Abstract
Plastics debris in the marine environment, including resin pellets, fragments and microscopic plastic fragments, contain organic contaminants, including polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons, petroleum hydrocarbons, organochlorine pesticides (2,2′-bis(p-chlorophenyl)-1,1,1-trichloroethane, hexachlorinated hexanes), polybrominated diphenylethers, alkylphenols and bisphenol A, at concentrations from sub ng g–1 to µg g–1. Some of these compounds are added during plastics manufacture, while others adsorb from the surrounding seawater. Concentrations of hydrophobic contaminants adsorbed on plastics showed distinct spatial variations reflecting global pollution patterns. Model calculations and experimental observations consistently show that polyethylene accumulates more organic contaminants than other plastics such as polypropylene and polyvinyl chloride. Both a mathematical model using equilibrium partitioning and experimental data have demonstrated the transfer of contaminants from plastic to organisms. A feeding experiment indicated that PCBs could transfer from contaminated plastics to streaked shearwater chicks. Plasticizers, other plastics additives and constitutional monomers also present potential threats in terrestrial environments because they can leach from waste disposal sites into groundwater and/or surface waters. Leaching and degradation of plasticizers and polymers are complex phenomena dependent on environmental conditions in the landfill and the chemical properties of each additive. Bisphenol A concentrations in leachates from municipal waste disposal sites in tropical Asia ranged from sub µg l–1 to mg l–1 and were correlated with the level of economic development.
Keywords: marine plastic debris, plastic resin pellet, microplastics, landfill leachate, endocrine-disrupting chemicals, persistent organic pollutants
1. Introduction
Plastics are considered to be biochemically inert materials that do not interact with the endocrine system because of their large molecular size, which prohibits their penetration through the cell membrane. However, plastic debris present in the marine environment (marine plastics) carry chemicals of smaller molecular size (MW < 1000). These chemicals can penetrate into cells, chemically interact with biologically important molecules and may disrupt the endocrine system. Such chemicals are categorized into two groups: (i) hydrophobic chemicals that are adsorbed from surrounding seawater owing to affinity of the chemicals for the hydrophobic surface of the plastics and (ii) additives, monomers and oligomers of the component molecules of the plastics. Many of the contaminants addressed herein have known biological consequences. For example, the plastic constitutional monomer bisphenol A (BPA) and alkylphenol additives exert oestrogenic effects (e.g. Sonnenschein & Soto 1998), while some phthalate plasticizers have been associated with reduced testosterone production (e.g. Foster 2006). A wide range of biological effects have been reported for polychlorinated biphenyls (PCBs; Neal 1985). Reviews of human and wildlife exposure to plastics additives are also available in this volume (Koch & Calafat 2009; Meeker et al. 2009; Oehlmann et al. 2009).
The objective of this paper is to review the phenomena by which plastics released to the environment serve as carriers of organic contaminants to wildlife. The first two sections describe leaching of contaminants from plastics in landfills. Section 2 reviews the migration and degradation of plasticizers (phthalates), additives (organotin compounds and nonylphenols (NP)) and monomers (BPA), while §3 focuses on landfill leachate as a source of plastics-derived endocrine-disrupting compounds. The following sections address the uptake of contaminants from the environment onto plastics. In §4, sorption is described mathematically and the model validated by experimental observations. Section 5 summarizes the types and quantities of contaminants found sorbed to plastics collected from the marine environment. The remaining sections emphasize plastics as a vector in the transport of contaminants to animals. Section 6 presents an overview of the transfer of plastic-derived contaminants to organisms. This is expanded in §7, which describes literature concerning the transport of contaminants to sediment-dwelling invertebrates. Finally, §8 reports initial experiments demonstrating transfer of contaminants from plastics to higher-trophic-level organisms (acronyms in this paper are listed in table 1).
Table 1.
List of acronyms.
| acronym | meaning |
|---|---|
| BD | brominated diphenylether congener |
| BDEs | brominated diphenylethers |
| BPA | bisphenol A |
| CB | chlorinated biphenyl congener |
| DDD | 2,2′-bis(p-chlorophenyl)-1,1-dischloroethane |
| DDE | 2,2′-bis(p-chlorophenyl)-1,1-dischloroethylene |
| DDT | 2,2′-bis(p-chlorophenyl)-1,1-trichloroethane |
| DDTs | DDT and its metabolites (i.e. DDD and DDE) |
| DEHP | diethylhexyl phthalate |
| DMP | dimethyl phthalate |
| DOC | dissolved organic carbon |
| E1 | estrone |
| E2 | oestradiol |
| E3 | estriol |
| EDCs | endocrine-disrupting chemicals |
| EEQ | oestradiol-equivalent concentration |
| FTIR | Fourier transform infrared spectroscopy |
| GC-ECD | gas chromatograph equipped with an electron capture detector |
| GDP | gross domestic products |
| HCHs | hexachlorocyclohexanes |
| HDPE | high-density polyethylene |
| HOCs | hydrophobic organic contaminants |
| MW | molecular weight |
| NOEC | no-effect concentration |
| NP | nonylphenol |
| OP | octylphenol |
| PAHs | polycyclic aromatic hydrocarbons |
| PBDEs | polybrominated diphenylethers |
| PCBs | polychlorinated biphenyls |
| PCE | tetrachloroethylene |
| PE | polyethylene |
| PVC | polyvinyl chloride |
| SML | sea-surface microlayer |
| SOM | sorbent organic matter |
| Tg | glass transition temperature |
| TNP | trisnonylphenolphosphites |
| UV | ultraviolet |
2. Release and degradation of additives and constitutional monomers from polymers
Organic compounds are used as additives in polymers to improve the properties of the resulting products. Release of the additives to the surrounding environment is an unwanted process for both the manufacturer and the environment, since loss of additives shortens polymer lifetime, e.g. loss of plasticizers lowers the tensile strength of polyvinyl chloride (PVC; Boyer 1951), and living organisms are exposed to the released additives. Phthalates, organotins and BPA, mentioned subsequently, have been shown to target nuclear hormone receptor signalling pathways (Grun & Blumberg 2007). The release may take place during the service life of the plastics or after their disposal, for example in landfills. Both the landfill compartment and other potential receptors such as sediments represent complex environments with multiple chemical and biological processes occurring concurrently.
The migration potential of an additive in a polymer depends on several parameters. The polymer itself has a three-dimensional porous structure in which the additives are dispersed. The pore diameter and the size of the additive are correlated such that smaller (lower molecular weight) additives move more easily through a polymer with bigger pore size. Additives that fit more exactly in the pores have a small but not insignificant capacity to migrate. Therefore, the pore size in the polymer and the size of the additive molecule are important parameters. Co-migration and temperature are positive migration factors as are certain physical–chemical properties of the additive and the surrounding environment. Release of a reactively bonded compound from a polymer requires cleavage of the covalent bond(s) before migration can take place. Therefore, loss of reactively bonded chemicals from the polymer resins is most probably because of release of unreacted constituents (see BPA below).
In landfills, plastics are exposed to an extraction solvent in the form of acidic (pH 5–6) leachates with high ionic strength and neutral or alkaline leachates containing high-molecular-weight organic compounds. The different leachates have not only different potentials to extract and transport, but also different biological populations with the potential to degrade or transform the released additives.
Plasticizers, which are the largest group of additives in polymers, range from molecular weights of approximately 200 to almost 700 g mol–1 and cover water solubility from g l–1 to sub-µg l–1. Phthalates (or more chemically correct, alkyl/aryl esters of 1,2-benzenedicarboxylic acid) are the most common plasticizers and may account for more than 60 per cent of polymers of PVC (Giam et al. 1984). Dimethyl phthalate (DMP) is fairly easily released from its resin, as soon as the DMP-containing product is landfilled, owing to its relatively high water solubility, i.e. there is a continuous depletion of DMP from the resin surface, and the negative concentration gradient from the inside to the surface causes the migration. In contrast, the higher-molecular-weight phthalates, such as diethylhexyl phthalate (DEHP), are more resistant to migration owing to their hydrophobicity, which causes less release from the polymer surface to leachate compared with DMP.
The importance of the surrounding medium for the extraction potential can be exemplified by the different degradation phases in a landfill. Acidic pH and high ionic strength of the leachate that surrounds waste materials lower the release potential of organic compounds, which make the initial acidogenic phase in a landfill's development a very poor extraction solvent for water-resistant plasticizers (Bauer et al. 1998; figure 1). In contrast, a neutral leachate, as found in landfills in the stable methanogenic phase, containing colloidal humic material, facilitates leaching and transport of non-soluble plasticizers owing to sorption to the dissolved organic carbon (DOC) fraction. Therefore, concentrations of phthalate esters in landfill leachates are highly correlated to the DOC content (Bauer & Herrmann 1998). As a consequence of the depletion of plasticizer from the polymer surface, migration from the inner part of the polymer product is enhanced. However, migration from the inner part to the outer surface seems to slow down and even stop as the polymer reaches its glass transition state (Ejlertsson et al. 2003). Then, new release of plasticizers only occurs if the brittle polymer structure fractures to expose new surfaces.
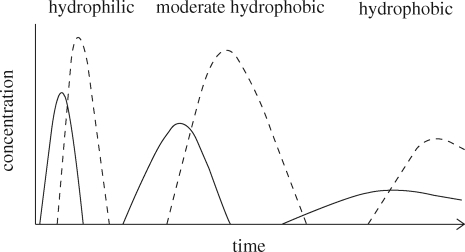
Figure 1.
Schematic appearance and concentration of a hydrophilic (left), moderate hydrophobic (middle) and hydrophobic (right) phthalic acid diester (solid lines) and respective monoester (dashed lines) in landfill leachate (modified from Jonsson 2003). The appearance of the diester is correlated to its depletion in the phthalate-containing product.
Degradation of phthalates is initiated by hydrolysis of the ester moiety to phthalic acid and the corresponding alcohols via the monoesters. In landfills, biotic hydrolysis is far more important than abiotic hydrolysis (Furtmann 1996; Staples et al. 1997) and takes place (i) at the surface of the original products, (ii) after they have been released from the products and dissolved in the leachate or (iii) following release from another surface to which they adsorbed after leaving the original resin. The most important hydrolysis scenario depends on the water solubility of the phthalate, i.e. the soluble phthalates are probably hydrolysed in the water phase and the hydrophobic phthalates are hydrolysed on to solid surfaces. Hydrolysis is strongly correlated to the methanogenic flora (Jonsson et al. 2003a, 2006; figure 2). Accumulation of the monoester occurs if the hydrolysis rate of the diester to the monoester is faster than that of the monoester to phthalic acid (Vavilin et al. 2005). In fact, phthalate monoester concentrations have been observed at higher concentrations than the corresponding diesters in landfill leachates (Jonsson et al. 2003b). If the phthalate diester is slowly released during a longer period including the methanogenic stage, the time period when the monoester is observed in the leachate is prolonged and the concentrations of the diester are consequently lower.
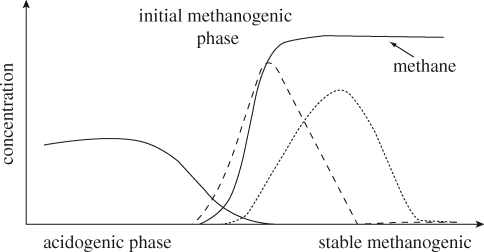
Figure 2.
Degradation of a phthalic acid diester (solid line) to its corresponding monoester (dashed line) and phthalic acid (dotted line) in a landfill developing from acidogenic to stable methanogenic phase. Also, the methane production is included (reproduced with permission from Jonsson 2003).
Organotin compounds are used as stabilizing additives in polymers, such as PVC, and they deserve special attention because of their toxicity such as deterioration of human immune function and endocrine disruption (Batt 2006). The stabilizers are added as high molecular mono- and dialkyltin carboxylates, mercaptides up to 0.54 per cent or, more common, mercaptans or sulphides up to 0.18 per cent, calculated as tin, in the polymer (Murphy et al. 2000; Batt 2006). The carboxylates and mercaptides are rapidly hydrolysed to their mono- and dialkyltin species, respectively, when in contact with water (Björn 2007). The alkyltins are also hydrolysed when they act as stabilizers within the polymer and are consequently released from the polymer surface as alkyltin chlorides. As for the phthalates, it seems likely that the main release of organotin compounds from plastic material occurs when a landfill turns methanogenic (Björn et al. 2007). It has been shown that the tin stabilizers are co-extracted from the polymer together with the phthalates. Therefore, organotins in flexible polymers are more readily released than from rigid ones. It should be noted that 90 per cent of the tin stabilizers are used in rigid PVC (ESPA 2002). However, at temperatures above the glass transition of the polymer, more organotin compounds are released than at temperatures below this point (Björn et al. 2007).
