Abstract
In the last decades, the availability of sophisticated analytical chemistry techniques has facilitated measuring trace levels of multiple environmental chemicals in human biological matrices (i.e. biomonitoring) with a high degree of accuracy and precision. As biomonitoring data have become readily available, interest in their interpretation has increased. We present an overview on the use of biomonitoring in exposure and risk assessment using phthalates and bisphenol A as examples of chemicals used in the manufacture of plastic goods. We present and review the most relevant research on biomarkers of exposure for phthalates and bisphenol A, including novel and most comprehensive biomonitoring data from Germany and the United States. We discuss several factors relevant for interpreting and understanding biomonitoring data, including selection of both biomarkers of exposure and human matrices, and toxicokinetic information.
Keywords: human biomonitoring, phthalates, bisphenol A, internal exposure, exposure assessment, risk assessment
1. Introduction
(a). Defining exposure, body burden and biological monitoring of exposure
In industrialized societies, humans are exposed to a wide spectrum of man-made chemicals. Besides occupational exposure scenarios, exposures can also occur through air, dust, water, food and using consumer and personal-care products. From all these sources, chemicals end up in the human body primarily via ingestion, inhalation or dermal absorption. Ambient monitoring aims at determining the variety of external exposure sources to selected environmental chemicals (Clark et al. 2003). By contrast, human biomonitoring1 determines internal exposure (i.e. body burden) by measuring the chemicals, their metabolites or specific reaction products in human specimens (e.g. urine or blood). Biomonitoring represents an integral measure of exposure from multiple sources and routes (Angerer et al. 2006; Needham et al. 2007).
Historically, exposure assessments relied on measuring the chemicals in environmental media and foodstuff; collecting survey/questionnaire data on personal lifestyle, product use and food consumption; calculating estimates of contact times and incorporated quantities; and making pharmacokinetic assumptions based on animal studies. Biomonitoring data permit a new approach to exposure assessment even when the quantity and quality of external exposures are unknown or ambiguous. Biomonitoring data can be used to compare exposures of the general population with special subpopulations and with toxicological animal data. Biomonitoring data also can be used in risk assessment and risk management; risk management, however, will not be discussed in this review. For risk assessment, biomonitoring/biomarker measurements are used to estimate dose, which can then be compared with toxicological parameters normally obtained from animal studies. One key task in interpreting biomonitoring data is to put in perspective exposure data with presumed toxic doses. Therefore, interpretation of human biomonitoring requires interdisciplinary expertise from several fields, including occupational and environmental medicine, toxicology, epidemiology, analytical and bioanalytical chemistry, exposure assessment, industrial hygiene, environmental fate/transport, pharmacology/pharmacokinetics and risk assessment.
The presence of biomarkers of exposure or their absolute concentration in biological specimens is not sufficient to establish whether exposures have reached toxic (or non-negligible) levels per se. In particular, ‘low-level' exposures can be critical when they refer to exposure doses close to the toxic animal doses, whereas ‘high-level' exposures can be regarded as less critical when toxic dose thresholds are considerably higher. Endocrine disrupting/modulating chemicals (EDCs) have generated considerable attention for their possible ‘low-dose' effects in mammals. The term ‘low-dose effects' is defined as ‘biological changes that occur at environmentally relevant exposure levels or at doses that are lower than those typically used in EPA’s standard toxicity testing paradigm' (NTP 2001).
Since the late 1990s, urinary concentrations of environmental chemicals, including EDCs (like phthalates and bisphenol A (BPA)) or their metabolites, have been measured as part of biomonitoring studies or programmes, and used as biomarkers to calculate exposure (doses) of the general population around the world (Blount et al. 2000; Koch et al. 2003b, 2007; Silva et al. 2004; CDC 2005; NTP 2008; Vandenberg et al. 2007; Calafat et al. 2008; Dekant & Voelkel 2008). Based on biomonitoring of exposure, calculated tolerable daily intakes (TDI) of certain endocrine disruptors in mg (kg body weight)−1 (and based on animal experiments) can be exceeded in humans. Therefore, these exposure levels have generated considerable attention for their potential effects in humans, in particular in susceptible subgroups such as pregnant women, children and young adolescents. Literature on phthalates and BPA research is exhaustive and cannot be covered comprehensively in this report (see also Talsness et al. (2009); Meeker et al. (2009); Thompson et al. (2009)). Nevertheless, we have attempted to present and review here the most relevant research on biomarkers of exposure for phthalates and BPA, to include novel biomonitoring data from Germany and the United States, and to interpret these biomonitoring data in terms of exposure and risk assessment.
2. Phthalates and bisphenol A
(a). Their use in plastic manufacture
Phthalates and BPA are long-standing man-made chemicals (used for more than 100 years) produced worldwide in more than 1 million tonnes each year. From an industrial chemist’s perspective, phthalates and BPA do not share many similarities except that their major (though not exclusive) field of application is in plastics manufacture.
The term phthalates describes a class of chemicals that are dialkyl- or alkylarylesters of 1,2-benzenedicarboxylic acid. Their industrial applications are related to the length of their ester chain. Phthalates with alkylchain lengths from 3 to 10 carbons are widely used as general-purpose plasticizers in polymers, primarily in polyvinyl chloride (PVC) resins. Within soft PVC, the plasticizing phthalate content can be up to 40 per cent. Typical products containing phthalates are floorings, roofings, wall coverings and cables, clothing, packaging materials and toys (David et al. 2001; EC 2008). Di-2-ethylhexyl phthalate (DEHP) is the major plasticizer for PVC-containing medical devices such as bags for blood or parenteral nutrition, tubings and catheters (FDA 2001). Because phthalates are not chemically bound to the polymer, they can leach or outgas into the surrounding media. Thus, phthalates can enter the environmental cycles or the human body directly. Phthalates also are used as industrial solvents and lubricants, additives in the textile industry, in pesticide formulations and as components in personal-care products (David et al. 2001; EC 2008). One of them, dibutyl phthalate (DBP), is also used in the pharmaceutical field as a constituent of the enteric coating of some medications (Hauser et al. 2004).
The ubiquitous presence of phthalates in the environment poses an analytical challenge: the phthalate blank problem. Some phthalates are detected even in the cleanest laboratory reagents, sampling equipment and analytical apparatus. These circumstances can hamper the reliable quantification of phthalates in real-life scenarios. As a result, all ambient monitoring data and all data in general related to the measures of low levels of phthalate diesters must be interpreted with caution because of possible external contamination. Early human studies using measures of phthalate diesters (and sometimes also of their hydrolytic monoester metabolites) were limited to highly exposed populations (Ching et al. 1981; Pollack et al. 1985; Dirven et al. 1993) or produced ambiguous results (Colon et al. 2000; McKee 2004). Progress in human biomonitoring has opened new possibilities in assessing phthalate exposures because most of the biomarkers used nowadays are specific metabolites (secondary or oxidized metabolites) that are not prone to contamination.
BPA is used mainly in manufacturing polycarbonate plastic and epoxy resins although other uses (e.g. PVC production and processing) are possible (EU 2003). BPA-derived products can be used in impact-resistant safety equipment and baby bottles, as protective coatings inside metal food containers, and as composites and sealants in dentistry (NTP 2008). Exposure to BPA results primarily from ingesting contaminated food (Kang et al. 2006; Vandenberg et al. 2007). Unlike phthalates, BPA represents a single compound. Further, BPA-produced polymers do not require plasticizers to gain desired flexibility and stability. Human exposure can occur either through the unreacted monomer in the polymer or through remobilized BPA from the final polymer. Therefore, although some phthalates and BPA are produced in rather comparable orders of magnitude, absolute exposure levels for BPA are probably orders of magnitude lower than for (some) phthalates.
(b). Suspected human endocrine disrupters
Phthalates and BPA have received considerable attention because of the various facets of their proven toxicity in animal studies and because of their ubiquitous presence in the environment and in humans.
In 1936, BPA was termed an ‘environmental oestrogen' when it was shown to stimulate the reproductive system by binding to oestrogen receptors in female rats (vom Saal et al. 2007). Exposure to high doses of BPA during pregnancy and/or lactation has adverse developmental effects, including reduced survival, birth weight, growth of offspring early in life and delayed onset of puberty in male and female rodents (NTP 2008). Mechanisms other than binding to the classical oestrogen receptors, which can stimulate cellular responses at low concentrations, also exist (Welshons et al. 2006).
In contrast to BPA, phthalates do not seem to act via direct hormonal mimicking. However, in rodents, some phthalates, namely butylbenzyl phthalate (BBzP), di-iso-butyl phthalate (DiBP), di-n-butyl phthalate (DnBP), dipentyl phthalate (DPeP), DEHP and diisononyl phthalate (DiNP) (Gray & Gangolli 1986; Gray et al. 2000; Borch et al. 2006; Howdeshell et al. 2007), can modulate the endogenous production of foetal testicular testosterone and influence insulin-like factor 3 and follicle-stimulating hormone production (Sharpe & Irvine 2004), resulting in functional and structural impairment of male reproduction and development (Gray et al. 2000; Lee et al. 2004; Tyl et al. 2004; Foster 2006).
3. Premises for human biomonitoring
(a). Selection of the biological matrix
After exposure, environmental chemicals may enter the body, reach the blood systemic circulation and distribute into various body compartments, where they can be in equilibrium with blood concentrations, secretion concentrations or both. To compare concentrations in blood and other matrices, information on partitioning of these chemicals from blood into tissues is needed, as well as basic information on metabolic pathways and on elimination (e.g. via urine or faeces) (Needham et al. 2005).
Blood (or its components) and urine are the most common matrices for biomonitoring. The use of unconventional matrices (e.g. breast milk, meconium, umbilical cord blood, amniotic fluid, seminal fluid, saliva) may be of interest for toxicological considerations (Barr et al. 2005), for assessing prenatal exposures (Whyatt & Barr 2001; Pichini et al. 2004) or for specific purposes (Palmeri et al. 2000; Kintz & Samyn 2002). However, both validated standard operating procedures and published reference ranges are lacking. Also, knowledge on the variability of these matrices related to demographic (e.g. age, gender, race), occupational and personal lifestyle factors is limited, and toxicokinetics are insufficiently described. Therefore, interpretation of biomonitoring data in these alternative matrices is difficult (Barr et al. 2005; Angerer et al. 2007).
