Abstract
Over the past five or six decades, contamination and pollution of the world’s enclosed seas, coastal waters and the wider open oceans by plastics and other synthetic, non-biodegradable materials (generally known as ‘marine debris’) has been an ever-increasing phenomenon. The sources of these polluting materials are both land- and marine-based, their origins may be local or distant, and the environmental consequences are many and varied. The more widely recognized problems are typically associated with entanglement, ingestion, suffocation and general debilitation, and are often related to stranding events and public perception. Among the less frequently recognized and recorded problems are global hazards to shipping, fisheries and other maritime activities. Today, there are rapidly developing research interests in the biota attracted to freely floating (i.e. pelagic) marine debris, commonly known as ‘hangers-on and hitch-hikers’ as well as material sinking to the sea floor despite being buoyant. Dispersal of aggressive alien and invasive species by these mechanisms leads one to reflect on the possibilities that ensuing invasions could endanger sensitive, or at-risk coastal environments (both marine and terrestrial) far from their native habitats.
Keywords: pelagic plastics, marine debris, entanglement and ingestion, hitch-hiking, alien invasions
1. Introduction
The environmental and other problems arising from indiscriminate disposal of plastics and other persistent synthetic materials (marine debris) into the global oceans and seas are chronic in nature rather than acute, and are long-recognized international problems (e.g. Mattlin & Cawthorn 1986; Thompson et al. 2009b). The endangering impacts of these materials on marine environments are many and are succinctly reviewed by Derraik (2002). These undesirable contaminants may have either land- or marine-based sources, although the latter is generally considered to be the more significant. Management, and preferably prevention, or at least reducing the problems created by marine debris are difficult to address. Available evidence suggests that the quantities involved are ever increasing and hence so is the magnitude of the resulting problems (see Barnes et al. 2009; Ryan et al. 2009). It has recently been estimated that the 1982 report of 8 million marine debris items entering the world’s oceans and seas each day now needs to be updated by being multiplied several fold (Barnes 2005). Even the most remote of localities of both Northern and Southern hemispheres are no longer immune from littering by marine debris: e.g. Antarctica and sub-Antarctic Islands of the Southern Ocean (Gregory et al. 1984; Eriksson & Burton 2003; Barnes & Milner 2005); North Pacific gyre (Moore 2003; Ebbesmeyer et al. 2007) and South Pacific Islands (Gregory 1999a). Nevertheless, and in contrast to the above comments, censuses at crudely 10 year intervals (mid-1970s, 1980s, 1990s, and mid-2000s in progress) of virgin plastic granules (pellets or nibs) suggest the quantities are slowly and steadily, but somewhat irregularly, declining on the shores of New Zealand, eastern Canada and possibly Bermuda (M. R. Gregory, unpublished). This may be a reflection of changes in handling and transport procedures rather than conscientious or focused efforts addressing the problem. Similar decreases in the composition of plastic litter in surface waters of the Atlantic and southwestern Indian oceans, and reductions in amounts ingested by several seabirds, have been reported recently (Ryan 2008; Ryan et al. 2009).
Many of the problems associated with marine debris attract considerable media and public attention. Foremost of these are the visual affront of unsightly discarded plastic and aesthetic values in general (figure 1). There are also tourist perceptions and emotive issues arising from widely published images of seabirds, marine mammals and fish entangled in abandoned or lost netting; furthermore, entanglement (figure 2) and ingestion (figure 3) may lead to death from starvation and debilitation, with a reduced quality of life and lowered reproductive performance (Laist 1987). Other impacts to receive limited attention are of no less importance, e.g. damage to subsistence fisheries (Nash 1992); hazards to recreational boating and larger commercial vessels; impact of plastic sheeting that blankets the biota of soft sediment, reef and rocky substrata (Uneputty & Evans 1997) as well as anoxia and hypoxia induced by inhibition of gas exchange between pore waters and overlying sea water (Goldberg 1997; Gregory & Andrady 2003).
Figure 1.
Debris (mainly plastic) collected during an annual beach clean at Mason Bay, South Island, New Zealand.
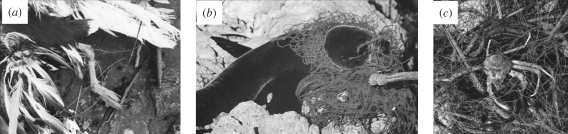
Figure 2.
Examples of entanglement from New Zealand that draw immediate public sympathy and anger: (a) Karoro (southern black-backed gull, Larus dominicanus) caught and hooked in nylon filament fishing line; (b) a New Zealand fur seal trapped in discarded netting and (c) Ghost fishing—derelict fishing gear dredged from >100 m on the Otago shelf.
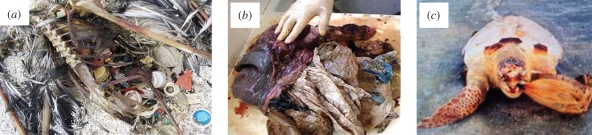
Figure 3.
Examples of ingestion: (a) Laysan Albatross (Phoebastria immutabilis, at Kure Atoll, Courtesy of AMRF); (b) plastic from the stomach of a young Minke whale (Balaenoptera acutorostrata) that had been washed ashore dead in France (Courtesy of G. Mauger & F. Kerleau, Groupe d’Études de Cétacés du Cotentin GECC) and (c) stranded sea turtle disgorging an inflated plastic bag. One infers that it has been mistaken for an edible jellyfish (medusoid).
2. Aesthetic values, entanglement, ingestion, smothering, ghost fishing, the wrack and beach cleaning
(a). Aesthetic values
Many of the litter problems associated with marine debris attract considerable media and public attention. Foremost of these is the visual affront of unsightly, discarded and/or accidentally lost plastic and other manufactured materials that tend to strand and concentrate along shorelines and sandy beaches (figure 1)—ones often of considerable recreational importance. There are also strongly emotive issues associated with both local beach users and tourist perceptions. Financial concerns over visitor numbers may also be a significant factor. Harshly critical public responses are common and may reflect personal observations or widely published and often harrowing images of seabirds, marine mammals and fish entangled in abandoned and beach-cast or lost netting (figure 2). Terrestrial vertebrates may also be snared or trapped in wrack debris. Where tidal range is moderate, and particularly during periods of consecutive spring high tides, unsightly littering material may be buried and hidden from view. Exhumation of litter may occur during later periods of higher wave activity (Williams & Tudor 2001a) and may also be cyclic in character. In addition to those factors identified previously (above) and later (below), concerns are commonly expressed about economic losses, health issues and harm to local biota, and otherwise general impressions of longer term deterioration in beach aesthetic values (e.g. Gabrielides 1995).
(b). Entanglement and ghost fishing
Laist (1997) has compiled a comprehensive list of marine species known to be impacted by entanglement (E) and ingestion (I). He identifies over 250 species, how impacted (E or I), the material involved, as well as location and source. The identified taxa include: turtles; penguins; albatrosses, petrels and shearwaters; shorebirds, skuas, gulls and auks; coastal birds other than seabirds; baleen whales, toothed whales and dolphins; earless or true seals, sea lions and fur seals; manatees and dugong; sea otters; fish and crustaceans.
Prior to the 1950s, rope and cordage used in all marine activities, including fisheries, was made of natural fibres—typically Indian or Manila hemp and cotton, and it was often strengthened with a coating of tar or strips of worn canvas. These materials lose their resilience in usage and if lost or discarded at sea tend to disintegrate quickly. For reasons summarized and simplified in table 1, over the past 50+ years these natural fibres have been replaced by nylon and other synthetic materials that are generally buoyant and far more endurable. The very properties that humankind find so desirable in plastic materials are also those responsible for the plethora of problems they are creating (globally) in marine environments.
Table 1.
