Abstract
One of the most ubiquitous and long-lasting recent changes to the surface of our planet is the accumulation and fragmentation of plastics. Within just a few decades since mass production of plastic products commenced in the 1950s, plastic debris has accumulated in terrestrial environments, in the open ocean, on shorelines of even the most remote islands and in the deep sea. Annual clean-up operations, costing millions of pounds sterling, are now organized in many countries and on every continent. Here we document global plastics production and the accumulation of plastic waste. While plastics typically constitute approximately 10 per cent of discarded waste, they represent a much greater proportion of the debris accumulating on shorelines.
Mega- and macro-plastics have accumulated in the highest densities in the Northern Hemisphere, adjacent to urban centres, in enclosed seas and at water convergences (fronts). We report lower densities on remote island shores, on the continental shelf seabed and the lowest densities (but still a documented presence) in the deep sea and Southern Ocean. The longevity of plastic is estimated to be hundreds to thousands of years, but is likely to be far longer in deep sea and non-surface polar environments. Plastic debris poses considerable threat by choking and starving wildlife, distributing non-native and potentially harmful organisms, absorbing toxic chemicals and degrading to micro-plastics that may subsequently be ingested. Well-established annual surveys on coasts and at sea have shown that trends in mega- and macro-plastic accumulation rates are no longer uniformly increasing: rather stable, increasing and decreasing trends have all been reported. The average size of plastic particles in the environment seems to be decreasing, and the abundance and global distribution of micro-plastic fragments have increased over the last few decades. However, the environmental consequences of such microscopic debris are still poorly understood.
Keywords: persistent organic pollutants, marine debris, plastic production, landfill, microplastic
1. Introduction
In the last half-century, there have been many drastic changes on the surface of the planet, but one of the most instantly observable is the ubiquity and abundance of plastic debris. Like many anthropogenic impacts on natural systems, it is one that, despite widespread recognition of the problem, is still growing and even if stopped immediately will persist for centuries. From what started as a perceived aesthetic problem of plastics littering towns, countryside, shores and even far out into the ocean soon emerged as causing the choking and entanglement of wildlife. The number of potentially harmful implications of plastic debris that have been identified has escalated and it is now realized that these items may also transport persistent organic pollutants (POPs; Mato et al. 2001), non-indigenous species to new locations (Barnes 2002) and distribute algae associated with red tides (Masó et al. 2003). Reports of accumulation of plastics spread rapidly in terms of the taxa influenced, geography and bathymetry of affected sites and countries beginning monitoring and beach clean-up operations. Schools and voluntary organizations have made annual coastal collections of stranded plastics; an important educational issue even on many of the planet's most remote islands. In some areas though, notably on the seabed, assessment of plastic accumulation has been relatively neglected (Goldberg 1994). Since 1990, the dumping of rubbish at sea from ships has been prohibited under the international shipping regulation MARPOL Annex V. A reduction of ship-derived plastic debris should therefore be expected, even if global use of plastics continues to increase. To gain an accurate and meaningful assessment of plastics and their influence, large-scale and long-term monitoring is needed across countries and environments (including the sea floor) and across a range of debris sizes. These can broadly be divided into macro-debris (>20 mm diameter), meso-debris (5–20 mm) and micro-debris (<5 mm); here we also use the term mega-debris (>100 mm) (see Ryan et al. 2009; Thompson et al. 2009).
Natural marine debris of some type (e.g. pumice) has floated on the surface of the global ocean for longer than life itself, but life greatly increased this through floating algae, shells, seeds, fruits and wood. Human activities and travel by water must have further greatly increased flotsam (e.g. by timber), but by far the biggest change in the potential for transport by debris came with the mass production of plastics. The accumulation of both macro- and micro-plastics has consistently increased on shores and in sediments for the last four decades (see Thompson et al. 2004; Barnes 2005, respectively). Their inexpensive, lightweight and durable properties have made plastic much more single use and ‘throw-away’ than previous synthetic artefacts. Such compounds do deteriorate in ultraviolet (UV) light, but haline environments and the cooling effect of the sea mean degradation require very long exposure times (Gregory 1999). Because plastics become fouled by marine organisms relatively quickly, the debris may also become shielded to some extent from UV light, and the persistence of this debris was recently illustrated by accounts that plastic swallowed by an albatross had originated from a plane shot down 60 years previously some 9600 km away (Weiss et al. 2006).
Mega-debris at sea was highlighted by tens of thousands of each of basketball shoes, hockey gloves and bath toys released from containers washed off of ships (Weiss et al. 2006). There are many sources for plastics accumulating in the environment from direct dropping and dumping of litter on land or at sea to blowing from landfill sites, losses in transport and accidents. Typically, 40–80% of mega- and macro-marine debris items are plastic, much of it packaging, carrier bags, footwear, cigarette lighters and other domestic items (Derraik 2002; Barnes 2005). A recent study by Ivar do Sul & Costa (2007) across Central and South America also found marine debris dominated by land-based plastic (though sometimes fishery gear can be abundant along continental shores as well). At more remote islands, fishing-related sources of debris are often more prevalent. Following establishment of ‘long-term’ monitoring surveys of stranded debris in the 1990s, there are now sufficient data to explore seasonal, annual and long-term patterns (e.g. Morishige et al. 2007).
Most waste plastics, including the large proportion used in single-use applications such as packaging, are disposed of in landfill sites. However, plastic persists in landfill sites and if not properly buried may later surface to become ‘debris’. Durability of plastic ensures that wherever it is, it does not ‘go-away’; that is, by placing plastics in landfill we may simply be storing a problem for the future. Although accumulation of plastics on land is important, little information is available on the amounts, rates, fate or impacts, whereas there has been a major effort to quantify impacts on shorelines and at sea. In this paper, we examine waste generation and disposal, together with the abundance, composition and fragmentation of plastic. We then consider temporal and spatial trends in accumulation of plastics on strandlines, the sea surface and at depth on the seabed. We assess published data and present new surveys and observations of spatial and temporal patterns to evaluate whether persistent marine debris, such as plastics, are still increasing and whether it varies geographically?
2. Anthropogenic waste and plastic accumulation in landfill
Plastics are present in most waste, and before trends in accumulation of plastic can be explained, it is important to first consider waste generation and disposal. Global production of plastics is estimated at 225 mt yr−1 (APME 2006). Waste composition data are useful to identify the relative quantity and types of plastic. As discussed in the contribution by Teuten et al. (2009), different plastics and resins have widely varying properties with respect to contaminant sorption and desorption.
(a). Waste generation
Waste is typically categorized based on its point of generation. Categories include municipal, commercial, industrial, agricultural and construction and demolition (C&D). However, there is ambiguity within these categories. For example, in the USA, municipal solid waste (MSW) includes that generated in residential, commercial and institutional (e.g. schools, government offices) sectors, while in other countries, MSW may include anything from residential waste only to all waste managed in the municipal system (e.g. C&D, non-hazardous industrial). This complexity is exacerbated by the fact that some municipal systems manage residual materials from the treatment of water and wastewater. This relatively heavy waste will distort the composition of dry wastes such as plastics.
Considering these multiple categories, it is difficult to compare waste composition between countries. Waste is typically classified by the agency in need of the information, and surveys are typically designed with specific goals. For example, a waste sort conducted to support planning of a recycling programme would identify commonly recycled plastics, including pigmented and translucent high-density polyethylene (HDPE) containers and clear and pigmented polyethylene terephthalate (PET), and classify the remaining plastics as ‘other’. These categories are useful in this (recycling) context, but are less complete for a study of plastics in the environment. Another confounding issue is that the types of plastics present vary between municipal, agricultural and C&D waste. Municipal waste is dominated by containers (e.g. drink bottles) and films (e.g. carrier bags, packaging sheets), agricultural waste may contain large quantities of a single film and C&D waste may contain polyvinyl chloride (PVC) pipe and large plastic containers. Thus, a municipal stream that contains 10 per cent (by mass) plastics is not equivalent to a C&D stream containing the same percentage.
Waste composition may also be presented on either an ‘as-generated’ or ‘as-discarded’ basis. The former includes all the waste generated in a particular sector, prior to separation for recycling, composting or other treatment. In contrast, ‘as-discarded’ indicates the waste remaining for disposal after the aforementioned separation. In areas with significant recycling programmes, the difference between waste generation and waste disposal could be 20–40%, and waste composition will change as recyclables are removed. If properly managed at the end of its useful life, plastic waste may be recycled, burned in combustion facilities to generate energy or buried in landfill. In each of these alternatives, the waste should be destroyed or contained, so that plastic is not released to the environment. The major release of plastics to the environment is the result of inappropriate waste management and improper human behaviour, e.g. littering (abandoning waste away from collection points). For example, plastic films can be released to the environment when not transported properly, and as a result of wind-blown litter at the point of burial in a landfill. Well-operated landfills include a daily cover over the waste consisting of soil or a synthetic material and fences surrounding the landfill to contain wind-blown debris.