Blood is the preferred matrix for biomonitoring of persistent compounds, and it is important when investigating the distribution, elimination and metabolism of non-persistent compounds. However, the relatively short half-lives of non-persistent compounds largely limit the use of blood for biomonitoring purposes at environmental exposure levels. Furthermore, amounts of blood available for analysis are normally limited, and blood collection is complicated and invasive (Needham & Sexton 2000). Nevertheless, information on the blood concentration of the compounds, obtained by using highly sensitive and selective detection techniques (i.e. mass spectrometry), could be useful for risk assessment.
In general, urine is the matrix of choice for biomonitoring of non-persistent chemicals, such as phthalates and BPA, because urinary concentrations of these compounds or their metabolites are higher than blood concentrations. Furthermore, urine is a relatively abundant matrix and its collection is generally simple and non-invasive (Needham & Sexton 2000). Daily urine volume, however, is related to liquid intake, physical exercise and individual health and lifestyle factors. This variability of either ‘diluted' or ‘concentrated' metabolite concentrations in urine has to be taken into consideration when interpreting biomonitoring urinary data. Furthermore, data from the National Health and Nutrition Examination Survey (NHANES), a US nationally representative cross-sectional study, showed significant variations in phthalate metabolite distributions depending on the time of day when urine samples were collected (Silva et al. 2004). Similarly, in the recent NHANES 2003–2004, variations existed in the geometric mean concentrations of BPA depending on the time of day of sample collection (Calafat et al. 2008). These observations, along with the non-persistent nature of phthalates and BPA, may reflect variability in exposures as a result of differences in factors such as diet, lifestyle and using products containing these compounds, which may contribute to the differences observed in urinary concentrations. Because collecting 24 h urine samples is not practical for epidemiological studies, consideration should be given to standardizing the time of sample collection. When the goal is to compare relative exposures to various chemicals across populations or among different studies, collecting first-morning urine samples may be preferred, although for chemicals for which diet is an important source of exposure (e.g. BPA) sampling a few hours after a meal may be preferable. However, studies designed to explore potential health risks of the chemicals should not restrict sample collection to first-morning urine samples because relevant exposure opportunities in the course of a day may be missed and result in exposure misclassification. At minimum, timing of the urine collection always should be recorded.
(b). Selection of biomarkers of exposure
Non-persistent chemicals such as phthalates and BPA are often metabolized to increase their hydrophilic character; both parent compound and metabolites can be excreted unchanged or can undergo phase II biotransformations (e.g. glucuronidation or sulphation). Any of these metabolites may be used as potential biomarkers of exposure.
(i). Bisphenol A
In humans, ingested BPA is not extensively metabolized by phase I modifications but is rapidly conjugated with glucuronic acid. Minor amounts of BPA might also be conjugated with sulphate. As a result, BPA is almost completely excreted in urine as a conjugate (Ye et al. 2005; CERHR 2007; Dekant & Voelkel 2008). After oral administration of a single dose (5 mg abs., 54–88 µg (kg body weight)−1 d−1) of d16-BPA to six adult volunteers, d16-BPA-glucuronide was the only metabolite detected in urine and blood; ingested d16-BPA was nearly completely recovered in urine as d16-BPA-glucuronide within 24 h (Volkel et al. 2002). Nevertheless, a fraction of the absorbed BPA may distribute to body storage site(s) such as adipose tissue, followed by a slow, low-level release of BPA into the bloodstream (Fernandez et al. 2007). BPA-glucuronide, which has a terminal half-life of <6 h (Volkel et al. 2002) and is rapidly excreted in urine, can potentially be used as a biomarker of exposure to BPA (Volkel et al. 2004, 2005; Ye et al. 2005). An important advantage of measuring the concentrations of the glucuronidated metabolite is that it excludes the potential external contamination by free BPA from the environment or BPA-containing materials in the sampling and analytical process. However, identifying and measuring conjugated species is challenging because conjugated standards are not always readily available, and trace-level analysis of conjugated species is especially difficult due to their chemical and physical properties. An alternative approach is to measure the total concentration of the compound (free plus conjugated species) after an enzymatic hydrolysis of the conjugate(s). In the case of BPA, special attention is required to avoid external contamination with the parent compound (Schonfelder et al. 2002; Volkel et al. 2005; Dekant & Voelkel 2008).
Currently, most population-based biomonitoring studies focus on determining the total concentration of BPA in urine (Volkel et al. 2005; Ye et al. 2005; Calafat et al. 2008; Dekant & Voelkel 2008). However, if the free form is the toxicologically active species, the concentrations of free BPA in circulating blood available to interact at the target organ(s) rather than the total urinary concentrations of BPA would be of special public health interest (Volkel et al. 2008). Ideally, in metabolism studies using mass spectrometry detection, stable isotope (D or 13C) labelled standards should be administered to exclude the possibility of external contamination. Both isotope labelled free and conjugated BPA should be determined in the specimens of interest (e.g. blood, tissue, urine) to ensure the highest specificity. Appropriate analytical standards (i.e. d16-BPA and 13C12-BPA) are commercially available; the synthesis of the glucuronides has been described (Volkel et al. 2002).
(ii). Phthalates
Metabolism and elimination of phthalates and, therefore, the selection of appropriate biomarkers as well as their interpretation is complex. In a first rapid step, which can occur at various stages/sites in the body (e.g. mouth or skin, stomach, intestines, blood), the phthalate diester is cleaved into the respective hydrolytic monoester. Therefore, blood concentrations of the intact phthalate diesters would be, if determined correctly, either very low, rather transient, or artifacts of analytical background contamination (Kessler et al. 2001, 2004; Koch et al. 2004a). In a second step, the alkyl chain of the resulting hydrolytic monoester can be modified by various oxidation reactions. In a third step, both the hydrolytic monoester and the oxidized secondary metabolites can be conjugated with glucuronic acid and finally excreted in urine. The extent of oxidative modification increases relative to the alkyl chain length of the phthalate monoester. Oxidative metabolites are more water soluble than the corresponding hydrolytic monoesters, which, in turn, have decreased water solubility when the alkyl chain length increases. Therefore, low-molecular weight phthalates (e.g. diethyl phthalate (DEP) and DBP) mostly metabolize to their hydrolytic monoesters (ATSDR 1995, 2001). By contrast, high-molecular weight phthalates with eight or more carbons in the alkyl chain (e.g. DEHP, DiNP) metabolize to their hydrolytic monoesters, which are extensively transformed to oxidative products (Albro & Lavenhar 1989; ATSDR 1997, 2002; Koch et al. 2005b; Silva et al. 2006a, 2007b; Kato et al. 2007; Koch & Angerer 2007).
Therefore, using the hydrolytic monoester metabolites as sole biomarkers to compare relative exposures can be misleading, especially when comparing the hydrolytic monoester concentrations of high- versus low-molecular weight phthalates (e.g. DBP versus DEHP). While approximately 70 per cent of an oral dose of DBP (four carbons in the alkyl chain) is excreted in urine as the hydrolytic monoester (Anderson et al. 2001), less than 10 per cent of DEHP (eight carbons in the alkyl chain) and less than 2 per cent of DiNP (nine carbons in the alkyl chain) are excreted as the hydrolytic monoesters (Koch et al. 2004a, 2005b; Preuss et al. 2005; Koch & Angerer 2007). Regarding diisodecyl phthalate (DiDP, with 10 carbons in the alkyl chain), no relevant concentrations of the hydrolytic monoester are excreted in urine (Kato et al. 2007; Silva et al. 2007a).
In recent years, research on the oxidative metabolism of phthalates, which initially was largely limited to DEHP and di-n-octyl phthalate, two phthalates with a defined chemical composition (Anderson et al. 2001; Koch et al. 2004a, 2005b; Preuss et al. 2005; Silva et al. 2005, 2006b; Calafat et al. 2006), has made considerable progress. As a result of this research, detailed toxicokinetic data exist on rodent and human metabolism, including metabolic conversion factors, distribution and elimination kinetics, and interspecies comparisons. However, most high-molecular weight phthalates are complex mixtures of isomers (e.g. DiNP, DiDP); their composition varies depending on the nature of the mixture of alcohols used for their synthesis, which, in turn, may vary depending upon the manufacturers or the manufacturing process. Thus, metabolism of isomeric high-molecular weight phthalates will result in multiple hydrolytic and oxidative monoesters. Recent research has focused on identifying and characterizing major isomers of these isomeric phthalates to derive suitable oxidative metabolites for biomonitoring (Calafat et al. 2006; Silva et al. 2006a; Kato et al. 2007; Koch & Angerer 2007). Using custom synthesized reference standards of specific oxidized metabolites of DiNP and DiDP, we are now able to assess human exposure to these phthalates (Calafat et al. 2006; Silva et al. 2006a; Kato et al. 2007; Koch & Angerer 2007; Wittassek & Angerer 2008). Table 1 summarizes selected phthalates and their respective metabolites (as biomarkers of exposure) that are currently being investigated in human biomonitoring studies.
Table 1.
