Abstract
Reduced clearance of amyloid-β (Aβ) from brain partly underlies increased Aβ brain accumulation in Alzheimer's disease (AD). The mechanistic basis for this pathology is unknown, but recent evidence suggests a neurovascular component in AD etiology. We show here that the ATP-driven pump, P-glycoprotein, specifically mediates efflux transport of Aβ from mouse brain capillaries into the vascular space, thus identifying a critical component of the Aβ brain efflux mechanism. We demonstrate in a transgenic mouse model of AD [human amyloid precursor protein (hAPP)-overexpressing mice; Tg2576 strain] that brain capillary P-glycoprotein expression and transport activity are substantially reduced compared with wild-type control mice, suggesting a mechanism by which Aβ accumulates in the brain in AD. It is noteworthy that dosing 12-week-old, asymptomatic hAPP mice over 7 days with pregnenolone-16α-carbonitrile to activate the nuclear receptor pregnane X receptor restores P-glycoprotein expression and transport activity in brain capillaries and significantly reduces brain Aβ levels compared with untreated control mice. Thus, targeting intracellular signals that up-regulate blood-brain barrier P-glycoprotein in the early stages of AD has the potential to increase Aβ clearance from the brain and reduce Aβ brain accumulation. This mechanism suggests a new therapeutic strategy in AD.
A hallmark of Alzheimer's disease (AD) is the accumulation of neurotoxic amyloid-β (Aβ) peptide within the brain. The Aβ transport-clearance hypothesis of AD proposed by Zlokovic and coworkers (Zlokovic and Frangione, 2003; Deane et al., 2004b; Zlokovic, 2005) states that reduced Aβ clearance (reduced Aβ efflux transport) from the brain underlies Aβ brain accumulation (see also Mooradian et al., 1997). This hypothesis suggests that the mechanism responsible for Aβ brain clearance itself could be a therapeutic target in AD.
Aβ clearance from brain to blood has to be a two-step process. Aβ must first pass through the abluminal (brain side) and then the luminal (blood side) plasma membranes of the brain capillary endothelial cells that comprise the blood-brain barrier. Given that Aβ is a peptide, both steps must be facilitated, involving receptors or transporters. At the abluminal membrane, the receptor low-density lipoprotein receptor-related protein 1 (LRP1) seems to be the major protein responsible for Aβ uptake from brain into capillary endothelial cells (Shibata et al., 2000; Deane et al., 2004a,b). However, the luminal membrane protein mediating the critical second step, Aβ efflux from the endothelial cells into the blood, has not been identified.
One candidate is P-glycoprotein, an ATP-driven efflux transporter that under normal physiological conditions is highly expressed at the luminal membrane of the brain capillary endothelium. This transporter handles a wide spectrum of nonpolar, therapeutic drugs, some of which are small polypeptide derivatives (Miller et al., 2008). Limited data with artificial model systems such as transporter-overexpressing cell lines of nonbrain endothelial origin and membrane vesicles from these cell lines suggest that P-glycoprotein can transport Aβ (Lam et al., 2001; Cirrito et al., 2005; Kuhnke et al., 2007), and one report suggests reduced Aβ efflux from the brain in P-glycoprotein-null mice (Cirrito et al., 2005). However, those mice also exhibit substantially reduced expression of LPR1 in cerebral vessels, so the involvement of blood-brain barrier P-glycoprotein in Aβ efflux in AD remains unclear.
Here, we establish a role for blood-brain barrier P-glycoprotein in the efflux transport of Aβ from the brain into capillary lumen. We use a transgenic mouse model of AD [human amyloid precursor protein (hAPP)-overexpressing mice; Tg2576 strain] to test the hypotheses that defective transport mediated by P-glycoprotein contributes to Aβ accumulation within the brain and that restoring such transport can reduce brain Aβ levels. We demonstrate here for the first time that P-glycoprotein mediates efflux transport of Aβ in intact brain capillaries. We show that P-glycoprotein expression and transport activity are substantially reduced in brain capillaries from hAPP mice. These experiments were done with 12-week-old transgenic mice, at a time when brain hAPP and human Aβ (hAβ) levels are substantial, but when there is not yet evidence of cognitive impairment (Hsiao et al., 1996; Kawarabayashi et al., 2001). Finally, and most importantly, using this AD model we show that in vivo activation of the nuclear receptor pregnane X receptor (PXR) over 7 days both restores P-glycoprotein expression and activity at the blood-brain barrier and significantly reduces Aβ brain levels.
These results suggest that up-regulating blood-brain barrier P-glycoprotein through PXR activation is a therapeutic strategy that could lower Aβ brain levels and delay onset and progression of AD.
Materials and Methods
Chemicals.
Antibodies against RAGE, human APP, human Aβ40, human Aβ42, and β-actin were purchased from Abcam, Inc. (Cambridge, MA). Note that in Western blots using the antibodies to detect human APP, human Aβ40, and human Aβ42, we observed a single band at the right molecular weight for each of these proteins, indicating that the antibodies used did not cross-react with other amyloid species and are thus specific for the corresponding amyloid isoform. C219 antibody against P-glycoprotein was from Signet Laboratories (Dedham, MA), LRP1 antibody was obtained from Calbiochem-Novabiochem (San Diego, CA), and PXR antibody was from BioLegend (San Diego, CA). Fluorescein-hAβ42 [fluorescein-βA(1–42)] was purchased from rPeptide (Bogart, GA), fumitremorgin C (FTC) was from Alexis Axxora (San Diego, CA), receptor-associated protein (RAP) was from Calbiochem-Novabiochem, and safflower oil was from MP Biomedicals (Solon, OH). [N-ε (4-nitrobenzofurazan-7-yl)-d-Lys8]-cyclosporine A (NBD-CSA) was custom-synthesized by R. Wenger (Basel, Switzerland) (Wenger, 1986). PSC833 (valspodar) was a kind gift from Novartis (Basel, Switzerland). Sulforhodamine 101 free acid, NaCN, mannitol, leukotriene C4 (LTC4), pregnenolone-16α-carbonitrile (PCN), and all other chemicals were of the highest grade and purchased from Sigma-Aldrich (St. Louis, MO).
Animals.
