Abstract
Plastic debris has significant environmental and economic impacts in marine systems. Monitoring is crucial to assess the efficacy of measures implemented to reduce the abundance of plastic debris, but it is complicated by large spatial and temporal heterogeneity in the amounts of plastic debris and by our limited understanding of the pathways followed by plastic debris and its long-term fate. To date, most monitoring has focused on beach surveys of stranded plastics and other litter. Infrequent surveys of the standing stock of litter on beaches provide crude estimates of debris types and abundance, but are biased by differential removal of litter items by beachcombing, cleanups and beach dynamics. Monitoring the accumulation of stranded debris provides an index of debris trends in adjacent waters, but is costly to undertake. At-sea sampling requires large sample sizes for statistical power to detect changes in abundance, given the high spatial and temporal heterogeneity. Another approach is to monitor the impacts of plastics. Seabirds and other marine organisms that accumulate plastics in their stomachs offer a cost-effective way to monitor the abundance and composition of small plastic litter. Changes in entanglement rates are harder to interpret, as they are sensitive to changes in population sizes of affected species. Monitoring waste disposal on ships and plastic debris levels in rivers and storm-water runoff is useful because it identifies the main sources of plastic debris entering the sea and can direct mitigation efforts. Different monitoring approaches are required to answer different questions, but attempts should be made to standardize approaches internationally.
Keywords: marine debris, mitigation, monitoring, plastic, seabirds, virgin pellets
1. Introduction
Two of the key characteristics that make plastics so useful—their light weight and durability—also make inappropriately handled waste plastics a significant environmental threat. Plastics are readily transported long distances from source areas and accumulate in sinks, mainly in the oceans, where they have a variety of significant environmental and economic impacts (Coe & Rogers 1997; Thompson et al. 2009a,b; UNEP 2005). Discarded plastics also affect terrestrial and fresh-water systems, including ingestion by and entanglement of animals, blocked drainage systems and aesthetic impacts. However, the literature on plastic pollution largely focuses on marine systems.
Most plastics break down slowly through a combination of photodegradation, oxidation and mechanical abrasion (Andrady 2003). Thick plastic items persist for decades, even when subject to direct sunlight, and survive even longer when shielded from UV radiation under water or in sediments. Except for expanded polystyrene, plastics take much longer to degrade in water than they do on land, mainly owing to the reduced UV exposure and lower temperatures found in aquatic habitats (Gregory & Andrady 2003). There has been a rapid increase in the amount of plastic litter in the marine environment (Ryan & Moloney 1993), linked to increases in the use of plastics. Even the development of the so-called ‘biodegradable’ plastics is not a long-term solution to the plastic litter problem, because many such materials contain only a proportion of biodegradable material such as starch, leaving behind microscopic plastic fragments (Klemchuk 1990).
Given the impacts of plastic litter (Gregory 2009), considerable effort has been made to remove waste plastic and other persistent debris from the environment. This removal may occur before it enters the sea, through litter collection and screening waste water systems (e.g. Marais & Armitage 2004) or, thereafter, through collections of litter from beaches (e.g. Ryan & Swanepoel 1996; Ocean Conservancy 2007), the seabed (e.g. Donohue et al. 2001) or at sea (Pichel et al. 2007). However, the most efficient and cost-effective solution is to reduce the release of plastics into the environment in the first place. Measures taken to achieve this goal include education, both of the general public and specific user groups (e.g. the plastics industry's Operation Clean Sweep to prevent loss of virgin pellets), and legislation (e.g. Annex V of MARPOL banning the dumping of plastics at sea). Assessing the efficacy of these measures requires monitoring both the amounts of plastic in the environment and the rates at which plastic litter enters the environment (Sheavly 2007).
Monitoring is a series of measures made to detect change in the state of a system (Goldsmith 1991). It is goal dependent, so the protocols used need to be tailored to the questions being asked. The main questions regarding plastic litter in the environment are:
What is the abundance, distribution and composition of plastic litter, and are these attributes changing over time?
What are the main sources of plastic litter, and are they changing over time?
What are the impacts of plastic litter (environmental and economic) and are they changing over time?
For all three questions, targets may be linked to specific mitigation measures and may operate at a range of spatial and temporal scales. For example, at a local scale, monitoring may assess whether the implementation of a screening system in an urban catchment reduces litter loads to target levels. At a larger scale, we might test whether initiatives to reduce the loss of virgin pellets have reduced the abundance of pellets at sea. Similarly, monitoring may track a broad suite of litter types or specific items that have particular impacts (e.g. high risk of entangling animals) or are indicators of specific sources of plastic litter.
This paper summarizes monitoring protocols used to measure changes in plastic debris with a focus on the marine environment because accumulation and impacts of plastic litter appear to be most serious in marine systems. We highlight the strengths and weaknesses of the various approaches and provide a set of best-practice guidelines for monitoring the abundance and impacts of plastics. Our paper links with that of Barnes et al. (2009) summarizing trends in plastics in the environment.
2. Plastics in marine ecosystems
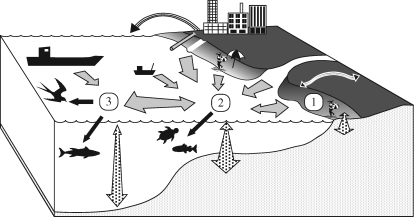
Plastics dominate marine debris (Coe & Rogers 1997; UNEP 2005). The proportion of plastic articles among litter increases with distance from source areas because they transport more easily than do more dense materials such as glass or metal and because they last longer than other low-density materials such as paper. Most plastics are less dense than water, but some are more dense (e.g. polyamide, polyterephthalate, polyvinyl chloride). Floating plastic debris has become a global problem because it is carried across ocean basins, contaminating even the most remote islands (Barnes 2002). Plastics in the marine environment derive from two main sources: rubbish dumped from ships at sea and land-based sources such as runoff from rivers, waste water systems, wind-blown litter and recreational litter left on beaches (Coe & Rogers 1997). To monitor plastic litter, we need to understand the dynamic linkages between litter sources and sinks (figure 1). As one moves offshore, there usually is an increase in the proportion of ship-based litter and a decrease in total litter loads, although aggregations may occur in mid-ocean gyres (Pichel et al. 2007). In addition, the suite of organisms exposed to plastic litter may differ between coastal and oceanic waters. Unfortunately, the rates at which plastic cycles through various pathways are largely unknown. The combination of multiple diffuse and point-source inputs and the non-random transportation of debris by winds and currents results in great temporal and spatial variability in litter loads. Such variability tends to mask long-term trends, requiring a non-confounded sampling design with sufficiently large replication in time and space. Superimposed on the dynamic system of plastic flux is the gradual fragmentation of large plastic articles over time. Plastic litter can be broadly divided into macrodebris (>20 mm diameter), meso-debris (2–20 mm) and micro-debris (<2 mm), although different authorities recommend subtly different size limits (e.g. Cheshire et al. 2009). There is little information on the rates at which different plastics degrade and fragment under different conditions (e.g. Pritchard 1997; Andrady 2003), nor is it clear what is the fate of all the plastic fragments (Thompson et al. 2004). We thus are challenged with trying to monitor a highly dynamic system about which our understanding is incomplete, and where the items being monitored appear and disappear in response to various societal, technological, environmental and political pressures.
Figure 1.
Schematic diagram showing the main sources and movement pathways for plastics in the marine environment, with sinks occurring (1) on beaches, (2) in coastal waters and their sediments and (3) in the open ocean. Curved arrows depict wind-blown litter, grey arrows water-borne litter, stippled arrows vertical movement through the water column (including burial in sediments) and black arrows ingestion by marine organisms.
Rees & Pond (1995) recognized three approaches to marine litter monitoring: (i) beach surveys, (ii) at-sea surveys and (iii) estimates of the amounts entering the sea. We summarize each of these approaches, starting with beach surveys, because they are often regarded as the simplest and most cost-effective way to monitor large-scale trends in marine litter (Dixon & Dixon 1981; Ribic et al. 1992; Rees & Pond 1995). We discuss the strengths and weaknesses of each approach and add a fourth one: evaluating trends in interactions between wildlife and plastic litter.