Primary (hydrolytic monoester) and secondary (oxidized monoester) phthalate metabolites used as reference standards in biological monitoring studies to quantify external exposure to the respective parent phthalates.a
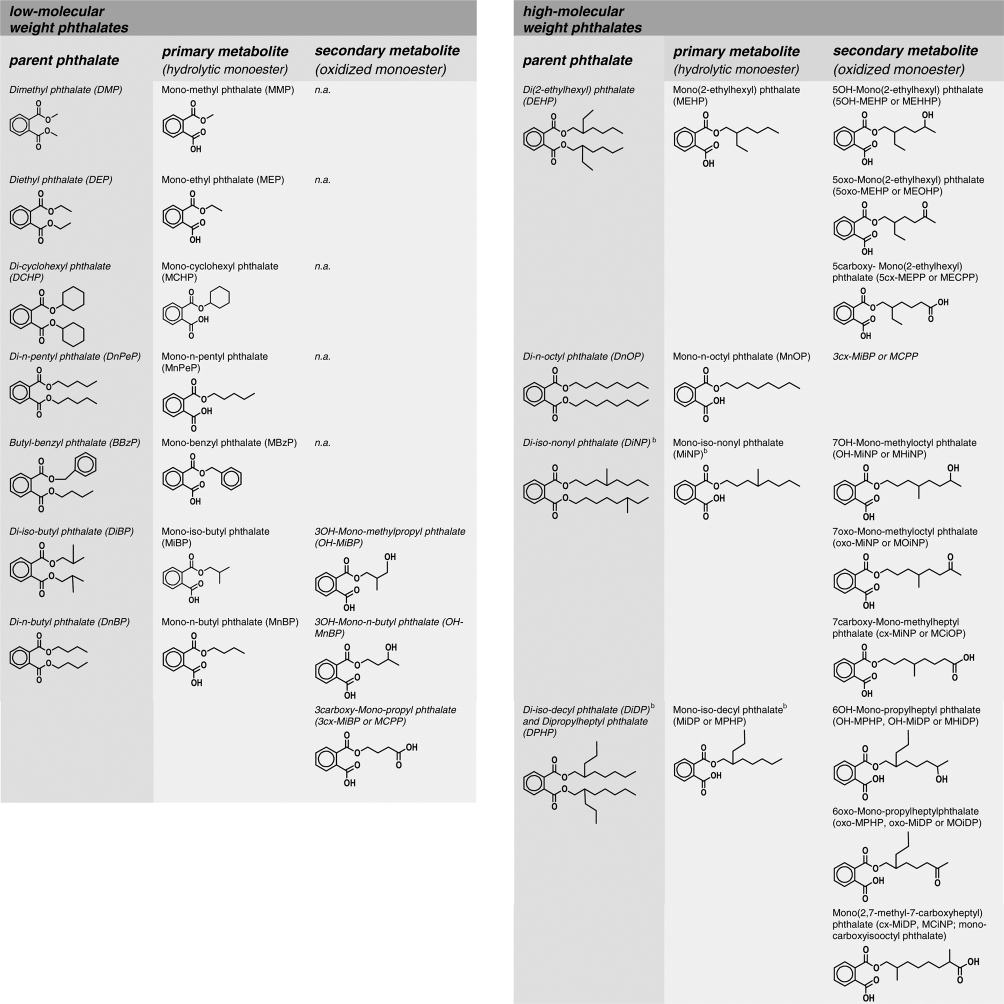 |
aBBzP, DiBP, DnBP, DPeP, DEHP and DiNP show endocrine disrupting/modulating potency in rodent studies (Gray & Gangolli 1986; Gray et al. 2000; Borch et al. 2006; Howdeshell et al. 2007).
bOnly one of a number of possible isomers is shown as an example.
Almost all phthalate diesters and metabolites are available both in their native as well as isotopically labelled (i.e. d4, 13C6 or 13C2) standards for reliable and state-of-the-art-trace analysis. As with BPA, isotopically labelled phthalate diester and metabolite standards are instrumental in metabolism experiments using mass spectrometry detection to exclude the omnipresent phthalate background exposure. Unless the monoesters are isotopically labelled, in blood and matrices other than urine, the concentrations of hydrolytic monoesters even though they can be determined accurately may include an unknown contribution from hydrolysis of contaminant phthalates by endogenous esterases (Kato et al. 2003, 2006; Calafat et al. 2004b; Mose et al. 2007). Therefore, the use of (unlabelled) hydrolytic monoesters as biomarkers of exposure in blood (also umbilical cord blood, placental tissue, mother's milk, amniotic fluid, meconium, saliva) generally should be avoided. This is particularly important when using archived specimens because details of the collection procedure (to ensure the absence of external contamination) may be unknown and treatment of the matrix with acid at the time of sample collection (to quench the enzymatic activity) may not have been feasible or recommended because, for example, of safety concerns. In these cases, biomonitoring results based on hydrolytic phthalate monoesters data have to be interpreted with caution or should, when possible, be verified with studies based on isotope labelled standards.
4. Biomonitoring programmes
Biomonitoring programmes are useful for investigating human exposure to phthalates and BPA, among other chemicals. One of these programmes, NHANES, is conducted annually in the United States by the Centers for Disease Control and Prevention and is designed to collect data on the health and nutritional status of the non-institutionalized, civilian US population (CDC 2003). The survey includes a physical examination, collecting a detailed medical history and collecting biological specimens (i.e. blood from participants 1 year of age or older and urine from participants ≥6 years old). Although biological specimens are used mostly for clinical and nutritional testing, some can be used to assess exposure to environmental chemicals. Data estimates from NHANES are probability based, and are representative of the US population. Therefore, NHANES data can be used to establish reference ranges for selected chemicals, provide exposure data for risk assessment (e.g. set intervention and research priorities, evaluate effectiveness of public health measures) and monitor exposure trends. Reference ranges can be used to assist epidemiologic investigations, to correlate the levels to other NHANES parameters/measurements (including potential health effects), and to identify (i) populations with the highest exposures, (ii) potential sources/routes of exposure, and (iii) chemicals with highest prevalence/frequency (Pirkle et al. 1995).
The German Environmental Surveys (GerESs), large-scale representative population studies for assessing the exposure of the German general population to environmental chemicals (e.g. lead, mercury, pentachlorophenol, PAHs, cotinine), have been conducted since the mid-1980s using questionnaires, human biomonitoring and both indoor and outdoor environmental samplings (http://www.umweltbundesamt.de/survey-e/index.htm). While previous surveys focused mostly on adults’ exposures, the ongoing GerES IV provides biomontioring data on children between 3 and 14 years of age. GerES IV is conducted in cooperation with the National Health Survey for Children and Adolescents by the Robert Koch Institute. Using data of the environmental and health surveys, it is possible to evaluate relations between environmental conditions and children’s health. In addition, the GerES IV study design also enables the direct comparison between ambient, biomonitoring and questionnaire data.
Even these comprehensive programmes have limitations: persons under 1 (NHANES) or 3 (GerES) years of age and older than 60 (NHANES) or 79 (GerES) years of age are not included for biomonitoring assessments, and no data are collected on foetal exposures. Therefore, a pressing need exists for assessing exposure during critical periods of development, a period of increased susceptibility to the potential adverse effects of environmental chemicals. Furthermore, these surveys, by design, are cross-sectional and do not intentionally include population groups that might be highly exposed to various point sources and could be examined to evaluate possible associations between high exposures and adverse health effects.
5. Data on internal exposures (body burden)
Since the late 1990s, there have been numerous reports suggesting that exposure to phthalates among the general population around the globe is widespread (Blount et al. 2000; Brock et al. 2002; Koch et al. 2003b, 2004b, 2005c; Becker et al. 2004; Silva et al. 2004; CDC 2005, 2008; Jonsson et al. 2005; Swan et al. 2005; Hauser et al. 2006; Adibi et al. 2008; Högberg et al. 2008; Sathyanarayana et al. 2008). Differences in exposure, as reflected by the concentrations of phthalate metabolites (mostly in urine), are evident both based on geographic and demographic variables.
For example, in a subset of samples collected for the GerES IV pilot study, younger children showed significantly higher internal exposures to the DEHP, DBP and BBzP metabolites than older children (Becker et al. 2004; Koch et al. 2007). Moreover, house dust concentrations of DEHP from the children’s homes and urine DEHP metabolite concentrations were not correlated. Also, urinary DEHP metabolite concentrations among children from households with PVC wall coverings or floorings were not significantly different from those among other children (Becker et al. 2004). These data suggest that neither phthalate plasticized PVC material in the children’s surroundings nor the actual concentration of DEHP in household dust (an often stated hypothesis) are the main contributors to the DEHP body burden in children. Foodstuff seems to be the major route of exposure to DEHP and the other high-molecular weight phthalates in adults. In a study involving 50 volunteers over 7 days, estimated DEHP intakes from the urinary concentrations correlated significantly with dietary DEHP intake from the day before, and the dietary intake calculated from food duplicates could quantitatively explain the internal DEHP exposure (Fromme et al. 2007). Nevertheless, for the youngest children, additional or specific phthalate exposure routes and sources might be of importance due to children’s behavioural patterns (e.g. crawling, mouthing habits). For the low-molecular weight phthalates, which can be found in personal-care products, associations between product use and hydrolytic monoester excretion both in children and adults have been reported (Duty et al. 2005; Sathyanarayana et al. 2008). Use of medications or nutritional supplements containing DBP in the enteric coating can also be a source of significant exposure (Hauser et al. 2004).
Interestingly, oxidative phthalate metabolism seems to be slightly favoured in neonates and young children compared with adults (Koch et al. 2006; Calafat & Needham 2008). This finding warrants additional research because the bioactivity of oxidized metabolites remains unclear. For the analytical chemist, this finding underlines the importance of measuring both hydrolytic and oxidative monoester metabolites whenever possible; for the biostatistician or epidemiologist, it stresses the need to include all metabolites for an integral interpretation; and for the toxicologist, it may shed light into possible mechanism(s) and mode(s) of action. When using phthalate metabolites to categorize human exposures, the preferred approach is to include both hydrolytic and oxidative monoester metabolites.
However, the wealth of biomonitoring data from general population studies does not suggest significant intra- or inter-individual differences in the metabolism of phthalates. The ratios of urinary concentrations of hydrolytic and oxidative metabolites are highly stable and comparable in all populations investigated. Therefore, the variations observed among metabolites of the same parent phthalate are probably more dependent on the time of exposure and the differences in elimination kinetics of the various metabolites than in significant polymorphisms of metabolizing enzymes (Koch et al. 2006).
In table 2, we present a brief (and not comprehensive) overview of biomonitoring urinary data on phthalate exposure in the United States and Germany. Our intent is to illustrate the rapid progress in biomarker development, point out potential differences in phthalate exposure in these two countries and also to discuss possible temporal changes in exposure. Since Blount et al. (2000) published their groundbreaking study on levels of seven urinary monoester metabolites in 289 US adults, the phthalate metabolite spectrum has steadily increased to up to 23 metabolites (as biomarkers of exposure to 11 phthalates). Moreover, not only the quantity but also the type of phthalate metabolites examined has evolved to include an increasing number of oxidative monoester metabolites in addition to the simple hydrolytic monoesters. Oxidative metabolites are of special importance for assessing exposure to the high-molecular weight phthalates. As an example, while NHANES data from 1999 to 2004 on the DiNP hydrolytic monoester mono-isononyl phthalate suggested that exposure to DiNP was non-existent (Silva et al. 2004; CDC 2005, 2008), the oxidative monoesters data suggest widespread human exposure both in the United States and Germany not only to DiNP but also DiDP (Silva et al. 2006c, 2007a; Wittassek & Angerer 2008). Oxidative metabolites of DnBP and DiBP, currently under investigation, may be a valuable complement to the hydrolytic monoesters, even though for these four carbon alkyl chain phthalates the monoester represents the main urinary metabolite (Silva et al. 2007b).