Male transgenic human APP-overexpressing mice [Tg2576 strain; 129S6.Cg-Tg(APPSWE)2576Kha] and corresponding male wild-type mice were purchased from Taconic Farms (Germantown, NY). Mice were 12 weeks old with an average body weight of 29 g for wild-type mice and 31 g for transgenic hAPP mice. Animals were kept under controlled environmental conditions (23°C, 35% relative humidity, 12-h dark-light cycle) with free access to tap water and standard rodent feed. After shipping, animals were allowed to adapt to the new environment for at least 1 week before experiments. Animal protocols were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of Minnesota (IACUC protocol 0801A25321; principal investigator Bjoern Bauer) and National Institutes of Health/National Institute on Environmental Health Sciences (IACUC protocol LPC99-14; principal investigator David Miller) and were in accordance with the Guides to Animal Use of the University of Minnesota, National Institutes of Health guidelines, Association for Assessment and Accreditation of Laboratory Animal Care regulations, and the U.S. Department of Agriculture Animal Welfare Act.
PCN Dosing and Facial Vein Bleeding.
Twenty hAPP transgenic mice received daily one injection of 25 mg/kg i.p. PCN for 7 days. Twenty hAPP transgenic controls and 20 wild-type mice received vehicle (safflower oil). Blood samples were taken by facial vein bleeding on days 0, 1, 3, and 5 from seven to eight animals per group. Twenty-four hours after the last injection, mice were euthanized by CO2 inhalation and decapitated, and trunk blood was collected. Brains from each animal were collected for Aβ determination and isolation of brain capillaries.
Brain Capillary Isolation.
Brain capillaries were isolated as described previously (Hartz et al., 2008). In brief, mice were euthanized by CO2 inhalation and then decapitated. Brains were removed, dissected, and homogenized in ice-cold phosphate-buffered saline (PBS) buffer (2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, 8.1 mM Na2HPO4, 0.9 mM CaCl2, and 0.5 mM MgCl2 supplemented with 5 mM d-glucose, 1 mM sodium pyruvate, pH 7.4). Ficoll was added to a final concentration of 15%, and the homogenate was centrifuged at 5,800g for 20 min at 4°C. After resuspending the pellet in 1% bovine serum albumin (BSA)/PBS, the capillary suspension was passed over a glass bead column. Capillaries adhering to the glass beads were collected by gentle agitation in 1% BSA/PBS. Capillaries were washed with BSA-free PBS and used for experiments.
P-Glycoprotein Transport Assay.
To determine P-glycoprotein transport activity, freshly isolated brain capillaries were incubated for 1 h at room temperature with the fluorescent P-glycoprotein-specific substrate NBD-CSA (2 μM in PBS buffer) (Hartz et al., 2008). For Aβ-transport studies, capillaries were incubated for 1 h at room temperature with 5 μM fluorescein-hAβ42 in PBS buffer. For each treatment, images of 10 capillaries were acquired by confocal microscopy [Nikon C1 LSC microscope unit, Nikon TE2000 inverted microscope, 40× oil immersion objective, numerical aperture 1.3, 488-nm line of an argon laser, Nikon Instruments Inc. (Melville, NY), or Zeiss LSM 510 META inverted confocal microscope, 40× water immersion objective, NA 1.2, 488-nm line of argon laser, Carl Zeiss Inc. (Thornwood, NY)]. Images were analyzed by quantitating luminal NBD-CSA fluorescence with Zeiss Image Examiner and Image J 1.41 software (Research Services Branch, National Institute of Mental Health/National Institutes of Health, Bethesda, MD). As before, specific, luminal NBD-CSA fluorescence was taken as the difference between total luminal fluorescence and fluorescence in the presence of the P-glycoprotein-specific inhibitor PSC833 (5 μM) (Hartz et al., 2008).
Sulforhodamine 101 Accumulation Assay.
Freshly isolated mouse brain capillaries were incubated with 2 μM sulforhodamine 101 for 1 h alone or transporter inhibitors as indicated. For each treatment group, confocal images (Nikon C1 LSC microscope unit, Nikon TE2000 inverted microscope, 40× oil immersion objective, numerical aperture 1.3, 488-nm line of an argon laser; Nikon Instruments Inc.) were obtained from 10 capillaries. Luminal sulforhodamine 101 fluorescence intensity was measured with Image J software (ver. 1.41; http://rsbweb.nih.gov/ij/index.html) as described previously (Bauer et al., 2008).
Aβ Immunostaining of Mouse Brain Capillaries.
Isolated mouse brain capillaries were fixed with 3% paraformaldehyde/0.25% glutaraldehyde for 15 min at room temperature. After washing with PBS, capillaries were permeabilized with 0.5% Triton X-100 for 30 min and washed with PBS. Capillaries were blocked with 1% BSA for 60 min and incubated overnight at 4°C with a 1:500 dilution of monoclonal antibody against hAβ40 or polyclonal antibody against hAβ42. Capillaries were washed and incubated with Alexa-Fluor 488-conjugated secondary IgG (1:750; Invitrogen, Carlsbad, CA) for 1 h at 37°C. Nuclei were counterstained with 2 μg/ml of propidium iodide. Negative controls for each treatment that were processed without primary antibody showed negligible background fluorescence (data not shown). Aβ immunofluorescence was visualized by confocal microscopy (Nikon C1 LSC microscope unit, Nikon TE2000 inverted microscope, 40× oil immersion objective, NA 1.3, 488 nm line of an argon laser, 543 nm line of a HeNe laser; Nikon Instruments Inc.). For each treatment, confocal images of 10 capillaries were acquired. Aβ membrane immunofluorescence for each capillary was quantitated with Image J software. A 10 × 10 grid was superimposed on each image, and fluorescence measurements of capillary membranes were taken between intersecting grid lines. Fluorescence intensity for each capillary was the mean of six measurements per capillary.
Western Blotting.
Protein expression levels from different tissues were analyzed by Western blotting as described previously (Hartz et al., 2008). Isolated capillaries were homogenized in lysis buffer (Sigma-Aldrich) containing Complete protease inhibitor (Roche Diagnostics, Mannheim, Germany). Homogenized samples were centrifuged at 10,000g for 15 min, and denucleated supernatants were centrifuged at 100,000g for 90 min to obtain brain capillary membranes. Brain capillary membranes were resuspended and stored at −80°C.
