Abstract
Components used in plastics, such as phthalates, bisphenol A (BPA), polybrominated diphenyl ethers (PBDE) and tetrabromobisphenol A (TBBPA), are detected in humans. In addition to their utility in plastics, an inadvertent characteristic of these chemicals is the ability to alter the endocrine system. Phthalates function as anti-androgens while the main action attributed to BPA is oestrogen-like activity. PBDE and TBBPA have been shown to disrupt thyroid hormone homeostasis while PBDEs also exhibit anti-androgen action. Experimental investigations in animals indicate a wide variety of effects associated with exposure to these compounds, causing concern regarding potential risk to human health. For example, the spectrum of effects following perinatal exposure of male rats to phthalates has remarkable similarities to the testicular dysgenesis syndrome in humans. Concentrations of BPA in the foetal mouse within the range of unconjugated BPA levels observed in human foetal blood have produced effects in animal experiments. Finally, thyroid hormones are essential for normal neurological development and reproductive function. Human body burdens of these chemicals are detected with high prevalence, and concentrations in young children, a group particularly sensitive to exogenous insults, are typically higher, indicating the need to decrease exposure to these compounds.
Keywords: plastic, endocrine disruptor, phthalates, bisphenol A, polybrominated diphenyl ether, tetrabromobisphenol A
1. Introduction
Due to the high-volume generation of plastics and low production costs, consumers typically use many plastic items only once before discarding them. Environmental concerns associated with plastic use are not only because of the amount of waste, but also the leaching of substances out of the plastic. Components used in plastics, such as bisphenol A (BPA), polybrominated diphenyl ethers (PBDE), tetrabromobisphenol A (TBBPA) and phthalates, are released from plastic products, and are also known as endocrine-disrupting compounds (EDCs) owing to their ability to modulate the endocrine system. The detection of EDCs in the environment, biota and humans is of concern due to their potential to interfere with the physiology of living organisms (see Oehlmann et al. 2009).
In general, EDCs may disrupt the endocrine system by competing with endogenous steroid hormone binding to receptors and hormone transport proteins or by altering the metabolism or synthesis of endogenous hormones, eventually influencing recruitment of transcription factors and altering gene expression in cells (Wetherill et al. 2007). This is of particular concern for the developing organism, as it is sensitive to changes in the hormonal milieu, or drug or chemical exposure, which can result in organizational changes that are permanent (Guillette et al. 1995). This is in contrast to the situation in adults where changes in steroid hormones often lead to activational changes that are transient. Epigenetic changes have been seen following early developmental exposure to EDCs in animal studies, characterized by modified methylation patterns of genes leading to altered gene expression and phenotypic changes (Skinner & Anway 2007).
Human exposures to EDCs during this particularly vulnerable developmental time frame have been documented in a number of studies showing contamination of human breast milk (Norén & Meironyté 2000; Sun et al. 2004; Main et al. 2006), foetal liver (Schecter et al. 2007), amniotic fluid (Ikezuki et al. 2002) and cord blood (Schönfelder et al. 2002; Guvenius et al. 2003). Data from animal and human studies suggest that EDCs may play a role in the development of cancer (Takashima et al. 2008), the reported decline in human sperm count (Mocarelli et al. 2008), temporal increases in the frequency of developmental abnormalities of the male reproductive tract (Sharpe & Skakkebaek 2008) and the trend towards precocious puberty in human females (Schoeters et al. 2008).
Phthalates function as plasticizers to give flexibility to high-molecular-weight polymers and are found in soft plastic products (the addition of phthalates makes brittle polyvinyl chloride (PVC) soft). In addition, phthalates are also used as chemical additives in gel capsules, cosmetics and other personal-care products. Bisphenol A is widely used in the production of epoxy resins, polycarbonate plastics and brominated flame retardants (BFRs). BPA is the monomer (as opposed to an additive) used for production of polycarbonate plastic intended for food and beverage contact and many other products; it is also used to make resins that line metal food and beverage cans. TBBPA and PBDEs make up approximately 59 and 33 per cent of the world market of BFRs (Law et al. 2006), respectively, and are incorporated in a wide variety of materials including plastics intended for electronics and appliances as well as fabrics.
This review will present some aspects of human exposure to these compounds and investigations describing effects in animal models. The potential for human health effects based on evidence derived from experimental studies in animals will be discussed for each of these chemicals.
2. Phthalates
(a). Human exposure
Phthalate esters have recently attracted the special attention of the scientific community, regulatory agencies and the general public as a consequence of their high production volume, widespread use as plasticizers and chemical additives and possible endocrine-related effects (Mylchreest et al. 1999). Phthalates can easily leach out of products to contaminate the external environment because they are not chemically bound to the plastic matrix or to other chemicals in formulations. Di-(2-ethylhexyl) phthalate (DEHP) is the most commonly used phthalate plasticizer for PVC (Matsumoto et al. 2002), but other compounds such as di-butyl phthalate (DBP), used as a fixative in cosmetics and other formulations, are also a cause of concern. Recent biomonitoring studies in the USA and Europe have detected relatively high levels of monoester metabolites of phthalates in the urine of the general population (Koch et al. 2004, 2006; Silva et al. 2004). In a recent study (Koch et al. 2006), the estimated median and 95th percentile of daily DEHP intake for a German population (n = 85) was 5.6 and 21 µg (kg bw–1) d–1, respectively. For children aged 3–14 years, these values were significantly higher—7.7 and 25 µg (kg bw–1) d–1 (n = 254). Other studies have confirmed these data (reviewed by Heudorf et al. 2007). In addition, critically ill patients and neonates hospitalized in intensive care units may be exposed to significantly higher doses of phthalates that migrate from medical devices such as blood bags, catheters and nasogastric and intravenous tubes (Koch et al. 2006). Considering that developing organisms are particularly vulnerable to the effects induced by phthalates, newborns undergoing medical treatment in intensive care units may be at increased risk when compared with the general population. In a pilot study conducted by Koch et al. (2006), the daily DEHP intake of 45 neonates who were treated with various medical procedures was calculated. The median and 95th percentile was 0.042 and 1.780 mg DEHP (kg bw–1) d–1, respectively, and the maximum calculated intake was 2.3 mg DEHP (kg bw–1) d–1.
(b). Experimental studies
Although phthalates display low general toxicity, exposure to certain compounds is associated with disruption of endocrine and reproductive functions in experimental animals. The male reproductive tract seems to be particularly sensitive to phthalate exposure. Treatment of adult male rats with high doses of certain phthalates (e.g. 2000 mg DEHP kg–1 d–1) results in rapid and severe changes in the testis (Gray & Gangolli 1986; Dostal et al. 1988). The observed alterations in spermatogenesis are thought to result from dysfunction in Sertoli cells, which cannot adequately provide physical and metabolic support to germ cells (Gray & Gangolli 1986). There is also experimental evidence showing that phthalates can target the Leydig cells and induce multiple hormonal disturbances (Akingbemi et al. 2004; Lin et al. 2008). However, most reproductive effects are not exerted by phthalate diesters themselves but rather by their active primary monoester metabolites formed in the liver, which are considered the proximate toxicants (Gray & Gangolli 1986). Recent evidence suggests that phthalates can also induce adverse responses in females following pre- and post-natal exposure (Grande et al. 2006, 2007; Gray et al. 2006).
Although most reproductive effects have been described in rats, phthalates can induce testicular injury in several other species including mice (Lamb et al. 1987), guinea pigs (Gray et al. 1982) and ferrets (Lake et al. 1976). However, some species, such as hamsters and non-human primates, seem to be less sensitive than rats (Gray et al. 1982; Foster et al. 1983; Kurata et al. 1998), and part of this variability may be attributed to differences in phthalate bioavailability (Foster et al. 1983; Ito et al. 2005). Accordingly, it has been argued that humans and non-human primates are less susceptible to the effects of phthalates, owing to the lower conversion of parent compounds into active monoester metabolites (Mckee et al. 2004). In a study by Kurata et al. (1998), no changes in testis weight or histopathology were observed in adult marmosets administered orally at 100, 500 or 2500 mg DEHP kg−1 d–1 for 13 weeks. More recently, the absence of testicular effects was also reported in young adult cynomolgus monkeys and juvenile marmosets exposed to high DEHP doses (500–2500 mg kg–1 d–1) (Pugh et al. 2000; Tomonari et al. 2006). However, non-human primates have not been extensively evaluated during foetal and neonatal periods, which represent developmental windows especially susceptible to exogenous insults. A recent work by Hallmark et al. (2007) indicates that neonatal marmosets treated orally with 500 mg kg–1 d–1 DBP respond similarly to rats in relation to changes in testosterone production and Leydig cell alterations, although marmosets seem to be able to reverse the suppression of testosterone production more efficiently than rats. Due to the lack of data on in utero and early post-natal exposures, it is not possible to draw conclusions regarding possible developmental effects in non-human primates.
In fact, the main concern involving phthalates is related to the effects induced during pre- and early post-natal development. Recent animal toxicity studies indicate that exposure to certain phthalates results in severe disorders in the developing male reproductive system, including defects in the external genitalia, undescended testes and testicular lesions. Reproductive toxicity induced during the perinatal period was reported for DEHP (Gray et al. 2000; Andrade et al. 2006a), DBP (Mylchreest et al. 1999), butyl benzyl phthalate (Gray et al. 2000; Nagao et al. 2000) and, to a lesser extent, for diisononyl phthalate (Gray et al. 2000). Male rat offspring exposed in utero or both in utero and during lactation to high phthalate doses (e.g. 500 mg DBP kg−1 d–1) display reproductive tract abnormalities compatible with disruption of androgen-dependent development and impaired testicular function (Mylchreest et al. 1999; Gray et al. 2000; Andrade et al. 2006a). The phenotypic alterations manifested in male offspring include cryptorchidism, hypospadias, atrophy or agenesis of sex accessory organs, testicular lesions (e.g. small fluid-filled testes), reduced daily sperm production, delayed preputial separation, permanent retention of nipples and decreased (feminized) anogenital distance.
Unlike other anti-androgens, which act by binding to the androgen receptor and thus inhibit its ability to respond to androgens, phthalates disrupt the development of androgen-dependent structures mainly by inhibiting foetal testicular testosterone biosynthesis (Parks et al. 2000; Wilson et al. 2004; Howdeshell et al. 2008). This effect is mediated by changes in gene expression of enzymes and proteins involved in testosterone production by foetal Leydig cells, including the steroidogenic acute regulatory (StAR) protein, which participates in the transport of cholesterol to the inner mitochondrial membrane, the step in the steroidogenic pathway that is considered to be rate-limiting (Shultz et al. 2001; Lehmann et al. 2004). Recently, the expression of another product of the foetal Leydig cell, insulin-like factor 3 (Insl3), has been shown to be reduced in phthalate-exposed animals (Wilson et al. 2004). Such an effect might explain the incidence of retention of testes in the abdomen (cryptorchidism) following phthalate exposure, as Insl3 is involved in the initial stages of testicular descent into the scrotum (Wilson et al. 2004; Foster 2006).
Testicular histopathology resulting from phthalate exposure is seen early in the foetal testis with the presence of dysgenetic areas characterized by malformed seminiferous cords containing multinucleated gonocytes and aggregates of Leydig cells (Barlow & Foster 2003; Fisher et al. 2003). In adult offspring, affected testes display reduced germ cell differentiation, Sertoli cell-only (SCO) tubules, Leydig cell aggregates and multinucleated giant germ cells (Gray et al. 2000; Fisher et al. 2003; Andrade et al. 2006b). Reductions in Sertoli cell number and/or proliferation have also been reported in neonatal rats treated with phthalates (Dostal et al. 1988; Li et al. 2000). However, this appears to be a transient effect, as no changes in the number of Sertoli cells are observed later in life (Dostal et al. 1988; Andrade et al. 2006b).
In both adult and developing males, disturbance of Leydig and Sertoli cell functions constitutes integral effects of phthalates. In addition, gene profiling data obtained by microarray analysis indicate that phthalates affect similar genetic targets in pre-pubertal and foetal rat testes (Lahousse et al. 2006). However, hormonal and local cell signalling perturbations during early development may irreversibly alter reproductive and endocrine functions in a manner that may not be predicted from post-natal exposure. In addition, although several target genes involved in the development and function of foetal Leydig and Sertoli cells have been identified so far (Shultz et al. 2001; Lehmann et al. 2004), the mechanisms by which phthalates alter the expression of these genes are currently unknown.