The alkyltin compounds may dealkylate to inorganic tin, methylate or demethylate in the landfill environment. It is likely that the microbial methylation capacity is greater at higher concentrations (more than 500 µg Sn l–1), while demethylation occurs at lower tin concentrations (below 100 µg Sn l–1; Björn 2007). Formation of tetramethyl tin changes the properties of the tin species radically, since this compound is very volatile.
Alkylphenols can be used as plasticizing additives or as stabilizers when added as derivatives of phosphites (e.g. trisnonylphenolphosphites: TNP). Upon oxidation and hydrolysis, alkylphenol phosphites are hydrolysed to the corresponding alkylphenol and phosphate, for example, TNP is readily oxidized and hydrolysed to NP under ambient conditions (Murata 1999). Since the alkylphenols and the phosphites are additives, the same reasoning can be applied for these compounds as for the phthalates. More precisely, compounds with shorter alkyl chains have higher leaching potential than longer alkyl chain analogues, and methanogenic leachates are more extractive than acidogenic leachates. However, unlike the phthalates, alkylphenol phosphites are only used in concentrations up to 3 per cent, compared with 60 per cent for the phthalates. Degradation studies of the pure alkylphenols performed under landfill conditions are scarce. However, alkylphenols seem to be the ultimate degradation product when alkylphenol ethoxylates are transformed under methanogenic conditions (e.g. Ejlertsson et al. 1999), suggesting that no further degradation occurs under anaerobic conditions (Maguire 1999).
Bisphenol A is, in contrast to the aforementioned compounds, mainly used as a building block of polycarbonate plastics, where the alkylphenol p-tert-butylphenol is added as a polymerization adjustor, or as a key constituent together with epichlorohydrin of epoxy resins. Also, BPA is used as additive in PVC, printer ink and some other products. Release of BPA under landfill conditions has not been reported as far as we know, but results from an analysis of landfill leachates suggest that the additive or unreacted BPA, owing to its more hydrophilic character, is readily released from its polymer during the early age of a landfill (Asakura et al. 2004, i.e. under acidogenic conditions as for the phthalate DMP). This is supported by leaching studies with water-containing acetic acid and ethanol (Kawamura et al. 1998), which is expected to mimic acidogenic leachates. Concerning degradation, complete mineralization has only been reported under aerobic conditions (e.g. Zhang et al. 2007). Bisphenol A is reported to be preserved in anaerobic sediments (e.g. Ying & Kookana 2003).
3. Phenolic endocrine-disrupting chemicals in leachates from municipal waste
Considerable amounts of plastics are disposed of in municipal landfills. As indicated earlier, certain additives and monomers can be released from plastic and will consequently be present in landfill leachate. Detection of BPA, phthalates and the alkylphenols NP and octylphenol (OP) in landfill leachate has been reported (Yasuhara et al. 1997; Yamamoto et al. 2001; Fromme et al. 2002; Coors et al. 2003; Jonsson et al. 2003c; Asakura et al. 2004; Deng et al. 2006). Although BPA concentrations varied depending on waste composition and landfill operation, concentrations of BPA in leachates ranged from ten to ten thousand µg l–1 from sites in the USA (Coors et al. 2003), Germany (Fromme et al. 2002) and Japan (Asakura et al. 2004). These concentrations were much higher than those detected in municipal sewage effluents (approx. 0.01–0.1 µg l–1, Fromme et al. 2002; Nakada et al. 2004), implying that untreated leachates from the landfills are potentially significant sources of BPA for the aquatic environment. Furthermore, the BPA concentrations in the leachates were up to seven orders of magnitude higher than the no-effect concentration (NOEC) of BPA for endocrine disruption in freshwater organisms (i.e. at 8 ng l–1 to induce malformations in female organs of a freshwater snail, Marisa cornuarietis; Schulte-Oehlmann et al. 2001). Significant concentrations of NP were also detected in landfill leachate sites (Asakura et al. 2004). However, the reported concentration ranges of NP (Asakura et al. 2004) were similar to those in municipal sewage effluents (Nakada et al. 2004).
Economic growth and industrialization bring larger amounts of plastics into society and may increase the amount of plastic waste. To investigate the effect of industrialization on the presence of endocrine-disrupting chemicals (EDCs) in landfill leachates, we measured plastic-derived chemicals in leachates from tropical Asian countries at different stages of economic growth. Leachate samples were collected from open dumps in Malaysia (Kuala Lumpur), Thailand (Bangkok), The Philippines (Manila), Vietnam (Hanoi, Can Tho), Cambodia (Phnom Penh, Angkor), Laos (Vientiane) and India (Kolkata) between 2002 and 2006. At all the sites, municipal wastes, including plastics, are buried. As a reference, leachate samples collected from a landfill site in Japan were also collected and analysed for the EDCs. Details of the analytical procedure were described by Nakada et al. (2004, 2006).
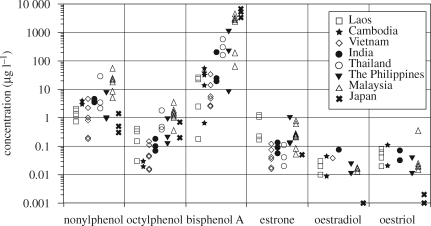
Concentrations of the EDCs in the leachates from sites in tropical Asia and Japan are shown in figure 3. Among the EDCs measured, BPA showed highest concentrations in the tropical Asian leachates, ranging from 0.18 to 4300 µg l–1. The highest concentrations were observed in leachates from Malaysia and were comparable to those from Japan. The concentration range of NP in the leachates (0.18–98 µg l–1) was lower than BPA but higher than OP (0.03–3.4 µg l–1). Bisphenol A concentrations were one to five orders of magnitude higher than those in sewage effluents (Nakada et al. 2004), whereas NP concentrations in the leachates were one to two orders of magnitude higher than those in effluents. This highlights the importance of the leachate as a source of BPA in aquatic environments. Bisphenol A in leachate could be derived from unreacted monomers in disposed polymers (polycarbonates and epoxy resins), degradation of the polymers and additives. In many landfill sites in industrialized countries, treatment facilities are installed and the environmental burden of these EDCs is reduced. High removal efficiency of BPA has been reported with aerobic treatment (99.3–99.7%, Kawagoshi et al. 2003; Asakura et al. 2004) and with membrane bioreactors (95.3%, Wintgens et al. 2003). However, because of high concentrations of BPA in raw leachates, even treated leachates showed higher BPA concentrations (0.11–30 µg l–1, Wintgens et al. 2003; Asakura et al. 2004) than the NOEC to freshwater organisms (0.008 µg l–1). Bisphenol A is more problematic in tropical Asian landfill sites with either no, or poorly functioning, leachate treatment facilities. Consequently, high concentrations of BPA were discharged to the surrounding environment (e.g. rivers, groundwater). Notably, BPA concentrations in water samples from a Malaysian pond, into which the leachate from the dump flowed, were an order of magnitude higher (i.e. approx. 11 µg l–1) than in the upstream inflowing river (0.45 µg l–1). This clearly demonstrates that waste-plastic-derived chemicals significantly increase the concentrations of EDCs in the environment.
Figure 3.
Concentrations of endocrine-disrupting chemicals (EDCs) in leachates from waste disposal sites in Asia.
High concentrations of natural oestrogens (estrone, E1: 0.127–1.00 µg l–1; oestradiol, E2: 0.002–0.0243 µg l–1) were also detected in the leachate from the tropical Asian countries. This is in contrast to leachate from a Japanese landfill site where relatively low concentrations of natural oestrogens (E1: <0.05 µg l–1; E2: <0.008 µg l–1) were detected. The natural oestrogens in the landfill leachates from the Southeast Asian countries could be derived from the disposal of human wastes and/or input from the faeces of scavengers living at the dumping sites. Based on the concentrations of individual EDCs and relative potency of endocrine disruption for individual compounds, oestrogenic activities of the individual compounds have been calculated and compared. The following relative potencies reported by Sumpter & Johnson (2005) for fish were used: NP (0.0025), OP (0.002), BPA (0.0004), E1 (0.3) and E2 (1.00). Oestradiol-equivalent concentrations (EEQ) were calculated by multiplying the concentrations of the individual compounds by their relative potency. The total EEQ ranged from 3.4 to 1355 ng-E2 l–1. The highest EEQs were observed in Malaysian, The Philippines and Thai leachates, where much higher concentrations of BPA were observed. In those leachates, BPA accounted for over 50 per cent of the total EEQ. This highlights the importance of BPA in terms of endocrine disruption caused by leachate. The abundance of BPA over natural oestrogens in the leachate contrasts with municipal wastewater effluents where natural oestrogens usually dominate over synthetic chemicals (e.g. Desbrow et al. 1998; Nakada et al. 2004).
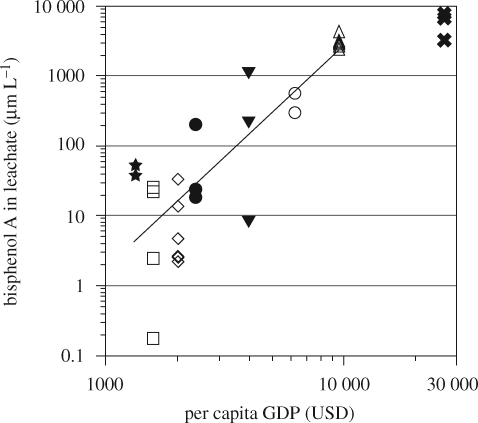
Among the countries investigated, the more industrialized countries (e.g. Malaysia and Thailand) had higher BPA concentrations in landfill leachate than less industrialized countries (e.g. Laos and Cambodia). To quantitatively express this trend, BPA concentrations were plotted against per capita gross domestic products (GDPs; Earth Trends 2007) in figure 4. Bisphenol A concentrations in the leachate show a significant positive correlation with per capita GDP of the tropical Asian countries (r2 = 0.66, n = 26, p < 0.0001). The most probable reason is that more industrialized countries use larger quantities of plastics resulting in the generation of more plastic waste. This suggests that economic growth in developing countries may increase the environmental prevalence of EDCs unless the leachate is collected and properly treated. To reduce the input of EDCs to the environment, the amount of waste plastics discarded should be decreased through reduction, recycling or other methods of disposal of plastic.
Figure 4.
Relationship between BPA concentrations in leachates from waste disposal sites and per capita GDP of Asian countries (r2 = 0.66, n = 26). Leachates from municipal waste disposal sites in capital and other major cities are plotted. See figure 3 for the symbols of the countries. Data are for countries except Japan.
4. Sorption and desorption of anthropogenic contaminants from plastics
Sorption and desorption are essential fate processes governing the distribution, persistence and ecological impact of hydrophobic anthropogenic contaminants in terrestrial and aquatic systems. Anthropogenic contaminants such as alkylbenzenes, chlorinated hydrocarbons, polycyclic aromatic hydrocarbons (PAHs) and PCBs are examples of compounds that will probably associate with sorbent organic matter (SOM) in the environment. The association of hydrophobic organic contaminants (HOCs) with SOM retards their transport and reduces their availability for biological and chemical transformation. Traditionally, the organic fraction of soils and sediments was considered to be the most important form of SOM in the environment, but recent studies documented the importance of plastics in sediments and debris collected from the marine environment (Colton et al. 1974; Mato et al. 2001; Ng & Obbard 2006; Rios et al. 2007). Hydrophobic organic contaminants were shown to have greater affinity for a range of plastics (polyethylene, polypropylene, PVC) compared with natural sediments (Teuten et al. 2007) and were detected on plastic pellets collected from the marine environment (Mato et al. 2001; Rios et al. 2007) as described in §5.
The extent and rate of HOC (de)sorption are influenced by factors including sorbent (i.e. SOM) properties, sorbate (i.e. HOC) properties, dissolved organic compounds in the aqueous phase, pH and temperature. The following discussion will focus on the effects of sorbent properties on sorption equilibrium and (de)sorption kinetics.
