Abstract
Objective
Compare effect of intravitreal triamcinolone acetonide with focal/grid photocoagulation on progression of diabetic retinopathy.
Methods
Exploratory analysis performed on subjects with diabetic macular edema randomly assigned to laser or intravitreal triamcinolone acetonide (1mg or 4mg). Fundus photographs were obtained at baseline, 1, 2 and 3 years.
Main Outcome Measures
Progression to proliferative diabetic retinopathy (PDR) or worsening of 2 or more severity levels on reading center masked assessment of 7-field fundus photographs, plus additional eyes that received panretinal photocoagulation (PRP) or had a vitreous hemorrhage.
Results
Cumulative probability of progression of retinopathy at 2 years was 31% (laser), 29% (1mg), and 21% (4mg), (compared with laser group, P=0.65 in 1mg group, and 0.005 in 4mg group). These differences appeared sustained at 3 years.
Conclusions
Intravitreal triamcinolone acetonide (4 mg) appeared to reduce the risk of progression of diabetic retinopathy. Given the exploratory nature of this analysis, and since intravitreal triamcinolone has cataract and glaucoma side effects, use of this treatment just to reduce the rates of progression of PDR or worsening of the level of diabetic retinopathy does not seem warranted at this time.
Introduction
Progression of diabetic retinopathy, especially the development of proliferative diabetic retinopathy (PDR) with retinal neovascularization at the disc (NVD) or elsewhere (NVE), can lead to severe visual loss and new onset blindness from vitreous hemorrhage or traction detachment of the retina if left untreated.1 Despite advances in the treatment of both diabetes and diabetic retinopathy, in the United States alone there are approximately 700,000 persons with PDR, with 63,000 new cases of proliferative retinopathy and 5,000 new cases of diabetes-induced legal blindness each year. The annual United States economic impact of retinopathy-associated morbidity, some of which is from PDR, is estimated to exceed $620 million.2
Multi-centered randomized clinical trials have demonstrated that glycemic control can reduce the risk of developing PDR.3,4 In addition, the Early Treatment Diabetic Retinopathy Study (ETDRS) has shown that high risk PDR treated with PRP markedly reduces the rate of severe visual loss when comparing treated eyes with the clinical course of untreated eyes..5, 6 The Early Treatment Diabetic Retinopathy Study demonstrated that PRP applied when an eye approaches or just reaches high risk PDR (3 or 4 high risk characteristics) reduces the risk of severe vision loss to less than 4%.7 Although remarkably effective at reducing visual loss if applied in a timely and appropriate manner, the treatment success depends on careful follow-up of eyes at risk for PDR so that PRP can be initiated promptly as an eye approaches or develops high risk PDR. Panretinal (scatter) photocoagulation is inherently destructive and is associated with side effects (including, for example, macular edema with transient or permanent central vision loss or diminished peripheral vision loss8), potential complications from misdirected or excessive burns, and progression of visual loss in nearly 5% of individuals despite appropriate treatment. Thus, identification of other treatments, beyond glycemic control and PRP, to reduce the risk of progression of retinopathy is desirable.
There is some rationale to consider whether corticosteroids could reduce the risk of progression of retinopathy, including development of proliferative diabetic retinopathy. For example, corticosteroids have been shown experimentally to down regulate vascular endothelial growth factor (VEGF) production and possibly reduce breakdown of the blood-retinal barrier. Similarly, steroids have anti-angiogenic properties, possibly due to attenuation of the effects of VEGF.9, 10 Furthermore, intravitreal triamcinolone acetonide has been used in the prevention of retinal neovascularization in animal studies11, 12 and clinically.13, 14
A randomized clinical trial15,16 by the Diabetic Retinopathy Clinical Research Network (DRCR.net) was designed to determine if 1 mg or 4 mg of intravitreal triamcinolone acetonide could reduce the risk of visual acuity loss from diabetic macular edema compared with focal/grid photocoagulation. At 2 years of follow up (the primary outcome visit for the trial), focal/grid photocoagulation was more effective with fewer side effects than 1 mg or 4 mg doses of preservative-free intravitreal triamcinolone in the treatment of diabetic macular edema. Progression of retinopathy was not the primary outcome in this trial, although change in retinopathy level was a planned secondary outcome. In addition, an exploratory analysis, as described below, was undertaken to determine whether intravitreal triamcinolone as given in this trial might reduce the risk of progression of retinopathy up to 3 years.