Summary of factors complicating and compromising analyses of marine entanglement (taken and adapted from Laist, D.W. 1996, p. 106). (Entanglement records are biased towards shoreline surveys. They may remain unpublished and/or be anecdotal in character: local and regional, as well as geographic and temporal comparisons will be difficult to evaluate.)
| detection and discovery | sampling and reporting biases |
|---|---|
| entanglements are isolated events scattered over wide areas | limited at-sea sampling and few long-term surveys |
| entangling debris often difficult to identify on active animals at sea; readily recognized when stranded | inconsistent sampling methodologies; strandings are an unknown portion of local and regional entanglements |
| dead animals difficult to see if floating just below sea surface and if concealed within matted debris mass | shore counts of live entangled animals are biased towards survivors sporting minor amounts of debris |
| entangled dead animals may disappear from view quickly through sinking and/or predation | some entanglements may involve interactions with active rather than derelict fishing gear |
Many marine animals (sea turtles, mammals, seabirds, fish and crustaceans) are either drawn to or accidentally entangled in netting, rope and monofilament lines that have their sources in discards and losses from commercial fishing activities. Set and drift nets are particularly hazardous. Many animals, if not most so caught, find it difficult to escape entanglement and are doomed to drown or die from injury, starvation and general debilitation. There are numerous reports of packing loops (cut and uncut) attracting the interest of seals and sea lions (e.g. Hanni & Pyle 2000, Southeast Farallon Island, California; Henderson 2001, Monk seals, Northwestern Hawaiian Islands; Page et al. 2004, Australian sea lions and New Zealand fur seals, Kangaroo Island, Australia; Boren et al. 2006, New Zealand fur seals, Kaikoura, NZ; Hofmeyr et al. 2006, Antarctic fur seals, Bouvetøya Island). Sharks also are often caught by ‘debris collars’ (e.g. Sazima et al. 2002). Recorded changing rates of entanglement in these studies are difficult to decipher, but it is evident that with intervention, individuals with severe wounds have a good chance of survival (see Page et al. 2004; Boren et al. 2006). Plastic packing loops may tighten and cut into flesh as animals grow, creating ‘lethal necklaces’ (figure 2b) ultimately leading to strangulation. Carelessly discarded plastic six-pack carriers may similarly capture fish and other wildlife; paperboard is not so endangering (Thompson & Côtė 1997). Other biologically harmful factors can include suppurating skin lesions, ulcerating body wounds, interruption of feeding activity and failed predator avoidance (Gregory 1991).
In recent years, sightings have regularly been made of pods of the endangered humpback whales travelling northwards along the east coast of the South Island of New Zealand and on their annual passage between Antarctic waters and tropical waters to the north. Over the last 7 or 8 years at least seven whales have had in tow a mass of tangled rope and other debris. In at least two instances the mass has included a crayfish (i.e. lobster) pot and a buoy with marker pole and flag. Attempts to free entanglements were successful in at least one instance and failed in others (D. Hayes, personal communication).
Tangled masses of relatively intact, but lost and abandoned or derelict, trawl net, gillnet, webbing and monofilament line can retain the ability to continue to capture target fish and other species for lengthy periods of time (Laist 1996; Carr & Harris 1997). This may lead to ghost fishing, with conspicuous mortality and catch losses. Comparable waste problems were associated with ‘drift-net’ fisheries in the South Pacific in the 1980s (Wright & Doulman 1991; Richards 1994). Ghost-net fishing is not restricted to surface or shallow waters. Over the past 20+ years, important seamount fisheries have developed around New Zealand and Tasmania and are known from elsewhere around the region (e.g. Koslow 1997; de Forges et al. 2000; O’Driscoll & Clark 2005). It is widely accepted that seamounts are fragile habitats. Trawl gear is today being deployed across steeply irregular, and often boulder-strewn, sea floor surfaces at depths typically lying between 500 and 1000/2000 m. Netting caught during passage across the seabed can cause considerable damage to seabed environments (e.g. deep water coral reefs), and if not recovered may remain there, out of sight, and continue ghost fishing almost indefinitely. The potential magnitude of disturbance to seabed environments can be likened to ‘forest clear cutting’ (Watling & Norse 1998).
(c). Ingestion
The literature on ingestion (and entanglement) of plastic items in marine debris is voluminous and often repetitive, and the widely reported environmental problems identified are global in character. These include: wounds (internal and external), suppurating skin lesions and ulcerating sores; blockage of digestive tract followed by satiation, starvation and general debilitation often leading to death; reduction in quality of life and reproductive capacity; drowning and limited predator avoidance; impairment of feeding capacity; and the possibility that plastic resin pellets may adsorb and concentrate potentially damaging toxic compounds from sea water (e.g. Gregory 1978, 1991; Laist 1997; Mato et al. 2001; see also the discussions in Oehlmann et al. 2009; Teuten et al. 2009).
Over 100 species of seabirds are known to ingest plastic artefacts and/or become entangled with them (Laist 1997). First local New Zealand recognition of high virgin plastic pellet concentrations on Auckland City beaches was made in the astral summer of 1971–1972 (Gregory 1977). Subsequent observations on remote beaches north of Auckland over the astral summers of 1972/1973/1974 and 1975 and examination of wrack along strandlines revealed a surprising abundance of plastic pellets and other marine debris. Observers quickly became aware of a developing environmental problem. Recording pellet quantities was a diversion during coastal studies along the extensive sandy beaches and dune fields of northern New Zealand. In subsequent years, ‘plastic pellet’ expeditions were made to beaches around the rest of the country (Gregory 1978). Occasionally, one came across beach-cast birds and attention was drawn to plastic pellets associated with disintegrating carcasses and also entangling monofilament line, often with attached fishhook. Over a 21-year period (1958–1977), observations were made of five prion species (Pachyptila) cast ashore on exposed beaches near Wellington (southern North Island). Gizzards and proventriculi were removed and examined. Harper & Fowler (1987) noted that the lightest birds carried the most pellets and concluded that the proportion of starved beach-cast prions suggested these birds would eat anything resembling food before they died. They also suggested that prions began ingesting plastic pellets by the early 1960s, and an accompanying graphical presentation shows irregular but rapid increases in the percentages of plastic carried in three prion species which grew significantly (from <5 to 25%) between 1960 and 1977 (Harper & Fowler 1987; table 2).
Table 2.
Occurrence of plastic pellets in five prion species collected from New Zealand beaches, between 1958 and 1977 (from Harper & Fowler 1987; p. 66).
| age class |
number with plastic pellets |
% total pellets |
||||||
|---|---|---|---|---|---|---|---|---|
| species | Imm | adult | gizzards examined | Imm | adult | Imm | adult | species status |
| broad-billed prion Pachyptila vittata | 170 | 140 | 310 | 18 | 33 | 10.6 | 23.6 | NZ resident |
| Salvin’s prion Pachyptila salvani | 651 | 12 | 663 | 133 | 0 | 20.4 | 0 | Indian Ocean migrant |
| Antarctic prion Pachyptila desolata | 29 | 6 | 35 | 4 | 1 | 13.8 | 16.7 | NZ sub-Antarctic migrant |
| thin-billed prion Pachyptila belcheri | 147 | 5 | 152 | 10 | 0 | 6.8 | 0 | Indian Ocean migrant |
| fairy prion Pachyptila tutur | 714 | 105 | 819 | 88 | 13 | 12.4 | 2.4 | NZ resident |
| totals | 1711 | 266 | 1979 | 253 | 47 | |||
Imm, immature, birds of the year; adults, all others. Age determined by bone ossification, gonad condition, bill and feet shrinkage, measurements, plumage.
Plastic materials of varying kinds had spread to all oceans and adjacent seas by the late 1970s or early 1980s and wide concern was being expressed over the amounts of cylindrical, virgin plastic pellets that are industrial feedstock, together with fragmented plastic particles of varying size and shape that were being ingested by pelagic seabirds (e.g. Shomura & Yoshida 1985). Over the past four or five decades, there have been numerous accounts of marine debris ingestion by a great variety of seabirds (see Appendix 2 in Laist 1997). Some representative examples typifying the global spread of plastic ingestion behaviour include red phalaropes (Connors & Smith 1982); 15 species of sea birds, Gough Island, South Atlantic Ocean (Furness 1985); Wilsons storm-petrels, Antarctica (Van Franeker & Bell 1988); storm-petrels, etc. (Blight & Burger 1997); short-tailed shearwaters, Bering Sea (Vlietstra & Parga 2002); southern giant petrels, Southern Atlantic Ocean (Copello & Quintana 2003); northern fulmars, Nunavut, Davis Strait (Mallory et al. 2006). Cadee (2002) has drawn attention to conspicuous bird pecking marks (possibly made by Northern Fulmars) in cuttlebones cast ashore on the Dutch coast near Texel. It was also noted that similar peck marks were common locally on beach-cast styrofoam and spongy plastic and it was suggested that fulmars were mistaking plastic artefacts for cuttlebone.