(c). Relevance of phthalate effects for humans
Interestingly, the spectrum of effects obtained following perinatal exposure of male rats to phthalates has remarkable similarities with the human testicular dysgenesis syndrome (TDS). According to Skakkebaek et al. (2001), the human TDS is characterized by low sperm counts, cryptorchidism, hypospadias and testicular cancer, and the clinical expression of these symptoms may vary with the severity of the syndrome. Accordingly, less severe manifestations would result in impaired spermatogenesis while other symptoms such as testicular cancer may be present in more severely affected individuals (Skakkebaek et al. 2001). Similar to the human TDS, the effects induced by phthalates in rats constitute a continuum of response with the most severe manifestations and highest incidence of reproductive tract malformations observed at high doses (Foster 2006). In utero exposure of rats to active phthalates such as DEHP and DBP has been suggested as a useful animal model for human TDS, as in both humans and rodents disturbance of foetal Leydig and Sertoli cell functions plays a major role in induction of TDS-like symptoms (Fisher et al. 2003).
However, the main question involving phthalates is whether the level of human exposure is sufficient to adversely impact male and/or female reproductive health. A study by Swan et al. (2005) reported an association between phthalate exposure and reduced anogenital distance in human infants, an effect that is also observed in rats. However, the range of doses typically used in animal studies is three to four orders of magnitude greater than the estimated daily exposure of humans. Only recently have researchers begun to investigate and report on possible biological changes at doses within the range of median human phthalate exposure (Lehmann et al. 2004; Andrade et al. 2006c; Lin et al. 2008).
Some studies indicate that treatment of rat dams with active phthalates may result in non-monotonic (biphasic) dose–responses for the activity/expression of enzymes and proteins involved in the biosynthesis of steroid hormones in the offspring. Lehmann et al. (2004) studied alterations in genes coding for steroidogenic enzymes in the foetal testis of rats exposed to DBP. In this study, the authors reported reductions in several genes at doses that approach maximum human exposure levels. In a recent study with DEHP, Andrade et al. (2006c) have shown a striking biphasic dose–response for aromatase enzyme activity in the brain of neonatal males, with low-dose inhibition and high-dose stimulation. Since aromatase is a key enzyme in the biosynthesis of oestrogens, phthalate exposure might be associated with disturbances of the normal balance between androgens and oestrogens.
In addition to studies using low phthalate doses, recent studies have been conducted in order to evaluate the possibility of cumulative effects, since humans are exposed to multiple phthalates that are known to affect male reproductive development in rats. Howdeshell et al. (2007, 2008) demonstrated that different phthalates can act in a cumulative, dose-additive manner to reduce the testicular testosterone production by the rat foetal testis. Rider et al. (2008) reported dose-additive responses on reproductive malformations and androgen-dependent organ weights with developmental exposure to a seven-chemical anti-androgenic mixture that included some active phthalates. Coadministration of no-effect doses of DEHP and DBP reduces testicular testosterone levels and results in misshapen seminiferous cords and gonocyte multinucleation in the rat foetal testis (Martino-Andrade et al. 2008).
(d). Conclusions
Overall, the resemblance of phthalate effects in rats to human reproductive disorders has raised concerns over a possible link between phthalate exposure and human disease, even though most reproductive tract abnormalities in animal studies have been reported at dosages that probably result in internal dose concentrations that are well above those observed in humans. However, recent evidence for effects at lower doses as well as the results of combined toxicity studies and some epidemiological data on possible associations between phthalate exposure and reproductive effects in humans have provided further evidence for concern. The investigation of phthalate effects in non-human primates during pre- and early post-natal periods, the possibility of cumulative effects at low doses and a better understanding of the mode of action of phthalates and its relevance to humans are critical issues that should be addressed in future experimental studies.
3. Bisphenol A
(a). Sources and amounts of human BPA exposure
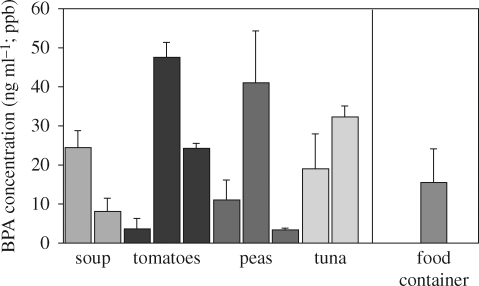
Bisphenol A was reported to be a synthetic oestrogen (relative potency was not assessed) in 1936 (Dodds & Lawson 1936), 2 years before the oestrogenic activity of the structurally and functionally related drug diethylstilbestrol (DES) was described (Dodds et al. 1938). In the 1950s, polymer chemists discovered that BPA molecules could be polymerized to make polycarbonate plastic. Bisphenol A is also the base compound used in the manufacture of the resin lining of food and beverage cans in the USA and many other countries, although the Japanese can industry changed the formulation of the plastic lining of cans in the late-1990s. This voluntary action was reported to be associated with a loss of the previous correlation between use of canned drinks and urine levels of BPA in Japanese students as well as over a 50 per cent decrease in BPA levels (Matsumoto et al. 2003). These latter findings suggest that use of BPA-based resins to line cans contributes significantly to the human body burden of BPA. Food contact items (can lining, food packaging and food and beverage containers) are thought to be the major contributors to the median and mean values of approximately 2–4 ng ml–1 of unconjugated BPA detected in adult and foetal serum (Vandenberg et al. 2007). Pure water was poured into food cans that had contained different products and a polycarbonate food storage container. The products were heated to 100°C for 24 h, and BPA was analysed by HPLC with CoulArraay detection. The data presented in figure 1 reveal that all products leached detectable levels of BPA, although there were differences in the amount of leaching from different manufacturers' products. As expected, the cans that had contained the acidic tomato sauce resulted in the highest BPA values, since acid accelerates hydrolysis of the ester bond linking BPA molecules in polycarbonate and resins.
Figure 1.
Bisphenol A (BPA) concentration in water placed into food cans that had contained different products. Two or three different brands of each product were examined. The cans were emptied, cleaned and rinsed with water that did not contain detectable levels of BPA. The 10 cans and a single new reusable ‘microwave safe’ polycarbonate food container were filled with HPLC-grade water and heated to 100°C for 24 h. Bisphenol A was extracted and analysed by HPLC with CoulArray detection (limit of detection was 0.01 ppb). Negative controls in glass bottles gave undetectable levels of BPA. Spiked samples in glass containers gave >95 per cent recovery. Significant leaching of BPA occurred from each type of product tested.
Another common use for BPA is as the monomer in dental sealants and composites used for fillings, from which BPA leaches in variable amounts and for different lengths of time depending on the product (Joskow et al. 2006). BPA is also an additive (referred to as a plasticizer) in PVC plastic products. For example, BPA has been reported to be present in PVC products such as stretch film (Lopez-Cervantes & Paseiro-Losada 2003). It is also used in printer ink and to coat paper used for receipts (referred to as ‘carbonless paper’). It is thus a major contaminant in recycled paper products (Vandenberg et al. 2007). Polycarbonate food storage and beverage containers (the hard, clear containers, which may be tinted in the case of sport water bottles or baby bottles) cause concern regarding their potential to leach BPA because they are re-usable (Nerin et al. 2003), and repeated use leads to an increase in leaching (Brede et al. 2003). Many of these containers are marketed for use in the microwave, despite the fact that heating is known to increase BPA leaching levels. It is a basic characteristic of the ester bond linking BPA molecules together in polycarbonate plastic and resins that the rate of breaking of the bond by hydrolysis increases with heat, releasing free BPA (Bae et al. 2002), and an increase in leaching also occurs as a result of either an increase or decrease in pH (Welshons et al. 2006; Vandenberg et al. 2007). When food or liquids (such as beer) are placed into a can, they are heated to a high temperature for sterilization. The consequence is that food and beverages in cans have variable levels of BPA based on whether the contents are lipophilic and/or acidic or alkaline (figure 1), all of which increase leaching (see the Environmental Working Group website for additional data on leaching of BPA from cans, at www.EWG.org).
A critical issue regarding routes of exposure to BPA was revealed by the Canadian Ministry of the Environment, which determined that BPA poses a threat to aquatic wildlife at current levels at which it is found in aquatic ecosystems (Canada 2008). This government report and other reviews (Vandenberg et al. 2007) identify that a number of studies show that BPA is leaching out of products being thrown into landfills and getting into ground water, contaminating rivers, streams and drinking water. While in an aerobic environment, BPA has a half-life of days, has a greater density than water and thus ends up in the sediment. In an anaerobic environment, BPA does not degrade. The Canadian Government is thus taking a regulatory approach to this problem that involves not only trying to limit the use of BPA in food and beverage containers, but also involves trying to deal with the problem of disposal of BPA-containing plastic that cannot be recycled to produce new BPA-based products.
The US Centers for Disease Control and Prevention (CDC) has measured BPA in the urine of people in the USA as part of the last national health survey (Calafat et al. 2008). The CDC reported that 93 per cent of people had detectable levels of BPA in their urine. Interestingly, the median and mean levels of unconjugated (parent) BPA reported in blood, as well as the lower and upper range reported for women and their foetuses at the time of parturition in Germany (Schönfelder et al. 2002), were virtually identical to values reported for total BPA in urine by the CDC. The findings by Schönfelder et al. are consistent with reports of blood levels of unconjugated BPA in people from other countries such as Japan (Vandenberg et al. 2007). In this review of the published literature, the authors determined that blood levels in humans identified by numerous analytical methods consistently showed approximately 10-fold higher median or mean levels of unconjugated BPA than average blood levels found throughout a 24 h period after laboratory rats were administered a BPA dose equal to the tolerable daily intake (TDI). The TDI corresponds to the amount of a chemical a person can be exposed to on a daily basis over an extended period of time (usually a lifetime) without suffering deleterious effects; this is also referred to by US regulatory agencies as the ‘reference dose' and ‘acceptable daily intake dose'.
Currently, a dose of 50 µg kg–1 d–1 is considered ‘safe' for daily human consumption by the US Environmental Protection Agency (EPA) (IRIS 1988) and Food and Drug Administration (FDA). Findings reported by Vandenberg et al. (2007) led to a consensus conclusion by 38 scientists who attended a US National Institutes of Health (NIH) sponsored conference on BPA (vom Saal et al. 2007) that current levels of human exposure to BPA already exceed the presumed safe daily exposure dose. The data presented in the review indicate that: (i) BPA is detected at the nanogram per millilitre (ppb) levels; (ii) unconjugated (bioactive) BPA is found in blood and (iii) the same levels are found in its conjugated (glucuronidated or sulphated) form in urine (Vandenberg et al. 2007). This is in sharp contrast to a review that concluded that BPA is rapidly and completely cleared from the blood of adults following oral absorption (Willhite et al. 2008). The conclusion that all ingested BPA is immediately metabolized (conjugated) to biologically inactive metabolites is thus not consistent with the published literature. The ‘back calculation' approach of estimating human exposure to BPA based on the assumption that all orally absorbed BPA is immediately cleared from blood into urine is an invalid approach if the chemical is not immediately and completely metabolized (Barr et al. 2005).
BPA is one of the highest production volume chemicals in commerce, with over 6 billion pounds produced in 2003 (Burridge 2003), and a significant increase in production volume was expected at that time. In order to accurately estimate human exposure to BPA, we would need to know what products it is used in, since without this knowledge we have no way to determine the potential for different routes of exposure. This is currently not possible as identification of the chemicals used in products, such as baby toys, food and beverage containers or paper products, is not required. The latter is interesting in that printer ink contains BPA, and the ‘carbonless paper' used to provide receipts for purchases is coated with BPA (the BPA reacts with dye in the paper in response to pressure or heat). Newspapers and carbonless paper could be significant sources of transdermal BPA exposure, and all recycled paper has BPA in it from these sources (Vandenberg et al. 2007). Dermal absorption of BPA from these potential sources of exposure has not been investigated.
(b). Adverse health effects of BPA in laboratory animals
There were three reports released in 2007 and 2008 in North America: two from the USA and one from Canada (discussed subsequently). The first was released as a consensus statement from an NIH-sponsored conference on BPA (vom Saal et al. 2007). This document was co-authored by 38 scientists who are experts in the field of endocrine disruption, and the majority of scientists at this conference had conducted research on BPA. The consensus document was a summary of the findings in five accompanying review articles that covered the entire published literature relating to the health effects of BPA as of the end of 2006 (Crain et al. 2007; Keri et al. 2007; Richter et al. 2007a,b; Vandenberg et al. 2007; Wetherill et al. 2007).