Methods
The methods for the DRCR.net trial comparing intravitreal triamcinolone and focal/grid photocoagulation have been published in detail elsewhere15,16 with the complete protocol available on line at www.drcr.net. In brief, eligible eyes were randomized to focal/grid photocoagulation, 1 mg intravitreal preservative-free triamcinolone acetonide in 0.05 cc as often as every 4 months, or 4 mg intravitreal preservative-free triamcinolone acetonide in 0.05 cc as often as every 4 months. Study eyes had diabetic macular edema with Optical Coherence Tomography central subfield thickness of at least 250 microns with a best-corrected visual acuity letter score between 73 (approximate Snellen equivalent 20/40) and 24 (approximate Snellen equivalent 20/320) inclusive following a protocol refraction and visual acuity measurement using an electronic visual acuity protocol.17 Study eyes could not have a history of PRP within 4 months prior to randomization or an anticipated need for PRP in the 4 months following randomization. Fundus photographs were obtained at baseline and annually to assess retinopathy level by masked graders.
During planned periodic review of adverse events, it was noted that the focal/grid photocoagulation treated eyes had a greater number of vitreous hemorrhage events than the 4 mg triamcinolone treated eyes. In order to explore if this were due to an effect on retinopathy progression, a hierarchy was created to identify all cases that may have progressed up to 3 years after randomization. Specifically, progression of retinopathy up to either of these time points was defined as follows: (1) cases that progressed from non-PDR (NPDR) (level 53 or lower) to PDR (level 61 or higher) on reading center grading of 7-standard stereoscopic fundus photographs where no PDR (<level 61) was identified at baseline, plus (2) additional cases that received PRP between baseline and follow-up (that were not identified in the first situation), plus (3) additional cases that had vitreous hemorrhage between baseline and follow-up (not already identified in the first two situations), plus (4) additional cases that worsened by 2 or more levels on the ETDRS diabetic retinopathy scale on reading center grading of fundus photographs. All randomized eyes were included in analyses, regardless of baseline retinopathy severity. Although eyes with PDR at baseline cannot meet the outcome by progressing from NPDR to PDR, they can still meet the definition of progression based on the PRP or vitreous hemorrhage criteria, or by worsening 2 or more retinopathy severity levels.
Cumulative probabilities of progression of retinopathy at each 4 month interval visit up to 36 months were calculated using the life-table method. One hundred sixteen eyes from subjects who discontinued the study prior to 2 years without meeting the outcome definition were censored in the interval after their last completed visit. Following analysis of the 2 year primary outcome data, the trial was stopped. Therefore, not every subject had the potential to complete post-2 year follow up. An additional 285 eyes from subjects who discontinued the study in the third year without meeting the outcome definition were censored prior to 3 years (265 which did not have the potential to complete 3 years of follow up, and 20 which did). If photograph data were missing (due to non-completion or non-gradable images) then the outcome definition depended solely on PRP and vitreous hemorrhage criteria. If neither of these were met, it was assumed the eye would not meet photograph criteria either and was considered no event. There were 66 eyes which completed 3 years of follow up, did not have PRP or vitreous hemorrhage, and had missing photograph data which potentially could have been an event. The proportional hazards model was used to compare treatment groups adjusting for baseline visual acuity, history of prior focal/grid photocoagulation, and baseline retinopathy severity. No substantial deviations from the proportional hazards assumption were detected. A robust sandwich estimate of the covariance matrix was used to account for correlation within subjects who had 2 study eyes.18 All reported P values are two-sided. Statistical analyses were conducted using SAS version 9.1 software (SAS Institute Inc, Cary, North Carolina).
Results
Between July 2004 and May 2006, 840 eyes from 693 subjects were enrolled in the study and randomly assigned to laser (N=330), 1 mg triamcinolone (N=256), or 4 mg triamcinolone (N=254). Ninety-five percent of subjects had type 2 diabetes, 49% were women, and mean age was 63 years. Seventy-three percent of eyes had NPDR, 27% had PDR on reading center grading of baseline fundus photographs, and 16% of eyes had prior PRP. Characteristics were similar among treatment groups.