Sorbent organic matter in the environment is composed of organic polymers that contain crystalline and amorphous regions. The crystalline region is characterized by molecules or segments of molecules that are regularly arranged in a crystal lattice. In contrast, the amorphous region has randomly arranged molecules, thus exhibiting a structure that is loose and flexible, and more similar to liquids. Sorption of HOCs generally occurs in the amorphous region, which is characterized on the basis of its internal structure as either glassy or rubbery. Hence, SOM can be envisioned as a mixture of glassy and rubbery polymers. The polymer segments of the glassy phases have higher cohesive forces and are more condensed, whereas those of the rubbery phases exhibit greater mobility and flexibility and can be visualized as a dynamic viscous liquid (Tobolsky & Mark 1980). A particular polymer can transition from the rubbery to the glassy state when it is cooled below its glass transition temperature (Tg). Based on Tg, a polymer is classified as rubbery or glassy at a given environmental temperature. At room temperature, polymers that have a low Tg (e.g. polyethylene has a Tg of −68°C) are rubbery, while those that have a high Tg (e.g. PVC has a Tg of 80°C) are glassy (Brandup et al. 1989). The polymer characteristics of the crystalline region lie between those of rubbery and glassy polymers owing to their unique structure. Crystalline polymers are moderately hard, yet flexible and strong (Treloar 1974).
Glassy polymers, because of their rigidity, possess long-lived, closed internal nanoscale pores that can serve as adsorption sites. The existence of amorphous polymer segments and internal nanovoids in glassy polymers results in HOC sorption by linear dissolution (partitioning/absorption) and by nonlinear hole-filling (adsorption) mechanisms (Xing & Pignatello 1997). Because of the dual sorption mechanisms, the sorptive behaviour of glassy polymers is normally described by the nonlinear Freundlich model (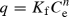 ), where q is the amount of the compound sorbed per unit mass of solid, Ce the aqueous-phase concentration at equilibrium, Kf the Freundlich constant related to the capacity of the sorbent material to sorb the sorbate and n the Freundlich exponent and an indicator of the site energy distribution of a sorbent (i.e. sorbent heterogeneity increases as n decreases from 1; Carter et al. 1995). Absorptive partitioning into an organic matrix is characterized by a linear sorption model (q = KpCe), where Kp is the partition coefficient. Weber et al. (1992) showed that nonlinear behaviour may be masked at high aqueous-phase concentrations, but can actually control the overall sorption behaviour at low aqueous-phase concentrations. At low-phase concentrations (<1–1.5% of aqueous solubility), HOCs are sorbed most favourably by regions or components of SOM that have the strongest affinity for that compound (Chiou & Kile 1998). As the high-affinity regions (characterized by nonlinear sorption isotherms) become saturated, HOC sorption is limited to less strongly sorbing regions (characterized by linear sorption isotherms).
), where q is the amount of the compound sorbed per unit mass of solid, Ce the aqueous-phase concentration at equilibrium, Kf the Freundlich constant related to the capacity of the sorbent material to sorb the sorbate and n the Freundlich exponent and an indicator of the site energy distribution of a sorbent (i.e. sorbent heterogeneity increases as n decreases from 1; Carter et al. 1995). Absorptive partitioning into an organic matrix is characterized by a linear sorption model (q = KpCe), where Kp is the partition coefficient. Weber et al. (1992) showed that nonlinear behaviour may be masked at high aqueous-phase concentrations, but can actually control the overall sorption behaviour at low aqueous-phase concentrations. At low-phase concentrations (<1–1.5% of aqueous solubility), HOCs are sorbed most favourably by regions or components of SOM that have the strongest affinity for that compound (Chiou & Kile 1998). As the high-affinity regions (characterized by nonlinear sorption isotherms) become saturated, HOC sorption is limited to less strongly sorbing regions (characterized by linear sorption isotherms).
(a). Model description
Desorption of HOCs from plastics can be described by a one-compartment polymer diffusion model. The model assumes that the HOC desorption rate is limited by diffusion through a single polymer phase, and each sorbent particle is a homogeneous polymeric sphere. Fick's second law of diffusion can be used to express HOC diffusion from plastic particles. In radial coordinates, Fick's second law of diffusion yields equation (4.1):
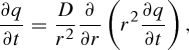 |
4.1 |
where D is the diffusion coefficient (L2/T), q the solid-phase concentration (sorbed HOC mass/sorbent mass), r the radial position in the sorbent particle (L) and t the time.
To solve equation (4.1), two-dimensionless variables, T and R, are introduced:
| 4.2 |
where a is the sorbent particle radius.
Therefore, the governing equation is transformed to:
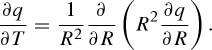 |
4.3 |
Initial and boundary conditions specific to the experimental method employed to estimate D are required to solve equation (4.3). For the initial condition, it was assumed that sorption equilibrium was attained prior to initiation of desorption and, therefore, the solid-phase concentration (q0) was uniform throughout the sorbent at the beginning of desorption tests, i.e.
| 4.4 |
The first boundary condition requires that symmetry is maintained at the particle centre at all times, i.e.
| 4.5 |
The second boundary condition specifies the solid-phase concentration at the external solid surface. For the results described here, in which volatile HOCs were tested, sorbents equilibrated with an aqueous phase were sparged continuously during the desorption test. Thus, the aqueous-phase HOC concentration was negligible (i.e. an infinite sink was approximated).
Assuming instantaneous equilibrium between the solid- and aqueous-phase concentrations at the external sorbent surface, the solid-phase concentration at the external particle boundary was therefore also zero, i.e.
| 4.6 |
A Crank–Nicholson finite-difference algorithm was developed to solve the one-compartment polymer diffusion model. The Newton–Raphson optimization routine was used to determine the diffusion coefficient (D) such that the mean square error between the model output and experimental data was minimized. The model requires the following input parameters: isotherm parameters (Kp for linear isotherms and Kf and n for nonlinear isotherms), particle radius, particle density, fractional uptake and initial estimate of D.
(b). Model application
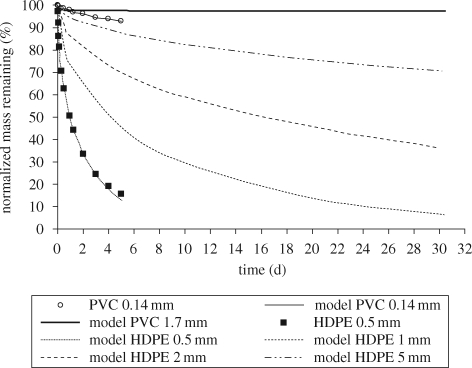
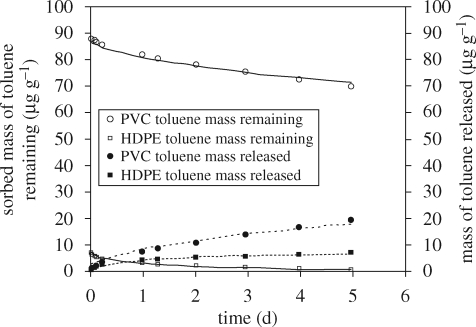
The validity of the one-compartment polymer diffusion model to simulate desorption kinetics of HOCs in homogeneous plastics was tested using toluene, o-xylene and tetrachloroethylene (PCE) as model HOCs, high-density polyethylene (HDPE) as a model rubbery polymer and PVC as a model glassy polymer. As shown in figure 5, model results agreed well with o-xylene desorption data from HDPE and PVC. Similarly, model results agreed well with toluene (figure 6) and PCE (not shown) desorption data. Table 2 summarizes inputs to the desorption model and the estimated D values for the tested HOCs.
Figure 5.
Comparison of o-xylene desorption data and one-compartment diffusion model fits as well as predictions of o-xylene desorption rates from PVC and HDPE spheres of different diameters. Desorption data were measured after six months of equilibration in ultrapure water.
Figure 6.
Effect of polymer type on sorbed toluene mass remaining and released per gram of sorbent. Lines represent model fits for sorption equilibrium liquid-phase concentration (Ce) of 100 µg l–1, Kp = 70.7 (µg kg–1)(l µg–1) for HDPE and Kf = 1663 (µg kg–1)(l µg–1), and n = 0.864 for PVC (Wu et al. 2001). The particle diameters of HDPE and PVC were 0.5 and 0.14 mm, respectively.
Table 2.
Model input parameters and estimates of toluene, o-xylene and PCE diffusivities in HDPE and PVC.
| isotherm parameters |
diffusion coefficienta |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| tolueneb |
o-xyleneb |
PCEc |
toluene | o-xylene | PCE | ||||||
| material | particle density (g cm–3) | mean particle diameter (µm) | K | nd | K | nd | K | nd | D | D | D |
| HDPE | 0.962 | 500 | 70.7e | 1.0 | 244.1 | 1.0 | 385 | 1.0 | 3.13 × 10–10 | 2.20 × 10–10 | 1.42 × 10–10 |
| PVC | 1.4 | 140 | 1663f | 0.864 | 4634 | 0.718 | 2951 | 0.918 | 4.33 × 10–13 | 4.22 × 10–14 | 1.50 × 10–14 |
acm2 s−1.
bValues from Wu et al. (2001).
cValues from Wagner (2003).
dDimensionless Freundlich exponent.
eKp for HDPE (µg kg−1)(l µg−1).
fKf for PVC (µg kg−1)(l µg−1)n.
In general, HOC diffusivities in plastics were higher in HDPE (D = 10−10 cm2 s−1) and lower in PVC (D = 10−13−10−14 cm2 s−1). The diffusivity of HOCs in PVC is reasonably consistent with the values observed by Berens (1989). For HDPE, the diffusivity of HOCs is an order of magnitude lower than values reported in literature, possibly because of differences in polymer composition and crystallinity, experimental conditions and uncertainties in estimates of diffusional length (Roger et al. 1960; Park et al. 1996; Sangam & Rowe, 2001; Joo et al. 2004). Typically, HDPE has 80–95 per cent crystallinity (Brandup et al. 1989) but the crystallinity of an HDPE geomembrane tested by Sangam & Rowe (2001) was only 47 per cent, which could account for the difference in D estimates. Moreover, uncertainties in diffusional length scale (i.e. film thickness or particle radius) could also affect the calculated diffusion coefficients. When the diffusion coefficients are normalized by diffusional length scale (D/a2), the normalized HOC diffusion coefficients determined for HDPE in this study have the same order of magnitude (10−2 d−1) as those in previous reports (Sangam & Rowe 2001; Joo et al. 2004).
The difference in HOC desorption rates observed between HDPE and PVC is consistent with their rubbery and glassy states. The polymeric organic matrix of glassy polymers such as PVC is more rigid than that of rubbery polymers such as HDPE. Because the relaxation speeds of glassy polymers are slow, diffusion of solute molecules into and out of the condensed and highly cross-linked organic matter is slow, which explains the smaller HOC diffusivities in glassy polymers (Brusseau et al. 1991; Pignatello & Xing 1996; Huang & Weber 1997, 1998). Moreover, nanovoids within glassy polymer matrices provide strong adsorption sites, and desorption of HOCs from these sites is generally activated (Pignatello & Xing 1996).
For the same input parameters and estimate of D, figure 5 illustrates the effect of diffusional length scale on o-xylene desorption rates. The particle sizes, for which model predictions are shown in figure 5, are representative of the size range of plastic pellets and fragments collected from the marine environment (Colton et al. 1974; Mato et al. 2001; Rios et al. 2007). In agreement with the inverse proportionality between desorption rate and the square of the sorbent particle radius, the results in figure 5 illustrate that HOC desorption rates decreased dramatically as the diffusional length scale increased. Additional model predictions showed that the time required for 50 per cent desorption of toluene, o-xylene and PCE from rubbery plastics (e.g. polyethylene and polypropylene) with a 1 mm particle diameter was 2.8, 4.0 and 6.2 days, respectively. When the particle diameter was doubled (2 mm), the half-lives increased to 11.3, 16.1 and 25.3 days, respectively. For glassy plastics (e.g. PVC and polystyrene) with a particle diameter of 0.2 mm, the time required for 50 per cent desorption of toluene, o-xylene and PCE was 85 days, 2.3 years and 6.5 years, respectively. The predicted half-life of o-xylene and PCE in PVC was >100 years when the particle diameter of glassy polymers was increased to 1.7 mm.
Figure 6 compares the mass of toluene released per unit mass of HDPE and PVC, assuming Ce = 100 µg l−1 and using a particle diameter of 0.5 mm for HDPE and 0.14 mm for PVC. Although toluene diffuses three orders of magnitude faster in HDPE, the amount of toluene released from PVC is greater than that released from HDPE. This is because the mass of toluene sorbed to PVC at equilibrium is greater than the mass of toluene sorbed to HDPE (Wu et al. 2001).
