Abstract
Silent information regulators are potent NAD+-dependent protein deacetylases, which have been shown to regulate gene silencing, muscle differentiation and DNA damage repair. Here, changes in the level and activity of sirtuin 1 (SIRT1) in response to exercise in groups of young and old rats were studied. There was an age-related increase in SIRT1 level, while exercise training significantly increased the relative activity of SIRT1. A strong inverse correlation was found between the nuclear activity of SIRT1 and the level of acetylated proteins. Exercise training induced SIRT1 activity due to the positive effect of exercise on the activity of nicotinamide phosphoribosyltransferase (NAMPT) and thereby the production of sirtuin-fueling NAD+. Exercise training normalized the age-associated shift in redox balance, since exercised animals had significantly lower levels of carbonylated proteins, expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. The age-associated increase in the level of SIRT6 was attenuated by exercise training. On the other hand, aging did not significantly increase the level of DNA damage, which was in line with the activity of 8-oxoguanine DNA glycosylase, while exercise training increased the level of this enzyme. Regular exercise decelerates the deleterious effects of the aging process via SIRT1-dependent pathways through the stimulation of NAD+ biosynthesis by NAMPT.
Keywords: Sirtuins, Exercise, DNA repair, Skeletal muscle, Aging
1. Introduction
Studies have revealed common regulatory mechanisms, including maintenance of genomic integrity, insulin growth factor-like signaling and silent information regulators, interact in concert with and influence complex pathways implicated in senescence/aging processes. Recent evidence suggests that among these factors, sirtuins are prominent regulators of aging from single cell organisms to mammals (Imai, 2009). Seven mammalian homologues of yeast Sir2 have been identified and shown to be dependent on nicotinamide adenine dinucleotide (NAD+), and thus closely linked to cell metabolism, energy production and DNA repair (Imai et al., 2000; Lombard et al., 2008). In support of these roles of NAD+ in the sirtuin pathway, the level/activity of nicotinamide phosphoribosyltransferase (NAMPT, also known as PBEF or Visfatin), a NAD+ biosynthetic enzyme, has been shown to extend the replicative lifespan of vascular smooth muscle cells via activation of SIRT1 (van der Veer et al., 2007). The NAD+/NADH ratio can also reflect the redox status (Ying, 2008) and it has been proven that ROS readily modify the activity of sirtuins (Furukawa et al., 2007).
SIRT1 can influence aging processes and many of the major diseases of aging, including metabolic disorders such as diabetes, or neurodegenerative diseases (Alzheimer’s and Parkinson’s), cancer and osteoporosis. Aging processes are orchestrated in part by powerful deacetylators SIRTs (Porcu and Chiarugi, 2005). For example, deacetylation of lysine residues of the histone tails by SIRT induces closed chromatin configuration and transcriptional silencing (Shahbazian and Grunstein, 2007). Besides histone deacetylation, SIRT1 targets a number of transcription factors such as nuclear factor κB, p53, peroxisome proliferator-activated receptor gamma coactivator-1α and MyoD, which are involved in inflammation, apoptosis, mitochondrial biogenesis, and skeletal muscle differentiation (Fulco et al., 2003; Lavu et al., 2008; Radak et al., 2004).
The age-associated shift in cellular redox state to an oxidized milieu can be characterized by the NAD+/NADH ratio, which has been shown to affect the activity of NAD+-dependent sirtuins. Indeed, increased ROS levels modulate SIRT1 expression (Fulco et al., 2003; Hipkiss, 2008) and the age-associated, organ-dependent changes in SIRT activities (Kwon and Ott, 2008). SIRT1, as well as SIRT6, are regulators of DNA repair (Mostoslavsky et al., 2006; Oberdoerffer et al., 2008; Wang et al., 2008). For example, SIRT6-deficient mice show degenerative processes which overlap with age-associated abnormalities due to deficiencies in maintaining genomic stability (Mostoslavsky et al., 2006).
Skeletal muscle, along with other tissues, accumulates increased levels of oxidative damage with aging (Radak et al., 2007, 2008). Regular exercise decreases the level of oxidative damage via increasing antioxidant potential of muscles and this change could be modulated by the activity and levels of SIRT1 (Ferrara et al., 2008; Radak et al., 2008; Suwa et al., 2008). To counteract increased generation of ROS, hypoxic conditions develop in muscle, resulting in increased expression of hypoxia-inducible factor-1α (HIF-1α). This expression is important in maintaining physiological redox conditions in skeletal muscle, especially in older mammals (Clanton, 2007; Mayr et al., 2008; Moller et al., 2001; O’Hagan et al., 2009). Regular endurance exercise-induced alteration in HIF-1α levels is associated with mitochondrial biogenesis and angiogenesis, the latter in consort with vascular endothelial growth factor (VEGF) (O’Hagan et al., 2009).
The present investigation has been designed to test the hypotheses that exercise training re-establishes physiologically relevant activity of SIRT1, which has been attenuated with aging. SIRT1’s total activity was significantly increased with training. Similarly, exercise training increased NAD+, NAMPT and mitochondrial uncoupling protein-3 (UCP3) levels/activities to levels comparable to those seen in skeletal muscle of young animals. These data suggest that regular exercise decelerates aging processes of skeletal muscle via SIRT1-dependent pathways.