As well as being entangled in discarded fishing gear, many marine vertebrate species have a record of regularly ingesting discarded plastic materials (see Laist 1996; Appendices 1 and 3). Several, if not most, sea turtle species are seriously threatened by ‘feeding on’ plastic and other marine debris (e.g. Hawaiian Islands, Balazs 1985; coastal Florida, Bjorndal et al. 1994; Western Mediterranean, Tomás et al. 2002; Paraíba, Brazil, Mascarenhas et al. 2004). Particular hazards are discarded and semi-inflated, floating plastic bags that are often mistaken for jelly fish (medusoids), which block the oesophagus (figure 3c). Manatee also have felt the undesirable impact of marine debris (e.g. Florida, Beck & Barros 1991). An unusual accumulation of small plastic particles recovered in the scats of fur seals from Macquarie Island has been recorded by Eriksson & Burton (2003). These were small, often angular in shape and buoyant, with surface striations, and could not be related to plastic pellet feedstock. It is suggested that the breakdown of larger, user plastic fragments was a response to being washed ashore and ground down by abrasion on high energy cobble beaches. Eriksson & Burton (2003, p. 380) furthermore hypothesized that the plastic particles were initially washed out to sea, before being size-selected and consumed by pelagic fish, and that the latter were the prey of fur seals.
(d). Smothering
Most plastic materials entering the marine environment are buoyant and float on the sea surface. It is therefore perhaps surprising to find that there are numerous reports of sunken marine debris of all kinds settling to the sea floor at all depths—from inter-tidal to abyssal environments; e.g. the Skagerrak (Hollström 1975); Tokyo Bay, Japan (Kanehiro et al. 1995); tidal flats, Ambon Bay, Indonesia (Uneputty & Evans 1997); Bristol Channel (Williams & Simmons 1997); European and Mediterranean waters (Galil et al. 1995; Stefatos et al. 1999; Galgani et al. 2000); Kodiak Island, Alaska (Hess et al. 1999); southern California Bight (Moore & Allen 2000); Hauraki Gulf, New Zealand (Backhurst & Cole 2000); Saronikos Gulf, Greece (Katsanevakis et al. 2007). Once these items reach the sea floor, particularly in deeper and still waters, they are doomed to a slow and yet permanent entombment.
Several authorities now consider that the sea floor is the ultimate sink for much marine debris (e.g. Williams et al. 1993; Goldberg 1997). The mechanisms by which these materials may reach the deep sea floor are poorly understood. Land-sourced materials are common on canyon floors of the western Mediterranean Sea. These can be tracked from the coast in their progressive passage to abyssal depths and at considerable distance offshore (Galgani et al. 2000). The pattern is strongly suggestive of rapid transport through near-shore zones and entrainment in bottom hugging currents (Williams et al. 2005). There is also evidence from the Rio de la Plata that bottom salinity fronts in estuarine environments may act as debris-accumulating barriers (Acha et al. 2003), similar to those associated with surface waters along convergence zones, oceanic fronts and eddies (e.g. Gregory 1999b). Furthermore, rapid and heavy fouling of floating plastic (and other objects) may so increase density that they sink to the sea floor. However, grazing organisms may episodically clean fouled surfaces leading to yo-yoing periods of submergence and resurfacing until permanent settlement to the sea floor occurs (Ye & Andrady 1991).
Sediment settling on pelagic plastic materials may also take them to the sea floor. Observations made in shallow, near-shore waters, by Backhurst & Cole (2000) and Katsanevakis et al. (2007), have confirmed that once there, gradual changes may occur in community structure and that the environment can no longer be considered pristine. Goldberg (1997) has suggested that the blanketing effects of plastic sheeting on the sea floor could lead to anoxia and hypoxia induced by inhibition of gas exchange between pore water and sea water. Furthermore, sediment settling on pelagic plastic materials and taking them to the sea floor can lead to the creation of artificial hardgrounds (e.g. Harms 1990). Following in a somewhat similar vein, Williams et al. (2005, p. 627), perhaps with a degree of irony, have claimed that benthic marine debris once settled on the sea floor could ‘… enhance or enrich local biodiversity in the short term, for in the long term it is doomed to permanent interment in a slowly accumulating sediment cover’. An interesting and disturbing aside that relates to settling rates of plastic items is Oshima’s (2000, p. 73) report of numerous white plastic shopping bags suspended upside down and freely drifting in the ocean at water depths of 2000 m—and looking like an assembly of ghosts.
(e). The wrack and beach cleaning
Natural flotsam, of both marine and terrestrial origin (seaweeds and plants) together with jetsam of indeterminate sources, tends to accumulate along high-tide strandlines, where it is commonly known as ‘the wrack’. These areas are often ephemeral, dynamic and seasonal environments and also tend to accumulate significant quantities of manufactured materials, in particular those made of plastic and other non-destructibles. As a consequence, wrack environments are commonly unsightly and the demands of local authorities to ‘clean up the mess’ are frequent and can be expensive (e.g. Ryan & Swanepoel 1996; Ballance et al. 2000). Often, and increasingly, the demands are for mechanical and complete removal of the strandline and any debris that is concentrated there. Llewellyn & Shackley (1996) demonstrated that a consequence of this may be the destruction of ecologically significant habitats. These habitats support rich and diverse marginal marine-to-terrestrial invertebrate biota and may also be visited by vertebrates, mostly birds—in New Zealand, and in many oceanic islands, it may typically be birds (and rats) but elsewhere it may include a number of scavenging small mammals (e.g. Llewellyn & Shackley 1996). Many local and managing authorities appear to accept blindly that damage from mechanical beach cleansing is cosmetic in character and that the strandline readily returns to its natural state. However, a recent and limited, small-scale cleaning experiment has concluded that while the near-surface meiofauna can quickly recover, repeated cleanings or deeper excavations ‘… may certainly result in much slower recolonization rates’ (Gheskiere et al. 2006). Commonly held opinions suggest beach clean-ups are short sighted, and a temporary cure at best, although with some educational values (Williams & Tudor 2001b). In part, the problems are being addressed through local activities of the Marine Conservation Society (a United Kingdom charity) and the European Blue Flag Scheme of beach evaluations and awards (e.g. Williams & Morgan 1995; Tudor & Williams 2006). A New Zealand example of problems with marine debris is informative (Gregory 1999b). At almost 47°S, Stewart Island’s Mason Bay is a spectacular, remote and isolated, c.10 km sandy beach that is open to the Southern Ocean and also faces into the Roaring Forties. The immediately close and offshore waters are intensely fished. The beach has been heavily fouled with marine debris dominated by fisheries-related items, most of which were from New Zealand sources. A minor, but significant, component came from Korea and Japan; rarer sources included Argentina, Australia, Belgium, Chile, France, Norway, Poland, Russia, Spain, South Africa and United Kingdom. Annual clean-ups have been organized since 1989 and it has been estimated that some 2–3 tonnes of debris was cleared each year. Disposal of the vast quantities collected is difficult (figure 1). After the 1989 exercise, a pyre was built on the beach and set alight with the aid of diesel and driftwood—this reduced the bulk to a quarter. Clinker and burnt remains were removed and placed in a pit set in dunes behind the beach. Since that time the collected marine debris has been placed at designated sites behind the fore-dunes. Local scarfing of fore-dunes has exposed once-buried plastic and other marine debris at several places. Strong on-shore winds blow shredded plastic bags and sheeting far inland to unsightly adorn and blanket the sparse coastal vegetation and may also be a contributor to environmental degradation of dune fields behind the beach. While burial may remove the unsightly debris from view in coastal settings of this kind, it cannot be considered a cure—in many instances it is at best a palliative.
3. Hangers-on and hitch-hiking aliens—invasive species
For untold millennia, floating, terrestrial plant matter, whether large and solitary tree trunks or smaller shrubs and stems with soil still attached, as well as matted masses of these materials, have freely voyaged, traversed and dispersed across the open oceans just as ‘sea beans’ (see Gunn & Dennis 1999, p. 3); logs, pumice and other natural flotsam continue so to do to this day. ‘Floating Islands’ with cargoes that include exotic plants and vertebrate animals have been recognized since medieval times (see Van Duzer 2004). Through the distant past to modern times, these materials have also attracted a diverse biota of sessile and motile marine organisms—freedom travellers (hitch-hikers and hangers-on if one likes). This process has been a mechanism in the slow trans-oceanic dispersal of marine and some terrestrial organisms; e.g. Wheeler’s (1916) report of ants carried in a floating log from the mainland of Brazil to offshore San Sebastian Island. Similarly, Ingólfsson (2000) has demonstrated that rafting on floating clumps of seaweed around Iceland may see inter-tidal species dispersed for considerable distances offshore. The hard surfaces of pelagic plastics provide an attractive and alternative substrate for a number of opportunistic colonizers. With the quantities of these synthetic and non-biodegradable materials in marine debris increasing manifold over the last five decades, dispersal will be accelerated and prospects for invasions by alien and possibly aggressive invasive species could be enhanced (e.g. Gregory 1978, 2004; Winston et al. 1997; Barnes 2002a,b) (examples illustrating some of the possibilities are provided in appendix A).
