Abstract
Age influences behavioral decisions such as reproductive timing and effort. In photoperiodic species, such age effects may be mediated, in part, by the individual's age-accrued experience with photostimulation. In female European starlings (Sturnus vulgaris) that do not differ in age, experimental manipulation of photostimulation experience (photoexperience) affects hypothalamic, pituitary, and gonadal activity associated with reproductive development. Does photoexperience also affect activity in forebrain regions involved in processing a social cue, the song of males, which can influence mate choice and reproductive timing in females? Female starlings prefer long songs over short songs in a mate-choice context, and, like that in other songbird species, their auditory telencephalon plays a major role in processing these signals. We manipulated the photoexperience of female starlings, photostimulated them, briefly exposed them to either long or short songs, and quantified the expression of the immediate-early gene ZENK (EGR-1) in the caudomedial nidopallium as a measure of activity in the auditory telencephalon. Using an information theoretic approach, we found higher ZENK immunoreactivity in females with prior photostimulation experience than in females experiencing photostimulation for the first time. We also found that long songs elicited greater ZENK immunoreactivity than short song did. We did not find an effect of the interaction between photoexperience and song length, suggesting that photoexperience does not affect forebrain ZENK-responsiveness to song quality. Thus, photoexperience affects activity in an area of the forebrain that processes social signals, an effect that we hypothesize mediates, in part, the effects of age on reproductive decisions in photoperiodic songbirds.
Keywords: age and aging, Akaike Information Criterion (AIC), birdsong, experience, photoperiodism
INTRODUCTION
According to life-history theory, an individual's age should affect its reproductive decisions. For example, the size or number of offspring should increase with the mother's age (Williams, 1966; Clutton-Brock, 1988). Numerous investigators have observed this and other effects of age on reproductive decisions and attributed the proximate control of these effects to age-accrued experiences that may influence how individuals subsequently respond to reproductive cues in the environment (Williams, 1966; Pugesek, 1981; Sæther, 1990; Forslund and Part, 1995; Sockman et al., 2006).
In some species, one experience that might help to mediate age-related change in reproductive decisions is experience with the process of photostimulation (photoexperience). The vast majority of temperate-zone bird species are photoperiodic, in that their behavioral and physiological responses to annual cycles in photoperiod enable the adaptive timing for the onset and cessation of reproductive activity (Nicholls et al., 1988; Hahn et al., 1997; Dawson et al., 2001). In most such species, young hatch under the long photophases of spring or summer in the photoperiodic state known as photorefractoriness (Williams et al., 1987; Williams et al., 1987), which is characterized as an insensitivity to the stimulatory effects of a long photophase (Hahn et al., 1997). Exposure to the short photophases of fall and winter then sensitizes them to the stimulatory effects of the long photophases they will experience the following spring, at which point they are said to be photostimulated (Dawson et al., 2001). Continued exposure to long photophases eventually leads back into a state of photorefractoriness (Nicholls et al., 1988), and the cycle continues.
In birds, first-year, inexperienced breeders often breed later in the season and have lower reproductive output than older, experienced breeders (Sæther, 1990; Rowe et al., 1994; Forslund and Part, 1995). One key difference between a first-year, photoperiodic breeder and one with prior breeding experience is their photoexperience; first-year breeders are naïve to photostimulation the first time they experience it, whereas older breeders have had prior experience with this process (i.e., are experienced) (Sockman et al., 2004).
When photoexperience is experimentally manipulated in female European starlings (Sturnus vulgaris) that do not differ in age, experienced individuals, like older individuals in natural populations, have more rapid and robust reproductive development than naïve individuals have (Sockman et al., 2004). Within four weeks of photostimulation (for the first time in naïve individuals and for the second time in experienced individuals), experienced individuals show greater body mass, higher concentrations of luteinizing hormone and vitellogenin circulating in the blood plasma, larger ovarian follicles, and a higher index of secretion of gonadotropin releasing hormone (GnRH) than naïve individuals show (Sockman et al., 2004). Thus, photoexperience influences the rate or magnitude of the hypothalamic, pituitary, and gonadal activity that characterizes the annual onset of reproductive development, possibly forming, in part, a basis for age-related differences in at least one reproductive decision, that of reproductive effort or timing.
Even in highly photoperiodic species, such as the European starling (Dawson, 2007), non-photoperiodic, ecological and social cues are also important in stimulating reproductive development (Wingfield and Farner, 1980; Wingfield, 1983). In female songbirds, one such social cue is male song, an acoustic signal important in species recognition, mate choice, and timing of reproduction (Hinde and Steel, 1978; McGregor, 1991; Ball and Bentley, 2000; Ball et al., 2006; Maney et al., 2007). Numerous studies on a variety of species have yielded considerable insight into how the songbird brain processes song cues (for review, see Ball et al., 2006; Sockman, 2007). Specifically, auditory input ascends from the periphery to the nucleus mesencephalicus lateralis pars dorsalis, which projects to the thalamic relay nucleus ovoidalis. Ovoidalis sends strong projections to Field L, an area of the songbird auditory telencephalon that is homologous to the mammalian primary auditory cortex. Field L projects to two adjacent areas of the auditory telencephalon analogous to the mammalian non-primary auditory cortex, the caudomedial mesopallium (CMM) and the caudomedial nidopallium (NCM), which themselves reciprocally connect (Vates et al., 1996) and show high-level, experience-dependent plasticity in the representation of learned songs (Gentner and Margoliash, 2003).
