Abstract
This review provides a critical analysis of the biological effects of the most widely used plasticizers, including dibutyl phthalate, diethylhexyl phthalate, dimethyl phthalate, butyl benzyl phthalate and bisphenol A (BPA), on wildlife, with a focus on annelids (both aquatic and terrestrial), molluscs, crustaceans, insects, fish and amphibians. Moreover, the paper provides novel data on the biological effects of some of these plasticizers in invertebrates, fish and amphibians. Phthalates and BPA have been shown to affect reproduction in all studied animal groups, to impair development in crustaceans and amphibians and to induce genetic aberrations. Molluscs, crustaceans and amphibians appear to be especially sensitive to these compounds, and biological effects are observed at environmentally relevant exposures in the low ng l−1 to µg l−1 range. In contrast, most effects in fish (except for disturbance in spermatogenesis) occur at higher concentrations. Most plasticizers appear to act by interfering with the functioning of various hormone systems, but some phthalates have wider pathways of disruption. Effect concentrations of plasticizers in laboratory experiments coincide with measured environmental concentrations, and thus there is a very real potential for effects of these chemicals on some wildlife populations. The most striking gaps in our current knowledge on the impacts of plasticizers on wildlife are the lack of data for long-term exposures to environmentally relevant concentrations and their ecotoxicity when part of complex mixtures. Furthermore, the hazard of plasticizers has been investigated in annelids, molluscs and arthropods only, and given the sensitivity of some invertebrates, effects assessments are warranted in other invertebrate phyla.
Keywords: bisphenol A, dibutyl phthalate, diethylhexyl phthalate, dimethyl phthalate, butyl benzyl phthalate, endocrine disruption
1. Introduction
The history of phthalate-based plasticizers and bisphenol A (BPA) dates back to the 1920s and 1930s, respectively. Phthalates have been applied as polyvinyl chloride (PVC) additives since 1926, but were also used for healthcare purposes as insect repellents and cercaricides (Ferguson et al. 1946). The development of BPA had a very different genesis, driven primarily by the search for synthetic oestrogens for clinical use (Cook et al. 1933). Dodds & Lawson (1936, 1938) demonstrated the oestrogenic activity of BPA in ovari-ectomized rats, but the formulation of a more effective synthetic oestrogen, diethylstilboestrol, at around the same time rendered BPA redundant as a clinical oestrogen (Dodds et al. 1938). In the 1950s, BPA was rediscovered when a Bayer chemist, Hermann Schnell, reacted BPA with phosgene to produce polycarbonate plastic, and its use in plastics has subsequently become its primary commercial application.
The emollient properties of phthalates and polymerization of BPA have driven their widespread use in the production of plastics (Thompson et al. 2009a,b). Today, phthalates and BPA are found in many mass-produced products including medical devices, food packaging, perfumes, cosmetics, children's toys, flooring materials, computers, CDs, etc. (Andrady & Neal 2009). Phthalates may constitute up to 50 per cent of the total weight of PVC plastics, and their worldwide annual production is approximately 2.7 million metric tonnes (Bauer & Herrmann 1997). For BPA, worldwide annual production is 2.5 million metric tonnes (Staples et al. 2002). Plasticizers work by reducing the chemical affinity between molecules when embedded between chains of plastic raw materials (or act as monomers in polycarbonate plastic), but as plasticizers are not especially stable in these products, they can leach out and thus end up in the environment. Phthalates are not classified as persistent compounds (Staples et al. 1997), but their occurrence in the environment has been reported widely, possibly arguing against a rapid biodegradation in some environments (Fatoki & Vernon 1990; Bauer & Herrmann 1997; Heemken et al. 2001; Fromme et al. 2002; Beauchesne et al. 2007). Bisphenol A is easily degraded (Howard 1989), but nevertheless regularly detected in aquatic ecosystems owing to its continuous release into the environment.
The European Union Environmental Risk Assessment (ERA) reports provide predicted environmental concentrations (PECs) for phthalates and BPA using the European Union System for the Evaluation of Substances (EUSES). The reliability of some of these PECs, however, is disputed as monitoring data from surface waters have found significantly higher environmental concentrations of phthalates. While the PEC for dibutyl phthalate (DBP) is 0.4 µg l−1 and 0.231 mg kg−1 (dry wt.) for water and sediments, respectively (EU 2004), Fatoki & Vernon (1990) report a maximum measured concentration of 33.5 µg l−1 in UK rivers. The ERA draft report calculates a PEC for diethylhexyl phthalate (DEHP) of 2.2 µg l−1 for water and 34 mg kg−1 (dry wt.) for sediments (EU 2006). This figure is exceeded in some German surface waters where measured concentrations range between 0.33 and 97.8 µg l−1 (Fromme et al. 2002) and in Japanese freshwaters and sediments where peak concentrations have been measured at 58 µg l−1 and 210 000 µg kg−1 (dry wt.), respectively (average concentrations in Japanese sediments range between 46 and 326 µg kg−1 (dry wt.; Naito et al. 2006). For di-isodecyl phthalate (DIDP), the EUSES-based calculations state PECs of 1.8 µg l−1 in surface waters and 32 mg kg−1 (dry wt.) in sediments (EU 2003a) and for di-isononyl phthalate (DINP), 0.7 µg l−1 in surface waters and 18 mg kg−1 (dry wt.) in sediments (EU 2003b). There is a lack of data on measured environmental concentrations of these compounds to confirm or refute these calculations.
Bisphenol A is present in many environmental compartments, including the aquatic environment, and enters water systems through point discharges, such as landfill leachates and sewage treatment plant effluents. Concentrations of BPA vary widely in the aquatic environment, but they may even reach concentrations up to 21 µg l−1 in freshwater systems (Crain et al. 2007). Concentrations of BPA in sediments are generally several orders of magnitude higher than those in the water column. For example, in the River Elbe in Germany, BPA was measured at 0.776 µg l−1 in the water compared with 343 µg kg−1 (dry wt.) in the sediment (Heemken et al. 2001). These findings contrast with a PEC of 0.12 µg l−1 for water and 1.6 µg kg−1 (dry wt.) for sediments as indicated by the ERA report for BPA (EU 2003c).
Heightening our concern about possible health implications of exposure to plasticizers, phthalates and BPA have been shown to bioaccumulate in organisms. Bioconcentration factors (BCFs) for plasticizers, however, appear to differ widely between species. Some of these differences relate to the type and nature of the plasticizers, but others relate to the differences in the experimental designs employed, diet, life and species' history and the tissues investigated. Bioconcentration factors reported in invertebrates are generally higher than those in vertebrates. As an example, for DEHP, BCFs reported are between 42 and 842 in different fish species, but 3600 for the amphipod Gammarus pseudolimnaueus, 2500 in the mussel Mytilus edulis and up to 5380 in the copepod Acartia tonsa (EU 2003d).
In this review paper, we analyse critically the effects on wildlife of the most prominent plasticizers, with a focus on effects in annelids (both aquatic and terrestrial), molluscs, crustaceans, insects, fish and amphibians. The paper further presents novel data from the authors on the biological effects of some plasticizers on animal species. The paper concludes with an analysis on the level of harm (and the uncertainties) associated with the discharge of plasticizers into the environment and identifies key research needs to address some of the uncertainties.
2. Material and methods
In this paper, a series of experiments are reported upon that provide novel data on the effects of butyl benzyl phthalate (BBP) on a fish (Danio rerio) and BPA on a crustacean (A. tonsa) and an amphibian (Xenopus laevis). These experiments are described briefly, as detailed information on study designs can be obtained from the references cited.
(a). Danio rerio (zebrafish)
A study was conducted to investigate the effects of BBP on reproduction in adult D. rerio. Fish were maintained in breeding colonies of six males and six females, according to Santos et al. (2007). The chemical exposure tanks were set up in duplicate and dosed with 0 (solvent controls with methanol <0.01‰), 3, 6 and 15 µg BBP l−1 and run for 20 days. The total number of eggs produced and the number of viable embryos at 8 h post fertilization (hpf) were determined according to Santos et al. (2007). At the end of the experiment, quantification of vitellogenin (VTG) in the plasma was performed according to Fenske et al. (2001), with an adjustment of the primary VTG antibody concentrations to 1 : 10 000 and a primary antibody incubation time of 30 min. One gonad from all parent males was dissected and immediately used for sperm quantity and motility assessments (as measures of sperm quality) using computer-assisted sperm analysis (CASA, v. 7V3B; Hobson Vision Ltd, UK) on a Hobson sperm tracker (Hobson Vision Ltd), according to the protocols and settings described in Santos et al. (2007).
Water samples were collected from each tank on days 1, 4, 7, 14 and 20, fixed with 5% methanol (Fisher, UK), extracted using Sep-Pack C18 cartridges (Waters Corporation, USA) and BBP concentrations of the extracted samples were determined by HPLC analysis (Shimadzu, USA). Samples were analysed through separation on a C18 column (Spherisorb ODS2) using an isocratic gradient of 70 per cent methanol in water, and the absorbance was monitored at 277 nm on a UV HPLC detector (Applied Biosystems, UK). Quantification was achieved by comparison of the absorbance of the BBP peak in each sample with the absorbance of serially diluted BBP standards (ranging from 0.0001 to 1 mg ml−1).
The statistical comparisons on the VTG and fecundity data were performed using one-way ANOVA followed by Tukey's multiple comparison post-test analysis (using Jandel Sigmastat 2.0 statistical software). The sperm data were arcsine transformed before a one-way ANOVA and Tukey's multiple comparison post-test analysis using Excel (Microsoft Excel 2002) and GraphPad Prism (v. 4.02, San Diego, CA, USA).
(b). Acartia tonsa (calanoid copepod, Crustacea)
Full life-cycle experiments were conducted with A. tonsa, starting with eggs and extending for three weeks to reproductively active adults, with BPA following an OECD guideline proposal (Kusk & Wollenberger 2007) with minor modifications. Animals were exposed in mass cultures (800 ml of medium/test solution with 400 copepods). Nominal BPA test concentrations were 10, 50, 100, 300, 1000 µg l−1. Semi-static exposures were performed at 20 ± 0.5°C with a photoperiod of 12 L : 12 D cycle using a synthetic seawater medium with a salinity of 18‰ (Kusk & Wollenberger 1999). On day 14, the number of eggs produced within a 24 h period was determined in isolated groups of 5 females (5 replicates per concentration, 10 control groups) and expressed as eggs per female. Eggs produced on day 18 were transferred to a clean medium (6 replicates per concentration, each containing 30 eggs) in order to observe the hatching rate and larval mortality of the second generation, determined on day 21. The larval development ratio (LDR), describing the proportion of animals completing metamorphosis from the nauplius to the copepodite morphology, was recorded on day 5. The LDR test (Wollenberger et al. 2005) was conducted in parallel to the exposure of the mass culture under the same exposure conditions, with nominal BPA concentrations of 30, 60, 125, 250, 500, 1000 µg l−1, with 4 replicates per concentration, each containing 30 copepods and 12 control groups.
(c). Xenopus laevis (African clawed frog)
The impacts of BPA on the thyroid system of X. laevis were determined as described previously for tetrabromobisphenol A by Jagnytsch et al. (2006). Briefly, the levels of thyroid receptor β (TRβ) mRNA were measured in the brain as biomarker for the bioavailability of thyroid hormones (THs) (Opitz et al. 2006). Bisphenol A was applied at concentrations ranging between 2.5 and 500 µg l−1 (2.5, 25, 250, 500 µg l−1) with 2 replicates per concentration and 20 tadpoles per replicate in a long-term Xenopus metamorphosis assay (XEMA 21 d) and at BPA concentrations of 100, 250 and 500 µg l−1 with and without 0.1 and 1.0 nM tri-iodothyronine (T3) in short-term exposures for 1, 2 and 3 days (starting with duplicate tanks, each containing 30 tadpoles/aquarium and subsampling 10 individuals in each replicate every day). The effects of exposure to BPA during larval development on sexual differentiation were also investigated, using a flow-through system, to the point of completion of metamorphosis (see Lutz et al. 2008 for details of protocol). Briefly, tadpoles were exposed to BPA at concentrations of 10−9, 10−8, 10−7 and 10−6 M (228 ng l−1 to 228 µg l−1) and to dilution water without BPA (negative control). A positive oestrogen control was also included (0.2 µg 17β-oestradiol l−1). In this study, there were 4 replicates for all treatments, and 25 individual tadpoles were exposed in each tank (n = 100 per treatment). The endpoints investigated were survival rate, time to completion of metamorphosis, weight, sex (based on gross morphology) and histology of testes. In addition, gene expression for hepatic insulin-like growth factor-I (IGF-I) and VTG mRNA was analysed in male X. laevis immediately at completion of metamorphosis using semi-quantitative RT–PCR, as described previously (Kloas et al. 1999).