3. Beach surveys
Much of what we know about the abundance, distribution and origin of plastic debris in the marine environment comes from surveys of litter stranded on beaches (Coe & Rogers 1997). Initial studies were baseline surveys that summarized the abundance, distribution and composition of litter in various regions. We cannot provide a comprehensive review of the more than 100 published surveys of beach macro-debris (see reviews by Pruter 1987; Derraik 2002), but there are some consistent patterns: plastics dominate beach litter in terms of numbers of items (Derraik 2002), and litter loads are greater close to urban areas (e.g. Garrity & Levings 1993; Willoughby et al. 1997), increasing with numbers of visitors to beaches (e.g. Frost & Cullen 1997). However, many authors note the difficulty in comparing data among studies. This difficulty is largely owing to differences in sampling protocols and the type of data recorded. Some studies record the numbers of items, some the mass of litter and some do both. Litter is categorized by the type of material, function or both. Most studies record only fairly large items, although the lower limit varies from 10 to 100 mm and is often not reported. Even more fundamentally, most studies record all litter between the sea and the highest strandline on the upper shore, whereas some measure litter within fixed areas, and others only sample specific strandlines (e.g. Velander & Mocogni 1999). Only a few studies have sampled buried litter, even though it may account for a substantial proportion of beach litter loads (Kusui & Noda 2003). In addition, the width of beach sampled varies greatly, from a few metres (e.g. Madzena & Lasiak 1997) to entire beaches >20 km long (e.g. Edyvane et al. 2004). Finally, most studies report standing stocks of litter, whereas others assess the rate of accumulation following removal of existing debris. This is a critical distinction that requires detailed discussion.
(a). Standing-stock surveys
Standing-stock surveys can show gross changes in the abundance and distribution of plastic litter (e.g. Ryan & Moloney 1990; Willoughby et al. 1997; figure 2), but there are significant problems with the interpretation of results. The amount of litter on a beach is determined by several factors in addition to the abundance of litter in adjacent coastal waters. These include local currents and circulation patterns, beach structure (slope, particle size, etc.), recent weather conditions and associated beach dynamics (burial or exposure of litter, especially on sandy beaches), local land-based sources (e.g. beach recreation, proximity to poorly managed landfill sites) and, at least for macro-debris, any formal or informal cleanup efforts (OSPAR Commission 2007a; Cheshire et al. 2009). Standing stocks of beach litter reflect the long-term balance between inputs (both local, land-based sources and strandings) and removal (through export, burial, degradation and cleanups). The few studies of beach-litter dynamics indicate that beach structure influences the rate of litter turnover, with fairly rapid turnover rates (3–12 months), although the fate of most items is unknown (Garrity & Levings 1993; Bowman et al. 1998). However, persistent accumulations probably occur at some beaches. Repeated measures of standing stocks at such beaches may reflect the gradual accumulation of long-lasting debris rather than provide an index of changes in the abundance of debris at sea.
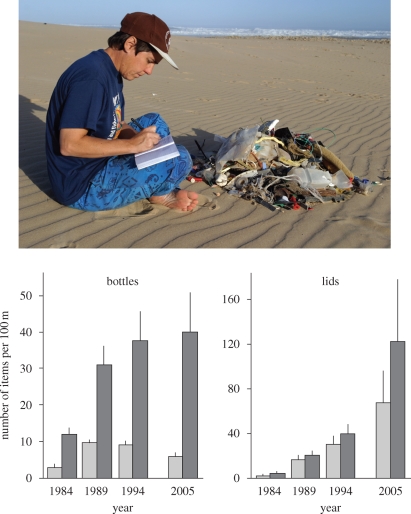
Figure 2.
Scoring litter collected from a 50 m stretch of beach (top) and trends in the abundance of plastic bottles and lids (mean and s.e.) at South African beaches sampled in 1984, 1989, 1994 and 2005. Light grey bars, 36 beaches with regular, municipal cleaning programmes; dark grey bars, 14 beaches with no formal cleaning programmes (P. G. Ryan & C. L. Moloney, unpublished data).
Some of the factors affecting litter inputs and removal are fairly constant and thus have little influence on monitoring programmes, but episodic events can mask long-term trends (e.g. storms carry large loads of land-based litter to sea and re-suspend buried litter). Of greater concern is that human activities typically change over time. Patterns of beach use tend to increase with growing human populations, coastal development and improved access. For example, many beaches categorized as ‘rural’ with little human influence in an initial survey of 50 South African beaches in 1984 (Ryan & Moloney 1990) have become resort beaches over the following two decades. There has been a concomitant increase in formal beach-cleaning efforts over this period (Ryan & Swanepoel 1996). As a result, the numbers of plastic bottles on beaches have stabilized at regularly cleaned beaches, but have continued to increase at seldom-cleaned beaches (figure 2). In comparison, small items such as lids that are often overlooked by cleaning teams have continued to increase at all beaches (figure 2). Beach cleanups increasingly are used as an educational tool (Storrier & McGlashan 2006; Ocean Conservancy 2007), altering litter loads on beaches, and thus need to be considered when monitoring changes in beach litter. Beachcombing also selectively removes items from all but the most remote beaches. For example, the abundance of fishing floats on uninhabited Inaccessible Island, central South Atlantic, is two orders of magnitude greater than similar beaches in South Africa (Ryan & Watkins 1988). Thus although standing-stock surveys can track changes in the composition of beach litter (Rees & Pond 1995), they are not sufficiently sensitive to monitor changes in macro-litter abundance (Escardó-Boomsma et al. 1995).
(b). Accumulation and loading rates
Many of the problems associated with monitoring trends in litter with standing-stock surveys are avoided by recording the rate at which litter accumulates on beaches. This requires an initial cleanup to remove all existing debris, followed by regular surveys that record and remove all newly arrived debris. Such surveys form the basis of major monitoring programmes established in the USA and western Europe (table 1). Such fine-scale studies can reveal surprising linkages between long-term patterns in litter accumulation rates and large-scale climatic cycles (Morishige et al. 2007). However, they require much more effort than do surveys of standing stocks, and substantial investment to conduct them routinely over a sufficiently large number of sites to track trends in debris abundance accurately (Sheavly 2007).
Table 1.
Comparison of survey protocols for monitoring the accumulation of beached litter in the USA (US Marine Debris Monitoring Program (USMDMP), Sheavly 2007) and Europe (Beach Litter Monitoring Programme, OSPAR Commission 2007a).
| USMDMP | OSPAR | |
|---|---|---|
| type of beach | sand/gravel | sand/gravel |
| beach slope | 15–45° (not steep) | — |
| beach length (m) | >500 | >1000 |
| length of beach surveyed (m) | 500 | 100 (all items) |
| 1000 (items >0.5 m across) | ||
| sample frequency (days) | 28 ± 3 | 90 (approx.)a |
| type of litter recorded | 31 indicator items | all debris (111 categories) |
| other criteria | no regular cleaning | distant from sources (rivers) |
| no impact on threatened species | visually/frequently littered |
aLitter is not removed at some OSPAR sites and some sites are also cleaned by local municipalities (Barbara Wenneker in litt.).
Escardó-Boomsma et al. (1995) made the distinction between loading rate (the amount of litter arriving on a beach) and net accumulation rate (the amount of litter that accumulates per unit of time). Turnover of plastic on beaches can happen rapidly. Scoring the amount accumulated at a predetermined interval will influence the estimate of accumulation rate. Most studies to date have sampled at monthly or quarterly intervals (e.g. Garrity & Levings 1993; Madzena & Lasiak 1997; table 1), but some more frequent surveys have been conducted (two weeks, Morishige et al. 2007; 3 days, Vauk & Schrey 1987; daily, Swanepoel 1995). There have been few attempts to assess the impact of sampling interval on estimates of accumulation rate. The US National Marine Debris Monitoring Program (USMDMP) found no difference in accumulation rate for samples collected at different intervals (Sheavly 2007), but failed to report the range of intervals tested. Walker et al. (1997) reported greater accumulation rates in winter, when sampling was monthly, than in summer, when litter could be sampled only at the end of the season; however, it was not possible to discriminate whether this was a sampling artefact or a seasonal difference in the rate of litter accumulation. The daily accumulation rate of all debris at two sites near Cape Town, South Africa, was 50–60% greater by mass and 100–600% greater by number compared with weekly sampling (Swanepoel 1995). This suggests that the loading rate of small items, in particular, is grossly underestimated by weekly sampling. The actual magnitude of this effect is dependent on turnover rates of debris, which are influenced by local conditions (Bowman et al. 1998) and the type of debris (in the Cape Town study, lighter debris such as foamed polystyrene turned over more rapidly, probably because it was blown away by the wind). Of course, frequent surveys fail to measure the rate of loss of stranded litter, but they provide the best proxy for at-sea abundance of litter.
Additional issues need to be considered when planning accumulation surveys. The initial cleanup is unlikely to locate all debris, so several collections may be necessary before the data reflect actual accumulation rates. In addition, there remain the problems of lateral drift from adjacent, uncleaned areas and exhumation of buried debris. Lateral drift may be minor (Garrity & Levings 1993) and can be addressed by cleaning buffer zones on either side of the monitoring area. Pilot studies tracking the movement of marked litter items should help determine the appropriate buffer width, which is likely to be site and season specific, linked to local current and wind conditions. Exhumation of buried debris is harder to tackle. Probably the best that can be achieved is to record major changes in beach profile. Such records might explain anomalies in the data series during subsequent analysis.