By quantifying the expression of the immediate-early gene ZENK (the avian homologue of and an acronym for zif-268, egr-1, NGFI-A, and Krox-24) after brief exposure to song stimuli, several investigators have demonstrated the important role the CMM and NCM play in processing song cues (for review, see Ball et al., 2006; Sockman, 2007). ZENK expression tends to be higher in individuals exposed to conspecific song than in those exposed to heterospecific song (Mello et al., 1992; Mello and Clayton, 1994). In the European starling, females prefer long songs over short songs in a mate-choice context (Eens et al., 1991; Mountjoy and Lemon, 1996; Eens, 1997; Gentner and Hulse, 2000), and long songs induce greater ZENK expression in the CMM and NCM than short songs do (Gentner et al., 2001; Sockman et al., 2002; Sockman et al., 2005; Sockman, 2007). These and similar results generated in other songbird and even non-songbird species (e.g., Eda-Fujiwara et al., 2003; Maney et al., 2003; Leitner et al., 2005) reveal a type of forebrain responsiveness to song variation that is relevant to mate-choice. Moreover, in the white-crowned sparrow (Zonotrichia leucophrys), ZENK expression in the CMM and NCM is positively correlated with the strength of the female's mate-choice behavior in response to the song to which she is exposed, suggesting a role for these areas of her brain in the reproductive decision of mate choice (Maney et al., 2003).
In European starlings, experience can affect activity of the auditory forebrain, in that the quality of the song environment to which the female has been recently exposed influences ZENK expression in the CMM and NCM (Sockman et al., 2002; Sockman et al., 2005; Sockman, 2007). Given (1) the important role photoexperience can play in the hypothalamic, pituitary, and gonadal activity that characterizes the annual onset of reproductive development (Sockman et al., 2004), (2) the potential role activity in the auditory forebrain might play in reproductive decisions (for review, see Sockman, 2007), and (3) the effects of some types of experience on activity in the auditory forebrain (Sockman et al., 2002; Gentner and Margoliash, 2003; Sockman et al., 2005; Sockman, 2007), we asked in this study whether photoexperience might influence neural activity in the auditory telencephalon of female starlings, as measured by ZENK expression. We predicted that, relative to naïve females, females experienced with photostimulation have elevated ZENK expression in the auditory forebrain. We also asked whether photoexperience influences the responsiveness of the female auditory forebrain to song quality, as measured by the difference between the ZENK response to long and to short songs (i.e., we asked whether photoexperience interacts with song length to influence forebrain ZENK expression).
METHODS
The Institutional Animal Care and Use Committee at Johns Hopkins University (Baltimore, U.S.A.), where we held the animals, approved the procedures described in this study. Many of the procedures were thoroughly described in a previous publication (Sockman et al., 2004). Below we summarize these procedures and also detail those specific to the present study. The previous (Sockman et al., 2004) and present study made use of the same individual birds.
Capture, Housing, and Initial Photosensitization
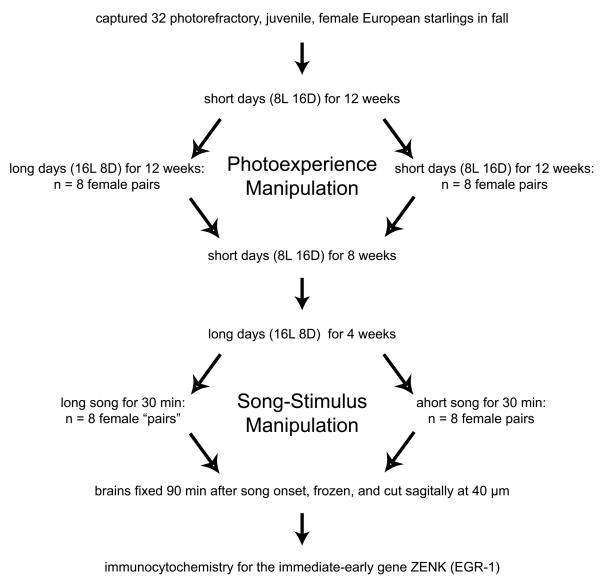
We captured European starlings in September 2001 on a farm near Baltimore, Maryland, U.S.A., determined by their brown speckled plumage that they had all hatched that spring or summer (Kessel, 1951), and held them on a daily photoperiod of 8 hours light and 16 hours dark (8L 16D) (Fig. 1). Throughout the study we provided them with ad libitum access to food and water. In mid-November, we anesthetized (ca. 5 mg secobarbitol injected i.m.) and sexed them by laparotomy.
Figure 1.
Experiment procedure. Because one female died during the photoexperience manipulation, one female “pair” in the song-stimulus manipulation was composed of only one female. For graphical simplicity, the two levels of the photoexperience manipulation and the two levels of the song-stimulus manipulation are depicted as spatially segregated. However, in the experiment, the replicates of one level of each manipulation were spatially interspersed with the replicates of the other level of each manipulation.
Photo-Experience Manipulation
Twelve weeks after capture and onset of 8L 16D, we randomly paired 32 females in 16, individually lit and ventilated sound-attenuation chambers, half on 8L 16D (naïve group) spatially interspersed in one room with the other half on 16L 8D (experienced group) (Fig. 1). Approximately 5 wk into this phase, one female in the experienced group died and was excluded from analysis. We periodically handled each individual in each group to determine the status of her feather molt and, as part of another study (Sockman et al., 2004), to weigh her and collect a blood sample.
Eight weeks of 8L 16D is typically sufficient to ensure starlings are photosensitive (Dawson, 1991), and 12 wk of 16L 8D is typically sufficient to drive starlings photorefractory (Dawson and Goldsmith, 1983). However, we confirmed photorefractoriness in the experienced group and lack of photorefractoriness in the naïve group by their differences in the progress of molt, the onset of which presages photorefractoriness due to the fact that both molt and photorefractoriness may be regulated by the same physiological mechanism (Dawson, 1998; Dawson and Sharp, 1998). All experienced and no naïve females initiated molt well in advance of the end of this photo-experience phase of the experiment (Sockman et al., 2004).
