Abstract
Spot 14 (THRSP, S14) is a nuclear protein involved in the regulation of genes required for fatty acid synthesis in normal and malignant mammary epithelial and adipose cells. Havartine and Bauman reported that conjugated linoleic acid (CLA) inhibits S14 gene expression in bovine mammary and mouse adipose tissues, and reduces milk fat production in cows. We hypothesized that CLA inhibits S14 gene expression in human breast cancer and liposarcoma cells, and that this will retard their growth. Exposure of T47D breast cancer cells to a mixture of CLA isomers reduced the expression of the S14 and fatty acid synthase (FAS) genes. The mixture caused a dose-related inhibition of T47D cell growth, as did pure c9, t11- and t10, c12-CLA, but not linoleic acid. Similar effects were observed in MDA-MB-231 breast cancer cells. Provision of 8 μM palmitate fully (CLA mix, t10, c12-CLA) or partially (c9, t11-CLA) reversed the antiproliferative effect in T47D cells. CLA likewise suppressed levels of S14 and FAS mRNAs in liposarcoma cells, and caused growth inhibition that was prevented by palmitic acid. CLA did not affect the growth of nonlipogenic HeLa cells or human fibroblasts. We conclude that, as in bovine mammary and mouse adipose cells, CLA suppresses S14 and FAS gene expression in human breast cancer and liposarcoma cells. Rescue from the antiproliferative effect of CLA by palmitic acid indicates that reduced tumor lipogenesis is a major mechanism for the anticancer effects of CLA.
Keywords: Spot 14, breast cancer, liposarcoma, tumor metabolism, fatty acids
Introduction
In cows, certain diets elicit a sharp decline in milk fat content termed milk fat depression (MFD; reviewed in (1)). Harvatine and Bauman performed cDNA microarray analysis of bovine mammary tissue during MFD, and observed reduced expression of genes coding lipogenic enzymes and key regulatory molecules including Spot 14 (THRSP, S14) (2). The authors postulated that MFD is mediated by conjugated linoleic acid (CLA), a systemically absorbed group of octadecadienoate isomers produced by bacteria in the rumen. Indeed, bovine MFD and its signature of suppressed expression of lipogenesis-related genes were reproduced by CLA administration. Moreover, published data also demonstrated reduced S14 mRNA content in adipose depots of CLA-fed mice (3).
CLA is known to inhibit the growth of breast cancer cells in tissue culture and of tumors in rodent breast cancer models (reviewed in (4)). For example, CLA slows the growth of MCF7 human breast cancer cells (5) and was chemopreventive in a chemically induced rat breast cancer model (6). CLA administered in the diet also substantially reduced metastasis in a breast cancer xenograft model in mice (7), and was chemopreventive in a carcinogen-induced breast cancer model in rats (8). CLA inhibited angiogenesis and vascular endothelial growth factor production in a mouse model (9), suggesting that these effects may contribute to its antitumor actions, although multiple candidate mechanisms for the anticancer effects of CLA have been proposed (4).
We previously demonstrated a role for S14 in the regulation of genes coding lipogenic enzymes in hepatocytes (10, 11). S14 is a nuclear protein that is induced physiologically in lipogenic tissues such as mammary gland, liver, and adipose, under circumstances that require brisk long-chain fatty acid synthesis, such as lactation in mammary epithelial cells. The S14 gene is also activated during adipose differentiation (12). S14 gene regulation is mediated by an array of promoter elements that transduce signals initiated by dietary substrates such as glucose, fuel-related hormones such as insulin and triiodothyronine, and a negative regulatory element for polyunsaturated fatty acids (reviewed in (13)).
In addition to its physiological role in metabolic regulation, S14 is a component of the virulent lipogenic phenotype observed in breast cancer (reviewed in (14)). Lipogenic breast tumors overexpress S14 and lipogenic enzymes, and depend on ongoing lipid synthesis for growth and survival. We demonstrated that S14 knockdown in breast cancer cells causes reduced lipogenesis and increased apoptosis (15), and that human primary breast tumors with high-level S14 expression are much more likely to recur (16). These observations, in concert with reduced S14 in CLA-treated bovine mammary gland and rodent adipose tissue discussed above, prompted us to determine whether CLA suppresses S14 gene expression in malignant human mammary epithelial and adipose cells. Our findings support this idea, and confirm the prediction that CLA would exert antiproliferative effects in both cell types. Moreover, our experiments directly support the hypothesis that the anticancer effects of CLA are largely mediated by its suppression of long-chain fatty acid synthesis.
Methods
Cell culture
We used T47D and MB-MDA-231 human breast cancer cells (ATCC), HeLa cells (ATCC), LiSa-2 (provided by Martin Wabitsch, University of Ulm, Germany) and SW872 (ATCC) human liposarcoma cells, and human fibroblasts (provided by Eva Rzucidlo, Dartmouth Medical School). Cells were grown in DMEM:F12 supplemented with 10% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA), 1% penicillin-streptomycin solution, and 2mM L-glutamine at 37°C in 95% air, 5% C02. Cells were seeded in 48 well plates (104 cells, 0.2 ml medium/well), and test media were added the following morning.