Accumulation studies are more labour-intensive than standing-stock surveys and require substantial resources to be conducted at multiple sites. Well-trained volunteers can assist with the process (Sheavly 2007). A less demanding alternative is to sample accumulation rates sporadically, recording accumulation on a daily or weekly basis for a period, then repeating the exercise several years hence (e.g. Velander & Mocogni 1998). However, consideration needs to be given to the variability in daily or weekly accumulation rates when deciding the appropriate duration for each bout of sampling. Daily sampling is more variable (coefficient of variation 47–60% by number, 61–86% by mass) than weekly sampling (CV 28–42% by number, 19–38% by mass; Swanepoel 1995; P. G. Ryan, unpublished data), suggesting that longer intervals between surveys buffer some of the short-term variability in accumulation rates linked to local conditions (e.g. wind direction and sea state).
(c). Sampling meso- and micro-plastics
Meso- and micro-debris on beaches differ from macro-debris, in that these categories of litter are less likely to derive directly from beach users and are not targeted by most cleanup efforts (floatation systems that separate out low-density debris are costly and not widely used). Accumulation studies are not feasible because they require a thorough initial cleanup. It is possible that changes in beach-cleaning regimes may cause a bias if a substantial proportion of small plastic fragments derive from the fragmentation of large litter items in situ, but this problem does not apply to virgin pellets. Repeated surveys of beach meso-debris can detect changes in their abundance (e.g. Ryan & Moloney 1990), but the results are hard to interpret if the goal is to detect changes in the amount of debris at sea. Further, without an understanding of the dynamics of meso-debris on beaches, it is hard to predict how a change in the at-sea abundance of debris will affect beach loads. If turnover in beach litter is rapid, one would expect a decrease in the amount of litter at sea to result in a decrease in beach load. However, if beaches accumulate meso-debris, a decrease in litter at sea would manifest in only a slowing in the rate of increase in beach load. Changes in the proportions of different debris types presumably indicate changes in their relative abundance at sea.
Another problem with sampling meso- and micro-debris on beaches is the design of representative sampling protocols. Sampling of micro-debris is still in its infancy, with baseline data largely confined to the presence/absence of different polymers (Thompson et al. 2004; Ng & Obbard 2006). Initial studies of meso-debris were also largely qualitative, sufficient only to detect gross changes in abundance (e.g. Gregory & Ryan 1997). Quantitative estimates of meso-debris have been obtained by sieving beach samples, typically to a depth of approximately 50 mm, and sorting samples including the use of floatation in sea water (e.g. Ryan & Moloney 1990; McDermid & McMullen 2004). However, little attention has been paid to sampling design and statistical power. Although debris is concentrated in strandlines, point sampling (Moore, S. L. et al. 2001; McDermid & McMullen 2004) is likely to miss old, buried lines. Sieving a strip transect from the most recent strandline to the back of the beach is a more reliable way to characterize meso-debris loads (e.g. Ryan & Moloney 1990).
(d). Best practice for beach surveys
The lack of consistency among surveys of beached plastics to date results in part from different goals, which include the need to assess the amount and composition of litter, to identify the sources of litter and to monitor changes in litter loads. Although these goals may favour different sampling approaches, there is need for greater standardization. The United Nations Environment Programme (UNEP) is currently developing a set of guidelines to standardize beach survey methods (Cheshire et al. 2009). The following recommendations are based on our best understanding of beach-litter dynamics and conform to the most commonly employed practice where possible.
For standing-stock and accumulation studies, the best approach is to record all litter from the sea edge to the highest strandline (in most cases, the edge of terrestrial vegetation). Ideally, both the numbers and mass of plastic items should be recorded, but counts may be sufficient for specific types of litter. Items should be identified as accurately as possible, allowing them to be categorized according to both composition and function. Methodologies should explicitly state the size range of litter items sampled. The minimum length of beach sampled is determined by the abundance of litter (de Araújo et al. 2006) and, for monitoring purposes, it should be determined by a power analysis based on estimates of variability in accumulation data from pilot studies and on considerations of the minimum rate of change to be detected (Ribic & Ganio 1996). Ideally, sample widths should be at least 50 m for standing stocks and 500 m for accumulation studies. Site selection is likely to be determined by the monitoring question (e.g. remote beaches track litter from ships and long-distance drift litter, whereas urban beaches track local inputs). However, it is important that sites be described adequately by recording substratum, slope, exposure to the open ocean, proximity to local litter sources, cleaning history, etc. (table 1). For most studies, it is best if no beach-cleaning takes place, because cleaning significantly alters the abundance and composition of debris (Moore, S. L. et al. 2001; Somerville et al. 2003). It is also crucial that the site can be relocated accurately for repeated sampling.
Comparisons of standing stocks have shown marked increases in some litter types (e.g. Ryan & Moloney 1990; Willoughby et al. 1997; figure 2), but this may reflect long-term accumulation rather than absolute increases in the amount of debris at sea. Accumulation studies are preferred because they demonstrate unambiguous trends in macro-debris abundance at sea (Ribic et al. 1992, 1997). Despite failing to demonstrate major changes in debris accumulation rates (Sheavly 2007), the USMDMP provides the best model for large-scale beach monitoring (table 1). However, it would be valuable to monitor a broader spectrum of litter types. Focusing on specific indicator groups can be useful (e.g. Józwiak 2005; Shigeru et al. 2006), but it may fail to detect important changes in non-target categories (Ribic 1998), including changes in plastic products in the waste stream resulting from novel packaging applications. Ideally, monitoring should take place at a network of sites, with analysis testing for common trends across sites.
Meso-debris should be sampled by a combination of sieving, dry picking and floatation to locate the greatest proportion of plastic litter. Fourier-transform infrared spectroscopy could be used to identify the polymer type of fragments collected. Surveys should sample the entire beach profile from the most recent strandline to the back of the shore to a depth of 50 mm. Minimum transect width is again determined by the abundance of litter, but should not be <0.5 m. Macro-debris sampled in this way can give an indication of buried litter loads compared with surface macro-debris (Kusui & Noda 2003).
4. Surveys at sea
If the primary goal is to monitor changes in the amount and composition of plastic debris at sea, direct surveys avoid many of the complications of beach dynamics and contamination by beach users. However, at-sea surveys are complicated by ocean current dynamics, shipboard disposal and accidental loss and are more costly and more challenging logistically, given the intensive sampling needed to detect subtle changes. Surveys at sea are also limited to assessing standing stocks rather than accumulation rates. Changes detected in the amounts of debris are the balance between inputs and losses and do not necessarily reflect the efficacy of mitigation measures to reduce losses of plastics into the environment.
(a). Floating and suspended debris
The abundance of floating plastics at sea can be estimated either by direct observation of large debris items (e.g. Day et al. 1990a; Matsumura & Nasu 1997; Thiel et al. 2003; Pichel et al. 2007) or by net trawls for smaller items (e.g. Carpenter & Smith 1972; Day & Shaw 1987; Ryan 1988a; Day et al. 1990b; Ogi et al. 1999; Moore, C. J. et al. 2001; Yamashita & Tanimura 2007). Direct observations rely on competent, motivated observers. Studies comparing detection ability show marked differences among observers (e.g. Ryan & Cooper 1989), which needs to be addressed if multiple observers are used to monitor debris at sea. Counts of litter at sea can be used to provide an index of abundance (number of items per unit distance) or an estimate of abundance based on fixed-width or line transects. Fixed-width transects assume that all debris is detected, which is unlikely unless transects are very narrow (e.g. Willoughby et al. 1997). For line transects, the perpendicular distance to each item has to be estimated to compensate for decreasing detection rate with distance from the observer (Buckland et al. 1993). This method assumes that the probability of detection on the transect line is 1, and there are problems with variable detection rates depending on sea state, light conditions and the size, colour and height above water of plastic objects. Observations should be conducted only on that side of the ship with the best viewing conditions. Separate detection curves should be estimated for different sea states, and studies should state the smallest size of items recorded.
Most surveys are conducted from ships or small boats, but aerial surveys also have been used to estimate the abundance of plastic litter at sea (Lecke-Mitchell & Mullin 1992) and to locate major aggregations of litter (Pichel et al. 2007). Aerial surveys cover large areas and are less prone to changes in litter detectability linked to wind strength and sea state, but they only detect large litter items. As with ship-based surveys, unless inter-observer effects can be strictly controlled, aerial surveys are more valuable for detecting spatial differences in abundance than for monitoring changes over time.