Pelagic plastic items are commonly colonized by a diversity of encrusting and fouling epibionts (e.g. figure 4). Most of these are sessile, hard-shelled or crustose organisms and dominated by bryozoans. Also included are barnacles, tube worms, foraminifera, coralline algae, hydroids and bivalve molluscs. Furthermore, they are also attractive substrates for a varied motile biota. The pseudo-planktic community that develops is comparable to that associated with Sargassum and other drifting seaweed, although with reduced species richness and diversity (Stevens et al. 1996; Winston et al. 1997). Flexible rope may also attract hangers-on (figure 4; see appendix A(ii)). Aggregations of marine debris can provide habitats suiting the larval and juvenile stages of numerous marine organisms. They may also attract free-living, ocean-roaming predators that often gather under fish aggregating devices, and where others simply sought a protective haven (see Winston et al. 1997, fig. 7.10). Aspects of floating substrata and colonizing biota are comprehensively reviewed in Theil & Gutow (2004).
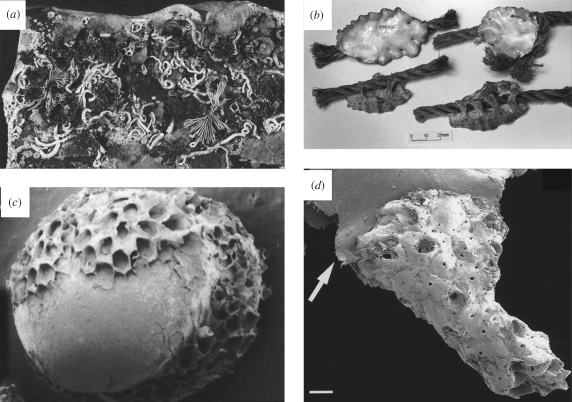
Figure 4.
Example of colonization and encrustation on plastic debris from the New Zealand coastline: (a) heavy and varied colonization of a plastic slab recovered (note the hard bodied encrustations and soft fleshy epibionts; (b) cuttings from a tangled mass of synthetic rope, carrying a cargo of the warm-water Indo-Pacific oyster, Lopha cristagalli, a species that is alien to New Zealand waters (appendix A(ii)); (c) plastic pellet (raw material for manufacture of plastic products) encrusted by the bryozoan Membranipora taberculata, see appendix A(i); (d) small bryozoan colony (Galeopsis mimicus) attached to a frayed plastic flake (arrowed) recovered from a depth of 393 m off the east coast shelf off the South Island (appendix A(xii)); scale bar 200 µm. Recently a tropical hermatypic coral has also been reported on a remote South Island shoreline (J. Lindqvist, personal communication).
4. New Zealand and the pacific sector of the Southern Ocean
In the Southern Hemisphere, a latitudinal gradient has been recognized in the extent to which drifting plastics are colonized by epibionts. Surface cover, particularly by bryozoans, as well as species richness and diversity is greatest at low latitudes (tropical and subtropical), decreasing through temperate mid latitudes and least in high (polar) latitudes (Barnes & Sanderson 2000; Barnes et al. 2006). Across the South Pacific Ocean and the Antarctic sector of the Southern Ocean, there are several important oceanic fronts along which marine debris tends to concentrate, e.g. Humbolt Front off Valparaiso, Chile (Bourne & Clark 1984). The southwards flowing East Australian Current rises in the Coral Sea. Eddies from it swing eastwards across Tasman Sea periodically bringing exotic tropical ‘sea beans’ to the shores of Aupouri Peninsula (northern New Zealand). These eddies also carry significant quantities of marine debris that originates from eastern Australia. The sources may be land-based or fisheries and other maritime activities and the cargo carried may include taxa alien to New Zealand.
Over 150 marine species are known from plastic debris stranding on the shores of northern New Zealand or as colonizers in experiments with moored plastic bottles suspended at from the surface at varying depths to 10 m (L. M. Stevens 1992, unpublished data). Most of the identified biota are hard-shelled or crustose organisms and are dominated by bryozoans (Stevens et al. in preparation). Around northern-most New Zealand, at least 60 bryozoan species have been identified. Of these, 28 had not been recorded previously—at first glance this suggests recent introductions. In truth it reflects lack of local research, as most of these taxa are known from eastern Australia and the Kermadec Ridge to the northeast of New Zealand (Gregory 1998). The cosmopolitan, warm-water, low-latitude bryozoan, Membranipora tuberculata (figure 4c) that now dominates beach-cast plastic items around northern New Zealand is a relatively recent arrival—perhaps from eastern Australia (Gregory 1978, 1998). The biota recorded from beached marine debris are strongly biased towards those taxa with hard and resistant parts. Recovery of freely drifting items in open waters as well as study over a nine-month period of moored panels has revealed the importance of colonization by a soft fleshy biota. This included a representative suite of well-known, northern New Zealand marine biota (e.g. brown and filamentous algae, hydroids, ascidians, sea anemones and sponges as well as motile organisms including crabs, amphipods, isopods, errant polychaetes, gastropods, limpets, chitons, echinoderms, sea slugs and sea cucumbers) (Winston et al. 1997; Stevens et al. in preparation). Soft and fleshy organisms disintegrate rapidly once out of water and left stranded and exposed to the harshness of beach environments. They are seldom recorded in beach surveys. Ye & Andrady (1991) have also recognized the importance of an adhering soft and fleshy biota.
Weakening eddies from the East Australian Current pass down the east coast of northern New Zealand, and off East Cape merge into the Subtropical Convergence zone. Remote Chatham Island, lying 850 km to the east of mainland New Zealand, sits virtually astride this zone. Marine debris is abundant on the island’s north- and west-facing shores (Gregory 1999b). Much of this comes from the local fishery and is generally clean of any attached biota. Nevertheless, some debris items support a varied suite of hitch-hikers and hangers-on. The degradation and weathering state of these materials as well as labelling suggests that these items have been afloat for some time and that they may have come from afar. Virgin plastic nibs, and for which there is no local (i.e. Chatham Island) source, are also common on these shores. The evidence for long-distance transport is irrefutable and the possibility of alien introductions must be acknowledged.
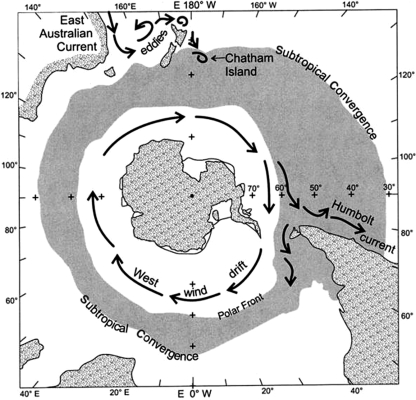
Following the separation of Antarctica from Gondwanaland, initiation in the Southern Ocean of the strong easterly-flowing Antarctic Circumpolar Current and development of the Polar Front some 30 million years ago, the Antarctic Continent has been effectively isolated (figure 5). As a consequence, the biota of shallow marine environments around the continent are highly endemic (Knox 1994). The Polar Frontal Zone (PFZ) (Antarctic Convergence) encircles the Antarctic Continent. Although noting records of pumice, dunnage and tree trunks escaping its clasp, Gregory et al. (1984), Gregory (1990) and Gregory & Ryan (1997) suggested that oceanic fronts, such as the Subtropical Convergence and Polar Front, were obstructions along which marine debris tended to collect and concentrate, and which would be difficult for it to cross. It is now appreciated that these obstructions are in reality somewhat ‘leaky barriers’ (see Barnes & Fraser 2003; Barnes et al. 2006, 2009; appendix A(vii)). However, the quantities of plastic trash and other debris here are many magnitudes less than the concentrations recorded by Moore (2003) in the North Pacific subtropical gyre.
Figure 5.
The Subtropical Convergence and strong easterly flowing Antarctic Circumpolar Polar Current are frontal zones and are ‘leaky barriers’ which some organisms are now traversing.
For marine debris with hitch-hiking aliens aboard, the possibilities of north–south (incursions) and south–north (excursions) transfers are probably greatest through disturbances and eddying as waters of the PFZ are forced through Drake Passage, as well as gyral circulation patterns that develop off the Weddell and Ross Seas. The recent report of 10 invertebrate species attached to plastic strapping that stranded on the ice-strewn shore of Adelaide Island (68°S) west of the Antarctic Peninsula exemplifies a developing problem (appendix A(viii)) (Barnes & Fraser 2003). Predicted climate changes and surface water warming of the Southern Ocean will only enhance the possibilities of this two-way latitudinal traffic. This is unlikely to be of benefit for endemic species that have been long isolated and are adapted and restricted to local cold-water environments.