Photosensitization followed by Photostimulation of All Females
Twelve weeks after transfer to the chambers (and transfer to 16L 8D for the experienced group), we removed all females from the chambers and placed them in open wire cages together in one room on 8L 16D (Fig. 1). After 8 wk, we changed the photoperiod to 16L 8D to simultaneously photostimulate naïve individuals for the first time and experienced individuals for the second time in their lives. To summarize, after holding half the females (naïve group) on a winter-like photoperiod (8L 16D) for 32 wk and passing the other half (experienced group) through a complete, simulated annual photocycle (8L 16D for 12 wk, 16L 8D for 12 wk, and then 8L 16D for 8 wk), we simultaneously photostimulated all of them. We then exposed them to male song (Fig. 1).
Song-Stimulus Manipulation
We exposed females to male song at a time in their photoperiodic cycle when they were likely to still be very responsive to reproductive stimuli, that is, at a time when hypothalamic neurons were still secreting GnRH. In first-year female starlings, mean hypothalamic GnRH concentrations do not decline (i.e., females do not begin regression of the hypothalamo-pituitary-gonadal axis) before 6 wk after transfer from 8L 16D to 18L 6D (Dawson et al., 1985). On 16L 8D, GnRH concentrations would decline even later. However, due to inter-individual variation (from photo-experience or other factors) and to be sure that measurements occurred when photo-induced hypothalamo-pituitary-gonadal capacity was still ascending, we exposed females to song stimuli after about 4 wk of photostimulation. Below are the details of the song exposure.
Beginning 3 wk and 6 days following photostimulation, one experienced and one naïve female were placed together in one of four sound attenuating chambers. Thirty minutes later, we exposed them to a 30 min short-song stimulus played through a speaker within the chamber and 60 min later anesthetized (6.5 mg secobarbitol injected i.m.) them and collected their brains for ZENK immunocytochemistry (Fig. 1). Thirty min after placing the first pair in the first chamber, we placed another pair in the second chamber and proceeded as described above, except we exposed them to a long-song stimulus instead of a short-song stimulus. We continued staggering female pairs every 30 min, alternating between short-song and long-song stimuli for each pair, until we had exposed eight pairs to song and collected their brains (2 pairs per chamber, each chamber balanced with respect to song stimulus). We repeated these procedures the following day on the remaining individuals.
Song Stimuli
Details of the song recordings have been described previously (Gentner and Hulse, 2000). A large library of complete songs was recorded from two laboratory-housed males, each directing song at a female. From each male's songs, 12 exemplars were selected, which, based on length, were divided into two sets of six: a long-song set and a short-song set, with mean song lengths of 55.2 and 26.0 sec, respectively, for one male and 55.6 and 25.4 sec for the other. Song sets were repeated in each file and enough silence inserted between songs to ensure that neither total song nor total silence duration differed between the long- and short-song sets. We exposed half the female pairs to the song sets from one male and half to those from the other. Because we used recordings from only two males, we cannot extend our conclusions to long and short songs in general (Kroodsma et al., 2001; Wiley, 2003). However, because a previous study showed that females in a mate-choice context preferred each of these exact long-song sets to each of these exact short-song sets (Gentner and Hulse, 2000), results are based on responses to stimuli known to differ in their attractiveness to females.
Immunocytochemistry and ZENK (EGR-1) Quantification
After sectioning brains on a cryostat at 40 μm in the sagittal plain, we performed immunocytochemistry for the protein ZENK as previously described by Sockman et al. (2002) (Fig. 1). Briefly, this immunocytochemistry procedure involved initially treating the acrolein-fixed tissue with a sodium borohydride solution, blocking endogenous peroxides with a hydrogen peroxide solution, and suppressing endogenous avidin and biotin binding activity with a blocking kit, before incubating for 40-48 hr at 4°C in the primary antibody, which we have previously validated for use in female European starlings (Sockman et al., 2002). We followed this with an incubation with biotinylated goat anti-rabbit secondary antibody to which an avidin-biotin horseradish peroxidase complex bound and which we colored with a nickel-enhanced diaminobenzidine tetrahydrachloride solution. We washed tissue in solutions of PBS or PBS with Triton-X-100 detergent (Thermo Fisher Scientific, Inc., Waltham, MA, U.S.A., Cat. No. BP151-500) between steps.
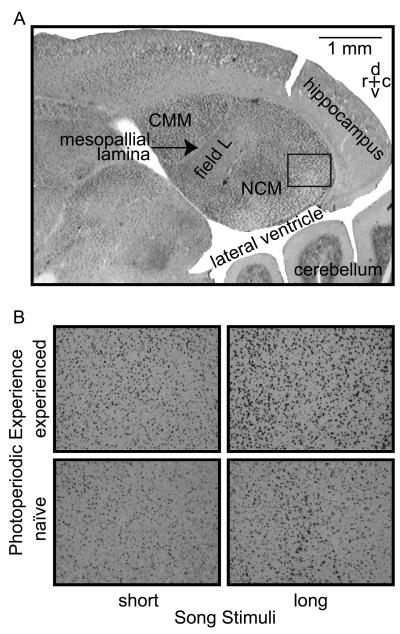
All image capture and quantification was conducted blind to the treatment of the animals. Using Köhler Illumination on a Leica DM4000 Digital Research Microscope with Leica DFC480 color digital camera, brightfield images of 683- × 512-μm areas were collected from the CMM and a dorsal (NCMd) and ventral (NCMv) region of the NCM (Fig. 2). These were magnified 320 times as 8-bit gray-scale images on an Apple Macintosh G5 dual-processor computer connected by firewire to the microscope camera. Of these brain areas, we present analyses of NCMd only, because of a bleached appearance and therefore lack of immunoreactivity (both specific and non-specific) in the CMM and NCMv of several females. There were no such problems for any of the females in any locations in the NCMd, which we henceforth refer to as the NCM. We do not know the reason for the histological problem, but it may have been due to limited penetration of the acrolein and therefore insufficient fixation of the regions that are furthest from the brain's surface (i.e., CMM and NCMv).