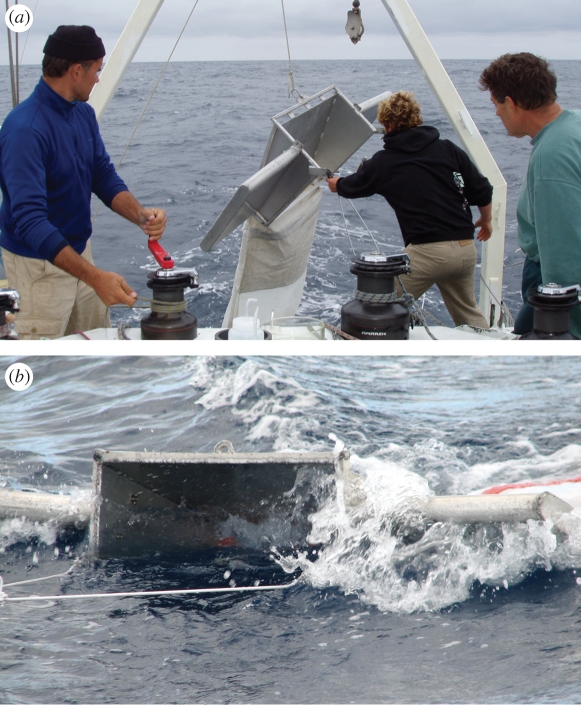
Net-based surveys are less subjective than direct observations but are limited regarding the area that can be sampled (net apertures 1–2 m and ships typically have to slow down to deploy nets, requiring dedicated ship's time). The plastic debris sampled is determined by net mesh size, with similar mesh sizes required to make meaningful comparisons among studies. Floating debris typically is sampled with a neuston or manta trawl net lined with 0.33 mm mesh (figure 3). Given the very high level of spatial clumping in marine litter (e.g. Ryan 1988a; Pichel et al. 2007), large numbers of net tows are required to adequately characterize the average abundance of litter at sea. Long-term changes in plastic meso-litter have been reported using surface net tows: in the North Pacific Subtropical Gyre in 1999, plastic abundance was 335 000 items km−2 and 5.1 kg km−2 (Moore, C. J. et al. 2001), roughly an order of magnitude greater than samples collected in the 1980s (Day et al. 1990a,b). Similar dramatic increases in plastic debris have been reported off Japan (Ogi et al. 1999). However, caution is needed in interpreting such findings, because of the problems of extreme spatial heterogeneity, and the need to compare samples from equivalent water masses.
Figure 3.
A manta trawl (a) being deployed from a research vessel and (b) being towed at sea to sample floating plastics.
To date, most studies have sampled floating plastic debris, but some plastics are more dense than seawater, making it important to sample mid-water and bottom loads of plastic debris. Suspended debris can be sampled with bongo nets with a 0.33 mm mesh (Lattin et al. 2004). Few such surveys have been conducted, but data from the eastern North Pacific suggest that the abundance of suspended plastic within 10–30 m of the sea surface averages two orders of magnitude less than that of surface plastics (AMRF, unpublished data). All subsurface net tows should be deployed with a flowmeter to assess the volume of water sampled. The continuous plankton recorder (CPR) offers a valuable subsurface tool to track changes in the distribution and composition of micro-plastic particles at sea, both spatially and temporally (Thompson et al. 2004).
(b). Litter on the seabed
Surveys of macro-debris loads on the seabed have been conducted with divers (e.g. Donohue et al. 2001; Nagelkerken et al. 2001), submersibles and remote-operated vehicles (Galgani et al. 2000) and trawl surveys (e.g. Galil et al. 1995; Galgani et al. 2000; Moore & Allen 2000; Lattin et al. 2004; OSPAR Commission 2007b). Perhaps somewhat surprisingly, plastics dominate macro-debris on the sea floor to an extent similar to which they dominate floating litter and beach debris. Just like stranded debris, plastic on the seabed aggregates locally in response to local sources and bottom topography (Galgani et al. 2000; Moore & Allen 2000). The amount of plastic litter is so great in some areas with large amounts of shipping traffic that initiatives have been started to clean the seabed with trawls (OSPAR Commission 2007b), despite concerns about the ecological impacts of trawling. To date, most studies have measured standing stocks of macro-debris, but some accumulation data have been obtained following cleanups of shallow reefs in Hawaii (Boland & Donohue 2003; Dameron et al. 2007). The rate of litter accumulation on these reefs is correlated with initial standing stock and is a function of reef exposure and depth (Dameron et al. 2007). There has been little attention to the abundance of meso- and micro-debris on the seabed. Epibenthic trawls have found substantial plastic loads just above the seabed in shallow coastal waters off southern California (Lattin et al. 2004). Bottom sediments in deeper waters can be sampled with a Van Veen grab or similar device. Micro-plastics have been found in subtidal sediments around the UK and Singapore (Thompson et al. 2004; Ng & Obbard 2006).
(c). Best practice for at-sea surveys
Effective monitoring of floating plastics at-sea requires huge sample sizes to overcome the very large spatial heterogeneity in plastic litter. Stratified random sampling can help with this issue, but it requires a priori categorization of water masses into the relevant sampling strata. If resources are available, probably the best tool is to sample with neuston nets with a 0.33 mm mesh. Direct observations, often using vessels of opportunity, are less resource-intensive, but are fraught with potential biases linked to differences in litter detectability. Such surveys provide only a crude index of the abundance of floating litter. Much less is known about the distribution and abundance of mid-water plastics, but they probably suffer the same sampling problems, with the added complication of even lower abundances. The CPR is a useful tool for long-term subsurface monitoring of micro-particles.
Monitoring changes in benthic plastic litter is functionally similar to beach surveys, with the added complication of working underwater. Divers can replicate beach sampling protocols in shallow water, but in deeper waters there are greater issues with quantitatively robust sampling owing to variation in trawl and grab efficiency (linked to substratum type and other local conditions). Trawl nets also become clogged, reducing their efficiency and thus underestimating actual plastic abundance. Remote cameras may provide a more objective sampling strategy for benthic litter.
5. Monitoring affected species
One of the major concerns about the accumulation of plastic debris in the ocean is the impact on the biota. Consequently, it is sensible to monitor the rates at which impacts occur, although it only provides information on specific types of plastic litter. The two main impacts are entanglement and ingestion, but monitoring other interactions with wildlife also can detect useful trends, such as the amount of litter incorporated in seabird nests (Hartwig et al. 2007).
(a). Entanglement
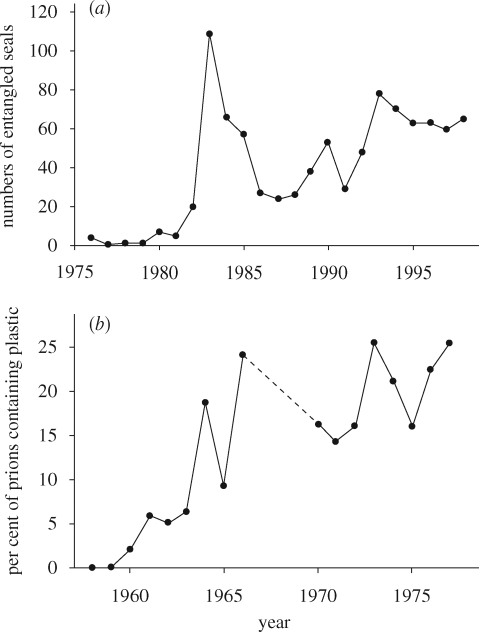
Entanglement is one of the more visible impacts of plastic debris, affecting a large number of marine and fresh-water species (Laist 1997). Monitoring the number of entangled organisms can indicate changes in the abundance of debris responsible for entanglements. For example, entanglement records for three species of seals from the Farallon Islands show a steady increase since the 1970s, but with a marked peak from 1983 to 1985 (Hanni & Pyle 2000; figure 4a). Camphuysen (2001) reported increased entanglement rates of Northern Gannets Morus bassanus in the North Sea from 1997 to 2000. Such data can be used to assess whether mitigation measures have been effective. There was no decrease in entanglement rates among Hawaiian Monk Seals Monachus schauinslandi following the introduction of MARPOL Annex V banning the disposal of plastics at sea (Henderson 2001), but most entanglements are from fishing gear lost at sea (not discarded). However, there was no decrease in entanglement of two seal species in Australia, despite efforts to reduce the amount of fishery debris being lost at sea (Page et al. 2004). At South Georgia, the proportion of entangled Antarctic fur seals Arctocephalus gazella halved following active promotion of MARPOL Annex V regulations, but the population of seals roughly doubled over the same period, suggesting that there was no decrease in the amount of litter at sea and that the total number of seals affected may have increased (Arnould & Croxall 1995). This example illustrates the need to interpret results with caution. Entanglement tends to be quite rare (Laist 1997), making it hard to obtain sufficient data to detect a significant change in the rate of entanglement. It is only really feasible to use as an index of plastic litter if there is constant search effort.
Figure 4.
Long-term trends in the impacts of plastics on marine animals: (a) numbers of entangled seals recorded annually on SE Farallon Island, California, 1976–1998 (reproduced with permission from Hanni & Pyle 2000); (b) proportions of prions Pachyptila spp. stranded on New Zealand beaches that had plastics in their stomachs, 1958–1977 (reproduced with permission from Harper & Fowler 1987).