2. Methods
2.1. Animals and training protocol
Twelve young (3 mo) and 12 old (26 mo) male Wistar rats were used in the study and grouped into young control (YC), young exercised (YE), old control (OC) and old exercised (OC). The investigation was carried out according to the requirements of The Guiding Principles for Care and Use of Animals, EU, and approved by the local ethics committee. Exercised rats were introduced to treadmill running for 3 days, then for the next 2 weeks the running speed was set to 10 m/min, on a 5% incline for 30 min. The running speed and duration of the exercise were gradually increased to 60% of VO2max of the animals. Therefore, on the last week of the 6 weeks training program, young animals ran at 22 m/min, at a 10% incline, for 60 min, whereas old animals ran at 13 m/min, and a 10% incline for 60 min. The animals were killed 2 days after the last exercise session to avoid the metabolic effects of the final run. Gactrocnemius muscle was carefully excised, homogenized in buffer containing 137 mM NaCl, 20 mM Tris-HCl pH 8.0, 2% NP 40, 10% glycerol and protease inhibitors. For some measurements, nuclear extracts were separated as described earlier (Radak et al., 2009). In brief, part of the gastrocnemius muscle samples were homogenized in buffer (HB) containing 20 mM Tris (pH 8.0), 1 mM EDTA, 1 mM dithiothereitol, 0.5 mM spermidine, 0.5 mM spermine, 50% glycerol, and protease inhibitors. The nuclear fraction was separated by differential centrifugation. To prepare nuclear fractions, the homogenate was centrifuged at 1000 × g for 10 min at 4 °C, and the pellet was suspended in HB and recentrifuged. The pellet was then re-suspended in HB with 0.5% NP40 and centrifuged. Next, the pellet was washed twice in HB. After centrifugation, the final nuclear pellet was rocked for 30 min after the addition of a 1/10 (vol/vol) of 2.5 M KCl and centrifuged at 14,000 rpm for 30 min. The supernatant was divided into aliquots and stored at −80 °C. The protein levels were measured using the BCA method.
2.2. Western blots
Ten to 50 μg of protein were electrophoresed on 8–12% (v/v) polyacrylamide SDS-PAGE gels. Proteins were electrotransferred onto PVDF membranes. The membranes were subsequently blocked and after blocking, PVDF membranes were incubated at room temperature with antibodies (1:500 #ab53517 Abcam SIRT1, 1:500 #S4322 Sigma SIRT6, 1:500 #ab37299 Abcam NAMPT (PBEF), 1:1000 #ab193 Abcam Acetylated protein, 1:200, #U7757 Sigma UCP3, 1:4000 #H 6536 Sigma HIF-1alpha, 1:500 #MAB1665 Chemicon VEGF, 1:200 #sc-7159 Santa-Cruz Cytocrome C, 1: 15000 #T6199 Sigma alpha-Tubulin, 1:150 #S7150 Chemicon anti-DNPH, and 1:500 #ab204 Abcam OGG1). After incubation with primary antibodies, membranes were washed in TBS-Tween-20 and incubated with HRP-conjugated secondary antibodies. After incubation with secondary antibody, membranes were repeatedly washed. Membranes were incubated with an ECL plus reagent (RPN 2132, Amershham) and protein bands were visualized on X-ray films. The bands were quantified by ImageJ software, and normalized to tubulin, which served as an internal control.
2.3. Detection of carbonylated proteins
Changes in oxidized protein levels were determined using an Oxyblot kit (Chemicon/Millipore Temecula, CA) according to the manufacturer recommendations. Briefly, proteins were derivatized with 4-dinitrophenylhydrazine (DNPH) for 15 min followed by incubation at room temperature with a neutralization buffer (Chemicon/Millipore). Derivatized proteins were electrophoresed on a 10% SDS-PAGE and blotted on Hybond PVDF membranes (Amersham, Piscataway, NJ). Blots were blocked with 5% non-fat dry milk (blocking buffer) in Dulbecco’s PBS containing 0.05% Tween 20 (PBS-T) for 3 h and incubated with anti-DNP primary antibody (1:150) (Chemicon/Millipore) overnight at 4 °C. After three washes with PBS-T, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (1:300) (Amersham, Piscataway, NJ). Immunocomplexes were visualized by chemiluminescence using the ECL kit (Amersham).
2.4. Assessment of SIRT1 activity
To measure SIRT1 deacetylase activity, Cyclex SIRT1/Sir2 Deacetylsase Fluorometric Assay Kit (Cyclex, CY-1151) was used according to the established protocol, including the separation of nuclear extract. In brief, 10 μl of cytosolic and nuclear extracts of quadriceps muscle were mixed with a reaction mixture (40 μl) containing 50 mM Tris–HCl pH 8.8, 4 mM MgCl2, 0.5 mM DTT, 0.25 mAU/ml Lysyl endpeptidase, 1 μM Trichostatin A, 20 μM Fluoro-Substrate Peptide, and 200 μM NAD+ in a microplate. The samples were mixed well and incubated for 10 min at RT and the fluorescence intensity (ex. 355 nm, em. 460 nm) was read for 2 h every 10 min and normalized by the protein content.
2.5. Measurement of NAD+/NADH level
Proteins were filtered through a 10 kD Microcon filter and applied to a NAD+/NADH Quantification kit (Bio Vision, K337-100) according to the given protocol. First, total NAD+ level was measured then NAD was decomposed by heating to 60 °C for 30 min, then cooled on ice and transferred to the microplate. Next, a 10 μl NADH developer was added to each well, mixed, and the optical density read at 450 nm every 30 min for 5 h. The NAD+ levels were calculated according to the manufacturer’s directions.