Figure 2.
(A) Photomicrograph of a sagittal section of the female European starling auditory telencephalon, depicting the location (rectangle) for quantification of ZENK-immunoreactivity. (B) Representative photomicrographs of ZENK-immunoreactivity for each of the four treatment groups. CMM, caudomedial mesopallium; NCM, caudomedial nidopallium; d, dorsal; v, ventral; r, rostral; c, caudal.
ZENK-immunoreactivity (ZENK-ir) was quantified in every fourth-cut section of tissue from near the midline to approximately 1120 μm bilateral, resulting in seven anatomical locations (sagittal sections) per hemisphere spanning previously described boundaries of NCM (Gentner et al., 2001). Using the software ImageJ (version 1.34s, National Institutes of Health), we determined — for one individual — a mean gray value for background (i.e., nonspecific) immunoreactivity, a threshold gray value for specific immunoreactivity, and a ratio between these two values to be used as a factor by which to multiply each of the other individuals' mean background gray values. This provided a threshold gray value for each individual that was adjusted according to the individual's mean background gray value. We then used an automatic ImageJ routine to determine the proportion of pixels that was above the individual's threshold gray-value. For analyses, we used the mean of the two hemispheres for each of the seven locations.
Analysis
Our data consisted of a combination of fixed (photoexperience, song stimulus, location) and hierarchically nested random (individual, song-exposure pair, and photoexperience pair) effects, each of which may differ from the others in its correlation structure. Therefore, we used a mixed-model framework (using the software Stata IC 10.0 for the Macintosh, Stata Corporation, College Station, Texas, U.S.A.), which is readily amenable to complex data sets with combinations of fixed and hierarchically structured random effects (Burton et al., 1998; Goldstein et al., 2002; Rabe-Hesketh and Skrondal, 2005). This mixed-modelling approach has the added advantage of estimating parameters with maximum likelihood procedures (we used restricted maximum likelihood), which are often more accurate and more powerful than the traditional least squares estimates used in analyses of variance and other linear models (e.g., Goldschmidt and Timm, 2003; Whitman, 2003; Orton and Lark, 2007). Specifically, we analyzed ZENK-ir as a function of photoexperience, song stimulus, location, and all their interactions, with measurement nested within individual female, individual female nested within photoexperience pair, and photoexperience pair nested within song pair, each as a random coefficient on location.
The goal of our analyses was as much to model our data efficiently, using the best subset of the predictors and interactions mentioned above, as it was to test statistical hypotheses. Traditional model selection (e.g., forward- or backward-stepping) procedures rely on arbitrary criteria (e.g., a P value less than or greater than 0.05 or 0.10) for the inclusion or exclusion of a factor. We opted for a more objective assessment of model fit by taking an information theoretic approach, in which we calculated the Akaike Information Criterion (AIC) (Burnham and Anderson, 2002) value for a null model containing only an intercept and for each model in which we added the different combinations of the predictors of interest and their interactions. A model's AIC value reflects its goodness of fit relative to its number of parameters, using the loglikelihood of the model while penalizing for each parameter. If the increase in loglikelihood offsets the increase in complexity with the addition of a parameter, then the AIC value declines, indicating a more efficient model. We calculated an AIC value for every combination of predictor and interaction, with the single stipulation that a model must contain all the lower order terms within any included higher order term. In other words, we did not test a model that contained an intercept, photoexperience, and the interaction between photoexperience and song stimulus, without also including song stimulus as a main effect (because it is part of the included interaction term). We then report the results from the statistical hypothesis tests conducted for only those predictors remaining in the single model with the lowest AIC value (i.e., the AIC-best model) (see Table 1). For additional information on using this mixed, multi-level modelling and information theoretic approach, see Sockman et al. (2008).
Table 1.
AIC values and parameter estimates for the modeling of ZENK-ir in the caudomedial nidopallium of adult female European starlings
| Predictor | AIC | Estimate | Standard error | P value |
|---|---|---|---|---|
| Intercept | −892.113 | 0.1424 | 0.0049 | < 0.001 |
| Photo | −907.392 | 0.0288 | 0.0051 | < 0.001 |
| Song | −903.521 | 0.0145 | 0.0053 | 0.006 |
| Location | −935.611 | −0.0132 | 0.0011 | < 0.001 |
| Photo × Song | −926.661 | |||
| Photo × Location | −914.129 | |||
| Song × Location | −900.121 | |||
| Photo × Song × Location | −889.889 |
ZENK-ir is the proportion of the quantified area of the caudomedial nidopallium that was immunoreactive for the immediate early gene ZENK. Photo is the photoperiodic experience, coded 0 for naïve and 1 for experienced. Song is the song stimulus, coded 0 for short songs and 1 for long songs. Location is an integer from 1-7 referring to the sagittal tissue section (1: approximately 160 μm from the midline; 7: approximately 1120 μm from the midline). Each AIC value applies to a model containing the predictor to its left and all predictors above it. For example, the AIC value of −926.661 applies to a model containing an intercept, photoperiodic experience, song stimulus, location, and the interaction between photoperiodic experience and song stimulus. Not shown but also assessed with AIC were numerous additional models, such as those in which predictors were introduced in alternate sequences (e.g., song stimulus before photoperiodic experience). None of these models were better (had a lower AIC value) than the lowest shown above. Estimates, standard errors, and P values are provided for those parameters in the final model only (i.e., the model with the lowest AIC value).