5. Discussion
The environmental, cultural, aesthetic, commercial and other problems arising from pelagic plastics in particular and varied marine debris items in general are manifold, widely acknowledged and often difficult to address (see discussion in Thompson et al. 2009a,b). For instance, in today’s world, ‘beach clean-ups’, whether by mechanical means and managed by local authorities, or following responses organized through public interest groups, have become phenomena of global proportions (see Ryan et al. 2009). The latter often involve tedious ‘hand picking’ and in some situations may endanger the health of participants. While recovery and/or collection of marine debris through ‘herding’ and use of barriers in harbour, port, estuary and near-shore settings is not uncommon, it is a difficult, if not nigh-on impossible, task for dispersed material afloat on the high seas. These approaches are not a panacea, for to date they do not seem to have led to any great reduction of marine debris materials afloat in the global oceans and enclosed seas or being cast ashore. I am of the opinion that attacking the source(s) at their varied places of origin may be the only viable approach in the longer term (see discussion in Thompson et al. 2009b). The possibility of long-distance slow dispersal of common ‘fouling organisms’ (marine and non-marine) through hitch-hiking, hanging-on and/or rafting has been recognized for some time (e.g. Wheeler 1916; WHOI 1952; Gregory 1978; Jokiel 1990) and the environmental importance of this process is now widely acknowledged (e.g. Barnes 2002a,b; Barnes et al. 2006). The possibility that pelagic plastics may be potential vectors in the dispersal of aggressive and invasive marine (and terrestrial) organisms that could endanger endemic biota now warrants serious consideration. The dangers are probably greatest where endemism is significant, such as in the remote tropical and mid-latitude islands of Oceania, and isolated sub-Antarctic islands. In a forthcoming era of global warming, shallow marine waters around Antarctica could be similarly threatened. Mechanisms for the evaluation of biosecurity and management of aggressive alien marine bioinvasions in the Southern Ocean are important recent developments (e.g. Lewis et al. 2004; Hewitt & Campbell 2007).
Despite numerous informal gatherings and beach clean-up exercises, local authority concerns, regional and international meetings, together with more formal conference settings, an ever-expanding volume of research literature (often repetitive), as well as attracting the interest of UNEP’s Regional Seas Programme since 1974, the environmental and multiple other problems associated with plastic-dominated marine debris appear to be ever expanding! For the present there seem to be no satisfactory and/or practical answers to the varied problems plastic debris creates in marine settings. Longer term successes of beach clean-ups and prescribed action plans are questionable. There are clearly needs for new approaches—foremost among these will probably be further development of biodegradable plastics with significantly reduced and tightly managed disintegration times (see Song et al. 2009; Thompson et al. 2009b).
Acknowledgements
My interest since the early 1970s in the varied problems associated with ‘marine debris’ was funded initially through University of Auckland’s research grants and later by the New Zealand Ministry of Environment research agenda, as well as the Marine Mammal Commission, Washington. I also acknowledge technical and other support over many years from R. Harris, K. Johnston, B. Curram and L. Cotterall. I have valued Allan Williams' commentaries on the topic of marine debris for many years and also appreciate the careful attention given to the manuscript by two anonymous referees.
Appendix A: A catalogue with examples of some invading species, not all are necessarily aggressive aliens (expanded from Gregory 2004)
(i) Membranipora tuberculata: This common bryozoan was not identified in New Zealand waters until the early 1970s when identifications around northern New Zealand were made. Specimens were found on plastic substrates including virgin plastic pellets (or nibs) and rarely on some larger artefacts. It was inferred that there had been eastwards dispersal from Australia across the northern Tasman sea by way of eddies in the East Australian Current (Gregory 1978). Later, L. M. Stevens (1992, unpublished data) was to report that it was abundant on both eastern and western shores around northernmost New Zealand. Several specimens were later noted (M. R. Gregory, unpublished) on occasional nibs found in concentrations on northern shores of Chatham Island. This island lies virtually astride the Subtropical Convergence where marine debris tends to gather and it is probably driven ashore by northerly winds (Gregory 1999a).
(ii) Lopha cristagalli: Numerous specimens of this common tropical water, Indo-Pacific oyster have been found attached to a tangled mass of synthetic rope stranded on a remote and isolated beach of Fiordland, southwestern New Zealand (Winston et al. 1997). The only previous local record of this taxon was in 1971 when a length of synthetic rope, hauled up from shallow water off Parengarenga Harbour in the far north, carried several recently dead specimens. It was suggested that their presence was due to an overseas fishing vessel (Gardner 1971). Recently, a similarly entangled mass of rope encrusted with a hermatypic coral has stranded at the same Fiordland locality (J. Lindqvist, personal communication).
(iii) Plastic toy boats: West’s (1981) report of a child’s small (<30 cm) plastic toy boat stranded on a small island (Tiritiri matangi) lying c. 4 km offshore in the Hauraki gulf near Auckland, New Zealand, is most informative. It was carrying a cargo with soil and the seeds of eight plant species. Of these, five were native and three exotic; one was of a species not known from the island and at least three were viable.
(iv) Thalamoporella evelinae: Bryozoans resembling this taxon, which is known from Brazil, arrived in significant quantities on Florida shores through attachment to pelagic plastic artefacts and later stranding on beaches where it had not been previously identified (Winston et al. 1997).
(v) Pinctata spp. A large blue fish crate with prominent Venezuelan markings stranding on Bermuda beaches with several single attached valves of this taxon suggests long-distance transport by way of the Gulf Stream (personal observation).
(vi) Diadumene lineata: In November 2000, numerous individuals of this widely recognized, aggressive and invasive inter-tidal sea anenome were discovered on derelict trawl netting in the lagoon of Pearl and Hermes Reef, Northwestern Hawaiian Islands. This is a cosmopolitan taxon that is native to Japan and had not been previously identified in Hawaiian waters. It was suggested that the net with its hitch-hiking cargo of D. lineata could have drifted from afar—possibly Japan (Zabin et al. 2004).
(vii) Mytilis galloprovincialis: This exotic smooth-shelled blue mussel arrived in Pearl Harbor, Hawaii (June 1998) as a component of the fouling community carried by USS Missouri. Apte et al. (2000) reported that spawning took place shortly thereafter and were later recruited to another ‘shipping vector’. They infer that a ‘stepping stone’ model between temperate latitudes could lead to dispersal and/or range expansion.
(viii) Adelaide Island, Antarctic Peninsula: Barnes & Fraser (2003) reported a plastic strapping band washing ashore, and on which were attached 10 species belonging to five phylla, including bryozoa, porifera, annelida, cnidaria and mollusca. It was suggested that this plastic artefact could have been afloat for at least 1 year.
(ix) Harmful microalgae: Masó et al.’s (2003) observations along the Catalan coast (northwestern Mediterranean) and suggestions that pelagic plastic debris could be a vector in the dispersal of harmful microalgae.
(x) Elminius modestus: This barnacle is endemic to Australasian waters. It arrived in southern England during the Second World War—perhaps through attachment to convoyed vessels. Subsequently, this aggressive and alien invasive taxon advanced northwards, colonizing rocky inter-tidal shores around the British Isles and also adjacent coasts of Europe. By 1978 it had reached the Shetland Islands. There are suggestions that in later years plastic substrates could have been implicated in this dispersal (Barnes & Milner 2005).
(xi) Macrobenthos, Ligurian Sea: In these waters, Aliani & Molcard (2003) have documented macrobenthic species colonizing plastic artefacts and occasional pieces of wood. Of the 14 stations sampled, the barnacle Lepas pectinata was present at 12 and the isopod Idotea metallica at 9. Hydroids and bryozoa were also common. They also noted that no alien species had been identified.
(xii) Galeopsis mimicus: This bryozoan was previously known from two sampling stations off the west coast of the South Island of New Zealand at water depths of 297 and 520 m. It is also known from >2000 km to the north and in water depths of 470–825 m. It has recently been identified on a small piece of frayed plastic substrate recovered from the top of a core taken c. 60 km off the Canterbury east coast at a depth of 393 m (figure 4d) (Carter & Gregory 2005).
(xiii) Giamardia trapesina: Long-distance dispersal in the sub-Antarctic and Southern Ocean waters through kelp rafting of brooding bivalves (Helmuth et al. 1994).
Footnotes
One contribution of 15 to a Theme Issue ‘Plastics, the environment and human health’.