Western blots were performed by using the Invitrogen NuPage Bis-Tris electrophoresis and blotting system. After protein electrophoresis and transfer, blotting membranes were blocked and incubated overnight with the primary antibody as indicated. Membranes were washed and incubated with horseradish peroxidase-conjugated ImmunoPure secondary IgG (1:15,000; Thermo Fisher Scientific, Waltham, MA) for 1 h. Proteins were detected by using SuperSignal West Pico Chemoluminescent Substrate (Thermo Fisher Scientific). Protein bands were visualized and recorded with a Bio-Rad Gel Doc 2000 gel documentation system (Bio-Rad Laboratories, Hercules, CA). QuantityOne 1-D software (vers. 4.6.5; Bio-Rad Laboratories) was used for densitometric analyses of band intensities and digital molecular weight analyses; molecular weight markers used were RPN800 (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) and NOVEX Sharp PS (Invitrogen). Linear adjustments of contrast and brightness were applied to entire Western blot images. None of the Western blots shown were modified by nonlinear adjustments.
ELISA for hAβ40 and hAβ42.
hAβ40 and hAβ42 was quantitated in plasma and brain samples by enzyme-linked immunosorbent assay (ELISA) (Invitrogen) according to the manufacturer's protocol. Plasma was obtained from blood samples by centrifugation at 5,000g for 5 min at 4°C. To determine hAβ40 plasma levels, samples were diluted 1:20 before analysis; to determine hAβ42 levels, samples were diluted 1:4 before analysis. hAβ40 and hAβ42 were extracted from brain tissue from APP and APP-PCN mice by homogenization with Tris-HCl buffer containing 5 M guanidine-HCl. Samples were diluted 1:20 in Dulbecco's PBS buffer containing 5% BSA and 0.03% Tween 20 and centrifuged at 16,000g for 20 min at 4°C. Supernatant was collected and diluted 1:1 with buffer before ELISA analysis.
Statistical Analysis.
Data are presented as mean ± S.E.M. Two-tailed unpaired Student's t test was used to evaluate differences between controls and treated groups; differences were considered to be statistically significant at P < 0.05.
Results
Blood-Brain Barrier P-Glycoprotein Transports hAβ42.
To investigate the role of P-glycoprotein in Aβ efflux at the blood-brain barrier, we used freshly isolated brain capillaries from normal wild-type mice. Our previous studies have shown that isolated brain capillaries from both mouse and rat are morphologically intact and remain metabolically active and functionally capable of ATP-driven transport for up to 12 h (Bauer et al., 2006; Hartz et al., 2008; data not shown). Thus, isolated brain capillaries closely mimic the blood-brain barrier in vivo and are well suited to study transport processes across the brain capillary endothelium.
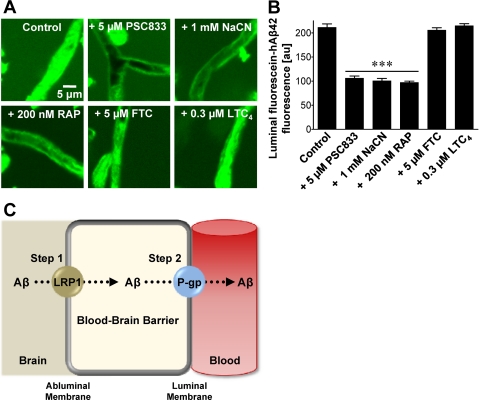
In the present study, we incubated isolated mouse brain capillaries with fluorescein-hAβ42 and measured its accumulation in capillary lumens by using confocal microscopy and quantitative imaging. Fluorescein-hAβ42 accumulated to high levels in the lumens of control capillaries, indicating active transport from bath to vascular space (Fig. 1, A and B; Supplemental Fig. 1). Luminal fluorescence was significantly (P < 0.001) reduced by the P-glycoprotein-specific inhibitors PSC833 (valspodar), XR9576 (tariquidar), ivermectin, cyclosporin A, and verapamil (Mayer et al., 1997; Fellner et al., 2002), the metabolic inhibitor NaCN, and the LRP1-specific inhibitor RAP. FTC, Ko143, LTC4, and probenecid, inhibitors of the ATP-driven efflux transporters, breast cancer resistance protein (BCRP), and multidrug resistance-associated proteins (MRPs) were without effect. These results are consistent with blood-brain barrier Aβ efflux being an active and ATP-dependent two-step process, involving LRP1-mediated Aβ uptake from brain into capillary endothelial cells followed by P-glycoprotein-mediated Aβ efflux from endothelium into blood (Fig. 1C).
Fig. 1.
P-glycoprotein mediates hAβ42 transport in mouse brain capillaries. A, representative confocal images of brain capillaries isolated from wild-type mice. Capillaries were incubated with 5 μM fluorescein-hAβ42 for 1 h alone (control) or with 5 μM fluorescein-hAβ42 plus PSC833 (P-glycoprotein inhibitor), NaCN (metabolic inhibitor), RAP (LRP1 inhibitor), FTC (BCRP inhibitor), or LTC4 (MRP inhibitor). B, capillary luminal fluorescein-hAβ42 fluorescence after image analysis. Residual fluorescence is caused by nonspecific binding (Hartz et al., 2008). Data represent mean ± S.E.M. for 10 capillaries from one preparation (pooled tissue from 10 wild-type mice). Shown are arbitrary fluorescence units (scale 0–255). ***, significantly lower than control, P < 0.001. C, proposed two-step mechanism of blood-brain barrier Aβ efflux involving the Aβ receptor LRP1, on the abluminal membrane and the efflux transporter, P-glycoprotein, on the luminal membrane of brain capillaries.
P-Glycoprotein Is Compromised at the Blood-Brain Barrier of hAPP Transgenic Mice.