RESULTS
We observed robust ZENK-ir in the NCM, regardless of photoexperience or song-stimulus treatment (Fig. 2). Nonetheless, there was considerable variation between individuals in NCM ZENK-ir. The most efficient model (i.e., that with the lowest AIC value) explaining this variation was one containing an intercept and the factors photoexperience, song stimulus, and location. AIC did not support the addition of any of the three possible two-way interaction terms or the three-way interaction in the model (Table 1).
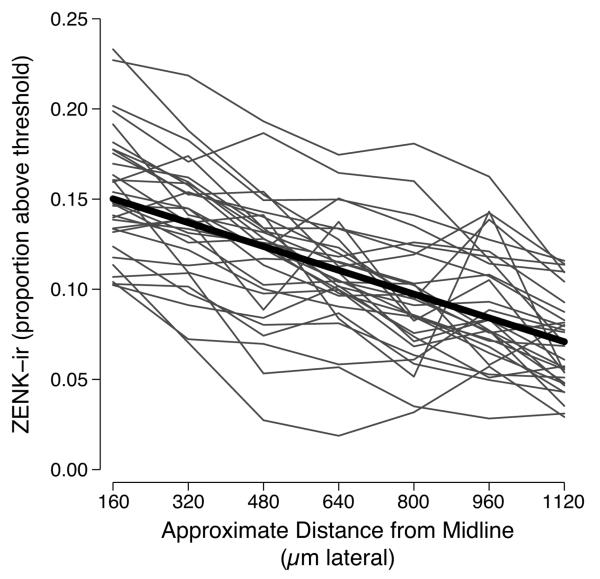
NCM ZENK-ir showed a general decline with increasing distance from the midline (Table 1, Fig. 3). However, we did not detect an effect of either photoexperience or song stimulus on the slope of this decline, as indicated by the lack of AIC support for the interactions between photoexperience and location, song and location, or photoexperience, song, and location together. Despite this general decline in ZENK-ir, we did observe some variation between the responses of individual females in this location effect. This between-female variation may be due to the true relationship between ZENK-ir and location or to the precision with which we matched locations (section numbers) between females, given the vagaries of sectioning heterogeneous tissue on a cryostat.
Figure 3.
ZENK-immunoreactivity in the caudomedial nidopallium of female European starlings relative to the distance from the midline. The dark line reflects the mean response of individual birds (light lines).
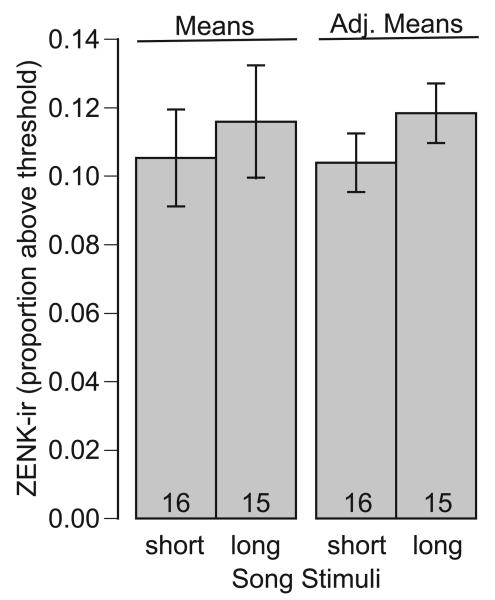
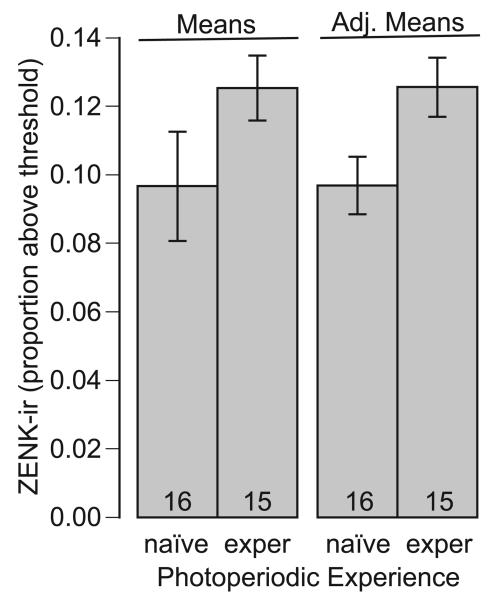
Exposure to the long-song stimulus induced greater NCM ZENK-ir than exposure to the short-song stimulus did (Table 1, Fig. 4). We found no evidence that this difference between responses to the two song stimuli was influenced by the lateral location in the NCM or by the individual's photoexperience, given the lack of AIC support for the inclusion of these interaction terms in the model. We also found that photoexperience affected NCM ZENK-ir (Table 1, Fig. 5). Females with prior experience with photostimulation showed greater ZENK-ir in the NCM than did females experiencing photostimulation for the first time. Again, we found no evidence that this difference was dependent on song stimulus or location, as indicated by the lack of AIC support for inclusion of these interaction terms in the model.
Figure 4.
ZENK-immunoreactivity in the caudomedial nidopallium of female European starlings relative to the type of song stimulus to which they were exposed. Both means and means adjusted to the response to other factors in the statistical model (photoexperience) are depicted, together with 95% confidence whiskers.