In summary, results from alkylbenzene and PCE desorption kinetic tests for glassy and rubbery polymers suggest that both sorbent and sorbate properties strongly influence HOC sorption uptake and desorption kinetics. Glassy polymers exhibit larger HOC sorption capacities and slower HOC release rates than rubbery polymers. Moreover, the size of the plastic pellet or fragment strongly affects the rate at which sorbed HOCs are released.
5. Types of contaminants detected in marine plastics
(a). Adsorption of contaminants to marine plastics from surrounding seawater
Carpenter et al. (1972) first detected PCBs in polystyrene spherules collected from Niantic Bay (northeastern Long Island Sound, USA). Although they suggested that the PCBs were adsorbed onto the plastic from the surrounding seawater, no supporting evidence was provided. After a 30 years break, Mato et al. (2001) triggered a series of systematic studies on toxic chemicals in marine plastics. They detected PCBs in polypropylene pellets from Japanese coasts with concentrations ranging from 4 to 117 ng g−1. They conducted a field adsorption experiment using virgin polypropylene pellets and demonstrated a significant and consistent increase in PCB concentrations throughout the 6 day experiment. This indicated that the source of the PCBs was ambient seawater and that adsorption to the pellet surfaces was the mechanism of enrichment. In another adsorption experiment, Mato et al. (2002) subjected polyethylene and polypropylene pellets to seawater for two weeks and found that polyethylene pellets adsorbed four times more PCBs than polypropylene pellets, indicating that polyethylene has higher affinity for hydrophobic compounds. This is consistent with field observation and experimental work described later and literature (e.g. Karapanagioti & Klontza 2008). Comparison of PCB concentrations in marine plastic resin pellets with those in seawater suggested their high degree of accumulation (apparent adsorption coefficient of 105–106).
Subsequently, Endo et al. (2005) conducted a detailed study of PCBs in beached resin pellets. They analysed PCB concentrations in individual pellets and observed a large (i.e. two orders of magnitude) piece-to-piece variation in PCB concentrations among the pellets. Variation in PCB concentrations may be caused by various factors including difference in materials, weathering and residence time in the sea. Endo et al. (2005) found that polyethylene pellets tended to have higher concentrations of PCBs than polypropylene, consistent with the results of the above adsorption experiment. They also indicated that yellowing and/or fouled pellets had higher concentrations of PCBs. Yellowing is derived from oxidation of phenolic additives to quinone-type degradation products and, therefore, is an indication of environmental residence time of the pellets. Based on their findings, they proposed a monitoring methodology ‘pellet watch' where beached yellowed and/or fouled polyethylene pellets are used to monitor coastal pollution by hydrophobic chemicals.
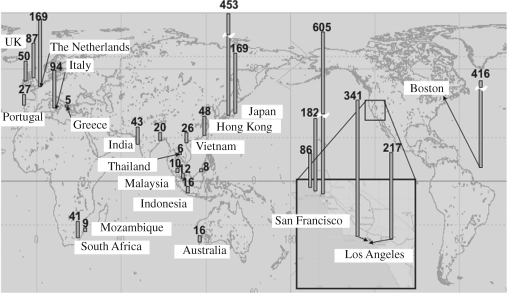
Based on the results, the monitoring was expanded to a global scale, named International Pellet Watch (http://www.tuat.ac.jp/~gaia/ipw/index.html). In this scheme, plastic resin pellets are collected on beaches by local volunteers and sent to the Tokyo University of Agriculture and Technology for analysis of a variety of HOCs. Through the activities of volunteers worldwide, and a network of scientists, 27 samples from 16 countries have been analysed. As shown in figure 7, PCB concentrations of beached plastic resin pellets were highest on the coasts of USA (San Francisco, Los Angeles and Boston), followed by Japan and Europe (The Netherlands, UK and Italy). In tropical Asia, Australia and southern Africa, PCB concentrations were much lower. This regional pattern reflects a difference in PCB usage, with larger amounts of PCBs used in the USA, western Europe and Japan, and minimal usage in tropical Asia, Australia and southern Africa. For example, more than half of the total global production of PCBs was used in the USA (Erickson 1997). Discharged PCBs have accumulated in coastal zones, particularly in sediments, which are likely to be resuspended into the water column. Correspondingly, higher concentrations of PCBs are still found in sediments, seawater and marine biota in the USA, western Europe and Japan. To examine the feasibility of a monitoring methodology employing plastic pellets, concentrations of PCBs in the pellets were compared with those in conventional biomonitoring organisms, i.e. mussels (green mussels, Perna viridis; blue mussels, Mytilus galloprovincialis) in the corresponding zones. Polychlorinated biphenyl concentrations in the beached resin pellets were highly correlated with those in mussels (R2 = 0.87). These data clearly demonstrate that beached resin pellets can be used to monitor pollution by hydrophobic chemicals on a global scale.
Figure 7.
Concentrations of PCBs (ng g−1 pellet) in beached plastic pellets. Polychlorinated biphenyl concentration = sum of concentrations of CB nos 66, 101, 110, 149, 118, 105, 153, 138, 128, 187, 180, 170, 206.
The hydrophobic surfaces of the resin pellets accumulate other chemicals in addition to PCBs. So far, 2,2′-bis(p-chlorophenyl)-1,1,1-trichloroethane (DDT) and its metabolites (DDE and DDD), hexachlorinated hexanes (HCHs), PAHs and hopanes have been detected in beached resin pellets. Interesting, regional distributions were observed. For example, higher concentrations of HCHs and DDTs were detected in South Africa and northern Vietnam, respectively, probably because of the current use of these chemicals as pesticides in these areas. These regional differences in contaminant concentrations in the resin pellets imply that ecological risks associated with the contaminants adsorbed to marine plastics will also vary among the areas.
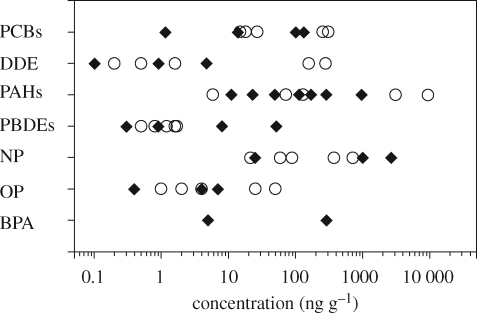
Considering potential effects on the marine ecosystem, plastic fragments, including microscopic fragments, are possibly more important than resin pellets because fragments are more abundant among marine plastic debris (e.g. Moore et al. 2001; McDermid & McMullen 2004; see also Barnes et al. 2009; Ryan et al. 2009). Similar to resin pellets, hydrophobic contaminants sorb to marine plastic fragments. Figure 8 shows the concentrations of various pollutants detected in plastic fragments collected from a beach near Tokyo, Japan and from the North Central Pacific Gyre (approx.1000 km off the west coast of the USA). The latter were floating plastic fragments and collected by a neuston net. The plastic fragments were sorted, and polyethylene fragments with various shapes and sizes ranging from 1 × 10 × 20 to 31 × 35 × 35 mm were extracted with dichloromethane by Soxhlet for analysis. To evaluate variability, several pools were analysed for each beach. As observed with the resin pellets, PCBs, DDE and PAHs were detected in plastic fragments from both areas. Concentrations of contaminants in polyethylene fragments were higher on the Japanese coast (PCBs: 12–254 ng g−1; DDE: 0.2–276 ng g−1; PAHs: <60–9370 ng g−1) than those in the Central Gyre (PCBs: 1–23 ng g−1; DDE: 0.1–4.7 ng g−1; PAHs: <100–959 ng g−1). This difference can be explained by regional differences in seawater concentrations of the contaminants (i.e. coast versus open ocean), since these compounds enter the aquatic environment predominantly from terrestrial runoff. Rios et al. (2007) reported similar concentrations of PCBs, DDE and PAHs in marine plastics (both resin pellets and fragments) collected from a wide variety of Pacific Ocean locations including the North Pacific Gyre, California and Hawaii. They also detected DDT, DDD, HCHs and n-alkanes.
Figure 8.
Concentrations of organic contaminants in marine plastic debris (fragments). Solid diamond: The North Pacific Central Gyre; open circle: Japanese coast of the Pacific Ocean. Polychlorinated biphenyls: sum of concentrations of CB nos 66, 101, 110, 149, 118, 105, 153, 138, 128, 187, 180, 170, 206; DDE: concentration of p, p′-dichlorodiphenyl dichloroethene; PAHs: sum of concentrations of phenanthrene, anthracene, methylphenanthrenes (substitution position: 3, 2, 9, 1), fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, benzo[ghi]perylene and coronene; PBDEs: concentration of BDE nos 3, 7, 15, 17, 28, 71, 49, 47, 66, 77, 100, 119, 99, 85, 126, 154, 153, 138, 183; NP: concentration of nonylphenols; OP: concentration of octylphenol; BPA: concentration of bisphenol A.
(b). Additive-derived chemicals
Plastics additives have also been measured on marine plastic debris. Nonylphenol, which forms from TNP degradation (§2), and is also present as an impurity in TNP (Gilbert et al. 1986), was detected in plastic resin pellets collected from 12 Japanese coasts and 7 Malaysian coasts (Mato et al. 2002). Nonylphenol concentrations ranged from 18 to 17 000 ng g−1. Interestingly, no difference in NP concentrations was observed between Japanese and Malaysian coasts, although PCB concentrations in the pellets from Japanese coasts were much higher than those from the Malaysian coast, probably because of the difference in pollution levels of PCBs in the coastal waters. Polypropylene pellets tended to have higher amounts of NP than polyethylene pellets (Mato et al. 2002), which is consistent with a higher prevalence of additives in polypropylene pellets.
Since additives are also compounded into plastics during moulding, fragments of post-consumer plastics may have more additive-derived chemicals than resin pellets. Nonylphenol, OP and BPA were detected in marine plastic fragments, as shown in figure 8. Interestingly, higher concentrations of NP (24.9–2660 µg g−1) were found in the polyethylene fragments from the open ocean (Central Gyre) than the Japanese coast, where higher concentrations of hydrophobic contaminants such as PCBs and PAHs were found. It is therefore likely that these NPs are derived from additives rather than adsorption from surrounding seawater. Significant concentrations of BPA (5–284 ng g−1) were also detected in the plastic samples from the Central Gyre, whereas no significant BPA was detected in plastic fragments from the Japanese coast (<25 ng g−1). Because only polyethylene plastic was sorted and analysed (i.e. no polycarbonate and epoxy resin was included), BPA detected in the marine plastic fragments is also likely to have been derived from an additive. Another important category of additive includes flame retardants such as polybrominated diphenyl ethers (PBDEs). Significant concentrations of PBDEs consisting of monobrominated to heptabrominated diphenyl ethers were detected in the plastic fragments both from the Japanese coast and the Central Gyre. Concentrations of PBDEs (sum of BDEs ranging from three to seven bromines) were 0.9–2.1 ng g−1 for the Japanese coast and 0.4–57 ng g−1 for Central Gyre. Since PBDEs are ubiquitous in coastal seawater and are hydrophobic, those on the plastic fragments may have sorbed from surrounding seawater. Alternatively, they may have been added to the plastics. In the plastic fragment samples from the Central Gyre, BD no. 183 was predominant. Since BD no. 183 is a major component of Octa-BDE, which is a flame retardant currently used in USA and not predominant in US coastal waters, this congener in the plastic fragment from the Central Gyre most probably originated from the flame retardant added to plastics. Polybrominated diphenyl ethers are suspected thyroid disruptors in wildlife and humans (WHO/IPCS 1994). Therefore, more research is needed to identify the sources of PBDEs in the plastic fragments.
6. Transfer of plastic-derived contaminants to organisms
Uptake of contaminants by organisms occurs by a variety of pathways, most commonly inhalation, dermal sorption and ingestion. Contaminant transfer to organisms from plastics may occur by any of these routes, and the major transport route will vary according to the organism and the physico-chemical properties of the contaminant. For most species, the predominant route of transfer of contaminants from plastics is likely to occur via plastic ingestion.