(b). Plastics production and recycling
Annual global consumption of the major plastic resins is considerable (Andrady & Neal 2009). Films (e.g. carrier bags, plastic sheets) are easiest to escape containment as wind-blown debris and are likely the major component of terrestrial plastic litter but plastic litter also includes discarded fishing equipment, food and beverage packaging and many other items that are present in the marine environment (Koutsodendris et al. 2008). Films are dominated by low-density polyethylene (LDPE)/linear LDPE (LLDPE). We present information on plastics in MSW in the USA and their management (table 1). The quantities recovered (i.e. for recycling) as a fraction of total discards shows that recycling rates are relatively low. In the USA, plastic recycling is largely limited to drink containers although local authorities continue to expand the types of plastics collected for recycling. In general, citizen participation rather than industrial capacity limits the quantities of plastics recycled. Efforts to provide incentives for recycling can increase the fraction recycled (Loughlin & Barlaz 2006).
Table 1.
Plastics production, recovery and disposal in the USA in 2005 (thousands of metric tonnes). Adopted from US EPA (2006). The data originated in reports of The American Plastics Council and include net imports. Plastic from the construction and agricultural sectors are not included in these quantities.
| generation of plastics in MSW | recovery | discards | |
|---|---|---|---|
| PET | 2600 | 491 | 2109 |
| HDPE | 5355 | 473 | 4882 |
| PVC | 1491 | 0 | 1491 |
| LDPE/LLDPE | 5864 | 173 | 5691 |
| polypropylene | 3636 | 9 | 3627 |
| polystyrene | 2355 | 0 | 2355 |
| other | 4982 | 355 | 4627 |
| total | 26 282 | 1500 | 24 782 |
In the USA, durable goods, products that last on average for >3 years and include items such as furniture and appliances, were the most important use for new plastics (figure 1). Non-durable goods, products that are consumed in <3 years such as trash bags and eating utensils, were the next biggest use category. In Europe, data on various packaging applications are typically combined rather than considered separately and hence disposable packaging represents the principal use of plastics (37%, PlasticsEurope 2008).
Figure 1.
Production of plastic products in the USA in 2005 (reproduced with permission from US EPA, 2006).
(c). The fraction of plastic in household waste
Plastics in the waste from various countries is estimated at approximately 10 per cent (of mass). Such estimates can only be used as an indication of plastics composition for several reasons. First, the data are not all from the same year. Second, where possible, data are on an ‘as-discarded’ basis to reflect the composition of waste after diversion for recycling. However, it is not always clear whether the data were reported ‘as-generated‘ or ‘as-discarded’. Third, the waste components included in national surveys vary within and between countries. For example, the US data are for wastes defined as MSW. Finally, country-specific data compiled for Europe (Eurostat 2007) are self-reported at the national level and are unlikely to have been generated using a consistent methodology. In the USA, plastics are estimated to comprise 11.8 and 16.3 per cent of MSW as-generated and as-discarded mass, respectively. The composition of discarded plastics is given in table 1 (US EPA 2006). In Europe, plastics are estimated to comprise 7 per cent of waste mass as-generated. Similarly, plastics were estimated to represent 5.8, 7.3, 8–10 and 10 per cent of waste mass in Singapore, Australia, the UK and Finland, respectively (Barlaz 2006; Burnley 2007; Sokka et al. 2007). Finally, plastics were estimated to comprise 4 and 13 per cent of waste in regions of China that use coal and natural gas, respectively, and the country-wide average for urban areas is projected to be 14 per cent plastics in 2030 (World Bank 2005). Despite the uncertainty, estimates from around the world are reasonably consistent in estimating plastics to comprise approximately 10 per cent of municipal waste mass. In contrast, plastics comprise 50–80% of the waste stranded on beaches, floating on the ocean surface and on the seabed (Gregory & Ryan 1997; Derraik 2002; Barnes 2005; Morishige et al. 2007).
3. Temporal and spatial trends in accumulation
(a). Ocean surface and beaches
Many plastics are buoyant (46%; US EPA 2006) and remain so until they become waterlogged or amass too much epibiota to float. Plastic items are commonly found at the sea surface or washed up on the shoreline. Mass production of plastics began in the 1950s, so less than a century ago we estimate that the amount of anthropogenic debris at sea would have been three to four orders of magnitude lower and restricted to much more degradable items. Some of the earliest accounts of plastic debris in the marine environment are of fragments and pellets ingested by seabirds in the 1960s (e.g. Kenyon & Kridler 1969; Harper & Fowler 1987), but now plastic mega- and macro-debris is routinely observed from boats everywhere on the planet. There has been a rapid and substantial increase in anthropogenic debris on the ocean surface and beaches over recent decades (e.g. Dixon & Dixon 1981; Derraik 2002; Barnes 2005), but of more pertinence now are the current spatial trends. Surveys of anthropogenic debris and clean-up operations have generally focused on the larger items along strandlines, and there is a wide geographical variability in the type of data available to examine potential trends. However in the last three of decades, it has become apparent that the raw material for making plastics, tiny pellets and micro-plastics have become more numerous (as marine debris) and, like larger pieces, these can travel considerable distances. Volunteer observations and collections in a growing number of nations are aiding our understanding of the scale and pattern of distribution of larger size fractions of plastics in the marine environment, but specialist examination is generally needed to investigate accumulation of micro-plastic, e.g. in sediments (Thompson et al. 2004). Beaches are the most easily accessible areas for studying marine debris (although such studies have some confounding factors), yet despite the establishment of many study sites, irregularity of sampling, differing protocol and observers have led to very few datasets spanning more than a decade (Barnes & Milner 2005).
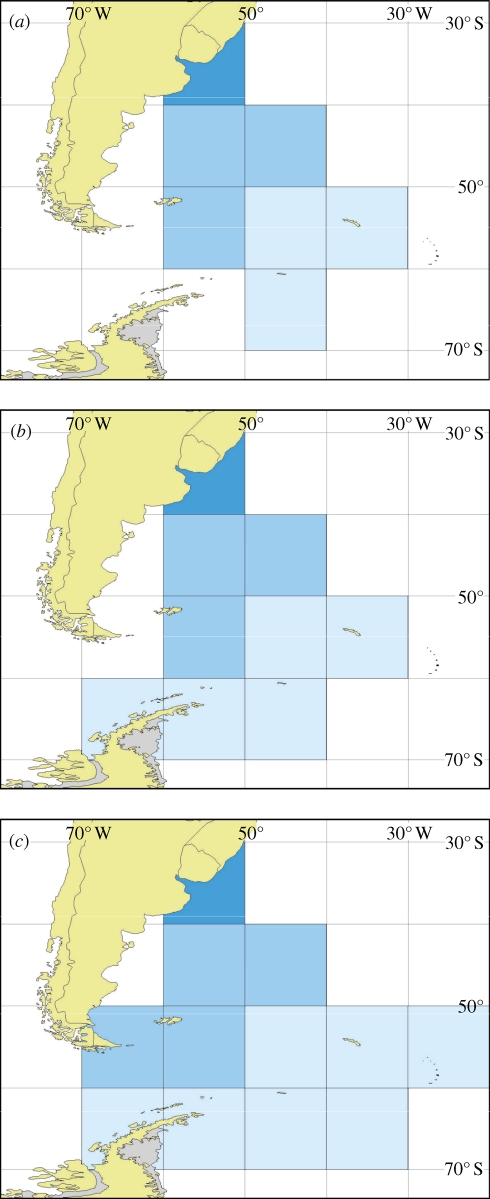
The distribution of plastic debris is very patchy at sea for a variety of reasons, including local wind and current conditions, coastline geography and the points of entry into the system such as urban areas and trade routes. For example, stranding of larger size fractions of plastics is between one and two orders of magnitude less per length of coastline on remote shores and at large spatial scales, abundance correlates very strongly (Pearson's correlation = 0.971, p < 0.001) with human population (per 10° latitude; Barnes 2005). Enclosed seas and semi-enclosed seas such as the Caribbean (Coe et al. 1997), typically have high densities of plastic debris but also considerable variability. High densities and variability can also be a feature of open ocean coastlines e.g. Brazil (Santos et al. 2005) and Hawaii (Dameron et al. 2007). One of the key sources of interannual variability seems to be changes in oceanic circulation driven by El Niňo events (Matsumura & Nasu 1997; Morishige et al. 2007). Typically about 2000 and 500 items of anthropogenic debris strand on north and south Atlantic Ocean shores (respectively) per linear kilometre per year, of which more than half is plastic (scaled up from surveys of items >1 cm in size along 200 m long beach sections; Barnes & Milner 2005). More than six times as much plastic strands in the Mediterranean Sea and less than six times as much strands in the Southern Ocean shores (Barnes & Milner 2005; table 2). Despite considerable variability in observation and accumulation rates of plastic debris, some temporal trends do emerge. Studies initiated in the 1980s and 1990s indicated that the rate of plastic stranding from oceanic sources showed a sustained and considerable increase over time (e.g. Ryan & Moloney 1993; Ribic et al. 1997; Torres & Jorquera 1999). Similarly, the occurrence of macro-plastics associated with wildlife (e.g. in bird nests and stomachs, entangling seals, strangling a wide variety of vertebrates or even used by hermit crabs instead of shells; Barnes 2005) also drastically increased. For example, between 1992 and 2005 the frequency of plastic garbage items in kittiwake nests increased from 39.3 to 57.2 per cent in northwest Denmark (Hartwig et al. 2007). Monitoring of strandings and effects on mega-fauna (such as birds) has now commenced on at least a few remote island shores in every ocean, and these, with negligible local sources of plastics, have revealed the scale at which anthropogenic debris is accumulating. Barnes (2005) found high levels but no consistent temporal trends in the abundance of anthropogenic debris on northern hemisphere shores compared with much lower levels, but increased densities through the 1980s, 1990s and early 2000s were reported in the southern hemisphere. The highest increases were at high southern latitudes (Barnes 2005). However, new data (reported here) show that patterns of stranding on islands are no longer clearly increasing and may be stabilizing, though often with a ‘noisy’ signal of annual variability (figure 2, see also Ryan et al. 2009). A similar lack of clear temporal trend in stranding densities of plastics is apparent in data collected intermittently at Ascension I., in the tropical Atlantic Ocean, and in the Falkland Is., south Atlantic Ocean (D. K. A. Barnes 2002, 2003 unpublished data). Approximately 27 per cent of macro-debris items stranding at Ascension I. was fishery-related, similar to remote Tern I. in the Hawaiian Is. (Morishige et al. 2007). This is much less than on shores adjacent to important fisheries e.g. in Brazil (Oigman-Pszczol & Creed 2007) or even sub-Antarctic Bird I. (Walker et al. 1997). Bird I. and Signy I. in the Southern Ocean (figure 2) have stranding densities of plastics an order of magnitude lower than remote localities at low latitudes, which in turn have at least an order of magnitude fewer plastics per kilometre than urban sites. Further south in the Southern Ocean, debris washes ashore much more rarely at Adelaide Island (west Antarctic Peninsula). The relatively consistent level of abundance for macro- and mega-debris at sea at high southern 1latitudes is supported by recent resurveys around the Drake Passage, Scotia Arc and northern Antarctic Peninsula (figure 3). Fifteen years after the first (Barnes & Milner 2005), the most recent survey of this area took place early in 2008 and will involve the first marine debris surveys of the south Bellingshausen and Amundsen seas. Visual surveys such as these are weaker as a source of data than surface-towed trawls but much more common and thus arguably comparable with data collected elsewhere, despite being semi-quantitative. Gregory et al. (1984) reported similarly low (on a global scale) levels of floating anthropogenic debris in the Ross Sea (Pacific sector) of the Southern Ocean. Observers from the University of Essex in conjunction with Greenpeace are currently undertaking repeat survey of plastics at sea in this area. As on surrounding strandlines, the north Atlantic Ocean and Pacific Ocean have high densities of floating plastic debris, especially at 20–40° N within a few hundred kilometres of the coast and in the gyre centres, e.g. between the tropical and subarctic waters (Matsumura & Nasu 1997). A recent (2005) survey of the subtropical convergence zone in this area showed plastic debris to be concentrating there remotely using satellite imagery (Pichel et al. 2007).