Figure 5.
ZENK-immunoreactivity in the caudomedial nidopallium of female European starlings relative to photoexperience (naïve vs. experienced). Both means and means adjusted to the response to other factors in the statistical model (song stimulus) are depicted, together with 95% confidence whiskers.
DISCUSSION
The proximate mechanisms by which age and experience influence reproductive decisions are poorly understood, but recent studies implicate a role for photostimulation experience (photoexperience) in photoperiodic species. Previously, we had shown in the European starling that photoexperience affects the hypothalamic, pituitary, and gonadal activity associated with the annual onset of reproductive development (Sockman et al., 2004). Here we provide evidence that photoexperience also influences activity in an area of the brain important in processing conspecific song, a supplementary, social cue likely influencing a host of reproductive decisions, including timing of reproduction, mate recognition, and mate choice (Hinde and Steel, 1978; McGregor, 1991; Ball and Bentley, 2000; Ball et al., 2006; Maney et al., 2007). Specifically, photoexperience elevated expression of the immediate early gene ZENK in the NCM of female starlings.
We have not provided evidence that photoexperience affects forebrain responsiveness to song itself, given that we did not include a no-song group in our experiment. Thus, although the ZENK expression we document could be song induced, it could also be independent of the song exposure. Still, given that ZENK expression in female white-crowned sparrows is correlated with the strength of mate-choice behavior (Maney et al., 2003), the elevated levels due to photoexperience are intriguing and may be related to changing responsiveness to song when females are making reproductive decisions or to a changing song-independent baseline level, which might influence the probability of ZENK expression reaching some hypothetical behaviorally relevant threshold when she eventually does hear song.
A limitation on the number of animals at our disposal and our primary interest in whether photoexperience influences forebrain responsiveness to song quality precluded the inclusion of a no-song group in this experiment. We did find that the preferred long songs give rise to greater ZENK expression in the auditory forebrain than do the less-preferred short songs, thus replicating two other studies in European starlings (Gentner et al., 2001; Sockman et al., 2002). However, we did not find that photoexperience influences the difference between ZENK expression in response to the preferred songs and that in response to non-preferred songs (i.e., we did not find compelling evidence for an effect of the interaction between photoexperience and song quality). Thus, we do not have evidence that photoexperience affects forebrain responsiveness to stimulus variation relevant to mate-choice. If ZENK expression in the female auditory forebrain is functionally tied to the particular choice of mate, then our lack of effect of the interaction between photoexperience and song quality leads to the prediction that photoexperience has little or no effect on which male a female is likely to chose based on his song alone (Sockman, 2007; Sockman and Salvante, 2008).
Based on the studies described below, the most likely mechanism by which photoexperience elevates ZENK expression in the auditory forebrain of female songbirds is one mediated by estradiol concentrations circulating in the blood plasma and possibly norepinephrine secretion in the auditory forebrain. Accordingly, we propose that experienced females have greater concentrations of estradiol circulating in their blood plasma and that estradiol acts on receptors in the auditory forebrain or in areas projecting to the auditory forebrain to influence ZENK expression through a noradrenergic-dependent process. Previously (Sockman et al., 2004), we showed that photoexperience elevates an index of secretion of GnRH, secretion of luteinizing hormone, ovarian follicle diameter, and circulating concentrations of the primary yolk-precursor protein, vitellogenin, the synthesis of which is estrogen-dependent (Christians and Williams, 1999; Williams, 1999; Williams, 1999). Although we did not measure estradiol in that study, it is very likely that, given the effects listed immediately above, estradiol was also elevated by photoexperience (Sockman et al., 2004).
Maney and colleagues have recently conducted a series of studies on female white-throated sparrows (Zonotrichia albicollis) that strongly implicates a requirement of high estradiol levels for the song-induced elevation of ZENK expression in the auditory forebrain (Maney et al., 2006; LeBlanc et al., 2007) and in other areas of the brain, including a network of nuclei involved in social behavior (Maney et al., 2008). Conspecific song elevates ZENK over frequency-matched tones in females with experimentally or naturally elevated estradiol concentrations but not in females with low estradiol concentrations (Maney et al., 2006; Maney et al., 2008). This ZENK-potentiating effect of estradiol may be mediated by catecholamine secretion in the auditory forebrain (LeBlanc et al., 2007; Lynch and Ball, 2008), in that experimental elevation of estradiol also elevates norepinephrine levels in the caudomedial mesopallium compared to control females with low estradiol levels [Sanford et al., unpubl data]. Moreover, in canaries (Serinus canaria), chemical lesion of forebrain noradrenergic fibers reduces song-induced elevation of ZENK in parts of the auditory forebrain (Lynch and Ball, 2008). It is possible that photoexperience elevates estradiol concentrations, which then elevate norepinephrine levels in the auditory forebrain and that norepinephrine modulates ZENK expression in response to song. This hypothesis would be consistent with data showing that the quality of the prevailing song environment, which also modulates song-induced ZENK expression in the female auditory forebrain (Sockman et al., 2002; Sockman et al., 2005), itself modulates norepinephrine secretion in the auditory forebrain (Sockman and Salvante, 2008) and with the hypothesis that norepinephrine and perhaps other monoamines play key roles in modulating song-induced reproductive decisions based on the context of the physical, ecological, or social environment (Ball et al., 2006; Sockman, 2007; Lynch and Ball, 2008; Sockman and Salvante, 2008). Still, we currently lack direct evidence that the effects of photoexperience on forebrain ZENK expression are mediated by estradiol and norepinephrine, and, thus, at this point, this mechanism is merely a hypothesis.