2.6. DNA damage and repair assays
The measurement of 8-hydroxy-2′-guanine (8-oxo-Gua) was done as previously described (Radak et al., 2002). The OGG1 assay was carried out according to the protocol outlined by (Radak et al., 2007). In brief, 20 pmol of synthetic substrate containing 8-oxo-Gua (Trevigen, Gaithersburg, MD, USA) were labeled with 32P at the 5′ end using polynucleotide T4 kinase (Boeringer Mannheim, Germany). For the nicking reaction, protein extract (2 μg) was mixed with 20 μl of a reaction mixture containing 0.5 M of N-[2-hydroxyethel]piperazine-N′-[ethanesulfonic acid], 0.1 M EDTA, 5 mM of dithiolthreitol, 400 mM KCl, purified BSA and labeled probe (approximately 2000 cpm). The reaction was carried out at 30 °C for 15 min and stopped by placing the mixture on ice. Next, 30 μl of chloroform were added, samples were centrifuged, and 15 μl of the aqueous layer were taken and mixed with loading buffer containing 90% formamide, 10 mM NaOH, and blue-orange dye. After three min heating at 95 °C, samples were chilled and loaded into a polyacrylamide gel (20%) with 7 M urea and 1 × TBE and run at 400 mV for 2 h. Radioactive signals of the cleavage product of the labeled substrate were quantified using a STORM Bioimaging Analyzer (Molecular Dynamics, USA). Radioactivity in the separated, cleaved product, and intact oligo bands was quantified with a PhosphpoImager (Molecular Dynamics) loaded with Image Quant software. The activity to repair 8-oxo-Gua was determined and expressed as a percentage of the substrate cleaved (Radak et al., 2007).
2.7. Statistical analyses
Statistical significance was assessed by one-way ANOVA, followed by Tukey’s post hoc test. The significance level was set at p < 0.05.
3. Results
3.1. Exercise increases the activity of SIRT1
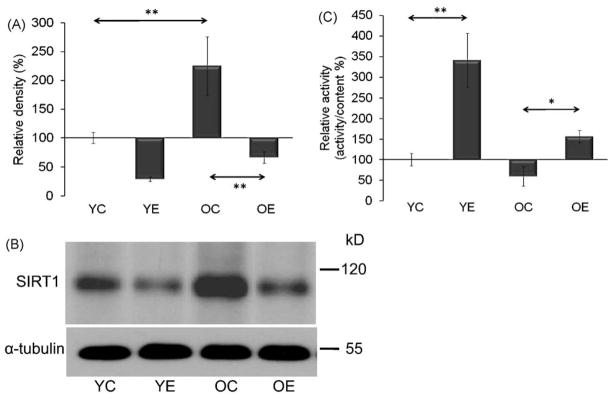
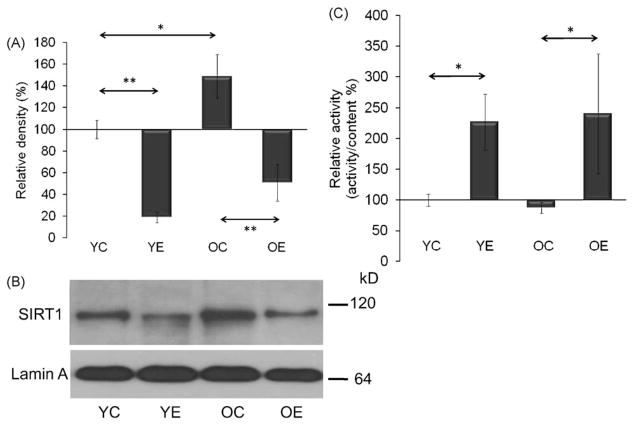
SIRT1 is localized in the cytosol and the nucleus. Therefore, the level and activity in both cell compartments were measured. Aging significantly (p < 0.01) increased the level of SIRT1 in the cytosol, while the specific activity of the enzyme did not change with aging (Fig. 1A–C). In the nucleus, similar phenomena were observed, i.e. an age-associated increase in the protein content of the enzyme with an unaltered relative activity (Fig. 2A–C). The age-associated increases in SIRT1 levels were decreased with exercise training (p < 0.01). However, exercise training increased the relative activity of SIRT1 in the cytoplasmic and nuclear extracts in both age groups (Figs. 1 and 2A–C).
Fig. 1.
Changes in cytosolic levels and activities of SIRT1 as a function of age and physical exercise. (A) Aging increases the levels of SIRT1. (B) Effect of exercise on SIRT1 levels in young and aged group of animals. Densities of the bands were normalized to tubulin which served as an internal control. (C) The graphical representation of SIRT1 activity. In panels (A)–(C): YC, young control; YE, young exercised; OC, old control; OE, old exercised. Values are means ± S.E. for six animals per group; *p < 0.05, **p < 0.01.
Fig. 2.
Change in nuclear SIRT1 levels (A panel) and activities (C panel) in muscles from young and aged animals. (B) Impact of exercise on nuclear levels of SIRT1. Densities of the bands were normalized to lamin A which served as an internal control of nucleus. In panels (A)–(C): YC, young control; YE, young exercised; OC, old control; OE, old exercised. Values are means ± S.E. for six animals per group; *p < 0.05, **p < 0.01.