Table 2.
Densities and proportion of plastics among benthic marine litter worldwide (per number of items). M, Mediterranean Sea; B, Baltic Sea; NA, North Atlantic; NP, northern Pacific Ocean; WP, western Pacific Ocean; T, trawling; PT, pole trawling; SA, South Atlantic.
| region | sea | method | item Ha–1 | plastic (%) | references |
|---|---|---|---|---|---|
| NA | Bay of Biscay | T | 1.42 ± 0.25 | 62.2 | Galgani et al. (1995a) |
| M | NW Mediterranean | T | 19.35 ± 6.33 | 77.1 | Galgani et al. (1995b) |
| B | Baltic Sea | T | 1.26 ± 0.82 | 35.7 | Galgani et al. (2000) |
| NA | North Sea | T | 1.56 ± 0.37 | 48.3 | Galgani et al. (2000) |
| NA | Channel East | T | 1.176 ± 0.067 | 84.6 | Galgani et al. (2000) |
| NA | Bay of Seine | T | 1.72 ± 0.058 | 89 | Galgani et al. (2000) |
| NA | Celtic Sea | T | 5.28 ± 2.47 | 29.5a | Galgani et al. (2000) |
| SA | Rio de la Plata | T | 0–15.09 | 74 | Acha et al. (2003) |
| M | Greece, 59 sites | T | 149 | 55.5 | Katsanevakis & Katsarou (2004) |
| M | Greece, Patras gulf | T | 0.89–2.40 | 79–83 | Stefatos et al. (1999) |
| M | W & S Greece | T | 0.72–4.37 | 55.9 | Koutsodendris et al. (2008) |
| M | Gulf of Lion | T | 1.43 ± 0.19 | 70.5 | Galgani et al. (2000) |
| M | East Corsica | T | 2.29 ± 0.72 | 45.8 | Galgani et al. (2000) |
| M | Adriatic Sea | T | 3.78 ± 2.51 | 69.5 | Galgani et al. (2000) |
| M | Sicily/Tunisia channel | T | 4.01 | 75 | Cannizarro et al. (1995) |
| M | Oriental basin | P T | 5.85–161.98 | 37 | Galil et al. (1995) |
| NP | Kodiak Island, Alaska | T | 0.11–1.47 | 47–59 | Hess et al. (1999) |
| NP | Oregon Coast | T | 1.49 | 26a | June (1990) |
| NP | Bering Sea | T | 0.075–0.51 | 27 | June (1990) |
| NP | Norton Sound | T | 2.49 | 49.0 | June (1990) |
| WP | Tokyo Bay | T | 2.70–5.50 | 40.1–41.6 | Kanehiro et al. (1995) |
| WP | Tokyo Bay | T | 1.85–3.38 | 48.3–58.9 | Kuriyama et al. (2003) |
| WP | Eastern China Sea | T | <5 | Lee et al. (2006) | |
| WP | South Sea of Korea | T | <10 | Lee et al. (2006) |
afishing area.
Figure 2.
Annual accumulation of all marine debris (predominantly plastic) on shores of selected islands with year. Data for Bird I. and Signy I. are from Walker et al. (1997); Convey et al. (2002) and CCAMLR. Data for Tern I. are from Morishige et al. (2007) and for the UK from Beachwatch 2006 (MCS 2007).
Figure 3.
Densities of all marine debris, predominantly plastic, at sea in the southwest Atlantic and Atlantic sector of the Southern Ocean by 10° latitude and longitude areas. Shades of light to dark blue code for [r1]densities 0–1, 2–10, 11–100, 101–1000 and 1001+ items square kilometre, respectively. The survey years are (a) April 1993, (b) April 2002 and (c) April 2006. Data from Barnes & Milner (2005) and reported here for the first time.
We know much less about the use by and distribution of organisms that hitch hike on plastics and other anthropogenic debris than about the debris itself. Macro- and mega-plastics have the potential to carry a wide range of species and support the growth of many to reproductive viability. The high abundance, lengthy durability and travel of plastics to even the most remote coasts make them a major potential vector for the dispersal of organisms (Gregory 2009). New data from surveys of marine debris stranding in the Seychelles in 2005 and 2006 showed that on some beaches >60 per cent of items carried fouling organisms, the highest reported anywhere (D. K. A. Barnes 2002 onwards, unpublished data). This is of significance because the prevailing currents that travel from north Australia and south Indonesia during summer (South Equatorial) and from Somalia, India and N. Indonesia during winter (Indian Monsoon) could potentially transport a very wide range of species to less biodiverse, mid-ocean islands. Recent surveys of marine debris at Ascension I. (reported here for the first time) found 38, 40 and 41 per cent of debris colonized by fauna in 2002, 2003 and 2005, respectively. Much of this had probably also travelled considerable distances given the prevailing currents come from the cape of South Africa. The likely response of many species to rapid regional warming is to move pole-ward to stay within their normal thermal envelope, but in previous phases of warming (interglacial periods), there were few vectors to travel on. Now plastic debris, ship hulls and other vectors make transport more rapid and frequent, and unprecedented warming at high latitudes also means that establishment success of potential invaders is likely to be higher.
(b). Seabeds from shallows to abyss
As at the surface, both in the open ocean and on strandlines, it is clear that the abundance and distribution of anthropogenic debris show considerable spatial variability. The geographical distribution of plastic debris is strongly influenced by hydrodynamics, geomorphology and human factors. Moreover, there is notable temporal, particularly seasonal, variation with a tendency for accumulation and concentration along coastal and particular geographical areas.
Under the weight of fouling by a wide variety of bacteria, algae, animals and accumulated sediment, plastics can sink to the seabed (R. C. T. 1997 unpublished data). Change in the nature, presence or abundance of anthropogenic debris on the sea floor is much less widely investigated than surface patterns. Studies that investigate seabed debris typically focus on continental shelves, and research into the deeper seabed, which forms about half the planet's surface, is restricted by sampling difficulties and cost. Patterns in even the shallow subtidal can differ substantially from the adjacent strandlines. Oigman-Pszczol & Creed (2007) found plastic to constitute a much greater proportion of debris on the nearshore Brazilian seabed than on the shore. While sonar does not enable discrimination of different types of debris, trawling (e.g. using Agassiz) is probably the most adequate method to date, particularly when mesh size and opening width can be manipulated (Goldberg 1994, 1995; Galgani & Andral 1998). Such nets are only semi-quantitative and because of their design for collecting epibenthos, probably underestimate the quantities of debris present. Therefore, pole trawling, with a constant mouth width, which works deeper in sediments, is considered the best approach. To date all off-shelf trawl data from submersibles have used this methodology. General strategies to investigate seabed debris are similar to methodology for benthic ecology and place more emphasis on the abundance and nature (e.g. bags, bottles, pieces of plastics) of items rather than their mass. Interpretation of trends is made difficult because the ageing of plastics at depth is not well researched and the fall of plastics to the seabed began long before specific scientific investigations started in the 1990s. Plastics have been found on the seabed of all seas and oceans across the planet, but macro-debris is still very rare in the Southern Ocean, particularly in deep water. For example a recent series of 32 Agassiz trawls and 29 epibenthic sledge tows (at 200–1500 m depth, B. A. S. 2006 unpublished data) around the most (human) visited area, the northern Antarctic Peninsula and Scotia Arc, found just one plastic piece and one metal shot. Large-scale evaluations of seabed debris distribution and densities anywhere are scarce (but see Galgani et al. 2000; Lee et al. 2006; Koutsodendris et al. 2008). However, there are a large number of small-scale studies that have investigated anthropogenic debris in coastal areas such as bays, estuaries and sounds (see table 2 and references therein).