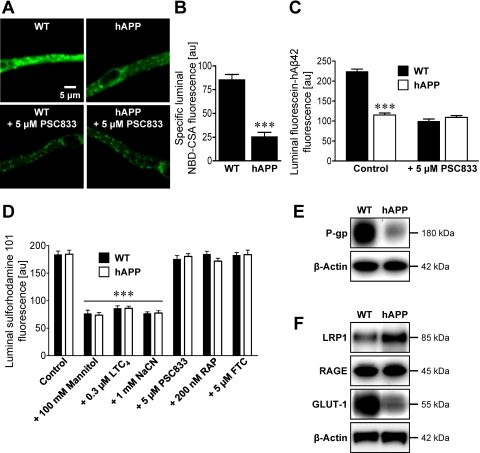
To test the hypothesis that P-glycoprotein is compromised at the blood-brain barrier in AD, we measured P-glycoprotein expression and transport activity in brain capillaries isolated from 12-week-old, male wild-type and hAPP transgenic mice (Tg2576; overexpressing hAPP with the Swedish mutation), a well established animal model of AD (Hsiao et al., 1996). At 12 weeks of age, hAPP transgenic mice exhibit accumulation of human Aβ in brain, but show no evidence of cognitive impairment, which does not start until approximately 6 months of age and then progressively worsens (Hsiao et al., 1996; Kawarabayashi et al., 2001). Figure 2A shows representative images of isolated brain capillaries from wild-type and hAPP mice that were incubated to steady state with the fluorescent dye NBD-CSA, a P-glycoprotein substrate. Using isolated brain capillaries from rats and mice we previously demonstrated that PSC833-sensitive luminal accumulation of this dye is a specific measure of P-glycoprotein transport activity (Hartz et al., 2008). In capillaries from wild-type mice, luminal NBD-CSA fluorescence was high and sensitive to P-glycoprotein inhibition with PSC833. Comparison of luminal NBD-CSA fluorescence in capillaries from wild-type and hAPP mice indicated a 70% decrease in P-glycoprotein transport activity for the latter (Fig. 2B). Consistent with Aβ being a P-glycoprotein substrate, isolated brain capillaries from hAPP mice exhibited substantially reduced fluorescein-hAβ42 transport (Fig. 2C).
Fig. 2.
P-glycoprotein expression and transport activity are reduced at the blood-brain barrier of hAPP mice. A, representative images of brain capillaries isolated from 12-week-old wild-type and hAPP mice. Capillaries were incubated with 2 μM NBD-CSA, a fluorescent P-glycoprotein-specific substrate, for 1 h alone or with PSC833. B, specific (PSC833-sensitive) luminal NBD-CSA fluorescence after image analysis of brain capillaries. C, luminal fluorescein-hAβ42 fluorescence in brain capillaries from wild-type and hAPP mice. D, luminal fluorescence of the MRP-specific, fluorescent substrate, sulforhodamine 101, in brain capillaries alone (control) or with mannitol (osmotic tight junction disruptor), LTC4 (MRP inhibitor), NaCN (metabolic inhibitor), PSC833 (P-glycoprotein inhibitor), RAP (LRP1 inhibitor), or FTC (BCRP inhibitor). Data in B–D are mean ± S.E.M. for 10 capillaries from one preparation (pooled tissue from 10–20 mice per group). Shown are arbitrary fluorescence units (scale 0–255). ***, significantly lower than control, P < 0.001. E and F, Western blots for P-glycoprotein (P-gp) (E) and LRP1, RAGE, and GLUT-1 (F) of brain capillary membranes from wild-type and hAPP mice. β-Actin was used as protein loading control (pooled tissue from 20 mice per group).
Reduced luminal accumulation of NBD-CSA and fluorescein-hAβ42 in brain capillary lumens from hAPP mice could reflect reduced P-glycoprotein expression or increased permeability of the tight junctions that separate endothelial cells within the endothelium (Hartz et al., 2004). If tight junctional permeability were altered, one would expect to see similar effects for all transporters that are capable of driving concentrative luminal substrate accumulation. In this regard, we recently demonstrated that transport of a fluorescent organic anion, sulforhodamine 101, into brain capillary lumens is mediated by another ATP-driven efflux pump, Mrp2 (multidrug resistance-associated protein isoform 2) (Bauer et al., 2008). When we compared the ability of brain capillaries from wild-type and hAPP mice with transport sulforhodamine 101, we found no difference (Fig. 2D, Supplemental Fig. 2). Unaltered luminal accumulation of sulforhodamine 101 indicates intact tight junctions in brain capillaries from hAPP mice. Thus, reduced luminal NBD-CSA and fluorescein-hAβ42 accumulation found in brain capillaries from hAPP mice is caused by reduced P-glycoprotein transport activity at the blood-brain barrier of these mice.
Consistent with reduced transport activity, we found a marked decrease of P-glycoprotein protein in brain capillary plasma membranes isolated from hAPP mice compared with membranes from wild-type controls (Fig. 2E). Western blot density measurements showed that P-glycoprotein levels were significantly decreased by approximately 60% [P-glycoprotein in hAPP mice: 39 ± 6% (S.E.M., P < 0.01) of wild-type control, P-glycoprotein levels normalized to β-actin]. Thus, despite the absence of cognitive impairment, 12-week-old hAPP mice display early biochemical and physiological changes in expression and transport activity of P-glycoprotein at the blood-brain barrier. In agreement with previous reports (Mooradian et al., 1997; Hooijmans et al., 2007), we also detected reduced protein expression of glucose transporter 1 (GLUT-1) in brain capillaries from hAPP mice. In contrast, in capillaries from hAPP mice, expression of the abluminal Aβ-uptake receptor LRP1 was slightly increased and that of the luminal Aβ-uptake receptor RAGE was unchanged (Fig. 2F). This indicates that decreased Aβ efflux transport in brain capillaries from hAPP mice was not caused by altered LRP1 or RAGE expression. Thus, these data suggest that the critical limiting step in Aβ brain clearance in AD seems to be Aβ transport across the luminal membrane into the vascular space.
PXR Activation Restores Blood-Brain Barrier P-Glycoprotein in hAPP Mice.