3. Results and discussion
(a). Effects of phthalates
Assessing the biological effects of phthalates such as DEHP in aquatic environments presents a significant challenge owing to its low water solubility and its tendency to form colloidal dispersions above 3 µg l−1. Many experiments exposing DEHP via the water at concentrations >3 µg l−1 have, nevertheless, clearly established concentration-related biological effects (Forget-Leray et al. 2005). Despite this, risk assessments of DEHP in Japan (Naito et al. 2006) and the EU (EU 2003d) have disregarded most studies adopting concentrations >3 µg l−1, and the Japanese assessment has set the threshold effect level (PNEC) to 3 µg DEHP l−1 (Naito et al. 2006). This omission needs addressing given the effects reported for this phthalate in this review. Working with other highly lipophilic phthalates presents similar solubility problems as for DEHP. Di-isononyl phthalate and DIDP have a log Kow of 8.8 and a water solubility of only 0.2 µg l−1 (EU 2003a,b), which makes them even more difficult to handle in aquatic media than DEHP.
(i). Annelids and molluscs
Despite their ecological importance, annelids and molluscs have received relatively little attention in assessing the effects of plasticizers compared with vertebrates or other invertebrates (especially crustaceans and insects; Weltje & Schulte-Oehlmann 2007). Early investigations on annelids and molluscs focused on bioaccumulation and acute toxicity of phthalates, but more recently, wider biological effects have been shown, including mitotic inhibition, induction of chromosomal aberrations and effects on larval development.
Considering soil-dwelling organisms, studies on the uptake and toxicity of the phthalates dimethyl phthalate (DMP), diethyl phthalate (DEP), DBP and DEHP (at a concentration of 5 mg kg−1 for 25 days) in the worm Eisenia fetida have shown that high molecular weight phthalates (DBP and DEHP) were incorporated but with relatively low accumulation factors (0.242–0.307 and 0.073–0.244, respectively), and DMP and DEP were apparently not taken up (Xiao-yu et al. 2005). It is possible that the lack of any uptake of DMP and DEP may be as a consequence of their higher microbial degradation rates and a faster metabolic degradation (Albro et al. 1993). Neuhauser et al. (1986) investigated the acute toxicity of phthalates and found similar LC50 values (1064–3335 mg kg−1 for a 14 day soil exposure) for DMP in four soil-dwelling annelid species (Allolobophora tuberculata, E. fetida, Eudrilus eugeniae, Perionyx excavatus). Together, these studies demonstrate that phthalates enter terrestrial worms, but they have a low acute toxicity. It is worth emphasizing that soils can receive very high inputs of phthalates, and the total amount of DEHP deposited in US landfills to 1992 has been estimated at 5 million metric tonnes.
Comparisons of the acute toxicity of several phthalate esters [DMP, DEP, DBP, BBP, di-n-hexyl-phthalate (DHP) and DEHP] in various aquatic organisms including the annelid Lumbriculus variegatus have shown that the higher molecular weight phthalates DHP and DEHP were not acutely toxic for the concentration range tested (<2.3–47.8 µg DHP l−1, 10.2–69.1 µg DEHP l−1; Call et al. 2001). The toxicity of lower molecular weight phthalates was positively correlated with their Kow, indicating non-polar narcosis as the underlying mode of action. The same study found L. variegatus was the species least sensitive to DMP; the 10 day LC50 (10 day) was 246, compared with 28.1 and 68.2 mg l−1 for the crustacean Hyalella azteca and the insect Chironomus tentans, respectively. In adult marine tubeworms Pomatoceros lamarckii, exposure to DMP was shown to decrease fertilization success at a threshold concentration of 1×10−5 M DMP (1.94 mg l−1) and induce a significant increase in the number of aberrations in chromosome separations in oocytes at anaphase at concentrations ≥1×10−7 M (19.4 µg l−1) (Dixon et al. 1999; Wilson et al. 2002). DMP has been shown to affect larval development in the polychaete worm at a threshold concentration ≥1 × 10−5 M (1.94 mg l−1).
The effects of phthalates have also been studied on various biochemical pathways such as antioxidant and peroxisomal marker enzymes in marine mussels (Mytilus galloprovincialis, Orbea et al. 2002; M. edulis, Cajaraville & Ortiz-Zarragoitia 2006). Exposure to 500 µg DEHP l−1 for 21 days was shown to result in a significant increase in the catalase and acyl-CoA oxidase (AOX) activity and an inhibition of the superoxide and manganate superoxide dismutase in M. galloprovincialis. The peroxisomal volume density in the digestive gland of M. edulis was significantly enhanced at 50 µg diallylphthalate (DAP) per litre after three weeks exposure, whereas the chemical had no significant effect on AOX activity in this species (Cajaraville & Ortiz-Zarragoitia 2006). DAP was able to decrease the phospho-protein level (a mussel VTG-like protein) but did not impair ovarian follicles, oocytes and spermatogenesis in M. edulis when exposed to 50 µg DAP l−1 for three weeks (Aarab et al. 2006). In another 21 day study, DAP was also shown to increase micronuclei frequency, a marker of genotoxicity, and fragmented-apoptotic cells in the gills of M. edulis at 50 µg DAP l−1 (Baršienė et al. 2006).
Very few studies have considered the effects of phthalates on annelid or mollusc populations, but Tagatz et al. (1986) found that exposure to DBP altered the community structure and colonization profile of the zoomacrobenthos under laboratory and field conditions. The densities of molluscs and echinoderms were reduced by 49 and 97 per cent, while no effects were seen in annelids, arthropods, chordates and other groups (at concentrations of 10–1000 mg kg−1 over eight weeks).
(ii). Crustaceans and insects
Lower molecular weight phthalates that are relatively water soluble (Howard et al. 1985) have been shown to have a lower acute and chronic toxicity in Daphnia magna. For example, 48 h immobilization tests with D. magna have shown IC50 values of 284, 22.0 and 6.78 mg l−1, for DMP, DEP and DBP, respectively (Jonsson & Baun 2003). Acute toxicity for phthalates, however, differs significantly for different species of crustaceans. Hyalella azteca is 10–20-fold more sensitive than D. magna, although the higher molecular weight phthalates DHP and DEHP did not exhibit toxic effects in H. azteca even at concentrations up to 22.5 µg DHP l−1 and 59.0 µg DEHP l−1 (Call et al. 2001). Other effects of DBP reported in crustaceans include decreased locomotor activity in Gammarus pulex at an exposure concentration of 500 µg l−1 (Thuren & Woin 1991).
In the Drosophila melanogaster BII cell line, DEP exhibited a weak ecdysteroid antagonistic activity (Dinan et al. 2001). However, the concentrations required to cause an effect were several orders of magnitude higher than that for 20-hydroxy ecdysone, thus classifying DEP as a very weak environmental anti-ecdysteroid only.
DEHP has been shown to induce sublethal effects in Chironomus riparius, and the exposure of larvae resulted in an increased female body volume at exposure concentrations of 0.3 µg l−1 (Kwak & Lee 2005). It also increased the expression of heat shock protein (HSP) and haemoglobin genes in exposed larvae of C. tentans at a concentration of 0.5 µg l−1 (Lee et al. 2006a), but the same compound was not acutely toxic to this species at concentrations up to 69.1 µg DEHP l−1 (Call et al. 2001). The significance of such effects on gene expression is not known, but in vertebrates, HSPs are involved in steroid hormone receptor stabilization and activation (Gillesby & Zacharewski 1998) and if they act similarly in arthropods, a change in the HSP70 titre might result in a disruption of the ecdysone axis by influencing the ecdysone receptor.
(iii). Fish
Given the widespread occurrence of phthalates in the aquatic environment, fish are also likely to be exposed to phthalates via the water column, food and/or via the sediments, depending on their ecological niche. In support of this, a study in wild fish in The Netherlands measured median concentrations of DEHP and DBP at 1.7 and 1.0 µg kg−1 (wet wt.), respectively, with 95th percentile values reaching up to 141 µg DEHP kg−1 and 26 µg DBP kg−1 (wet wt.) (Peijnenburg & Struijs 2006). Laboratory studies have further shown that phthalates bioconcentrate in fish. For example, the BCF for DEHP was 120 in the carp and for BBP, 9.4 in bluegill sunfish (Staples et al. 1997). The presence of phthalates in wild fish and their ability to concentrate in body tissues demonstrate their potential to induce adverse effects on the health of wild populations, but there has been little bearing on this expectation.
Very few studies have comprehensively addressed the biological effects of phthalates on fish, and in vivo studies have reported mixed findings. BBP has been reported to be oestrogenic and induces VTG synthesis in male fathead minnow (Pimephales promelas) at a concentration of 100 µg l−1 via the water (Harries et al. 2000), and, similarly, to induce VTG in carp (Cyprinus carpio) exposed to 1–20 mg DEP l−1 over a period of four weeks (Barse etal. 2007). In salmon (Salmo salar), exposure to 1500 mg DEHP kg−1 in the food for a prolonged period (four months) during early life resulted in a small incidence of intersex (Norman et al. 2007). These in vivo effects are consistent with the mode of action of BBP and DEHP established in vitro where they have been shown to bind to the oestrogen receptor (ER) and elicit a weakly oestrogenic response in fish cell cultures (Jobling et al. 1995) and in the yeast oestrogen screen (YES; containing the human ER) (Harris et al. 1997). In female Japanese medaka (Oryzias latipes), exposure to concentrations in excess of 1 µg DEHP l−1 caused a marked reduction in both VTG and in the percentage of females containing mature oocytes, suggesting an anti-oestrogenic mode of action (Kim et al. 2002). This anti-oestrogenic effect presumably arises through competition with the endogenous oestrogens for the receptor. In vitro receptor interaction studies have also shown that BBP and DEHP possess anti-androgenic activity (Sohoni & Sumpter 1998). Other in vivo effects of phthalates in fish include effects on the metabolic pathways involved in steroid biosynthesis and metabolism. In a study by Thibaut & Porte (2004), DEHP and DBP altered the activity of enzymes involved in the synthesis of endogenous steroid hormones and their metabolism in carp, effects that can lead to the alteration of the balance between endogenous oestrogens and androgens, and thus alterations in their reproductive physiology. These findings are supported by studies in mammals in which phthalates have also been shown to alter the expression and enzymatic activity of enzymes involved in the synthesis and metabolism of sex steroids in the testis (Liu et al. 2005). In fish, exposures to low µg l−1 concentrations of DEP have been shown to induce alterations in a number of enzymes in the liver and muscle, including phosphatases and transaminases, showing a disruptive effect on general metabolic functions (Barse et al. 2007). Other studies have also reported that, potentially, the most relevant mechanism of action of phthalates is via binding to the peroxisome proliferator-activated receptors (PPARs) and modulation of the transcriptional events coordinated by this molecular pathway, which includes steroid hormone metabolism (Fan et al. 2004).