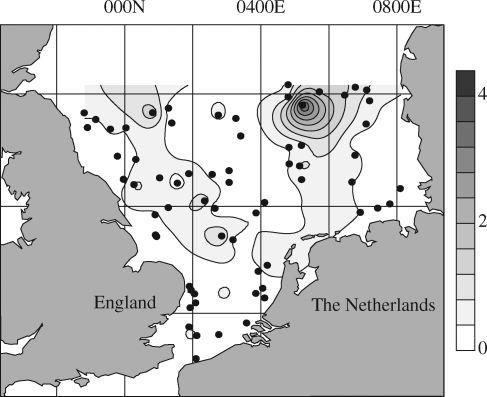
The abundance of plastic debris is very dependent on location, with values ranging from 0 to 7290 items per hectare (Ha) (although an extreme find of 10 110 anthropogenic items Ha–1 was found in 1998 at one position, 43º42′84″ N, 7º22′98″ E using a pole trawl). Assessments of abundance clearly demonstrate the domination of this debris by plastics, as at more than half the study sites plastics constituted >50 per cent of debris (table 2). Of the areas investigated to date, Mediterranean sites tend to show the greatest densities owing to the combination of a densely populated coastline and shipping in coastal waters and a lack of dispersion of plastics because of limited tidal flow or water circulation. In general, bottom debris tends to become trapped in areas of low circulation and high sediment accumulation in contrast to floating debris, which accumulates in frontal areas. Debris that reaches the seabed may already have been transported considerable distance, only sinking when weighed down by fouling. The consequence is an accumulation of plastics debris in bays rather than the open sea (Hess et al. 1999; Stefatos et al. 1999). Some accumulation zones in the Atlantic Sea and the Mediterranean Sea have very high debris densities despite being far from coasts. These densities relate to the consequence of large-scale residual ocean circulation patterns. There are higher densities in particular areas such as around rocks and wrecks or in depressions or channels (Galgani et al. 1996). In the North Sea (figure 4), accumulation of plastics 320 km offshore from Denmark (Galgani et al. 2000) is a consequence of several factors. These include the eddying circulation in the central North Sea (Delhez & Martin 1992) and long-term circulation of water from the gulf stream transporting plastics northwards (Breton & Salomon 1995) and to the convergence zone of seabed sediment movements, owing to local decreases in turbidity and turbulence (Tappin et al. 1997).
Figure 4.
Plastic debris on the seabed from the southern North Sea (North Atlantic) in 1999. Plastics were counted after 30 min trawl time (16 m mouth, 20 mm mesh) at 64 stations (•) on the continental shelf. Results are given as items Ha–1 (10 000 m2).
Large rivers are responsible for substantial inputs of debris to the sea bed (Williams & Simmons 1997). They can transport waste out to sea because of their high flow rate and the strength of bottom currents. In smaller rivers, the displacement is slight, and waste can be found in zones adjacent to or in the estuaries and is often coincident with fronts (Acha et al. 2003). Patterns of debris transport should therefore be linked to river flow strength and may follow patterns similar to deposition of sediment load (often depositing only small amounts of material immediately along the coast).
Deep submarine extensions of coastal rivers also influence the distribution of seabed debris. In some areas, local water movements transport plastics away from the coast to accumulate in zones of high sedimentation. Under these conditions, the distal deltas of rivers can fan out in deeper waters, creating areas of high accumulation (Galgani et al. 1996). Continental shelves often have lower concentrations of debris since most of the anthropogenic debris in the outer shelf originates from coasts to shelves that are washed offshore by currents associated with river plumes. Data from the shelf areas off the River Rhone (Galgani et al. 1995b) and California (Moore & Allen 2000) show that circulation can be strongly, locally influenced by storm water events. The accumulation of plastics in coastal canyons may also be related to strong currents occurring in the upper part of canyons, which decrease rapidly in deeper areas resulting from increased confinement. Accordingly, debris distribution seems to be more temporally stable. An inevitable effect of this is the presence of greater amounts of debris in deeper shelf waters than in coastal waters (Galgani et al. 1996, 2000).
A wide variety of human activities contribute to these patterns of seabed debris distribution, including proximity to fishing activities, urban development and tourism. Also with plastic as a main component, debris from the fishing industry is prevalent in fishing areas (Kanehiro et al. 1995; Galgani et al. 2000). This type of material accounts for a high percentage of debris, for example up to 72 per cent in eastern China Sea (Lee et al. 2006) and 65 per cent in the Celtic sea (Galgani et al. 2000). Finally, fishing gear was also the dominant source of both plastic and overall debris in California (Moore & Allen 2000).
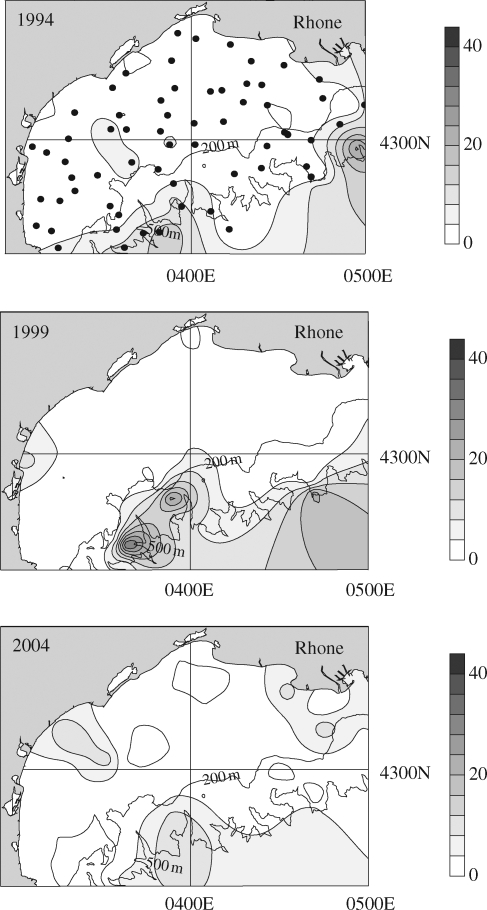
Investigations using submersibles at depths beyond the continental shelf usually consider the number of items per linear kilometre because of variability in transect width. They have revealed substantial quantities of debris (figure 5). Besides the high densities found in coastal canyons (up to 112 items per kilometre and 70% plastics), plastics and other anthropogenic debris were found widely dispersed at slope and abyssal depths (Galgani et al. 2000). Deployment of a remotely operated vehicle submarine in the Fram Strait (Arctic) (Galgani & Lecornu 2004) revealed 0.2–0.9 pieces of plastic per linear kilometre at Hausgarten (2500 m). On dives between 5500 and 6770 m, 15 items of debris were observed, of which 13 were plastic, probably carried there by the Norwegian current in the North Atlantic. At such latitude and bathymetry, there is negligible human activity, suggesting long-distance transport of debris. Even more than on the sea surface or strandlines of remote locations, such as in the Southern Ocean, accumulation trends in the deep sea are of special concern. Most polymers are highly persistent in the marine environment and only degrade slowly via photo-catalysis when exposed to UV radiation (Andrady 2003). Estimates for the longevity of plastics are variable but are believed to be in the range of hundreds or even thousands of years depending on the physical and chemical properties of the polymer, but this is likely to be greatly increased at depth where oxygen concentrations are low and light is absent. We know little about trends in accumulation of debris in the deep sea as studies are rare, but the data we have indicate considerable variability. For example, in some areas, such as the Bay of Tokyo, debris densities decreased from 1996 to 2003 (Kanehiro et al. 1995; Kuriyama et al. 2003). In contrast, abundance remained stable in the Gulf of Lion, France during a similar period (figure 6). Furthermore in some areas around Greece, the abundance of debris at depth has increased over the last 8 years (Stefatos et al. 1999; Koutsodendris et al. 2008). Interpretation of temporal trends is also complicated by annual variations in debris transport, such as seasonal changes in flow rate of rivers. Other seasonal factors include variation in the position of water fronts, the intensity of currents, swell, winds and upwelling, which influence both the distribution and densities. Nevertheless, if we extrapolate from existing data, it would appear that in the Mediterranean Sea as a whole there are about 3 × 109 debris items (floating or sunk), of which 70–80% are plastic. New initiatives to minimize littering and to reduce, reuse and recycle plastic should ultimately reduce plastic input into the sea, although usage is still very high. However, fragmentation of macro- and mega-plastics to micro-plastic pieces will also contribute to future trends in the abundance of visible plastics.