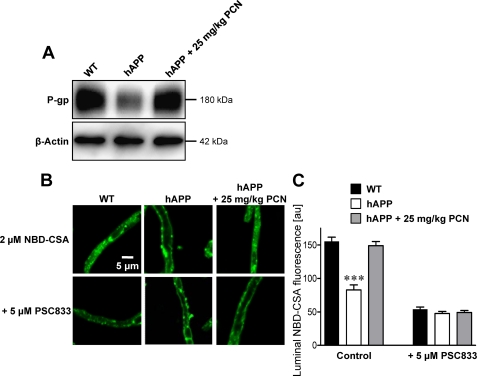
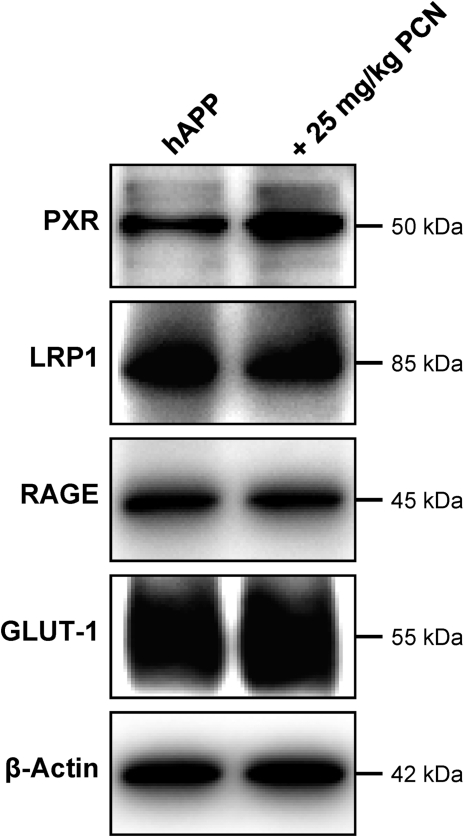
We previously demonstrated that blood-brain barrier P-glycoprotein can be up-regulated by in vivo exposure to ligands that activate PXR, a nuclear receptor that controls expression of drug metabolizing enzymes and efflux transporters (Kliewer et al., 1998; Geick et al., 2001; Bauer et al., 2004, 2006). In preliminary experiments we found that PXR protein was expressed in brain capillaries from wild-type and hAPP mice, but expression was slightly lower in capillaries from hAPP mice (Supplemental Fig. 3). To determine whether PXR activation in vivo could restore P-glycoprotein expression and transport activity at the blood-brain barrier, we dosed hAPP mice with the prototypical rodent PXR ligand, PCN, at 25 mg/kg i.p. once a day for 7 days (Kliewer et al., 1998). PCN dosing of hAPP mice increased P-glycoprotein expression in brain capillary membranes to levels observed in untreated wild-type mice [Fig. 3A; hAPP: 56 ± 8% (S.E.M.; P < 0.03) of control; hAPP+PCN: 92 ± 9% (S.E.M.; not statistically significant, P = 0.49) of control as determined by optical density measurements; P-glycoprotein levels normalized to β-actin]. Consistent with increased protein expression, specific (PSC833-sensitive) P-glycoprotein transport activity in brain capillaries from PCN-treated hAPP mice was restored to levels in wild-type mice for both NBD-CSA and fluorescein-hAβ42 (Figs. 3, B and C and 4). PXR protein expression in brain capillaries was slightly increased by PCN dosing as reported previously (Pascussi et al., 2000); LRP1, RAGE, and GLUT-1 protein levels were unaffected (Fig. 5). Thus, PCN dosing of hAPP mice selectively up-regulated P-glycoprotein at the blood-brain barrier and the newly synthesized transporter protein was functionally active.
Fig. 3.
PXR activation in hAPP mice restores P-glycoprotein expression and transport of NBD-CSA. A, P-glycoprotein (P-gp) Western blot of brain capillary membranes isolated from vehicle-treated wild-type and hAPP mice and hAPP mice dosed with 25 mg/kg PCN once a day for 7 days. β-Actin was used as protein loading control (pooled tissue from 20 mice per group). B, accumulation of NBD-CSA in brain capillaries from vehicle-treated wild-type and hAPP mice and PCN-treated hAPP mice. Capillaries were incubated with 2 μM NBD-CSA for 1 h alone or with PSC833. C, data after capillary image analysis. Data are mean ± S.E.M. for 10 capillaries from one preparation (pooled tissue from 20 mice per group). Shown are arbitrary fluorescence units (scale 0–255). ***, significantly lower than control, P < 0.001.
Fig. 4.
PXR activation in hAPP mice restores P-glycoprotein-mediated hAβ42 transport. A, representative images of brain capillaries isolated from wild-type, hAPP, and PCN-treated hAPP mice. Capillaries were incubated with 5 μM fluorescein-hAβ42 for 1 h. B, data after capillary digital image analysis. Data are mean ± S.E.M. for 10 capillaries from one preparation (pooled tissue from 20 mice per group). Shown are arbitrary fluorescence units (scale 0–255). ***, significantly lower than control, P < 0.001.
Fig. 5.
Western blots for indicated proteins of capillary membranes from vehicle- and PCN-treated hAPP mice (pooled tissue from 20 mice per group).
Restoring Blood-Brain Barrier P-Glycoprotein Reduces Aβ Brain Levels.
We next determined the consequences of restoring blood-brain barrier P-glycoprotein on hAβ levels in hAPP mice. We initially measured hAβ40 and hAβ42 in plasma by ELISA but found no difference between untreated hAPP mice and PCN-treated hAPP mice over the 7-day period of PCN dosing (Supplemental Fig. 4, A and B). This finding is consistent with rapid Aβ clearance from blood by filtration at the kidney.
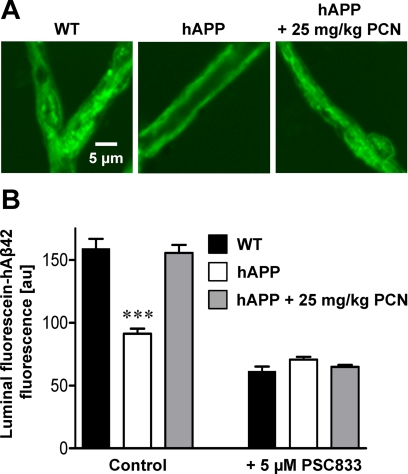
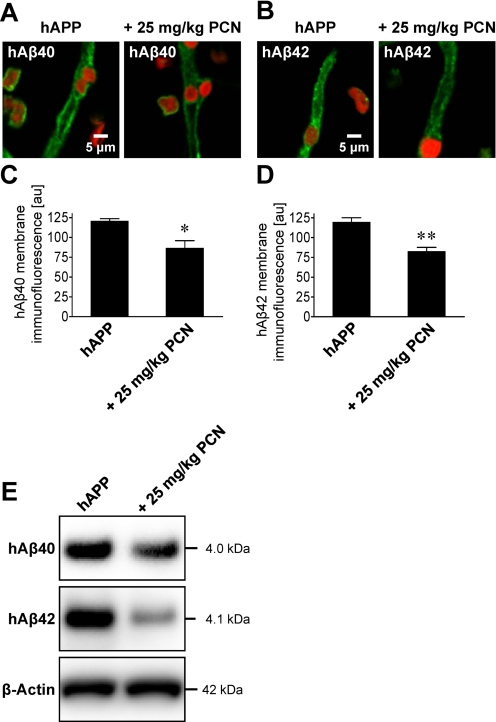
An early sign of AD is cerebral amyloid angiopathy, characterized by Aβ deposition in brain capillary membranes (Greenberg et al., 2004; Iadecola, 2004; Zlokovic, 2008). To measure capillary-associated Aβ, we isolated brain capillaries from hAPP control and PCN-dosed mice and immunostained for hAβ40 and hAβ42. Capillaries from untreated hAPP mice stained positive for both Aβ peptides (Fig. 6, A–D). Seven days of PCN treatment significantly decreased membrane immunofluorescence of both peptides in brain capillaries from hAPP mice by 28% for hAβ40 (P < 0.05) and 31% for hAβ42 (P < 0.01) compared with capillaries from untreated hAPP mice (Fig. 6, A–D). Western blotting of brain capillary membranes also showed reduced levels of hAβ40 and hAβ42 in hAPP mice treated with PCN compared with untreated hAPP mice [Fig. 6E; hAβ40: 82 ± 1.4% (S.E.M.; P < 0.05) of control, hAβ42: 45 ± 4.4% (S.E.M.; P < 0.001) of control as determined by optical density measurements; hAβ levels normalized to β-actin]. Thus, PCN dosing clearly reduced capillary-associated Aβ, suggesting reduced angiopathy.