(i). Adult exposure, experimental findings and relevance for human health
There is extensive published literature showing the effects of acute exposure to very low doses of BPA in adult experimental animals: rats, mice and various aquatic species (Richter et al. 2007a,b). However, an issue that has generated much confusion is the rate at which BPA is metabolized. As described earlier, if one makes the assumption that virtually all BPA exposure is via an oral route and that virtually all BPA is immediately conjugated in the liver after absorption from the gut (transported from the gut to the liver via the direct hepatic portal vessels), then of course there would be no concern about BPA posing a threat to adults. However, this assumption is not consistent with the published biomonitoring literature regarding levels of unconjugated BPA in human blood (Vandenberg et al. 2007). Importantly, no rodent or human experiment has been conducted that involves chronic exposure, although the human biomonitoring data suggest virtual continuous exposure (Calafat et al. 2008). This is a major gap that needs to be filled.
The issue of route of exposure to BPA has generated significant controversy, since the default assumption is that oral exposure accounts for most (or virtually all) BPA exposure in humans. It is well known that exposure to BPA via injection, which does not result in the first-pass liver metabolism (glucuronidation/sulphation) that would occur following oral exposure, results in about a 10–20-fold higher amount of BPA in the blood relative to levels following oral administration (Vandenberg et al. 2007). However, if BPA is injected into rodents at doses of BPA that are thousands of times lower than the lowest adverse effect level (LOAEL) of 50 mg kg–1 d–1 that was used to establish the safe dose of 50 µg kg–1 d–1, it is logical to assume that findings from studies that used injection as the route of administration are also relevant to assessing the potential health hazards posed by BPA, while recognizing that a correction for differences in pharmacokinetics based on route of administration is required. Just a few examples of effects of low doses of BPA in adult rodents are a significant stimulation of insulin secretion followed by insulin resistance in mice (Ropero et al. 2008), a significant decrease in daily sperm production in rats (Sakaue et al. 2001), a decrease in maternal behaviour in mice (Palanza et al. 2002) and disruption of hippocampal synapses, leading to the appearance of a brain typical of that seen in senility in both rats and monkeys (MacLusky et al. 2005; Leranth et al. 2007, 2008).
Related to the fact that type 2 diabetes is increasing in many regions of the world is the finding that exposure of adult mice to a low oral dose of BPA (10 µg kg–1 d–1) resulted in stimulation of insulin secretion that was mediated by oestrogen receptor (ER) alpha, occurring as a rapid response mediated by the extracellular signal-regulated kinases 1 and 2 (ERK1/2) pathway; and that the prolonged hypersecretion of insulin was followed by insulin resistance and postprandial hyperinsulinaemia (Alonso-Magdalena et al. 2005, 2008; Ropero et al. 2008). The fact that these very-low-dose studies of BPA are confirmed in cell culture studies that reveal molecular pathways that mediate effects of BPA in the low parts per trillion range has been reviewed (Wetherill et al. 2007). A consensus conclusion from the NIH-sponsored meeting on BPA was that the more recently discovered rapid-response pathway (as opposed to the nuclear ERs acting as ligand-activated transcription factors) may mediate many, but not all, of the low-dose effects of BPA (vom Saal et al. 2007).
The prediction from mechanistic studies in mice concerning the molecular pathways by which very low doses of BPA stimulate insulin production and secretion, which is then followed by insulin resistance (Ropero et al. 2008), leads to the fact that BPA may be related to elevated blood insulin and glucose, as well as insulin resistance. In addition, Hugo et al. (2008) reported that human adipocytes removed from different regions showed a marked suppression of the critical regulatory cytokine adiponectin, with the maximum response occurring at 1 nM (0.23 ppb), directly in the range of human exposure to BPA. A decrease in adiponectin is related to an increased risk for type 2 diabetes and cardiovascular disease and heart attack (Beltowski et al. 2008). It is thus of considerable interest that Lang et al. (2008) reported that in an analysis of data from 1455 people examined for BPA levels in urine as part of the US National Health and Nutrition Examination Survey (NHANES) conducted in 2003–2004, they found a significant relationship between urine levels of BPA and cardiovascular disease, type 2 diabetes and abnormalities in liver enzymes. This report, suggesting links between BPA and some of the most significant and economically burdensome human diseases, is based on a cross-sectional study and therefore cannot establish causality, but the fact that these findings are related to other studies that identify plausible mechanisms by which BPA at current levels of human exposure could result in these diseases greatly strengthens the importance of the findings (vom Saal & Myers 2008).
(ii). Developmental exposure, experimental findings and relevance for human health
The greatest concern regarding exposure to BPA is during development: foetuses, neonates, infants, children and adolescents. The laboratory animal research on BPA is unique in that there are now hundreds of studies that have examined doses of BPA within the range of human exposure rather than the more typical approach in regulatory toxicology of only testing a few doses that are typically thousands of times higher than human exposure levels. The surprise associated with the first ‘low dose' publication on the effects of BPA in laboratory mice in 1997 (Nagel et al. 1997) was that effects on the reproductive system in male offspring were found at a daily oral dose to pregnant mice that was 25 000 times lower than had ever been examined, and 25 times below the still current safe daily exposure dose according to the US FDA and US EPA, as well as the European Food Safety Authority (EFSA 2006). The lowest dose of BPA that had previously been examined was 50 mg kg–1 d–1 in some traditional very-high-dose toxicological studies conducted in the1980s. The 50 mg kg–1 d–1 was thus the LOAEL, and this dose was divided by 10 to ‘estimate' the no observable adverse effect level (NOAEL) and divided by 1000 for the EPA to estimate that 50 µg kg−1 d−1 was safe for daily human exposure (IRIS 1988). Numerous reviews have been published discussing the assumptions used in estimating safe exposure levels for endocrine-disrupting chemicals such as BPA (vom Saal & Sheehan 1998; Markey et al. 2003; Welshons et al. 2003, 2006; vom Saal & Hughes 2005; Myers & vom Saal 2008).
One of the main concerns with the adverse effects reported in response to developmental exposure to very low doses of BPA (that produce blood levels in animals below those in humans) is that they all relate to disease trends in humans (table 1). For example, there is an obesity epidemic in many regions of the world, and developmental exposure to BPA increases body weight later in life (Howdeshell et al. 1999; Takai et al. 2000; Rubin et al. 2001). In addition, neonatal exposure to a low dose of DES (1 µg kg−1 d−1) stimulated a subsequent increase in body weight and fat in CD-1 mice, while a dose 1000 times higher resulted in a significant decrease in body weight (Newbold et al. 2004).
Table 1.
Prenatal–neonatal exposure of mice and rats to BPA at human exposure levels in relation to human health trends.
| effects in mice and rats | human health trends |
|---|---|
| prostate hyperplasia and cancer | prostate cancer increase |
| mammary hyperplasia and cancer | breast cancer increase |
| abnormal urethra/obstruction | hypospadias |
| sperm count decrease | sperm count decrease |
| early puberty in females | early sexual maturation |
| ovarian cysts/uterine fibroids | polycystic ovary syndrome/uterine fibroids |
| abnormal oocyte chromosomes | miscarriage |
| body weight increase | obesity increase |
| insulin resistance | type 2 diabetes |
| hyperactivity/impaired learning | attention deficit hyperactivity disorder |
Relevance for human health. The largest literature on the adverse effects of BPA exposure during development concerns adverse effects on brain structure, chemistry and behaviour (Richter et al. 2007a,b). One of the most interesting aspects of this literature is that there is a consistent finding of a loss of sex differences in brain structure, chemistry and behaviour owing to foetal/neonatal exposure to low doses of BPA. BPA thus appears to interfere with the normal processes that govern sexual differentiation, with brain changes reported in both males and females, depending on the outcome measured (Fujimoto et al. 2006; Rubin et al. 2006; Palanza et al. 2008). The implications at the population level for disruption of normal socio-sexual behaviours have not been extensively studied, although there are reports of changes in play behaviour (Dessi-Fulgheri et al. 2002) as well as other socio-sexual behaviours (Farabollini et al. 2002) that could impact population dynamics.
There are also numerous studies of the effects of low doses of BPA on the development of the female (Soto et al. 2008) and male reproductive organs in female rats and mice. Findings include chromosomal abnormalities in oocytes in females (Susiarjo et al. 2007), and long-term effects on accessory reproductive organs that are not observed until mid-life, such as uterine fibroids and para-ovarian cysts (Newbold et al. 2007). Other studies have shown that very low doses of BPA during pre-natal or neonatal development can result in permanent effects in male rats and mice. Low doses of BPA cause a decrease in daily sperm production and an increase in prostate size (vom Saal et al. 1998), an increase in prostatic androgen receptors (Gupta 2000; Richter et al. 2007a,b) and a progression from hyperplasia of prostate basal (stem) cells in the primary prostatic ducts during foetal life (Timms et al. 2005) to basal cell squamous metaplasia in adulthood (Ogura et al. 2007) and eventually to early stage prostate cancer (prostatic interepithelial neoplasia or PIN) in response to adult administration of testosterone and oestradiol (Ho et al. 2006).
(c). Conclusions
The required testing of chemicals for regulatory purposes is not aimed at understanding molecular mechanisms and focuses on effects occurring at high doses that are typically not relevant for human exposure scenarios. Over the last 10 years, there has been a dramatic shift in the approach of scientists with regard to investigating doses that are relevant for human exposure to study the effect of BPA in laboratory animals. This has led to a totally unique toxicological literature revealing extensive evidence that effects in laboratory animals are occurring at blood levels that are lower than those found in the average person in a developed country. This is clearly of great concern for possible impact on human health.
The assumptions used in chemical risk assessments, such as all dose–response curves are monotonic, and there is a threshold dose below which no effect occurs at which the system is ‘off', clearly do not apply to endogenous hormones, hormonally active drugs or hormonally active chemicals. How can there be a threshold at which no oestrogen responses occur for a chemical that is adding oestrogenic activity to a system that is already ‘on' at zero dose of the exogenous chemical (Sheehan et al. 1999; Sheehan 2005)? Also, all hormones show responses that are non-monotonic, and non-monotonic dose–response curves have been reported in many BPA studies (Richter et al. 2007a,b; Alonso-Magdalena et al. 2008). The need to integrate concepts of endocrinology in the assumptions underlying chemical risk assessments will some day result in the development of a new system, and the data from findings with low doses of BPA will play an important role in this paradigm shift (Myers & vom Saal 2008).
4. Brominated flame retardants
(a). Uses of TBBPA
Brominated flame retardants function by increasing the time between ignition of a fire and flash over, which is the point when enough heat is generated to cause combustion of flammable materials. TBBPA is the classical halogenated flame retardant chemically bonded to epoxy and polycarbonate resins. It is present in printed circuit boards and casings used in personal computers, printers, fax machines and copiers. Dimethyl TBBPA is added to acrylonitrile–butadiene–styrene (ABS) resin and high impact polystyrene (HIP). This derivative is blended with the polymers, meaning that it exists free in the chemical matrix. ABS resins have a wide variety of applications including automotive parts, pipes and fittings, and domestic and office appliances. Polystyrene is used in packaging, electrical and electronic equipment enclosures for televisions, furniture and construction materials. Another derivative, bis (2-hydroxyethyl ether) TBBPA is used as a flame retardant for paper and textile adhesives and coatings (Alaee et al. 2003).
(b). Exposure to TBBPA
Products with both additive and chemically bonded forms of TBBPA have been shown to release TBBPA into the environment (Birnbaum & Staskal 2004), resulting in detection in sewage sludge (Oberg et al. 2002), soil, sediments, birds, fish (Morris et al. 2004) and air from different occupational settings (Sjödin et al. 2001). TBBPA serves as a source of environmental BPA as it has been shown to break down to BPA in marine sediments (European Union Risk Assessment Report 2005).
Although studies indicate high first-pass metabolism of TBBPA in rats and humans owing to rapid conjugation with glucuronic acid and elimination in the bile (Kuester et al. 2007), TBBPA has been detected in cow and human milk (Thomsen et al. 2002a; Antignac et al. 2008), human serum (e.g. Hayama et al. 2004), human adipose tissue (Johnson-Restrepo et al. 2008) and umbilical cord serum (Antignac et al. 2008). Evaluation of BFRs in archived human serum samples in Norway showed a temporal increase in concentrations of six PBDE congeners and TBBPA over the period 1977–1999. The concentrations for different age groups were relatively similar, except for the 0–4 year olds who had 1.6–3.5 times higher serum concentrations (Thomsen et al. 2002b).
(c). Experimental studies on TBBPA
There are only a few published studies regarding the toxicology of TBBPA. Information regarding the endocrine-disrupting potential of TBBPA mainly comes from in vitro studies and in vivo studies performed in quail, fish and tadpoles; however, two papers describing a study in the rodent model have been recently published.