Fatty acids
Media containing a mixture of CLA isomers (Sigma-Aldrich, St. Louis, MO catalog #O5507), pure CLA isomers (cis 9, trans 11 or trans 10, cis 12; Nu-Chek Prep, Elysian, MN), linoleate, or palmitate (Sigma-Aldrich) were prepared as described by Ip and coworkers (17) using delipidated albumin (Sigma-Aldrich catalog #A7888) for solublization.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA (500 ng) harvested in Trizol was used as template using protocols and primers as previously described (15). Data were corrected for the cyclophilin mRNA content of each sample.
Cell growth
The number of viable cells/well was estimated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (18).
Plasmid transfection
Cells were plated at 50% confluence in 75 cm2 flasks and were transfected with 8 μg plasmid DNA in 48 μl Fugene (Roche, Basel, Switzerland) the next morning in media without antibiotics. Plasmids contained a 4003 bp fragment of the proximal human S14 gene promoter (kindly supplied by Cary Mariash, U. MN) or 157 bp of the human FAS gene promoter ((19); kindly supplied by Johan Swinnen, Leuven, Belgium) driving firefly luciferase expression. In order to ensure uniform transfection efficiency, cells were trypsinized, mixed, and redistributed into 6 well plates 8 h after transfection. Extracts were prepared 40 h later in 250 μl/well reporter lysis buffer (Promega, Madison, WI) and luciferase activity/mg protein was determined in a LMaxII384 luminometer (Molecular Devices, Toronto, Canada).
Lipid chromatography
HPLC analysis of CLA isomers was based upon the Ag+-HPLC method of Cross and coworkers (20). We used a Varian HPLC system with model 210 pumps, a model 410 autosampler, and a Varian Pro Star model 345 detector. Separation was achieved using two Varian (Palo Alto, CA) ChromSpher Lipid 5 (4.6mm × 250mm) columns in series. The mobile phase consisted of 2.5% acetic acid and acetonitrile (0.0-0.1%) in hexane, and was pumped at 1ml/min. Columns were maintained at 30° C using the Varian 410 autosampler oven and an Eppendorf (Mississauga, Canada) CH-30 heater, controlled by a TC-50 programmer. Eluates were monitored at 234 nm. Data were acquired using Varian LC workstation software version 6.3.
Statistics
All experiments were repeated at least once. Comparison of two groups was by two-tailed unpaired Student's “t” test, and between more than two groups was by two-way analysis of variance.
Results
Effects of CLA on S14 and FAS gene expression in T47D breast cancer cells
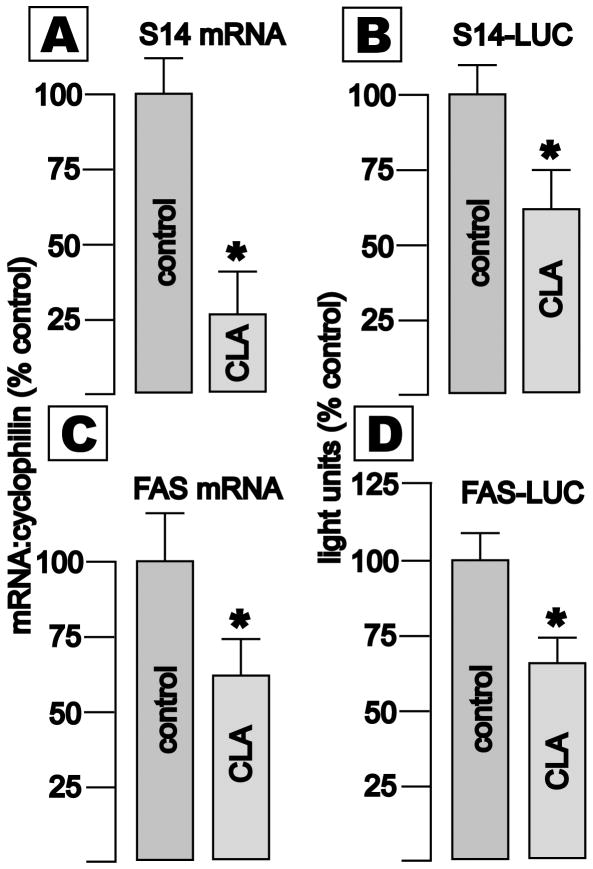
Real time RT-PCR revealed that expression of S14 and FAS mRNAs, relative to mRNA coding cyclophilin, was reduced by 74 and 38%, respectively, after exposure to a mixture of CLAs (64 μM total) for 96 h (Fig. 1, panels A and C, *p < 0.05). Luciferase activity driven by the proximal human S14 or FAS gene promoters was also significantly reduced (Fig. 1, panels B and D). Thus, CLA-mediated reductions in the cellular content of S14 and FAS mRNAs in bovine mammary and murine adipose cells are recapitulated in the malignant human counterparts of those cell types.
Fig. 1. Effects of CLA on expression of the S14 and FAS genes in T47D breast cancer cells.
Cells were seeded in normal media or media containing 64 μM CLA (Sigma mixture) × 5 d. Levels of mRNA coding S14 (panel A) or FAS (panel C), were determined by real time RT-PCR. Values (Mean +/- SEM, 8 wells/group, *p<0.05) are normalized to cyclophilin mRNA, and to the control groups. Transcriptional activity was determined by transient transfection of a plasmid containing the proximal 4003 bp of the human S14 promoter (panel B) or 157 bp of the human fatty acid synthase promoter (panel D) driving luciferase expression. Cells were seeded in 75 cm2 plates, transfected, and grown overnight in normal media. The following day cells were trypsinized and seeded into 6 well plates, and control media or media containing 128 μM CLA were added. Cells were harvested 3 d later. Luciferase activity, corrected for protein content, is shown (mean +/- SEM, 4 wells/group, *p<0.05).