Table 2.
Selected biomonitoring data on phthalate exposure in the United States and Germany (median (95th percentile); concentrations are reported in µg l−1).
| study | Silva et al. (2004)a | CDC (2005)a | Silva et al. (2006c, 2007a,b) | Becker et al. (2004); Koch et al. (2007b) | Wittassek et al. (2007b) | unpublished data | |
| sample origin | NHANES | NHANES | NHANES | GerES | ESBHum | Germany | |
| n | 2541 | 2782 | 2605/129 | 254 | 60 | 45 | |
| age | ≥6 years | ≥6 years | ≥6 yearsb; DiDP and DiNP: adults | 3–14 years | 20–29 years | adults | |
| urine | spot | spot | spot | first morning | 24 h | first morning | |
| sampling year | 1999–2000 | 2001–2002 | 2003–2004 | 2001–2002 | 2003 | 2007 | |
| DiDP/DPHP | OH-MiDP | — | — | 4.9 (70.6) | — | — | 1.0 (4.0) |
| oxo-MiDP | — | — | 1.2 (15.0) | — | — | 0.2 (1.1) | |
| cx-MiDP | — | — | 4.4 (104.4) | — | — | 0.7 (2.6) | |
| MiDP | — | — | <LOQ (<LOQ) | — | — | — | |
| DiNP | OH-MiNP | — | — | 13.2 (43.7) | — | 2.3 (13.3) | 4.7 (16.8) |
| oxo-MiNP | — | — | 1.2 (6.6) | — | 1.6 (10.4) | 1.7 (6.7) | |
| cx-MiNP | — | — | 8.4 (46.2) | — | — | 5.3 (15.5) | |
| MiNP | <LOQ (3.5) | <LOQ (<LOQ) | <LOQ (<LOQ)b | — | — | <LOQ (<LOQ) | |
| DEHP | 5OH-MEHP | — | 20.1 (192) | 21.1 (266)b | 52.1 (188) | 13.4 (38.8) | 13.9 (42.9) |
| 5oxo-MEHP | — | 14.0 (120) | 14.4 (157)b | 41.4 (139) | 12.2 (34.9) | 8.2 (21.5) | |
| 5cx-MEPP | — | — | 33.0 (337)b | — | 17.5 (60.6) | 11.5 (35.0) | |
| MEHP | 3.2 (23.8) | 4.1 (38.9) | 1.9 (31.0)b | 7.2 (29.7) | 4.6 (25.2) | 1.8 (8.5) | |
| DnOP | MnOP | <LOQ (2.9) | <LOQ (<LOQ) | <LOQ (<LOQ)b | <LOQ (<LOQ) | — | <LOQ (<LOQ) |
| DnBP | MnBP | 26.0 (149) | 20.4 (108) | 23.2 (121)b | 166 (624) | 50.8 (161) | 12.6 (43.5) |
| 3OH-MnBP | — | — | — | — | — | 1.8 (4.5) | |
| MCPP | — | 3.0 (14.6) | 3.1 (15.2)b | — | — | 0.7 (3.1) | |
| DiBP | MiBP | — | 2.6 (17.9) | 4.1 (21.3)b | — | 30.4 (124) | 22.4 (101) |
| OH-MiBP | — | — | — | — | — | 13.8 (62.4) | |
| DcHP | McHP | <LOQ (1.0) | <LOQ (0.4) | <LOQ (0.2)b | — | — | <LOQ (<LOQ) |
| BBzP | MBzP | 15.3 (103) | 15.7 (122) | 14.3 (101)b | 18.8 (123) | 5.9 (24.8) | 2.5 (8.4) |
| DPeP | MPeP | — | — | — | — | — | < LOQ (0.3) |
| DEP | MEP | 164 (2840) | 169 (2500) | 174 (2660)b | — | — | 77.5 (396) |
| DMP | MMP | — | 1.5 (9.8) | 1.2 (16.3)b | — | — | <LOQ (17.2) |
aCDC Third National Report on Human Exposure to Environmental Chemicals.
bNational Health and Nutrition Examination Survey. 2003–2004 Laboratory Files. Lab 24 Urinary Phthalates. See http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/lab03_04.htm (accessed 12 February 2008).
Important differences in study design (e.g. seasonal versus throughout-the-year collection, first-morning voids versus non-first-morning voids, convenience sampling versus nationally representative sampling) exist for the United States and German data presented in table 2. Therefore, comparisons must be conducted with caution. Nevertheless, these data suggest that phthalate exposures are within the same order of magnitude in the two countries, although some differences in the exposure to certain phthalates may exist. For example, exposure to DEP and BBzP appears to be somewhat higher in the United States than in Germany, while exposure to DiBP is higher in Germany. Furthermore, in the United States the levels of exposure appear to be rather similar during the time window investigated (1999–2004). By contrast, in Germany, DEHP, DnBP and BBzP exposures seem to be declining, while DiNP exposure appears to be on the rise. These latter observations are in agreement with recent developments in Europe regarding toxic labelling and use restrictions for some phthalates, and with a rapid shift in production volumes of various phthalates (e.g. decreased production of DEHP and increased production of DiNP and DiDP/DPHP) (EU 2004, 2005, 2006; Helm 2007).
Like for the phthalates, in the last decade, data on the urinary concentrations of BPA in selected populations of various countries, including NHANES in the United States, have become available (Ouchi & Watanabe 2002; Kim et al. 2003; Matsumoto et al. 2003; Yang et al. 2003; Arakawa et al. 2004; Calafat et al. 2005; Liu et al. 2005; Volkel et al. 2005; Miyamoto & Kotake 2006; Yang et al. 2006; NTP 2008; Vandenberg et al. 2007; Wolff et al. 2007; Dekant & Voelkel 2008). These data suggest that human exposure to BPA is widespread (NTP 2008 and references therein), but the urinary concentrations of BPA measured in several populations show some variation. BPA was detected in about 93 per cent of the samples collected from NHANES 2003–2004 participants ≥6 years of age; the geometric mean and 95th percentile concentrations were 2.6 µg l−1 (2.6 µg (g creatinine)−1) and 15.9 µg l−1 (11.2 µg (g creatinine)−1), respectively (Calafat et al. 2008). BPA-glucuronide was detected in all samples collected from 48 female Japanese college students; the median concentration was 1.2 µg l−1 (0.77 µg (g creatinine)−1) (Ouchi & Watanabe 2002). By contrast, concentrations of BPA in 7 males and 12 females in Germany were <1.14 µg l−1 (Volkel et al. 2005), whereas the geometric mean concentration of BPA in a group of 73 adult Koreans was 9.54 µg l−1 (8.91 µg (g creatinine)−1) (Yang et al. 2003). Differences in the exposure to BPA may exist geographically, but the differences could also be caused, at least in part, by differences in study design.
In summary, NHANES, GerES and biomonitoring data from other studies suggest that exposure to both BPA and phthalates is widespread although important differences across selected demographic groups exist. Of interest, young children have higher concentrations of most phthalate metabolites (except monoethyl phthalate) and BPA than adolescents and adults (Koch et al. 2003b, 2007; Silva et al. 2004; CDC 2005, 2008; Wittassek et al. 2007a; Calafat et al. 2008). This finding can be explained by the children’s higher food consumption or air inhalation, or both, in relation to their weight than those of adolescents or adults. The differences also could relate to differences in exposure pathways or in absorption, distribution, metabolism or excretion of these environmental chemicals. All these biomonitoring findings highlight the need for additional research to identify the sources and routes of exposure to BPA and phthalates and the need for epidemiologic studies to target health outcomes, especially in susceptible populations (e.g. children, pregnant women).
6. Estimating daily intakes
Historically, calculations of exposure estimates for phthalates and BPA were generally indirect (i.e. relying on surveys of product use, measuring the concentrations of these chemicals in various media, estimating human contact and pharmacokinetic assumptions based on animal data). With the advent of biomonitoring, direct methods using urinary concentrations of BPA or phthalate metabolites as biomarkers of exposure are routine. These methods may provide the most accurate assessments because biomonitoring data represent an integrative measure of exposure from multiple sources and routes.
The average concentrations of BPA in selected populations worldwide are quite similar (i.e. in the low µg l−1 range for the sum of free plus conjugated (total) species of BPA), although some variability exists (CERHR 2007; Vandenberg et al. 2007; Calafat et al. 2008; Dekant & Voelkel 2008). From these urinary data, estimated mean exposures are in the range of 0.01–0.05 µg kg−1 d−1 for adults and somewhat higher (∼0.07 µg kg−1 d−1) for children (CERHR 2007; Dekant & Voelkel 2008); estimated worst-case exposures for adults are 0.1–0.13 µg kg−1 d−1 (Dekant & Voelkel 2008). Indirect estimates of mean exposure to BPA range from <1 to ∼10 µg kg−1 d−1 (CERHR 2007). This comparison suggests that both indirect and biomarker-based methods produced BPA mean estimates below the current US EPA reference dose (RfD) and the European Union TDI (both 50 µg kg−1 d−1). Biomonitoring data are unavailable for infants. However, for children 6 years of age or older, NHANES 2003–2004 data suggest that ambient exposures to BPA, although about three times higher than for adults, are lower than the RfD and TDI.
An integral part of daily intake estimation based on phthalates biomonitoring data is the need for reliable urinary excretion fractions (fue) for each metabolite. The fue are related to the ingested amount of the parent phthalate over 24 h after oral application (table 3). For these calculations, the extrapolation from using urinary concentration data collected from one spot urine sample to reflect the 24 h exposure has the highest uncertainty, although all proposed approaches to account for this fact (e.g. urine volume or creatinine correction) produce similar results (David 2000; Kohn et al. 2000; Koch et al. 2003a, 2007; Wittassek & Angerer 2008). Furthermore, dose calculations based on spot urine samples and on 24 h urine samples produced very comparable results (Wittassek et al. 2007a). Additional factors must be considered for children to account for their fast growth (Remer et al. 2002; Koch et al. 2007; Wittassek et al. 2007a).
Table 3.