Figure 5.
Accumulation of debris in deep sea environments. Submersible observations in Mediterranean canyons (a and b: plastic bottles at 1000 m depth at two different locations in the Marseille canyon, 43°03′00″ N, 05°00′00″ E) and above the polar circle, under ice floe (c and d: individual plastic bags, 2200–2600 m depth at Hausgarten, Fram strait, 79°03′80″ N, 04°11′60″ E).
Figure 6.
Plastic debris on the sea floor from the Gulf of Lion (Mediterranean Sea, France) between 1994 and 2004. Plastics were counted after 60 min trawl time (net = 16 m mouth, 10 mm mesh) at 65 stations (•) located on the continental shelf and adjacent canyons (down to 800 m) from the gulf. Results are given as items Ha–1 (10 000 m2). Top plot shows the sampling stations from 1994–2004.
4. Fragmentation of plastics in the environment
The longevity of plastics is a matter for some debate, and estimates range from hundreds to thousands of years. It is considered that (with the exception of materials that have been incinerated) all of the conventional plastic that has ever been introduced into the environment still remains to date unmineralized either as whole items or as fragments (Thompson et al. 2005). However, since we have only been mass-producing conventional plastics for around 60 years, it is too early to say exactly how long these materials will persist. Despite the durability of these polymers, plastic items are fragmenting in the environment as a consequence of prolonged exposure to UV light and physical abrasion (Colton et al. 1974; Gregory 1978; Andrady 2003; Thompson et al. 2004). This is particularly evident on shorelines where photo-degradation and abrasion through wave action make plastic items brittle, increasing their fragmentation.
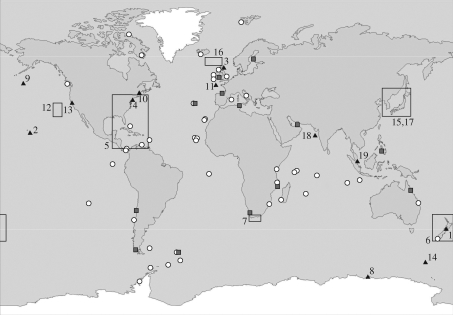
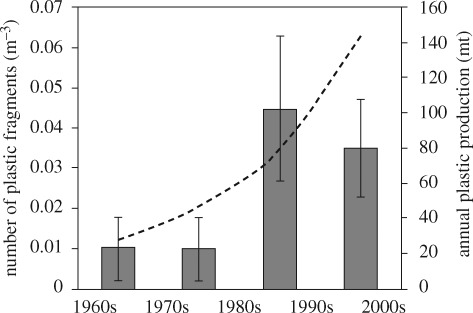
Some of the first evidence of accumulation of plastic fragments in the environment came indirectly from examination of the gut contents of sea birds in the 1960s (e.g. Kenyon & Kridler 1969). Later, in the early 1970s, small fragments of plastic were observed in seawater collected with plankton samples from the North Sea (Buchanan 1971) and were subsequently reported on much broader scales in the northwestern Atlantic (Colton et al. 1974). There have since been numerous reports of fragments in the oceans, on the seabed and on shorelines worldwide (figure 7), and there is clear evidence that the abundance of these fragments is increasing (figure 8). The UK Marine Conservation Society, which organizes annual voluntary beach cleaning on shores all around the UK, reports a 30 per cent increase in the abundance of large fragments (1–50 cm in size) and a 20 per cent increase in the abundance of smaller fragments (<1 cm) between 1998 and 2006 (MCS 2007). On shorelines close to Plymouth, one of us (R. C. T.) recently recorded strandline material with >10 per cent (10.89 ± 0.67, mean ± s.d.) by weight of plastic fragments and pieces (including some pre-production plastic pellets, which are used to manufacture plastic products). In 2004, Thompson et al. (2004) reported on the abundance of even smaller fragments of plastic, some just 20 µm, in diameter, which had accumulated on shorelines around the UK. Using plankton samples archived by the Sir Alistair Hardy Foundation for Ocean Science, it was evident that the abundance of this microscopic debris had increased significantly in recent years (figure 8). Similar fragments have since been identified from shorelines worldwide (figure 7), and in terms of numerical abundance, micro-plastic can constitute over 80 per cent of intertidal plastic debris at some locations (Browne et al. 2007).
Figure 7.
Reports of plastic fragments in the marine environment presented in chronological order: 1, Harper & Fowler (1987) report on plastic (mainly pre-production pellets) ingested by seabirds since 1960; 2, plastic fragments found in body cavity of dead laysan albatrosses during 1966 survey (Kenyon & Kridler 1969); 3, synthetic fibres in medium plankton net hauls (size not specified) (Buchanan 1971); 4, polystyrene spherules (average 500 µm) in coastal waters (Carpenter et al. 1972); 5, particles, spheres and discs (1–5 mm) in surface waters (Colton et al. 1974); 6, resin pellets (approx. 5 mm) on shoreline (Gregory 1978); 7, temporal trends in abundance and composition of plastic on beaches 1984–1989 (Ryan & Moloney 1990); 8, plastic particles (approx. 3 mm) in gut of storm petrels (van Franeker & Bell 1988); 9, fragments (≥500 µm) at sea surface (Shaw & Day 1994); 10, micro-plastic fibres (≥20 µm) in sewage sludge (Habib et al. 1996); 11, fragments in deep sea (size not specified) (Galgani et al. 2000); 12, fragments (≥350 µm) at sea surface (Moore et al. 2001a); 13, fragments and resin pellets on shoreline (size not specified) (Moore et al. 2001b); 14, fragments (≥1 mm) in scats of fur seals (Eriksson & Burton 2003); 15, fragments (≥1 mm) on beaches (Kusui & Noda 2003); 16, micro-plastics (≥20 µm) in surface waters and on beaches (Thompson et al. 2004); 17, resin pellets (approx. 5 mm) on beaches (Endo et al. 2005); 18, micro-plastics (≥10 µm) on shorelines near ship breaking yards (Reddy et al. 2006); 19, micro-plastics in surface waters and sediments (≥1.6 µm) (Ng & Obbard 2006). Red squares show distribution of micro-plastics (≥ 20 µm) in intertidal sediments (R. C. Thompson et al. 2003–2007 unpublished data). White dots show mega- and macro-plastic strandline surveys (Barnes 2002, 2005).
Figure 8.
Microscopic plastic in surface waters, collected with continuous plankton recorder, revealed a significant increase in abundance when samples from the 1960s and 1970s were compared with the 1980s and 1990s (F3,3 = 14.42, p < 0.05). Global production of plastic overlain for comparison (APME 2006). Grey boxes, number of plastic fibres (m−3); dashed line, plastic produced per year (million tonnes). (Reproduced with permission from Thompson et al. 2004.)
Fragments of plastic can be identified using Fourier transform infrared (FT-IR) spectroscopy to match spectra obtained from unknown debris items to those of known polymers. Using this approach, a range of common polymers including polypropylene, polyethylene and polyester have been identified as fragments and microscopic fragments. These materials have a wide range of domestic and industrial uses from rope and packaging to clothing, and it seems likely that the fragments are forming from the breakdown of a wide range of everyday plastic products (Thompson et al. 2004). In addition to this ‘natural’ deterioration, it has been suggested that plastic items are also deliberately being shredded on board some ships in order that plastic waste can be concealed in food waste discharged at sea (van Franeker et al. 2004, 2005). The abundance of small items of plastic is further increased by the use of plastic particles as scrubbers and abrasives in commercial cleaning applications (Gregory 1996) and by spillage of pre-production plastic pellets (approx. 5 mm in diameter) and powders such as those used for rotomoulding (approx. 300 µm in diameter) (e.g. Carpenter et al. 1972; Colton et al. 1974; Gregory 1978). Hence, it is apparent that small items of plastic are entering the environment directly and that larger items of debris are fragmenting.
The accumulation of plastic fragments is of particular concern because they are difficult to remove from the environment and because they have the potential to be ingested by a much wider range of organisms than larger items of debris. Marine mammals, turtles and numerous other organisms are known to ingest large items of plastic including bags and bottles (Laist 1997; Derraik 2002). Smaller fragments can be ingested by birds, fish and invertebrates (Thompson et al. 2004; van Franeker et al. 2005). Upon ingestion, it is possible that these small fragments may present a physical hazard in a similar way to larger items of debris by clogging feeding appendages or the digestive system (Laist 1997; Derraik 2002). Microscopic fragments are also be taken up from the gut into other body tissues (Browne et al. 2008). In addition to concerns about the physical hazards presented by this debris, it has also been suggested that plastics could transfer harmful chemicals to living organisms (e.g. Oehlmann et al. 2009; Talsness et al. 2009; Koch & Calafat 2009). A range of chemicals are used as additives in the manufacture of plastics. These increase the functionality of the plastics, but some such as phthalate plasticizers and brominated flame retardants are potentially harmful and have been associated with carcinogenic and endocrine disrupting effects (Teuten et al. 2009). In seawater, plastics are also known to sorb and concentrate contaminants, which have arisen in the environment from other sources. These contaminants include persistent organic ‘pollutants’ such as polychlorinated biphenyls (PCBs), dichlorodiphenyldichloroethylene (DDE), nonylphenol and phenanthrene, which can become several orders of magnitude more concentrated on the surface of plastic debris than in the surrounding seawater (Mato et al. 2001). It has been widely suggested that these sorbed contaminants and the chemicals additives that are used in manufacture could subsequently be released if the plastics are ingested (Teuten et al. 2009). Small and microscopic plastic fragments present a likely route for the transfer of these chemicals because they have a much greater surface area to volume ratio than larger items of debris from which they have originated and because of their size they are available to a wide range of organisms, including deposit feeders such as the lug worm, Arenicola marina, that feed by stripping organic matter from particulates (Mayer et al. 1997; Voparil et al. 2004). Recent in vitro modelling studies predict that even very small quantities of micro-plastic have the potential to significantly increase the transport of phenanthrene to A. marina (Teuten et al. 2007) and work in this volume has examined the uptake of contaminants from plastics by birds (Teuten et al. 2009).