(i). Thyroid hormone effects
An increase in thyronine (T3) in female rat offspring and a reduction in circulating total thyroxine (T4) were observed in both sexes in a reproductive study with exposure to TBBPA in food, beginning 70 or 14 days prior to mating of F0 males and females, respectively, and which continued throughout gestation and lactation up to 14 weeks of age at necropsy (Van der Ven et al. 2008). Thyroid hormone concentrations were similarly altered at 12 weeks of age in a subacute toxicity study (28 days of repeated dosing of TBBPA) with a significant decrease in T4 and increase in T3 in males. Parallel changes were observed in females; however, they were not statistically significant (Van der Ven et al. 2008). In the same reproductive experiment, appraisal of brainstem auditory evoked potentials (BAEP), a means to detect effects on hearing, indicated changes in hearing latency and hearing threshold similar to previous reports on developmental exposure to polychlorinated biphenyls (PCBs) (Lilienthal et al. 2008). It is plausible that the changes in thyroid hormone status mediated these effects as thyroid hormones play a crucial role in the development of auditory function, and the benchmark dose levels for changes in thyroid hormones (2.3–30.8 mg kg−1 d−1) and BAEP (1–40 mg kg−1 d−1) were in the same range (Van der Ven et al. 2008).
(ii). Binding to transthyretin
TBBPA has an even closer structural relationship to T4 than PCBs. In vitro competitive binding assays demonstrated that TBBPA binding to human transthyretin (TTR) is more potent than the natural ligand (Meerts et al. 2000; Hamers et al. 2006). It is theorized that these compounds may decrease serum T4 concentrations by displacing it from the carrier proteins, leading to increased clearance. This displacement could also make more T4 available for deiodination, leading to increased formation of T3 or reverse T3.
(iii). Binding to thyroid hormone receptor and thyroid hormone activity
During development, most tadpole tissues are influenced by thyroid hormones, making them suitable to evaluate potential disruption of thyroid homeostasis. An in vivo study simultaneously exposing Rana rugosa tadpoles to T3 and TBBPA found TBBPA suppression of T3 induced tail shortening, indicating a thyroid hormone antagonist effect (Kitamura et al. 2005a). While only mild effects on larval development were observed, a study in Xenopus laevis tadpoles also suggested thyroid hormone antagonism by TBBPA (Jagnytsch et al. 2006). In vitro, TBBPA competes with T3 binding to a nuclear suspension from rat pituitary Mt/T/e-2 cells (Kitamura et al. 2002) and was shown to inhibit the activity of T3 in Chinese hamster ovary cells transfected with human TRα or TRß (Kitamura et al. 2005a).
(iv). Sex steroid effects
An increase in pituitary weight in male offspring was noted in the reproductive study (Van der Ven et al. 2008), which correlates with previously published in vitro findings from Kitamura et al. (2002, 2005b), showing that TBBPA increased proliferation of GH3 cells (rat pituitary cell line) and growth hormone production. Ghisari & Bonefeld-Jorgensen (2005) also observed that TBBPA stimulates GH3 cell growth, which was inhibited by the anti-oestrogen ICI 182 780, suggesting that this effect is ER-mediated.
In vitro studies indicate that the lower brominated forms of TBBPA have higher affinity with the ER than the higher brominated analogues (Samuelsen et al. 2001). In MCF-7 human breast cancer cells, TBBPA functioned as a partial ER agonist (Olsen et al. 2003) and demonstrated mixed agonist/antagonist activities in estrogen responsive element (ERE)-luciferase reporter gene assays. The oestrogenic activity was confirmed in vivo using the uterotrophic assay (Kitamura et al. 2005b).
Inhibition of oestradiol sulphation by TBBPA has been reported in vitro (Kester et al. 2002; Hamers et al. 2006), which suggests the potential for impaired elimination in vivo leading to increased biologically active oestradiol concentrations. Environmentally relevant TBBPA concentrations have been shown to decrease reproductive success in zebra fish (Kuiper et al. 2007) and inhibition of oestradiol metabolism in lake trout ( Jurgella et al. 2006). In male Japanese quail, however, no oestrogenic effects after embryonic exposure to TBBPA were observed for reproductive behaviour, plasma testosterone and testicular morphology (Berg et al. 2001; Halldin et al. 2005).
(d). Products with PBDEs
Two (penta and octa formulations) of the three main commercial mixtures of PBDEs added to polymers for the manufacture of goods are no longer in production. The penta mixture was applied to polyurethane foams used in furniture, mattresses, carpet pads and automobile seats and styrene plastics used for electrical appliances and flame-retardant textiles. The octa-technical mixture was blended with ABS resins used in automobile electronics, home and office appliances and high-impact sport equipment and toys. The deca product is added to a variety of polymers, and examples of end products include fabric backings and housings for electronics (Frederiksen et al. 2008).
(e). Exposure to PBDE
The presence of PBDEs in breast milk, adipose tissue and serum has been confirmed in several studies (as reviewed in Frederiksen et al. 2009). An examination of a cohort of 4-year-old children revealed that breast-fed children have 6.5 times higher average body burden of total PBDEs than formula-fed children (Carrizo et al. 2007). Detection of PBDEs in liver tissue of human foetuses (Schecter et al. 2007) and in cord blood (e.g. Antignac et al. 2008) demonstrates that in utero exposure is taking place. On a pro-kilogram basis, studies have described higher levels of exposure to PBDEs for children than adults (as reviewed in Frederiksen et al. 2009).
(f). Animal studies investigating developmental exposure to PBDEs
There are more published studies regarding effects following in vivo exposure to PBDEs than for TBBPA. Animal studies have revealed the potential for endocrine disruption and effects on neurobehaviour and the reproductive system.
(i). Thyroid hormone
In rodent studies, perinatal or peripubertal exposure to PBDEs causes a reduction in T4 while effects on T3 and TSH are less consistent. Perinatal exposure to 1, 10 or 30 mg kg−1 d−1 DE-71 (commercial penta-mixture) (Zhou et al. 2002) or 18 mg kg−1 d−1 (Ellis-Hutchings et al. 2006) did not alter T3 concentrations. However, a concomitant decrease in T3 and an increase in thyroid stimulating hormone (TSH) in males only were observed following peripubertal exposure to 30 or 60 mg kg−1 d−1 DE-71 (Stoker et al. 2004), and another study reported a decrease in T3 in female rats following exposure to 100 or 300 mg kg−1 d−1 DE-71 or 60 or 100 mg kg−1 d−1 DE-79 (octa-commercial mixture) on post-natal day (PND) 28–32 (Zhou et al. 2001). Administration of 300 µg kg−1 d−1 BDE-99 (2,2′,4,4′,5-pentabromodiphenyl ether) on gestation day (gd) 6 resulted in a transient decrease in T3 in male offspring on PND 1 with reductions in T4 occurring in both male and female offspring on PND 22 (Kuriyama et al. 2007).
(ii). Effects on sex steroids and the reproductive system
Following gestational exposure, in vivo oestrogen activity for BDE-99 has been indicated by increased expression of mRNA of oestrogen responsive genes in the uterus (Ceccatelli et al. 2006). Alterations in the sex hormone profile have been reported, including reductions in circulating oestradiol and testosterone in adult male offspring (Lilienthal et al. 2006) and lower circulating oestradiol concentrations without changes in whole ovarian aromatase activity in young female rat offspring exposed to 700 µg kg−1 of BDE-47 on gd 6 (Talsness et al. 2008). Possible impairment of androgen function following high-dose peripubertal exposure to DE-71 was indicated by postponed puberty and delayed growth of androgen-dependent tissues in male rats without an effect on circulating testosterone concentrations (Stoker et al. 2004). Additional in vitro studies by the same group suggest that the observed effects may be because of binding of some of the congeners in the mixture acting as antagonists to the androgen receptor (Stoker et al. 2005).
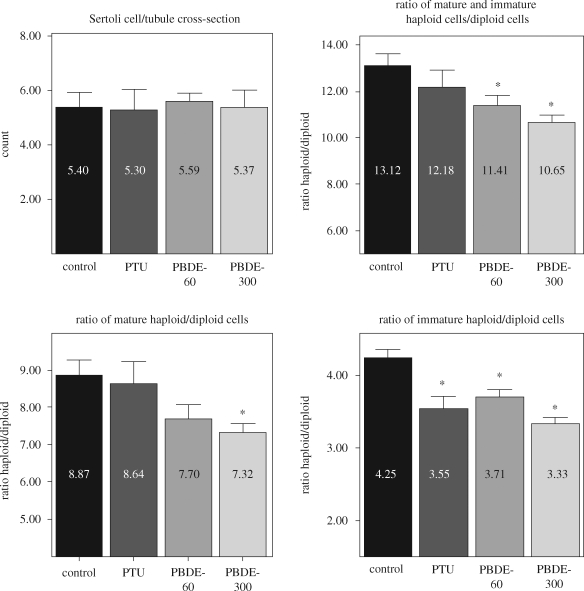
Functional changes to the gonads have been observed following in utero exposure to PBDEs. Administration of an agent used to treat hyperthyroidism, 6-n-propyl-2-thiouracil (5 mg l−1 drinking water on gd 7–21), or 60 or 300 µg BDE-99 kg−1 on gd 6 (Kuriyama et al. 2007) resulted in a reduction in spermatid and sperm counts without an influence on circulating testosterone concentrations in adult F1 males (Kuriyama et al. 2005). Whether the impaired spermatogenesis is accompanied by changes in the testicular cell population based on DNA ploidy (flow cytometry analysis) was examined in isolated testicular cells from the other testis (12 per group) of the same animals. DNA staining was performed by suspending the cells in phosphate buffered saline with 100 µg ml−1 ribonuclease A and 50 µg ml−1 propidium iodide. Propidium iodide fluorescence was measured at 620 nm to differentiate between mature haploid cells (elongated spermatid), immature haploid cells (round/elongating spermatid) and diploid cells (spermatogonia, spermatocyte, Sertoli cells, Leydig cells). The number of Sertoli cells in the seminiferous epithelium is related to sperm production since each cell supports a finite number of germ cells (Russel et al. 1990). This cell number was also determined in histological sections from other F1 males (six per group) on PND 110. Sertoli cell nucleoli were counted in 25 round or nearly round seminiferous tubule cross-sections chosen at random, and 25 Sertoli cell nucleoli diameters were measured for each animal. These counts were corrected for section thickness and the smallest recognizable nucleolar profile (cap section) as described previously (Russel et al. 1990).
A statistically significant decrease in the ratio of haploid/diploid cells was observed in the PBDE-99 group (figure 2), which corroborates the reduced number of spermatid/sperm counts observed. We observed slight differences in the ratio of mature haploid (mostly elongated spermatid)/diploid cells in treated animals, and this effect was more pronounced when the ratio of immature haploid (round/elongating spermatid)/diploid cells was compared with controls. No change in the number of Sertoli cells/seminiferous tubule cross-section was observed among the groups (figure 2), indicating that the reduction in haploid cells is because of another cause and supporting the finding that the changes in these ratios are owing to reductions in the haploid populations. The observed impaired spermatogenesis in male offspring exposed to low-dose BDE-99 during development (Kuriyama et al. 2005) has been confirmed by flow cytometry analysis and is not mediated by reduced numbers of Sertoli cells. Alternatively, a hormonal-dependent mechanism can be proposed as sperm production is dependent on permissive actions of follicle stimulating hormone (FSH) and testosterone and, therefore, luteinizing hormone (LH). Although significant differences in testosterone and LH concentrations in serum from adult animals were not found, the possibility of hormonal changes during early development cannot be ruled out. Functional changes in the seminiferous epithelium leading to impairment of normal spermatogenesis in adult rat offspring can also be speculated.
Figure 2.
Number of Sertoli cells per tubule cross-section in adult male offspring (F1) on PND 110 and flow cytometry analysis of testicular cell population on the basis of their DNA ploidy in adult rats on PND 140. Gravid female rats were administered per gavage either vehicle (control) or 60 or 300 µg BDE-99 (kg bw)−1 (PBDE-60 or PBDE-300) on gd 6. A fourth group received 5 mg 6-n-propyl-2-thiouracil (PTU) l−1 in drinking water from gd 7–21. ANOVA followed by Dunnett t-test, *p < 0.05.
In adult female offspring, ultrastructural changes in the ovary following low-dose gestational exposure to either PBDE-99 (Talsness et al. 2005) or BDE-47 (Talsness et al. 2008) and effects on folliculogenesis after administration of 1 or 10 mg kg−1 d−1 of BDE-99 during gestation (Lilienthal et al. 2006) were observed. Altered folliculogenesis evaluated around the time of puberty after low-dose exposure to BDE-47 during gestation has also been reported (Talsness et al. 2008). The changes in the latter study were characterized by a reduction in antral follicle numbers and circulating estradiol concentrations. Whether these changes are because of thyroid hormone disruption or alterations in gonadotropins and/or sex hormones is unclear.