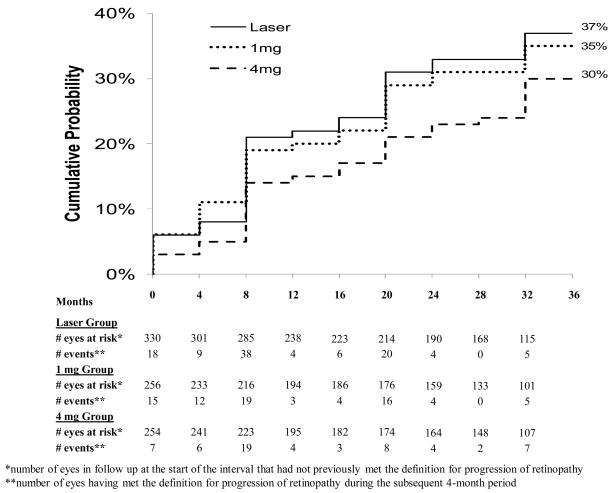
The cumulative probability of progression of retinopathy up to 1 year was 21% in the laser group, 19% in the 1 mg group, and 14% in the 4 mg group, up to 2 years was 31%, 29%, and 21%, and up to 3 years was 37%, 35%, and 30%, in the three groups, respectively (Figure 1; [at one year, P=0.71 comparing the laser and 1 mg groups, P=0.03 comparing the laser and 4 mg groups, and P=0.08 comparing the 1 mg and 4 mg groups, at 2 years P= 0.64, 0.005, and 0.03, and at 3 years P= 0.73, 0.02, and 0.07, respectively]).
Figure 1.
Cumulative probability of progression of retinopathy by treatment group over time defined as progression from non-proliferative diabetic retinopathy at baseline to proliferative diabetic retinopathy up to the given follow-up visit based on reading center assessment of fundus photographs, worsened by 2 or more levels up to the given follow-up visit on the ETDRS diabetic retinopathy scale based on reading center assessment of fundus photographs, panretinal photocoagulation received up to the given follow-up visit, or vitreous hemorrhage reported up to the given follow-up visit. Cumulative probabilities calculated using the life-table method. Subjects who dropped prior to an event were censored in the interval after their last completed visit.
Progression of retinopathy partitioned into a stepwise hierarchy of criteria to meet the outcome definition or treating each criterion as a separate outcome measure produced similar trends, although to a lesser degree than the combined definition (Table 1). Results were consistent when progression of retinopathy was evaluated within subgroups of eyes that had NPDR versus PDR at baseline, were psuedophakic at baseline, or according to the number of randomized treatments received, with a smaller cumulative probability of progression of retinopathy in the 4 mg group than the laser and 1 mg groups up to 1, 2 and 3 years within every level of subgroup evaluated (data not shown).
Table 1.
Progression of Retinopathy by Treatment Group – Cumulative Probabilities* up to 1 Year, 2 Years, and 3 Years
| 1 Year | 2 Years | 3 Years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Laser N=330 | IVT 1mg N=256 | IVT 4mg N=254 | Laser N=330 | IVT 1mg N=256 | IVT 4mg N=254 | Laser N=330 | IVT 1mg N=256 | IVT 4mg N=254 | |
| A: Total cases that progressed from NPDR to PDR from baseline up to 1, 2, and 3 years based on reading center assessment of fundus photographs | 9% | 7% | 4% | 15% | 11% | 8% | 19% | 14% | 16% |
| B: Total/Additional (not counted in A) cases that received PRP up to 1,2, and 3 years | 7%/5% | 6%/3% | 3%/3% | 14%/9% | 10%/6% | 9%/5% | 18%/10% | 11%/7% | 12%/6% |
| C: Total/Additional (not counted in A or B) cases that reported vitreous hemorrhage up to 1, 2, and 3 years | 11%/6% | 13%/8% | 7%/6% | 16%/6% | 16%/9% | 11%/5% | 19%/6% | 20%/12% | 15%/5% |
| D: Total/Additional (not counted in A, B or C) cases that worsened by 2 or more levels on the ETDRS diabetic retinopathy scale from baseline up to 1,2, and 3 years based on reading center assessment of fundus photographs | 9%/1% | 6%/1% | 4%/1% | 15%/1% | 11%/3% | 11%/3% | 17%/2% | 15%/2% | 17%/3% |
| Progression of Retinopathy: Total of A+B+C+D | 21% | 19% | 14% | 31% | 29% | 21% | 37% | 35% | 30% |
Cumulative probabilities calculated using the life-table method. Subjects who dropped prior to an event were censored in the interval after their last completed visit.
There were 72 subjects who had one eye assigned to the laser group and one eye in the 1 mg triamcinolone group and 75 subjects who had one eye in the laser group and one eye in the 4 mg triamcinolone group. For the laser-1 mg subjects, 13% met the definition of progression of retinopathy by 3 years in the laser eye but not the 1 mg eye, and 17% met the definition in the 1 mg eye but not the laser eye. For the laser-4 mg subjects, 21% met the definition by 3 years in the laser eye but not the 4 mg eye, compared with 7%, in the 4 mg eye but not the laser eye.