Given current levels of production and the quantities of plastic that are already present in the environment, it seems inevitable that the abundance of plastic fragments will continue to increase for the foreseeable future. More work is therefore needed to model the environmental consequences of this debris and to produce environmental risk assessment models to predict the transport of a range of contaminants by fragments of common polymers (Thompson et al. 2005; Thompson 2006; Teuten et al. 2007).
5. Summary and conclusions
Less than 60 years ago, the mass production of plastics started and now most items that people use, virtually anywhere on the planet are partly or wholly made of this inexpensive, durable material. Plastics have transformed the surface of the planet, far beyond areas of human population density—fragments of all sizes are ubiquitous in soils to lake beds, from remote Antarctic island shores to tropical seabeds. Plastics turn up in bird nests, are worn by hermit crabs instead of shells and are present in turtle stomachs. Humans generate considerable amounts of waste and the quantities are increasing as standards of living and the population increase. Although quantities vary between countries, approximately 10 per cent of solid waste is plastic. Up to 80 per cent or sometimes more of the waste that accumulates on land, shorelines, the ocean surface or seabed is plastic. The most common items are plastic films, such as carrier bags, which are easily wind blown, as well as discarded fishing equipment and food and beverage packaging. Strandline surveys (beach-cleaning operations) are now organized in many countries and provide information about temporal and spatial trends. However, these surveys typically only provide data on coarse trends and larger items. There is considerable variation in methodology between regions and between investigators, and more valuable and comparable data could be obtained by standardizing monitoring approaches (Ryan et al. 2009). Accumulation rates vary widely with many factors such as proximity of urban settlements, shore use, prevailing wind and ocean currents and region. There were dramatic increases in quantities of mega- and macro-plastic debris in the northern hemisphere up to the 1990s. Quantities of debris in the oceans appear to have stabilized over the last decade but have increased on shorelines. However, this could indicate quantities of debris entering the sea are declining, but the material already in the sea is progressively being deposited on the shore or sinking to the deep. Accumulation rates are much lower in the Southern Hemisphere but are still increasing significantly, although repeat surveys on remote Antarctic islands and ocean areas suggest stabilization over the last decade. Fouled by organisms and sediment, plastics can sink and form an even higher proportion of human waste reaching the seabed, and quantities in excess of tens of thousands of items square kilometres have been reported. As on beaches and the ocean surface, enclosed seas such as the Mediterranean have the highest densities, but investigations in deeper waters have shown that high accumulation rates can stretch far (hundreds of kilometres) from the coast, particularly adjacent to large river mouths or in canyons. As in surface environments, trends of debris accumulation on the seabed increase at some locations, but are stable or decreasing at other sites. Quantities of debris in the oceans appear to have stabilized in the oceans over the last decade but have increased on shorelines. The problem of plastic fragments has taken on increased importance in the last few decades. From the first reports in the 1970s, it was only a few years before the widespread finding of plastic including reports of microscopic fragments (20 µm in diameter). The abundance of microscopic fragments was greater in the 1980s and 1990s than in previous decades. It has also been suggested that plastic waste is deliberately being shredded into fragments to conceal and discarded at sea. Plastics of all sizes are now reaching the most remote and deepest parts of the planet, and although we have much better knowledge of their sources, quantities and distribution, we still understand little about their longevity and affects on organisms. Further, we have made little progress in reducing the release of plastic to the environment (see discussion in Thompson et al. 2009). Temporal trends of macro-plastics on remote islands suggest that regulations to reduce dumping at sea have been successful to some extent. However, our sustained demand for plastic means that contamination of the environment by micro-plastic pieces seems set to increase. In addition, future sampling may reveal increasing quantities of debris in the planet's least known habitat, the deep sea.
Acknowledgements
The authors would like to thank past marine debris observers on beaches and ships who have generously given up their time and effort to recording items. The authors would also like to thank Alison Cook for help in preparation of figure 7; also Mark Brown and Stuart Niven for analysis of micro-plastic data in figure 7.
Footnotes
One contribution of 15 to a Theme Issue ‘Plastics, the environment and human health’.
References
- Acha E., Hermes W., Mianzan A., Iribarne C., Domingo A., Gagliardini C., Carlos Lasta A., Pedro Daleo D.2003The role of the Rıo de la Plata bottom salinity front in accumulating debris. Mar. Pollut. Bull. 46, 197–202 (doi:10.1016/cS0025-326X(02)00356-9) [DOI] [PubMed] [Google Scholar]
- Andrady A. L.2003Plastics in the environment. In Plastics in the environment (ed. Andrady A. L.), p. 762 New Jersey, NJ: John Wiley & Sons [Google Scholar]
- Andrady A. L., Neal M. A.2009Applications and societal benefits of plastics. Phil. Trans. R. Soc. B 364, 1977–1984 (doi:10.1098/rstb.2008.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- APME 2006An analysis of plastics production, demand and recovery in Europe Brussels, Belgium: Association of Plastics Manufacturers [Google Scholar]
- Barlaz M. A.2006Forest products decomposition in municipal solid waste landfills. Waste Manag. 26, 321–333 (doi:10.1016/j.wasman.2005.11.002) [DOI] [PubMed] [Google Scholar]
- Barnes D. K. A.2002Invasions by marine life on plastic debris. Nature 416, 808–809 (doi:10.1038/416808a) [DOI] [PubMed] [Google Scholar]
- Barnes D. K. A.2005Remote islands reveal rapid rise of Southern Hemisphere sea debris. Dir. Sci. 5, 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. K. A., Milner P.2005Drifting plastic and its consequences for sessile organism dispersal in the Atlantic Ocean. Mar. Biol. 146, 815–825 (doi:10.1007/s00227-004-1474-8) [Google Scholar]
- Breton M., Salomon J. C.1995A long term advection-dispersion model for the channel and southern North Sea. J. Mar. Syst. 6, 495–513 (doi:10.1016/0924-7963(95)00020-P) [Google Scholar]
- Browne M. A., Galloway T., Thompson R.2007Microplastic—an emerging contaminant of potential concern. Integr. Environ. Assess. Manag. 3, 559–566 [DOI] [PubMed] [Google Scholar]
- Browne M. A., Dissanayake A., Galloway T. S., Lowe D. M., Thompson R. C.2008Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 42, 5026–5031 (doi:10.1021/es800249a) [DOI] [PubMed] [Google Scholar]
- Buchanan J. B.1971Pollution by synthetic fibres. Mar. Pollut. Bull. 2, 23 (doi:10.1016/0025-326X(71)90136-6) [Google Scholar]
- Burnley S. J.2007A review of municipal solid waste composition in the United Kingdom. Waste Manag. 27, 1274–1285 (doi:10.1016/j.wasman.2006.06.018) [DOI] [PubMed] [Google Scholar]
- Cannizarro L., Garofalo G., Giusto G., Rizzo P., Levi D.1995. Qualitative and quantitative estimate of solid waste in the channel of Sicily. In Proc. Second Int. Conf. on the Mediterranean Coastal Environment, MED-COAST 95 (ed. Ozhan E.), Tarragona, Spain, 24–27 October [Google Scholar]
- Carpenter E. J., Anderson S. J., Harvey G. R., Miklas H. P., Bradford B. P.1972Polystyrene spherules in coastal waters. Science 178, 749–750 (doi:10.1126/science.178.4062.749) [DOI] [PubMed] [Google Scholar]
- Coe J. M., Andersson S., Rogers D. B.1997Marine debris in the Caribbean region. In Marine debris: sources, impact and solutions (eds Coe J. M., Rogers D. B.), pp. 25–34 New York, NY: Springer Verlag [Google Scholar]