References
- Acha E. M., Mianzan H. W., Iribarne O., Gagliardini D. A., Lasta C., Daleo P.2003The role of the Rio de la Plata bottom salinity front in accumulating debris. Mar. Pollut. Bull. 46, 197–202 (doi:10.1016/S0025-326X(02)00356-9) [DOI] [PubMed] [Google Scholar]
- Aliani S., Molcard A.2003Hitch-hiking on floating marine debris: macrobenthic species in the Western Mediterranean Sea. Hydrobiologia 503, 59–67 (doi:10.1023/B:HYDR.0000008480.95045.26) [Google Scholar]
- Apte S., Holland B. S., Godwin L. S., Gardner J. P. A.2000Jumping ship: a stepping stone event mediating transfer of a non-indigenous species via a potentially unsuitable environment. Biol. Inv. 2, 75–79 (doi:10.1023/A:1010024818644) [Google Scholar]
- Backhurst M. K., Cole R. G.2000Subtidal benthic marine litter at Kawau Island, north-eastern New Zealand. J. Environ. Manage. 60, 227–237 (doi:10.1006/jema.2000.0381) [Google Scholar]
- Balazs G. H.1985Impact of ocean debris on marine turtles: entanglement and ingestion. In Proc. of the Workshop on the Fate and Impact of Marine Debris, 27–29 November 1984, Honolulu, Hawaii (eds Shomura R. S., Yoshida) H. O., pp. 387–429 US Dept. Commerce, NOAA Tech. Memo; NMFS, NOAA-TM-NMFS-SWFS-54 [Google Scholar]
- Ballance A., Tyan P. G., Turpie J. K.2000How much is a clean beach worth? The impact of litter on beach users in the Cape Peninsula, South Africa. S. Afr. J. Sci. 96, 210–213 [Google Scholar]
- Barnes D. K. A.2002aInvasions by marine life on plastic debris. Nature 416, 808–809 (doi:10.1038/416808a) [DOI] [PubMed] [Google Scholar]
- Barnes D. K. A.2002bHuman rubbish assists alien invasions. Dir. Sci. 1, 107–112 (doi:10.1100/tsw.2002.879) [Google Scholar]
- Barnes D. K. A.2005Remote Islands reveal rapid rise of Southern Hemisphere, sea debris. Sci. World J. 5, 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. K. A., Fraser K. P. P.2003Rafting by five phyla on man-made flotsam in the Southern Ocean. Mar. Ecol. Prog. Ser. 262, 281–289 (doi:10.3354/meps262289) [Google Scholar]
- Barnes D. K. A., Milner P.2005Drifting plastic and its consequences for sessile organism dispersal in the Atlantic Ocean. Mar. Biol. 146, 815–825 (doi:10.1007/s00227-004-1474-8) [Google Scholar]
- Barnes D. K. A., Sanderson W. G.2000Latitudinal patterns of colonization of marine debris. In Proc. of the 11th Int. Bryozoology Assoc. Conf., Chicago (eds Herrera-Cubilla A., Jackson J. B. C.), pp. 154–160 Smithsonian Tropical Research Institute [Google Scholar]
- Barnes D. K. A., Hodgson D. A., Convey P., Allen C. S., Clarke A.2006Incursion and excursion of Antarctic Biota: past, present and future. Glob. Ecol. Biogeogr. 15, 121–142 (doi:10.1111/j.1466-822X.2006.00216.x) [Google Scholar]
- Barnes D. K. A., Galgani F., Thompson R. C., Barlaz M.2009Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998 (doi:10.1098/rstb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. A., Barros N. B.1991The impact of debris on the Florida Manatee. Mar. Pollut. Bull. 22, 508–510 (doi:10.1016/0025-326X(91)90406-I) [Google Scholar]
- Bjorndal K. A., Bolten A. B., Laguex C. J.1994Ingestion of marine debris by juvenile turtles in coastal Florida habitats. Mar. Pollut. Bull. 28, 154–158 (doi:10.1016/0025-326X(94)90391-3) [Google Scholar]
- Blight L. K., Burger A. E.1997Occurrence of plastic particles in sea-birds from the eastern North Pacific. Mar. Pollut. Bull. 34, 323–325 (doi:10.1016/S0025-326X(96)00095-1) [Google Scholar]
- Boren L. J., Morrissey M., Muller C. G., Gemmell N. J.2006Entanglement of New Zealand fur seals in man-made debris at Kaikoura, New Zealand. Mar. Pollut. Bull. 52, 442–446 (doi:10.1016/j.marpolbul.2005.12.003) [DOI] [PubMed] [Google Scholar]
- Bourne W. R. P., Clark G. C.1984The occurrence of birds and garbage at the Humboldt Front off Valparaiso, Chile. Mar. Pollut. Bull. 15, 343–344 (doi:10.1016/0025-326X(84)90493-4) [Google Scholar]
- Cadee G. C.2002Seabirds and floating plastic debris. Mar. Pollut. Bull. 44, 1294–1299 (doi:10.1016/S0025-326X(02)00264-3) [DOI] [PubMed] [Google Scholar]
- Carr H. A., Harris J.1997Ghost-fishing gear: have fishing practices during the past few years reduced the impact? In Marine debris, sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 141–151 New York, NY: Springer-Verlag [Google Scholar]
- Carter R., Gregory M. R.2005Bryozoan encrusted plastic from the continental slope: eastern South Island, New Zealand. N. Z. Nat. Sci. 30, 49–55 [Google Scholar]
- Connors P. J., Smith K. G.1982Oceanic plastic particle pollution: suspected effect on fat deposition in Red Phalropes. Mar. Pollut. Bull. 13, 18–20 (doi:10.1016/0025-326X(82)90490-8) [Google Scholar]
- Copello S., Quintana F.2003Marine debris ingestion by Southern Giant Petrels and its potential relationships with fisheries in the Southern Atlantic Ocean. Mar. Pollut. Bull. 46, 1513–1515 (doi:10.1016/S0025-326X(03)00312-6) [DOI] [PubMed] [Google Scholar]
- de Forges B. D., Koslow J. A., Poore G. C. B.2000Diversity and endemism of the benthic seamount fauna in the southwest Pacific. Nature 405, 944–947 (doi:10.1038/35016066) [DOI] [PubMed] [Google Scholar]
- Derraik J. G. B.2002The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 44, 842–852 (doi:10.1016/S0025-326X(02)00220-5) [DOI] [PubMed] [Google Scholar]
- Ebbesmeyer C. C., Ingraham W. J., Jr, Royer T. C., Grosch C. E.2007Tub toys orbit the Pacific Subarctic gyre. EOS, Trans. Am. Geophys. Union 88, 1&4 [Google Scholar]
- Eriksson C., Burton H.2003Origins and biological accumulation of small plastic particles in Fur Seals from Macquarie Island. Ambio 32, 380–385 [DOI] [PubMed] [Google Scholar]
- Furness R. W.1985Ingestion of plastic particles by seabirds at Gough Island, South Atlantic Ocean. Environ. Pollut. Ser A 38, 261–272 (doi:10.1016/0143-1471(85)90131-X) [Google Scholar]
- Gabrielides G. P.1995Pollution of the Mediterranean Sea. Water Sci. Technol. 32, 9–10 (doi:10.1016/0273-1223(96)00070-4) [Google Scholar]
- Galgani F., et al. 2000Litter on the sea floor along the European coasts. Mar. Pollut. Bull. 40, 516–527 (doi:10.1016/S0025-326X(99)00234-9) [Google Scholar]
- Galil B. S., Golik A., Turkay M.1995Litter at the bottom of the sea: a sea bed survey in the Eastern Mediterranean. Mar. Pollut. Bull. 30, 22–24 (doi:10.1016/0025-326X(94)00103-G) [Google Scholar]
- Gardner N. N.1971Lopha cristagali (Linne). Poirieria 5, 104 [Google Scholar]
- Gheskiere T., Madda V., Greet P., Steven D.2006Are strandline meiofaunal assemblages affected by a once-only mechanical; beach cleaning? Experimental findings. Mar. Environ. Res. 61, 245–264 (doi:10.1016/j.marenvres.2005.10.003) [DOI] [PubMed] [Google Scholar]
- Goldberg E. D.1997Plasticizing the seafloor: an overview. Environ. Technol. 18, 195–202 (doi:10.1080/09593331808616527) [Google Scholar]
- Gregory M. R.1977Plastic pellets on New Zealand beaches. Mar. Pollut. Bull. 9, 82–84 (doi:10.1016/0025-326X(77)90193-X) [Google Scholar]
- Gregory M. R.1978Accumulation and distribution of virgin plastic granules on New Zealand beaches. N. Z. J. Mar. Freshwater Res. 12, 399–414 [Google Scholar]
- Gregory M. R.1990Environmental and pollution aspects. In Antarctic sector of the Pacific. (ed. Glasby G. P.). Elsevier Oceanography Series, 51, pp. 291–324 [Google Scholar]