Excretion fractionsa (fue) of phthalate metabolites in human urine related to the ingested amount of the parent phthalate determined 24 h after oral application.
| phthalate diester | phthalate metabolite |
fue |
reference | |
|---|---|---|---|---|
| DEP | MEP | 69%b |
Anderson et al. (2001) | |
| DnBP | MnBP | 69% |
Anderson et al. (2001) | |
| DiBP | MiBP | 69%b |
Anderson et al. (2001) | |
| BBzP | MBzP | 73% |
Anderson et al. (2001) | |
| DEHP | MEHP | 5.9% | sum: 62.7%c,d | Koch et al. (2004a, 2005b) |
| 5OH-MEHP | 23.3% | |||
| 5oxo-MEHP | 15.0% | |||
| 5cx-MEPP | 18.5% | |||
| DiNP | MiNP | 2.1% | sum: 39.6%c,d | Koch & Angerer (2007) |
| OH-MiNP | 18.4% | |||
| oxo-MiNP | 10.0% | |||
| cx-MiNP | 9.1% | |||
| DiDP/DPHP | OH-MiDP | n.a. | sum: 34%c,d | Wittassek & Angerer (2008) |
| oxo-MiDP | n.a. | |||
| cx-MiDP | n.a. | |||
aThe excretion fractions must not be confused with the total percentage of the applied dose excreted in urine. Because additional metabolites besides the investigated ones are excreted and excretion is not fully completed after 24 h, the total percentage of the applied dose excreted in urine is always higher than the corresponding excretion fraction (fue) for each metabolite.
bNo excretion factor available; factor determined for MnBP.
cSignificant amounts of oxidized metabolites are, due to their longer half-lives of elimination, still excreted on the second day after exposure, which are not included in this percentage of renally excreted dose.
dOxidative metabolites with more than one oxidative modification and breakdown products by β-oxidation have been described previously (Silva et al. 2006c,d, 2007a), which are not included in this percentage of renally excreted dose.
First daily intake calculations based on urinary phthalate monoester data by Blount et al. (2000) were performed by David (2000) and Kohn et al. (2000). In table 4, we present a selection of phthalate daily intake calculations for the general US and German population based on phthalate metabolite concentrations. In all these cases, the mean/median exposures for the general population were in the same range as exposures estimated from ambient data (Clark et al. 2003), and below levels determined to be safe for daily exposures (RfD and TDI). However, the upper percentiles of DBP and DEHP urinary metabolite concentrations suggested that for some people, these daily phthalate intakes might be substantially higher than previously assumed and even close to or exceeding the RfD and TDI (David 2000; Kohn et al. 2000; Koch et al. 2003a). Furthermore, special situations including using DBP-containing medications (Hauser et al. 2004), platelet donation (Koch et al. 2005a) or intensive medical interventions (Calafat et al. 2004a; Koch et al. 2006; Weuve et al. 2006) can result in daily intakes that exceed the RfD or TDI for long periods of time and/or are close to levels where first toxic effects have been observed in animals. The toxicological significance of reaching the RfD and/or TDI of multiple phthalate exposures among susceptible subpopulations (e.g. children, pregnant women), of the time when these high exposures occurred (e.g. prenatal exposures) and of co-exposures to other EDCs remains unclear and warrants further investigation (Hotchkiss et al. 2004; Carruthers & Foster 2005; Foster 2006; Gray et al. 2006; Howdeshell et al. 2007; Rider et al. 2008).
Table 4.
Daily intakes (µg (kg body weight)−1 d−1) of phthalates for the German and US American general population deduced from urinary metabolite concentrations; values are median (95th percentile) (maximum).
| study | Marsee et al. (2006) | Kohn et al. (2000)a | Koch et al. (2003a) | Wittassek et al. (2007a)b; Koch et al. (2007)b | Wittassek et al. (2007b) | ||
| country of origin | USA | USA | Germany | Germany | Germany | ||
| sample origin | pregnant women, SFF IIc | NHANES III | general population | GerES IV | ESBHumd | ||
| sampling year | 1999–2002 | 1988–1994 | 2002 | 2001–2002 | 1988–2003 | ||
| urine | spot | spot | first morning | first morning | 24 h | ||
| n | 214 | 289 | 85 | 239 | 632 | ||
| age (years) | n.a. | 20–60 | 7–63 | 2–14 | 20–29e | ||
| RfDf | TDIg | ||||||
| DEP | 6.64 (112) (1260) | 12 (110) (320) | 2.32 (22.1) (69.3) | — | — | 800 | — |
| BBzP | 0.5 (2.47) (15.53) | 0.88 (4.0) (29) | 0.6 (2.5) (4.5) | 0.42 (2.67) (13.9) | 0.26 (1.6) (27.3) | 200 | 500 |
| DnBP | 0.84 (2.33) (5.86) | 1.5 (7.2) (110)h | 5.22 (16.2) (22.6) | 4.07 (14.9) (76.4) | 4.1 (19.1) (116) | 100 | 10 |
| DiBP | 0.12 (0.41) (2.90) | — | — | — | 1.4 (5.7) (29.0) | — | — |
| DEHP | 1.32 (9.32) (41.1)i | 0.71 (3.6) (46) | 4.6 (17.0) (58.2)j | 4.3 (15.2) (140)j | 3.5 (10.1) (39.8)j | 20 | 50 |
| DiNP | — | <LOD (1.7) (22) | — | — | 0.29 (1.7) (20.2)k | — | 150 |
aBased on the same biomonitoring data by Blount et al. (2000), David (2000) used a slightly different calculation model with very similar results.
bOf the two calculation models used, the creatinine model was chosen for purpose of comparison, the volume model data (not shown) gave higher values by a factor of approximately 2.
cStudy for Future Families, a multicenter pregnancy cohort study.
dTaken from the German Environmental Specimen Bank for Human Tissues. The German ESB is an archive of environmental specimens from representative ecosystems as well as humans for monitoring and evaluating the general quality of the environment as well as the body load with chemicals in selected human populations in Germany.
eStudents only.
gTolerable daily intake, European Food Safety Authority (EFSA 2005).
hSum of DnBP and DiBP.
iBased on MEHP only. Based on the secondary metabolites, DEHP intakes were as follows: 1.7 (10.7) (144).
jBased on the hydrolytic monoester and the three oxidized metabolites, metabolic conversion factors adopted from (Koch et al. 2005b).
kBased on three oxidized metabolites.
7. Risk assessment based on biomonitoring data
Daily intake calculations based on biomonitoring data allow the comparison of individual (or group) exposures with doses determined to be harmful in toxicological studies. More importantly, although estimated including some assumptions (e.g. daily urine volume or creatinine excretion, uniform metabolism), these dose calculations reflect real exposures rather than exposure hypothetically possible based on environmental or questionnaire information. Therefore, biomonitoring-based intake estimates complement intake estimates based on ambient monitoring or on model calculations, which are often either based on worst-case assumptions or on limited knowledge of the variety or extent of external exposure pathways (Wormuth et al. 2006).
Another approach in terms of using biomonitoring data for risk assessment is to directly or retrospectively correlate internal exposures with biological or health effects. The reported associations between urinary concentrations of some phthalate metabolites and anogenital distance, as a measure of certain biological outcomes, suggested for the first time that subtle detrimental effects of phthalate exposure might be observed among the general population (Swan et al. 2005). However, these preliminary study results need confirmation on larger populations (Swan et al. 2005, 2006; Swan 2008; Mcewen & Renner 2006).
It is out of the scope of this review to engage into the ongoing scientific debate around the interpretation of the evidence from toxicological studies related to potential low-dose effects of BPA (NTP 2001; EU 2003; Gray et al. 2004; vom Saal & Hughes 2005; Goodman et al. 2006) or the discussion on the cumulative toxicity of phthalates among each other and in combination with other endocrine disruptors (Hotchkiss et al. 2004; Gray et al. 2006; Howdeshell et al. 2007; Rider et al. 2008). Nevertheless, we hope that this overview can be used to promote research in the various fields of environmental medicine to better understand the toxicological evaluation of exposures to phthalates and BPA on an individual and a population basis (see also discussions in Talsness et al. (2009) and Meeker et al. (2009)).
8. Conclusions
Although phthalates and BPA have been in commerce for decades, human studies using biomonitoring are relatively recent (e.g. conducted within the last 5–10 years). Further epidemiologic studies are needed to advance our understanding of potential human health risks of phthalates and BPA; these studies could include the examination of subtle changes in humans (e.g. anogenital distance) when assessing the effects of environmental exposures to these compounds. Studies also are needed to identify the phthalate metabolites and BPA species relevant to human health, paying special attention to potentially vulnerable segments of the population (e.g. children, women of reproductive age, minorities). Additional research to identify the relative contributions of the numerous sources and routes by which humans are exposed to these compounds is needed, so measures to reduce exposure, if necessary, can be implemented.
Acknowledgements
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Endnote
One contribution of 15 to a Theme Issue ‘Plastics, the environment and human health’.
The term ‘biological monitoring' or ‘biomonitoring' encompasses biomonitoring of exposure, effect and susceptibility within the exposure–disease continuum (Angerer & Weiss 2002; Weis et al. 2005). In this paper, biomonitoring always refers to biomonitoring of exposure.