Discussion
This exploratory analysis suggests that 4 mg of intravitreal triamcinolone acetonide as given in this trial can reduce the risk of progression of retinopathy through 3 years. The effect was sustained between years 1 and 2 and then between years 2 and 3 even though most eyes did not receive corticosteroids every 4 months in the second year and fewer than 50% received any corticosteroids in the third year. Theoretically, it is possible the reduction in risk of retinopathy progression may have been even greater if intravitreal triamcinolone had been given more frequently between years 1 and 3 of follow-up. On the other hand, it is possible that eyes with less severe or resolved edema may have had injections discontinued, and such eyes may have been healthier and thus did not progress at a faster rate in the absence of repeat injections.
It is unlikely that the observed difference between the 4 mg triamcinolone group and the focal/grid photocoagulation group is attributable to an increase in retinopathy progression caused by focal/grid photocoagulation. Based on data from the ETDRS, there does not appear to be a difference in risk of developing PDR in eyes with mild to severe non-proliferative diabetic retinopathy between focally treated eyes and untreated eyes (11.1% in the group assigned to immediate focal/grid photocoagulation and 10.8% in the group assigned to deferred focal/grid photocoagulation, at 1 year [personal communication, Frederick L. Ferris, MD]). In addition, the fact that the cumulative incidence in the laser group and 1 mg intravitreal triamcinolone group appeared similar, even though most eyes in the 1 mg group did not receive focal/grid photocoagulation, provides further evidence to suggest that it is unlikely that the 4 mg effect was a result of an increased risk of progression of retinopathy in the laser group.
While there are numerous basic science findings to support the hypothesis that anti-inflammatory medications, such as corticosteroids, could reduce the risk of an eye progressing to PDR,19–22 there are limited data to support this hypothesis from the clinical trial literature. A similar finding was observed in a trial that evaluated the effect of sustained release fluocinolone acetonide intravitreal implants on diabetic retinopathy levels in patients with diabetic macular edema in which 197 subjects were randomized to receive either a 0.59 mg fluocinolone acetonide implant or standard of care (repeat laser or observation).23 There was a higher rate of improvement and a lower rate of worsening in diabetic retinopathy severity in the fluocinolone acetonide implanted group than in the standard of care group (P<0.001 at 6 months, P<0.002 at 1 year, P=0.012 at 2 years, and P<0.015 at 3 years post implantation) (personal communication, P. Andrew Pearson, MD).
Some of the strengths of the current investigation include the prospective collection of fundus photograph and treatment data as well as randomization by treatment group. Analyses of the fundus photographs were completed by reading center graders providing uniform assessment across 88 clinical sites from which the data were obtained. The graders also were masked to treatment assignment. In addition, when evaluating the subgroup of subjects who had one eye in the laser group and one eye in the 4 mg triamcinolone group, presumably controlling for the impact of systemic factors on progression to PDR, similar outcomes in favor of the 4 mg group reducing the risk of progression to PDR were noted.
There are a number of potential weaknesses of this study. The study protocol was not designed primarily to determine the effect of intravitreal corticosteroids on preventing the progression of retinopathy, and the analyses presented were not planned secondary outcomes before the onset of the study, although the concept was considered as the analysis plan at the onset of the study included comparing the change in retinopathy levels on fundus photographs. Also, approximately 14% of subjects were censored prior to the two year visit, and an additional 34% between the 2 and 3 year visits (of which only 7% had the potential to complete the 3 year visit due to early closeout of the study). Furthermore, interpretation of these results should be tempered by the recognition that the 4 mg triamcinolone group was more likely to have cataract (51% and 64% of phakic eyes had a cataract extraction by 2 and 3 years, respectively in the 4 mg group, compared with 23% and 35%, respectively in the 1 mg group and 13% and 21%, respectively in the laser group). This cataract could obscure identification of PDR by the Reading Center. Cataract also could obscure identification of PDR by the treating ophthalmologist, resulting in less likelihood to proceed with PRP. However, this possibility seems unlikely as the view through most cataracts, at the time of surgery, is usually sufficient to identify PDR by retina specialists. Also, cataract development, depending on its severity, is not likely to influence perception of vitreous hemorrhage by a subject or identification of vitreous hemorrhage by the treating ophthalmologist. Also, failure to identify PDR by the ophthalmologist potentially should have increased the possibility of vitreous hemorrhage events from untreated (unrecognized) PDR. However, the observed proportion with vitreous hemorrhage was less in the 4 mg group than focal/grid group. Finally, the investigators were not masked with respect to whether the subject received intravitreal triamcinolone or focal/grid photocoagulation, and 2 of the 4 components of the primary outcome for this analysis were determined by the investigator; however, investigators were masked as to the dose of triamcinolone.