Ag+HPLC of CLA preparations
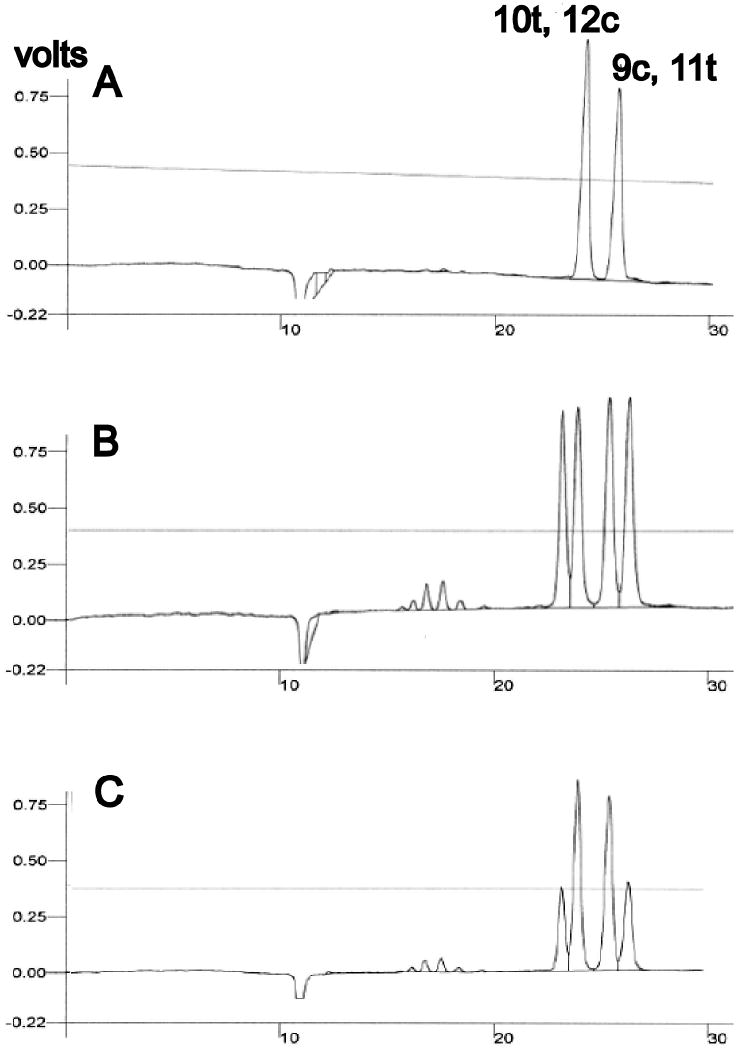
After ascertaining the retention times of pure preparations of 10t, 12c- and 9c, 11t-CLA (data not shown), we analyzed a mixture of these two isomers (Fig. 2, panel A). This demonstrated that our HPLC system produced clear separation of these CLAs. We then chromatographed the frequently used CLA mixture employed in the experiment depicted in Fig. 1. This revealed several minor and 4 major peaks, including two peaks with retention times virtually the same as those of the pure 10t, 12c- and 9c, 11t-CLA isomers (Fig 2, panel B). Analysis of a mixture of the Sigma CLA preparation spiked with pure 10t, 12c- and 9c, 11t-CLA isomers revealed augmentation of the second and third major peaks observed in the Sigma mixture, indicating that they represented 10t, 12c- and 9c, 11t-CLA, respectively (Fig. 2, panel C). We calculated the ratio of the 10t, 12c:9c, 11t isomers in the Sigma mixture to be 0.89.
Fig. 2. Ag+HPLC of CLA preparations.
Panel A: After assessing the retention times of pure 10t, 12c- and 9c, 11t-CLAs, a 1:1 mixture of them was chromatographed, demonstrating resolution of the two isomers. Panel B: Analysis of the commercial CLA mixture (Sigma) reveals four major peaks. Panel C: Chromatography of an aliquot of the commercial mixture spiked with equal amounts of the pure CLA isomers reveals augmentation of the second and third major peaks observed in the Sigma mix, indicating that they represent the 10t, 12c- and 9c, 11t-CLA isomers, respectively.