Exposures to phthalates have also been shown to alter behaviour in fish. Exposure to 100 µg BBP l−1, via the water, caused alterations in shoaling and feeding behaviour in three-spined stickleback (Gasterosteus aculeatus) (Wibe et al. 2002, 2004), and exposure to 5 mg DEP l−1 caused alterations in the general behaviour of common carp (Barse et al. 2007). It should be noted that these studies have employed very high exposure concentrations, and these are unlikely to occur in the water column in part owing to their low solubility. Exceptions to this may be for fish living in/closely associated with the sediments in heavily contaminated environments. Exposures of fish to lower levels of phthalates have generally found no adverse effects. As an example, exposure of Japanese medaka to 21.9 and 19.3 µg g−1 DINP and DIDP, respectively, via the diet, with a daily feeding regime of 5 per cent of the body weight, failed to identify any effects on reproduction (gonad somatic index, egg production, embryo survival and sex ratios), growth or survival in a multi-generational study (14 days post fertilization (dpf) to 140 dpf of the F1 generation) (Patyna et al. 2006). Similarly, exposure of medaka to concentrations up to 5 mg DEHP l−1 for 90 days post-hatch did not affect germ cell development (Metcalfe et al. 2001).
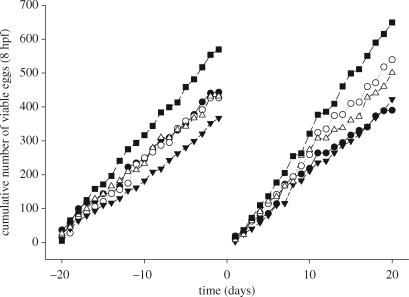
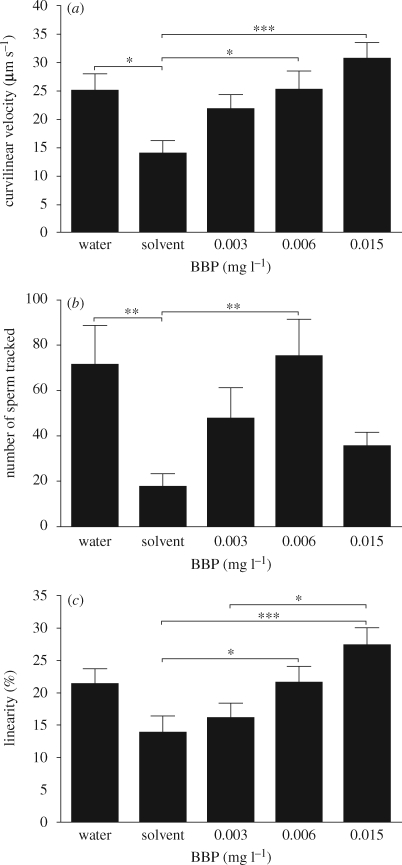
In our exposure of adult breeding zebrafish to BBP, measured concentrations in the tank water were 3, 6 and 15 µg l−1 over the exposure period. Exposure to BBP did not affect the concentration of VTG in the plasma at any of the concentrations tested in males (VTG concentrations in the plasma were 1310 ± 255; 1500 ± 358; 948 ± 141; 1300 ± 318; 1640 ± 220 ng ml−1 for the water control, solvent control, 3, 6 and 15 µg l−1 BBP, respectively; n = 9–12 males per treatment group). Similarly, there was no evidence of any chemical effect on the number of eggs spawned; the total number of eggs spawned per colony over the assessment period ranged between 4109 and 7578 with a maximum of 727 eggs spawned by a single colony on a single day, with a mean number of eggs spawned per female per day (including days with no eggs) of 22.2 ± 1.4; 21.3 ± 1.4; 18.4 ± 0.8; 21.6 ± 1.7; 28.5 ± 1.4 prior to exposure and 19.5 ± 1.6; 27.0 ± 1.7; 21.1 ± 1.2; 25.0 ± 2.1; 32.5 ± 1.4 during the exposure for the water control, solvent control, 3, 6 and 15 µg BBP l−1, respectively. Furthermore, there were no effects of BBP on the mean percentage viability of embryos at 8 hpf (figure 1). These results are in agreement with reports in the literature, where VTG induction in males, or reproductive effects on females, has been induced only at exposure concentrations that far exceed the measured concentrations in our study. In contrast, however, we found consistent changes in parameters of sperm quality in BBP-exposed male zebrafish. The solvent adopted (methanol) had a negative effect on zebrafish sperm motility compared with the water control after 20 days of exposure (mean curvilinear velocity of 14 µm s−1 (mean) and 25 µm s−1, respectively, p < 0.05, figure 2a), and BBP exposure resulted in a concentration-dependent enhancement in curvilinear velocity of the solvent-affected sperm. At the highest concentration tested (15 µg BBP l−1), the average curvilinear velocity of the sperm was restored to levels similar to those found in the water control tanks (31 µm s−1; figure 2a). Both the number of sperm tracked and the linearity of the sperm movement showed patterns similar to those described for the curvilinear velocity (figure 2b,c).
Figure 1.
Danio rerio. Cumulative number of viable eggs spawned in colonies during a 20 day period prior to BBP exposure and a 20 day period during BBP exposure. Symbols represent average results for two colonies per treatment: filled circle, water control; open circle, solvent control; inverted filled triangle, 0.003 mg l−1 BBP; open triangle, 0.006 mg l−1 BBP; filled square, 0.015 mg l−1 BBP.
Figure 2.
Danio rerio. Effects of exposure to BBP for 20 days on sperm quality: (a) the curvilinear velocity, (b) the number of sperm tracked and (c) sperm linearity. Bars represent mean ± SEM and are given for the first four intervals of tracking (i.e. sperm motility): 0–15, 16–30, 31–45 and 46–60 s. Chemical concentrations are measured in the tank water. Data were arcsine transformed before statistical analysis (one-way ANOVA and Tukey's multiple comparison post-test; *p < 0.05; **p < 0.01; ***p < 0.001).
The ability of BBP to reverse the adverse effect of the methanol on sperm number and motility is a novel finding, and we hypothesize that this may have resulted from an interaction of BBP with the PPAR receptor, which leads to increased oxidative stress in the testis, as demonstrated in mammals for phthalates (reviewed in Corton & Lapinskas 2005). In the final stages of sperm development, maturation and activation are crucially dependent on the oxidative balance within the seminiferous tubes and in the sperm itself. Exposure to methanol may have reduced the free oxygen radicals required to promote sperm maturation and activation, and this effect may have been reversed by the increase in free radicals caused by the BBP exposure. The ethanol metabolite, acetaldehyde, has been shown to block the action of PPARs in vitro (Galli et al. 2001), suggesting a mechanism by which sperm motility might be reduced in fish exposed to a solvent. Exposure to BBP in the solvent carrier may counterbalance this effect, restoring the normal motility of the sperm. The increase in oxidative stress within the testis as a result of phthalate exposure has been previously observed in mammalian systems, including in rats (Kasahara et al. 2002), and this effect was shown to be mediated through activation of PPAR pathways.
(iv). Amphibians
Studies on phthalates in amphibians (reviewed in Kloas 2002) are less extensive than those in fish and some invertebrates. The effects of plasticizers in amphibians have been focused on their toxicity, their potential to induce teratogenic effects and their endocrine-disrupting activities, most notably effects on reproduction, sexual differentiation and the thyroid system. Focusing on toxicity, Larsson & Thurén (1987) found that exposure of Rana arvalis eggs to DEHP via sediment decreased successful hatchings with increasing concentrations and deduced a no observed effect concentration (NOEC) of 10 mg kg−1 fresh weight. Studies with DBP using the frog embryo teratogenesis assay Xenopus (FETAX) have shown developmental malformations at 500 µg l−1 and enhanced mortality rates at concentrations of 10 and 15 mg l−1 (Lee et al. 2005).
DBP has also been shown to affect sexual differentiation in male Rana rugosa tadpoles, delaying gonadal development (Ohtani et al. 2000) and altering the spermatogenesis process in eight-month-old X. laevis after exposure during larval development (at 1–10 mg DBP l−1 from stage 52 to completion of metamorphosis; Lee & Veeramachaneni 2005). Studies in Xenopus investigating the potential for phthalates to bind to the ER via competitive displacement experiments with cytosolic liver preparations have found that DEP has only a moderate affinity for the ER (Lutz & Kloas 1999). Parallel in vitro studies using X. laevis primary hepatocytes, however, have shown that DBP, BBP and butylphthalyl-butylglycolate had no oestrogenic activity, deduced by measuring VTG gene expression (Kloas et al. 1999; Lutz et al. 2005) and VTG synthesis (Nomura et al. 2006) for a concentration range between 4×10−7 and 10−4 M (110 µg l−1 to 33.6 mg l−1).
(b). Effects of bisphenol A
(i). Annelids and molluscs
There is only one study reporting effects of BPA in annelids (Biggers & Laufer 2004), where BPA induced metamorphosis and settlement in 2-day-old Capitella capitata metatrochophore larvae (a marine polychaete), following a 1 h exposure at 11.4 µg l−1. In contrast, there has been considerably more research investigating the effects of BPA in molluscs, where most has been focused on gastropods and bivalves. Oehlmann et al. (2000) have reported that exposure of ramshorn snails (Marisa cornuarietis) to BPA induced a superfeminization syndrome at all nominal concentrations tested (1, 5, 25, 100 µg BPA l−1). Superfemales are characterized by additional sex organs, enlarged accessory sex glands, gross malformations of the pallial oviduct, enhanced egg production outside the main spawning season and increased female mortality (13.3–15.7% in exposed snails compared with control mortality of 3.8%). Effects were concentration dependent (except for mortality) and statistically significant at every test concentration. Limitations on this first study included the lack of analytical confirmation of the exposure regimes, a lack of replication and the absence of a positive control. However, two follow-up exposures employing these features confirmed the original findings (Schulte-Oehlmann et al. 2001; Oehlmann et al. 2006). Schulte-Oehlmann et al. (2001) investigated the effects of BPA at different phases of the reproductive cycle at concentrations of 0.05, 0.1, 0.25, 0.5 and 1.0 µg l−1 over 180 days. Before and after the main spawning season, superfemales were observed with oviduct malformations and a significantly increased egg production in all treated groups except the lowest test concentration. During the peak spawning period, no effect of BPA was seen on spawning levels, probably because spawning activity in the controls increased six-fold at this time, masking any effect seen for pre- and post-spawning periods. Calculated NOEC and EC10 values in this experiment were 7.9 and 13.9 ng BPA l−1 for egg production, respectively, based on measured concentrations in the tanks.
Oehlmann et al. (2006) demonstrated that the effects of BPA on reproductive output and oviduct malformations in the ramshorn snail were temperature dependent. Sexually mature snails (2 replicates of 30) were exposed to 0.25, 0.5, 1, and 5 µg BPA l−1 for five months outside the main spawning season at either 20 or 27°C. As was seen previously, exposure to BPA at 20°C produced significantly more eggs compared with controls. The calculated EC10 value was 14.8 ng l−1 for egg production, supporting the results from Schulte-Oehlmann etal. (2001). In contrast, at 27°C, none of the treatment groups produced more eggs per female compared with controls. At this temperature, however, reproductive output in controls was considerably higher, probably masking the stimulatory effects of BPA that occurred at lower temperatures. In contrast with these findings, Forbes et al. (2007) found no effects of BPA on fecundity, mortality and hatchability in M. cornuarietis when exposed to 0, 0.1, 1.0, 16, 160 or 640 µg BPA l−1 for 12 weeks. However, in that study, snails were exposed at temperatures of 25 ± 1°C, potentially masking the effects of BPA. Furthermore, the study also lacked a positive control, making accurate interpretations on these data difficult.
The mud snail Potamopyrgus antipodarum has also been shown to be sensitive to BPA when exposed via sediments with a stimulation in embryo production at an EC50 of 5.67 µg kg−1 and an EC10 of 0.19 µg kg−1 after four weeks of exposure (Duft et al. 2003). In another study on mud snails, exposure via the water to 1, 5, 25 and 100 µg BPA l−1 also stimulated embryo production (and additionally for the ethinyloestradiol-positive controls) with significant effects at a concentration of 5 µg BPA l−1 (NOEC 1 µg BPA l−1; Jobling et al. 2004).