More than 180 species of animals have been documented to ingest plastic debris, including birds, fish, turtles and marine mammals (Laist 1997). Small plastic pieces floating on the ocean surface are mistaken for food by both fish and birds, while turtles eat suspended plastic bags, which they may mistake for jellyfish. To some extent, plastic ingestion by marine mammals may also be indirect, occurring by ingestion of fish that have eaten plastic (Eriksson & Burton 2003). In addition, plankton trawls recover substantial quantities of plastic (Moore et al. 2001), and plankton-feeding mammals such as mysticetes (baleen whales) are also at risk of plastic ingestion (see Gregory 2009). Temporal studies suggest that plastic ingested by birds has been increasing since the 1960s (Robards et al. 1995; Vlietstra & Parga 2002; Ryan et al. 2009). Since few of these ingestion studies date later than the early 1990s, it is difficult to assess the effect of the enactment of later legislation such as MARPOL Annex V, which came into effect in 1989 and is intended to restrict further the disposal of plastics at sea (Derraik 2002). However, Cadée (2002) reported that 80 per cent of plastic debris on the Dutch coast had peck marks made by birds, indicating widespread sampling of plastic by birds. Ingestion of plastics has many detrimental consequences, including gastrointestinal blockages (Baird & Hooker 2000), ulceration (Fry et al. 1987), internal perforation and death (Mascarenhas et al. 2004).
A lesser-studied problem is the transfer of contaminants from ingested plastics to organisms. Sections 2 and 5 detail the presence of a selection of organic and organometallic contaminants in plastics. In addition, a number of platinum group metals have demonstrated a high affinity for plastics (Cobelo-Garcia et al. 2007). This suggests a high potential for plastics to transport such chemicals. The nature and environmental significance of this transport are then partly determined by the subsequent fate of the plastic. Sorption of contaminants to plastics may also inhibit contaminant biodegradation. For example, microbial degradation of phenanthrene has been shown to be reduced by a factor of 6 when associated with polyethylene (Hatzinger & Alexander 1997). Thus, plastics not only have the potential to transport contaminants, but they may also increase their environmental persistence. Ingestion of plastics with sorbed contaminants has been suggested as a possible exposure route for contaminants (Fry et al. 1987; Ryan et al. 1988; Mato et al. 2001; Thompson et al. 2004). In support of this hypothesis, the quantity of the contaminant desorbed from the plastic was greatly enhanced by the presence of surfactants and organic matter (Sakai et al. 2000), suggesting that increased leaching of contaminants will occur under gastric conditions. Furthermore, acidic gastric conditions may enhance desorption of metals bound to plastics. Finally, a positive correlation has been observed between the mass of ingested plastic and the PCB concentration in the fat tissue of birds (great shearwaters; Puffinus gravis) (Ryan et al. 1988). Since plastics are known to accumulate PCBs in the environment (§5), this correlation supports plastic-mediated transfer of contaminants to organisms.
7. Transfer of contaminants from plastics to lower trophic organisms
Microscopic plastic debris as small as 20 µm (Thompson et al. 2004) also litters the global environment (Thompson et al. 2004; Ng & Obbard 2006; Reddy et al. 2006). This material enters the environment both directly from use as ‘scrubbers' in cleaning products and as abrasive beads for cleaning ships, and indirectly from deterioration of brittle, weathered macroscopic plastics (Derraik 2002). To investigate the possibility for microscopic plastics to transport contaminants to benthic organisms, lugworms (Arenicola marina) were exposed to contaminant-sorbed plastics. Phenanthrene (a PAH), tetrabromodiphenyl ether (a PBDE), triclosan (antimicrobial) and NP were sorbed to microscopic PVC particles at environmentally relevant concentrations. Lugworms were exposed to sediment containing these contaminated plastics (5% w/w) for 10 days. At the end of the trial, contaminant concentrations in the lugworm tissue were significantly higher than that in the sediment. While this demonstrated that contaminants can be transferred from plastics to benthic organisms (R. C. Thompson 2004–2007, unpublished data), the mechanism remains unclear. Some plastic debris is comparable in size to sediment and suspended particulate matter and is ingested by invertebrates with varying feeding patterns. Sediment-ingesting lugworms, filter-feeding barnacles (Semibalanus balanoides) and amphipods (Orchestia gammarellus), which eat decaying organic matter, have all been shown to ingest plastic fragments (Thompson et al. 2004). Therefore, ingestion of contaminant-sorbed plastics may be a route of contaminant transport to these organisms. Indeed, digestive surfactants isolated from deposit feeders have been shown to enhance desorption of pollutants including PCBs and PAHs (Voparil & Mayer 2000; Ahrens et al. 2001).
Perhaps more important is the potential for invertebrates to uptake contaminants passively, as described by equilibrium partitioning. Contaminants accumulate in the tissues of some organisms as a consequence of the organism reaching equilibrium with their surrounding environment. Determination of the equilibrium concentration of a contaminant between the animal lipids and their environment (e.g. the surrounding water, sediment, soil) is an effective method for estimating contaminant burden in biota (Di Toro et al. 1991; European Chemicals Bureau 2003; Reichenberg & Mayer 2006). This method has been widely applied to explain accumulation of both organic and inorganic contaminants in sediment and soil-dwelling organisms (European Chemicals Bureau 2003). Uptake probably occurs through both the skin and the gut, but ultimately the organism reaches equilibrium with its environment.
Using the equilibrium partitioning model, the effect of contaminant uptake from plastic into the lugworm was examined (Teuten et al. 2007). Distribution coefficients (Kd) were measured in vitro for sorption of phenanthrene to a selection of sediments and plastics including polyethylene, polypropylene and PVC from seawater. Unsurprisingly perhaps, the measured Kd values were up to three orders of magnitude higher for sorption to plastic than to sediment, with uptake onto polyethylene exceeding that onto the other plastics by an order of magnitude (Teuten et al. 2007). These measurements were then used to estimate the amount of phenanthrene expected to accumulate in lugworm tissue in the presence of plastic. Addition of clean polyethylene to sandy sediment, at plastic concentrations reported in the environment, was predicted to decrease the amount of phenanthrene in lugworm tissue by 13 per cent, compared with accumulation in the absence of plastic (Teuten et al. 2007). This can be viewed as ‘scavenging' of the phenanthrene by the highly sorbent plastic, which reduces the equilibrium concentrations in the other phases, including the organism. However, owing to the high uptake of contaminants onto plastics and the longevity of plastics in the environment, it is unlikely that plastic debris will remain ‘clean’ for any extended period of time. Hence any predicted beneficial effect of plastics in reducing contaminant concentrations in benthic organisms will likely be short-lived, if it operates at all (Teuten et al. 2007). Many plastics float in the sea-surface microlayer (SML), where contaminant concentrations can be highly enriched over concentrations in the bulk water column (Wurl & Obbard 2004). Further fouling of floating plastics causes them to become negatively buoyant (Ye & Andrady 1991), which may result in sedimentation, and thus act as a transporter of contaminants into offshore as well as strandline sediments.
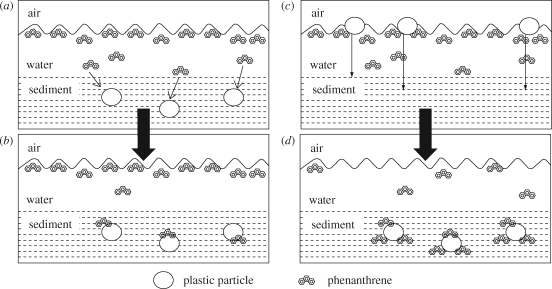
Equilibrium partitioning was also used to examine the effect of scavenging of phenanthrene in the SML, followed by deposition of the contaminated plastics into sediments, on subsequent phenanthrene accumulation by lugworms living in the sediment. Figure 9 displays a schematic in which this transfer mechanism is compared with direct addition of clean plastic to the sediment, as described earlier. The calculations showed that only a small quantity of plastic would be required to sorb a significant amount of phenanthrene from the SML and transport it into the sediment. This would increase total phenanthrene in the benthic environment and was predicted to result in higher lugworm tissue concentrations of phenanthrene. Compared with plastic-free sediment, addition of only 1 ppm of phenanthrene-contaminated polyethylene to sandy sediment was predicted to increase the phenanthrene tissue concentration of the worms by 80 per cent. This, however, has yet to be verified by in vivo experiment.
Figure 9.
Schematic illustrating the additional effects of plastics in the transport of phenanthrene: (a) sorption of phenanthrene to clean plastic in sediment resulting in (b) subsequent accumulation of phenanthrene in the sediment, compared with (c) sorption of phenanthrene to plastic in the SML and subsequent sinking resulting in (d) accumulation of phenanthrene in the sediment. Note that (c)→(d) results in higher sediment phenanthrene concentrations than (a)→(b). Although not shown in the schematic, sorption to the sediment also occurs (reproduced with permission from Teuten et al. 2007). Copyright 2007 (American Chemical Society).
Little is known about the effect of modification of plastics by ‘weathering’ on the sorption of contaminants to plastics, but photo-oxidation is likely to be important as photolytic reactions are known to modify plastic surfaces (Neidlinger & Schissel 1987). For example, polymer chain scission ultimately leads to cracking, thus increasing the surface area of the plastics (Neidlinger & Schissel 1987). This may then increase uptake of contaminants. Reaction with oxygen increases the surface polarity of some plastics (Neidlinger & Schissel 1987), decreasing affinity for hydrophobic contaminants. The results of a series of experiments, complementing the above work and designed to investigate the effect of photo-oxidation on sorption of contaminants to polymers, are illustrated in figure 9.
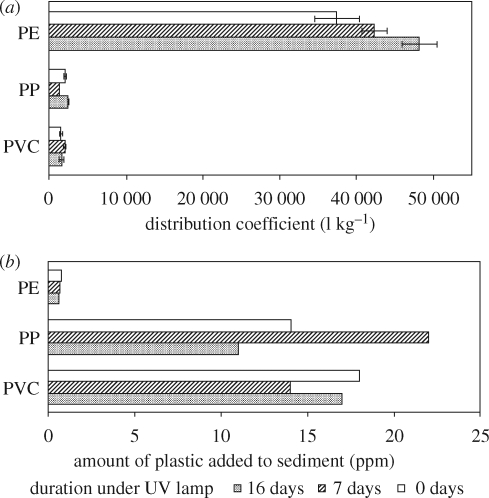
Granules (150–200 µm) of three high-production plastics (polyethylene, polypropylene and PVC) were artificially weathered using a filtered xenon lamp (Heraeus Suntest CPS), while maintaining the temperature at 25 ± 3°C in a glycerol bath connected to a Coolflow CFT-33 refrigerated recirculator. The spectrum of this lamp is similar to that of natural sunlight (Ali et al. 1995). The plastics were agitated daily to maximize exposure to radiation from the lamp. Calibration of the lamp by optometry indicated that 1.08 h of exposure time was equivalent to 1 day of Florida sunlight (30°N, 12 h) (West 2007). Plastics were exposed to the lamp for 7 and 16 days. This was determined to be equivalent to 208 and 460 days of exposure to sunlight at 30°N. The plastics did not appear visibly altered by exposure to the lamp. Aged polypropylene showed an additional absorption at approximately 1750 cm−1 as determined by Fourier transform infrared spectroscopy (FTIR), characteristic of the presence of a carbonyl group (C = O), presumably because of oxidation. A similar absorption was faintly visible in the FTIR spectrum of polyethylene aged for 16 days. Distribution coefficients (Kd) were determined for sorption of phenanthrene to these artificially weathered polymers, and tissue concentrations in lugworms were estimated using equilibrium partitioning, as described previously (Teuten et al. 2007).
Figure 10 shows the measured Kd for sorption of phenanthrene to plastics from seawater and the predicted amount of contaminated plastic required in sediment with 0.2 per cent organic carbon to give an 80 per cent increase in lugworm tissue, compared with plastic-free sediment. For polyethylene, a clear and statistically significant increase in Kd with length of exposure to light was observed (figure 10). The results for the other plastics were quite variable. However, in all cases Kd is lower for sorption to virgin plastic than to plastic exposed to the xenon lamp for 16 days, so that less of the weathered plastic would be required to transport phenanthrene to lugworms. The most likely explanation for the variation is that two competing surface changes occur on photo-oxidation, giving opposite effects. An increase in surface area will increase Kd, while an increase in surface oxidation might decrease Kd for hydrophobic organic compounds. The increase in oxidation is particularly evident for exposed polypropylene, for which FTIR analysis indicated the appearance of a carbonyl group arising from reaction of oxygen with the polymer. This correlated with a decrease in Kd after 7 days light exposure, indicating a reduction in the amount of phenanthrene sorbed to polypropylene. Accurate determination of the surface area of the ultraviolet (UV)-exposed polymers was not possible with the amounts used. Further work involving light treatment would be advantageous. These variable results are consistent with work by Endo et al. (2005) who observed no significant relationship between PCB concentrations in beached plastic resin pellets and their carbonyl index.
Figure 10.