Effect of CLA preparations on the growth of lipogenic breast cancer cells
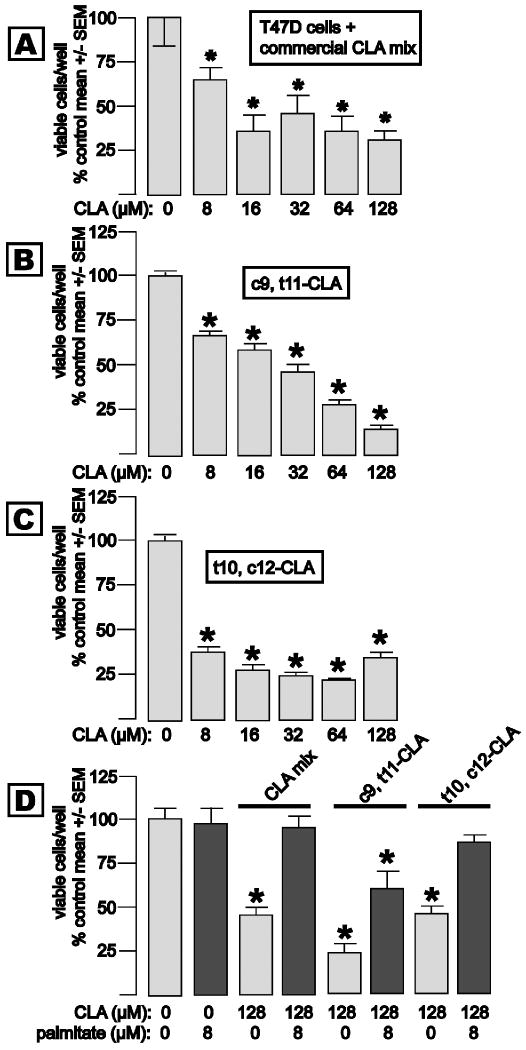
T47D cells have sex steroid receptors and exhibit progestin-inducible S14 and FAS gene expression (21), and are growth-inhibited by S14 knockdown (15). Exposure of T47D cells to media containing 8 to 128 μM of the commercially-available CLA mixture for 5 d caused a stepwise reduction in T47D cell accumulation that was evident at 8 μM, and maximal at 16 μM CLA (Fig. 3, panel A). T47D cell proliferation was also inhibited by pure c9, t11-CLA, with a stepwise reduction in cell accumulation across the 8-128 μM concentration range (Fig. 3, panel B). Pure t10, c12-CLA caused reduced T47D cell growth that was near-maximal at 8 μM (Fig. 3, panel C). Palmitic acid is the principle product of de novo long-chain fatty acid synthesis. In order to determine whether the observed CLA-mediated suppression of genes related to long-chain fatty acid synthesis contributes to the inhibition of cell growth, we added palmitic acid, with or without CLA, to culture media in concert with 128 μM of each of the CLA preparations (Fig. 3, panel D). Exposure to the commercial CLA mixture for 5 d reduced T47D cell accumulation by 54%, and provision of 8 μM palmitic acid rescued the cells from growth inhibition, whereas addition of palmitic acid alone did not affect growth. Analogous experiments using pure CLA isomers demonstrated a partial (c9, t11-CLA) and full rescue (t10, c12-CLA) of the cells from growth inhibition by palmitate, respectively. We thus conclude that reduced expression of lipogenesis-related genes, and a consequent depletion of long-chain fatty acids, is a major mechanism for inhibition of breast cancer cell growth by CLA, and that the t10, c12 isomer is the most potent of the isomers tested in this regard.
Fig. 3. Effects of CLA on the growth of lipogenic T47D breast cancer cells.
Cells were plated in normal media, and exposed to media containing the indicated concentrations of CLA the next day. Viable cell mass was determined in the MTT assay (Mean +/- SEM, 8 wells/group, *p<0.05). Panel A: Exposure to the commercial CLA mixture × 5 d caused a dose related growth inhibition that was evident at 8 μM and maximal at 16 μM CLA. Panel B: Incubation with 8 to128 μM pure c9, t11-CLA caused a dose related inhibition of T47D cell growth. Panel C: Incubation with 8 to128 μM pure t10, c12-CLA caused a dose related inhibition of T47D cell growth that was maximal at 8-16 μM CLA. Panel D: Growth inhibition induced by 72 h exposure to 64 or 128 μM of each CLA preparation is completely (CLA mix, t10, c12-CLA) or partially (c9, t11-CLA) prevented by coadministration of 8 μM palmitic acid.
We also examined the antiproliferative activity of CLA isomers in MDA-MB-231 cells, which lack receptors for sex steroids and express very low levels of Her2/neu (22, 23), and thus represent the aggressive “triple negative” breast cancer phenotype (Fig. 4). As was observed for T47D cells, both c9, t11- (panel A) and t10, c12-CLA (panel B) inhibited MDA-MB-231 cell growth, and the t10,c12 isomer was more potent.
Fig. 4. Effect of CLA isomers on the growth of breast cancer cells lacking sex steroid and trastuzumab receptors (MDA-MB-231.
Cells were treated as in the experiment depicted in Fig. 3, and exposed to the indicated concentrations of c9, t11- (panel A) or t10, c12-CLA (panel B).
S14 and FAS mRNAs in CLA-treated liposarcoma cells
We previously demonstrated liposarcoma cells to exhibit an adipogenic gene expression signature and, as is the case for breast cancer cells, to require fatty acids for growth (24). The reported inhibition of S14 gene expression by CLA in mouse adipose tissue (3) thus prompted the prediction that it would likewise affect liposarcoma cells. The impact of CLA on S14 and FAS gene expression in LiSa2 liposarcoma cells is shown in Fig. 5. Cells were treated with control media or media containing 128 μM CLA for 4 d, at which time total RNA was isolated and analyzed for S14 (panel A) or FAS mRNAs (panel B). As observed in breast cancer cells, CLA caused significant reductions in the cellular content of these mRNAs in liposarcoma cells.
Fig. 5. CLA suppresses S14 and FAS gene expression in LiSa2 liposarcoma cells.
Cells (20,000/well) were seeded in normal media or media containing 128 μM CLA mixture × 5 d. Relative levels of mRNA coding S14 (panel A) or FAS (panel B) were determined by real time RT-PCR. Values (Mean +/- SEM, 8 wells/group *p<0.05) are corrected for the expression of cyclophilin mRNA, and are normalized to the control groups.