Bisphenol A effects have also been studied in bivalves, notably Mytilus species. In M. edulis, BPA (at 50 µg l−1 for three weeks) has been shown to increase the phospho-protein levels, induce spawning in both sexes, damage oocytes and ovarian follicles (Aarab et al. 2006) and induce increased micronuclei frequency in gill cells (Baršienė et al. 2006). Canesi et al. (2005) showed that BPA at a nominal concentration of 25 nM (5.7 µg BPA l−1) in the haemolymph caused a significant lysosomal membrane destabilization in haemocytes in M. galloprovincialis. Canesi et al. (2007a) evaluated the lysosomal membrane destabilization in extracted haemocytes of the same species and reported an NOEC of 1 µM and a corresponding EC50 value of 34.5 µM (7.87 mg l−1). Furthermore, BPA induced significant changes in the phosphorylation of MAP kinases and STAT factors, resulting in a decrease in phosphorylation of the stress-activated p38 MAPK and CREB-like transcription factor (cAMP-responsive element binding protein). The MAP-kinase pathway is important in embryogenesis, cell growth, differentiation and apoptosis, and disturbances of STAT signalling favour tumour development by compromising the immune surveillance. Thus the findings of Canesi et al. (2007a) may have significant implications for mussel health. Canesi et al. (2007b) also reported altered patterns of gene expression and activity of enzymes involved in the redox balance following an injection of BPA into the posterior adductor muscle (at nominal doses of 3, 15 and 60 ng BPA g−1 dry wt.). In the digestive gland, an increased expression of the Mytilus ER (MeER2) was observed at the lowest test concentration 24 h after injection, whereas results for the higher test concentrations were not significant. The positive control 17β-oestradiol had the same effect on both receptors (MeER1, MeER2). Metallothionein MT20 gene expression was significantly downregulated; catalase, GSH transferase and GSSG reductase exhibited a changed activity pattern and total glutathione content was enhanced at all test concentrations. Unlike for the phthalate DAP, a study by Cajaraville & Ortiz-Zarragoitia (2006) found no effects of BPA on AOX activity or peroxisomal volume density.
(ii). Crustaceans and insects
Bisphenol A has been shown to be acutely toxic in the high µg l−1 range in crustaceans and aquatic insects. In D. magna, nominal IC/LC50 values vary widely, at between 240 and 12 800 µg l−1 (Chen et al. 2002; Hirano et al. 2004; Park & Choi 2007). The lowest acute value, an IC50 for D. magna of 240 µg l−1, for 24 h (Park & Choi 2007), is remarkably low compared with other studies and also compared with studies for effects on reproduction in D. magna (Brennan et al. 2006). Differences in chemical sensitivity have been shown to occur between strains of D. magna (Baird et al. 1991); therefore, Park & Choi (2007) may have been working with an especially sensitive strain. Applying the Comet assay to this sensitive strain, DNA breakage occurred at 2.1 µg BPA l−1 (Park & Choi 2007).
Generally in D. magna, chronic BPA effects are observed at concentrations slightly below those that are acutely toxic. Bisphenol A inhibits reproduction and development rates with an NOEC of 1000 µg l−1 (Brennan et al. 2006) and affected intermolt duration at 9000 µg l−1 (Mu et al. 2005). In the marine copepods A. tonsa and Tigriopus japonicus, BPA has been shown to inhibit development at 100 and 0.1 µg l−1, respectively (Andersen et al. 2001; Marcial et al. 2003). A significant stimulation of larval development in A. tonsa was, however, observed at 12.5–50 µg l−1 (Mariager 2001), which was also seen in our own experiments at 30 and 60 µg l−1, reported in this paper. In 10-day-old A. tonsa, there was an initial increased egg production at 20 µg l−1 BPA (Andersen et al. 1999), possibly indicating an accelerated rate of maturation owing to a higher development rate of the early life stages at this exposure concentration. In our experiments with fully mature A. tonsa (14 days old), egg production was reduced at a lowest observed effect concentration (LOEC) of 300 µg BPA l−1. Furthermore, in subsequent female offspring derived from exposed parents (to all test concentrations of BPA down to and including 50 µg l−1), the hatching rate was significantly reduced. Mortality in second-generation larvae was also significantly higher compared with controls. These results indicate a maternal transfer of BPA into the developing eggs and/or a disrupting effect during oogenesis. In conclusion, these datasets show that concentrations of BPA between 10 and 60 µg l−1 in the water stimulated development and growth of A. tonsa, while concentrations of 100 µg l−1 and higher inhibited development, growth and egg production.
In studies on C. riparius exposed to BPA via spiked sediments, emergence of second-generation individuals was delayed (at a water phase concentration of 78 ng BPA l−1 and higher, Watts et al. 2001). This observation may have resulted from effects of BPA on the levels of HSP, which has been observed in Gammarus fossarum exposed to BPA (Schirling et al. 2006). In this study, a 34 day exposure to 50 and 500 µg BPA l−1 accelerated maturation of oocytes, reduced the number of offspring produced and resulted in reduced levels of HSP. However, in C. tentans, the expression of HSP70 genes has been shown to be stimulated at concentrations down to 8 µg BPA l−1 (the lowest concentration applied; Lee et al. 2006a). In the D. melanogaster BII cell line, BPA was shown to act as an ecdysteroid antagonist, but only at a high exposure concentration of 22.8 mg l−1 (Dinan et al. 2001). Studies combining BPA with other chemicals have shown interactive effects, affecting the number of embryo abnormalities and offspring emergence (Mu et al. 2005; Wang et al. 2005). In summary, for studies in aquatic arthropods, BPA appears to interfere with their hormone systems—possibly by affecting the synthesis of HSPs and/or by interfering with the ecdysone receptor itself—and causes adverse effects at concentrations in the low µg l−1 range.
(iii). Fish
Studies in vitro using the YES have clearly established that BPA has the ability to act as an oestrogenic substance in vertebrates by directly binding and activating the ER (Harris et al. 1997). In parallel, studies using a human breast cancer cell line (MCF7) have demonstrated that BPA is able to elicit an oestrogenic response similar to that induced by natural oestrogens (Olea et al. 1996). In these studies, the concentrations required to cause an effect, however, were several orders of magnitude higher than that for oestradiol, thus classifying BPA as a weak environmental oestrogen in vertebrates.
The presence of BPA in rivers and streams at relatively high concentrations (up to 21 µg l−1, Crain et al. 2007) has led to significant research efforts investigating its biological effects in fish. Consistent with these findings, BPA has been shown to have feminizing effects in vivo and to induce VTG and/or zona radiata proteins (ZRPs) synthesis in a diverse range of fish species (carp, 100 µg l−1, Mandich et al. 2007; fathead minnow, 160 µg l−1, Brian et al. 2005; Sohoni et al. 2001; cod, 50 µg l−1, Larsen et al. 2006; medaka, 1000 µg l−1, Ishibashi et al. 2005; rainbow trout, 500 µg l−1, Lindholst et al. 2001). These concentrations, however, have not been reported in the aquatic environment. In vivo studies have shown that BPA affects numerous other biological processes and in many cases at concentrations that have been found in the aquatic environment. These effects include disruptions in androgen and oestrogen synthesis and metabolism. Studies in carp have reported that exposure to low concentrations of BPA (1–10 µg l−1) decreases the ratio of oestrogen to androgen in the plasma, whereas exposure to high concentrations (1000 µg l−1) increases it (Mandich et al. 2007). Alterations in concentrations of circulating steroids have also been reported in juvenile turbot (Psetta maxima), exposed to 59 µg BPA l−1 (Labadie & Budzinski 2006). This effect is likely to result from alterations in the levels of steroidogenic enzymes, or their enzymatic activity catalysing the synthesis and/or metabolism of these hormones and/or displacement of oestradiol from sex steroid-binding globulins (reviewed in Crain et al. 2007). The biological consequences of induced changes in the ratio between oestrogens and androgens are wide ranging and may include masculinization or feminization of organisms, and/or alterations in other processes regulated by these hormones (including growth, bone morphogenesis, insulin signalling, neural development, cell division and apoptosis).
Studies in fish, as in some invertebrates, have also provided evidence for differential species sensitivities for exposure to BPA. For example, in exposures of Atlantic cod (Gadus morhua) and turbot (Scophthalmus maximus) to 59 µg BPA l−1 via the water, VTG and ZRP were more highly induced in the cod than in turbot (Larsen et al. 2006). A possible reason for the differences in species sensitivity is in the rate of metabolic transformation of BPA. Supporting this argument, BPA has been shown to be metabolized and eliminated much more rapidly in the zebrafish (D. rerio) than in the rainbow trout (Oncorhynchus mykiss) (Lindholst et al. 2003).
BPA has also been shown to induce alterations in gonadal development and gamete quality in fish, including at concentrations found in the environment. These effects include alterations in the progression of germ cell development in fathead minnow (1 µg BPA l−1, Sohoni et al. 2001), alterations in the gonadal structure in male carp (from 1 µg BPA l−1) and an enhancement of atresia in oocytes in female carp (1 µg BPA l−1, Mandich et al. 2007). In addition, in brown trout (Salmo trutta f. fario), exposures to BPA has been shown to cause reduced sperm quality (1.75–2.40 µg BPA l−1), delayed ovulation in females (1.75 µg BPA l−1) and inhibition of ovulation in females (5 µg BPA l−1, Lahnsteiner et al. 2005). The effects observed for brown trout may have population-level implications as they would lead to a delay in breeding, with the risk that offspring would be produced at less favourable seasonal periods. In carp, exposure to very high levels of BPA (1 mg l−1) has also been shown to induce intersex (Mandich et al. 2007). Another reported physiological effect in turbot exposed to BPA (at 50 µg BPA l−1 for three weeks) is an increased micronuclei frequency in erythrocytes, demonstrating the capability of this compound to induce DNA damage in fish (Bolognesi et al. 2006).
Gene expression studies have been employed with good success for identifying the molecular mechanisms leading to the biological effects of BPA in fish. Studies in Kryptolebias marmoratus (formerly Rivulus m.) have shown that exposure to BPA induces changes in the expression of genes associated with oestrogen signalling, including cytochrome P450 aromatase A and B and ERα but not ERβ or androgen receptor (Lee et al. 2006b; Seo et al. 2006). Similarly, BPA has been shown to induce the expression of ERα in the anal fin of Japanese medaka (O. latipes) (Hayashi et al. 2007). Studies on carp (C. carpio) exposed to BPA employing gene arrays have reported alterations in the expression of many of the same genes induced by exposure to natural and synthetic oestrogens (oestradiol and ethinyloestradiol), but also some unique genes too (Moens et al. 2006, 2007). Studies on K. marmoratus following exposure to BPA also demonstrated differential expression of large sets of genes and, in particular, genes belonging to the Gene Ontologies representing catalytic activity and binding (Lee et al. 2007). These studies however, have generally employed high BPA exposure concentrations. BPA has also been shown to modulate the somatostatin system in the brain of teleosts, resulting in deregulation of neuro-hormonal functions linked to the reproductive system (Alo et al. 2005). Taken together, these molecular mechanistic studies highlight that BPA appears to act predominantly, but not exclusively, through oestrogen signalling pathways in exerting its biological effects on fish.
The biological effects induced in laboratory exposures to BPA are consistent with some phenotypes observed in wild fish living in waters receiving high levels of effluent discharges and containing relatively high concentrations of BPA. Although BPA does not appear to bioconcentrate in fish and can degrade rapidly in the environment (Staples et al. 1998), it can be metabolized into biologically more active compounds (Ishibashi et al. 2005). Overall, the available datasets support the contention that BPA may contribute to adverse reproductive health effects in wild fish populations inhabiting contaminated sites.
(iv). Amphibians
BPA has been shown to induce a wide range of adverse effects in amphibians. Teratogenic effects have been induced in X. laevis embryos exposed from stage 6 onwards (Oka et al. 2003), and they include crooked vertebrae, abnormal development of head and abdomen to stage 40 (at an exposure of 2 × 10−5 M (4.6 mg l−1)), as well as apoptosis in the central nervous system. When exposures are started later than stage 10, however, no adverse impacts of BPA on survival or development occur (Iwamuro et al. 2003; Pickford et al. 2003; Levy et al. 2004). Exposure of Rana temporaria to BPA in combination with another stressor, ultraviolet-B (UVB) radiation, has been shown to induce an enhanced effect on tadpole mortality (Koponen & Kukkonen 2002). The mechanism for this is unknown, but it is not due to an effect of UVB radiation on the accumulation or depuration kinetics of BPA (Koponen et al. 2007).