(b). Plastic ingestion
Ingestion of plastic debris occurs much more frequently than entanglement, with almost all individuals of some species containing ingested plastic (e.g. Ryan 1987; Laist 1997; Robards et al. 1997). Indeed, the first indication that plastics were a problem in marine systems came when plastic fragments were found in the stomachs of seabirds in the 1960s. Prions Pachyptila spp. in New Zealand showed a steady increase in the incidence of plastic ingestion from the 1960s to the 1970s (Harper & Fowler 1987; figure 4b), and 74 per cent of albatross Phoebastria spp. chicks found dead on Laysan Island, Hawaii, contained plastics in 1965 (Kenyon & Kridler 1969). Seabirds that accumulate plastics in their stomachs (especially petrels and storm-petrels, Procellariiformes) are useful indicators of changes in the amount and composition of plastic debris at sea. They collect debris over large areas and can be sampled with little cost by examining the stomach contents of beached birds (Harper & Fowler 1987), birds killed accidentally by fishing activities (Mallory et al. 2006; Ryan 2008) or by examining regurgitated pellets of predators that feed on seabirds (Ryan 2008).
Plastic loads in birds reflect regional differences in the abundance of marine debris (Day et al. 1985; van Franeker 1985; van Franeker & Bell 1985; Spear et al. 1995). For example, the amount of ingested plastic in Northern Fulmars Fulmarus glacialis in the North Atlantic is greatest in highly contaminated waters of the North Sea, where almost all birds contain some plastic (van Franeker et al. 2005), decreasing to only 36 per cent of birds in arctic Canada (Mallory et al. 2006). Even within the North Sea, regional differences are apparent, with average plastic loads doubling from the Faroe Islands to Scotland and doubling again from Scotland to the southern North Sea (van Franeker et al. 2005). These regional differences suggest that there are marked differences in the abundance of meso-debris between these regions, provided that the foraging areas of birds are largely confined to these regions.
For monitoring purposes, it is important to understand the factors that influence the amount of plastic in birds' stomachs (Ryan 2008). Seabirds select the types of plastic fragments they ingest (Day et al. 1985; Ryan 1987), but comparisons within species should have a consistent bias. Plastic loads in Northern Fulmars found dead on the Dutch coast are affected by age but not by sex, season, level of starvation or cause of death (van Franeker et al. 2005). Young birds typically contain more plastic than adults, probably because of the transfer of plastic from parents to their offspring (Ryan 1988b) exacerbated by poor discrimination of suitable food items by naive birds (Day et al. 1985). Another issue is how ingested plastic loads change with increases in the abundance of plastic at sea. Is there a linear relationship, or do birds reach a point where they become saturated? This question has not been resolved (Ryan 2008).
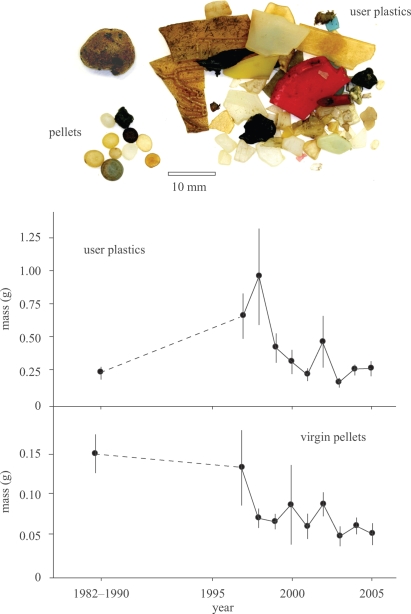
Monitoring of plastic loads in seabirds initially showed increases in plastic ingestion from the 1960s to the 1980s (figure 4b; Day et al. 1985; Moser & Lee 1992), but plastic loads have stabilized or decreased more recently, with significant changes in the composition of ingested plastic. In the North Pacific, plastic loads in Short-tailed Shearwaters Puffinus tenuirostris did not change significantly from the 1970s to the late-1990s, but virgin pellets were replaced by fragments of user plastics, decreasing from 55–73% in the 1970s to 33 per cent in the 1990s (Vlietstra & Parga 2002). Similar decreases in the proportions of virgin pellets have been found in five other seabirds foraging in the Atlantic and southern Indian Oceans from the 1980s to 2000s (Ryan 2008). Among Northern Fulmars stranded on Dutch beaches, there has been a long-term decrease in the mass of virgin pellets over the last 20 yr and, after peaking in the 1990s, the total mass of ingested plastic has decreased over the last 10 yr (figure 5). There thus appears to have been a global decrease in the abundance of virgin pellets at sea over the last two decades, which, with the exception of fulmars in the North Sea, has been offset to a degree by increases in user plastics. These insights have been obtained at a fraction of the cost of ship-based surveys of meso-debris abundance at sea.
Figure 5.
Typical plastics from a Northern Fulmar stomach (top panel) and trends in the average mass (±s.e.) of user plastic and virgin plastic pellets in Northern Fulmars stranded on Dutch beaches in 1982–1990 (n = 69) and 1997–2005 (n = 580).
6. Monitoring inputs
The most direct measure of success in the campaign against plastic pollution is to monitor the amounts of waste plastic entering the marine environment. This is no trivial undertaking, given the wide range of sources of plastic debris. Two main sources of plastic debris can be considered separately: ship- and land-based (Coe & Rogers 1997).
(a). Ship-based sources
Initial concerns about debris in marine environments focused largely on ship-based sources. Early attempts to assess the amount of waste disposed of by vessels at sea (Dixon & Dixon 1981; Pruter 1987) provided crude estimates of the amount dumped (Rees & Pond 1995), but there have been no formal estimates since MARPOL Annex V came into force in 1988. Estimates of compliance with the ban on disposal at sea imposed on signatories of MARPOL Annex V have been obtained from the use of port reception facilities (Carpenter & Macgill 2005). In addition, independent fishery observers can be tasked to report on disposal practices on fishing vessels (Jones 1995; Walker et al. 1997).
(b). Land-based sources
Although some plastic debris is transported by wind, most land-based litter is carried by water via rivers and storm-water. Few attempts to quantify the magnitude of litter in runoff have been published in the primary literature (see papers in Coe & Rogers 1997), but numerous studies have been conducted, often as part of programmes to educate the public to the dangers of inappropriate disposal of plastics. Most studies quantify plastic loads in runoff by sampling litter captured in a variety of filtering systems (e.g. Durrum 1997; Armitage & Roseboom 2000) or in customized nets (table 2). However, one study in Melbourne, Australia, released marked litter items in street-side storm-water drains and then used schoolchildren to locate them on surrounding beaches, thus establishing the link between street litter and beach litter.
Table 2.
Sampling protocols used to assess plastic debris loads in urban runoff by the Algalita Marine Research Foundation. Water-based samples are collected upstream of debris booms, above the influence of the tidal prism.
| habitat sampled | collection device | net aperture (m) | mesh size (mm) |
|---|---|---|---|
| surface; mid-stream | manta trawl | 0.9 × 0.15 | 0.33 |
| surface, edge | hand net | 0.46 × 0.25 | 0.5 or 0.8 |
| mid-water | weighted net | 0.46 × 0.25 | 0.33 |
| bed load | streambed sampler | 0.15 × 0.15 | 0.33 |
| bottom sediments | scoop | 15 l bucketa | — |
aFifteen litres of bottom sediments are collected to a depth of 100 mm; in cement-lined canals, samples are collected where the concrete bottom terminates and natural bottom begins.
To date, most studies of litter in urban runoff have focused on macro-debris (e.g. Marais & Armitage 2004; Marais et al. 2004). The most comprehensive survey of small plastic litter has been conducted by the Algalita Marine Research Foundation (AMRF) in southern California (www.algalita.org; table 2), which recorded up to 81 g m−3 of small plastic items in storm-water discharges. A key challenge is to cope with the great temporal heterogeneity in plastic loads linked to rainfall events. Litter loads build up between rain events and are then flushed into the receiving water body. Sampling high-flow events is complicated by clogging of sampling nets or grids. Passive litter traps risk blocking drains or losing samples through the tearing of nets. Surveys of litter on land can also provide useful information on debris abundance. The AMRF conducted site inspections of plastic converters in the Los Angeles basin to assess pellet spillages, then re-surveyed these businesses following the implementation of best management practices. This intervention reduced pellet spillage by 80 per cent.
7. Conclusions
Just as multiple initiatives are needed to tackle the marine litter problem (Coe & Rogers 1997), diverse approaches are required to monitor the abundance of plastics in marine environments. For any monitoring programme, the objectives must be clearly stated, the methodology clearly defined and quality control implemented to ensure quality data. Sampling design needs to be cognizant of the dynamics of plastic in the environment. Debris monitoring is complicated by large spatial and temporal heterogeneity in the amounts of plastic debris. Pilot studies should be used to estimate variability in sample data, and then power analysis should assess the numbers of samples necessary to detect a predetermined change.
