin
- NMDA
- N-methyl-d-aspartate
- NMDAR
- N-methyl-d-aspartate receptor
- PTX
- picrotoxin
- ANOVA
- analysis of variance
- IP
- immunoprecipitation
- FV
- fiber volley
- PPF
- paired-pulse facilitation
- CGP55845
- (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride
- QX314
- N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium
- ACSF
- artificial cerebral spinal fluid.
References
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. (2008) Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci 28:9092–9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. (2006) Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci 9:636–641 [DOI] [PubMed] [Google Scholar]
- Billa SK, Sinha N, Rudrabhatla SR, Morón JA. (2009) Extinction of morphine-dependent conditioned behavior is associated with increased phosphorylation of the GluR1 subunit of AMPA receptors at hippocampal synapses. Eur J Neurosci 29:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. (2003) Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300:1751–1755 [DOI] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. (2000) Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27:107–119 [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. (2006) Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol 16:288–297 [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. (2007) Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8:101–113 [DOI] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW. (2009) Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse 63:224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. (2000) Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28:511–525 [DOI] [PubMed] [Google Scholar]
- Frade JG, Barbosa RM, Laranjinha J. (2009) Stimulation of NMDA and AMPA glutamate receptors elicits distinct concentration dynamics of nitric oxide in rat hippocampal slices. Hippocampus 19:603–611 [DOI] [PubMed] [Google Scholar]
- Gray EE, Fink AE, Sariñana J, Vissel B, O'Dell TJ. (2007) Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol 98:2488–2492 [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. (2009) Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1–S845 phosphorylation. Proc Natl Acad Sci USA 106:20033–20038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8:844–858 [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. (2000) Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405:955–959 [DOI] [PubMed] [Google Scholar]
- Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW. (2007) Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J 26:4879–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126 [DOI] [PubMed] [Google Scholar]
- Morón JA, Abul-Husn NS, Rozenfeld R, Dolios G, Wang R, Devi LA. (2007) Morphine administration alters the profile of hippocampal postsynaptic density-associated proteins: a proteomics study focusing on endocytic proteins. Mol Cell Proteomics 6:29–42 [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Isaac JT, Collingridge GL. (2004) Multiple, developmentally regulated expression mechanisms of long-term potentiation at CA1 synapses. J Neurosci 24:4903–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piëch V, Sheng M. (2001) Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci 4:917–926 [DOI] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9:602–604 [DOI] [PubMed] [Google Scholar]
- Shen F, Meredith GE, Napier TC. (2006) Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci 26:11041–11051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105:331–343 [DOI] [PubMed] [Google Scholar]
- Sprengel R. (2006) Role of AMPA receptors in synaptic plasticity. Cell Tissue Res 326:447–455 [DOI] [PubMed] [Google Scholar]
- Tóth K, McBain CJ. (1998) Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci 1:572–578 [DOI] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. (2008) Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem 106:1780–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120 [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. (2001) Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292:1175–1178 [DOI] [PubMed] [Google Scholar]
- Wang YT. (2008) Probing the role of AMPAR endocytosis and long-term depression in behavioural sensitization: relevance to treatment of brain disorders, including drug addiction. Br J Pharmacol 153 (Suppl 1):S389–S395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Numberger M, Zhang S, Dingledine R. (1997) Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci 17:9393–9406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. (2005) Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 5:9–19 [DOI] [PubMed] [Google Scholar]
- Zhong W, Dong Z, Tian M, Cao J, Xu T, Xu L, Luo J. (2006) Opiate withdrawal induces dynamic expressions of AMPA receptors and its regulatory molecule CaMKIIalpha in hippocampal synapses. Life Sci 79:861–869 [DOI] [PubMed] [Google Scholar]