BPA has been reported to affect sexual development, causing sex reversal at 10−7 M (22.8 µg l−1) in semi-static exposures (Kloas et al. 1999; Levy et al. 2004), but under flow-through conditions (at an exposure to BPA up to 500 µg l−1), no such feminizing effects were found (Pickford et al. 2003). In the new data presented here, no effects of exposure to BPA (using a flow-through system and spanning concentrations from 228 ng l−1 to 228 µg l−1) were seen on gross morphological sex; in the controls and BPA treatments there were between 45 and 50 per cent males and 50 and 55 per cent females, and in the oestradiol (E2)-positive control (0.2 µg l−1) there were 19 per cent males, 72 per cent females and 9 per cent mixed sex. However, detailed histological analysis of the testes (n varies between 19 in the E2 treatment and 47 in 10−6 M BPA treatment)—examining the complete gonad by serial sections—identified effects for the BPA treatments. At 10−9, 10−8, 10−7 and 10−6 M (228 ng l−1 and 2.28, 22.8 and 228 µg l−1), BPA testicular lacunae occurred at frequencies of 15, 20, 10 and 25 per cent and oogonia with frequencies of 40, 60, 55 and 60 per cent, demonstrating oestrogenic effects even at 10−9 M BPA (228 ng l−1). In addition, BPA also affected growth and body weight; these were significantly increased in males exposed to concentrations of 10−8 M BPA (2.28 µg l−1) and above (p < 0.05; Mann–Whitney U-test). This effect on growth was supported by the induction of hepatic IGF-I expression at 10−8 M BPA (2.28 µg l−1) and above. In these treatments, there was a significant induction of VTG gene expression in liver at 10−6 M BPA (228 µg l−1).
In competitive displacement experiments with cytosolic liver extracts of X. laevis, BPA was shown to bind only moderately to amphibian ER (Lutz & Kloas 1999). Similarly in VTG- and ER-mRNA induction studies with primary hepatocytes from male X. laevis, BPA was stimulatory at 10−7 M (22.8 µg l−1) compared with 10−9 M (272 ng l−1) for E2 (Kloas et al. 1999; Lutz et al. 2005). In another, longer term in vitro study, induction of VTG occurred only at a BPA concentration of 1.1 × 10−5 M (2.5 mg l−1) (compared with 4.1 × 10−11 M (11.1 ng l−1) in the E2 positive control; Nomura et al. 2006). Both experimental approaches indicate that BPA is moderately to weakly oestrogenic for VTG and ER induction. BPA has also been shown to significantly reduce basal water absorption of male pelvic patches in the Japanese tree frog, Hyla japonica, a known oestrogenic response (Kohno et al. 2004).
BPA also affects the thyroid system in amphibians. Exposure to 10−5 M BPA (2.28 mg l−1) has been shown to inhibit spontaneous and TH-induced metamorphic changes in X. laevis and suppress TRβ gene expression both in vivo and in vitro (Iwamuro et al. 2003). More recently, in vitro tail cultures of X. laevis have shown to respond to BPA, reducing T3-induced gene expression of TRα and TRβ at exposures as low as 10−7 M (22.8 µg l−1). In contrast, the T3-induced suppression of RXRγ was only moderately affected by exposure to BPA alone (Iwamuro et al. 2006). Similar results for effects of BPA on T3-induced metamorphic changes were found by Goto et al. (2006) studying Silurana tropicalis and R. rugosa. In the skin of Rana tagoi, expression of the antimicrobial peptides preprotemporins (normally upregulated by TH) has been shown to be reduced markedly following BPA treatment at 10−8 M (2.28 µg l−1) (Ohnuma et al. 2006), and using transgenic X. laevis embryos bearing a TH/bZIP-green fluorescent protein construct, T3-induced fluorescence was shown to be inhibited by BPA at a concentration of 10−5 M (2.28 mg l−1; Fini et al. 2007).
4. Conclusion
The datasets reported upon here show that phthalates and BPA can affect both development and reproduction in a wide range of wildlife species. Reproductive and developmental disturbances include alterations in the number of offspring produced and/or reduced hatching success, disruption of larval development and, in insects, delayed emergence. Interphyla differences in responses to plasticizers occur, and as an example, in amphibians, phthalates and BPA disrupt thyroid function impacting on larval development, but no such effects have been reported thus far for fish, even though TH is deposited into fish oocytes and subsequently promotes and regulates larval metamorphosis. Similarly in molluscs, plasticizers have not been found to affect embryo development. There are clear differences in species sensitivity to the effects of plasticizers too. Molluscs, crustaceans and amphibians appear more responsive, with most effects induced in exposures via the water in the low ng l−1 to µg l−1 concentration range. In contrast, effects in fish are generally induced in the higher µg l−1 and mg l−1 range, with the exception of disruption of spermatogenesis by BPA, that occurs in the low µg l−1 range. These taxon-specific effects hamper meaningful comparisons of the relative sensitivities of different phyla to plasticizers.
Given that the biological effect concentrations for plasticizers seen in the laboratory coincide with environmental concentrations, some wildlife populations are probably impacted. To date, however, studies investigating population effects have not been reported, and this constitutes a significant knowledge gap. Furthermore, for invertebrate phyla most highly sensitive to the biological effects of specific plasticizers, long-term exposures should be considered as a research priority. However, only a very limited number of invertebrate phyla have been tested for the effects of phthalates and BPA, and representatives from other, presently neglected, taxa should be considered in the future, including terrestrial species. Mixture effects of plasticizers, as of many other environmental pollutants, are also a much neglected area of study, and given that there are likely to be additive effects, this warrants investigations to assess more accurately their impacts in the environment (see discussion in Koch & Calafat 2009; Teuten et al. 2009; Thompson et al. 2009b). As a final note, increasingly, investigations into the mechanisms of action of plasticizers are finding that some (e.g. phthalates) can have multiple interaction sites in the body, affecting a wide range of biological processes (see discussion in Meeker et al. 2009; Talsness et al. 2009). Thus, a more thorough understanding of the modes of action of these chemicals will help in developing a more comprehensive understanding of their potential for harm and in the identification of the species most vulnerable to their biological effects.
Acknowledgements
This work was financially supported by the European Union (COMPRENDO project, contract EVK1-CT-2002-00129). E.M.S. and G.C.P. were funded by the Natural Environment Research Council (NERC), grant ref NE/C507661/1, under the Post-genomics and Proteomics research programme and under the Environmental Genomics Thematic (grant ref: NER/T/S/2002/00182), and by the Biotechnology and Biological Sciences Research Council (BBSRC; grant ref: 9/S15001), UK to C.R.T.
Footnotes
One contribution of 15 to a Theme Issue ‘Plastics, the environment and human health’.
REFERENCES
- Aarab N., Lemaire-Gony S., Unruh E., Hansen P. D., Larsen B. K., Andersen O. K., Narbonne J. F.2006Preliminary study of responses in mussel (Mytilus edulis) exposed to bisphenol A, diallyl phthalate and tetrabromodiphenyl ether. Aquat. Toxicol. 78, 86–92 (doi:10.1016/j.aquatox.2006.02.021) [DOI] [PubMed] [Google Scholar]
- Albro P. W., Corbett J. T., Schroeder J. L.1993The metabolism of di-(2-ethylhexyl)phthalate in the earthworm Lumbricus terrestris. Comp. Biochem. Physiol. C 104, 335–344 (doi:10.1016/0742-8413(93)90045-M) [DOI] [PubMed] [Google Scholar]
- Alo R., Facciolo R. M., Madeo M., Giusi G., Carelli A., Canonaco M.2005Effects of the xenoestrogen bisphenol A in diencephalic regions of the teleost fish Coris julis occur preferentially via distinct somatostatin receptor subtypes. Brain Res. Bull. 65, 267–273 (doi:10.1016/j.brainresbull.2005.01.006) [DOI] [PubMed] [Google Scholar]
- Andersen H. R., Halling-Sorensen B., Kusk K. O.1999A parameter for detecting estrogenic exposure in the copepod Acartia tonsa. Ecotoxicol. Environ. Saf. 44, 56–61 (doi:10.1006/eesa.1999.1800) [DOI] [PubMed] [Google Scholar]
- Andersen H. R., Wollenberger L., Halling-Sorensen B., Kusk K. O.2001Development of copepod nauplii to copepodites—a parameter for chronic toxicity including endocrine disruption. Environ. Toxicol. Chem. 20, 2821–2829 (doi:10.1897/1551-5028(2001)020<2821:DOCNTC>2.0.CO;2) [PubMed] [Google Scholar]
- Andrady A. L., Neal M. A.2009Applications and societal benefits of plastics. Phil. Trans. R. Soc. B 364, 1977–1984 (doi:10.1098/rstb.2008.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird D. J., Barber I., Bradley M., Soares A. M. V. M., Calow P.1991A comparative study of genotype sensitivity to acute toxic stress using clones of Daphnia magna Straus. Ecotoxicol. Environ. Saf. 21, 257–265 (doi:10.1016/0147-6513(91)90064-V) [DOI] [PubMed] [Google Scholar]
- Barse A. V., Chakrabarti T., Ghosh T. K., Pal A. K., Jadhao S. B.2007Endocrine disruption and metabolic changes following exposure of Cyprinus carpio to diethyl phthalate. Pest. Biochem. Physiol. 88, 36–42 (doi:10.1016/j.pestbp.2006.08.009) [Google Scholar]
- Baršienė J., Šyvokienė J., Bjornstad A.2006Induction of micronuclei and other nuclear abnormalities in mussels exposed to bisphenol A, diallyl phthalate and tetrabromodiphenyl ether-47. Aquat. Toxicol. 78, 105–108 (doi:10.1016/j.aquatox.2006.02.023) [DOI] [PubMed] [Google Scholar]
- Bauer M. J., Herrmann R.1997Estimation of the environmental contamination by phthalic acid esters leaching from household wastes. Sci. Total Environ. 208, 49–57 (doi:10.1016/S0048-9697(97)00272-6) [DOI] [PubMed] [Google Scholar]
- Beauchesne I., Barnabé S., Cooper D. G., Nicell J. A.2007Plasticizers and related toxic degradation products in wastewater sludges. In IWA Specialist Conf. Biosolids, Moncton, New Brunswick, Canada, 24–27 June [DOI] [PubMed] [Google Scholar]
- Biggers W. J., Laufer H.2004Identification of juvenile hormone-active alkylphenols in the lobster Homarus americanus and in marine sediments. Biol. Bull. 206, 13–24 (doi:10.2307/1543194) [DOI] [PubMed] [Google Scholar]
- Bolognesi C., Perrone E., Roggieri P., Pampanin D. M., Sciutto A.2006Assessment of micronuclei induction in peripheral erythrocytes of fish exposed to xenobiotics under controlled conditions. Aquat. Toxicol. 78, 93–98 (doi:10.1016/j.aquatox.2006.02.015) [DOI] [PubMed] [Google Scholar]