Fig. 6.
Restoring P-glycoprotein in hAPP mice reduces Aβ levels in brain capillaries. A and B, representative images of hAβ40-immunostained (A) and hAβ42-immunostained (B) brain capillaries from vehicle- and PCN-treated hAPP mice. C and D, data from membrane hAβ-immunofluorescence analysis. Data are mean ± S.E.M. for 10 capillaries (pooled tissue from 20 mice per group). Shown are arbitrary fluorescence units (scale 0–255). *, significantly lower than control, P < 0.05; **, significantly lower than control, P < 0.01. E, hAβ40 and hAβ42 Western blots of brain capillary membranes.
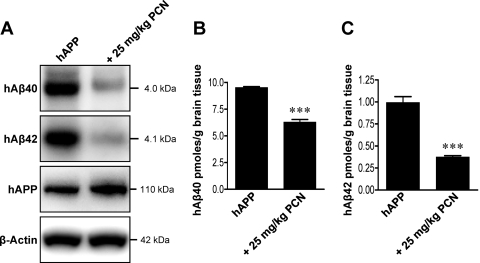
Finally, Western blots and ELISA analysis showed significantly (P < 0.001) reduced hAβ40 and hAβ42 levels in brain tissue of hAPP mice dosed with PCN compared with untreated hAPP control mice (Fig. 7). Optical density measurements of Western blots revealed that PCN dosing reduced brain levels of hAβ40 and hAβ42 by approximately 50 and 60%, respectively [Fig. 7A; hAβ40: 50 ± 4.5% (S.E.M., P < 0.001) of control; hAβ42: 36 ± 1.0% (S.E.M., P < 0.001) of control; hAβ levels normalized to β-actin]. This was confirmed by ELISA measurements that demonstrated that brain levels of hAβ40 and hAβ42 were reduced by approximately 35 and 60%, respectively [Fig. 7, B and C; hAβ40: 66 ± 3% (S.E.M., P < 0.001) of control; hAβ42: 38 ± 2% (S.E.M., P < 0.001) of control]. It is noteworthy that hAPP brain levels were not affected (Fig. 7A), indicating that PCN-induced PXR activation did not change Aβ precursor levels. Thus, activation of PXR increased blood-brain barrier P-glycoprotein expression and transport activity, which acutely reduced hAβ40 and hAβ42 brain levels in hAPP mice.
Fig. 7.
Restoring P-glycoprotein in hAPP mice reduces Aβ brain levels. A, hAβ40, hAβ42, and hAPP Western blots of total brain from vehicle- and PCN-treated hAPP mice. B and C, hAβ40 (B) and hAβ42 (C) ELISA analysis of brain tissue from vehicle- and PCN-treated hAPP mice (pooled tissue from 19 vehicle-treated hAPP mice and 20 PCN-treated hAPP mice). ***, significantly lower than control, P < 0.001.
Discussion
The present study reconciles three disparate observations, each of which suggests that P-glycoprotein is a critical component of the Aβ brain clearance mechanism: 1) cell lines that overexpress human P-glycoprotein transport Aβ (Lam et al., 2001; Kuhnke et al., 2007; Tai et al., 2009); 2) injecting a P-glycoprotein inhibitor into hAPP transgenic mice increases Aβ brain levels (Cirrito et al., 2005); and 3) brain deposition of Aβ in elderly, nondemented patients is inversely correlated with brain capillary expression of P-glycoprotein (Vogelgesang et al., 2002, 2004). Indeed, we provide here compelling evidence for both P-glycoprotein contributing significantly to Aβ efflux across the luminal membrane of the brain capillary endothelium and defective P-glycoprotein-mediated Aβ efflux transport in a mouse model of AD.
Recent studies and our current work argue for an important neurovascular component in AD etiology. They show that at least three plasma membrane proteins facilitate Aβ movement across the brain capillary endothelium: RAGE, LRP1, and P-glycoprotein (Fig. 1C) (present study; Deane et al., 2003, 2004b). Note that a recent publication suggests that the blood-brain barrier ATP-driven efflux pump BCRP could affect brain levels of Aβ (Xiong et al., 2009). This work indicates that BCRP transports Aβ in BCRP-overexpressing cell lines, Aβ accumulates in the brain of BCRP null mice, and BCRP expression is increased in brain samples from patients with AD and AD brains from two AD mouse models. However, the latter results are contrary to what one would expect if BCRP contributed to Aβ efflux from the brain and reduced BCRP-mediated efflux contributed to Aβ accumulation. In addition, the findings by Xiong et al. (2009) in patients were made after AD diagnosis, which is based on cognitive symptoms. Likewise, based on the age of the AD mice used in the study by Xiong et al. (2009) (3XTg and Tg-SwDI models), cognitive symptoms were already evident in those animals. In the present study, we used hAPP mice at 12 weeks of age, a time well before cognitive symptoms and presumably complex neuropathology set in (Hsiao et al., 1996; Kawarabayashi et al., 2001). Because we found no evidence for BCRP-mediated Aβ efflux in intact mouse brain capillaries, increased BCRP expression may appear at later stages of the disease, be model-dependent, or both. Thus, at this time the role of BCRP in AD etiology remains unclear.