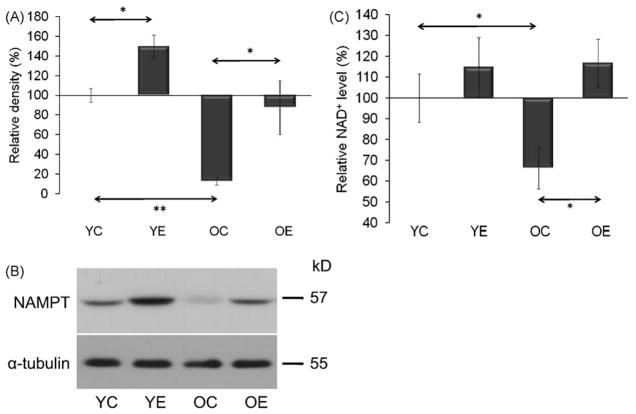
The levels of NAMPT, one of the rate limiting enzymes in NAD+ biosynthesis, decreased in the aged group of animals (p < 0.01) (Fig. 3A and B). In accordance with this finding, NAD+ levels were significantly lower in skeletal muscles of aged animals (Fig. 3C). Exercise significantly (p < 0.05) increased NAMPT levels in young and old groups. It is noteworthy that NAMPT levels in old-exercised animals demonstrated a wide range of variation but the average levels were nearly identical with levels found in young control rats. Importantly, these data suggest that the age-associated decreases in NAD+ and NAMPT levels were reversed with regular exercise, leading to increased specific activity of SIRT1.
Fig. 3.
Decreases in NAD+ and NAMPT levels were re-established by exercise training in aged animals. (A) Changes in the levels of NAD+ biosynthetic enzyme NAMPT. (B) Exercise increases NAMPT level in muscles of young and aged animals. Densities of the bands were normalized to tubulin which served as an internal control. (C) Exercise training prevented the age-dependent decrease in NAD+ level. In panels (A)–(C): YC, young control; YE, young exercised; OC, old control; OE, old exercised. Values are means ± S.E. for six animals per group; *p < 0.05, **p < 0.01.
3.2. Aging-related SIRT1-associated processes are attenuated by exercise
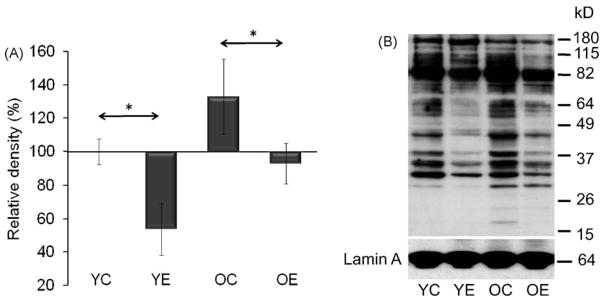
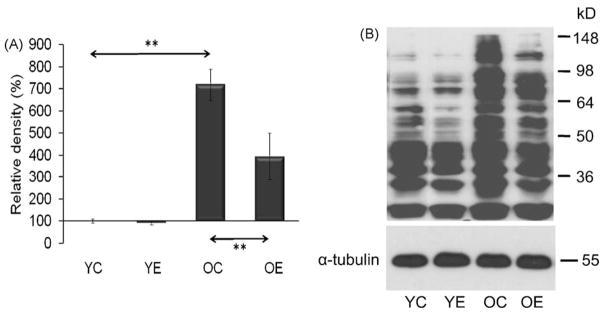
The overall acetylation level in the nuclear fractions was assessed by Western blot analysis. The rate of acetylated lysine residues showed significant (p < 0.05) differences between control and exercised animals both in young and old groups (Fig. 4A and B). Generally, exercise decreased overall levels of acetylated proteins and these data are in good agreement (or consistent) with increased activity of SIRT1 by exercise. Importantly, acetylation profiles of nuclear proteins from old-exercised animals resembled those of young controls (Fig. 4A and B).
Fig. 4.
Change in levels of acetylated proteins in nuclear lysates. (A) Graphical deceptions of acetylated protein levels in age and exercise groups. (B) Abundance of acetylated proteins in nuclear lysates from muscle. Densities of the bands were normalized to lamin A which served as an internal control of nucleus. In panels (A) and (B): YC, young control; YE, young exercised; OC, old control; OE, old exercised. Values are means ± S.E. for six animals per group; *p < 0.05.
The majority of cellular ROS are byproducts of mitochondrial respiration. UCP3 has been shown to decrease the generation of reactive oxygen species (Jiang et al., 2009). In addition, UCP3 is abundantly expressed in skeletal muscle and its activity can be regulated by SIRT1 (Amat et al., 2007). As shown in Fig. 5A and B, UCP3 levels were decreased in skeletal muscle of aged animals. Exercise appears to increase the UCP3 levels, which narrowed the gap between young and old animals (Fig. 5A and B). In control experiments, the mitochondrial levels of cytochrome c (cyt-c) showed no significant differences with age, and exercise did not alter cyt-c levels (data not shown). These data confirm the results that exercise increases expression of UCP3 that may explain lower oxidative stress levels in trained old groups of animals (Anastasiou and Krek, 2006).
Fig. 5.