- Brennan S. J., Brougham C. A., Roche J. J., Fogarty A. M.2006Multi-generational effects of four selected environmental oestrogens on Daphnia magna. Chemosphere 64, 49–55 (doi:10.1016/j.chemosphere.2005.11.046) [DOI] [PubMed] [Google Scholar]
- Brian J. V., et al. 2005Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ. Health Perspect. 113, 721–728 (doi:10.1289/ehp.7598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajaraville M. P., Ortiz-Zarragoitia M.2006Specificity of the peroxisome proliferation response in mussels exposed to environmental pollutants. Aquat. Toxicol. 78, 117–123 (doi:10.1016/j.aquatox.2006.02.016) [DOI] [PubMed] [Google Scholar]
- Call D. J., et al. 2001An assessment of the toxicity of phthalate esters to freshwater benthos. 1. Aqueous exposures. Environ. Toxicol. Chem. 20, 1798–1804 (doi:10.1897/1551-5028(2001)020<1798:AAOTTO>2.0.CO;2) [PubMed] [Google Scholar]
- Canesi L., Betti M., Lorussoa L. C., Ciaccia C., Gallob G.2005‘In vivo’ effects of bisphenol A in Mytilus hemocytes: modulation of kinase-mediated signalling pathways. Aquat. Toxicol. 71, 73–84 (doi:10.1016/j.aquatox.2004.10.011) [DOI] [PubMed] [Google Scholar]
- Canesi L., Lorussoa L. C., Ciaccia C., Betti M., Rocchi M., Poiana G., Marcomini A.2007aImmunomodulation of Mytilus hemocytes by individual estrogenic chemicals and environmentally relevant mixtures of estrogens: in vitro and in vivo studies. Aquat. Toxicol. 81, 36–44 (doi:10.1016/j.aquatox.2006.10.010) [DOI] [PubMed] [Google Scholar]
- Canesi L., Borghi C., Ciacci C., Fabbri R., Vergani L., Gallo G.2007bBisphenol A alters gene expression and functional parameters in molluscan hepatopancreas. Mol. Cell. Endocrinol. 276, 36–44 (doi:10.1016/j.mce.2007.06.002) [DOI] [PubMed] [Google Scholar]
- Chen M. Y., Ike M., Fujita M.2002Acute toxicity, mutagenicity, and estrogenicity of bisphenol A and other bisphenols. Environ. Toxicol. 17, 80–86 (doi:10.1002/tox.10035) [DOI] [PubMed] [Google Scholar]
- Cook J. W., Dodds E. C., Hewett C. J.1933A synthetic oestrus-exciting compound. Nature 131, 56–57 (doi:10.1038/131056b0) [Google Scholar]
- Corton J. C., Lapinskas P. J.2005Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol. Sci. 83, 4–17 (doi:10.1093/toxsci/kfi011) [DOI] [PubMed] [Google Scholar]
- Crain D. A., Eriksen M., Iguchi T., Jobling S., Laufer H., LeBlanc G. A., Guillette L. J.2007An ecological assessment of bisphenol A: evidence from comparative biology. Reprod. Toxicol. 24, 225–239 (doi:10.1016/j.reprotox.2007.05.008) [DOI] [PubMed] [Google Scholar]
- Dinan L., Bourne P., Whiting P., Dhadialla T. S., Hutchinson T. H.2001Screening of environmental contaminants for ecdysteroid agonist and antagonist activity using the Drosophila melanogaster B-II cell in vitro assay. Environ. Toxicol. Chem. 20, 2038–2046 (doi:10.1897/1551-5028(2001)020<2038:SOECFE>2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Dixon D. R., Wilson J. T., Pascoe P. L., Parry J. M.1999Anaphase aberrations in the embryos of the marine tubeworm Pomatoceros lamarckii (Polychaeta: Serpulidae): a new in vivo test assay for detecting aneugens and clastogens in the marine environment. Mutagenesis 14, 375–383 (doi:10.1093/mutage/14.4.375) [DOI] [PubMed] [Google Scholar]
- Dodds E. C., Lawson W.1936Synthetic oestrogenic agents without the phenanthrene nucleus. Nature 137, 996 (doi:10.1038/137996a0) [Google Scholar]
- Dodds E. C., Lawson W.1938Molecular structure in relation to oestrogenic activity. Compounds without a phenanthrene nucleus. Proc. R. Soc. Lond. B 125, 222–232 (doi:10.1098/rspb.1938.0023) [Google Scholar]
- Dodds E. C., Goldberg L., Lawson W., Robinson R.1938Estrogenic activity of certain synthetic compounds. Nature 141, 247–248 (doi:10.1038/141247b0) [Google Scholar]
- Duft M., Schulte-Oehlmann U., Weltje L., Tillmann M., Oehlmann J.2003Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mudsnail Potamopyrgus antipodarum. Aquat. Toxicol. 64, 437–449 (doi:10.1016/S0166-445X(03)00102-4) [DOI] [PubMed] [Google Scholar]
- EU 2003aEuropean Union Risk Assessment Report for 1,2-benzenedicarboxylic acid, di-C9-11-branched alkyl esters, C10-rich and di-'isodecyl' phthalate (DIDP). Luxembourg: Office for Official Publications of the European Communities [Google Scholar]
- EU 2003bEuropean Union Risk Assessment Report for 1,2-benzenedicarboxylic acid, di-C8-10-branched alkyl esters, C9-rich and di-‘isononyl' phthalate (DINP). Luxembourg: Office for Official Publications of the European Communities [Google Scholar]
- EU 2003cEuropean Union Risk Assessment Report for 4,4′-isopropylidenediphenol (bisphenol-A). Luxembourg: Office for Official Publications of the European Communities [Google Scholar]
- EU 2003dEuropean Union Risk Assessment Report Draft. Bis(2-ethylhexyl)phthalate (DINP). Luxembourg: Office for Official Publications of the European Communities [Google Scholar]
- EU 2004European Union Risk Assessment Report for dibutyl phthalate with addendum to the environmental section 2004. Luxembourg: Office for Official Publications of the European Communities [Google Scholar]
- EU 2006European Union Risk Assessment Report for bis(2-ethylhexyl) phthalate. Luxembourg: Office for Official Publications of the European Communities; Draft of March 2006 [Google Scholar]
- Fan L. Q., You L., Brown-Borg H., Brown S., Edwards R. J., Corton J. C.2004Regulation of phase I and phase II steroid metabolism enzymes by PPARα activators. Toxicology 204, 109–121 (doi:10.1016/j.tox.2004.06.018) [DOI] [PubMed] [Google Scholar]
- Fatoki O. S., Vernon F.1990Phthalates esters in rivers of the Greater Manchester area, UK. Sci. Total Environ. 95, 227–232 (doi:10.1016/0048-9697(90)90067-5) [Google Scholar]
- Fenske M., van Aerle R., Brack S., Tyler C. R., Segner H.2001Development and validation of a homologous zebrafish (Danio rerio Hamilton-Buchanan) vitellogenin enzyme-linked immunosorbent assay (ELISA) and its application for studies on estrogenic chemicals. Comp. Biochem. Physiol. C 129, 217–232 [DOI] [PubMed] [Google Scholar]
- Ferguson M. S., Graham O. H., Bang F. B., Hairston N. G.1946Studies on Schistosomiasis japonica. V. Protection experiments against Schistosomiasis japonica. Am. J. Epidemiol. 44, 367–378 [DOI] [PubMed] [Google Scholar]
- Fini J. B., Le Mevel S., Turque N., Palmier K., Zalko D., Cravedi J. P., Demeneix B.2007An in vivo multiwell-based fluorescent screen for monitoring vertebrate thyroid hormone disruption. Environ. Sci. Technol. 41, 5908–5914 (doi:10.1021/es0704129) [DOI] [PubMed] [Google Scholar]
- Forbes V. E., Aufderheide J., Warbritton R., van der Hoevend N., Caspers N.2007Does bisphenol A induce superfeminization in Marisa cornuarietis? Part II: toxicity test results and requirements for statistical power analyses. Ecotoxicol. Environ. Saf. 66, 319–325 (doi:10.1016/j.ecoenv.2006.09.001) [DOI] [PubMed] [Google Scholar]
- Forget-Leray J., Landriau I., Minier C., Leboulenger F.2005Impact of endocrine toxicants on survival, development, and reproduction of the estuarine copepod Eurytemora affinis (Poppe). Ecotoxicol. Environ. Saf. 60, 288–294 (doi:10.1016/j.ecoenv.2004.06.008) [DOI] [PubMed] [Google Scholar]
- Fromme H., Küchler T., Otto T., Pilz K., Müller J., Wenzel A.2002Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 36, 1429–1438 (doi:10.1016/S0043-1354(01)00367-0) [DOI] [PubMed] [Google Scholar]
- Galli A., Pinaire J., Fischer M., Dorris R., Crabb D. W.2001The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism—a novel mechanism for the development of ethanol-induced fatty liver. J. Biol. Chem. 276, 68–75 (doi:10.1074/jbc.M008791200) [DOI] [PubMed] [Google Scholar]
- Gillesby B. E., Zacharewski T. R.1998Exoestrogens: mechanisms of action and strategies for identification and assessment. Environ. Toxicol. Chem. 17, 3–14 (doi:10.1897/1551-5028(1998)017<0003:EMOAAS>2.3.CO;2) [Google Scholar]
- Goto Y., Kitamura S., Kashiwagi K., Oofusa K., Tooi O., Yoshizato K., Sato J., Ohta S., Kashiwagi A.2006Suppression of amphibian metamorphosis by bisphenol A and related chemical substances. J. Health Sci. 52, 160–168 (doi:10.1248/jhs.52.160) [Google Scholar]
- Harries J. E., Runnalls T., Hill E., Harris C. A., Maddix S., Sumpter J. P., Tyler C. R.2000Development of a reproductive performance test for endocrine disrupting chemicals using pair-breeding fathead minnows (Pimephales promelas). Environ. Sci. Technol. 34, 3003–3011 (doi:10.1021/es991292a) [Google Scholar]
- Harris C. A., Henttu P., Parker M. G., Sumpter J. P.1997The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 105, 802–811 (doi:10.2307/3433697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Nishimoto A., Oshima N., Iwamuro S.2007Expression of the estrogen receptor alpha gene in the anal fin of Japanese medaka, Oryzias latipes, by environmental concentrations of bisphenol A. J. Toxicol. Sci. 32, 91–96 (doi:10.2131/jts.32.91) [DOI] [PubMed] [Google Scholar]
- Heemken O. P., Reincke H., Stachel B., Theobald N.2001The occurrence of xenoestrogens in the Elbe river and the North Sea. Chemosphere 45, 245–259 (doi:10.1016/S0045-6535(00)00570-1) [DOI] [PubMed] [Google Scholar]
- Hirano M., Ishibashi H., Matsumura N., Nagoa Y., Watanabe N., Watanabe A., Onikura N., Kishi K., Arizono K.2004Acute toxicity responses of two crustaceans, Americamysis bahia and Daphnia magna, to endocrine disrupters. J. Health Sci. 50, 97–100 (doi:10.1248/jhs.50.97) [Google Scholar]
- Howard P. H. (ed.) 1989Handbook of environmental fate and exposure data for organic chemicals, vol. I: large production and priority pollutants. Boca Raton, FL: Lewis Publishers [Google Scholar]
- Howard P. H., Banerjee S., Robillard K. H.1985Measurement of water solubilities, octanol water partition-coefficients and vapor-pressures of commercial phthalate-esters. Environ. Toxicol. Chem. 4, 653–661 (doi:10.1897/1552-8618(1985)4[653:MOWSWP]2.0.CO;2) [Google Scholar]
- Ishibashi H., Watanabe N., Matsumura N., Hirano M., Nagao Y., Shiratsuchi H., Kohra S., Yoshihara S., Arizono K.2005Toxicity to early life stages and an estrogenic effect of a bisphenol A metabolite, 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on the medaka (Oryzias latipes). Life Sci. 77, 2643–2655 (doi:10.1016/j.lfs.2005.03.025) [DOI] [PubMed] [Google Scholar]