- Colton J. B., Knapp F. D., Burns B. R.1974Plastic particles in surface waters of the Northwestern Atlantic. Science 185, 491–497 (doi:10.1126/science.185.4150.491) [DOI] [PubMed] [Google Scholar]
- Convey P., Barnes D. K. A., Morton A.2002Debris accumulation on oceanic island shores of the Scotia Arc, Antarctica. Polar Biol. 25, 612–617 [Google Scholar]
- Dameron O. J., Parke M., Albins M. A., Brainard R. E.2007Marine debris accumulation in the Northwestern Hawaiian Islands: an examination of rates and processes. Mar. Pollut. Bull. 53, 423–433 [DOI] [PubMed] [Google Scholar]
- Delhez E., Martin G.1992Preliminary results on 3-D baroclinic models of the mesoscale and macroscale circulations on the North-western European continental shelf. J. Mar. Syst. 3, 423–440 (doi:10.1016/0924-7963(92)90014-Y) [Google Scholar]
- Derraik J. G. B.2002The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 44, 842–852 (doi:10.1016/S0025-326X(02)00220-5) [DOI] [PubMed] [Google Scholar]
- Dixon T. R., Dixon T. J.1981Marine litter surveillance. Mar. Pollut. Bull. 12, 289–295 (doi:10.1016/0025-326X(81)90078-3) [Google Scholar]
- Endo S., Takizawa R., Okuda K., Takada H., Chiba K., Kanehiro H., Ogi H., Yamashita R., Date T.2005Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar. Pollut. Bull. 50, 1103–1114 (doi:10.1016/j.marpolbul.2005.04.030) [DOI] [PubMed] [Google Scholar]
- Eriksson C., Burton H.2003Origins and biological accumulation of small plastic particles in fur seals from Macquarie Island. Ambio 32, 380–384 [DOI] [PubMed] [Google Scholar]
- Eurostat 2007. Waste generated and treated in Europe 1995–2003. See http://epp.eurostat.cec.eu.int/cache/ITY_OFFPUB/KS-55-03-471/EN/KS-55-03-471-EN.PDF (accessed 4 December 2007)
- Galgani F., Andral B.1998Methods for evaluating debris on the deep sea floor. OCEANS'98/IEEE/OEC Conference, Nice 28/09-01/10/983, 1512–1521 [Google Scholar]
- Galgani F., Lecornu F.2004Debris on the sea floor at ‘Hausgarten’: in the expedition ARKTIS XIX/3 of the research vessel POLARSTERN in 2003. Berichte Polar Meeresforsch. 488, 260–262 [Google Scholar]
- Galgani F., Burgeot T., Bocquene G., Vincent F., Leaute J. P.1995aAbundance of debris on the continental shelf of the Bay of Biscaye and in the Seine Bay. Mar. Pollut. Bull. 30, 58–62 (doi:10.1016/0025-326X(94)00101-E) [Google Scholar]
- Galgani F., Jaunet S., Campillo A., Guenegan X., His E.1995bDistribution and abundance of debris on the continental shelf of the North-western Mediterranean Sea. Mar. Pollut. Bull. 30, 713–717 (doi:10.1016/0025-326X(95)00055-R) [Google Scholar]
- Galgani F., Souplet A., Cadiou Y.1996Accumulation of debris on the deep sea floor of the French Mediterranean coast. Mar. Ecol. Progr. Ser. 142, 225–234 (doi:10.3354/meps142225) [Google Scholar]
- Galgani F., et al. 2000Litter on the sea floor along European coasts. Mar. Pollut. Bull. 40, 516–527 (doi:10.1016/S0025-326X(99)00234-9) [Google Scholar]
- Galil B. S., Golik A., Tuerkay M.1995Litter at the bottom of the sea. A sea-bed survey in the eastern Mediterranean Sea. Mar. Pollut. Bull. 30, 22–24 (doi:10.1016/0025-326X(94)00103-G) [Google Scholar]
- Goldberg E.1994Diamonds and plastics are forever? Editorial. Mar. Pollut. Bull. 28, 466 (doi:10.1016/0025-326X(94)90511-8) [Google Scholar]
- Goldberg E.1995Emerging problems in the coastal zone for the twenty-first century. Mar. Pollut. Bull. 31, 152–158 (doi:10.1016/0025-326X(95)00102-S) [Google Scholar]
- Gregory M. R.1978Accumulation and distribution of virgin plastic granules on New Zealand beaches. N. Z. J. Mar. Freshwat. Res. 12, 339–414 [Google Scholar]
- Gregory M. R.1996Plastic ‘scrubbers’ in hand cleansers: a further (and minor) source for marine pollution identified. Mar. Pollut. Bull. 32, 867–871 (doi:10.1016/S0025-326X(96)00047-1) [Google Scholar]
- Gregory M. R.1999Plastics and South Pacific Island shores: environmental implications. Ocean Coastal Manag. 42, 603–615 (doi:10.1016/S0964-5691(99)00036-8) [Google Scholar]
- Gregory M. R.2009Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Phil. Trans. R. Soc. B 364, 2013–2025 (doi:10.1098/rstb.2008.0265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M. R., Ryan P.1997Pelagic plastics and other seaborne persistent synthetic debris: a review of Southern Hemisphere perspectives. In Marine debris: sources, impacts and solutions (eds Coe J., Rogers D.), pp. 49–66.New York, NY: Springer Verlag [Google Scholar]
- Gregory M. R., Kirk R. M., Mabin M. C. G.1984Pelagic tar, oil, plastics and other litter in surface waters of the New Zealand sector of the Southern Ocean and on Ross Dependency shores. N. Z. Antarct. Record 6, 12–28 [Google Scholar]
- Habib B., Locke D. C., Cannone L. J.1996Synthetic fibers as indicators of municipal sewage sludge, sludge products and sewage treatment plant effluents. Water Air Soil Pollut. 103, 1–8 (doi:10.1023/A:1004908110793) [Google Scholar]
- Harper P. C., Fowler J. A.1987Plastic pellets in New Zealand storm-killed prions (Pachyptila spp.), 1958–1977. Notornis 34, 65–70 [Google Scholar]
- Hartwig E., Clemens T., Heckroth M.2007Plastic debris as nesting material in a Kittiwake-(Rissa tridactyla)-colony at the Jammerbugt, Northwest Denmark. Mar. Pollut. Bull. 54, 595–597 (doi:10.1016/j.marpolbul.2007.01.027) [DOI] [PubMed] [Google Scholar]
- Hess N., Ribic C., Vining Y.1999Benthic marine debris, with an emphasis on fishery-related items, surrounding Kodiak Island, Alaska, 1994–1996. Mar. Pollut. Bull. 38, 885–890 (doi:10.1016/S0025-326X(99)00087-9) [Google Scholar]
- Ivar do Sul J. A., Costa M. A.2007Marine debris review for Latin America and the Wider Caribbean Region: from the 1970s until now, and where do we go from here? Mar. Pollut. Bull. 54, 1087–1104 [DOI] [PubMed] [Google Scholar]
- June J. A.1990Type, source, and abundance of trawl-caught debris of Oregon, in the Eastern Bering Sea, and in Norton Sound in 1988. In Proc. Second Int. Conf. Marine Debris (eds Shomura R. S., Godfrey M. L.), pp. 279–301 NMFS-SWF-SC-154, US Department of Commerce, NOAA Technical Memo [Google Scholar]
- Kanehiro H., Tokai T., Matuda K.1995Marine litter composition and distribution on the sea-bed of Tokyo Bay. Fish. Eng. 31, 195–199 [Google Scholar]
- Katsanevakis S., Katsarou A.2004Influences on the distribution of marine debris on the seafloor of shallow coastal areas in Greece (Eastern Mediterranean). Water Air Soil Pollut. 159, 325–337 (doi:10.1023/B:WATE.0000049183.17150.df) [Google Scholar]
- Kenyon K. W., Kridler E.1969Laysan Albatross swallow indigestible matter. Auk 86, 339–343 [Google Scholar]
- Koch H. M., Calafat A. M.2009Human body burdens of chemicals used in plastic manufacture. Phil. Trans. R. Soc. B 364, 2063–2078 (doi:10.1098/rstb.2008.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsodendris A., Papatheodorou A., Kougiourouki O., Georgiadis M.2008Benthic marine litter in four Gulfs in Greece, Eastern Mediterranean; abundance, composition and source identification. Est. Coast. Shelf Sci. 77, 501–512 (doi:10.1016/j.ecss.2007.10.011) [Google Scholar]
- Kuriyama Y., Tokai T., Tabata K., Kanehiro H.2003Distribution and composition of litter on seabed of Tokyo Gulf and its age analysis. Nippon Suisan Gakkaishi 69, 770–781 [Google Scholar]
- Kusui T., Noda M.2003International survey on the distribution of stranded and buried litter on beaches along the Sea of Japan. Mar. Pollut. Bull. 47, 175–179 (doi:10.1016/S0025-326X(02)00478-2) [DOI] [PubMed] [Google Scholar]
- Laist D. W.1997Impacts of marine debris: entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In Marine debris: sources, impacts and solutions (eds Coe J. M., Rogers B. D.), pp. 99–141.Berlin, Germany: Springer [Google Scholar]
- Lee D., Hyeon-Seo C., Sun-Beom J.2006Distribution characteristics of marine litter on the sea bed of the East China Sea and the South Sea of Korea. Est. Coast. Shelf Sci. 70, 187–194 (doi:10.1016/j.ecss.2006.06.003) [Google Scholar]
- Loughlin D. H., Barlaz M. A.2006Policies for strengthening markets for recyclables: a worldwide perspective. Environ. Sci. Technol. 36, 287–326 [Google Scholar]
- Masó M., Garcés J., Pagès F., Camp J.2003Drifting plastic debris as a potential vector for dispersing Harmful Algal Blooms (HAB) species. Sci. Mar. 67, 107–111 [Google Scholar]