On the other hand, the Aβ receptors RAGE and LRP1 have been shown to contribute to Aβ trafficking across the brain capillary endothelium, but not at early stages of the disease (Deane et al., 2003, 2004b). In both AD mouse models and patients with AD in whom cognitive symptoms are evident, RAGE has been shown to be increased and LRP1 to be decreased (Deane et al., 2003, 2004a; Donahue et al., 2006). In the present study with 12-week-old asymptomatic hAPP mice, unchanged protein expression of the luminal Aβ uptake receptor RAGE in brain capillaries suggests that Aβ uptake from blood into the endothelium may not be increased before the onset of cognitive impairment in AD. Slightly increased expression of the abluminal Aβ uptake receptor LRP1 suggests increased Aβ uptake from brain into the endothelium, again in very early stages of the disease, which may contribute to Aβ accumulation within the capillary endothelium in cerebral amyloid angiopathy. However, such changes in expression of these receptors cannot underlie reduced brain efflux of Aβ in the early-stage AD mouse model used in the present study. Thus, our results point to P-glycoprotein as the final step in clearing Aβ from the brain. They indicate that efflux mediated by P-glycoprotein may well be the limiting factor in Aβ brain clearance and the critical step that is defective in AD. Thus, reduced blood-brain barrier P-glycoprotein expression in AD would be a major contributor to Aβ brain accumulation. These results have two important implications for how we view the disease.
First, the present findings indicate that reduced P-glycoprotein expression at the blood-brain barrier is an early biochemical manifestation of AD pathology that occurs before cognitive symptoms are evident. The mechanism by which the disease signals the loss of P-glycoprotein is unknown. In the early stages of the disease, accumulation of Aβ42 levels in the brain capillary plasma membrane could directly impair P-glycoprotein function, lead to Aβ accumulation, and reduce P-glycoprotein expression. In this regard, our unpublished experiments show that exposure of rat or mouse brain capillaries to low levels of Aβ40 selectively removes P-glycoprotein from the plasma membrane and sends it to the proteasome, which implies that a particularly pernicious positive feedback loop drives the loss of efflux transporter expression, leading to a further increase in Aβ brain levels. We posit that this loop contributes to the progressive nature of AD, at least in the early stages. Second, P-glycoprotein is one transporter for which few endogenous substrates have been identified (King et al., 2001). Our results indicate that Aβ is one such substrate and that P-glycoprotein is responsible for the final critical step in Aβ efflux across the blood-brain barrier (Fig. 1C). P-glycoprotein is also a major protective element of the blood-brain barrier, limiting a large number of xenobiotics, including many therapeutic drugs, from entering into the central nervous system (Schinkel et al., 1996; Fellner et al., 2002). Thus, substantially reduced protein expression and efflux transport activity of blood-brain barrier P-glycoprotein in AD could have significant consequences for patients with AD. These include increased brain uptake of xenobiotics, altered dose response relationships for therapeutic drugs, and increased neurotoxicity for those drugs that exhibit a narrow therapeutic index and neurotoxicants that are P-glycoprotein substrates. All of these could aggravate the progression of AD.
It is clear from the present experiments with hAPP transgenic mice that targeting PXR, a ligand-activated nuclear receptor that modulates blood-brain barrier P-glycoprotein (Bauer et al., 2004, 2006), restores transporter expression and function, which in turn reduces brain Aβ burden. PXR is activated by a number of drugs and dietary constituents, and potent ligands for human PXR include the antibiotic, rifampicin, and the St. John's Wort constituent hyperforin (Jones et al., 2000; Watkins et al., 2001). We previously showed that rifampicin dosing of transgenic mice expressing human PXR increased expression and transport activity of P-glycoprotein at the blood-brain barrier (Bauer et al., 2006). In these mice, the rifampicin dose was adjusted so that free plasma levels of the drug were comparable with those in patients receiving rifampicin treatment. In this regard, a recent clinical trial showed that rifampicin dosing lessened cognitive decline in patients with AD over the 12-month treatment period (Loeb et al., 2004). The mechanistic basis for this observation is not known, but rifampicin activation of PXR leading to induction of blood-brain barrier P-glycoprotein is a likely possibility. These findings and the present results indicate that PXR activation to increase blood-brain barrier P-glycoprotein could be used in the clinic to increase Aβ brain efflux and transport and lower Aβ brain burden. This therapeutic strategy implies PXR protein expression in human brain capillaries. Although PXR mRNA has previously been detected in human whole brain and brain capillaries (Lamba et al., 2004; Dauchy et al., 2009), PXR protein expression has not yet been demonstrated. However, our study provides proof of principle, and we anticipate that up-regulation of P-glycoprotein expression through PXR or other signaling pathways has the potential to increase Aβ brain efflux transport and lower Aβ brain burden.
Finally, although brain accumulation of Aβ is not the only major contributor to cognitive impairment in AD (Iadecola, 2004; Blennow et al., 2006), reducing Aβ accumulation in the transgenic hAPP (Tg2576) mouse model does delay pathology (Karlnoski et al., 2009). It remains to be seen to what extent a general, long-term strategy of targeting signals that up-regulate blood-brain barrier P-glycoprotein will reduce brain Aβ burden over the long term and thus prove to be a useful therapeutic strategy for delaying the onset of AD and slowing the progression of the disease.
Supplementary Material
Acknowledgments
We thank Destiny Sykes, Jonathan Lucking, Sylvia Notenboom, and Anton Pekcec for technical assistance; David Armstrong and James Putney for valuable comments; and Emily Madole for editorial assistance.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This research was supported in part by the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences [Grant Z01-ES080048]; a Duluth Medical Research Institute Grant; and University of Minnesota College of Pharmacy startup funds.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061754.