References
- Adibi J. J., et al. 2008Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 116, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro P. W., Lavenhar S. R.1989Metabolism of di(2-ethylhexyl)phthalate. Drug Metab. Rev. 21, 13–34 (doi:10.3109/03602538909029953) [DOI] [PubMed] [Google Scholar]
- Anderson W. A. C., Castle L., Scotter M. J., Massey R. C., Springall C.2001A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit. Contam. 18, 1068–1074 (doi:10.1080/02652030110050113) [DOI] [PubMed] [Google Scholar]
- Angerer J., Weiss T. (eds) 2002Biological monitoring: prospects in occupational and environmental medicine Weinheim, Germany: Wiley-CH [Google Scholar]
- Angerer J., Bird M. G., Burke T. A., Doerrer N. G., Needham L., Robison S. H., Sheldon L., Zenick H.2006Strategic biomonitoring initiatives: moving the science forward. Toxicol. Sci. 93, 3–10 (doi:10.1093/toxsci/kfl042) [DOI] [PubMed] [Google Scholar]
- Angerer J., Ewers U., Wilhelm M.2007Human biomonitoring: state of the art. Int. J. Hyg. Environ. Health 210, 201–228 (doi:10.1016/j.ijheh.2007.01.024) [DOI] [PubMed] [Google Scholar]
- Arakawa C., Fujimaki K., Yoshinaga J., Imai H., Serizawa S., Shiraishi H.2004Daily urinary excretion of bisphenol A. Environ. Health Prev. Med. 9, 22–26 (doi:10.1265/ehpm.9.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR 1995Toxicological profile for diethyl phthalate (DEP) Atlanta, GA: Agency for Toxic Substances and Disease Registry; See http://www.atsdr.cdc.gov/toxprofiles/tp73.html (accessed 16 May 2008) [PubMed] [Google Scholar]
- ATSDR 1997Toxicological profile for di-n-octyl phthalate (DNOP) Atlanta, GA: Agency for Toxic Substances and Disease Registry; See http://www.atsdr.cdc.gov/toxprofiles/tp95.html (accessed 26 July 2004) [Google Scholar]
- ATSDR 2001Toxicological profile for di-n-butyl phthalate (DBP) Atlanta, GA: Agency for Toxic Substances and Disease Registry; See http://www.atsdr.cdc.gov/toxprofiles/tp135.html (accessed 26 July 2004) [PubMed] [Google Scholar]
- ATSDR 2002Toxicological profile for di(2-ethylhexyl)phthalate (DEHP) Atlanta, GA: Agency for Toxic Substances and Disease Registry; See http://www.atsdr.cdc.gov/toxprofiles/tp9.html (accessed 26 July 2004) [PubMed] [Google Scholar]
- Barr D. B., Wang R. Y., Needham L. L.2005Biologic monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the National Children’s Study. Environ. Health Perspect. 113, 1083–1091 (doi:10.1289/ehp.7617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K., et al. 2004DEHP metabolites in urine of children and DEHP in house dust. Int. J. Hyg. Environ. Health 207, 409–417 (doi:10.1078/1438-4639-00309) [DOI] [PubMed] [Google Scholar]
- Blount B. C., Silva M. J., Caudill S. P., Needham L. L., Pirkle J. L., Sampson E. J., Lucier G. W., Jackson R. J., Brock J. W.2000Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 108, 979–982 (doi:10.2307/3435058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch J., Axelstad M., Vinggaard A. M., Dalgaard M.2006Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis. Toxicol. Lett. 163, 183–190 (doi:10.1016/j.toxlet.2005.10.020) [DOI] [PubMed] [Google Scholar]
- Brock J. W., Caudill S. P., Silva M. J., Needham L. L., Hilborn E. D.2002Phthalate monoesters levels in the urine of young children. Bull. Environ. Contam. Toxicol. 68, 309–314 (doi:10.1007/s001280255) [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Needham L. L.2008Factors affecting the evaluation of biomonitoring data for human exposure assessment. Int. J. Androl. 31, 139–143 (doi:10.1111/j.1365-2605.2007.00826.x) [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Needham L. L., Silva M. J., Lambert G.2004aExposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics 113, e429–e434 (doi:10.1542/peds.113.5.e429) [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Slakman A. R., Silva M. J., Herbert A. R., Needham L. L.2004bAutomated solid phase extraction and quantitative analysis of human milk for 13 phthalate metabolites. J. Chromatogr. B 805, 49–56 (doi:10.1016/j.jchromb.2004.02.006) [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Kuklenyik Z., Reidy J. A., Caudill S. P., Ekong J., Needham L. L.2005Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 113, 391–395 (doi:10.1289/ehp.7534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A. M., Silva M. J., Reidy J. A., Gray L. E., Samandar E., Preau J. L., Herbert A. R., Needham L. L.2006Mono-(3-carboxypropyl) phthalate, a metabolite of di-n-octyl phthalate. J. Toxicol. Environ. Health A 69, 215–227 (doi:10.1080/15287390500227381) [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Ye X. Y., Wong L. Y., Reidy J. A., Needham L. L.2008Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 116, 39–44 (doi:10.1289/ehp.10753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers C. M., Foster P. M. D.2005Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res B Dev Reprod Toxicol 74, 277–285 (doi:10.1002/bdrb.20050) [DOI] [PubMed] [Google Scholar]
- CDC 2003National Health and Nutrition Examination Survey. National Center for Health Statistics; See http://www.cdc.gov/nchs/nhanes.htm (accessed 11 August 2003) [Google Scholar]
- CDC 2005Third National Report on Human Exposure to Environmental Chemicals Atlanta, GA: Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences; See http://www.cdc.gov/exposurereport/3rd/pdf/thirdreport.pdf (accessed 11 April 2007) [Google Scholar]
- CERHR 2007NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Bisphenol A Research Triangle Park, NC: National Toxicology Program, U.S. Department of Health and Human Services; See http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPAFinalEPVF112607.pdf (accessed 26 November 2007) [Google Scholar]
- Ching N. P. H., Jham G. N., Subbarayan C., Bowen D. V., Smit A. L. C., Grossi C. E., Hicks R. G., Field F. H., Nealon T. F.1981Gas chromatographic-mass spectrometric detection of circulating plasticizers in surgical patients. J. Chromatogr. 222, 171–177 [DOI] [PubMed] [Google Scholar]
- Clark K., Cousins I., MacKay D.2003Assessment of critical exposure pathways. In The handbook of environmental chemistry, 3Q. Phthalate esters (ed. Staples C.), pp. 227–262, New York, NY: Springer [Google Scholar]
- Colon I., Caro D., Bourdony C. J., Rosario O.2000Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ. Health Perspect. 108, 895–900 (doi:10.2307/3434999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R. M.2000Exposure to phthalate esters. Environ. Health Perspect. 108, A440 (doi:10.2307/3435032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R. M., McKee R. H., Butala J. H., Barter R. A., Kayser M.2001Esters of aromatic mono-, di-, and tricarboxylic acids, aromatic diacids, and di-, tri-, or polyalcohols. In Patty’s Toxicology, vol 6 (eds Bingham E., Cohrssen B., Powell C. H.), pp. 635–932, 5th edn.New York, NY: John Wiley & Sons [Google Scholar]
- Dekant W., Voelkel W.2008Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol. Appl. Pharmacol. 228, 114–134 (doi:10.1016/j.taap.2007.12.008) [DOI] [PubMed] [Google Scholar]
- Dirven H. A. A. M., van den Broek P. H. H., Arends A. M. M., Nordkamp H. H., de Lepper A. J. G. M., Henderson P. Th., Jongeneelen F. J.1993Metabolites of the plasticizer di(2-ethylhexyl)phthalate in urine samples of workers in polyvinylchloride processing industries. Int. Arch. Occup. Environ. Health 64, 549–554 (doi:10.1007/BF00517699) [DOI] [PubMed] [Google Scholar]
- Duty S. M., Ackerman R. M., Calafat A. M., Hauser R.2005Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ. Health Perspect. 113, 1530–1535 (doi:10.1289/ehp.8083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC 2008Risk assessment reports on phthalates. European Commission. European Chemicals Bureau. See http://ecb.jrc.it/home.php?CONTENU=/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/ (accessed 12 January 2008) [Google Scholar]
- EFSA 2005Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). European Food Safety Authority. See http://www.efsa.europa.eu/EFSA/ScientificPanels/efsa_locale-1178620753812_AFC.htm (accessed 12 January 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA 1990Integrated Risk Information System (IRIS). Dibutyl phthalate (CASRN 84-74-2). U.S. Environmental Protection Agency; See http://www.epa.gov/ncea/iris/subst/0038.htm (accessed 12 January 2008) [Google Scholar]
- EPA 1993aIntegrated Risk Information System (IRIS). Butyl benzyl phthalate (CASRN 85-68-7). U.S. Environmental Protection Agency; See http://www.epa.gov/ncea/iris/subst/0293.htm (accessed 12 January 2008) [Google Scholar]
- EPA 1993bIntegrated Risk Information System (IRIS). Di(2-ethylhexyl)phthalate (DEHP) (CASRN 117-81-7). U.S. Environmental Protection Agency; See http://www.epa.gov/IRIS/subst/0014.htm (accessed 12 January 2008) [Google Scholar]
- EPA 1993cIntegrated Risk Information System (IRIS). Diethyl phthalate (CASRN 84-66-2). U.S. Environmental Protection Agency; See http://www.epa.gov/iris/subst/0226.htm (accessed 12 January 2008) [Google Scholar]
- EU 2003EU Risk Assessment report-BPA. European Union. See http://ecb.jrc.it/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/REPORT/bisphenolareport325.pdf (accessed 12 April 2008.) [Google Scholar]
- EU 2004Directive 2004/93/EC. Council, European Parliament. See http://eur-lex.europa.eu. [Google Scholar]
- EU 2005Directive 2005/84/EC. Council, European Parliament. See http://eur-lex.europa.eu. [Google Scholar]
- EU 2006Directive 2005/90/EC. Council, European Parliament. See http://eur-lex.europa.eu. [Google Scholar]
- FDA 2001Safety assessment of di(2-ethylhexyl)phthalate (DEHP) released from PVC medical devices Rockville, MD: Center for Devices and Radiological Health, U.S. Food and Drug Administration; See http://www.fda.gov/cdrh/ost/dehp-pvc.pdf (accessed 11 August 2003) [Google Scholar]
- Fernandez M. F., Arrebola J. P., Taoufiki J., Navalon A., Ballesteros O., Pulgar R., Vilchez J. L., Olea N.2007Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 24, 259–264 (doi:10.1016/j.reprotox.2007.06.007) [DOI] [PubMed] [Google Scholar]
- Foster P. M. D.2006Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 29, 140–147 (doi:10.1111/j.1365-2605.2005.00563.x) [DOI] [PubMed] [Google Scholar]