Previously, we have published evidence that degree of photosensitivity (also likely influencing plasma estradiol concentrations) may influence ZENK expression in the auditory forebrain of female starlings (Sockman et al., 2002). Due to the design of that experiment, we could not rule out other factors such as the passage of time. In the presently described experiment, however, we can rule out other factors (with a probability reflected in our P values) as being solely responsible for the difference between our two photoexperience groups. Thus, regardless of the mechanisms or behavioral ramifications, our findings may be the first to demonstrate an effect of photoperiodic experience on gene expression in the forebrain of any organism and, in particular, on gene expression in the auditory forebrain of an organism that relies heavily on auditory cues when making reproductive decisions.
Acknowledgments
Contract grant sponsors: NICHD (National Institute of Child Health and Human Development) and NINDS (National Institute of Neurological Disorders and Stroke); contract grant numbers: F32 41854 to K.W.S. and R01 35467 to G.F.B.
We thank S.R. Vora for assistance in data collection, T.Q. Gentner for constructing the song stimuli, and T. P. Hahn and J. Balthazart for their insight on study design.
REFERENCES
- Ball GF, Bentley GE. Neuroendocrine mechanisms mediating the photoperiodic and social regulation of seasonal reproduction in birds. In: Wallen K, Schneider JE, editors. Reproduction in Context: Social and Environmental Influences on Reproductive Physiology and Behavior. Massachusetts: MIT Press; Cambridge: 2000. pp. 129–158. [Google Scholar]
- Ball GF, Sockman KW, Duffy DL, Gentner TQ. A neuroethological approach to song behavior and perception in European starlings: interrelationships among testosterone, neuroanatomy, immediate early gene expression, and immune function. Adv Stud Behav. 2006;36:59–121. (doi: 10.1016/S0065-3454(06)36002-0) [Google Scholar]
- Burnham HP, Anderson DR. Model Selection and Multimodel Inference. Springer-Verlag; New York: 2002. [Google Scholar]
- Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modeling. Stat Med. 1998;17:1261–1291. doi: 10.1002/(sici)1097-0258(19980615)17:11<1261::aid-sim846>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Christians JK, Williams TD. Effects of exogenous 17β-estradiol on the reproductive physiology and reproductive performance of European starlings (Sturnus vulgaris) J Exper Biol. 1999;202:2679–2685. doi: 10.1242/jeb.202.19.2679. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. Reproductive success. In: Clutton-Brock TH, editor. Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. University of Chicago Press; Chicago: 1988. pp. 472–485. [Google Scholar]
- Dawson A. Effect of daylength on the rate of recovery of photosensitivity in male starlings (Sturnus vulgaris) J Reprod Fertil. 1991;93:521–524. doi: 10.1530/jrf.0.0930521. [DOI] [PubMed] [Google Scholar]
- Dawson A. Photoperiodic control of the termination of breeding and the induction of moult in house sparrows Passer domesticus. Ibis. 1998;140:35–40. [Google Scholar]
- Dawson A. Seasonality in a temperate zone bird can be entrained by near equatorial photoperiods. Proc R Soc Lond B. 2007;274:721–725. doi: 10.1098/rspb.2006.0067. (doi: 10.1098/rspb.2006.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Follett BK, Goldsmith AR, Nicholls TJ. Hypothalamic gonadotrophin-releasing hormone and pituitary and plasma FSH and prolactin during photostimulation and photorefractoriness in intact and thyroidectomized starlings (Sturnus vulgaris) J Endocrinol. 1985;105:71–77. doi: 10.1677/joe.0.1050071. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith AR. Plasma prolactin and gonadotrophins during gonadal development and the onset of photorefractoriness in male and female starlings (Sturnus vulgaris) on artificial photoperiods. J Endocrinol. 1983;97:253–260. doi: 10.1677/joe.0.0970253. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythm. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Dawson A, Sharp PJ. The role of prolactin in the development of reproductive photorefractoriness and postnuptial molt in the European starling (Sturnus vulgaris) Endocrinology. 1998;139:485–490. doi: 10.1210/endo.139.2.5701. [DOI] [PubMed] [Google Scholar]
- Eda-Fujiwara H, Satoh R, Bolhuis JJ, Kimura T. Neuronal activation in female budgerigars is localized and related to male song complexity. Eur J Neurosci. 2003;17:149–154. doi: 10.1046/j.1460-9568.2003.02414.x. [DOI] [PubMed] [Google Scholar]
- Eens M. Understanding the complex song of the European starling: an integrated ethological approach. Adv Stud Behav. 1997;26:355–434. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Male song as a cue for mate choice in the European starling. Behaviour. 1991;116:210–238. [Google Scholar]
- Forslund P, Part T. Age and reproduction in birds - hypotheses and tests. Trends Ecol Evol. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH. Female European starling preference and choice for variation in conspecific male song. Anim Behav. 2000;59:443–458. doi: 10.1006/anbe.1999.1313. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature. 2003;424:669–674. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L, Timm NH. A comparison of noniterative generalized least squares and iterative maximum likelihood estimators when testing hypotheses in random coefficient growth curve models. Comm Stat Simul Comp. 2003;32:297–318. (doi: 10.1081/sac-120017493) [Google Scholar]
- Goldstein H, Browne W, Rasbash J. Partitioning variation in multilevel models. Understanding Statistics. 2002;1:223–231. [Google Scholar]
- Hahn TP, Boswell T, Wingfield JC, Ball GF. Temporal flexibility in avian reproduction: patterns and mechanisms. Curr Ornithol. 1997;14:39–80. [Google Scholar]