The animal data thus far suggest that PBDEs may affect the reproductive system via alterations in thyroid hormone profile and/or sex steroid action. Aberrations in thyroid hormone status are known to adversely affect reproduction. In animal studies, thyroid hormone has been shown to play an integral role in testicular development (Cooke et al. 1992) and affect ovarian follicular maturation (Baldridge et al. 2004).
(iii). Neurobehaviour
The period of rapid brain growth represents a particularly vulnerable developmental window to insults and is characterized by a dramatic increase in the number of cells and myelination, cell migration, dendritic and axonal growth and the formation of neural connections. Exposure to PBDEs during the brain growth spurt of mice modified spontaneous motor behaviour and habituation to new surroundings (e.g. Viberg et al. 2006) and altered levels of proteins involved in brain maturation (Viberg et al. 2008). Other studies have found changes in spontaneous motor activity following early post-natal exposure to mice, as well as effects on one measure of the ontogeny of sensorimotor integration (Rice et al. 2007) and subtle differences in neuromotor development (Branchi et al. 2002; Gee & Moser 2008). In addition, examination of cognitive function revealed deficits as mice exhibited impaired learning and memory on the Morris water maze test following early post-natal PBDE exposure (Viberg et al. 2003).
Administration of BDE-99 to rats during gestation at a dose (300 ug kg−1 on gd 6) resulting in dam adipose tissue concentrations (Kuriyama et al. 2007) only slightly higher than those reported for this congener in humans (Johnson-Restrepo et al. 2005) led to hyperactivity in the rat offspring (Kuriyama et al. 2005).
(g). Relevance of exposure to flame retardants for human health
(i). Reproduction
It is known that diseases of the thyroid gland affect the reproductive capacity of women. In particular, hypothyroid women may exhibit anovulation, and hypothyroidism is associated with hyperprolactinaemia, which inhibits the release of pituitary gonadotropin and gonadal steroids.
Although less is known about the role of thyroid hormones in the development of the reproductive system, it is believed that they are important for gonad development in both sexes (Cooke et al. 2004). Animal studies and in vitro studies demonstrate that TBBPA and PBDEs interfere with thyroid hormone action, and the function of both the ovary and the testis has been altered following in utero exposure to PBDEs. Thyroid hormones indirectly affect sperm production by regulating the number of Sertoli cells, which act as nurse cells for developing sperm. It should be noted, however, that the new data presented here indicate that the observed reduction in sperm count following gestational BDE-99 exposure was not associated with changes in Sertoli cell number. In the ovary, thyroid hormone receptors are present in granulosa cells, which produce steroids and provide growth factors interacting with the oocyte.
In addition, alterations in sex steroid concentrations or function and LH levels have been reported in animal studies for PBDEs, indicating the potential to adversely affect human reproduction. Two epidemiological studies indicate that exposure may be associated with adverse affects on the testis. Preliminary data from a pilot study performed in Japan suggest an inverse relationship between BDE-153 (2,2′4,4′,5,5′-hexabromodiphenyl ether) serum concentrations and sperm concentration in young Japanese males (Akutsu et al. 2008). A larger study relating information regarding in utero and early developmental exposure to PBDEs with cryptorchidism involved 86 Danish and Finnish newborn boy–mother pairs. Associations between PBDE contamination of human breast milk and cryptorchidism and increased gonadotropin release to support normal testosterone production were observed (Main et al. 2007). Although there was no significant difference in the placental concentrations, the sum of PBDEs in breast milk was higher in cryptorchid boys than in controls and was positively correlated with infant serum LH concentration. The authors suggest that the lack of correlation between the PBDE concentrations in the placental and breast milk samples may be that the placenta represents the situation at delivery as one would find in a single blood sample and not the long-term exposure.
Although the exposure time frames are not comparable, an increase in LH was also reported for adult male rats exposed to 60 mg kg−1 d−1 of DE-71 for 3 days (Stoker et al. 2005). As indicated previously, Insl3 and androgen play roles in testicular descent. LH regulates testosterone production and plays a role in the differentiation of Leydig cells, which must reach a certain stage before production of Insl3 occurs (Sadeghian et al. 2005). The rate of this maturation process in rats has been reported to be influenced by T3 (Mendis-Handagama et al. 2007). Alterations in thyroid hormone profile or changes in the status of the hypothalamo–pituitary–testicular axis could influence testicular descent by impacting Insl3 formation.
(ii). Neurodevelopment
Thyroid hormone is crucial for growth and development and in particular, for neurodevelopment. Decreases in foetal and maternal thyroid hormone are known to impact neuropsychological development in humans, and impaired achievement on neuropsychological tests can occur even when maternal hypothyroidism is subclinical (Haddow et al. 1999). An evaluation of human thyroid hormone status and PBDE exposure was performed in China. TSH and serum concentrations of PBDEs were found to be significantly increased in subjects living close to an electronic waste site compared with those living 50 km away, suggesting hormone disruption (Yuan et al. 2008).
Aberrations in thyroid hormone homeostasis caused by PBDEs and TBBPA raise concerns regarding their potential to influence child neurodevelopment. Animal studies indicate changes in behaviour following exposure to PBDEs during a critical developmental window, which may be mediated through changes in thyroid hormones or direct neurotoxicity.
Data regarding neurotoxicants indicate that there is a continuum of toxic outcomes at low doses, i.e. chronic daily exposures, which do not induce overt clinical symptoms (Grandjean & Landrigan 2006). The authors suggest that neurodevelopmental disorders associated with exposure to known human neurotoxicants and untested chemicals have resulted in a veiled pandemic, incurring significant costs to society because of lowered productivity and reductions in intelligence (Grandjean & Landrigan 2006).
5. General conclusions
Exposure of humans to pharmaceuticals is deliberate, with the intention of achieving a desired effect. Development and testing of medications involves a series of evaluations culminating in human clinical trials before marketing is approved. This is quite different from the situation with chemicals, whose presence in biota and humans is inadvertent. In the field of toxicology, information regarding potential human health effects is mainly derived from experimental studies and, when available, from epidemiological studies. Difficulties are not only encountered with extrapolation from animal models to humans, but epidemiological studies are also thwarted by drawbacks such as controlling for confounding factors. In particular, subjects are exposed to an assortment of chemicals on a daily basis and, often, lack of data regarding the extent of exposure at what may have been the critical time frame. One of the goals of toxicology is to identify effects in animal models with the aim to lower the risks of negatively impacting human health. Implicit in this task is that toxicological data, derived from animal studies indicating a potential for adverse effects, serve as a basis to limit exposure before effects appear or are confirmed in humans. The evidence from animal studies on single exposures to the chemicals discussed here suggests the potential for risk to human health. Moreover, data derived from co-exposure studies support the contention that the assortment of chemicals to which we are exposed on a daily basis increases the likelihood of health effects. The high prevalence of body burdens of these chemicals and simultaneous exposure to a number of substances, in conjunction with the fact that the highest concentrations have been demonstrated in the developing young, a sensitive subpopulation of society, indicate the need to decrease the exposure to these compounds.
Acknowledgements
We would like to thank Brigitte Woelffel, Zeynep Akkoc and Lars Niemann for their excellent technical assistance. Support for the previously unpublished data on BDE-99 was through a grant from the Umweltbundesamt (Federal Environment Agency, grant No. 29965221/04).
Footnotes
All authors contributed equally to this review.
One contribution of 15 to a Theme Issue ‘Plastics, the environment and human health’.
References
- Akingbemi B. T., Ge R., Klinefelter G. R., Zirkin B. R., Hardy M. P.2004Phthalate-induced Leydig cell hyperplasia is associated with multiple endocrine disturbances. Proc. Natl Acad. Sci. USA 101, 775–780 (doi:10.1073/pnas.0305977101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akutsu K., Takatori S., Nozawa S., Yoshiike M., Nakazawa H., Hayakawa K., Makino T., Iwamoto T.2008Polybrominated diphenyl ethers in human serum and sperm quality. Bull. Environ. Contam. Toxicol 80, 345–350 (doi:10.1007/s00128-008-9370-4) [DOI] [PubMed] [Google Scholar]
- Alaee M., Arias P., Sjödin A., Bergman A.2003An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 29, 683–689 (doi:10.1016/S0160-4120(03)00121-1) [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P., Laribi O., Ropero A. B., Fuentes E., Ripoll C., Soria B., Nadal A.2005Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ. Health Perspect. 113, 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P., Morimoto S., Ripoll C., Fuentes E., Nadal A.2006The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 114, 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P., Ropero A. B., Carrera M. P., Cederroth C. R., Baquie M., Gauthier B. R., Nef S., Stefani E., Nadal A.2008Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE 3, e2069 (doi:10.1371/journal.pone.0002069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade A. J., Grande S. W., Talsness C. E., Grote K., Golombiewski A., Sterner-Kock A., Chahoud I.2006aA dose–response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): effects on androgenic status, developmental landmarks and testicular histology in male offspring rats. Toxicology 225, 64–74 (doi:10.1016/j.tox.2006.05.007) [DOI] [PubMed] [Google Scholar]
- Andrade A. J., Grande S. W., Talsness C. E., Gericke C., Grote K., Golombiewski A., Sterner-Kock A., Chahoud I.2006bA dose response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult male offspring rats. Toxicology 228, 85–97 (doi:10.1016/j.tox.2006.08.020) [DOI] [PubMed] [Google Scholar]
- Andrade A. J., Grande S. W., Talsness C. E., Grote K., Chahoud I.2006cA dose–response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose–response and low dose effects on rat brain aromatase activity. Toxicology 227, 185–192 (doi:10.1016/j.tox.2006.07.022) [DOI] [PubMed] [Google Scholar]
- Antignac J. P., et al. 2008Exposure assessment of fetus and newborn to brominated flame retardants in France: preliminary data. Mol. Nutr. Food Res. 52, 258–265 (doi:10.1002/mnfr.200700077) [DOI] [PubMed] [Google Scholar]
- Bae B., Jeong J. H., Lee S. J.2002The quantification and characterization of endocrine disruptor bisphenol-A leaching from epoxy resin. Water Sci. Technol. 46, 381–387 [PubMed] [Google Scholar]
- Baldridge M. G., Stahl R. L., Gerstenberger S. L., Tripoli V., Hutz R. J.2004In utero and lactational exposure of Long-Evans rats to ammonium perchlorate (AP) disrupts ovarian follicle maturation. Reprod. Toxicol. 19, 155–161 (doi:10.1016/j.reprotox.2004.07.002) [DOI] [PubMed] [Google Scholar]
- Barlow N. J., Foster P. M.2003Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to di(n-butyl) phthalate. Toxicol. Pathol. 31, 397–410 [DOI] [PubMed] [Google Scholar]
- Barr D. B., Wang R. Y., Needham L. L.2005Biologic monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the National Children's Study. Environ. Health Perspect. 113, 1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltowski J., Jamroz-Wisniewska A., Widomska S.2008Adiponectin and its role in cardiovascular diseases. Cardiovasc. Hematol. Disord. Drug Targets 8, 7–46 (doi:10.2174/187152908783884920) [DOI] [PubMed] [Google Scholar]
- Berg C., Halldin K., Brunström B.2001Effects of bisphenol A and tetrabromobisphenol A on sex organ development in quail and chicken embryos. Environ. Toxicol. Chem. 20, 2836–2840 (doi:10.1897/1551-5028(2001)020<2836:EOBAAT>2.0.CO;2) [PubMed] [Google Scholar]
- Birnbaum L. S., Staskal D. F.2004Brominated flame retardants: cause for concern? Environ. Health Perspect. 112, 9–17 (doi:10.1289/chp.6559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I., Alleva E., Costa L. G.2002Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicology 23, 375–384 (doi:10.1016/S0161-813X(02)00078-5) [DOI] [PubMed] [Google Scholar]
- Brede C., Fjeldal P., Skjevrak I., Herikstad H.2003Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 20, 684–689 (doi:10.1080/0265203031000119061) [DOI] [PubMed] [Google Scholar]
- Burridge E.2003Bisphenol A: product profile. Eur. Chem. News 14–20, 17 [Google Scholar]
- Calafat A. M., Ye X., Wong L. Y., Reidy J. A., Needham L. L.2008Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 116, 39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada 2008Draft screening assessment for the challenge phenol, 4,4′-(1-methylethylidene)bis-(bisphenol A). Chemical Abstracts Service Registry Number 80-05-7. See http://www.ec.gc.ca/substances/ese/eng/challenge/batch2/batch2_80-05-7.cfm.