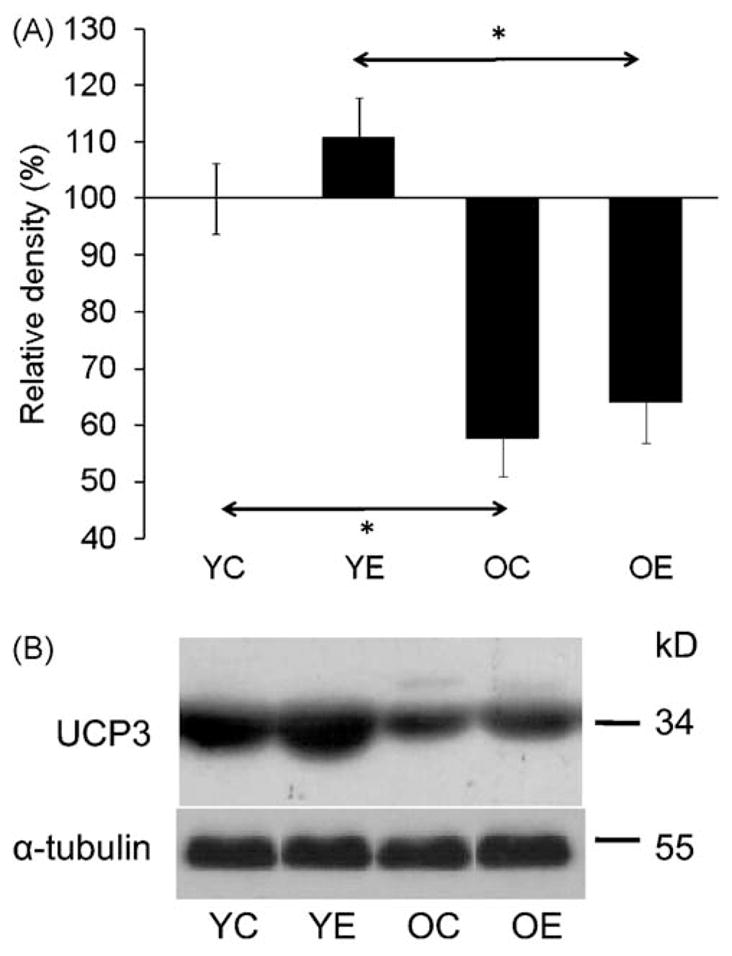
UCP3 levels in response to exercise in young and aged animals. (A) Graphical illustration of results from densitometry. (B) Changes at protein levels for UCP3 in muscles of control and exercised group of animals. Densities of the bands were normalized to tubulin which served as an internal control. In panel (A) and (B): YC, young control; YE, young exercised; OC, old control; OE, old exercised. Values are means ± S.E. for six animals per group; *p < 0.05.
3.3. Exercise attenuates the impact of oxidative stress
Oxidative stress to cells/tissues is commonly assessed by the levels of reactive carbonyl derivatives, the levels of which are increased with age (Figueiredo et al., 2009; Kayali et al., 2007). As well, significant increases have been observed in protein carbonyl levels in muscles of aged animals. In young animals, exercise did not impact significantly on the levels of cabonylated proteins. Importantly, in aged animals exercise significantly (p < 0.01) decreased levels of oxidatively modified proteins (Fig. 6A and B).
Fig. 6.
Change in levels of carbonylated proteins in response to age and exercise. (A) Graphical illustration of results from densitometry. (B) Representative auto radiogram of carbonylated proteins from muscles of control and exercised animals. Densities of the bands were normalized to tubulin which served as an internal control. In panels (A) and (B): YC, young control; YE, young exercised; OC, old control; OE, old exercised. Values are means ± S.E. for six animals per group; **p < 0.01.
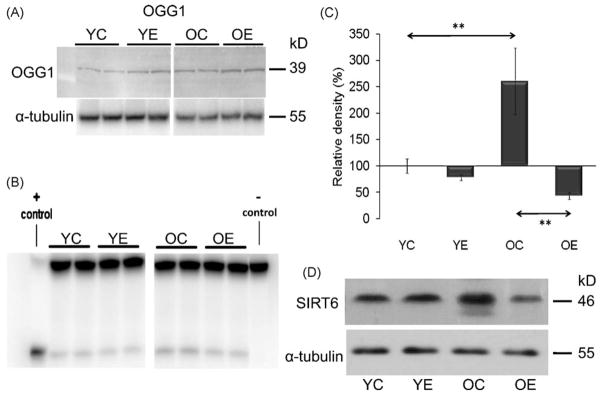
Although there were significant differences in protein carbonylation levels between old and young animals, no significant alterations in the levels of 8-ox-Gua were observed in the DNA. The 8-ox-Gua levels were as follows YC: 0.87 ± 0.23, YE: 0.79 ± 0.17, OC: 0.76 ± 0.15, OE: 0.77 ± 0.27 (8-ox-Gua per 10−5 guanine base). This DNA base lesion is repaired primarily by OGG1 (Bohr et al., 2002; Hazra et al., 2002), whose protein level tend to increase in the nuclear lysates of muscle in exercised young and aged animals [relative band-density: YC: 100 ± 08, YE: 143 ± 16, OC: 138 ± 19, OE: 182 ± 25; Fig. 7A]. The changes in the activity of OGG1 did not reach the significant level (7B). Strong functional relationship between OGG1 and SIRT6 can not be suggested from the peresenet findigs, although both OGG1 and SIRT6 protein content increased as a result of aging (Fig. 7C and D).
Fig. 7.