- Gregory M. R.1991The hazards of persistent marine pollution: drift plastics and conservation islands. J. R. Soc. N. Z. 21, 83–100 [Google Scholar]
- Gregory M. R.1998Pelagic plastics and marine invaders. Aliens, 7, 6–7 [Google Scholar]
- Gregory M. R.1999aPlastics and South Pacific island shores. Ocean Coastal Manage. 42, 603–615 (doi:10.1016/S0964-5691(99)00036-8) [Google Scholar]
- Gregory M. R.1999bMarine debris: notes from Chatham Island, and Mason and Doughboy Bays, Stewart Island. Tane 37, 201–210 [Google Scholar]
- Gregory M. R.2004Marine debris: hangers-on and hitch-hiking aliens. In Derelict fishing gear and related marine debris: an educational outreach seminar among APEC partners. Seminar Proc., 13–16 January 2004, Honolulu, Hawaii pp. 40–44 [Google Scholar]
- Gregory M. R., Andrady A. L.2003Plastics in the marine environment. In Plastics and the environment (ed. Andrady A. L.), pp. 379–401 Hoboken, NJ: John Wiley and Sons, Inc [Google Scholar]
- Gregory M. R., Ryan P. G.1997Pelagic plastics and other seaborne persistent synthetic debris: a revue of Southern Hemisphere perspectives. In Marine Debris, sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 49–66 New York, NY: Springer-Verlag [Google Scholar]
- Gregory M. R., Kirk R. M., Mabin M. C. G.1984Plastics and other litter in surface waters of the New Zealand sector of the Southern Ocean and on Ross Dependency shores. N. Z. Antarct. Rec. 7, 12–28 [Google Scholar]
- Gunn C. R., Dennis J. V.1999. World guide to tropical drift seeds and fruits Malabar, FL: Kreiger Publishing Company, 240 p [Google Scholar]
- Hanni K. D., Pyle P.2000Entanglement of Pinnipeds in synthetic materials at South-east Farallon Island, California, 1976–1998. Mar. Pollut. Bull. 40, 1076–1081 (doi:10.1016/S0025-326X(00)00050-3) [Google Scholar]
- Harms J.1990Marine plastic litter as an artificial hard bottom fouling ground. Helgoläender Meersuntersuchungen 44, 503 (doi:10.1007/BF02365483) [Google Scholar]
- Harper P. C., Fowler J. A.1987Plastic pellets in New Zealand storm-killed prions (Pachyptila spp.) 1958–1977. Notornis 34, 65–70 [Google Scholar]
- Helmuth B. R., Veit R., Holberton R.1994Long-distance dispersal of a SubAntarctic brooding bivalve (Gaimardia trapesina) by kelp rafting. Mar. Biol. 120, 421–426 (doi:10.1007/BF00680216) [Google Scholar]
- Henderson J. R.2001A pre- and post-MARPOL Annex V summary of Hawaiian monk seal entanglements and marine debris accumulation in the Northwestern Hawaiian Islands, 1982–1998. Mar. Pollut. Bull. 42, 584–589 (doi:10.1016/S0025-326X(00)00204-6) [DOI] [PubMed] [Google Scholar]
- Hess N. A., Ribic C. A., Vining I.1999Benthic marine debris with an emphasis on fisheries-related items, surrounding Kodiak Island, Alaska, 1994–1996. Mar. Pollut. Bull. 38, 885–890 (doi:10.1016/S0025-326X(99)00087-9) [Google Scholar]
- Hewitt C. L., Campbell M. L.2007Mechanisms for the prevention of marine bioinvasions for better biosecurity. Mar. Pollut. Bull. 55, 395–401 (doi:10.1016/j.marpolbul.2007.01.005) [DOI] [PubMed] [Google Scholar]
- Hofmeyr G. J. G., Bester M. N., Kirkman S. P., Lydersen C., Kovacs K. M.2006Entanglement of Antarctic fur seals at Bouvetóya, Southern Ocean. Mar. Pollut. Bull. 52, 1077–1080 (doi:10.1016/j.marpolbul.2006.05.003) [DOI] [PubMed] [Google Scholar]
- Hollström A.1975Plastic films on the bottom of the Skagerrak. Nature 255, 622–623 (doi:10.1038/255622a0) [Google Scholar]
- Ingólfsson A.2000Colonization of floating seaweed by pelagic and subtidal benthic animals in south-western Iceland. Hydrobiologia 440, 181–189 (doi:10.1023/A:1004119126869) [Google Scholar]
- Jokiel P. L.1990Long-distance dispersal by rafting: reemergence of an old hypothesis. Endeavour 14, 66–73 (doi:10.1016/0160-9327(90)90074-2) [Google Scholar]
- Kanehiro H., Tokai T., Matuda K.1995Marine litter composition and distribution on the sea-bed of Tokyo Bay, Japan. Fish. Eng. 31, 195–199 [Google Scholar]
- Katsanevakis S., Verriopoulos G., Nicolaidou A., Thessalou-Legaki M.2007Effect of marine litter on the benthic megafauna of coastal soft bottoms: a manipulative field experiment. Mar. Pollut. Bull. 54, 771–778 (doi:10.1016/j.marpolbul.2006.12.016) [DOI] [PubMed] [Google Scholar]
- Knox G. A.1994The biology of the Southern Ocean Cambridge, UK: Cambridge University Press, 444 p. [Google Scholar]
- Koslow J. A.1997Seamounts and the ecology of deep-sea fisheries. Am. Sci. 85, 168–175 [Google Scholar]
- Laist D. W.1987Overview of the biological effects of lost and discarded plastic debris in the marine environment. Mar. Pollut. Bull. 18, 319–326 (doi:10.1016/S0025-326X(87)80019-X) [Google Scholar]
- Laist D. W.1996Marine debris entanglement and ghost fishing: a cryptic and significant type of bycatch. Alaska Sea Grant College Progarm Report no. 96-03. pp. 33–39, University of Alaska, Fairbanks, AK [Google Scholar]
- Laist D. W.1997Impacts of marine debris: entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In Marine debris, sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 99–139 New York, NY: Springer-Verlag [Google Scholar]
- Lewis P. N., Riddle M. J., Smith S. D. A.2005Assisted passage or passive drift: a comparison of alternative transport mechanisms for non-indigenous coastal species into the Southern Ocean. Antarct. Sci. 17, 183–191 (doi:10.1017/S0954102005002580) [Google Scholar]
- Llewellyn P. J., Shackley S. E.1996The effects of mechanical beach-cleaning on invertebrate populations. Br. Wildl. 7, 147–155 [Google Scholar]
- Mallory M. L., Robertson G. J., Moenting A.2006Marine plastic debris in northern fulmars from Davis Strait, Nanavut. Can. Mar. Pollut. Bull. 52, 800–815 [DOI] [PubMed] [Google Scholar]
- Mascarenhas A., Santos R., Zeppelini D.2004Plastic debris by sea turtle in Paraíba. Mar. Pollut. Bull. 49, 354–355 (doi:10.1016/j.marpolbul.2004.05.006) [DOI] [PubMed] [Google Scholar]
- Masó M., Garcés E., Pagès F., Camp J.2003Drifting plastic debris as a potential vector for dispersing harmful algal bloom (HAB) species. Sci. Mar. 67, 107–111 [Google Scholar]
- Mato Y., Isobe T., Takada H., Kahnehiro H., Ohtake C., Kaminuma O.2001Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 35, 318–324 (doi:10.1021/es0010498) [DOI] [PubMed] [Google Scholar]
- Mattlin R. H., Cawthorn M. W.1986Marine debris—an international problem. N. Z. Environ. 51, 3–6 [Google Scholar]
- Moore C.2003Trashed: across the Pacific Ocean, plastics, plastics, everywhere. Nat. Hist. 112, 46–51 [Google Scholar]
- Moore S. L., Allen M. J.2000Distribution of anthropogenic and natural debris on the mainland shelf of the southern California Bight. Mar. Pollut. Bull. 40, 83–88 (doi:10.1016/S0025-326X(99)00175-7) [Google Scholar]
- Nash A.1992Impacts of marine debris on subsistence fishermen—an exploratory study. Mar. Pollut. Bull. 24, 150–156 (doi:10.1016/0025-326X(92)90243-Y) [Google Scholar]
- O’Driscoll R. L., Clark M. R.2005Quantifying the relative intensity of fishing on New Zealand Seamounts. N. Z. J. Mar. Freshwater Res. 39, 839–850 [Google Scholar]
- Oehlmann J., et al. 2009A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 364, 2047–2062 (doi:10.1098/rstb.2008.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima S.2000Towards a ‘Visual Sea’. Hydro Int. 4, 73 [Google Scholar]