- Fromme H., Gruber L., Schlurnmer M., Wz G., Bohmer S., Angerer J., Mayer R., Liebl B., Bolte G.2007Intake of phthalates and di(2-ethylhexyl)adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ. Int. 33, 1012–1020 (doi:10.1016/j.envint.2007.05.006) [DOI] [PubMed] [Google Scholar]
- Goodman J. E., McConnell E. E., Sipes I. G., Witorsch R. J., Slayton T. M., Yu C. J., Lewis A. S., Rhomberg L. R.2006An updated weight of the evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit. Rev. Toxicol. 36, 387–457 (doi:10.1080/10408440600758317) [DOI] [PubMed] [Google Scholar]
- Gray T. J. B., Gangolli S. D.1986Aspects of the testicular toxicity of phthalate-esters. Environ. Health Perspect. 65, 229–235 (doi:10.2307/3430187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. E., Ostby J., Furr J., Price M., Veeramachaneni D. N. R., Parks L.2000Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 58, 350–365 (doi:10.1093/toxsci/58.2.350) [DOI] [PubMed] [Google Scholar]
- Gray G. M., Cohen J. T., Cunha G., Hughes C., McConnell E. E., Rhomberg L., Sipes I. G., Mattison D.2004Weight of the evidence evaluation of low-dose reproductive and developmental effects of bisphenol A. Hum. Ecol. Risk Assess. 10, 875–921 (doi:10.1080/10807030490513883) [Google Scholar]
- Gray L. E., Wilson V. S., Stoker T., Lambright C., Furr J., Noriega N., Howdeshell K., Ankley G. T., Guillette L.2006Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int. J. Androl. 29, 96–104 (doi:10.1111/j.1365-2605.2005.00636.x) [DOI] [PubMed] [Google Scholar]
- Hauser R., Duty S., Godfrey-Bailey L., Calafat A. M.2004Medications as a source of human exposure to phthalates. Environ. Health Perspect. 112, 751–753 (doi:10.1289/ehp.6804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R., Meeker J. D., Duty S., Silva M. J., Calafat A. M.2006Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology 17, 682–691 (doi:10.1097/01.ede.0000235996.89953.d7) [DOI] [PubMed] [Google Scholar]
- Helm D.2007Correlation between production amounts of DEHP and daily intake. Sci. Total Environ. 388, 389–391 [DOI] [PubMed] [Google Scholar]
- Högberg J., et al. 2008Phthalate diesters and their metabolites in human breast milk, blood and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 116, 334–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss A. K., Parks-Saldutti L. G., Ostby J. S., Lambright C., Furr J., Vandenbergh J. G., Gray L. E.2004A mixture of the ‘antiandrogens' linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol. Reprod. 71, 1852–1861 (doi:10.1095/biolreprod.104.031674) [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Furr J., Lambright C. R., Rider C. V., Wilson V. S., Gray L. E.2007Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol. Sci. 99, 190–202 (doi:10.1093/toxsci/kfm069) [DOI] [PubMed] [Google Scholar]
- Jonsson B. A. G., Richthoff J., Rylander L., Giwercman A., Hagmar L.2005Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology 16, 487–493 (doi:10.1097/01.ede.0000164555.19041.01) [DOI] [PubMed] [Google Scholar]
- Kang J. H., Kondo F., Katayama Y.2006Human exposure to bisphenol A. Toxicology 226, 79–89 (doi:10.1016/j.tox.2006.06.009) [DOI] [PubMed] [Google Scholar]
- Kato K., Silva M. J., Brock J. W., Reidy J. A., Malek N. A., Hodge C. C., Nakazawa H., Needham L. L., Barr D. B.2003Quantitative detection of nine phthalate metabolites in human serum using reversed-phase high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Anal. Toxicol. 27, 284–289 [DOI] [PubMed] [Google Scholar]
- Kato K., Silva M. J., Needham L. L., Calafat A. M.2006Quantifying phthalate metabolites in human meconium and semen using automated off-line solid-phase extraction coupled with on-line SPE and isotope-dilution high-performance liquid chromatography-tandem mass spectrometry. Anal. Chem. 78, 6651–6655 (doi:10.1021/ac0608220) [DOI] [PubMed] [Google Scholar]
- Kato K., Silva M. J., Wolf C., Gray L. E., Needham L. L., Calafat A. M.2007Urinary metabolites of diisodecyl phthalate in rats. Toxicology 236, 114–122 (doi:10.1016/j.tox.2007.04.009) [DOI] [PubMed] [Google Scholar]
- Kessler W., Phokha W., Csanady G. A., Filser J. G.2001No background concentrations of di(2-ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in blood of rats. Arch. Toxicol. 75, 62–64 (doi:10.1007/s002040000198) [DOI] [PubMed] [Google Scholar]
- Kessler W., Numtip W., Grote K., Csanady G. A., Chahoud I., Filser J. G.2004Blood burden of di(2-ethylhexyl) phthalate and its primary metabolite mono(2-ethylhexyl) phthalate in pregnant and nonpregnant rats and marmosets. Toxicol. Appl. Pharmacol. 195, 142–153 (doi:10.1016/j.taap.2003.11.014) [DOI] [PubMed] [Google Scholar]
- Kim Y. H., Kim C. S., Park S., Han S. Y., Pyo M. Y., Yang M. H.2003Gender differences in the levels of bisphenol A metabolites in urine. Biochem. Biophys. Res. Commun. 312, 441–448 (doi:10.1016/j.bbrc.2003.10.135) [DOI] [PubMed] [Google Scholar]
- Kintz P., Samyn N.2002Use of alternative specimens: drugs of abuse in saliva and doping agents in hair. Ther. Drug Monit. 24, 239–246 (doi:10.1097/00007691-200204000-00006) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Angerer J.2007Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int. J. Hyg. Environ. Health 210, 9–19 (doi:10.1016/j.ijheh.2006.11.008) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Drexler H., Angerer J.2003aAn estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int. J. Hyg. Environ. Health 206, 1–7 (doi:10.1078/1438-4639-00205) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Rossbach B., Drexler H., Angerer J.2003bInternal exposure of the general population to DEHP and other phthalates—determination of secondary and primary phthalate monoester metabolites in urine. Environ. Res. 93, 177–185 (doi:10.1016/S0013-9351(03)00083-5) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Bolt H. M., Angerer J.2004aDi(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch. Toxicol. 78, 123–130 (doi:10.1007/s00204-003-0522-3) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Drexler H., Angerer J.2004bInternal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP). Int. J. Hyg. Environ. Health 207, 15–22 (doi:10.1078/1438-4639-00270) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Angerer J., Drexler H., Eckstein R., Weisbach V.2005aDi(2-ethylhexyl)phthalate (DEHP) exposure of voluntary plasma and platelet donors. Int. J. Hyg. Environ. Health 208, 489–498 (doi:10.1016/j.ijheh.2005.07.001) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Bolt H. M., Preuss R., Angerer J.2005bNew metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol. 79, 367–376 (doi:10.1007/s00204-004-0642-4) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Preuss R., Drexler H., Angerer J.2005cExposure of nursery school children and their parents and teachers to di-n-butylphthalate and butylbenzylphthalate. Int. Arch. Occup. Environ. Health 78, 223–229 (doi:10.1007/s00420-004-0570-x) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Preuss R., Angerer J.2006Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure: an update and latest results. Int. J. Androl. 29, 155–165 (doi:10.1111/j.1365-2605.2005.00607.x) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Becker K., Wittassek M., Seiwert M., Angerer J., Kolossa-Gehring M.2007Di-n-butylphthalate and butylbenzylphthalate—urinary metabolite levels and estimated daily intakes: pilot study for the German Environmental Survey on children. J. Exp. Sci. Environ. Epidemiol. 17, 378–387 (doi:10.1038/sj.jes.7500526) [DOI] [PubMed] [Google Scholar]
- Kohn M. C., Parham F., Masten S. A., Portier C. J., Shelby M. D., Brock J. W., Needham L. L.2000Human exposure estimates for phthalates. Environ. Health Perspect. 108, A440–A442 (doi:10.2307/3435033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. Y., Shibutani M., Takagi H., Kato N., Takigami S., Uneyama C., Hirose M.2004Diverse developmental toxicity of di-n-butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology 203, 221–238 (doi:10.1016/j.tox.2004.06.013) [DOI] [PubMed] [Google Scholar]
- Liu Z. S., Wolff M. S., Moline J.2005Analysis of environmental biomarkers in urine using an electrochemical detector. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 819, 155–159 (doi:10.1016/j.jchromb.2005.02.005) [DOI] [PubMed] [Google Scholar]
- Marsee K., Woodruff T. J., Axelrad D. A., Calafat A. M., Swan S. H.2006Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ. Health Perspect. 114, 805–809 (doi:10.1289/ehp.8663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Kunugita N., Kitagawa K., Isse T., Oyama T., Foureman G. L., Morita M., Kawamoto T.2003Bisphenol A levels in human urine. Environ. Health Perspect. 111, 101–104 (doi:10.1289/ehp.5512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen G. N., Renner G.2006Validity of anogenital distance as a marker of in utero phthalate exposure. Environ. Health Perspect. 114, A19–A20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee R. H.2004Phthalate exposure and early thelarche. Environ. Health Perspect. 112, A541–A543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Sathyanarayana S., Swan S. H.2009Phthalates and other additives in plastics: human exposure and associated health outcomes. Phil. Trans. R. Soc. B 364, 2097–2113 (doi:10.1098/rstb.2008.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Kotake M.2006Estimation of daily bisphenol A intake of Japanese individuals with emphasis on uncertainty and variability. Environ. Sci. 13, 15–29 [PubMed] [Google Scholar]
- Mose T., Mortensen G. K., Hedegaard M., Knudsen L. E.2007Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod. Toxicol. 23, 83–91 (doi:10.1016/j.reprotox.2006.08.006) [DOI] [PubMed] [Google Scholar]
- NTP 2001Final report of the endocrine disruptors low-dose peer review panel. See http://ntp.niehs.nih.gov/ntp/htdocs/liason/LowDosePeerFinalRpt.pdf (accessed 24 April 2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP 2008NTP Brief on Bisphenol A [CAS NO. 80-05-07]. See http://cerhr.niehs.nih.gov/chemicals/bisphenol/bisphenol.pdf (accessed 24 April 2009) [Google Scholar]
- Needham L. L., Sexton K.2000Assessing children’s exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J. Expo. Anal. Environ. Epidemiol. 10, 611–629 (doi:10.1038/sj.jea.7500142) [DOI] [PubMed] [Google Scholar]
- Needham L. L., Patterson D. G., Barr D. B., Grainger J., Calafat A. M.2005Uses of speciation techniques in biomonitoring for assessing human exposure to organic environmental chemicals. Anal. Bioanal. Chem. 381, 397–404 (doi:10.1007/s00216-004-2975-5) [DOI] [PubMed] [Google Scholar]