- Carrizo D., Grimalt J. O., Ribas-Fito N., Sunyer J., Torrent M.2007Influence of breastfeeding in the accumulation of polybrominated diphenyl ethers during the first years of child growth. Environ. Sci. Technol. 41, 4907–4912 (doi:10.1021/es070217u) [DOI] [PubMed] [Google Scholar]
- Ceccatelli R., Faass O., Schlumpf M., Lichtensteiger W.2006Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE-99 and PCB. Toxicology 220, 104–116 (doi:10.1016/j.tox.2005.12.004) [DOI] [PubMed] [Google Scholar]
- Cooke P. S., Porcelli J., Hess R. A.1992Induction of increased testis growth and sperm production in adult rats by neonatal administration of the goitrogen propylthiouracil (PTU): the critical period. Biol. Reprod. 46, 146–154 (doi:10.1095/biolreprod46.1.146) [DOI] [PubMed] [Google Scholar]
- Cooke P. S., Holsberger D. R., Witorsch R. J., Sylvester P. W., Meredith J. M., Treinen K. A., Chapin R. E.2004Thyroid hormone, glucocorticoids, and prolactin at the nexus of physiology, reproduction, and toxicology. Toxicol. Appl. Pharmacol. 194, 309–335 (doi:10.1016/j.taap.2003.09.016) [DOI] [PubMed] [Google Scholar]
- Crain D. A., Eriksen M., Iguchi T., Jobling S., Laufer H., LeBlanc G. A., Guillette L. J., Jr2007An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod. Toxicol. 24, 225–239 (doi:10.1016/j.reprotox.2007.05.008) [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F., Porrini S., Farabollini F.2002Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ. Health Perspect. 110Suppl. 3, 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds E. C., Lawson W.1936Synthetic oestrogenic agents without the phenanthrene nucleus. Nature 137, 996 (doi:10.1038/137996a0) [Google Scholar]
- Dodds E. C., Lawson W., Noble R. L.1938Biological effects of the synthetic oestrogenic substance 4: 4′-dihydroxy- a: B-dimethylstilbene. Lancet 234, 1389–1391 [Google Scholar]
- Dostal L. A., Chapin R. E., Stefanski S. A., Harris M. W., Schwetz B. A.1988Testicular toxicity and reduced Sertoli cell numbers in neonatal rats by di(2-ethylhexyl)phthalate and the recovery of fertility as adults. Toxicol. Appl. Pharmacol. 95, 104–121 (doi:10.1016/S0041-008X(88)80012-7) [DOI] [PubMed] [Google Scholar]
- EFSA 2006Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to 2,2-BIS(4-HYDROXYPHENYL)PROPANE (Bisphenol A). EFSA J. 428, 1–76 [Google Scholar]
- Ellis-Hutchings R. G., Cherr G. N., Hanna L. A., Keen C. L.2006Polybrominated diphenyl ether induced alterations in vitamin A and thyroid hormone concentrations in the rat during lactation and early postnatal development. Toxicol. Appl. Pharmacol. 215, 135–145 (doi:10.1016/j.taap.2006.02.008) [DOI] [PubMed] [Google Scholar]
- Environmental Working Group 2007Bisphenol A: toxic plastics chemical in canned food. See www.erg.org/node/20933
- European Union Risk Assessment Report 2005European Union Risk Assessment Report on 2,2′,6,6′-tetrabromo-4,4′-isopropylene dipenol (tetrabromobisphenol-A). CAS no. 79-94-7, EINECS no. 201-236-9, European Chemicals Bureau, Ispra, Italy [Google Scholar]
- Farabollini F., Porrini S., Della Seta D., Bianchi F., Dessi-Fulgheri F.2002Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ. Health Perspect. 110Suppl. 3, 409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. S., Macpherson S., Marchetti N., Sharpe R. M.2003Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. 18, 1383–1394 (doi:10.1093/humrep/deg273) [DOI] [PubMed] [Google Scholar]
- Foster P. M.2006Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 29, 140–147 (doi:10.1111/j.1365-2605.2005.00563.x) [DOI] [PubMed] [Google Scholar]
- Foster P. M., Cook M. W., Thomas L. V., Walters D. G., Gangolli S. D.1983Differences in urinary metabolic profile from di-n-butyl phthalate-treated rats and hamsters. A possible explanation for species differences in susceptibility to testicular atrophy. Drug Metab. Dispos. 11, 59–61 [PubMed] [Google Scholar]
- Frederiksen M., Vorkamp K., Thomsen M., Knudsen L. E.2009Human internal and external exposure to PBDEs—a review of levels and sources. Int. J. Hyg. Environ. Health 212, 109–135 (doi:10.1016/j.ijheh.2008.04.005) [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Kubo K., Aou S.2006Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 1068, 49–55 (doi:10.1016/j.brainres.2005.11.028) [DOI] [PubMed] [Google Scholar]
- Gee J. R., Moser V. C.2008Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol. Teratol. 30, 79–87 (doi:10.1016/j.ntt.2007.11.001) [DOI] [PubMed] [Google Scholar]
- Ghisari M., Bonefeld-Jorgensen E. C.2005Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol. Cell Endocrinol. 244, 31–41 (doi:10.1016/j.mce.2005.01.013) [DOI] [PubMed] [Google Scholar]
- Grande S. W., Andrade A. J., Talsness C. E., Grote K., Chahoud I.2006A dose–response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicol. Sci. 91, 247–254 (doi:10.1093/toxsci/kfj128) [DOI] [PubMed] [Google Scholar]
- Grande S. W., Andrade A. J., Talsness C. E., Grote K., Golombiewski A., Sterner-Kock A., Chahoud I.2007A dose–response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult female offspring rats. Toxicology 229, 114–122 (doi:10.1016/j.tox.2006.10.005) [DOI] [PubMed] [Google Scholar]
- Grandjean P., Landrigan P. J.2006Developmental neurotoxicity of industrial chemicals. Lancet 368, 2167–2178 (doi:10.1016/S0140-6736(06)69665-7) [DOI] [PubMed] [Google Scholar]
- Gray T. J., Gangolli S. D.1986Aspects of the testicular toxicity of phthalate esters. Environ. Health Perspect. 65, 229–235 (doi:10.2307/3430187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray T. J., Rowland I. R., Foster P. M., Gangolli S. D.1982Species differences in the testicular toxicity of phthalate esters. Toxicol. Lett. 11, 141–147 (doi:10.1016/0378-4274(82)90119-9) [DOI] [PubMed] [Google Scholar]
- Gray L. E., Ostby J., Furr J., Price M., Veeramachanemi D. N., Parks L.2000Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 58, 350–365 (doi:10.1093/toxsci/58.2.350) [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Laskey J., Ostby J.2006Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female Long Evans hooded rats. Toxicol. Sci. 93, 189–195 (doi:10.1093/toxsci/kfl035) [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Crain D. A., Rooney A. A., Pickford D. B.1995Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ. Health Perspect. 103, 157–164 (doi:10.2307/3432527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta C.2000Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc. Soc. Exp. Biol. Med. 224, 61–68 (doi:10.1046/j.1525-1373.2000.22402.x) [DOI] [PubMed] [Google Scholar]
- Guvenius D. M., Aronsson A., Ekman-Ordeberg G., Bergman A., Norén K.2003Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorphenol. Environ. Health Perspect. 111, 1235–1241 (doi:10.1289/ehp.5946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow J. E., et al. 1999Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 341, 549–555 (doi:10.1056/NEJM199908193410801) [DOI] [PubMed] [Google Scholar]
- Halldin K., Axelsson J., Brunström B.2005Effects of endocrine modulators on sexual differentiation and reproductive function in male Japanese quail. Brain Res. Bull. 65, 211–218 (doi:10.1016/j.brainresbull.2004.11.020) [DOI] [PubMed] [Google Scholar]
- Hallmark N., et al. 2007Effects of monobutyl- and di-(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ. Health Perspect. 115, 390–396 (doi:10.1289/ehp.9490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers T., Kamstra J. H., Sonneveld E., Murk A. J., Kester M. H. A., Andersson P. L., Legler J., Brouwer A.2006In vitro profiling of the endocrine disrupting potency of brominated flame retardants. Toxicol. Sci. 92, 157–173 (doi:10.1093/toxsci/kfj187) [DOI] [PubMed] [Google Scholar]
- Hayama T., Yoshida H., Onimaru S., Yonekura S., Kuroki H., Todoroki K., Nohta H., Yamaguchi M.2004Determination of tetrabromobisphenol A in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 809, 131–136 (doi:10.1016/j.jchromb.2004.06.013) [DOI] [PubMed] [Google Scholar]
- Heudorf U., Mersch-Sundermann V., Angerer J.2007Phthalates: toxicology and exposure. Int. J. Hyg. Environ. Health 210, 623–634 (doi:10.1016/j.ijheh.2007.07.011) [DOI] [PubMed] [Google Scholar]
- Ho S. M., Tang W. Y., Belmonte de Frausto J., Prins G. S.2006Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 66, 5624–5632 (doi:10.1158/0008-5472.CAN-06-0516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell K. L., Hotchkiss A. K., Thayer K. A., Vandenbergh J. G., vom Saal F. S.1999Exposure to bisphenol A advances puberty. Nature 401, 763–764 [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Furr J., Lambright C. R., Rider C. V., Wilson V. S., Gray L. E., Jr2007Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol. Sci. 99, 190–202 (doi:10.1093/toxsci/kfm069) [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Wilson V. S., Furr J., Lambright C. R., Rider C. V., Blystone C. R., Hotchkiss A. K., Gray L. E., Jr2008A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague Dawley rat in a cumulative, dose additive manner. Toxicol. Sci. 105, 153–165 (doi:10.1093/toxsci/kfn077) [DOI] [PubMed] [Google Scholar]
- Hugo E. R., Brandebourg T. D., Woo J. G., Loftus J., Alexander J. W., Ben-Jonathan N.2008Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health Perspect. 116, 1642–1647 (doi:10.1289/ehp.11537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezuki Y., Tsutsumi O., Takai Y., Kamei Y., Taketani Y.2002Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 11, 2839–2841 [DOI] [PubMed] [Google Scholar]
- IRIS 1988. ‘Bisphenol A. (CASRN 80-05-7)'. 2002 US-EPA Integrated Risk Information System Substance file. See http://www.epa.gov/iris/subst/0356.htm. [Google Scholar]
- Ito Y., Yokota H., Wang R., Yamanoshita O., Ichihara G., Wang H., Kurata Y., Takagi K., Nakajima T.2005Species differences in the metabolism of di(2-ethylhexyl) phthalate (DEHP) in several organs of mice, rats, and marmosets. Arch. Toxicol. 79, 147–154 (doi:10.1007/s00204-004-0615-7) [DOI] [PubMed] [Google Scholar]
- Jagnytsch O., Opitz R., Lutz I., Kloas W.2006Effects of tetrabromopbisphenol A on larval development and thyroid hormone-regulated biomarkers of the amphibian Xenopus laevis. Environ. Res. 101, 340–348 (doi:10.1016/j.envres.2005.09.006) [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B., Kurunthachalam K., Rapaport D. P., Rodan B. D.2005Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ. Sci. Technol. 39, 5177–5182 (doi:10.1021/es050399x) [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B., Adams D. H., Kannan K.2008Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere 70, 1935–1944 (doi:10.1016/j.chemosphere.2007.10.002) [DOI] [PubMed] [Google Scholar]
- Joskow R., Barr D. B., Barr J. R., Calafat A. M., Needham L. L., Rubin C.2006Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J. Am. Dent. Assoc. 137, 353–362 [DOI] [PubMed] [Google Scholar]
- Jurgella G. F., Marwah A., Malison J. A., Peterson R., Barry T. P.2006Effects of xenobiotics and steroids on renal and hepatic estrogen metabolism in lake trout. Gen. Comp. Endocrinol. 148, 273–281 (doi:10.1016/j.ygcen.2006.03.011) [DOI] [PubMed] [Google Scholar]
- Keri R. A., Ho S. M., Hunt P. A., Knudsen K. E., Soto A. M., Prins G. S.2007An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod. Toxicol. 24, 240–252 (doi:10.1016/j.reprotox.2007.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester M. H. A., et al. 2002Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J. Clin. Endocrinol. Metab. 87, 1142–1150 (doi:10.1210/jc.87.3.1142) [DOI] [PubMed] [Google Scholar]
- Kitamura S., Jinno N., Ohta S., Kuroki H., Fujimoto N.2002Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem. Biophys. Res. Commun. 293, 554–559 (doi:10.1016/S0006-291X(02)00262-0) [DOI] [PubMed] [Google Scholar]