- Page B., et al. 2004Entanglement of Australian sea lions and New Zealand fur seals in lost fishing gear and other marine debris before and after Government and industry attempts to reduce the problem. Mar. Pollut. Bull. 49, 33–42 (doi:10.1016/j.marpolbul.2004.01.006) [DOI] [PubMed] [Google Scholar]
- Richards A. H.1994Problems of drift-net fisheries in the South Pacific. Mar. Pollut. Bull. 29, 106–111 (doi:10.1016/0025-326X(94)90433-2) [Google Scholar]
- Ryan P. G.2008Seabirds indicate changes in the composition of plastic litter in the Atlantic and south-western Indian Oceans. Mar. Pollut. Bull. 56, 1406–1409 (doi:10.1016/j.marpolbul.2008.05.004) [DOI] [PubMed] [Google Scholar]
- Ryan P. G., Swanepoel D.1996Cleaning beaches; sweeping the rubbish under the carpet. S. Afr. J. Sci. 92, 163–165 [Google Scholar]
- Ryan P. G., Moore C. J., van Franeker J. A., Moloney C. L.2009Monitoring the abundance of plastic debris in the marine environment. Phil. Trans. R. Soc. B 364, 1999–2012 (doi:10.1098/rstb.2008.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazima I., Gadig O. B. F., Namora R. C., Motta F. S.2002Plastic debris collars on juvenile carcharhinid sharks (Rhizoprionodon lalandii) in southwest Atlantic. Mar. Pollut. Bull. 44, 1147–1149 (doi:10.1016/S0025-326X(02)00141-8) [DOI] [PubMed] [Google Scholar]
- Shomura R. S., Yoshida H. O. (eds) 1985Proc. of the Workshop on the Fate and Impact of Marine Debris, 26–29 November 1984, Honolulu, Hawaii, U.S. Dep. Commer., NOAA. Tech. Memo; NMFS, NOAA-TM-NMFS-SWFC-54 [Google Scholar]
- Song J. H., Murphy R. J., Narayan R., Davies G. B. H.2009Biodegradable and compostable alternatives to conventional plastics. Phil. Trans. R. Soc. B 364, 2127–2139 (doi:10.1098/rstb.2008.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefatos A., Chararampakis M., Papatheodorou G., Ferentinos G.1999Marine debris on the sea floor of the Mediterranean sea: examples from two enclosed Gulfs in Western Greece. Mar. Pollut. Bull. 36, 389–393 (doi:10.1016/S0025-326X(98)00141-6) [Google Scholar]
- Stevens L. M., Gregory M. R., Foster B. A.1996Fouling bryozoa on pelagic and moored plastics from northern New Zealand. In Bryzoans in space and time. Proc. of the 10th Int. Bryozoology Conf. (eds Gordon D. P., Smith A. M., Grant-Mackie J. A.), pp. 321–340 Wellington, New Zealand: National Institute of Water & Atmospheric Research Ltd [Google Scholar]
- Stevens L. M., Gregory M. R., Foster B. A.In preparation The epibionts and associated biota of pelagic plastic debris from northern New Zealand. [Google Scholar]
- Teuten E. L., et al. 2009Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 364, 2027–2045 (doi:10.1098/rstb.2008.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil M., Guto L.2005The ecology of rafting in the marine environment. 1. The floating substrata. In Oceanography and marine biology (eds Gibson R. N., Atkinson R. J. A., Gordon J. D. M.), vol. 42, pp. 181–264 [Google Scholar]
- Tomás J., Guitart R., Mateo R., Raga J. A.2002Marine debris ingestion in loggerhead sea turtles, Caretta carettahe, from the western Mediterranean. Mar. Pollut. Bull. 44, 211–216 (doi:10.1016/S0025-326X(01)00236-3) [DOI] [PubMed] [Google Scholar]
- Thompson M. E., Cóté W. A.1997Potential effects of discarded Triton paperboard six-pack carriers on fish. Mar. Pollut. Bull. 34, 135–137 (doi:10.1016/S0025-326X(96)00128-2) [Google Scholar]
- Thompson R. C., Swan S. H., Moore C. J., vom Saal F. S.2009aOur plastic age. Phil. Trans. R. Soc. B 364, 1973–1976 (doi:10.1098/rstb.2009.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Moore C. J., vom Saal F. S., Swan S. H.2009bPlastics, the environment and human health: current consensus and future trends. Phil. Trans. R. Soc. B 364, 2153–2166 (doi:10.1098/rstb.2009.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor D. T., Williams A. T.2006A rationale for beach selection by the public on the coast of Wales, UK. Area 38, 153–164 (doi:10.1111/j.1475-4762.2006.00684.x) [Google Scholar]
- Uneputty P., Evans S. M.1997The impact of plastic debris on the biota of tidal flats in Ambon Bay (Eastern Indonesia). Mar. Environ. Res. 44, 233–242 (doi:10.1016/S0141-1136(97)00002-0) [Google Scholar]
- Van Duzer C.2004Floating islands: a global bibliography. pp. 204p, Los Altos Hills, CA: Cantor Press , 204 p.(12 plates) [Google Scholar]
- Van Franeker J. A., Bell P. J.1988Plastic ingestion by petrels breeding in Antarctica. Mar. Pollut. Bull. 19, 672–674 (doi:10.1016/0025-326X(88)90388-8) [Google Scholar]
- Vlietstra L. S., Parga J. A.2002Long-term changes in the type, but not amount, of ingested plastic particles in short-tailed shearwaters in the southeastern Bering Sea. Mar. Pollut. Bull. 44, 945–955 (doi:10.1016/S0025-326X(02)00130-3) [DOI] [PubMed] [Google Scholar]
- Watling L., Norse E. A.1998Disturbance of seabed by mobile fishing gear: a comparison to forest clearcutting. Conserv. Biol. 12, 1180–1197 (doi:10.1046/j.1523-1739.1998.0120061180.x) [Google Scholar]
- West C. J.1981The significance of small plastic boats as seed dispersal agents. Tane 27, 175 [Google Scholar]
- Wheeler W. M.1916Ants carried in a floating log from the Brazilian mainland to San Sabastian Island. Psyche 23, 180–183 (doi:10.1155/1916/59414) [Google Scholar]
- WHOI (Woods Hole Oceanographic Institution) 1952Marine fouling and its prevention, pp. 338 Annapolis, MD: United States Naval Institute [Google Scholar]
- Williams A. T., Morgan R.1995Beach awards and rating systems. Shore Beach 63, 29–33 [Google Scholar]
- Williams A. T., Simmons S. L.1997Estuarine litter at the river/beach interface in the Bristol Channel, UK. J. Coastal Res. 13, 1159–1165 [Google Scholar]
- Williams A. T., Simmons S. L., Fricker A.1993Off-shore sinks of marine litter: a new problem. Mar. Pollut. Bull. 26, 404–405 (doi:10.1016/0025-326X(93)90192-M) [Google Scholar]
- Williams A. T., Tudor D. T.2001aLitter burial and exhumation: spatial and temporal distribution on a cobble pocket beach. Mar. Pollut. Bull. 42, 1031–1039 (doi:10.1016/S0025-326X(01)00058-3) [DOI] [PubMed] [Google Scholar]
- Williams A. T., Tudor D. T.2001bTemporal trends in litter dynamics at a pebble pocket beach. J. Coastal Res. 17, 137–145 [Google Scholar]
- Williams A. T., Tudor D. T., Gregory M. R.2005Marine debris—onshore, offshore, seafloor litter. In Encylopedia of Coastal Science (Encyclopedia of Earth Sciences Series) (ed. Schwartz M. L.), pp. 623–628 Berlin, Germany: Springer [Google Scholar]
- Winston J. E., Gregory M. R., Stevens L. M.1997Encrusters, epibionts, and other biota associated with pelagic plastics: a review. In Marine debris, sources, impacts, and solutions (eds Coe J. M., Rogers D. B.), pp. 81–97 New York, NY: Springer-Verlag [Google Scholar]
- Wright A., Doulman D. J.1991Drift-net fishing in the South Pacific: from controversy to management. Mar. Policy 15, 303–337 (doi:10.1016/0308-597X(91)90081-L) [Google Scholar]
- Ye S., Andrady A. L.1991Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar. Pollut. Bull. 22, 608–613 (doi:10.1016/0025-326X(91)90249-R) [Google Scholar]
- Zabin C. J., Carlton J. T., Goodwin L. S.2004First report of the Asian sea anenome Diadumene lineata from the Hawaiian Islands. Bishop Museum Occasional Paper no. 79, 56–61 [Google Scholar]