CLA impairs the growth of liposarcoma cells
The effect of 128 μM CLA on the growth of LiSa2 cells is shown in Fig. 6. Cells were exposed to pure c9, t11- (panel A) or t10, c12-CLA (panel B) for 4 days. As was the case for the breast cancer cells, the t10, c12 isomer was a more potent inhibitor of liposarcoma cell growth. The CLA mixture also inhibited LiSa-2 cell growth (panel C), and the cells were rescued from this effect by the provision of palmitic acid. In preliminary experiments, we found that LiSa-2 cells required a higher concentration of palmitic acid to restore growth in the presence of CLA than did the breast cancer cells. We observed a similar inhibition of growth and rescue by palmitic acid in the SW872 liposarcoma cell line (data not shown).
Fig. 6. CLA inhibits liposarcoma cell growth, and rescued by palmitate.

Lisa-2 cells were grown in control media or media containing the indicated concentrations of CLA for 4 days, at which time viable cells/well were estimated in the MTT assay. Data are mean ± SEM, 8 wells/group; * denotes p<0.05. Panel A: c9, t11-CLA significantly inhibited LiSa-2 cell growth only at the highest concentration tested. Panel B: The t10, c12-CLA isomer caused a stepwise reduction in LiSa-2 cell growth across the 8 to 128 μM concentration range. Panel C: The commercially-available CLA mixture inhibited LiSa-2 cell growth, and this effect was prevented by the addition of palmitic acid.
Specificity of CLA-mediated growth inhibition
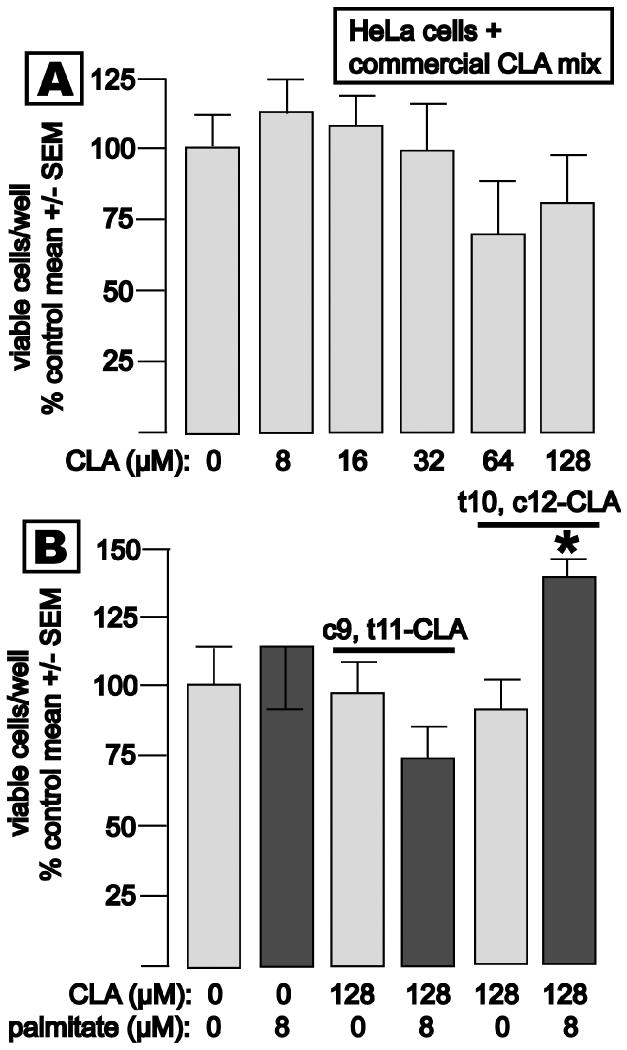
The lack of effect of 8-128 μM of the commercial CLA mixture on the growth of non-lipogenic, malignant epithelial cells (HeLa, derived from carcinoma of the uterine cervix) is shown in Fig. 7, panel A. Exposure of HeLa cells to pure c9, t11- or t10, c12-CLA likewise had no impact on proliferation (panel B). Interestingly, the combination of palmitate and t10, c12-CLA elicited a small but statistically significant enhancement of HeLa cell growth.
Fig. 7. CLA does not inhibit proliferation of nonlipogenic HeLa cells.
Cells were treated as described for Fig. 3. Panel A: HeLa cell growth was not significantly inhibited by up to 128 μM CLA mixture for 5 d. Panel B: Neither 128 μM c9, t11- or t10, c12-CLA inhibited HeLa cell growth. Paradoxically, 8 μM palmitic acid significantly accelerated HeLa cell growth in the presence of t10, c12-CLA.
As an additional, mesenchymal control for the impact of CLA on liposarcoma cell growth, we exposed human fibroblasts to 64 or 128 μM CLA, with or without concurrent addition of palmitate (32 μM) for 5 d. The MTT assay did not reveal any difference in viable cells/well in any of the groups (8 wells/group, data not shown). We also exposed T47D breast cancer cells to linoleic acid (8 to 128 μM × 5 d) to control for potential nonspecific effects of di-unsaturated fatty acids on cancer cells, and this also did not inhibit cell growth (data not shown).