By selecting beaches at varying distances from major litter sources, beach surveys can provide useful insights into the origins of plastic debris. Monitoring of stranded litter should concentrate on estimating the accumulation rate of debris on beaches, because this gives a measure of the amount of litter at sea. However, this effort adds considerably to the cost of beach surveys. Accumulation rates are sensitive to sampling interval; frequent sampling reduces biases owing to rapid debris turnover, but estimates are more variable than longer sampling intervals, requiring greater sampling effort. At-sea sampling is also extremely expensive, but net-based samples suggest that there have been marked increases in plastic litter in accumulation zones in oceanic gyres. Seabirds that accumulate plastic in their stomachs provide an inexpensive, powerful tool to monitor changes in the abundance and composition of small plastic debris at regional scales. Monitoring the incidence of entanglement also is useful, provided that search effort is constant and that interpretation acknowledges changes in the population size of affected organisms.
Assessing the magnitude of debris sources is complicated by the large number of point and diffuse sources of plastic debris (figure 1). However, this approach has the advantage of assessing directly whether specific mitigation efforts are having the desired effect. Dumping of ship-based litter can be assessed by independent observers on vessels and by monitoring the use of port reception facilities. Land-based sources can be monitored by sampling rivers, storm-water run-off and other key sources. In tandem with appropriate education programmes, measurement of sources can be effective in promoting changes in disposal practices that ultimately reduce the amounts of plastic entering the environment.
Acknowledgements
We thank our colleagues and many volunteers who assisted with data collection and tolerated the sometimes smelly business of collecting beached birds and sorting litter. P.G.R. and C.L.M. received financial support from the National Research Foundation and the Plastics Federation of South Africa. C.J.M. and the Algalita Foundation received financial support for ocean sampling aboard ORV Alguita from the Will J. Reid Foundation. Fulmar monitoring in The Netherlands was commissioned by the Ministry of Transport, Public Works and Water Management (VenW) and is possible thanks to volunteer assistance from the Dutch Seabird Group (NZG). North Sea wide monitoring was established by EU Interreg support and is continued with funds from NYK Group Europe Ltd and Chevron Upstream Europe.
Footnotes
One contribution of 15 to a Theme Issue ‘Plastics, the environment and human health’.
References
- Andrady A. L. (ed.) 2003Plastics and the environment. New York: Wiley [Google Scholar]
- Armitage N., Roseboom A.2000The removal of urban litter from stormwater conduits and streams: paper 1—the quantities involved and catchment litter management options. Water SA 26, 181–187 [Google Scholar]
- Arnould J. P. Y., Croxall J. P.1995Trends in entanglement of Antarctic fur seals (Arctocephalus gazella) in man-made debris at South Georgia. Mar. Pollut. Bull. 30, 707–712 (doi:10.1016/0025-326X(95)00054-Q) [Google Scholar]
- Barnes D. K. A.2002Invasions by marine life on plastic debris. Nature 416, 808–809 (doi:10.1038/416808a) [DOI] [PubMed] [Google Scholar]
- Barnes D. K. A., Galgani F., Thompson R. C., Barlaz M.2009Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998 (doi:10.1098/rstb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland R. C., Donohue M. J.2003Marine debris accumulation in the nearshore marine habitat of the endangered Hawaiian monk seal, Monachus schauinslandi 1999–2001. Mar. Pollut. Bull. 46, 1385–1394 (doi:10.1016/S0025-326X(03)00291-1) [DOI] [PubMed] [Google Scholar]
- Bowman D., Manor-Samsonov N., Golik A.1998Dynamics of litter pollution on Israeli Mediterranean beaches: a budgetary, litter flux approach. J. Coastal Res. 14, 418–432 [Google Scholar]
- Buckland S. T., Anderson D. R., Burnham K. P., Laake J. L.1993Distance sampling: estimating abundance of biological populations. London, UK: Chapman and Hall [Google Scholar]
- Camphuysen C. J.2001Northern Gannets Morus bassanus found dead in the Netherlands, 1970–2000. Atlantic Seabirds 3, 15–30 [Google Scholar]
- Carpenter A., Macgill S. M.2005The EU Directive on port reception facilities for ship-generated waste and cargo residues: the results of a second survey on the provision and uptake of facilities in North Sea ports. Mar. Pollut. Bull. 50, 1541–1547 (doi:10.1016/j.marpolbul.2005.06.021) [DOI] [PubMed] [Google Scholar]
- Carpenter E. J., Anderson S. J., Harvey G. R., Miklas H. P., Peck B. B.1972Polystyrene spherules in coastal waters. Science 178, 749–750 (doi:10.1126/science.178.4062.749) [DOI] [PubMed] [Google Scholar]
- Cheshire A. C., et al. UNEP/IOC Guidelines on survey and monitoring of marine litter. 2009 UNEP Regional Seas Rpts & Studies, No. 186; IOC Tech. Ser. No. 83. [Google Scholar]
- Coe J. M., Rogers D. B. (eds) 1997Marine debris: sources, impacts, and solutions. New York, NY: Springer-Verlag [Google Scholar]
- Dameron O. J., Parke M., Albins M. A., Brainard R.2007Marine debris accumulation in the northwestern Hawaiian Islands: an examination of rates and processes. Mar. Pollut. Bull. 54, 423–433 (doi:10.1016/j.marpolbul.2006.11.019) [DOI] [PubMed] [Google Scholar]
- Day R. H., Shaw D. G.1987Patterns of abundance of pelagic plastic and tar in the North Pacific Ocean, 1976–1985. Mar. Pollut. Bull. 18, 311–316 (doi:10.1016/S0025-326X(87)80017-6) [Google Scholar]
- Day R. H., Wehle D. H. S., Coleman F. C.1985Ingestion of plastic pollutants by marine birds. In Proc. Workshop on the Fate and Impact of Marine Debris (eds Shomura R. S., Yoshida H. O.), pp. 344–386 US Dept Commerce: NOAA Tech. Mem., NOAA-TM-NMFS-SWFSC-54 [Google Scholar]
- Day R. H., Shaw D. G., Ignell S. E.1990aThe quantitative distribution and characteristics of marine debris in the North Pacific Ocean, 1984–1988. In Proc. Second International Conference on Marine Debris (eds Shomura R. S., Godfrey M. L.), pp. 182–211 US Dept Commerce: NOAA Tech. Mem., NOAA-TM-NMFS-SWFSC-154 [Google Scholar]
- Day R. H., Shaw D. G., Ignell S. E.1990bThe quantitative distribution and characteristics of neuston plastic in the North Pacific Ocean, 1984–1988. In Proc. Second International Conference on Marine Debris (eds Shomura R. S., Godfrey M. L.), pp. 247–266 US Dept Commerce: NOAA Tech. Mem.,NOAA-TM-NMFS-SWFSC-154 [Google Scholar]
- de Araújo M. C. B., Santos P. J. P., Costa M. F.2006Ideal width of transects for monitoring source-related categories of plastics on beaches. Mar. Pollut. Bull. 52, 957–961 [DOI] [PubMed] [Google Scholar]
- Derraik J. G. B.2002The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 44, 842–852 (doi:10.1016/S0025-326X(02)00220-5) [DOI] [PubMed] [Google Scholar]
- Dixon T. R., Dixon T. J.1981Marine litter surveillance. Mar. Pollut. Bull. 12, 289–295 (doi:10.1016/0025-326X(81)90078-3) [Google Scholar]
- Donohue M., Boland R. C., Sramek C. M., Antonelis G. A.2001Derelict fishing gear in the northwestern Hawaiian Islands: diving surveys and debris removal in 1999 confirm threat to coral reef ecosystems. Mar. Pollut. Bull. 42, 1301–1312 (doi:10.1016/S0025-326X(01)00139-4) [DOI] [PubMed] [Google Scholar]
- Durrum E.1997The control of floating debris in an urban river. In Marine debris: sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 351–358 New York, NY: Springer-Verlag [Google Scholar]
- Edyvane K. S., Dalgetty A., Hone P. W., Higham J. S., Wace N. M.2004Long-term marine litter monitoring in the remote Great Australian Bight, South Australia. Mar. Pollut. Bull. 48, 1060–1075 (doi:10.1016/j.marpolbul.2003.12.012) [DOI] [PubMed] [Google Scholar]