OGG1 and SIRT6 levels and activities in resting and exercised young and aged animals. (A) Representative auto radiogram of OGG1 level. Densities of the bands were normalized to tubulin which served as an internal control. (B) Representative auto radiogram of OGG1 activity. (C) Graphical illustration of results from densitometry of SIRT6 level. (D) Representative auto radiograms from aged and/or exercised animals. In panel (A)–(D): YC, young control; YE, young exercised; OC, old control; OE, old exercised. Values are means ± S.E. for six animals per group; **p < 0.01.
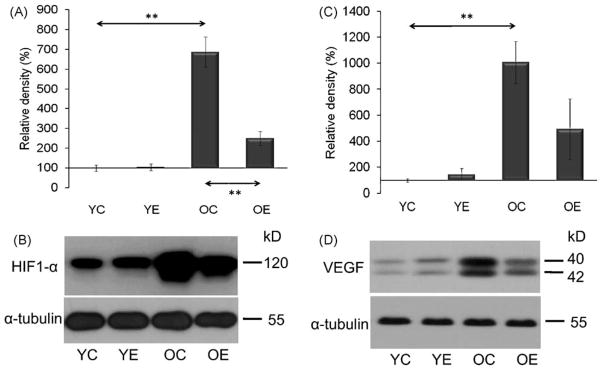
Physical exercise induces numerous adaptive responses that increase the oxidative and metabolic capacity of skeletal muscle, upregulation of HIF-1α expression being one of the important factors (Mason et al., 2007). Indeed, in the present model, the age-associated shift in redox balance (as shown by increased levels of carbonylated proteins; Fig. 6A and B) greatly increased the levels of HIF-1α in muscle samples of old animals (Fig. 8A and B). HIF-1α levels were similar in young control and exercised animals. HIF-1α is believed to induce vascularization, via VEGF, and the latter showed a positive correlation with HIF-1α [r = 0.815; Fig. 8C and D] in young and aged animals. These data suggest that regular exercise training decreases the difference between young and old rats in regard to oxidative stress markers.
Fig. 8.
Aging increased the level of HIF-1α (A and B) and VEGF (C and D) and these increases were significantly attenuated by exercise training. Densities of the bands were normalized to tubulin which served as an internal control. In panels (A)–(D): YC, young control; YE, young exercised; OC, old control; OE, old exercised. Values are means ± S.E. for six animals per group; **p < 0.01.
4. Discussion
SIRT1 is a unique protein deacetylator and because of its dependence on NAD, it is closely linked to cellular metabolism (Bhakat et al., 2006; Canto et al., 2009; Sakamoto et al., 2009). Indeed, changes in SIRT1 activity by agents, such as caloric restriction or resveratrol or SRT501, have been shown to increase lifespan via a wide range of processes, including suppressed apoptosis, and inflammation, or enhanced DNA repair (Pallas et al., 2009; Porcu and Chiarugi, 2005). Although, the beneficial effects of sirtuin activators on mammals are still unclear, some data indicate that sirtuins do play both protective and pro-aging roles (Li et al., 2008). Despite a significant interest in the role of SIRT1 in the aging process, little is known about the effects of exercise and aging on SIRT1-mediated processes in various tissues and specifically in skeletal muscle.
The present results show that aging increases the levels, but decreases the relative activities of SIRT1 in cytosolic and nuclear extracts. Therefore, it appears that the activity per molecule of SIRT1 is lower in the skeletal muscle of older rats, suggesting that there are more inactive or less active molecules in these animals. It is noteworthy that aging differentially affects SIRT1 levels in various organs (Kwon and Ott, 2008). These observations are in line with the finding that cytoplasmic aggregation of SIRT1 with decreased protein activity was found in the nucleus of human skeletal muscle in sporadic inclusion-body myositis subjects (Nogalska et al., 2008). Furthermore, over-expression exacerbated, while low to moderate expression of SIRT1 attenuated age-dependent increases in cardiac hypertrophy, apoptosis/fibrosis, cardiac dysfunction, and expression of senescence markers in transgenic mouse hearts (Alcendor et al., 2007). It is also possible that an age-associated drop in NAD+ content, which could be due to the decreased levels of NAMPT, results in a compensatory mechanism within the cell, which leads to an increased production of SIRT1. Regardless of the exact mechanism leading to the age-associated increase in SIRT1 levels, it is clear that regular exercise significantly increases the relative activity of SIRT1 in skeletal muscle of both old and young animals.
The present data, indeed, also reveal that aging decreases the levels of NAMPT, one of the key enzymes to produce NAD+ (Yang and Sauve, 2006). Decreased production of NAD+ can readily result in metabolic alteration of the cell, causing exhaustion of energy production (Breen et al., 2008; Skokowa et al., 2009). It is generally believed that genotoxic stress kills cells by depleting nuclear and cytosolic NAD+ pools (Burkle, 2005) due to extensive use of NAD+ for DNA repair process. The present data reveal that aging is associated with decreased levels of NAD+, which decreases the rate of cell survival in response to a variety of stressors (Burkle, 2005). On the other hand, increased levels of NAMPT have been shown to significantly increase resistance to cell death (Yang et al., 2007) in line with our observations with exercise training that is beneficial for cellular metabolisms. Therefore, one of the novel findings of this work is that in order to maintain SIRT1 activity in skeletal muscle of aged animals, aging-associated losses of NAD+ and NAMPT levels can be prevented with exercise training.