- Hinde RA, Steel E. The influence of daylength and male vocalizations on the estrogen-dependent behavior of female canaries and budgerigars, with discussion of data from other species. Adv Stud Behav. 1978;8:39–73. [Google Scholar]
- Kessel B. Criteria for sexing and aging European starlings (Sturnus vulgaris) Bird Banding. 1951;22:16–23. [Google Scholar]
- Kroodsma DE, Byers BE, Goodale E, Johnson S, Liu W-C. Pseudoreplication in playback experiments, revisited a decade later. Anim Behav. 2001;61:1029–1033. [Google Scholar]
- LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. (doi: 10.1016/j.brainres.2007.06.086) [DOI] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64:275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Ball GF. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain Behav Evolut. 2008;72:207–214. doi: 10.1159/000157357. (doi: 10.1159/000157357) [DOI] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lake JI, Lange HS, O'Brien S. Rapid neuroendocrine responses to auditory courtship signals. Endocrinology. 2007;148:5614–5623. doi: 10.1210/en.2007-0879. (doi: 10.1210/en.2007-0879) [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Maney DL, MacDougall-Shackleton EA, MacDougall-Shackleton SA, Ball GF, Hahn TP. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. J Comp Physiol A. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- McGregor PK. The singer and the song: on the receiving end of bird song. Biol Rev. 1991;66:57–81. [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy DJ, Lemon RE. Female choice for complex song in the European starling: a field experiment. Behav Ecol Sociobiol. 1996;38:65–71. [Google Scholar]
- Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Orton TG, Lark RM. Estimating the local mean for Bayesian maximum entropy by generalized least squares and maximum likelihood, and an application to the spatial analysis of a censored soil variable. Eur J Soil Sci. 2007;58:60–73. (doi: 10.1111/j.1365-2389.2006.00800.x) [Google Scholar]
- Pugesek BH. Increased reproductive effort with age in the California gull (Larus californicus) Science. 1981;212:822–823. doi: 10.1126/science.212.4496.822. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. Stata Press; College Station, Texas: 2005. p. 317. [Google Scholar]
- Rowe L, Ludwig D, Schluter D. Time, condition, and the seasonal decline of avian clutch size. Am Nat. 1994;143:698–722. [Google Scholar]
- Sæther B-E. Age-specific variation in reproductive performance of birds. Curr Ornithol. 1990;7:251–283. [Google Scholar]
- Sockman KW. Neural orchestration of mate-choice plasticity in songbirds. J Ornithol. 2007;148:S225–S230. (doi: 10.1007/s10336-007-0151-3) [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc R Soc Lond B. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Complementary neural systems for the experience-dependent integration of mate-choice cues in the European starling. J Neurobiol. 2005;62:72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Develop Neurobiol. 2008;68:656–668. doi: 10.1002/dneu.20611. (doi: 10.1002/dneu.20611) [DOI] [PubMed] [Google Scholar]
- Sockman KW, Sharp PJ, Schwabl H. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility in clutch size, incubation behaviour, and yolk-androgen deposition. Biol Rev. 2006;81:629–666. doi: 10.1017/S1464793106007147. (doi: 10.1017/S1464793106007147) [DOI] [PubMed] [Google Scholar]
- Sockman KW, Weiss J, Webster MS, Talbott V, Schwabl H. Sex-specific effects of yolk-androgens on growth of nestling American kestrels. Behav Ecol Sociobiol. 2008;62:617–625. (doi: 10.1007/s00265-007-0486-z) [Google Scholar]
- Sockman KW, Williams TD, Dawson A, Ball GF. Prior experience with photostimulation enhances photo-induced reproductive development in female European starlings: a possible basis for the age-related increase in avian reproductive performance. Biol Reprod. 2004;71:979–986. doi: 10.1095/biolreprod.104.029751. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Whitman CS. Accelerated life test calculations using the method of maximum likelihood: an improvement over least squares. Microelect Rel. 2003;43:859–864. (doi: 10.1016/s0026-2714(03)00071-4) [Google Scholar]
- Wiley RH. Is there an ideal behavioural experiment? Anim Behav. 2003;66:585–588. [Google Scholar]
- Williams GC. Adaptation and Natural Selection. Princeton University Press; Princeton, New Jersey: 1966. [Google Scholar]
- Williams TD. Avian reproduction. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. Vol. 1. Academic Press; San Diego, California: 1999. pp. 325–336. [Google Scholar]
- Williams TD. Parental and first generation effects of exogenous 17β-estradiol on reproductive performance of female zebra finches (Taeniopygia guttata) Horm Behav. 1999;35:135–143. doi: 10.1006/hbeh.1998.1506. [DOI] [PubMed] [Google Scholar]
- Williams TD, Dawson A, Nicholls TJ, Goldsmith AR. Reproductive endocrinology of free-living nestling and juvenile starlings, Sturnus vulgaris; an altricial species. J Zool. 1987;212:619–628. [Google Scholar]
- Williams TD, Dawson A, Nicholls TJ, Goldsmith AR. Short days induce premature reproductive maturation in juvenile starlings, Sturnus vulgaris. J Reprod Fertil. 1987;80:327–333. doi: 10.1530/jrf.0.0800327. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Environmental and endocrine control of avian reproduction: an ecological approach. In: Mikami S-i, Homma K, Wada M., editors. Avian Endocrinology: Environmental and Ecological Perspectives. Japan Scientific Society Press; Tokyo: 1983. pp. 265–288. [Google Scholar]
- Wingfield JC, Farner DS. Control of seasonal reproduction in temperate-zone birds. Prog Reprod Biol. 1980;5:62–101. [Google Scholar]