Use of this intravitreal corticosteroid preparation to reduce the likelihood of progression of retinopathy is not warranted at this time because of the increased risk of glaucoma and cataract associated with intravitreal steroid use. Any treatment to be used routinely to prevent PDR likely needs to be relatively safe since PDR can be treated successfully and safely with PRP. Nevertheless, further investigation on the role of pharmacotherapy for reducing the incidence of progression of retinopathy appears warranted.
Acknowledgments
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY14269, EY14229.
Footnotes
A published list of the Diabetic Retinopathy Clinical Research Network investigators and staff participating in this protocol can be found in Ophthalmology 2008;115:1447–9, 1449 e1–10 with a current list available at www.drcr.net.
The funding organization participated in oversight of the conduct of the study and review of the manuscript but not directly in the design of the study, the conduct of the study, data collection, data management, data analysis, interpretation of the data, or preparation of the manuscript.
Allergan, Inc. provided the triamcinolone and topical antibiotics after successfully competing for a request for proposals issued by DRCR.net for a company to provide a preservative-free triamcinolone for the study. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol. Allergan, Inc. has provided unrestricted funds to DRCR.net for its discretionary use.
A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net
An address for reprints is not provided.
References
- 1.The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81(4):383–96. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- 2.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839–41. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complication Trial Research Group. The effect of intensive diabetic treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 5.Ferris FL, 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984;28 (Suppl):452–61. doi: 10.1016/0039-6257(84)90227-3. [DOI] [PubMed] [Google Scholar]
- 6.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report no. 4. Int Ophthalmol Clin. 1987;27(4):265–72. doi: 10.1097/00004397-198702740-00006. [DOI] [PubMed] [Google Scholar]
- 7.Chew EY, Ferris FL, 3rd, Csaky KG, et al. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: the early treatment diabetic retinopathy follow-up study. Ophthalmology. 2003;110:1683–9. doi: 10.1016/S0161-6420(03)00579-7. [DOI] [PubMed] [Google Scholar]
- 8.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98:766–85. [PubMed] [Google Scholar]
- 9.Diaz-Florez L, Gutierrez R, Varela H. Angiogenesis: an update. Histol Histopathol. 1994;9:807–43. [PubMed] [Google Scholar]
- 10.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 11.Antoszyk AN, Gottlieb JL, Machemer R, Hatchell DL. The effects of intravitreal triamcinolone acetonide on experimental pre-retinal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1993;231:34–40. doi: 10.1007/BF01681698. [DOI] [PubMed] [Google Scholar]
- 12.Danis RP, Bingaman DP, Yang Y, Ladd B. Inhibition of preretinal and optic nerve head neovascularization in pigs by intravitreal triamcinolone acetonide. Ophthalmology. 1996;103:2099–104. doi: 10.1016/s0161-6420(96)30383-7. [DOI] [PubMed] [Google Scholar]
- 13.Challa JK, Gillies MC, Penfold PL, et al. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol. 1998;26:277–81. doi: 10.1111/j.1442-9071.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 14.Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Aust N Z J Ophthalmol. 1995;23:293–8. doi: 10.1111/j.1442-9071.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 15.Ip MS, Bressler SB, Antoszyk AN, et al. A randomized trial comparing intravitreal triamcinolone and laser photocoagulation for diabetic macular edema: Baseline features. Retina. 2008;28(7):919–30. doi: 10.1097/IAE.0b013e31818144a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–9. 9, e1–10. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. Dordrecht: Kluwer Academic; 1992. pp. 237–47. [Google Scholar]
- 19.Gardner TW, Antonetti DA. Novel potential mechanisms for diabetic macular edema: leveraging new investigational approaches. Curr Diab Rep. 2008;8(4):263–9. doi: 10.1007/s11892-008-0047-5. [DOI] [PubMed] [Google Scholar]
- 20.King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8 Suppl):1527–34. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- 21.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30(2):65–84. doi: 10.1007/s00281-008-0111-x. [DOI] [PubMed] [Google Scholar]
- 22.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson P, Levy B, Comstock T Fluocinolone Acetonide Implant Study Group. Fluocinolone Acetonide Intravitreal Implant to Treat Diabetic Macular Edema: 3–Year Results of a Multi–Center Clinical Trial. 2006;47 E-Abstract 5442. [Google Scholar]