Abstract
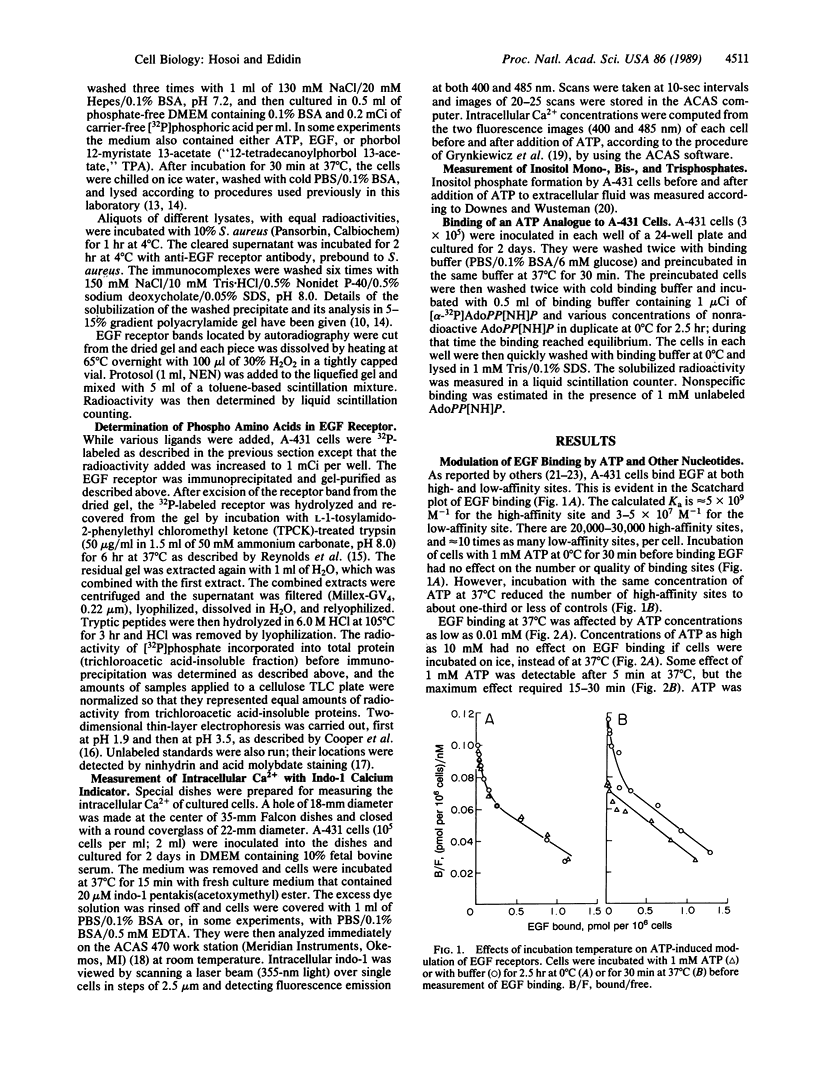
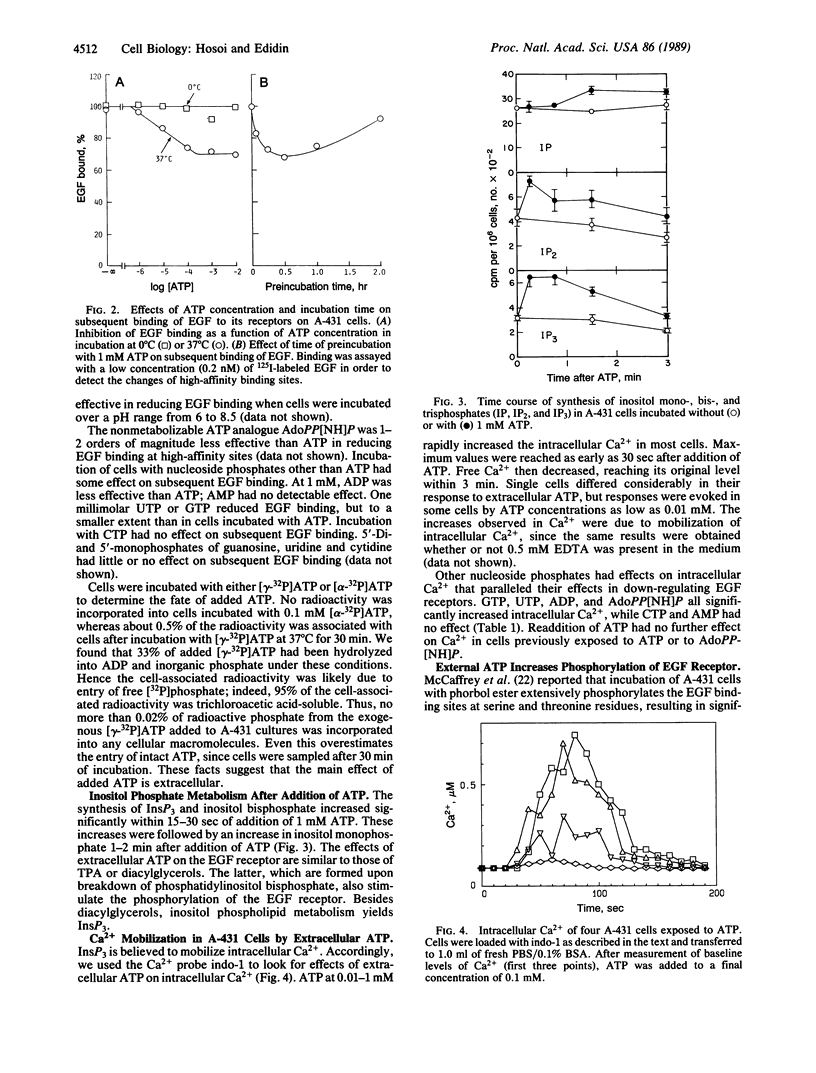
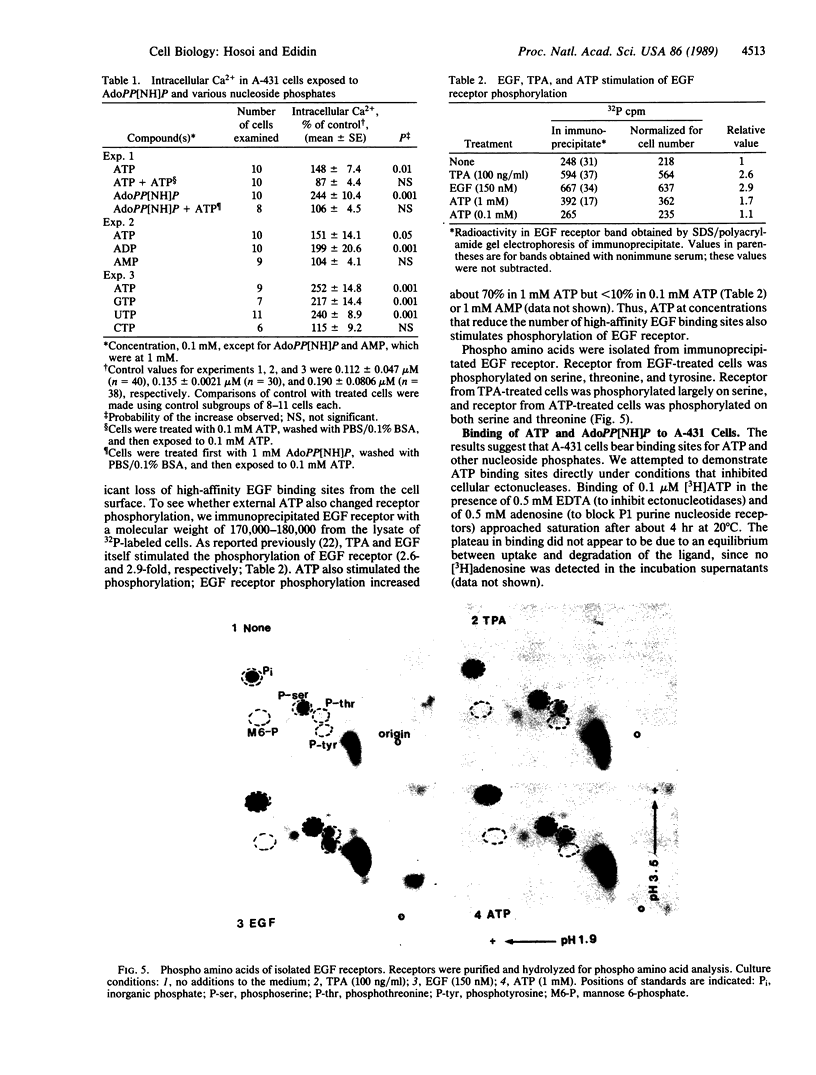
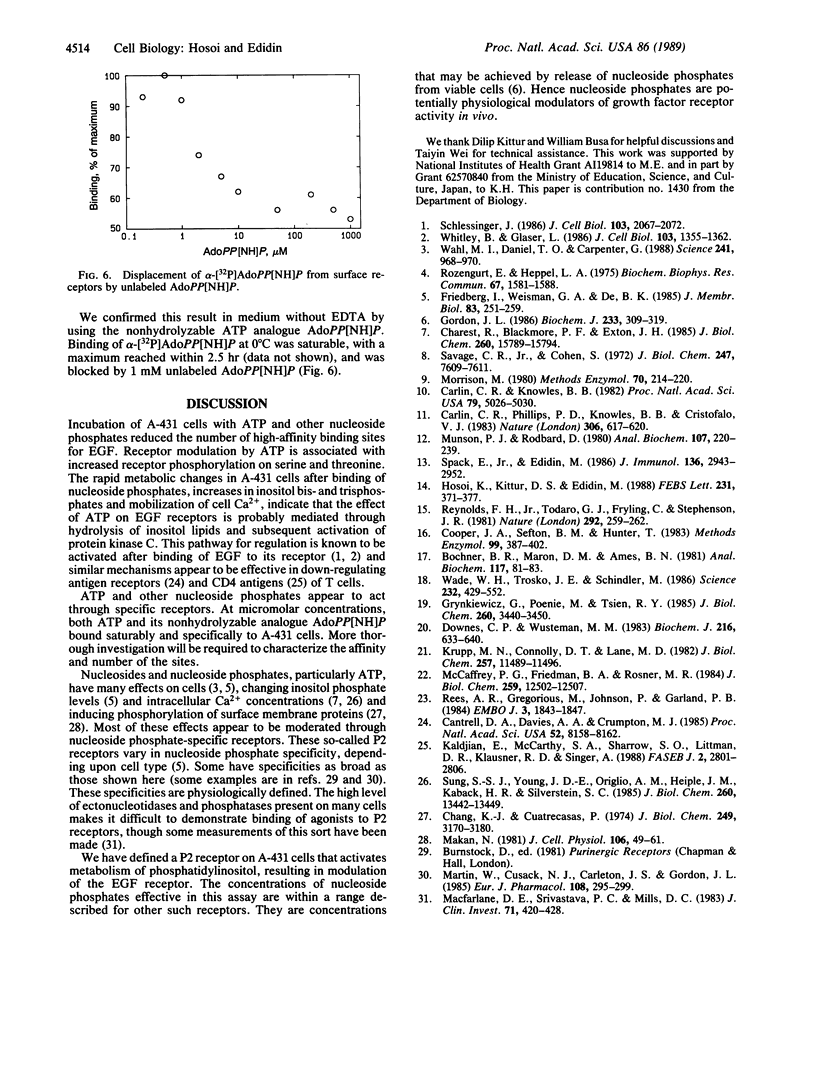
The binding of epidermal growth factor (EGF) by A-431 human epidermoid carcinoma cells was reduced after exposure of the cells to low concentrations (0.01-1 mM) of ATP and other nucleoside 5'-triphosphates at 37 degrees C, but not at 0 degree C. This was due to loss of high-affinity EGF binding sites. The modulation was associated with transient increases in inositol phosphate synthesis and intracellular Ca2+ and with phosphorylation of the EGF receptor on serine and threonine. There was no evidence for entry of labeled ATP into the cells. ATP appeared to bind to specific cell surface receptors. Such binding was demonstrated directly with the nonmetabolizable ATP analogue adenosine 5'-[beta,gamma-imido]triphosphate.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner B. R., Maron D. M., Ames B. N. Detection of phosphate esters on chromatograms: an improved reagent. Anal Biochem. 1981 Oct;117(1):81–83. doi: 10.1016/0003-2697(81)90695-3. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Davies A. A., Crumpton M. J. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Knowles B. B. Identity of human epidermal growth factor (EGF) receptor with glycoprotein SA-7: evidence for differential phosphorylation of the two components of the EGF receptor from A431 cells. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5026–5030. doi: 10.1073/pnas.79.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Phillips P. D., Knowles B. B., Cristofalo V. J. Diminished in vitro tyrosine kinase activity of the EGF receptor of senescent human fibroblasts. Nature. 1983 Dec 8;306(5943):617–620. doi: 10.1038/306617a0. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Cuatrecasas P. Adenosine triphosphate-dependent inhibition of insulin-stimulated glucose transport in fat cells. Possible role of membrane phosphorylation. J Biol Chem. 1974 May 25;249(10):3170–3180. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg I., Weisman G. A., De B. K. Permeability change in transformed mouse fibroblasts caused by ionophores, and its relationship to membrane permeabilization by exogenous ATP. J Membr Biol. 1985;83(3):251–259. doi: 10.1007/BF01868699. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hosoi K., Kittur D. S., Edidin M. Modulation of HLA antigens in response to the binding of epidermal growth factor by A431 cells. FEBS Lett. 1988 Apr 25;231(2):371–377. doi: 10.1016/0014-5793(88)80852-4. [DOI] [PubMed] [Google Scholar]
- Kaldjian E., McCarthy S. A., Sharrow S. O., Littman D. R., Klausner R. D., Singer A. Nonequivalent effects of PKC activation by PMA on murine CD4 and CD8 cell-surface expression. FASEB J. 1988 Sep;2(12):2801–2806. doi: 10.1096/fasebj.2.12.3261700. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Connolly D. T., Lane M. D. Synthesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11489–11496. [PubMed] [Google Scholar]