Discussion
Several common human cancers frequently exhibit a lipogenic phenotype, manifest by high levels of fatty acid-synthesizing enzymes and dependence on long-chain fatty acids for cell growth and survival (reviewed in (25) and (26)). As discussed in the introduction, we previously identified S14 as a key component of this phenotype in breast cancer. Evidence supporting this includes S14 gene amplification in breast tumors (27), reduced lipogenesis and growth of breast cancer cells in S14 knockdown experiments (15) and a strong correlation between high S14 content and poor prognosis in primary clinical breast tumors (16). We have also recently characterized lipid metabolism in human liposarcoma cells and its impact on proliferation (24). These cells exhibit a pattern of gene expression similar to that of fully-differentiated adipocytes, and thus also express key genes related to long-chain fatty acid synthesis, including S14. As is the case for lipogenic breast cancer cells, liposarcoma cells depend on de novo lipid synthesis for growth and survival.
Identification of S14 as a target gene in both CLA-induced MFD in dairy cows and in adipose tissue of CLA-treated mice prompted the hypothesis that reduced S14 expression would occur after exposure of malignant human mammary epithelium (exemplified by lipogenic T47D and MDA-MB-231 breast cancer cells) and transformed adipose cells (exemplified by LiSa-2 and SW872 liposarcoma cells). The major observation of the current study was that CLA suppresses S14 and FAS expression in both tumor cell types, and that, as anticipated from our work with siRNA-mediated S14 knockdown in breast cancer cells (15) and triterpenoid-induced inhibition of S14 and FAS expression in liposarcoma cells (24), it inhibits their growth as well.
Attempts to target components of the lipogenic pathway in cancer have focused primarily on pharmacological inhibition of FAS enzyme activity. Currently available FAS inhibitors include Cerulenin and its analog C75 (reviewed in (28)), as well as Orlistat, a drug approved by the F.D.A. for oral administration for the treatment of obesity (29, 30). It was previously proposed that the apoptotic effect of FAS inhibition resulted from accumulation of its substrate, malonyl CoA, which is an inhibitor of fatty acid oxidation because it down-regulates the rate-determining enzyme of mitochondrial fatty acid uptake, carnitine-palmitoyl acyl transferase I (31, 32). Recent studies, however, show that targeting ATP citrate-lyase, acetyl CoA-carboxylase, or S14 is also cytotoxic (15, 33-35). Moreover, provision of palmitate, the principal end-product of the pathway, rescues the tumor cells in several experimental systems, including those reported here. It thus appears that fatty acid deficiency causes the growth inhibition and/or cell death. Overall, our data support this formulation and our experiments demonstrating rescue by palmitic acid strongly indicate that some or all of the long-recognized anticancer effects of CLA are mediated through its impact on lipid metabolism.
We have proposed that S14 and lipogenesis are particularly important for breast cancer metastasis, as opposed to tumorigenesis per se (14). Our observation that primary tumors with high expression of S14 are far more likely to recur than those with low expression supports this idea (16), as does the assignment of S14 to the “metastasis signature” in a genetically engineered mouse model of breast cancer (36). Given that our data indicate that S14 is a target gene for CLA in breast cancer cells, it is notable that CLA has shown a striking anti-metastasis effect in a mouse breast cancer model (7).
Several published studies have employed the commercially-available CLA mixture that we used, and our identification of the second and third major peaks observed on Ag+-HPLC as c9, t11- and t10, c12-CLA confirms the findings of Cross and coworkers (20). Biomedically relevant effects of CLA have been attributed primarily to these two isomers, and both have been shown to have anticancer effects (8, 37). Our data in T47D and MDA-MB-231 breast cancer cells are thus consistent with this formulation in that both isomers suppressed cell growth. The t10, c12 form is believed to be more potent for inhibition of lipid metabolism in adipocytes and reduction of body fat content in vivo (reviewed in (38)). Interestingly, we found the t10, c12 isomer to be a more potent inhibitor of T47D and MDA-MB-231 cell growth than was c9, t11-CLA (Figs. 3 and 4). This is consistent with the findings of Ip and coworkers in an in vivo carcinogen-induced rat breast cancer model, where the two CLA isomers exerted comparable chemopreventive effects, but tissue concentrations of t10, c12-CLA were much lower than those of the c9, t11 isomer, indicating it to be more potent (8). More recently, however, Ip and colleagues reported enhanced tumor growth and metastasis in a genetically engineered mouse model of Her2/neu-induced breast cancer (39). It remains unclear whether this is a species-related difference in response, or if it is related to the expression of Her2/neu by the tumor model.
The observed complete rescue of T47D cells from the growth inhibition produced by t10, c12-CLA by palmitate strongly supports the idea that the antiproliferative action of this isomer is mediated by suppression of lipid synthesis, and we thus propose that these two actions of t10, c12-CLA (inhibition of lipid metabolism, suppression of cell growth) are inseparable. The lack of an effect of CLA on HeLa cell growth is consistent with this conclusion, as HeLa cells are deficient in de novo lipogenesis (40), and appear to rely on lipoprotein lipase-mediated lipolysis for their lipid supply (14). The failure of palmitic acid to stimulate T47D cell growth by itself indicates that the rescue was indeed mediated by repletion of fatty acids, rather than an additive effect of two mechanistically unrelated stimuli. The inability of palmitic acid to totally reverse the growth inhibition produced by c9, t11-CLA suggests that that isomer may have additional mechanisms of action at the 128 mμM concentration employed.