Protein carbonyl levels were significantly higher in aged animals. This oxidative damage may partially inhibit SIRT1 activity similarly to many other proteins (Radak et al., 2008). It is also interesting to note that both a major residue of protein oxidation (carbonylation) and acetylation are lysine, suggesting that higher carbonylation would decrease acetylation of the same residue, thus potentially influencing the activity of modified proteins (A. Nakamura, personal communication).
Aging has been suggested to cause changes in mitochondrial function, especially in the electron transport chain, leading to increased ROS generation (Figueiredo et al., 2009). UCP3 lowers mitochondrial membrane potential and protects muscle cells against an overload of fatty acids, and decreases excessive production of ROS (Jiang et al., 2009). The present data revealed that the decreases in UCP3 level as a result of aging, as has been previously observed by others (Hoeks et al., 2006; Kontani et al., 2002), which could be one of the reason of age-associated increase in ROS production. Here we report an age-related increase in SIRT1 and decrease in UCP3 levels, which supports the finding of Amat et al. (2007), who described that SIRT1 represses UCP3 gene expression. Exercise training mimics the effects of caloric restriction as both result in increases in the protein content of UCP3 (Bevilacqua et al., 2005).
SIRT6 deficiency causes the most striking phenotype among all the sirtuin knockouts, with slow rate of growth and sensitivity to genotoxic agents (Lombard et al., 2008). SIRT6 was identified as a chromatin-associated protein, which influences the efficiency of DNA repair probably through its interaction with DNA polymerase beta (Mostoslavsky et al., 2006), which fills the remaining gap in the DNA chain after the removal of 5′-deoxyribose phosphate, before the final steps in BER are completed by DNA ligase. 8-oxoG is one of the most characterized DNA damage primarily repaired by OGG1. An age-associated increase in 8-oxo-Gua level was not observed in the gastrocnemius muscle, unlike in an earlier report (Radak et al., 2002, 2007). Different strains and ages of rats used in the two studies could explain the dissimilar results. On the other hand, age-associated increases in SIRT6 and OGG1 protein levels were noted, which does not rule out that indeed SIRT6 can regulate OGG1.
The incision activity of OGG1 is significantly increased by acetylation (Bhakat et al., 2006). The findigs of the current study do not allow to confirm the exact role of SIRT1 or SIRT6 in the regulation of OGG1.
The other novel finding in the present study is the age-associated increase in HIF-1α and VEGF levels. Interestingly, exercise training decreased the HIF-1α levels in the old group, which appears to be contradictory to the understanding that HIF-1α mediates exercise-induced oxidative adaptation to skeletal muscle (Freyssenet, 2007). Nevertheless, recent findings suggest that HIF-1α negatively effects mitochondrial adaptation (Mason et al., 2007). Moreover, in accordance with the present data others have reported that exercise training decreases HIF-1α expression in skeletal muscle (Lundby et al., 2006). Sensing of changes in oxygen levels and induction of HIF-1α were shown to be dependent on mitochondrially generated ROS (but independent of oxidative phosphorylation) (Brunelle et al., 2005). Therefore, the redox sensitive HIF-1α level is increased in the skeletal muscle of older animals, indicating an inadequate oxygen supply. This observation correlates well with increased levels of VEGF, which are transcriptionally dependent on HIF-1α. Exercise training decreased levels of both HIF-1α and VEGF.
In conclusion, exercise training increases the activity, but not the level of SIRT1 in muscle of both young and old animals. Aging results in decreases in NAMPT and NAD content, and these processes are significantly attenuated by exercise training, providing a possible explanation of increased SIRT1 activity. Aging is associated with increased SIRT6 and OGG1 level, which is reversed by exercise. Regular exercise training prevents the age-induced increase in HIF-1α and VEGF levels in skeletal muscle of rats, most likely by the normalization of cellular redox milieu. Overall, the data suggest that regular exercise training attenuates the aging process of skeletal muscle via the NAMPT-NAD+-SIRT1 pathway.
Acknowledgments
The present work was supported by Hungarian grants: ETT 38388, TéT JAP13/02, awarded to Z. Radák, AG 021830 (to I. Boldogh) from the U.S. NIH/NIA and grants from the Finnish Ministry of Education to MA.