(a) Distribution coefficients (Kd) for sorption of phenanthrene to UV-treated plastics from seawater. (b) Amount of phenanthrene-sorbed plastic required in sediment (0.2% organic carbon) to increase lugworm tissue concentration by 80%, compared with plastic-free sediment, predicted using equilibrium partitioning as described previously (Teuten et al. 2007). Plastics were exposed to a UV lamp for 9 and 16 days, equivalent to 208 and 460 days in natural sunlight. Note that plastic concentrations in sediment are well below the maximum reported amount of 81 ppm (Reddy et al. 2006).
The discussion mentioned earlier illustrates that transfer of contaminants from plastics to biota is a complex phenomenon relying on a variety of processes. For many lower-trophic-level organisms, the extent of contaminant uptake from plastics will be determined by equilibrium partitioning with the surrounding environment. This may be facilitated by active desorption of the contaminants in vivo, for example, by solubilization in digestive fluids. The nature and the history of the plastic will also be important in governing transfer of contaminants to organisms.
8. Transfer of contaminants to higher-trophic-level organisms and biomagnification
In the previous sections, we have demonstrated that chemicals adsorbed to the plastics may be released to digestive fluid and can be transferred to the tissue of organisms. However, the situation for higher-trophic-level organisms is more complex because of ‘biomagnification'. Tissue concentrations of hydrophobic and poorly metabolizable contaminants, such as PCBs, are amplified through the food web. Higher-trophic-level organisms (e.g. seabirds) are exposed to highly enriched concentrations of hydrophobic contaminants via their prey (e.g. fish). Therefore, ingested plastics (i.e. anthropogenic prey) compete with the natural prey in terms of contaminant burden to the predator. To assess the potential hazard of ingested plastic-derived chemicals to the predator, chemicals present in the natural prey should be considered. This section reports the preliminary results of a field experiment in which seabirds were fed with plastic resin pellets to examine the transfer of contaminants from the plastic to the seabird.
Chicks of streaked shearwater (Calonectris leucomelas) were fed with natural fish and kept in cages located at Mikurajima Island, a natural breeding ground of the bird. Eight 40-day-old chicks were used for the experiment. Among them, five individuals were fed with polyethylene resin pellets collected from Kasai seaside park in Tokyo Bay that contained significant amounts of PCBs as described later. Forty pellets (approx. 1 g) were mixed with the natural prey (Japanese sand lance: Ammodytes personatus) at the beginning of the experiment (day 0) and fed to each of the five chicks. Some of the pellets were not fed to the birds and were stored for PCB determination. As a control, three individuals were fed with the Japanese sand lance without plastic resin pellets. For both plastic-feeding and control settings, approximately 10–120 g wet of the fish was fed to each chick daily. Some of the fish were stored in a freezer until the PCB measurement. The experiment continued for 42 days and every 7 days preen gland oil, which is secreted from the preen gland located at the base of the tail feathers, was collected from the live chicks. Polychlorinated biphenyls in the resin pellets, the fish and the preen gland oil were analysed through solvent-extraction, chromatographic purification and a gas chromatograph equipped with an electron capture detector (GC-ECD). Twenty-four PCB congeners (IUPAC numbers 8/5, 18, 28, 52, 44, 66/95, 90/101, 110/77, 118, 132/153, 105, 138/160, 187, 128, 180, 170/190 and 206) were identified and quantified. The total concentration of these congeners is expressed as total PCB in the present study. The details of the analytical procedure for the plastic pellets and fish were described in Endo et al. (2005) and for preen gland oil in Yamashita et al. (2007).
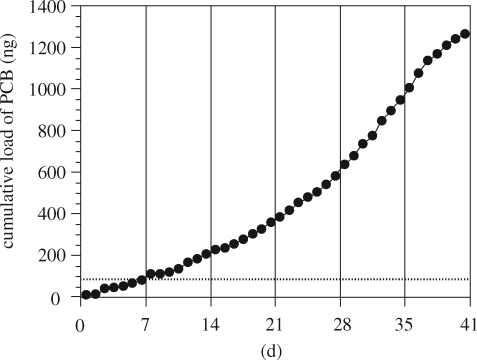
In the pellets from Tokyo Bay, PCBs were detected at concentrations ranging from 51 to 562 ng g−1, with a mean of 97 ng g−1. Based on the number of pellets fed to the streaked shearwaters, each chick is estimated to have been exposed to approximately 100 ng of PCBs. Significant concentrations of PCBs were also detected in the fish fed to the chicks, because these fish bioconcentrate PCBs through their prey (e.g. copepods). Polychlorinated biphenyl concentrations in the fish ranged from 0.298 to 0.706 ng g−1 wet (mean: 0.451 ng g−1 wet). The PCBs in the fish should be compared with those in the pellets to evaluate the contribution of the plastics to the total PCB burden of the seabird. Based on the weight of daily feeding of fish (approx. 10–120 g), daily exposure of PCBs from the fish is calculated to be approximately 15 ng. This ‘daily' exposure (approx. 15 ng) is lower than those from the resin pellets (approx. 100 ng). However, plastic was fed once (day 0) during the experiment, while fish were fed daily throughout the experiment. Therefore, in the early stage of the experiment (days 0–7), PCB exposure from the pellets dominated those from the fish, whereas after Day 7, PCB exposure from the fish overcame that from the plastics (figure 11). This suggests that potential effects of fed-pellet-derived PCBs could be observed in the early stage of the experiment.
Figure 11.
Loads of total PCBs in chicks. Closed circles: cumulative load from fish (Japanese sand lance: Ammodytes personatus); dotted line: load from plastic resin pellets.
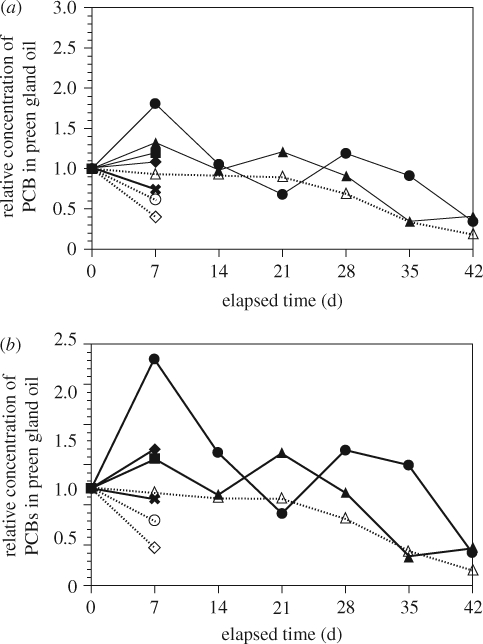
Figure 12 shows the preliminary results on the time-course of PCB concentrations in the preen gland oil from the streaked shearwater. It has been previously demonstrated that PCBs are accumulated in the preen gland oil owing to their hydrophobic nature and that the PCB concentrations in the oil reflect PCB levels in the internal tissue (i.e. abdominal adipose; Yamashita et al. 2007). Preen gland oil can be taken from a live bird without injury or stress. It provides a practical and useful tool to monitor PCB concentrations in biological tissue of ‘live' seabirds over time. Figure 12 displays the relative concentrations of PCBs in the individual chicks. For each chick, the preen gland oil PCB concentration is normalized to that at day 0. The PCB concentrations in preen gland oil increased through day 7 for the plastic-feeding setting, whereas no such increase was observed in the control. On the other hand, in the later stage, PCB concentrations in preen gland oil showed no difference between the plastic-feeding and the control settings (figure 12a). This is consistent with a larger PCB dose from the plastics than from fish-only in the early stage of the experiment (through day 7). These results suggest that plastic-derived PCBs were transferred to the biological tissue of the seabird. However, the difference between the plastic-feeding chicks and the control was not statistically significant.
Figure 12.
Time-course of PCBs in preen gland oil of the chicks during the feeding experiment: (a) total PCBs* and (b) lower chlorinated congeners**. *Total PCBs: sum of CB nos 8, 5, 28, 52, 44, 90, 101, 110, 77, 118, 132, 153, 138, 160, 187, 128, 180, 170, 190, and 206; **lower chlorinated congeners: sum of CB nos 8, 5, 18, 28, 52, 44, 66, 95. Polychlorinated biphenyl concentrations are normalized to those on day 0 on each series. Closed symbols and solid lines: plastic-feeding setting; open symbols and dotted lines: control setting. Replicate chicks no. 1 (closed square), 5 (cross), 8 (closed diamond), 10 (closed circle), 14 (closed triangle), 4 (open circle), 9 (open triangle), 18 (open diamond) were analysed. For chicks 1, 5, 8, 4, 18, samples were analysed on day 0 and day 7 only.
To obtain clearer evidence on the nature of the transfer, we focused on lower chlorinated congeners, because lower chlorinated congeners were relatively rich in plastic resin pellets compared with the fish (figure SI in the electronic supplementary material). The difference was probably because of biomagnification of higher chlorinated congeners in fish through the food web. Lower chlorinated congeners are more easily metabolized and selectively depleted when PCBs move through the food chain and, consequently, are less abundant in the fish tissue. Plastic resin pellets, however, just concentrate (partition) PCB congeners from seawater (not through the food web) and no metabolic process occurs, therefore lower chlorinated congeners that could be subject to biological degradation are not depleted. Lower chlorinated congeners can be regarded as a sensitive tracer to detect the contribution from plastic-derived PCBs. As illustrated in figure 12b, concentrations of lower chlorinated congeners in the plastic-feeding chicks increased up to three times from day 0 to day 7, whereas no increase in the congeners was observed for the control. This difference was statistically significant (p < 0.05, two-tailed t-test), demonstrating that transfer of PCBs, especially lower chlorinated congeners, occurs from ingested plastics to the biological tissue of the organisms that intake the plastics.
In higher-trophic-level organisms, plastic-derived contaminants compete with the biomagnified contaminants through the food web. However, marine plastics may act as a more important source of phenolic additive-derived chemicals (i.e. NP, OP and BPA) to higher-trophic-level organisms. Biomagnification of phenolic compounds through the food chain is unlikely as their hydrophilic group makes them easier to metabolize. Several studies suggest that biomagnification does not play an important role in the transfer of NP to animals and birds at higher trophic levels (e.g. Hu et al. 2005; Takeuchi et al. 2009). Thus, ingestion of marine plastics could be a direct and important route of phenolic chemicals to higher animals such as seabirds. Studies on the burden of the additive-derived chemicals to organisms, their transfer to organisms, and potential adverse effects are all needed.
9. Conclusions
This work reviews an expanse of literature addressing the role of plastics in the release and transport of environmental contaminants. Plastics can act as a source of environmental contaminants. For example, in the landfill environment, many additives and constitutional monomers leach out of plastics, and the discharged leachate can introduce plastic-derived contaminants into the environment. Concentrations of BPA and NP were significantly higher in landfill leachate than in wastewater effluent, and BPA accounted for more than half of the oestrogenic activity of the leachate.
Plastics also sorb and concentrate organic contaminants from the marine environment. The extent of uptake varies among plastics; polyethylene has a higher contaminant diffusivity and exhibits greater uptake of contaminants than other plastics including polypropylene and PVC. Ultraviolet weathering also appears to affect the uptake of contaminants by plastics. Unfortunately, our understanding of how to evaluate the risks associated with chemicals derived from marine plastics is poor. However, evidence is emerging that plastics with environmental contaminants can transport these compounds to organisms at various trophic levels.
Finally, these findings warn us that we should not underestimate the environmental impact of discarded plastics. As plastics production and usage continue to increase, particularly in economically developing countries, the environmental implications of their disposal should be carefully considered to avoid inadvertent release, magnification and transport of contaminants (Thompson et al. 2009a,b).
Footnotes
One contribution of 15 to a Theme Issue ‘Plastics, the environment and human health’.