- Escardó-Boomsma J., O'Hara K., Ribic C. A.1995National Marine Debris Monitoring Program, vols 1–2 Washington, DC: US EPA Office of Water [Google Scholar]
- Frost A., Cullen M.1997Marine debris on northern New South Wales beaches (Australia): sources and the role of beach usage. Mar. Pollut. Bull. 34, 348–352 (doi:10.1016/S0025-326X(96)00149-X) [Google Scholar]
- Galgani F., et al. 2000Litter on the sea floor along European coasts. Mar. Pollut. Bull. 40, 516–527 (doi:10.1016/S0025-326X(99)00234-9) [Google Scholar]
- Galil B. S., Golik A., Türkay M.1995Litter at the bottom of the sea: a sea bed survey in the eastern Mediterranean. Mar. Pollut. Bull. 30, 22–24 (doi:10.1016/0025-326X(94)00103-G) [Google Scholar]
- Garrity S. D., Levings S. C.1993Marine debris along the Caribbean coast of Panama. Mar. Pollut. Bull. 26, 317–324 (doi:10.1016/0025-326X(93)90574-4) [Google Scholar]
- Goldsmith F. B. (ed.) 1991Monitoring for conservation and ecology London, UK: Chapman & Hall [Google Scholar]
- Gregory M. R.2009Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Phil. Trans. R. Soc. B 364, 2013–2025 (doi:10.1098/rstb.2008.0265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M. R., Andrady A. L.2003Plastics in the marine environment. In Plastics and the environment (ed. Andrady A. L.), pp. 379–402 New York, NY: Wiley [Google Scholar]
- Gregory M. R., Ryan P. G.1997Pelagic plastics and other seaborne persistent synthetic debris: a review of Southern Hemisphere perspectives. In Marine debris: sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 49–66 New York, NY: Springer-Verlag [Google Scholar]
- Hanni K. D., Pyle P.2000Entanglement of pinnipeds in synthetic materials at South-east Farallon Island, California, 1976–1998. Mar. Pollut. Bull. 40, 1076–1081 (doi:10.1016/S0025-326X(00)00050-3) [Google Scholar]
- Harper P. C., Fowler J. A.1987Plastic pellets in New Zealand storm-killed prions (Pachyptila spp.), 1958–1977. Notornis 34, 65–70 [Google Scholar]
- Hartwig E., Clemens T., Heckroth M.2007Plastic debris as nesting material in a Kittiwake (Rissa tridactyla) colony at the Jammerbugt, Northwest Denmark. Mar. Pollut. Bull 54, 595–597 (doi:10.1016/j.marpolbul.2007.01.027) [DOI] [PubMed] [Google Scholar]
- Henderson J. R.2001A pre- and post-MARPOL Annex V summary of Hawaiian Monk Seal entanglements and marine debris accumulation in the northwestern Hawaiian Islands, 1982–1998. Mar. Pollut. Bull. 42, 584–589 (doi:10.1016/S0025-326X(00)00204-6) [DOI] [PubMed] [Google Scholar]
- Jones M. M.1995Fishing debris in the Australian marine environment. Mar. Pollut. Bull. 30, 25–33 (doi:10.1016/0025-326X(94)00108-L) [Google Scholar]
- Józwiak T.2005Tendencies in the numbers of beverage containers on the Polish coast in the decade from 1992 to 2001. Mar. Pollut. Bull. 50, 87–90 (doi:10.1016/j.marpolbul.2004.11.011) [DOI] [PubMed] [Google Scholar]
- Kenyon K. W., Kridler E.1969Laysan Albatross swallow indigestible matter. Auk 86, 339–343 [Google Scholar]
- Klemchuk P. P.1990Degradable plastics: a critical review. Polym. Degrad. Stab. 27, 183–202 (doi:10.1016/0141-3910(90)90108-J) [Google Scholar]
- Kusui T., Noda M.2003International survey on the distribution of stranded and buried litter on beaches along the Sea of Japan. Mar. Pollut. Bull. 47, 175–179 (doi:10.1016/S0025-326X(02)00478-2) [DOI] [PubMed] [Google Scholar]
- Laist D. W.1997Impacts of marine debris: entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In Marine debris: sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 99–140 New York, NY: Springer-Verlag [Google Scholar]
- Lattin G. L., Moore C. J., Zellers A. F., Moore S. L., Weisberg S. B.2004A comparison of neustonic plastic and zooplankton at different depths near the southern Californian shore. Mar. Pollut. Bull. 49, 291–294 (doi:10.1016/j.marpolbul.2004.01.020) [DOI] [PubMed] [Google Scholar]
- Lecke-Mitchell K. M., Mullin K.1992Distribution and abundance of large floating plastic in the north-central Gulf of Mexico. Mar. Pollut. Bull. 24, 598–601 (doi:10.1016/0025-326X(92)90279-F) [Google Scholar]
- Madzena A., Lasiak T.1997Spatial and temporal variations in beach litter on the Transkei coast of South Africa. Mar. Pollut. Bull. 34, 900–907 (doi:10.1016/S0025-326X(97)00052-0) [Google Scholar]
- Mallory M. L., Robertson G. J., Moenting A.2006Marine plastic debris in Northern Fulmars from Davis Straight, Nunavut, Canada. Mar. Pollut. Bull. 52, 813–815 (doi:10.1016/j.marpolbul.2006.04.005) [DOI] [PubMed] [Google Scholar]
- Marais M., Armitage N.2004The measurement and reduction of urban litter entering stormwater drainage systems: paper 2—strategies for reducing the litter in the stormwater drainage systems. Water SA 30, 469–482 [Google Scholar]
- Marais M., Armitage N., Wise C.2004The measurement and reduction of urban litter entering stormwater drainage systems: paper 1—quantifying the problem using the City of Cape Town as a case study. Water SA 30, 483–492 [Google Scholar]
- Matsumura S., Nasu K.1997Distribution of floating debris in the North Pacific Ocean: sighting surveys 1986–1991. In Marine debris: sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 15–24 New York, NY: Springer-Verlag [Google Scholar]
- McDermid K. J., McMullen T. L.2004Quantitative analysis of small-plastic debris on beaches in the Hawaiian archipelago. Mar. Pollut. Bull. 48, 790–794 (doi:10.1016/j.marpolbul.2003.10.017) [DOI] [PubMed] [Google Scholar]
- Moore C. J.2008Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ. Res. 108, 131–139 (doi:10.1016/j.envres.2008.07.025) [DOI] [PubMed] [Google Scholar]
- Moore S. L., Allen M. J.2000Distribution of anthropogenic and natural debris on the mainland shelf of the southern California Bight. Mar. Pollut. Bull. 40, 83–88 (doi:10.1016/S0025-326X(99)00175-7) [Google Scholar]
- Moore C. J., Moore S. L., Leecaster M. K., Weisberg S. B.2001A comparison of plastic and plankton in the North Pacific central gyre. Mar. Pollut. Bull. 42, 1297–1300 (doi:10.1016/S0025-326X(01)00114-X) [DOI] [PubMed] [Google Scholar]
- Moore S. L., Gregorio D., Carreon M., Weisberg S. B., Leecaster M. K.2001Composition and distribution of beach debris in Orange County, California. Mar. Pollut. Bull. 42, 241–245 (doi:10.1016/S0025-326X(00)00148-X) [DOI] [PubMed] [Google Scholar]
- Morishige C., Donohue M. J., Flint E., Swenson C., Woolaway C.2007Factors affecting marine debris deposition at French Frigate Shoals, Northwestern Hawaiian Islands Marine National Monument, 1990–2006. Mar. Pollut. Bull. 54, 1162–1169 (doi:10.1016/j.marpolbul.2007.04.014) [DOI] [PubMed] [Google Scholar]
- Moser M. L., Lee D. S.1992A fourteen-year survey of plastic ingestion by western North Atlantic seabirds. Colon. Waterbirds 15, 83–94 (doi:10.2307/1521357) [Google Scholar]
- Nagelkerken I., Wiltjer G. A. M. T., Debrot A. O., Pors J. L. P. J.2001Baseline study of submerged marine debris at beaches in Curacao, West Indies. Mar. Pollut. Bull. 42, 786–789 (doi:10.1016/S0025-326X(01)00091-1) [DOI] [PubMed] [Google Scholar]
- Ng K. L., Obbard J. P.2006Prevalence of microplastics in Singapore's coastal marine environment. Mar. Pollut. Bull. 52, 761–767 (doi:10.1016/j.marpolbul.2005.11.017) [DOI] [PubMed] [Google Scholar]
- Ocean Conservancy 2007International Coastal Cleanup Report 2006: a world of difference. Washington, DC, USA: Ocean Conservancy [Google Scholar]
- Ogi H., Baba N., Ishihara S., Shibata Y.1999Sampling of plastic pellets by two types of neuston net and plastic pollution in the sea. Bull. Faculty Fisheries, Hokkaido Univ. 50, 77–91 [Google Scholar]
- OSPAR Commission 2007aOSPAR pilot project on monitoring marine beach litter: monitoring of marine litter on beaches in the OSPAR region .London, UK: OSPAR Commission [Google Scholar]
- OSPAR Commission 2007bBackground report on fishing-for-litter activities in the OSPAR region. London, UK: OSPAR Commission [Google Scholar]