- Aβ
- amyloid-β
- hAβ
- human Aβ
- AD
- Alzheimer's disease
- BCRP
- breast cancer resistance protein
- FTC
- fumitremorgin C
- hAPP
- human amyloid precursor protein
- LTC4
- leukotriene C4
- LRP1
- low-density lipoprotein receptor-related protein 1
- MRP
- multidrug resistance-associated protein
- NBD-CSA
- [N-ε (4-nitrobenzofurazan-7-yl)-d-Lys8]-cyclosporine A
- PCN
- pregnenolone-16α-carbonitrile
- PSC833
- valspodar
- PXR
- pregnane X receptor
- RAGE
- receptor for advanced glycation end products
- RAP
- receptor-associated protein
- PBS
- phosphate-buffered saline
- BSA
- bovine serum albumin
- IACUC
- Institutional Animal Care and Use Committee
- ELISA
- enzyme-linked immunosorbent assay
- GLUT-1
- glucose transporter 1.
References
- Bauer B, Hartz AM, Fricker G, Miller DS. (2004) Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 66:413–419 [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. (2008) Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab 28:1222–1234 [DOI] [PubMed] [Google Scholar]
- Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. (2006) In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol 70:1212–1219 [DOI] [PubMed] [Google Scholar]
- Blennow K, de Leon MJ, Zetterberg H. (2006) Alzheimer's disease. Lancet 368:387–403 [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, et al. (2005) P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J Clin Invest 115:3285–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, Scherrmann JM, De Waziers I, Declèves X. (2009) Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol 77:897–909 [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al. (2003) RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9:907–913 [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. (2004a) LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43:333–344 [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Zlokovic BV. (2004b) RAGE (yin) versus LRP (yang) balance regulates Alzheimer amyloid β-peptide clearance through transport across the blood-brain barrier. Stroke 35 (Suppl 1):2628–2631 [DOI] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, et al. (2006) RAGE, LRP-1, and amyloid-β protein in Alzheimer's disease. Acta Neuropathol 112:405–415 [DOI] [PubMed] [Google Scholar]
- Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruss T, Bernhardt G, Graeff C, Färber L, Gschaidmeier H, et al. (2002) Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest 110:1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geick A, Eichelbaum M, Burk O. (2001) Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem 276:14581–14587 [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Gurol ME, Rosand J, Smith EE. (2004) Amyloid angiopathy-related vascular cognitive impairment. Stroke 35 (Suppl 1):2616–2619 [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. (2008) Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J 22:2723–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. (2004) Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol 66:387–394 [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Graven C, Dederen PJ, Tanila H, van Groen T, Kiliaan AJ. (2007) Amyloid β deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brains of aged APP/PS1 mice. Brain Res 1181:93–103 [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. (1996) Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274:99–102 [DOI] [PubMed] [Google Scholar]
- Iadecola C. (2004) Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 5:347–360 [DOI] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, et al. (2000) The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14:27–39 [DOI] [PubMed] [Google Scholar]
- Karlnoski RA, Rosenthal A, Kobayashi D, Pons J, Alamed J, Mercer M, Li Q, Gordon MN, Gottschall PE, Morgan D. (2009) Suppression of amyloid deposition leads to long-term reductions in Alzheimer's pathologies in Tg2576 mice. J Neurosci 29:4964–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. (2001) Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci 21:372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Su W, Chang A, Zuckerman A, Pasternak GW. (2001) Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci 4:268–274 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, et al. (1998) An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92:73–82 [DOI] [PubMed] [Google Scholar]
- Kuhnke D, Jedlitschky G, Grube M, Krohn M, Jucker M, Mosyagin I, Cascorbi I, Walker LC, Kroemer HK, Warzok RW, et al. (2007) MDR1-P-glycoprotein (ABCB1) mediates transport of Alzheimer's amyloid-β peptides: implications for the mechanisms of Aβ clearance at the blood-brain barrier. Brain Pathol 17:347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, Reiner PB. (2001) β-Amyloid efflux mediated by p-glycoprotein. J Neurochem 76:1121–1128 [DOI] [PubMed] [Google Scholar]
- Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. (2004) PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol 199:251–265 [DOI] [PubMed] [Google Scholar]
- Loeb MB, Molloy DW, Smieja M, Standish T, Goldsmith CH, Mahony J, Smith S, Borrie M, Decoteau E, Davidson W, et al. (2004) A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer's disease. J Am Geriatr Soc 52:381–387 [DOI] [PubMed] [Google Scholar]
- Mayer U, Wagenaar E, Dorobek B, Beijnen JH, Borst P, Schinkel AH. (1997) Full blockade of intestinal P-glycoprotein and extensive inhibition of blood-brain barrier P-glycoprotein by oral treatment of mice with PSC833. J Clin Invest 100:2430–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AM. (2008) Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev 60:196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian AD, Chung HC, Shah GN. (1997) GLUT-1 expression in the cerebra of patients with Alzheimer's disease. Neurobiol Aging 18:469–474 [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ. (2000) Dexamethasone induces pregnane X receptor and retinoid X receptor-α expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol 58:361–372 [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. (1996) P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 97:2517–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, et al. (2000) Clearance of Alzheimer's amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106:1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Loughlin AJ, Male DK, Romero IA. (2009) P-glycoprotein and breast cancer resistance protein restrict apical-to-basolateral permeability of human brain endothelium to amyloid-β. J Cereb Blood Flow Metab 29:1079–1083 [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, Kunert-Keil C, Walker LC, Warzok RW. (2002) Deposition of Alzheimer's β-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly nondemented humans. Pharmacogenetics 12:535–541 [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Warzok RW, Cascorbi I, Kunert-Keil C, Schroeder E, Kroemer HK, Siegmund W, Walker LC, Pahnke J. (2004) The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer's disease. Curr Alzheimer Res 1:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. (2001) The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292:2329–2333 [DOI] [PubMed] [Google Scholar]
- Wenger RM. (1986) Cyclosporine and analogues—isolation and synthesis—mechanism of action and structural requirements for pharmacological activity. Fortschr Chem Org Naturst 50:123–136 [DOI] [PubMed] [Google Scholar]
- Xiong H, Callaghan D, Jones A, Bai J, Rasquinha I, Smith C, Pei K, Walker D, Lue LF, Stanimirovic D, et al. (2009) ABCG2 is up-regulated in Alzheimer's brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Aβ(1–40) peptides. J Neurosci 29:5463–5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. (2005) Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci 28:202–208 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Frangione B. (2003) Transport-clearance hypothesis for Alzheimer's disease and potential therapeutic implications, in Aβ Metabolism in Alzheimer's Disease (Saido TC. ed) pp 114–122, Landes Bioscience, Georgetown, TX: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.