References
- Ahrens M. J., Hertz J., Lamoureux E. M., Lopez G. R., McElroy A. E., Brownawell B. J.2001The role of digestive surfactants in determining bioavailability of sediment-bound hydrophobic organic contaminants to 2 deposit-feeding polychaetes. Mar. Ecol. Prog. Ser. 212, 145–157 (doi:10.3354/meps212145) [Google Scholar]
- Ali L. N., Mantoura R. F., Rowland S. J.1995The dissolution and photodegradation of Kuwaiti crude oil in seawater. Part 2: a laboratory photodegradation apparatus and photodegradation kinetics of a model seawater soluble hydrocarbon (phenanthrene). Mar. Environ. Res. 40, 319–335 (doi:10.1016/0141-1136(94)00149-J) [Google Scholar]
- Asakura H., Matsuto T., Tanaka N.2004Behavior of endocrine-disrupting chemicals in leachate from MWS landfill sites in Japan. Waste Manag. 24, 613–622 (doi:10.1016/j.wasman.2004.02.004) [DOI] [PubMed] [Google Scholar]
- Baird R. W., Hooker S. K.2000Ingestion of plastic and unusual prey by a juvenile porpoise. Mar. Pollut. Bull. 40, 719–720 (doi:10.1016/S0025-326X(00)00051-5) [Google Scholar]
- Barnes D. K. A., Galgani F., Thompson R. C., Barlaz M.2009Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998 (doi:10.1098/rstb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt J. M.2006The world of organotin chemicals: applications, substituents, and the environment. Organotin Environmental Program Association (ORTEPA). See http://ortepa.org/WorldofOrganotinChemicals.pdf. [Google Scholar]
- Bauer M. J., Herrmann R.1998Dissolved organic carbon as the main carrier of phthalic acid esters in municipal landfill leachates. Waste Manag. Res. 16, 446–454 (doi:10.1177/0734242X9801600507) [Google Scholar]
- Bauer M. J., Hermann R., Martin A., Zellmann H.1998Chemodynamics, transport behaviour and treatment of phthalic acid esters in municipal landfill leachates. Water Sci. Technol. 38, 185–192 [Google Scholar]
- Berens A. R.1989Sorption of organic liquids and vapors by rigid PVC. J. Appl. Polym. Sci. 37, 901–913 (doi:10.1002/app.1989.070370405) [Google Scholar]
- Björn A.2007Microbial transformation of organotin compounds under simulated landfill conditions (Diss.). Linköping Studies in Arts and Science no. 415, Linköping University, Sweden [Google Scholar]
- Björn A., Hörsing M., Karlsson A., Mersiowsky I., Ejlertsson J.2007Impacts of temperature on the leaching of organotin compounds from poly(vinyl chloride) plastics—a study conducted under simulated landfill conditions. J. Vinyl Addit. Technol. 13, 176–188 (doi:10.1002/vnl.20131) [Google Scholar]
- Boyer R. F.1951Relation of tensile strength to brittle temperature in plasticized polymers. J. Appl. Phys. 12, 723–728 [Google Scholar]
- Brandup J., Immergnt E. H., Grulke E. A.1989Polymer handbook, p. 206 New York, NY: Wiley [Google Scholar]
- Brusseau M. L., Jessup R. E., Rao P. S. C.1991Nonequilibrium sorption of organic chemicals: elucidation of rate-limiting processes. Environ. Sci. Technol. 25, 134–142 (doi:10.1021/es00013a015) [Google Scholar]
- Cadée G. C.2002Seabirds and floating plastic debris. Mar. Pollut. Bull. 44, 1294–1295 (doi:10.1016/S0025-326X(02)00264-3) [DOI] [PubMed] [Google Scholar]
- Carpenter E. J., Anderson S. J., Harvey G. R., Miklas H. P., Peck B. B.1972Polystyrene spherules in coastal waters. Science 178, 749–750 (doi:10.1126/science.178.4062.749) [DOI] [PubMed] [Google Scholar]
- Carter M. C., Kilduff J. E., Weber W. J.1995Site energy distribution analysis of preloaded adsorbents. Environ. Sci. Technol. 29, 1773–1780 (doi:10.1021/es00007a013) [DOI] [PubMed] [Google Scholar]
- Chiou C. T., Kile D. E.1998Deviations from sorption linearity on soils of polar and nonpolar organic compounds at low relative concentrations. Environ. Sci. Technol. 32, 338–343 (doi:10.1021/es970608g) [Google Scholar]
- Cobelo-Garcia A., Turner A., Millward G. E., Couceiro F.2007Behaviour of palladium(II), platinum(IV), and rhodium(III) in artificial and natural waters: influence of reactor surface and geochemistry on metal recovery. Anal. Chim. Acta 585, 202–210 [DOI] [PubMed] [Google Scholar]
- Colton J. B., Knapp F. D., Burns B. R.1974Plastic particles in surface waters of the Northwestern Atlantic. Science 185, 491–497 (doi:10.1126/science.185.4150.491) [DOI] [PubMed] [Google Scholar]
- Coors A., Jones P. D., Giesy J. P., Ratte H. T.2003Removal of estrogenic activity from municipal waste landfill leachate assessed with a bioassay based on reporter gene expression. Environ. Sci. Technol. 37, 3430–3434 (doi:10.1021/es0300158) [DOI] [PubMed] [Google Scholar]
- Deng L., Liu Y., Chen P., Wang L., Deng N.2006Determination of trace bisphenol A in leachate by solid phase microextraction coupled with high performance liquid chromatography. Anal. Lett. 39, 395–404 (doi:10.1080/00032710500477183) [Google Scholar]
- Derraik J. G. B.2002The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull 44, 842–852 (doi:10.1016/S0025-326X(02)00220-5) [DOI] [PubMed] [Google Scholar]
- Desbrow C., Routledge E. J., Brighty G. C., Sumpter J. P., Waldock M.1998Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ. Sci. Technol. 32, 1549–1558 (doi:10.1021/es9707973) [Google Scholar]
- Di Toro D. M., et al. 1991Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. Environ. Toxicol. Chem. 10, 1541–1583 (doi:10.1897/1552-8618(1991)10[1541:TBFESQ]2.0.CO;2) [Google Scholar]
- Earth Trends Country Profiles. 2007. See http://earthtrends.wri.org/country_profiles/index.php?theme=5 .
- Ejlertsson J., Nilsson M. L., Kylin H., Bergman A., Karlson L., Öquist M., Svensson B. H.1999Anaerobic degradation of nonylphenol mono- and diethoxylates in digestor sludge, landfill municipal solid waste and landfill sludge. Environ. Sci. Technol. 33, 301–306 (doi:10.1021/es980669u) [Google Scholar]
- Ejlertsson E., Karlsson A., Lagerkvist A., Hjertberg T., Svensson B. H.2003Effects of co-disposal of wastes containing organic pollutants with municipal solid waste—a landfill simulation study. Adv. Environ. Res. 7, 949–960 (doi:10.1016/S1093-0191(02)00099-0) [Google Scholar]
- Endo S., Takizawa R., Okuda K., Takada H., Chiba K., Kanehiro H., Ogi H., Yamashita R., Date T.2005Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar. Pollut. Bull. 50, 1103–1114 (doi:10.1016/j.marpolbul.2005.04.030) [DOI] [PubMed] [Google Scholar]
- Erickson M. D.1997Analytical Chemistry of PCBs Boca Raton, CA: Lewis Publisher [Google Scholar]
- Eriksson C., Burton H.2003Origins and biological accumulation of small plastic particles in fur seals from Macquarie Island. Ambio 32, 380–384 [DOI] [PubMed] [Google Scholar]
- ESPA. Use, exposure and loss data for the use of mono/di-tin compounds as PVC stabilizers. Communication from the European Stabilizers Producers Association; 2002. [Google Scholar]
- European Chemicals Bureau. Technical guide document on risk assessment. Institute for Health and Consumer Protection; 2003. [Google Scholar]
- Foster P. M. D.2006Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 29, 140–147 (doi:10.1111/j.1365-2605.2005.00563.x) [DOI] [PubMed] [Google Scholar]
- Fromme H., Kuchler T., Otto T., Pilz K., Muller J., Wenzel A.2002Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 36, 1429–1438 (doi:10.1016/S0043-1354(01)00367-0) [DOI] [PubMed] [Google Scholar]
- Fry D. M., Fefer S. I., Sileo L.1987Ingestion of plastic by laysan albatrosses and wedge-tailed shearwaters in the Hawaiian Islands. Mar. Pollut. Bull. 18, 339–343 (doi:10.1016/S0025-326X(87)80022-X) [Google Scholar]
- Furtmann K.1996Phthalates in the aquatic environment, p. 136 Bruxelles, Belgium: ECPI [Google Scholar]
- Giam C. S., Atlas A., Powers J. M. A., Leonard J. E.1984Phthalic acid esters. In Handbook of environmental chemistry, vol. 3 (ed. Hutzinger O.), pp. 67–140 New York, NY: Springer Verlag [Google Scholar]
- Gilbert J., Stain J. R., McGuinness J. D.1986Compositional analysis of commercial PVC bottles and studies of aspects of specific and overall migration into foods and stimulants. Food Addit. Contam. 3, 133–144 [DOI] [PubMed] [Google Scholar]
- Gregory M. R.2009Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Phil. Trans. R. Soc. B 364, 2013–2025 (doi:10.1098/rstb.2008.0265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F., Blumberg B.2007Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev. Endocr. Metab. Disord. 8, 161–171 (doi:10.1007/s11154-007-9049-x) [DOI] [PubMed] [Google Scholar]
- Hatzinger P. B., Alexander M.1997Biodegradation of organic compounds sequestered in organic solids or in nanopores within silica crystals. Environ. Toxicol. Chem. 16, 2215–2221 (doi:10.1897/1551-5028(1997)016<2215:BOOCSI>2.3.CO;2) [Google Scholar]
- Hu J., Jin F., Wan Y., Yang M., An L., An W., Tao S.2005Trophodynamic behavior of 4-nonylphenol and nonylphenol polyethoxylate in a marine aquatic food web from Bohai Bay, North China: comparison to DDTs. Environ. Sci. Technol. 39, 4801–4807 (doi:10.1021/es048735h) [DOI] [PubMed] [Google Scholar]
- Huang W. L., Webber J. W.1997A distribution reactivity model for sorption by soils and sediments. 10. Relationships between desorption, hysteresis, and the chemical characteristics of organic domains. Environ. Sci. Technol. 131, 2562–2569 [Google Scholar]
- Huang W. L., Yu H., Weber W. J.1998Hysteresis in sorption and desorption of hydrophobic organic contaminants by soils and sediments. Environ. Sci. Technol. 32, 3549–3555 (doi:10.1021/es970764n) [Google Scholar]
- Jonsson S.2003Phthalates in landfill leachates—a signature of their degradation: analytical aspects and toxicological considerations (Diss.). Linköping Studies in Arts and Science no. 268, Linköping University, Sweden [Google Scholar]
- Jonsson S., Ejlertsson J., Svensson B. H.2003aBehaviour of mono and diesters of o-phthalic acid in leachates released during digestion of municipal solid waste under landfill conditions. Adv. Environ. Res. 7, 181–192 [Google Scholar]
- Jonsson S., Ejlertsson J., Ledin A., Mersiowsky I., Svensson B. H.2003bMono- and diesters from o-phthalic acid in leachates from different European landfills. Water Res. 37, 609–617 (doi:10.1016/S0043-1354(02)00304-4) [DOI] [PubMed] [Google Scholar]
- Jonsson S., Vavilin V. A., Svensson B. H.2006Phthalate hydrolysis under landfill conditions. Water Sci. Technol. 53, 119–127 [DOI] [PubMed] [Google Scholar]
- Joo J. C., Kim J. Y., Nam K.2004Mass transfer of organic compounds in dilute aqueous solutions in high density polyethylene geomembranes. J. Environ. Eng. 130, 175–183 [Google Scholar]
- Karapanagioti H. K., Klontza I.2008Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on lesvos island beaches (Greece). Mar. Environ. Res 65, 283–290 [DOI] [PubMed] [Google Scholar]
- Kawagoshi Y., Fujita Y., Kishi I., Fukunaga I.2003Estrogenic chemicals and estrogenic activity in leachate from municipal waste landfill determined by yeast two-hybrid assay. J. Environ. Monit. 5, 269–274 (doi:10.1039/b210962j) [DOI] [PubMed] [Google Scholar]
- Kawamura Y., Koyama Y., Takeda Y., Yamada T.1998Migration of bisphenol A from poly-carbonate products. J. Food Hyg. Soc. Jpn 99, 206–212 [Google Scholar]
- Koch H. M., Calafat A. M.2009Human body burdens of chemicals used in plastic manufacture. Phil. Trans. R. Soc. B 364, 2063–2078 (doi:10.1098/rstb.2008.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laist D. W.1997Impacts of marine debris: entanglement of marine life in debris including a comprehensive list of species with entanglement and ingestion records. In Marine debris—sources, impacts and solutions (eds Coe J. M., Rogers D. B.), pp. 99–140 Berlin: Springer [Google Scholar]