- Needham L. L., Calafat A. M., Barr D. B.2007Uses and issues of biomonitoring. Int. J. Hyg. Environ. Health 210, 229–238 (doi:10.1016/j.ijheh.2006.11.002) [DOI] [PubMed] [Google Scholar]
- Ouchi K., Watanabe S.2002Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 780, 365–370 (doi:10.1016/S1570-0232(02)00547-0) [DOI] [PubMed] [Google Scholar]
- Palmeri A., Pichini S., Pacifici R., Zuccaro P., Lopez A.2000Drugs in nails—physiology, pharmacokinetics and forensic toxicology. Clin. Pharm. 38, 95–110 (doi:10.2165/00003088-200038020-00001) [DOI] [PubMed] [Google Scholar]
- Pichini S., Pacifici R., Pellegrini M., Marchei E., Lozano J., Murillo J., Vall O., Garcia-Algar O.2004Development and validation of a high-performance liquid chromatography—mass spectrometry assay for determination of amphetamine, methamphetamine, and methylenedioxy derivatives in meconium. Anal. Chem. 76, 2124–2132 (doi:10.1021/ac035419x) [DOI] [PubMed] [Google Scholar]
- Pirkle J. L., Needham L. L., Sexton K.1995Improving exposure assessment by monitoring human tissues for toxic chemicals. J. Expo. Anal. Environ. Epidemiol. 5, 405–424 [PubMed] [Google Scholar]
- Pollack G. M., Buchanan J. F., Slaughter R. L., Kohli R. K., Shen D. D.1985Circulating concentrations of di(2-ethylhexyl) phthalate and its de-esterified phthalic-acid products following plasticizer exposure in patients receiving hemodialysis. Toxicol. Appl. Pharmacol. 79, 257–267 (doi:10.1016/0041-008X(85)90347-3) [DOI] [PubMed] [Google Scholar]
- Preuss R., Koch H. M., Angerer J.2005Biological monitoring of the five major metabolites of di-(2-ethylhexyl)phthalate (DEHP) in human urine using column-switching liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 816, 269–280 (doi:10.1016/j.jchromb.2004.11.048) [DOI] [PubMed] [Google Scholar]
- Remer T., Neubert A., Maser-Gluth C.2002Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am. J. Clin. Nutr. 75, 561–569 [DOI] [PubMed] [Google Scholar]
- Rider C. V., Furr J., Wilson V. S., Gray L. E.2008A mixture of seven antiandrogens induces reproductive malformations in rats. Int. J. Androl. 31, 249–262 (doi:10.1111/j.1365-2605.2007.00859.x) [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S., Karr C. J., Lozano P., Brown E., Calafat A. M., Liu F., Swan S. H.2008Baby care products: possible sources of infant phthalate exposure. Pediatrics 121, E260–E268 (doi:10.1542/peds.2006-3766) [DOI] [PubMed] [Google Scholar]
- Schonfelder G., Wittfoht W., Hopp H., Talsness C. E., Paul M., Chahoud I.2002Parent bisphenol A accumulation in the human maternal–fetal–placental unit. Environ. Health Perspect. 110, A703–A707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M., Irvine D. S.2004How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? Br. Med. J. 328, 447–451 (doi:10.1136/bmj.328.7437.447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J., Barr D. B., Reidy J. A., Malek N. A., Hodge C. C., Caudill S. P., Brock J. W., Needham L. L., Calafat A. M.2004Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 112, 331–338 (doi:10.1289/ehp.6723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J., Kato K., Gray E. L., Wolf C., Needham L. L., Calafat A. M.2005Urinary metabolites of di-n-octyl phthalate in rats. Toxicology 210, 123–133 (doi:10.1016/j.tox.2005.01.012) [DOI] [PubMed] [Google Scholar]
- Silva M. J., Kato K., Wolf C., Samandar E., Silva S. S., Gray E. L., Needham L. L., Calafat A. M.2006aUrinary biomarkers of di-isononyl phthalate in rats. Toxicology 223, 101–112 (doi:10.1016/j.tox.2006.03.005) [DOI] [PubMed] [Google Scholar]
- Silva M. J., Reidy A., Preau J. L., Samandar E., Needham L. L., Calafat A. M.2006bMeasurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers 11, 1–13 (doi:10.1080/13547500500382868) [DOI] [PubMed] [Google Scholar]
- Silva M. J., Reidy J. A., Preau J. L., Needham L. L., Calafat A. M.2006cOxidative metabolites of diisononyl phthalate as biomarkers for human exposure assessment. Environ. Health Perspect. 114, 1158–1161 (doi:10.1289/ehp.8865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J., Samandar E., Preau J. L., Needham L. L., Calafat A. M.2006dUrinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 219, 22–32 (doi:10.1016/j.tox.2005.10.018) [DOI] [PubMed] [Google Scholar]
- Silva M. J., Reidy J. A., Kato K., Preau J. L., Needham L. L., Calafat A. M.2007aAssessment of human exposure to di-isodecyl phthalate using oxidative metabolites as biomarkers. Biomarkers 12, 133–144 (doi:10.1080/13547500601066915) [DOI] [PubMed] [Google Scholar]
- Silva M. J., Samandar E., Reidy J. A., Hauser R., Needham L. L., Calafat A. M.2007bMetabolite profiles of di-n-butyl phthalate in humans and rats. Environ. Sci. Technol. 41, 7576–7580 (doi:10.1021/es071142x) [DOI] [PubMed] [Google Scholar]
- Swan S. H., et al. 2005Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 113, 1056–1061 (doi:10.1289/ehp.8100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H., Main K., Kruse R., Stewart S., Redmon B., Ternand C., Sullivan S.2006Anogenital distance and phthalate exposure: Swan et al. respond. Environ. Health Perspect. 114, A20–A21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H.2008Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 108, 177–184 (doi:10.1016/j.envres.2008.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsness C. E., Andrade A. J. M., Kuriyama S. N., Taylor J. A., vom Saal F. S.2009Components of plastic: experimental studies in animals and relevance for human health. Phil. Trans. R. Soc. B 364, 2079–2096 (doi:10.1098/rstb.2008.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Moore C. J., vom Saal F. S., Swan S. H.2009Plastics, the environment and human health: current consensus and future trends. Phil. Trans. R. Soc. B 364, 2153–2166 (doi:10.1098/rstb.2009.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyl R. W., Myers C. B., Marr M. C., Fail P. A., Seely J. C., Brine D. R., Barter R. A., Butala J. H.2004Reproductive toxicity evaluation of dietary butyl benzyl phthalate (BBP) in rats. Reprod. Toxicol 18, 241–264 (doi:10.1016/j.reprotox.2003.10.006) [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Hauser R., Marcus M., Olea N., Welshons W. V.2007Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177 (doi:10.1016/j.reprotox.2007.07.010) [DOI] [PubMed] [Google Scholar]
- Volkel W., Colnot T., Csanady G. A., Filser J. G., Dekant W.2002Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 15, 1281–1287 (doi:10.1021/tx025548t) [DOI] [PubMed] [Google Scholar]
- Volkel W., Bittner N., Dekant W.2004Detection of bisphenol A in human urine by LC-MS/MS. Toxicol. Appl. Pharmacol. 197, 190 [Google Scholar]
- Volkel W., Bittner N., Dekant W.2005Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 33, 1748–1757 (doi:10.1124/dmd.105.005454) [DOI] [PubMed] [Google Scholar]
- Volkel W., Kiranoglu M., Fromme H.2008Determination of free and total bisphenol A in human urine to assess daily uptake as a basis for a valid risk assessment. Toxicol. Lett. 179, 155–162 (doi:10.1016/j.toxlet.2008.05.002) [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Hughes C.2005An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113, 926–933 (doi:10.1289/ehp.7713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal F. S., et al. 2007Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 24, 131–138 (doi:10.1016/j.reprotox.2007.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis B. K., et al. 2005Personalized exposure assessment: promising approaches for human environmental health research. Environ. Health Perspect. 113, 840–848 (doi:10.1289/ehp.7651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons W. V., Nagel S. C., vom Saal F. S.2006Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147, S56–S69 (doi:10.1210/en.2005-1159) [DOI] [PubMed] [Google Scholar]
- Weuve J., Sanchez B. N., Calafat A. M., Schettler T., Green R. A., Hu H., Hauser R.2006Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ. Health Perspect. 114, 1424–1431 (doi:10.1289/ehp.8926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt R. M., Barr D. B.2001Measurement of organophosphate metabolites in postpartum meconium as a potential biomarker of prenatal exposure: a validation study. Environ. Health Perspect. 109, 417–420 (doi:10.2307/3454902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M., Angerer J.2008Phthalates: metabolism and exposure. Int. J. Androl. 31, 131–136 (doi:10.1111/j.1365-2605.2007.00837.x) [DOI] [PubMed] [Google Scholar]
- Wittassek M., Heger W., Koch H. M., Becker K., Angerer J., Kolossa-Gehring M.2007aDaily intake of di(2-ethylhexyl)phthalate (DEHP) by German children—a comparison of two estimation models based on urinary DEHP metabolite levels. Int. J. Hyg. Environ. Health 210, 35–42 (doi:10.1016/j.ijheh.2006.11.009) [DOI] [PubMed] [Google Scholar]
- Wittassek M., Wiesmuller G. A., Koch H. M., Eckard R., Dobler L., Muller J., Angerer J., Schluter C.2007bInternal phthalate exposure over the last two decades: a retrospective human biomonitoring study. Int. J. Hyg. Environ. Health 210, 319–333 (doi:10.1016/j.ijheh.2007.01.037) [DOI] [PubMed] [Google Scholar]
- Wolff M. S., et al. 2007Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ. Health Perspect. 115, 116–121 (doi:10.1289/ehp.9488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormuth M., Scheringer M., Vollenweider M., Hungerbuhler K.2006What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 26, 803–824 (doi:10.1111/j.1539-6924.2006.00770.x) [DOI] [PubMed] [Google Scholar]
- Yang M. H., Kim S. Y., Lee S. M., Chang S. S., Kawamoto T., Jang J. Y., Ahn Y. O.2003Biological monitoring of bisphenol A in a Korean population. Arch. Environ. Contam. Toxicol. 44, 546–551 (doi:10.1007/s00244-002-2124-0) [DOI] [PubMed] [Google Scholar]
- Yang M., Kim S. Y., Chang S. S., Lee I. S., Kawamoto T.2006Urinary concentrations of bisphenol A in relation to biomarkers of sensitivity and effect and endocrine-related health effects. Environ. Mol. Mutagen. 47, 571–578 (doi:10.1002/em.20230) [DOI] [PubMed] [Google Scholar]
- Ye X. Y., Kuklenyik Z., Needham L. L., Calafat A. M.2005Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 383, 638–644 (doi:10.1007/s00216-005-0019-4) [DOI] [PubMed] [Google Scholar]