- Iwamuro S., Sakakibara M., Terao M., Ozawa A., Kurobe C., Shigeura T., Kato M., Kikuyama S.2003Teratogenic and anti-metamorphic effects of bisphenol A on embryonic and larval Xenopus laevis. Gen. Comp. Endocrinol. 133, 189–198 (doi:10.1016/S0016-6480(03)00188-6) [DOI] [PubMed] [Google Scholar]
- Iwamuro S., Yamada M., Kato M., Kikuyama S.2006Effects of bisphenol A on thyroid hormone-dependent up-regulation of thyroid hormone receptor alpha and beta down-regulation of retinoid X receptor gamma in Xenopus tail culture. Life Sci. 79, 2165–2171 (doi:10.1016/j.lfs.2006.07.013) [DOI] [PubMed] [Google Scholar]
- Jagnytsch O., Opitz R., Lutz I., Kloas W.2006Effects of tetrabromobisphenol A on larval development and thyroid hormone regulated biomarkers of the amphibian Xenopus laevis. Environ. Res. 101, 340–348 (doi:10.1016/j.envres.2005.09.006) [DOI] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P.1995A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ. Health Perspect. 103, 582–587 (doi:10.2307/3432434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S., Casey D., Rodgers-Gray T., Oehlmann J., Schulte-Oehlmann U., Pawlowski S., Braunbeck T., Turner A. P., Tyler C. R.2004Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat. Toxicol. 66, 207–222 (doi:10.1016/j.aquatox.2004.01.002) [DOI] [PubMed] [Google Scholar]
- Jonsson S., Baun A.2003Toxicity of mono- and diesters of o-phthalic esters to a crustacean, a green alga, and a bacterium. Environ. Toxicol. Chem. 22, 3037–3043 (doi:10.1897/02-548) [DOI] [PubMed] [Google Scholar]
- Kasahara E., Sato E. F., Miyoshi M., Konaka R., Hiramoto K., Sasaki J., Tokuda M., Nakano Y., Inoue M.2002Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem. J. 365, 849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. J., Kim J. W., Lee S. K.2002Inhibition of oocyte development in Japanese medaka (Oryzias latipes) exposed to di-2-ethylhexyl phthalate. Environ. Int. 28, 359–365 (doi:10.1016/S0160-4120(02)00058-2) [DOI] [PubMed] [Google Scholar]
- Kloas W.2002Amphibians as model for the study of endocrine disrupters. Int. Rev. Cytol. 216, 1–57 (doi:10.1016/S0074-7696(02)16002-5) [DOI] [PubMed] [Google Scholar]
- Kloas W., Lutz I., Einspanier R.1999Amphibians as model to study endocrine disruptors: II. Estrogenic activity of environmental chemicals in vitro and in vivo. Sci. Total Environ. 225, 59–68 (doi:10.1016/S0048-9697(99)80017-5) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Calafat A. M.2009Human body burdens of chemicals used in plastic manufacture. Phil. Trans. R. Soc. B 364, 2063–2078 (doi:10.1098/rstb.2008.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S., Fujime M., Kamishima Y., Iguchi T.2004Sexually dimorphic basal water absorption at the isolated pelvic patch of Japanese tree frog, Hyla japonica. J. Exp. Zool. A 301, 428–438 [DOI] [PubMed] [Google Scholar]
- Koponen P. S., Kukkonen J. V. K.2002Effects of bisphenol A and artificial UVB radiation on the early development of Rana temporaria. J. Toxicol. Environ. Health A 65, 947–959 (doi:10.1080/00984100290071180) [DOI] [PubMed] [Google Scholar]
- Koponen P. S., Tuikka A., Kukkonen J. V. K.2007Effects of ultraviolet-B radiation and larval growth on toxicokinetics of waterborne bisphenol A in common frog (Rana temporaria) larvae. Chemosphere 66, 1323–1328 (doi:10.1016/j.chemosphere.2006.07.018) [DOI] [PubMed] [Google Scholar]
- Kusk K. O., Wollenberger L.1999Fully defined saltwater medium for cultivation of and toxicity testing with marine copepod Acartia tonsa. Environ. Toxicol. Chem. 20, 2821–282911764166 [Google Scholar]
- Kusk K. O., Wollenberger L.2007Towards an internationally harmonized test method for reproductive and developmental effects of endocrine disrupters in marine copepods. Ecotoxicology 16, 183–195 (doi:10.1007/s10646-006-0112-2) [DOI] [PubMed] [Google Scholar]
- Kwak I. S., Lee W.2005Endpoint for DEHP exposure assessment in Chironomus riparius. Bull. Environ. Contam. Toxicol. 74, 1179–1185 (doi:10.1007/s00128-005-0705-0) [DOI] [PubMed] [Google Scholar]
- Labadie P., Budzinski H.2006Alteration of steroid hormone balance in juvenile turbot (Psetta maxima) exposed to nonylphenol, bisphenol A, tetrabromodiphenyl ether 47, diallylphthalate, oil, and oil spiked with alkylphenols. Arch. Environ. Contam. Toxicol. 50, 552–561 (doi:10.1007/s00244-005-1043-2) [DOI] [PubMed] [Google Scholar]
- Lahnsteiner F., Berger B., Kletzl M., Weismann T.2005Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario. Aquat. Toxicol. 75, 213–224 (doi:10.1016/j.aquatox.2005.08.004) [DOI] [PubMed] [Google Scholar]
- Larsen B. K., Bjornstad A., Sundt R. C., Taban I. C., Pampanin D. M., Andersen O. K.2006Comparison of protein expression in plasma from nonylphenol and bisphenol A-exposed Atlantic cod (Gadus morhua) and turbot (Scophthalmus maximus) by use of SELDI-TOF. Aquat. Toxicol. 78, 25–33 (doi:10.1016/j.aquatox.2006.02.026) [DOI] [PubMed] [Google Scholar]
- Larsson P., Thurén A.1987Di-2-ethylhexylphthalate inhibits the hatching of frog eggs and is bioaccumulates by tadpoles. Environ. Toxicol. Chem. 6, 417–422 (doi:10.1897/1552-8618(1987)6[417:DITHOF]2.0.CO;2) [Google Scholar]
- Lee S. K., Veeramachaneni D. N. R.2005Subchronic exposure to low concentrations of di-n-butyl phthalate disrupts spermatogenesis in Xenopus laevis frogs. Toxicol. Sci. 84, 394–407 (doi:10.1093/toxsci/kfi087) [DOI] [PubMed] [Google Scholar]
- Lee S. K., Owens G. A., Veeramachaneni D. N.2005Exposure to low concentrations of di-n-butyl phthalate during embryogenesis reduces survivability and impairs development of Xenopus laevis frogs. J. Toxicol. Environ. Health A 68, 763–772 (doi:10.1080/15287390590930243) [DOI] [PubMed] [Google Scholar]
- Lee S. M., Lee S. B., Park C. H., Choi J.2006aExpression of heat shock protein and hemoglobin genes in Chironomus tentans (Diptera, chironomidae) larvae exposed to various environmental pollutants: a potential biomarker of freshwater monitoring. Chemosphere 65, 1074–1081 (doi:10.1016/j.chemosphere.2006.02.042) [DOI] [PubMed] [Google Scholar]
- Lee Y. M., Seo J. S., Kim I. C., Yoon Y. D., Lee J. S.2006bEndocrine disrupting chemicals (bisphenol A, 4-nonylphenol, 4-tert-octylphenol) modulate expression of two distinct cytochrome P450 aromatase genes differently in gender types of the hermaphroditic fish Rivulus marmoratus. Biochem. Biophys. Res. Commun. 345, 894–903 (doi:10.1016/j.bbrc.2006.04.137) [DOI] [PubMed] [Google Scholar]
- Lee Y. M., Rhee J. S., Hwang D. S., Kim I. C., Raisuddin S., Lee J. S.2007Mining of biomarker genes from expressed sequence tags and differential display reverse transcriptase–polymerase chain reaction in the self-fertilizing fish, Kryptolebias marmoratus, and their expression patterns in response to exposure to an endocrine-disrupting alkylphenol, bisphenol A. Mol. Cells 23, 287–303 [PubMed] [Google Scholar]
- Levy G., Lutz I., Krüger A., Kloas W.2004Bisphenol A induces feminization in Xenopus laevis tadpoles. Environ. Res. 94, 102–111 (doi:10.1016/S0013-9351(03)00086-0) [DOI] [PubMed] [Google Scholar]
- Lindholst C., Pedersen S. N., Bjerregaard P.2001Uptake, metabolism and excretion of bisphenol A in the rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 55, 75–84 (doi:10.1016/S0166-445X(01)00157-6) [DOI] [PubMed] [Google Scholar]
- Lindholst C., Wynne P. M., Marriott P., Pedersen S. N., Bjerregaard P.2003Metabolism of bisphenol A in zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss) in relation to estrogenic response. Comp. Biochem. Physiol. C 135, 169–177 [DOI] [PubMed] [Google Scholar]
- Liu K. J., Lehmann K. P., Sar M., Young S. S., Gaido K. W.2005Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol. Reprod. 73, 180–192 (doi:10.1095/biolreprod.104.039404) [DOI] [PubMed] [Google Scholar]
- Lutz I., Kloas W.1999Amphibians as model to study endocrine disruptors: I. Environmental pollution and estrogen receptor binding. Sci. Total Environ. 225, 49–57 (doi:10.1016/S0048-9697(99)80016-3) [DOI] [PubMed] [Google Scholar]
- Lutz I., Blödt S., Kloas W.2005Regulation of estrogen receptors in primary cultured hepatocytes of the amphibian Xenopus laevis as estrogenic biomarker and its application in environmental monitoring. Comp. Biochem. Physiol. 141, 384–392 [DOI] [PubMed] [Google Scholar]
- Lutz I., Kloas W., Springer T. A., Holden L. R., Wolf J. C., Krüger H. O., Hosmer A. J.2008Development, standardization and refinement of procedures for evaluating effects of endocrine active compounds on development and sexual differentiation of Xenopus laevis. Anal. Bioanal. Chem 390, 2031–2048 (doi:10.1007/s00216-008-1973-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandich A., Bottero S., Benfenati E., Cevasco A., Erratico C., Maggioni S., Massari A., Pedemonte F., Vigano L.2007In vivo exposure of carp to graded concentrations of bisphenol A. Gen. Comp. Endocrinol. 153, 15–24 (doi:10.1016/j.ygcen.2007.01.004) [DOI] [PubMed] [Google Scholar]
- Marcial H. S., Hagiwara A., Snell T. W.2003Estrogenic compounds affect development of harpacticoid copepod Tigriopus japonicus. Environ. Toxicol. Chem. 22, 3025–3030 (doi:10.1897/02-622) [DOI] [PubMed] [Google Scholar]
- Mariager L.2001Effects of environmental endocrine disrupters on a freshwater and a marine crustacean. Master thesis, Aarhus University Dept. Zool., Institute of Biological Sciences, Aarhus, Denmark [Google Scholar]
- Meeker J. D., Sathyanarayana S., Swan S. H.2009Phthalates and other additives in plastics: human exposure and associated health outcomes. Phil. Trans. R. Soc. B 364, 2097–2113 (doi:10.1098/rstb.2008.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe C. D., Metcalfe T. L., Kiparissis Y., Koenig B. G., Khan C., Hughes R. J., Croley T. R., March R. E., Potter T.2001Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 20, 297–308 (doi:10.1897/1551-5028(2001)020<0297:EPOCDI>2.0.CO;2) [PubMed] [Google Scholar]
- Moens L. N., van der Ven K., Van Remortel P., Del-Favero J., De Coen W. M.2006Expression profiling of endocrine-disrupting compounds using a customized Cyprinus carpio cDNA microarray. Toxicol. Sci. 93, 298–310 (doi:10.1093/toxsci/kfl057) [DOI] [PubMed] [Google Scholar]
- Moens L. N., van der Ven K., Van Remortel R., Del-Favero J., De Coen W. M.2007Gene expression analysis of estrogenic compounds in the liver of common carp (Cyprinus carpio) using a custom cDNA microarray. J. Biochem. Mol. Toxicol. 21, 299–311 (doi:10.1002/jbt.20190) [DOI] [PubMed] [Google Scholar]
- Mu X. Y., Rider C. V., Hwang G. S., Hoy H., LeBlanc G. A.2005Covert signal disruption: anti-ecdysteroidal activity of bisphenol A involves cross talk between signalling pathways. Environ. Toxicol. Chem. 24, 146–152 (doi:10.1897/04-063R.1) [DOI] [PubMed] [Google Scholar]