- Macfarlane D. E., Srivastava P. C., Mills D. C. 2-Methylthioadenosine[beta-32P]diphosphate. An agonist and radioligand for the receptor that inhibits the accumulation of cyclic AMP in intact blood platelets. J Clin Invest. 1983 Mar;71(3):420–428. doi: 10.1172/JCI110786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makan N. R. Passive membrane permeability to small molecules and ions in transformed mammalian cells: probable role of surface phosphorylation. J Cell Physiol. 1981 Jan;106(1):49–61. doi: 10.1002/jcp.1041060107. [DOI] [PubMed] [Google Scholar]
- Martin W., Cusack N. J., Carleton J. S., Gordon J. L. Specificity of P2-purinoceptor that mediates endothelium-dependent relaxation of the pig aorta. Eur J Pharmacol. 1985 Feb 5;108(3):295–299. doi: 10.1016/0014-2999(85)90452-2. [DOI] [PubMed] [Google Scholar]
- McCaffrey P. G., Friedman B., Rosner M. R. Diacylglycerol modulates binding and phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984 Oct 25;259(20):12502–12507. [PubMed] [Google Scholar]
- Morrison M. Lactoperoxidase-catalyzed iodination as a tool for investigation of proteins. Methods Enzymol. 1980;70(A):214–220. doi: 10.1016/s0076-6879(80)70051-4. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Rees A. R., Gregoriou M., Johnson P., Garland P. B. High affinity epidermal growth factor receptors on the surface of A431 cells have restricted lateral diffusion. EMBO J. 1984 Aug;3(8):1843–1847. doi: 10.1002/j.1460-2075.1984.tb02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Todaro G. J., Fryling C., Stephenson J. R. Human transforming growth factors induce tyrosine phosphorylation of EGF receptors. Nature. 1981 Jul 16;292(5820):259–262. doi: 10.1038/292259a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. A Specific effect of external ATP on the permeability of transformed 3T3 cells. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1581–1588. doi: 10.1016/0006-291x(75)90207-7. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spack E., Jr, Edidin M. The class I MHC antigens of erythrocytes: a serologic and biochemical study. J Immunol. 1986 Apr 15;136(8):2943–2952. [PubMed] [Google Scholar]
- Sung S. S., Young J. D., Origlio A. M., Heiple J. M., Kaback H. R., Silverstein S. C. Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+], and inhibits phagocytosis in mouse macrophages. J Biol Chem. 1985 Nov 5;260(25):13442–13449. [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Whiteley B., Glaser L. Epidermal growth factor (EGF) promotes phosphorylation at threonine-654 of the EGF receptor: possible role of protein kinase C in homologous regulation of the EGF receptor. J Cell Biol. 1986 Oct;103(4):1355–1362. doi: 10.1083/jcb.103.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]