We conclude that, as in bovine mammary and mouse adipose cells, CLA suppresses S14 and FAS gene expression in human breast cancer and liposarcoma cells. Rescue from the antiproliferative effect of CLA by palmitic acid indicates that the observed reduction in expression of the S14 and FAS genes, with a resultant decline in tumor cell lipogenesis is a major mechanism for the anticancer effects of CLA. CLA, particularly the t10, c12 isomer, may thus be a useful therapy for some lipogenic tumors, such as breast cancer and liposarcoma, in humans. The aforementioned report of enhanced tumor growth in a murine Her2/neu driven breast cancer model treated with t10, c12-CLA, however, dictates caution in the administration this isomer to patients with Her2/neu positive tumors (39). Little is known regarding CLA pharmacokinetics and safety in humans, although a 1.5 y trial of its impact on body weight in obese subjects revealed no toxicity (41).
Acknowledgments
We thank Martin Wabitsch for providing liposarcoma cells, and Cary Mariash and Johan Swinnen for providing reporter gene constructs.
Grant support: This work was supported by NIH grant 2 R01 CA126618 (W.B.K.), The Hitchcock Foundation (W.B.K.), and a Norris Cotton Cancer Center Prouty Grant (B.L.E. & L.D.L) and NIH CA23108 (A.E., B.L.E., L.D.L.).
Abbreviations employed
- CLA
conjugated linoleic acid
- DNA
deoxyribonucleic acid
- FAS
fatty acid synthase
- HPLC
high performance liquid chromatography
- MFD
milk fat depression
- mRNA
messenger ribonucleic acid
- RT-PCR
reverse transcriptase polymerase chain reaction
- S14
Spot 14
References
- 1.Bauman D, Grinari J. Nutritional regulation of milk fat synthesis. Ann Rev Nutr. 2003;23:203–207. doi: 10.1146/annurev.nutr.23.011702.073408. [DOI] [PubMed] [Google Scholar]
- 2.Harvatine K, Bauman D. SREBP1 and thyroid hormone responsive spot 14 (S14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA. J Nutrition. 2006;136:2468–2474. doi: 10.1093/jn/136.10.2468. [DOI] [PubMed] [Google Scholar]
- 3.House R, Cassady J, Eisen E, Eling T, Collins J, Grissom S, Odle J. Functional genomic characterization of delipidation elicited by trans-10, cis-12-conjugated linoleic acid (t10,c12-CLA) in a polygenic obese line of mice. Physiol Genomics. 2005;21:351–361. doi: 10.1152/physiolgenomics.00244.2004. [DOI] [PubMed] [Google Scholar]
- 4.Belury M. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995–2998. doi: 10.1093/jn/131.10.2995. [DOI] [PubMed] [Google Scholar]
- 5.Durgam V, Fernandes G. The growth inhibitory effect of conjugated linoleic acid on MCF-7 cells is related to estrogen response system. Cancer Letters. 1997;116:121–130. doi: 10.1016/s0304-3835(97)00192-4. [DOI] [PubMed] [Google Scholar]
- 6.Ip C, Singh M, Thompson H, Scimeca J. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212–1215. [PubMed] [Google Scholar]
- 7.Hubbard N, Lim D, Summers L, Erikson K. Reduction of murine mammary tumor metatstasis by conjugated linoleic acid. Cancer Letters. 2000;150:93–100. doi: 10.1016/s0304-3835(99)00379-1. [DOI] [PubMed] [Google Scholar]
- 8.Ip C, Dong Y, Ip M, Banni S, Carta G, Angioni E, Murru E, Spada S, Melis M, Saebo A. Conjugated linoleic acid isomers and mammary cancer prevention. Nutrition and Cancer. 2002;43:52–58. doi: 10.1207/S15327914NC431_6. [DOI] [PubMed] [Google Scholar]
- 9.Ip M, Masso-Welch P, Ip C. Prevention of mammary cancer with conjugated linoleic acid: role of the stroma and epithelium. J Mamm Gland Biol Neopl. 2003;8:103–118. doi: 10.1023/a:1025739506536. [DOI] [PubMed] [Google Scholar]
- 10.Kinlaw W, Church J, Harmon J, Mariash C. Direct evidence for a role of the “spot 14” protein in the regulation of lipid synthesis. J Biol Chem. 1995;270:16615–16618. doi: 10.1074/jbc.270.28.16615. [DOI] [PubMed] [Google Scholar]
- 11.Brown SB, Maloney M, Kinlaw WB. “Spot 14” protein functions at the pretranslational level in the regulation of hepatic metabolism by thyroid hormone and glucose. J Biol Chem. 1997;272:2163–2166. [PubMed] [Google Scholar]
- 12.Lepar G, Jump D. Hormonal regulation of the S14 gene in 3T3-F442A cells. Mol Endocrinol. 1989;3:1207–1214. doi: 10.1210/mend-3-8-1207. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham B, Moncur J, Huntington J, Kinlaw WB. “Spot 14” protein: a metabolic integrator in normal and neoplastic cells. Thyroid. 1998;8:815–825. doi: 10.1089/thy.1998.8.815. [DOI] [PubMed] [Google Scholar]
- 14.Kinlaw W, Quinn J, Wells W, Roser-Jones C, Moncur J. S14 in breast cancer: a marker of aggressive disease and a potential therapeutic target. Endocrinology. 2006;147:4048–4055. doi: 10.1210/en.2006-0463. [DOI] [PubMed] [Google Scholar]
- 15.Martel P, Bingham C, McGraw C, Baker C, Morganelli P, Meng M, A J, Moncur J, Kinlaw WB. S14 protein in breast cancer cells: direct evidence for regulation by SREBP-1c, superinduction with progestin, and implication in cell growth. Experimental Cell Research. 2005;312:278–288. doi: 10.1016/j.yexcr.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Wells W, Schwartz G, Morganelli P, Cole B, Chambers J, Kinlaw WB. Expression of “Spot 14” (THRSP) predicts disease free survival in invasive breast cancer: immunohistochemical analysis of a new molecular marker. Breast Cancer Research and Treatment. 2006;98:231–240. doi: 10.1007/s10549-005-9154-z. [DOI] [PubMed] [Google Scholar]