- Naito W., Gamo Y., Yoshida K.2006Screening-level risk assessment of di(2-ethylhexyl) phthalate for aquatic organisms using monitoring data in Japan. Environ. Monit. Assess. 115, 451–471 (doi:10.1007/s10661-006-7239-8) [DOI] [PubMed] [Google Scholar]
- Neuhauser E. F., Durkin P. R., Malecki M. R., Anatra M.1986Comparative toxicity of ten organic chemicals to four earthworm species. Comp. Biochem. Physiol. 83, 197–200 (doi:10.1016/0742-8413(86)90036-8) [DOI] [PubMed] [Google Scholar]
- Nomura Y., Mitsui N., Bhawal U. K., Sawajiri M., Tooi O., Takahashi T., Okazaki M.2006Estrogenic activity of phthalate esters by in vitro VTG assay using primary-cultured Xenopus hepatocytes. Dent. Mater. J. 25, 533–537 [DOI] [PubMed] [Google Scholar]
- Norman A., Borjeson H., David F., Tienpont B., Norrgren L.2007Studies of uptake, elimination, and late effects in Atlantic salmon (Salmo salar) dietary exposed to di-2-ethylhexyl phthalate (DEHP) during early life. Arch. Environ. Contam. Toxicol. 52, 235–242 (doi:10.1007/s00244-005-5089-y) [DOI] [PubMed] [Google Scholar]
- Oehlmann J., Schulte-Oehlmann U., Tillmann M., Markert B.2000Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part I: bisphenol A and octylphenol as xeno-estrogens. Ecotoxicology 9, 383–397 (doi:10.1023/A:1008972518019) [DOI] [PubMed] [Google Scholar]
- Oehlmann J., Schulte-Oehlmann U., Bachmann J., Oetken M., Lutz I., Kloas W., Ternes T. A.2006Bisphenol A induces superfeminization in the ramshorn snail Marisa cornuarietis (Gastropoda: Prosobranchia) at environmentally relevant concentrations. Environ. Health Perspect. 114, 127–133 (doi:10.1289/ehp.8065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma A., Conlon J. M., Kawasaki H., Iwamuro S.2006Developmental and triiodothyronine-induced expression of genes encoding preprotemporins in the skin of Tago's brown frog Rana tagoi. Gen. Comp. Endocrinol. 146, 242–250 (doi:10.1016/j.ygcen.2005.11.015) [DOI] [PubMed] [Google Scholar]
- Ohtani H., Miura I., Ichikawa Y.2000Effects of dibutyl phthalate as an environmental endocrine disrupter on gonadal sex differentiation of genetic males of the frog Rana rugosa. Environ. Health Perspect. 108, 1189–1193 (doi:10.2307/3434832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Adati N., Shinkai T., Sakuma K., Nishimura T., Kurose K.2003Bisphenol A induces apoptosis in central neural cells during early development of Xenopus laevis. Biochem. Biophys. Res. Commun. 312, 877–882 (doi:10.1016/j.bbrc.2003.10.199) [DOI] [PubMed] [Google Scholar]
- Olea N., Pulgar R., Perez P., Oleaserrano F., Rivas A., NovilloFertrell A., Pedraza V., Soto A. M., Sonnenschein C.1996Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 104, 298–305 (doi:10.2307/3432888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz R., Lutz I., Nguyen N., Scanlan T., Kloas W.2006The analysis of thyroid hormone receptor βA mRNA expression in Xenopus laevis tadpoles as a means to detect agonism and antagonism of thyroid hormone action. Toxicol. Appl. Pharmacol. 212, 1–13 (doi:10.1016/j.taap.2005.06.014) [DOI] [PubMed] [Google Scholar]
- Orbea A., Ortiz-Zarragoitia M., Cajaraville M. P.2002Interactive effects of benzo(a)pyrene and cadmium and effects of di(2-ethylhexyl) phthalate on antioxidant and peroxisomal enzymes and peroxisomal volume density in the digestive gland of mussel Mytilus galloprovincialis Lmk. Biomarkers 7, 33–48 [DOI] [PubMed] [Google Scholar]
- Park S. Y., Choi J.2007Cytotoxicity, genotoxicity and ecotoxicity assay using human cell and environmental species for the screening of the risk from pollutant exposure. Environ. Int. 33, 817–822 (doi:10.1016/j.envint.2007.03.014) [DOI] [PubMed] [Google Scholar]
- Patyna P. J., Brown R. P., Davi R. A., Letinski D. J., Thomas P. E., Cooper K. R., Parkerton T. F.2006Hazard evaluation of diisononyl phthalate and diisodecyl phthalate in a Japanese medaka multigenerational assay. Ecotoxicol. Environ. Saf. 65, 36–47 (doi:10.1016/j.ecoenv.2005.05.023) [DOI] [PubMed] [Google Scholar]
- Peijnenburg W., Struijs J.2006Occurrence of phthalate esters in the environment of The Netherlands. Ecotoxicol. Environ. Saf. 63, 204–215 (doi:10.1016/j.ecoenv.2005.07.023) [DOI] [PubMed] [Google Scholar]
- Pickford D. B., Hetheridge M. J., Caunter J. E., Hall A. T., Hutchinson T. H.2003Assessing chronic toxicity of bisphenol A to larvae of the African clawed frog (Xenopus laevis) in a flow-through exposure system. Chemosphere 53, 223–235 (doi:10.1016/S0045-6535(03)00308-4) [DOI] [PubMed] [Google Scholar]
- Santos E. M., Paull G. C., Van Look K. J. W., Workman V. L., Holt W. V., Van Aerle R., Kille P., Tyler C. R.2007Gonadal transcriptome responses and physiological consequences of exposure to oestrogen in breeding zebrafish (Danio rerio). Aquat. Toxicol. 83, 134–142 (doi:10.1016/j.aquatox.2007.03.019) [DOI] [PubMed] [Google Scholar]
- Schirling M., Jungmann D., Ladewig V., Ludwichowski K. U., Nagel R., Köhler H. R., Triebskorn R.2006Bisphenol A in artificial indoor streams: II. Stress response and gonad histology in Gammarus fossarum (Amphipoda). Ecotoxicol. 15, 143–156 (doi:10.1007/s10646-005-0044-2) [DOI] [PubMed] [Google Scholar]
- Schulte-Oehlmann U., Tillmann M., Casey D., Duft M., Markert B., Oehlmann J.2001Östrogenartige Wirkungen von Bisphenol A auf Vorderkiemerschnecken (Mollusca: Gastropoda: Prosobranchia). UWSF—Z. Umweltchem. Ökotox. 13, 319–333 [Google Scholar]
- Seo J. S., Lee Y. M., Jung S. O., Kim I. C., Yoon Y. D., Lee J. S.2006Nonylphenol modulates expression of androgen receptor and estrogen receptor genes differently in gender types of the hermaphroditic fish Rivulus marmoratus. Biochem. Biophys. Res. Commun. 346, 213–223 (doi:10.1016/j.bbrc.2006.05.123) [DOI] [PubMed] [Google Scholar]
- Sohoni P., Sumpter J. P.1998Several environmental oestrogens are also anti-androgens. J. Endocrinol. 158, 327–339 (doi:10.1677/joe.0.1580327) [DOI] [PubMed] [Google Scholar]
- Sohoni P., et al. 2001Reproductive effects of long-term exposure to bisphenol A in the fathead minnow (Pimephales promelas). Environ. Sci. Technol. 35, 2917–2925 (doi:10.1021/es000198n) [DOI] [PubMed] [Google Scholar]
- Staples C. A., Peterson D. R., Parkerton T. F., Adams W. J.1997The environmental fate of phthalates esters: a literature review. Chemosphere 35, 667–749 [Google Scholar]
- Staples C. A., Dorn P. B., Klecka G. M., O'Block S. T., Harris L. R.1998A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36, 2149–2173 (doi:10.1016/S0045-6535(97)10133-3) [DOI] [PubMed] [Google Scholar]
- Staples C. A., Woodburn K., Caspers N., Hall A. T., Klečka G. M.2002A weight of evidence approach to the aquatic hazard assessment of bisphenol A. Hum. Ecol. Risk Assess. 8, 1083–1105 (doi:10.1080/1080-700291905837) [Google Scholar]
- Tagatz M. E., Plaia G. R., Deans C. H.1986Toxicity of dibutyl phthalate-contaminated sediment to laboratory- and field-colonized estuarine benthic communities. Bull. Environ. Contam. Toxicol. 37, 141–150 (doi:10.1007/BF01607741) [DOI] [PubMed] [Google Scholar]
- Talsness C. E., Andrade A. J. M., Kuriyama S. N., Taylor J. A., vom Saal F. S.2009Components of plastic: experimental studies in animals and relevance for human health. Phil. Trans. R. Soc. B 364, 2079–2096 (doi:10.1098/rstb.2008.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuten E. L., et al. 2009Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 364, 2027–2045 (doi:10.1098/rstb.2008.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut R., Porte C.2004Effects of endocrine disrupters on sex steroid synthesis and metabolism pathways in fish. J. Steroid Biochem. Mol. Biol. 92, 485–494 (doi:10.1016/j.jsbmb.2004.10.008) [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Moore C. J., vom Saal F. S., Swan S. H.2009aPlastics, the environment and human health: current consensus and future trends. Phil. Trans. R. Soc. B 364, 2153–2166 (doi:10.1098/rstb.2009.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Swan S. H., Moore C. J., vom Saal F. S.2009bOur plastic age. Phil. Trans. R. Soc. B 364, 1973–1976 (doi:10.1098/rstb.2009.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuren A., Woin P.1991Effects of phthalate-esters on the locomotor-activity of the fresh water amphipod Gammarus pulex. Bull. Environ. Contam. Toxicol. 46, 159–166 (doi:10.1007/BF01688270) [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Olmstead A. W., Li H., LeBlanc G. A.2005The screening of chemicals for juvenoid-related endocrine activity using the water flea Daphnia magna. Aquat. Toxicol. 74, 193–204 (doi:10.1016/j.aquatox.2005.05.010) [DOI] [PubMed] [Google Scholar]
- Watts M. M., Pascoe D., Carroll K.2001Chronic exposure to 17α-ethinylestradiol and bisphenol A—effects on development and reproduction in the freshwater invertebrate Chironomus riparius (Diptera: Chironomidae). Aquat. Toxicol. 55, 113–124 (doi:10.1016/S0166-445X(01)00148-5) [DOI] [PubMed] [Google Scholar]
- Weltje L., Schulte-Oehlmann U.2007The seven year itch—progress in research on endocrine disruption in aquatic invertebrates since 1999. Ecotoxicology 16, 1–3 (doi:10.1007/s10646-006-0116-y) [Google Scholar]
- Wibe A. E., Billing A., Rosenqvist G., Jenssen B. M.2002Butyl benzyl phthalate affects shoaling behavior and bottom-dwelling behavior in threespine stickleback. Environ. Res. 89, 180–187 (doi:10.1006/enrs.2002.4360) [DOI] [PubMed] [Google Scholar]
- Wibe A. E., Fjeld E., Rosenqvist G., Jenssen B. M.2004Postexposure effects of DDE and butylbenzylphthalate on feeding behavior in threespine stickleback. Ecotoxicol. Environ. Saf. 57, 213–219 (doi:10.1016/S0147-6513(03)00005-8) [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Dixon D. R., Dixon L. R. J.2002Numerical chromosomal aberrations in the early life-history stages of a marine tubeworm, Pomatoceros lamarckii (Polychaeta: Serpulidae). Aquat. Toxicol. 59, 163–175 (doi:10.1016/S0166-445X(01)00249-1) [DOI] [PubMed] [Google Scholar]
- Wollenberger L., Dinan L., Breitholtz M.2005Brominated flame retardants: activities in a crustacean development test and in an ecdysteroid screening assay. Environ. Toxicol. Chem. 24, 400–407 (doi:10.1897/03-629.1) [DOI] [PubMed] [Google Scholar]
- Xiao-yu H., Bei W., Shuzhen Z., Xiao-quan S.2005Bioavailability of phthalate congeners to earthworms (Eisenia fetida) in artificially contaminated soils. Ecotoxicol. Environ. Saf. 62, 26–34 (doi:10.1016/j.ecoenv.2005.02.012) [DOI] [PubMed] [Google Scholar]