References
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–34076. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- Anastasiou D, Krek W. SIRT1: linking adaptive cellular responses to aging-associated changes in organismal physiology. Physiology (Bethesda) 2006;21:404–410. doi: 10.1152/physiol.00031.2006. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005;289:E429–E438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- Bhakat KK, Mokkapati SK, Boldogh I, Hazra TK, Mitra S. Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo. Mol Cell Biol. 2006;26:1654–1665. doi: 10.1128/MCB.26.5.1654-1665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr VA, Stevnsner T, de Souza-Pinto NC. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286:127–134. doi: 10.1016/s0378-1119(01)00813-7. [DOI] [PubMed] [Google Scholar]
- Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Burkle A. Poly(ADP-ribose). The most elaborate metabolite of NAD+ FEBS J. 2005;272:4576–4589. doi: 10.1111/j.1742-4658.2005.04864.x. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton TL. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol. 2007;102:2379–2388. doi: 10.1152/japplphysiol.01298.2006. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- Figueiredo PA, Powers SK, Ferreira RM, Appell HJ, Duarte JA. Aging impairs skeletal muscle mitochondrial bioenergetic function. J Gerontol A: Biol Sci Med Sci. 2009;64:21–33. doi: 10.1093/gerona/gln048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssenet D. Energy sensing and regulation of gene expression in skeletal muscle. J Appl Physiol. 2007;102:529–540. doi: 10.1152/japplphysiol.01126.2005. [DOI] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20:45–54. doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- Hazra TK, Izumi T, Boldogh I, Imhoff B, Kow YW, Jaruga P, Dizdaroglu M, Mitra S. Identification and characterization of a human DNA glycosylase for repair of modified bases in oxidatively damaged DNA. Proc Natl Acad Sci USA. 2002;99:3523–3528. doi: 10.1073/pnas.062053799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss AR. Energy metabolism, altered proteins, sirtuins and ageing: converging mechanisms? Biogerontology. 2008;9:49–55. doi: 10.1007/s10522-007-9110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeks J, Hesselink MK, Schrauwen P. Involvement of UCP3 in mild uncoupling and lipotoxicity. Exp Gerontol. 2006;41:658–662. doi: 10.1016/j.exger.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Jiang N, Zhang G, Bo H, Qu J, Ma G, Cao D, Wen L, Liu S, Ji LL, Zhang Y. Upregulation of uncoupling protein-3 in skeletal muscle during exercise: a potential antioxidant function. Free Radic Biol Med. 2009;46:138–145. doi: 10.1016/j.freeradbiomed.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Kayali R, Cakatay U, Uzun H, Genc H. Gender difference as regards myocardial protein oxidation in aged rats: male rats have increased oxidative protein damage. Biogerontology. 2007;8:653–661. doi: 10.1007/s10522-007-9107-5. [DOI] [PubMed] [Google Scholar]
- Kontani Y, Wang Z, Furuyama T, Sato Y, Mori N, Yamashita H. Effects of aging and denervation on the expression of uncoupling proteins in slow- and fast-twitch muscles of rats. J Biochem. 2002;132:309–315. doi: 10.1093/oxfordjournals.jbchem.a003225. [DOI] [PubMed] [Google Scholar]
- Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci. 2008;33:517–525. doi: 10.1016/j.tibs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins—novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Schwer B, Alt FW, Mostoslavsky R. SIRT6 in DNA repair, metabolism and ageing. J Intern Med. 2008;263:128–141. doi: 10.1111/j.1365-2796.2007.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Gassmann M, Pilegaard H. Regular endurance training reduces the exercise induced HIF-1alpha and HIF-2alpha mRNA expression in human skeletal muscle in normoxic conditions. Eur J Appl Physiol. 2006;96:363–369. doi: 10.1007/s00421-005-0085-5. [DOI] [PubMed] [Google Scholar]
- Mason SD, Rundqvist H, Papandreou I, Duh R, McNulty WJ, Howlett RA, Olfert IM, Sundberg CJ, Denko NC, Poellinger L, Johnson RS. HIF-1alpha in endurance training: suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2059–R2069. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- Mayr M, Sidibe A, Zampetaki A. The paradox of hypoxic and oxidative stress in atherosclerosis. J Am Coll Cardiol. 2008;51:1266–1267. doi: 10.1016/j.jacc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Moller P, Loft S, Lundby C, Olsen NV. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001;15:1181–1186. doi: 10.1096/fj.00-0703com. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Nogalska A, D’Agostino C, Engel WK, Davies KJ, Askanas V. Decreased SIRT1 deacetylase activity in sporadic inclusion-body myositis muscle fibers. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.021. [DOI] [PubMed] [Google Scholar]
- O’Hagan KA, Cocchiglia S, Zhdanov AV, Tambawala MM, Cummins EP, Monfared M, Agbor TA, Garvey JF, Papkovsky DB, Taylor CT, Allan BB. PGC-1{alpha} is coupled to HIF-1{alpha}-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci USA. 2009;106:2188–2193. doi: 10.1073/pnas.0808801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M, Casadesus G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6:70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005;26:94–103. doi: 10.1016/j.tips.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Radak Z, Atalay M, Jakus J, Boldogh I, Davies K, Goto S. Exercise improves import of 8-oxoguanine DNA glycosylase into the mitochondrial matrix of skeletal muscle and enhances the relative activity. Free Radic Biol Med. 2009;46:238–243. doi: 10.1016/j.freeradbiomed.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18:749–750. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kumagai S, Nakamoto H, Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. J Appl Physiol. 2007;102:1696–1701. doi: 10.1152/japplphysiol.01051.2006. [DOI] [PubMed] [Google Scholar]
- Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Iwasaki K, Sugiyama H, Tsuji Y. Role of the tumor suppressor PTEN in antioxidant responsive element-mediated transcription and associated histone modifications. Mol Biol Cell. 2009;20:1606–1617. doi: 10.1091/mbc.E08-07-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Imai S. From heterochromatin islands to the NAD world: a hierarchical view of aging through the functions of mammalian Sirt1 and systemic NAD biosynthesis. Biochim Biophys Acta. 2009;1790:997–1004. doi: 10.1016/j.bbagen.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, Brechlin AM, Schambach A, Hinrichsen L, Meyer G, Gaestel M, Stanulla M, Tong Q, Welte K. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism. 2008;57:986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- van der Veer E, Ho C, O’Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, Zheng ZM, Appella E, Wang XW, Ried T, Deng CX. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, de Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Sauve AA. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006;8:E632–E643. doi: 10.1208/aapsj080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]