- Page B., et al. 2004Entanglement of Australasian sea lions and New Zealand fur seals in lost fishing gear and other marine debris before and after government and industry attempts to reduce the problem. Mar. Pollut. Bull. 49, 33–42 (doi:10.1016/j.marpolbul.2004.01.006) [DOI] [PubMed] [Google Scholar]
- Pichel W. G., Churnside J. H., Veenstra T. S., Foley D. G., Friedman K. S., Brainard R. E., Nicoll J. B., Zheng Q., Clemente-Colón P.2007Marine debris collects within the North Pacific Subtropical Convergence Zone. Mar. Pollut. Bull. 54, 1207–1211 (doi:10.1016/j.marpolbul.2007.04.010) [DOI] [PubMed] [Google Scholar]
- Pritchard G.1997Plastics additives: an A-Z reference .London, UK: Chapman & Hall [Google Scholar]
- Pruter A. T.1987Sources, quantities and distribution of persisten plastics in the marine environment. Mar. Pollut. Bull. 18, 305–310 (doi:10.1016/S0025-326X(87)80016-4) [Google Scholar]
- Rees G., Pond K.1995Marine litter monitoring programmes—a review of methods with special reference to national surveys. Mar. Pollut. Bull. 30, 103–108 (doi:10.1016/0025-326X(94)00192-C) [Google Scholar]
- Ribic C. A.1998Use of indicator items to monitor marine debris on a New Jersey beach from 1991 to 1996. Mar. Pollut. Bull. 36, 887–891 (doi:10.1016/S0025-326X(98)00064-2) [Google Scholar]
- Ribic C. A., Ganio L. M.1996Power analysis for beach surveys of marine debris. Mar. Pollut. Bull. 32, 554–557 (doi:10.1016/0025-326X(96)84575-9) [Google Scholar]
- Ribic C. A., Dixon T. R., Vining I.1992Marine debris survey manual NOAA Technical Report NMFS 108, US Department of Commerce, Springfield, VA [Google Scholar]
- Ribic C. A., Johnson S. W., Cole C. A.1997Distribution, type, accumulation, and source of marine debris in the US 1989–1993. In Marine debris: sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 35–47 New York, NY: Springer-Verlag [Google Scholar]
- Robards M. D., Gould P. J., Piatt J. F.1997The highest global concentrations and increased abundance of oceanic plastic debris in the North Pacific: evidence from seabirds. In Marine debris: sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 99–140 New York, NY: Springer-Verlag [Google Scholar]
- Ryan P. G.1987The incidence and characteristics of plastic particles ingested by seabirds. Mar. Environ. Res. 23, 175–206 (doi:10.1016/0141-1136(87)90028-6) [Google Scholar]
- Ryan P. G.1988aThe characteristics and distribution of plastic particles at the sea-surface off the southwestern Cape Province, South Africa. Mar. Environ. Res. 25, 249–273 (doi:10.1016/0141-1136(88)90015-3) [Google Scholar]
- Ryan P. G.1988bIntraspecific variation in plastic ingestion by seabirds and the flux of plastic through seabird populations. Condor 90, 446–452 (doi:10.2307/1368572) [Google Scholar]
- Ryan P. G.2008Seabirds indicate decreases in plastic pellet litter in the Atlantic and south-western Indian Ocean. Mar. Pollut. Bull. 56, 1406–1409 (doi:10.1016/j.marpolbul.2008.05.004) [DOI] [PubMed] [Google Scholar]
- Ryan P. G., Cooper J.1989Observer precision and bird conspicuousness during counts of birds at sea. S. Afr. J. Mar. Sci. 8, 271–276 [Google Scholar]
- Ryan P. G., Moloney C. L.1990Plastic and other artefacts on South African beaches: temporal trends in abundance and composition. S. Afr. J. Sci. 86, 450–452 [Google Scholar]
- Ryan P. G., Moloney C. L.1993Marine litter keeps increasing. Nature 361, 23 (doi:10.1038/361023a0)8421491 [Google Scholar]
- Ryan P. G., Swanepoel D.1996Cleaning beaches: sweeping litter under the carpet. S. Afr. J. Sci. 92, 275–276 [Google Scholar]
- Ryan P. G., Watkins B. P.1988Accumulation of stranded plastic objects and other artefacts at Inaccessible Island, central South Atlantic Ocean. S. Afr. J. Antarct. Res. 18, 11–13 [Google Scholar]
- Sheavly S. B.2007National Marine Debris Monitoring Program: final program report, data analysis and summary. Washington, DC, USA: Ocean Conservancy [Google Scholar]
- Shigeru F., Azusa K., Haruyuki K.2006Monitoring marine debris using disposable lighters as an indicator. J. Japan Soc. Waste Manage. Experts 17, 117–124 [Google Scholar]
- Somerville S. E., Miller K. L., Mair J. M.2003Assessment of the aesthetic quality of a selection of beaches in the Firth of Forth, Scotland. Mar. Pollut. Bull. 46, 1184–1190 (doi:10.1016/S0025-326X(03)00126-7) [DOI] [PubMed] [Google Scholar]
- Spear L. B., Ainley D. G., Ribic C. A.1995Incidence of plastic in seabirds from the Tropical Pacific, 1984–1991: relation with distribution of species, sex, age, season, year and body weight. Mar. Environ. Res. 40, 123–146 (doi:10.1016/0141-1136(94)00140-K) [Google Scholar]
- Storrier K. L., McGlashan D. J.2006Development and management of a coastal litter campaign: the voluntary coastal partnership approach. Mar. Policy 30, 189–196 (doi:10.1016/j.marpol.2005.01.002) [Google Scholar]
- Swanepoel D.1995An analysis of beach debris accumulation in Table Bay, Cape Town, South Africa. MSc thesis, University of Cape Town, Cape Town [Google Scholar]
- Thiel M., Hinojosa I., Vásquez N., Macaya E.2003Floating marine debris in coastal waters of the SE-Pacific (Chile). Mar. Pollut. Bull. 46, 224–231 (doi:10.1016/S0025-326X(02)00365-X) [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Olsen Y., Mitchell R. P., Davis A., Rowland S. J., John A. W. G., McGonigle D., Russell A. E.2004Lost at sea: where is all the plastic? Science 304, 838 (doi:10.1126/science.1094559) [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Swan S. H., Moore C. J., vom Saal F. S.2009aOur plastic age. Phil. Trans. R. Soc. B 364, 1973–1976 (doi:10.1098/rstb.2009.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Moore C. J., vom Saal F. S., Swan S. H.2009bPlastics, the environment and human health: current consensus and future trends. Phil. Trans. R. Soc. B 364, 2153–2166 (doi:10.1098/rstb.2009.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP 2005Marine litter, an analytical overview. Nairobi, Kenya: United Nations Environment Programme [Google Scholar]
- van Franeker J. A.1985Plastic ingestion in the North Atlantic Fulmar. Mar. Pollut. Bull. 16, 367–369 [Google Scholar]
- van Franeker J. A., Bell P. J.1985Plastic ingestion by petrels breeding in Antarctica. Mar. Pollut. Bull. 19, 672–674 [Google Scholar]
- van Franeker J. A., et al. 2005. ‘Save the North Sea’ Fulmar Study 2002–2004: a regional pilot project for the Fulmar-Litter-EcoQO in the OSPAR area. Alterra-rapport 1162, Alterra, Wageningen. See www.zeevogelgroep.nl .
- Vauk G. J. M., Schrey E.1987Litter pollution from ships in the German Bight. Mar. Pollut. Bull. 18, 316–319 (doi:10.1016/S0025-326X(87)80018-8) [Google Scholar]
- Velander K., Mocogni M.1998Maritime litter and sewage contamination at Cramond Beach Edinburgh—a comparative study. Mar. Pollut. Bull. 38, 1134–1140 (doi:10.1016/S0025-326X(99)00143-5) [Google Scholar]
- Velander K., Mocogni M.1999Beach litter sampling strategies: is there a ‘best’ method? Mar. Pollut. Bull. 38, 1134–1140 (doi:10.1016/S0025-326X(99)00143-5) [Google Scholar]
- Vlietstra L. S., Parga J. A.2002Long-term changes in the type, but not amount, of ingested plastic particles in short-tailed shearwaters in the southeastern Bering Sea. Mar. Pollut. Bull. 44, 945–955 (doi:10.1016/S0025-326X(02)00130-3) [DOI] [PubMed] [Google Scholar]
- Walker T. R., Reid K., Arnould J. P. Y., Croxall J. P.1997Marine debris surveys at Bird Island, South Georgia 1990–1995. Mar. Pollut. Bull. 34, 61–65 (doi:10.1016/S0025-326X(96)00053-7) [Google Scholar]
- Willoughby N. G., Sangkoyo H., Lakaseru B. O.1997Beach litter: an increasing and changing problem for Indonesia. Mar. Pollut. Bull. 34, 469–478 (doi:10.1016/S0025-326X(96)00141-5) [Google Scholar]
- Yamashita R., Tanimura A.2007Floating plastic in the Kuroshio Current area, western North Pacific Ocean. Mar. Pollut. Bull. 54, 485–488 (doi:10.1016/j.marpolbul.2006.11.012) [DOI] [PubMed] [Google Scholar]