- 17.Ip M, Masso-Welch P, Shoemaker S, Shea-Elton W, Ip C. Conjugated linoleic acid inhibits proliferation and induces apoptosis of normal rat mammary epithelial cells in primary culture. Exp Cell Res. 1999;250:22–34. doi: 10.1006/excr.1999.4499. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorometric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Swinnen J, Ulrix W, Heyns W, Verhoven G. Coordinate regulation of lipogenic gene expression by androgens: evidence for a cascade mechanism involving sterol regulatory element binding proteins. PNAS. 1997;94:12975–12980. doi: 10.1073/pnas.94.24.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cross R, Ostrowska E, Muralitharan M, Dunshea F. Mixed mode retention and the use of competing acid for the Ag+-HPLC analysis of underivatized conjugated linoleic acids. J High Res Cromatogr. 2000;23:317–323. [Google Scholar]
- 21.Heemers H, Vanderhoydonc F, Heyns W, Verhoeven G, Swinnen J. Progestins and androgens increase expression of spot 14 in T47-D breast tumor cells. BBRC. 2000;269:209–212. doi: 10.1006/bbrc.2000.2262. [DOI] [PubMed] [Google Scholar]
- 22.Cailleau R, Olive M, Crueiger Q. Long term human breast cancer cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 23.Hollywood D, Hurst H. A novel transcription factor, OB2-1, is required for overexpression of the proto-oncogene c-eb-2 in mammary tumor lines. EMBO J. 1993;12:2369–2375. doi: 10.1002/j.1460-2075.1993.tb05891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes D, Martel P, Kinlaw W, Eisenberg B. The synthetic triterpenoid CDDO-Im inhibits fatty acid synthase expression and has antiproliferative and proapoptotic effects in human liposarcoma cells. Cancer Invest. 2008;26:118–127. doi: 10.1080/07357900701522612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swinnen J, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 26.Menendez J, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 27.Moncur J, Park J, Mohandes TK, Memoli V, Kinlaw W. The “spot 14” gene resides on the telomeric end of the 11q13 amplicon and is expressed in lipogenic breast cancers. PNAS. 1998;95:6989–6994. doi: 10.1073/pnas.95.12.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhajda F. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 29.Kridel S, Axelrod F, Rozenkrantz N, Smith J. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 30.Menendez J, Vellon L, Lupu R. Antitumoral actions of the anti-obesity drug orlistat (Xenical) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erb B-2) oncogene. Ann Oncol. 2005;16:1253–1267. doi: 10.1093/annonc/mdi239. [DOI] [PubMed] [Google Scholar]
- 31.Pizer E, Thupari J, Han W, Pinn M, Chrest F, Frehywot G, Townsend C, Kuhajda F. Malonyl-Coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- 32.Thupari J, Pinn M, Kuhajda F. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. BBRC. 2001;285:217–223. doi: 10.1006/bbrc.2001.5146. [DOI] [PubMed] [Google Scholar]
- 33.Hatsivassiliou G, Zhao F, Bauer D, Andreadis C, Shaw A, Dhanak D, Hingorani S, Tuveson D, Thompson C. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Brusselmans K, De Schrijver E, Verhoeven G, Swinnen J. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-a gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–6725. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- 35.Chajes V, Cambot M, Moreau K, Lenoir G, Joulin V. Acetyl-CoA carboxylase-a is essential to breast cancer cell survival. Cancer Res. 2006;66:5287–5294. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Crawford N, Lukes L, Finney R, Lancaster M, Hunter K. Metastasis predictive signature profiles pre-exist in normal tissues. Clin Exp Metastasis. 2005;22:593–603. doi: 10.1007/s10585-005-6244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem. 2006;17:789–810. doi: 10.1016/j.jnutbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Pariza M, Park Y, Cook M. The biologically active isomers of conjugated linoleic acid. Progr Lipid Res. 2001;40:283–298. doi: 10.1016/s0163-7827(01)00008-x. [DOI] [PubMed] [Google Scholar]
- 39.Ip M, Sibel O, Masso-Welch A, Ip C, Meng X, Ou L, Shoemaker S. The t10,c12 isomer of conjugated linoleic acid stimulates mammary tumorigenesis in transgenic mice over-expressing erbB2 in the mammary epithelium. Carcinogenesis. 2007;28:1269–1276. doi: 10.1093/carcin/bgm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oskouian B. Overexpression of fatty acid synthase in SKBR3 breast cancer cell line is mediated via a transcriptional mechanism. Cancer Letters. 2000;149:43–51. doi: 10.1016/s0304-3835(99)00342-0. [DOI] [PubMed] [Google Scholar]
- 41.Gaullier J, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H, Gudmundsen O. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr. 2005;135:778–784. doi: 10.1093/jn/135.4.778. [DOI] [PubMed] [Google Scholar]