- Mato Y., Isobe T., Takada H., Kanehiro H., Ohtake C., Kaminuma T.2001Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 35, 318–324 (doi:10.1021/es0010498) [DOI] [PubMed] [Google Scholar]
- Matsumura S., Nasu K.1997Distribution of floating debris in the North pacific Ocean: sighting surveys 1986–1991. In Marine debris: sources, impact and solutions (eds Coe J. M., Rogers D. B.), pp. 15–24.New York, NY: Springer Verlag [Google Scholar]
- Mayer L. M., Schick L. L., Self R. F. L., Jumars P. A., Findlay R. H., Chen Z., Sampson S.1997Digestive environments of benthic macroinvertebrate guts: enzymes, surfactants and dissolved organic matter. J. Mar. Res. 55, 785–812 (doi:10.1357/0022240973224247) [Google Scholar]
- MCS 2007Beachwatch 2006—the 14th annual Beach litter survey report. Marine Conservation Society, Ross on Wye [Google Scholar]
- Moore S. L., Allen M. J.2000Distribution of anthropogenic and natural litter on the mainland shelf of the Southern California Bight. Mar. Pollut. Bull. 40, 83–88 (doi:10.1016/S0025-326X(99)00175-7) [Google Scholar]
- Moore C. J., Moore S. L., Leecaster M. K., Weisberg S. B.2001aA comparison of plastic and plankton in the North Pacific Central Gyre. Mar. Pollut. Bull. 42, 1297–1300 (doi:10.1016/S0025-326X(01)00114-X) [DOI] [PubMed] [Google Scholar]
- Moore S. L., Gregorio D., Carreon M., Weisberg S. B., Leecaster M. K.2001bComposition and distribution of beach debris in Orange County, California. Mar. Pollut. Bull. 42, 241–245 (doi:10.1016/S0025-326X(00)00148-X) [DOI] [PubMed] [Google Scholar]
- Morishige C., Donohue M. J., Flint E., Swenson C., Woolaway C.2007Factors affecting marine debris deposition at French Frigate Shoals, Northwestern Hawaiian Islands Marine National Monument, 1900–2006. Mar. Pollut. Bull. 54, 1162–1169 (doi:10.1016/j.marpolbul.2007.04.014) [DOI] [PubMed] [Google Scholar]
- Ng K. L., Obbard J. P.2006Prevalence of microplastics in Singapore's coastal marine environment. Mar. Pollut. Bull. 52, 761–767 (doi:10.1016/j.marpolbul.2005.11.017) [DOI] [PubMed] [Google Scholar]
- Oehlmann J., et al. 2009A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 364, 2047–2062 (doi:10.1098/rstb.2008.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oigman-Pszczol S. S., Creed J. C.2007Quantification and classification of marine litter on beaches along Armação dos Búzios, Rio de Janeiro, Brazil. J. Coastal Res. 23, 421–428 (doi:10.2112/1551-5036(2007)23[421:QACOML]2.0.CO;2) [Google Scholar]
- Pichel W. G., et al. 2007Detection of marine debris in the North Pacific Subtropical Convergence Zone. Mar. Pollut. Bull. 54, 1207–1211 (doi:10.1016/j.marpolbul.2007.04.010) [DOI] [PubMed] [Google Scholar]
- Plastics Europe 2008Annual report 2007, p. 36 Brussels, Belgium: Association of Plastics Manufacturers [Google Scholar]
- Reddy M. S., Basha S., Adimurthy S., Ramachandraiah G.2006Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Est. Coast. Shelf Sci. 68, 656–660 (doi:10.1016/j.ecss.2006.03.018) [Google Scholar]
- Ribic C. A., Johnson S. W., Cole C. A.1997Distribution, type, accumulation, and source of marine debris in the United States, 1989–1993. In Marine debris: sources, impact and solutions (eds Coe J. M., Rogers D. B.), pp. 35–48.New York, NY: Springer Verlag [Google Scholar]
- Ryan P. G., Moloney C. L.1993Marine litter keeps increasing. Nature 361, 23 (doi:10.1038/361023a0)8421491 [Google Scholar]
- Ryan P. G., Moore C. J., van Franeker J. A., Moloney C. L.2009Monitoring the abundance of plastic debris in the marine environment. Phil. Trans. R. Soc. B 364, 1999–2012 (doi:10.1098/rstb.2008.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos I. R., Friedrich A. C., Baretto F. P.2005Overseas garbage pollution on beaches of northeast Brazil. Mar. Pollut. Bull. 50, 783–786 (doi:10.1016/j.marpolbul.2005.04.044) [DOI] [PubMed] [Google Scholar]
- Shaw D. G., Day R. H.1994Color and form-dependent loss of plastic micro-debris from the North Pacific-Ocean. Mar. Pollut. Bull. 28, 39–43 (doi:10.1016/0025-326X(94)90184-8) [Google Scholar]
- Sokka L., Antikainen R., Kauppi P. E.2007Municipal solid waste production and composition in Finland—changes in the period 1960–2002 and prospects until 2020. Resour. Conserv. Recy. 50, 475–488 (doi:10.1016/j.resconrec.2007.01.011) [Google Scholar]
- Stefatos M., Charalampakis M., Papatheodorou G., Ferentinos G.1999Marine debris on the sea-floor of the Mediterranean Sea: examples from two enclosed gulfs in Western Greece. Mar. Pollut. Bull. 36, 389–393 [Google Scholar]
- Talsness C. E., Andrade A. J. M., Kuriyama S. N., Taylor J. A., vom Saal F. S.2009Components of plastic: experimental studies in animals and relevance for human health. Phil. Trans. R. Soc. B 364, 2079–2096 (doi:10.1098/rstb.2008.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappin A., Burton J. D., Millward G. E., Statham P. J.1997A numerical transport model for predicting the distributions of Cd, Cu, Ni, Pb an Zn in the southern North Sea: the sensitivity of model results to uncertainties in the magnitudes of metal inputs. J. Mar. Syst. 13, 173–204 (doi:10.1016/S0924-7963(96)00112-1) [Google Scholar]
- Teuten E. L., Rowland S. J., Galloway T. S., Thompson R. C.2007Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 41, 7759–7764 (doi:10.1021/es071737s) [DOI] [PubMed] [Google Scholar]
- Teuten E. L., et al. 2009Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 364, 2027–2045 (doi:10.1098/rstb.2008.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C.2006Plastic debris in the marine environment: consequences and solutions. In Marine nature conservation in Europe. (eds Krause J. C., von Nordheim H., Brager S.) pp. 107–116 Stralsund, Germany: Federal Agency for Nature Conservation [Google Scholar]
- Thompson R. C., Olsen Y., Mitchell R. P., Davis A., Rowland S. J., John A. W. G., McGonigle D., Russell A. E.2004Lost at sea: where is all the plastic? Science 304, 838 (doi:10.1126/science.1094559) [DOI] [PubMed] [Google Scholar]
- Thompson R., Moore C., Andrady A., Gregory M., Takada H., Weisberg S.2005New directions in plastic debris. Science 310, 1117. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Moore C. J., vom Saal F. S., Swan S. H.2009Plastics, the environment and human health: current consensus and future trends. Phil. Trans. R. Soc. B 364, 2153–2166 (doi:10.1098/rstb.2009.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres D., Jorquera D.1999Synthesis of marine debris survey at Cape Shirreff, Livingston Island, during the Antarctic season 1998/99. CCAMLR-XVIII/BG/39, CCAMLR, Hobart, Australia [Google Scholar]
- US EPA 2006Municipal Solid Waste in the United States: 2005 facts and figures. EPA530-R-06-011, United States Environmental Protection Agency, Office of Solid Waste, Washington, DC [Google Scholar]
- van Franeker J. A., Bell P. J.1988Plastic ingestion by petrels breeding in Antarctica. Mar. Pollut. Bull. 19, 672–674 [Google Scholar]
- van Franeker J. A., Meijboom A., de Jong M. L.2004Marine litter monitoring by Northern Fulmars in the Netherlands 1982–2003. Alterra, 1093, Wageningen [Google Scholar]
- Van Franeker J. A., et al. 2005. ‘Save the North Sea’ Fulmar Study 2002–2004: a regional pilot project for the Fulmar-Litter-EcoQO in the OSPAR area. Alterra-rapport 1162, Alterra, Wageningen: See www.zeevogelgroep.nl [Google Scholar]
- Voparil I. M., Burgess R. M., Mayer L. M., Tien R., Cantwell M. G., Ryba S. A.2004Digestive bioavailability to a deposit feeder (Arenicola marina) of polycyclic aromatic hydrocarbons associated with anthropogenic particles. Environ. Toxicol. Chem. 23, 2618–2626 (doi:10.1897/03-357) [DOI] [PubMed] [Google Scholar]
- Walker T. R., Reid K., Arnould J. P. Y., Croxall J.1997Marine debris surveys at Bird Island, South Georgia 1990–1995. Mar. Pollut. Bull. 34, 61–65 (doi:10.1016/S0025-326X(96)00053-7) [Google Scholar]
- Weiss K. R., McFarling U. L., Loomis R.2006Plague of plastic chokes the seas. Los Angeles Times 2 August [Google Scholar]
- Williams A., Simmons S.1997Estuarine litter at the estuarine/beach interface in the Bristol Channel. J. Coast. Res. 13, 1159–1165 [Google Scholar]
- World Bank 2005Waste Management in China: issues and recommendations. Working Paper No. 9, Urban Development Working Papers, East Asia Infrastructure Development [Google Scholar]