- Kitamura S., et al. 2005aAnti-thyroid hormonal activity of tetrabromobisphenol A, a flame retardant, and related compounds: affinity to the mammalian thyroid hormone receptor, and effect on tadpole metamorphosis. Life Sci. 76, 1589–1601 (doi:10.1016/j.lfs.2004.08.030) [DOI] [PubMed] [Google Scholar]
- Kitamura S., et al. 2005bComparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 84, 249–259 (doi:10.1093/toxsci/kfi074) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Drexler H., Angerer J.2004Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP). Int. J. Hyg. Environ. Health 207, 15–22 (doi:10.1078/1438-4639-00270) [DOI] [PubMed] [Google Scholar]
- Koch H. M., Preuss R., Angerer J.2006Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure—an update and latest results. Int. J. Androl. 29, 155–165 (doi:10.1111/j.1365-2605.2005.00607.x) [DOI] [PubMed] [Google Scholar]
- Kuester R. K., Sólyom A. M., Rodriguez V. P., Sipes I. G.2007The effects of dose, route, and repeated dosing on the disposition and kinetics of tetrabromobisphenol A in male F-344 rats. Toxicol. Sci. 96, 237–245 (doi:10.1093/toxsci/kfm006) [DOI] [PubMed] [Google Scholar]
- Kuiper R. V., van den Brandhof E. J., Leonards P. E. G., van der Ven L. T. M., Wester P. W., Vos J. G.2007Toxicity of tetrabromobisphenol A (TBBPA) in zebrafish (Danio rerio) in a partial life cycle test. Arch. Toxicol. 81, 1–9 (doi:10.1007/s00204-006-0117-x) [DOI] [PubMed] [Google Scholar]
- Kurata Y., Kidachi F., Yokoyama M., Toyota N., Tsuchitani M., Katoh M.1998Subchronic toxicity of di(2-ethylhexyl)phthalate in common marmosets: lack of hepatic peroxisome proliferation, testicular atrophy, or pancreatic acinar cell hyperplasia. Toxicol. Sci. 42, 49–56 [DOI] [PubMed] [Google Scholar]
- Kuriyama S. N., Talsness C. E., Grote K., Chahoud I.2005Developmental exposure to low dose PBDE 99: effects on male fertility and neurobehavior in rat offspring. Environ. Health Perspect. 113, 149–154 (doi:10.1289/ehp.7421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S. N., Wanner A., Fidalgo-Neto A. A., Talsness C. E., Koerner W., Chahoud I.2007Developmental exposure to low-dose PBDE-99: tissue distribution and thyroid hormone levels. Toxicology 242, 80–90 (doi:10.1016/j.tox.2007.09.011) [DOI] [PubMed] [Google Scholar]
- Lahousse S. A., Wallace D. G., Liu D., Gaido K. W., Johnson K. J.2006Testicular gene expression profiling following prepubertal rat mono-(2-ethylhexyl) phthalate exposure suggests a common initial genetic response at fetal and prepubertal ages. Toxicol. Sci. 93, 369–381 (doi:10.1093/toxsci/kfl049) [DOI] [PubMed] [Google Scholar]
- Lake B. G., Brantom P. G., Gangolli S. D., Butterworth K. R., Grasso P.1976Studies on the effects of orally administered di-(2-ethylhexyl) phthalate in the ferret. Toxicology 6, 341–356 (doi:10.1016/0300-483X(76)90038-X) [DOI] [PubMed] [Google Scholar]
- Lamb J. C., Chapin R. E., Teague J., Lawton A. D., Reel J. R.1987Reproductive effects of four phthalic acid esters in the mouse. Toxicol. Appl. Pharmacol. 88, 255–269 (doi:10.1016/0041-008X(87)90011-1) [DOI] [PubMed] [Google Scholar]
- Lang I. A., Galloway T. S., Scarlett A., Henley W. E., Depledge M., Wallace R. B., Melzer D.2008Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300, 1303–1310 (doi:10.1001/jama.300.11.1303) [DOI] [PubMed] [Google Scholar]
- Law R. J., Allchin C. R., de Boer J., Covaci A., Herzke D., Lepom P., Morris S., Tronczynski J., de Wit C. A.2006Levels and trends of brominated flame retardants in the European environment. Chemosphere 64, 187–208 (doi:10.1016/j.chemosphere.2005.12.007) [DOI] [PubMed] [Google Scholar]
- Lehmann K. P., Phillips S., Sar M., Foster P. M., Gaido K. W.2004Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol. Sci. 81, 60–68 (doi:10.1093/toxsci/kfh169) [DOI] [PubMed] [Google Scholar]
- Leranth C., Szigeti-Buck K., Maclusky N. J., Hajszan T.2007Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology 149, 988–994 (doi:10.1210/en.2007-1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C., Hajszan T., Szigeti-Buck K., Bober J., MacLusky N. J.2008Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc. Natl Acad. Sci. USA 105, 14 187–14 191 (doi:10.1073/pnas.0806139105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. H., Jester W. F., Jr, Laslett A. L., Orth J. M.2000A single dose of di-(2-ethylhexyl) phthalate in neonatal rats alters gonocytes, reduces Sertoli cell proliferation, and decreases cyclin D2 expression. Toxicol. Appl. Pharmacol. 166, 222–229 (doi:10.1006/taap.2000.8972) [DOI] [PubMed] [Google Scholar]
- Lilienthal H., Hack A., Roth-Härer A., Wichert Grande S., Talsness C. E.2006Effects of developmental exposure to 2,2′,4,4′,5-pentabromodipheyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ. Health. Perspect. 114, 194–201 (doi:10.1289/ehp.8391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal H., Verwer C. M., van der Ven L. T., Piersma A. H., Vos J. G.2008Exposure to tetrabromobisphenol A (TBBPA) in Wistar rats: neurobehavioral effects in offspring from a one-generation reproduction study. Toxicology 246, 45–54 (doi:10.1016/j.tox.2008.01.007) [DOI] [PubMed] [Google Scholar]
- Lin H., et al. 2008Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc. Natl Acad. Sci. USA 105, 7218–7222 (doi:10.1073/pnas.0709260105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Cervantes J., Paseiro-Losada P.2003Determination of bisphenol A in, and its migration from, PVC stretch film used for food packaging. Food Addit. Contam. 20, 596–606 [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Hajszan T., Leranth C.2005The environmental estrogen bisphenol A inhibits estrogen-induced hippocampal synaptogenesis. Environ. Health Perspect. 113, 675–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main K. M., et al. 2006Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 114, 270–276 (doi:10.1289/ehp.8075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main K. M., Kiviranta H., Virtanen H. E., Sundqvist E., Tuomisto J. T., Tuomisto J., Vartiainen T., Skakkebaek N. E., Toppari J.2007Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ. Health Perspect. 115, 1519–1526 (doi:10.1289/ehp.9924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey C. M., Coombs M. A., Sonnenschein C., Soto A. M.2003Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol. Dev. 5, 67–75 (doi:10.1046/j.1525-142X.2003.03011.x) [DOI] [PubMed] [Google Scholar]
- Martino-Andrade A. J., Morais R. N., Botelho G. G., Muller G., Grande S. W., Carpentieri G. B., Leão G. M., Dalsenter P. R.2008Coadministration of active phthalates results in disruption of foetal testicular function in rats. Int. J. Androl. [Epub ahead of print]. (doi:10.1111/j.1365-2605.2008.00939.x) [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Yokota H., Yuasa A.2002Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ. Health Perspect. 110, 193–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Kunugita N., Kitagawa K., Isse T., Oyama T., Foureman G. L., Morita M., Kawamoto T.2003Bisphenol A levels in human urine. Environ. Health Perspect. 111, 101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee R. H., Butala J. H., David R. M., Gans G.2004NTP Center for the evaluation of risks to human reproduction reports on phthalates: addressing the data gaps. Reprod. Toxicol. 18, 1–22 (doi:10.1016/j.reprotox.2003.09.002) [DOI] [PubMed] [Google Scholar]
- Meerts I. A. T. M., van Zanden J. J., Luijks E. A. C., van Leeuwen-Bol I., Göran M., Jakobsson E., Bergman Å., Brouwer A.2000Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 56, 95–104 (doi:10.1093/toxsci/56.1.95) [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama S. M., Ariyaratne H. B., Mrkonjich L., Ivell R.2007Expression of insulin-like peptide 3 in the postnatal rat Leydig cell lineage: timing and effects of triiodothyronine-treatment. Reproduction 133, 479–485 (doi:10.1530/REP-06-0238) [DOI] [PubMed] [Google Scholar]
- Mocarelli P., et al. 2008Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ. Health Perspect. 116, 70–77 (doi:10.1289/ehp.10399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S., et al. 2004The distribution and fate of HBCD and TBBP-A brominated flame retardants in North Sea estuaries and aquatic food webs. Environ. Sci. Technol. 38, 5497–5504 (doi:10.1021/es049640i) [DOI] [PubMed] [Google Scholar]
- Myers J. P., vom Saal F. S.2008Time to update environmental regulations. San Francisco Medi. 81, 30–31 [Google Scholar]
- Mylchreest E., Sar M., Cattley R. C., Foster P. M.1999Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol. Appl. Pharmacol. 156, 81–95 (doi:10.1006/taap.1999.8643) [DOI] [PubMed] [Google Scholar]
- Nagao T., Ohta R., Marumo H., Shindo T., Yoshimura S., Ono H.2000Effect of butyl benzyl phthalate in Sprague–Dawley rats after gavage administration: a two-generation reproductive study. Reprod. Toxicol. 14, 513–532 (doi:10.1016/S0890-6238(00)00105-2) [DOI] [PubMed] [Google Scholar]
- Nagel S. C., vom Saal F. S., Thayer K. A., Dhar M. G., Boechler M., Welshons W. V.1997Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ. Health Perspect. 105, 70–76 (doi:10.2307/3433065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerin C., Fernandez C., Domeno C., Salafranca J.2003Determination of potential migrants in polycarbonate containers used for microwave ovens by high-performance liquid chromatography with ultraviolet and fluorescence detection. J. Agric. Food Chem. 51, 5647–5653 (doi:10.1021/jf034330p) [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Jefferson W. N., Padilla-Banks E., Haseman J.2004Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reprod. Toxicol. 18, 399–406 (doi:10.1016/j.reprotox.2004.01.007) [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Jefferson W. N., Padilla-Banks E.2007Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 24, 253–258 (doi:10.1016/j.reprotox.2007.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norén K., Meironyté D.2000Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere 40, 1111–1123 (doi:10.1016/S0045-6535(99)00360-4) [DOI] [PubMed] [Google Scholar]
- Oberg K., Warman K., Oberg T.2002Distribution and levels of brominated flame retardants in sewage sludge. Chemosphere 48, 805–809 (doi:10.1016/S0045-6535(02)00113-3) [DOI] [PubMed] [Google Scholar]
- Oehlmann J., et al. 2009A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 364, 2047–2062 (doi:10.1098/rstb.2008.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y., Ishii K., Kanda H., Kanai M., Arima K., Wang Y., Sugimura Y.2007Bisphenol A induces permanent squamous change in mouse prostatic epithelium. Differentiation 75, 745–756 (doi:10.1111/j.1432-0436.2007.00177.x) [DOI] [PubMed] [Google Scholar]
- Olsen C. M., Meussen-Elholm E. T. M., Samuelsen M., Holme J. A., Hongslo J. K.2003Effects of the environmental oestrogens bisphenol A, tetrachlorobisphenol A, tetrabromobisphenol A, 4-hydroxybiphenyl and 4,4′-dihydroxybiphenyl on oestrogen receptor binding, cell proliferation and regulation of oestrogen sensitive proteins in the human breast cancer cell line MCF-7. Pharmacol. Toxicol. 92, 180–188 (doi:10.1034/j.1600-0773.2003.920408.x) [DOI] [PubMed] [Google Scholar]
- Palanza P., Howdeshell K. L., Parmigiani S., vom Saal F. S.2002Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ. Health Perspect. 110, 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P. L., Gioiosa L., Parmigiani S., vom Saal F. S.2008Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ. Res. 108, 150–157 (doi:10.1016/j.envres.2008.07.023) [DOI] [PubMed] [Google Scholar]
- Parks L. G., Ostby J. S., Lambright C. R., Abbott B. D., Klinefelter G. R., Barlow N. J., Gray L. E.2000The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 5, 339–349 [DOI] [PubMed] [Google Scholar]
- Pugh G., Jr, Isenberg J. S., Kamendulis L. M., Ackley D. C., Clare L. J., Brown R., Lington A. W., Smith J. H., Klauning J. E.2000Effects of di-isononyl phthalate, di-2-ethylhexyl phthalate, and clofibrate in cynomolgus monkeys. Toxicol. Sci. 56, 181–188 (doi:10.1093/toxsci/56.1.181) [DOI] [PubMed] [Google Scholar]