- Maguire R. J.1999Review on the persistence of nonylphenol and nonylphenol ethoxylates in aquatic environments. Water Qual. Res. J. Can. 34, 37–78 [Google Scholar]
- Mascarenhas R., Santos R., Zeppelini D.2004Plastic ingestion by sea turtle in Paraiba, Brazil. Mar. Pollut. Bull. 49, 354–355 (doi:10.1016/j.marpolbul.2004.05.006) [DOI] [PubMed] [Google Scholar]
- Mato Y., Isobe T., Takada H., Kanehiro H., Ohtake C., Kaminuma T.2001Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 35, 318–324 (doi:10.1021/es0010498) [DOI] [PubMed] [Google Scholar]
- Mato Y., Takada H., Zakaria M. P., Kuriyama Y., Kanehiro H.2002Toxic chemicals contained in plastic resin pellets in the marine environment—spatial difference in pollutant concentrations and the effects of resin type. Kankyo Kagakukaishi 15, 415–423 [Google Scholar]
- McDermid K. J., McMullen T. L.2004Quantitative analysis of small-plastic debris on beaches in the Hawaiian archipelago. Mar. Pollut. Bull. 48, 790–794 (doi:10.1016/j.marpolbul.2003.10.017) [DOI] [PubMed] [Google Scholar]
- Meeker J. D., Sathyanarayana S., Swan S. H.2009Phthalates and other additives in plastics: human exposure and associated health outcomes. Phil. Trans. R. Soc. B 364, 2097–2113 (doi:10.1098/rstb.2008.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. J., Moore S. L., Leecaster M. K., Weisberg S. B.2001A comparison of plastic and plankton in the North Pacific Central Gyre. Mar. Pollut. Bull. 42, 1297–1300 (doi:10.1016/S0025-326X(01)00114-X) [DOI] [PubMed] [Google Scholar]
- Murata T.1999The endocrine disruptors contained in the plastics. Chem. Eng. 63, 305–309 [Google Scholar]
- Murphy S. R., Bertelo R., Ringwood M., Cochran M.2000Improved organotin stabilizers: continuing health and environmental research. J. Vinyl Addit. Technol. 6, 104–108 (doi:10.1002/vnl.10232) [Google Scholar]
- Nakada N., Nyunoya H., Nakamura M., Hara A., Iguchi T., Takada H.2004Identification of estrogenic compounds in wastewater effluent. Environ. Toxicol. Chem. 23, 2807–2815 (doi:10.1897/03-699.1) [DOI] [PubMed] [Google Scholar]
- Nakada N., Tanishima T., Shinohara H., Kiri K., Takada H.2006Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 40, 3297–3303 (doi:10.1016/j.watres.2006.06.039) [DOI] [PubMed] [Google Scholar]
- Neal R. A.1985Mechanisms of the biological effects of PCBs, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans in experimental animals. Environ. Health Perspect. 60, 41–46 (doi:10.2307/3429943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidlinger H. H., Schissel P.1987Polymers in solar technologies. In Polymers for advanced technologies. IUPAC international symposium (ed. Lewin W.). New York, NY: VCH [Google Scholar]
- Ng K. L., Obbard J. P.2006Prevalence of microplastics in Singapore's coastal marine environment. Mar. Pollut. Bull. 52, 761–767 (doi:10.1016/j.marpolbul.2005.11.017) [DOI] [PubMed] [Google Scholar]
- Oehlmann J., et al. 2009A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 364, 2047–2062 (doi:10.1098/rstb.2008.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. K., Sakti J. P., Hoopes J. A.1996Transport of organic compounds in thermoplastic geomembranes. I: mathematical model. J. Environ. Eng. 122, 800–806 [Google Scholar]
- Pignatello J. J., Xing B.1996Mechanisms of slow sorption of organic chemicals to natural particles. Environ. Sci. Technol. 30, 1–11 (doi:10.1021/es940683g) [Google Scholar]
- Reddy M. S., Basha S., Adimurthy S., Ramachandraiah G.2006Description of the small plastic fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Est. Coast. Shelf Sci. 68, 656–660 (doi:10.1016/j.ecss.2006.03.018) [Google Scholar]
- Reichenberg F., Mayer P.2006Two complementary sides of bioavailability: accessibility and chemical activity of organic contaminants in sediments and soils. Environ. Toxicol. Chem. 25, 1239–1245 (doi:10.1897/05-458R.1) [DOI] [PubMed] [Google Scholar]
- Rios L. M., Moore C., Jones P. R.2007Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 54, 1230–1237 (doi:10.1016/j.marpolbul.2007.03.022) [DOI] [PubMed] [Google Scholar]
- Robards M. D., Piatt J. F., Wohl K. D.1995Increasing frequency of plastic particles ingested by seabirds in the subarctic North Pacific. Mar. Pollut. Bull. 30, 151–157 (doi:10.1016/0025-326X(94)00121-O) [Google Scholar]
- Rogers C. E., Stanett V., Szwarc M.1960The sorption, diffusion, and permeation of organic vapors in polyethylene. J. Polym. Sci. 45, 61–82 (doi:10.1002/pol.1960.1204514506) [Google Scholar]
- Ryan P. G., Connell A. D., Gardener B. D.1988Plastic ingestion and PCBs in seabirds: is there a relationship? Mar. Pollut. Bull. 19, 174–176 (doi:10.1016/0025-326X(88)90674-1) [Google Scholar]
- Ryan P. G., Moore C. J., van Franeker J. A., Moloney C. L.2009Monitoring the abundance of plastic debris in the marine environment. Phil. Trans. R. Soc. B 364, 1999–2012 (doi:10.1098/rstb.2008.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S., Urano S., Takatsuki H.2000Leaching behavior of PCBs and PCDDs/DFs from some waste materials. Waste Manag. 20, 241–247 (doi:10.1016/S0956-053X(99)00316-5) [Google Scholar]
- Sangam H. P., Rowe K.2001Migration of dilute aqueous organic pollutants through HDPE geomembranes. Geotext. Geomembr. 19, 329–357 (doi:10.1016/S0266-1144(01)00013-9) [Google Scholar]
- Schulte-Oehlmann U., Tillmann M., Casey D., Duft M., Markert B., Oehlmann J.2001Xeon-estrogenic effects of bisphenol A in prosobranchs (mollusca: Gaastropoda: Prosbranchia). Z. Umweltchem. Okotox. 13, 319–333 [Google Scholar]
- Sonnenschein C., Soto A. M.1998An updated review of environmental estrogen and androgen mimics and antagonists. J. Steriod Biochem. Mol. Biol. 65, 143–150 (doi:10.1016/S0960-0760(98)00027-2) [DOI] [PubMed] [Google Scholar]
- Staples C. A., Peterson D. R., Parkerton T. F., Adams W. J.1997The environmental fate of phthalates: a literature review. Chemosphere 35, 667–749 [Google Scholar]
- Sumpter J. P., Johnson A. C.2005Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment. Environ. Sci. Technol. 39, 4321–4332 (doi:10.1021/es048504a) [DOI] [PubMed] [Google Scholar]
- Takeuchi I., Miyoshi N., Mizukawa K., Takada H., Ikemoto T., Omori K., Tsuchiya K.2009Biomagnification profiles of polycyclic aromatic hydrocarbons, alkylphenols and polychlorinated biphenyls in Tokyo Bay elucidated by δ 13C and δ 15 N isotope ratios as guides to trophic web structure. Mar. Pollut. Bull 58, 663–671 [DOI] [PubMed] [Google Scholar]
- Teuten E. L., Rowland S. J., Galloway T. S., Thompson R. C.2007Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 41, 7759–7764 (doi:10.1021/es071737s) [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Olsen Y., Mitchell R. P., Davis A., Rowland S. J., John A. W. G., McGonigle D., Russell A. E.2004Lost at sea: where is all the plastic? Science 304, 838 (doi:10.1126/science.1094559) [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Swan S. H., Moore C. J., vom Saal F. S.2009aOur plastic age. Phil. Trans R. Soc B 364, 1973–1976 (doi:10.1098/rstb.2009.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Moore C. J., vom Saal F. S., Swan S. H.2009bPlastics, the environment and human health: current consensus and future trends. Phil. Trans R. Soc B 364, 2153–2166 (doi:10.1098/rstb.2009.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobolsky A. V., Mark H. F.1980Polymer science material (ed. Robert E.). Huntington, New York, NY: Krieger Publishing Company [Google Scholar]
- Treloar L. R. G.1974Introduction to polymer science London and Winchester, UK: Wykeham Publications (London) Ltd [Google Scholar]
- Vavilin V. A., Jonsson S., Svensson B. H.2005Kinetic analysis of the transformation of phthalate esters in a series of stoichiometric reactions in anaerobic wastes. Appl. Microbiol. Biotechnol. 69, 474–484 (doi:10.1007/s00253-005-0061-3) [DOI] [PubMed] [Google Scholar]
- Vlietstra L. S., Parga J. A.2002Long-term changes in the type, but not amount, of ingested plastic particles in short-tailed shearwaters in the southeastern Bering Sea. Mar. Pollut. Bull. 44, 945–955 (doi:10.1016/S0025-326X(02)00130-3) [DOI] [PubMed] [Google Scholar]
- Voparil I. M., Mayer L. A.2000Dissolution of polycyclic aromatic hydrocarbons into the lugworm's (Arenicola marina) digestive fluids. Environ. Sci. Technol. 34, 1221–1228 (doi:10.1021/es990885i) [Google Scholar]
- Wagner T.2003. Factors controlling hydrophobic organic contaminant sorption to and desorption from municipal solid waste. Masters thesis, North Carolina State University [Google Scholar]
- Weber W. J., McGinley P. M., Katz L. E.1992A distributed reactivity for sorption by soils sediments. 1. Conceptual basis and equilibrium assessments. Environ. Sci. Technol. 26, 1955–1962 (doi:10.1021/es00034a012) [Google Scholar]
- West C.2007The photodegradation of diazepam and its human metabolites in water PhD thesis, University of Plymouth, Plymouth, UK [Google Scholar]
- WHO/IPCS 1994Brominated diphenyl ethers. Environmental Health Criteria 162 In Proceedings of the International Programme on Chemical Safety Geneva, Switzerland: WHO/IPCS [Google Scholar]
- Wintgens T., Gallenkemper M., Melin T.2003Occurrence and removal of endocrine disrupters in landfill leachate treatment plants. Water Sci. Technol. 48, 127–134 [PubMed] [Google Scholar]
- Wu B., Taylor C. M., Knappe D. R. U., Nanny M. A., Barlaz M. A.2001Factors controlling alkylbenzene sorption to municipal solid waste. Environ. Sci. Technol. 35, 4569–4576 (doi:10.1021/es010893a) [DOI] [PubMed] [Google Scholar]
- Wurl O., Obbard J. P.2004A review of pollutants in the sea-surface microlayer (SML): a unique habitat for marine organisms. Mar. Pollut. Bull. 48, 1016–1030 (doi:10.1016/j.marpolbul.2004.03.016) [DOI] [PubMed] [Google Scholar]
- Xing B. S., Pignatello J. J.1997Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter. Environ. Sci. Technol. 31, 792–799 (doi:10.1021/es960481f) [Google Scholar]
- Yamamoto T., Yasuhara A., Shiraishi H., Nakasugi O.2001Bisphenol A in hazardous waste landfill leachates. Chemosphere 42, 415–418 (doi:10.1016/S0045-6535(00)00079-5) [DOI] [PubMed] [Google Scholar]
- Yamashita R., Takada H., Murakami M., Fukuwaka M., Watanuki Y.2007Evaluation of noninvasive approach for monitoring PCB pollution of seabirds using preen gland oil. Environ. Sci. Technol. 41, 4901–4906 (doi:10.1021/es0701863) [DOI] [PubMed] [Google Scholar]
- Yasuhara A., et al. 1997Determination of organic components in leachates from hazardous waste disposal sites in Japan by gas chromatography-mass spectrometry. J. Chromatogr. A 774, 321–332 (doi:10.1016/S0021-9673(97)00078-2) [Google Scholar]
- Ye S., Andrady A. L.1991Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar. Pollut. Bull. 22, 608–613 (doi:10.1016/0025-326X(91)90249-R) [Google Scholar]
- Ying G.-G., Kookana R. S.2003Degradation of five selected endocrine-disrupting chemicals in seawater and marine sediment. Environ. Sci. Technol. 37, 1256–1260 (doi:10.1021/es0262232) [Google Scholar]
- Zhang C., Zeng G., Yuan L., Yu J., Li J., Huang G., Xi B., Liu H.2007Aerobic degradation of bisphenol A by Achromobacter xylosoxidans strain B-16 isolated from compost leachate of municipal solid waste. Chemosphere 68, 181–190 (doi:10.1016/j.chemosphere.2006.12.012) [DOI] [PubMed] [Google Scholar]