- Rice D. C., Reeve E. A., Herlihy A., Zoeller R. T., Thompson W. D., Markowski V. P.2007Developmental delays and locomotor activity in the C47BL6/L mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol. Teratol. 29, 511–520 (doi:10.1016/n.ntt.2007.03.061) [DOI] [PubMed] [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S.2007aIn vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 24, 199–224 (doi:10.1016/j.reprotox.2007.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. A., Taylor J. A., Ruhlen R. R., Welshons W. V., vom Saal F. S.2007bEstradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate cells. Environ. Health Perspect. 115, 902–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider C. V., Furr J., Wilson V. S., Gray L. E., Jr2008A mixture of seven antiandrogens induces reproductive malformations in rats. Int. J. Androl. 31, 249–262 (doi:10.1111/j.1365-2605.2007.00859.x) [DOI] [PubMed] [Google Scholar]
- Ropero A. B., Alonso-Magdalena P., Garcia-Garcia E., Ripoll C., Fuentes E., Nadal A.2008Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int. J. Androl. 31, 194–200 (doi:10.1111/j.1365-2605.2007.00832.x) [DOI] [PubMed] [Google Scholar]
- Rubin B. S., Murray M. K., Bamassa D. A., King J. C., Soto A. M.2001Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 109, 675–680 (doi:10.2307/3454783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. S., Lenkowski J. R., Schaeberle C. M., Vandenberg L. N., Ronsheim P. M., Soto A. M.2006Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology 147, 3681–3691 (doi:10.1210/en.2006-0189) [DOI] [PubMed] [Google Scholar]
- Russel L. D., Ettlin R. A., Sinha Hikkin A. P., Clegg E. D.1990Histological and histopathological evaluation of the testis, pp. 210–266 Clearwater: Cache River Press [Google Scholar]
- Sadeghian H., Anand-Ivell R., Balvers M., Relan V., Ivell R.2005Constitutive regulation of the Insl3 gene in rat Leydig cells. Mol. Cell Endocrinol. 241, 10–20 (doi:10.1016/j.mce.2005.03.017) [DOI] [PubMed] [Google Scholar]
- Sakaue M., Ohsako S., Ishimura R., Kurosawa S., Kurohmaru M., Hayashi Y., Aoki Y., Yonemoto J., Tohyama C.2001Bisphenol A affects spermatogenesis in the adult rat even at a low dose. J. Occup. Health 43, 185–190 (doi:10.1539/joh.43.185) [Google Scholar]
- Samuelsen M., Olsen C., Holme J. A., Meussen-Elholm E., Bergmann A., Hongslo J. K.2001Estrogen-like properties of brominated analogs of bisphenol A in the MCF-7 human breast cancer cell line. Cell Biol. Toxicol. 17, 139–151 (doi:10.1023/A:1011974012602) [DOI] [PubMed] [Google Scholar]
- Schecter A., Johnson-Welch S., Tung K. C., Harris T., Papke O., Rosen R.2007Polybrominated diphenyl ether (PBDE) levels in livers of U.S. human fetuses and newborns. J. Toxicol. Environ. Health A 70, 1–6 (doi:10.1080/15287390600748369) [DOI] [PubMed] [Google Scholar]
- Schoeters G., Den Hond E., Dhooge W., van Larebeke N., Leijs M.2008Endocrine disruptors and abnormalities of pubertal development. Basic Clin. Pharmacol. Toxicol. 102, 168–175 (doi:10.1111/j.1742-7843.2007.00180.x) [DOI] [PubMed] [Google Scholar]
- Schönfelder G., Wittfoht W., Hopp H., Talsness C. E., Paul M., Chahoud I.2002Parent bisphenol A accumulation in human maternal-fetal-placental unit. Environ. Health Perspect. 110, A703–A707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E.2008Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil. Steril. 89, 33–38 (doi:10.1016/j.fertnstert.2007.12.026) [DOI] [PubMed] [Google Scholar]
- Sheehan D. M.2005No-threshold dose–response curves for nongenotoxic chemicals: findings and applications for risk assessment. Environ. Res. 100, 93–99 (doi:10.1016/j.envres.2005.09.002) [DOI] [PubMed] [Google Scholar]
- Sheehan D. M., Willingham E., Gaylor D., Bergeron J. M., Crews D.1999No threshold dose for estradiol-induced sex reversal of turtle embryos: how little is too much? Environ. Health Perspect. 107, 155–159 (doi:10.2307/3434373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz V. D., Phillips S., Sar M., Foster P. M., Gaido K. W.2001Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate. Toxicol. Sci. 64, 233–242 (doi:10.1093/toxsci/64.2.233) [DOI] [PubMed] [Google Scholar]
- Silva M. J., Barr D. B., Reidy J. A., Malek N. A., Hodge C. C., Caudill S. P., Brock J. W., Needham L. L., Calafat A. M.2004Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 112, 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A., Carlsson H., Thuresson K., Sjölin S., Bergman Å., Ostman C.2001Flame retardants in indoor air at an electronics recycling plant, and at other work environments. Environ. Sci. Technol. 35, 448–454 (doi:10.1021/es000077n) [DOI] [PubMed] [Google Scholar]
- Skakkebaek N. E., Rajpert-De Meyts E., Main K. M.2001Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Anway M. D.2007Epigenetic transgenerational actions of vinclozolin on the development of disease and cancer. Crit. Rev. Oncog. 13, 75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Vandenberg L. N., Maffini M. V., Sonnenschein C.2008Does breast cancer start in the womb? Basic Clin. Pharmacol. Toxicol. 102, 125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker T. E., Laws S. C., Crofton K. M., Hedge J. M., Ferrell J. M., Cooper R. L.2004Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol. Sci. 78, 144–155 (doi:10.1093/toxsci/kfh029) [DOI] [PubMed] [Google Scholar]
- Stoker T. E., Cooper R. L., Lambright C. S., Wilson V. S., Furr J., Gray L. E.2005In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol. Appl. Pharmacol. 207, 78–88 (doi:10.1016/j.taap.2005.05.010) [DOI] [PubMed] [Google Scholar]
- Sun Y., Irie M., Kishikawa N., Wada M., Kuroda N., Nakashima K.2004Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed. Chromatogr. 18, 501–507 (doi:10.1002/bmc.345) [DOI] [PubMed] [Google Scholar]
- Susiarjo M., Hassold T. J., Freeman E., Hunt P. A.2007Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 3, 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H., et al. 2005Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 113, 1056–1061 (doi:10.1289/ehp.8100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Tsutsumi O., Ikezuki Y., Kamei Y., Osuga Y., Yano T., Taketan Y.2000Preimplantation exposure to bisphenol A advances postnatal development. Reprod. Toxicol. 15, 71–74 (doi:10.1016/S0890-6238(00)00119-2) [DOI] [PubMed] [Google Scholar]
- Takashima K., Ito Y., Gonzalez F. J., Nakajima T.2008Different mechanisms of DEHP-induced hepatocellular adenoma tumorigenesis in wild-type and Pparα-null mice. J. Occup. Health 50, 169–180 (doi:10.1539/joh.L7105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsness C. E., Shakibaei M., Kuriyama S. N., Grande S. W., Sterner-Kock A., Schnitker P., de Souza C., Grote K., Chahoud I.2005Ultrastructural changes observed in rat ovaries following in utero and lactational exposure to low doses of a polybrominated flame retardant. Toxicol. Lett. 157, 189–202 (doi:10.1016/j.toxlet.2005.02.001) [DOI] [PubMed] [Google Scholar]
- Talsness C. E., Kuriyama S. N., Sterner-Kock A., Schnitker P., Grande S. W., Shakibaei M., Andrade A., Grote K., Chahoud I.2008In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ. Health Perspect. 116, 308–314 (doi:10.1289/ehp.10536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C., Leknes H., Lundanes E., Becher G.2002aA new method for determination of halogenated flame retardants in human milk using solid-phase extraction. J. Anal. Toxicol. 26, 129–137 [DOI] [PubMed] [Google Scholar]
- Thomsen C., Lundanes E., Becher G.2002bBrominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environ. Sci. Technol. 36, 1414–1418 (doi:10.1021/es0102282) [DOI] [PubMed] [Google Scholar]
- Timms B. G., Howdeshell K. L., Barton L., Bradley S., Richter C. A., vom Saal F. S.2005Estrogenic chemicals in plastic and oral contraceptives disrupt development of the mouse prostate and urethra. Proc. Natl Acad. Sci. USA 102, 7014–7019 (doi:10.1073/pnas.0502544102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonari Y., Kurata Y., David R. M., Gans G., Kawasuso T., Katoh M.2006Effect of di(2-ethylhexyl) phthalate (DEHP) on genital organs from juvenile common marmosets: I. Morphological and biochemical investigation in 65-week toxicity study. J. Toxicol. Environ. Health A 69, 1651–1672 (doi:10.1080/15287390600630054) [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Hauser R., Marcus M., Olea N., Welshons W. V.2007Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177 (doi:10.1016/j.reprotox.2007.07.010) [DOI] [PubMed] [Google Scholar]
- Van der Ven L. T., et al. 2008Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study. Toxicology 245, 76–89 (doi:10.1016/j.tox.2007.12.009) [DOI] [PubMed] [Google Scholar]
- Viberg H., Fredriksson A., Eriksson P.2003Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 192, 95–106 (doi:10.1016/S0041-008X(03)00217-5) [DOI] [PubMed] [Google Scholar]
- Viberg H., Johansson N., Fredriksson A., Eriksson J., Marsh G., Eriksson P.2006Neonatal exposure to higher brominated diphenyl ethers, heptabromo-(PBDE 183), octabromo- (PBDE 203) or nonabromodiphenyl ether (PBDE 206), impairs spontaneous behaviour, and learning and memory functions of adult mice. Toxicol. Sci. 92, 211–218 (doi:10.1093/toxsci/kfj196) [DOI] [PubMed] [Google Scholar]
- Viberg H., Mundy W., Eriksson P.2008Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth, and synaptogenesis. Neurotoxicology 29, 152–159 (doi:10.1016/j.neuro.2007.10.007) [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Hughes C.2005An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113, 926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal F. S., Myers J. P.2008Bisphenol A and risk of metabolic disorders. JAMA 300, 1353–1355 (doi:10.1001/jama.300.11.1353) [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Sheehan D. M.1998Challenging risk assessment. Forum Appl. Res. Public Pol. 13, 11–18 [Google Scholar]
- vom Saal F. S., Cooke P. S., Buchanan D. L., Palanza P., Thayer K. A., Nagel S. C., Parmigiani S., Welshons W. V.1998A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol. Ind. Health 14, 239–260 [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., et al. 2007Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 24, 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons W. V., Thayer K. A., Judy B. M., Taylor J. A., Curran E. M., vom Saal F. S.2003Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 111, 994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons W. V., Nagel S. C., vom Saal F. S.2006Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147Suppl. 6, S56–S69 [DOI] [PubMed] [Google Scholar]
- Wetherill Y. B., Akingbemi B. T., Kanno J., McLachlan J. A., Nadal A., Sonnenschein C., Watson C. S., Zoeller R. T., Belcher S. M.2007In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 24, 178–198 (doi:10.1016/j.reprotox.2007.05.010) [DOI] [PubMed] [Google Scholar]
- Willhite C. C., Ball G. L., McLellan C. J.2008Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J. Toxicol. Environ. Health 11, 69–146 [DOI] [PubMed] [Google Scholar]
- Wilson V. S., Lambright C., Furr J., Ostby J., Wood C., Held G., Gray L. E.2004Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol. Lett. 146, 207–215 (doi:10.1016/j.toxlet.2003.09.012) [DOI] [PubMed] [Google Scholar]
- Yuan J., et al. 2008Elevated serum polybrominated diphenyl ethers and thyroid-stimulating hormone associated with lymphocytic micronuclei in Chinese workers from an E-waste dismantling site. Environ. Sci. Technol. 42, 2195–2200 (doi:10.1021/es702295f) [DOI] [PubMed] [Google Scholar]
- Zhou T., Ross D. G., DeVito M. J., Crofton K. M.2001Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol. Sci. 61, 76–82 (doi:10.1093/toxsci/61.1.76) [DOI] [PubMed] [Google Scholar]
- Zhou T., Taylor M. M., DeVito M. J., Crofton K. M.2002Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci. 66, 105–116 (doi:10.1093/toxsci/66.1.105) [DOI] [PubMed] [Google Scholar]