Abstract
This article explains the history, from 1600 BC to 2008, of materials that are today termed ‘plastics’. It includes production volumes and current consumption patterns of five main commodity plastics: polypropylene, polyethylene, polyvinyl chloride, polystyrene and polyethylene terephthalate. The use of additives to modify the properties of these plastics and any associated safety, in use, issues for the resulting polymeric materials are described. A comparison is made with the thermal and barrier properties of other materials to demonstrate the versatility of plastics. Societal benefits for health, safety, energy saving and material conservation are described, and the particular advantages of plastics in society are outlined. Concerns relating to littering and trends in recycling of plastics are also described. Finally, we give predictions for some of the potential applications of plastic over the next 20 years.
Keywords: plastic, society, history, polymers, development
Humans have benefited from the use of polymers since approximately 1600 BC when the ancient Mesoamericans first processed natural rubber into balls, figurines and bands (Hosler et al. 1999). In the intervening years, man has relied increasingly on plastics and rubber, first experimenting with natural polymers, horn, waxes, natural rubber and resins, until the nineteenth century, when the development of modern thermoplastics began.
In 1839, Goodyear invented vulcanized rubber, and Eduard Simon, a German apothecary, discovered polystyrene (PS). Developmental work continued through the nineteenth century on natural/synthetic polymers producing such notables as celluloid for billiard balls, polyvinyl chloride (PVC), which is used in myriad applications, and viscose (rayon) for clothing. Development of modern plastics really expanded in the first 50 years of the twentieth century, with at least 15 new classes of polymers being synthesized. The success of plastics as a material has been substantial; they have proved versatile for use in a range of types and forms, including natural polymers, modified natural polymers, thermosetting plastics, thermoplastics and, more recently, biodegradable plastics. Plastics have a range of unique properties: they can be used at a very wide range of temperatures, are chemical- and light-resistant and they are very strong and tough, but can be easily worked as a hot melt. It is this range of properties together with their low cost that has driven the annual worldwide demand for plastics to reach 245 million tonnes (PlasticsEurope 2008) today. Even at a somewhat conservative annual growth rate of 5 per cent, a continuation of this trend suggests that at least 308 million tonnes of plastics will be consumed annually worldwide by 2010 (PlasticsEurope 2008). This projected growth is mainly attributed to increasing public demand for plastics. Here we summarize the main types of plastic in use today.
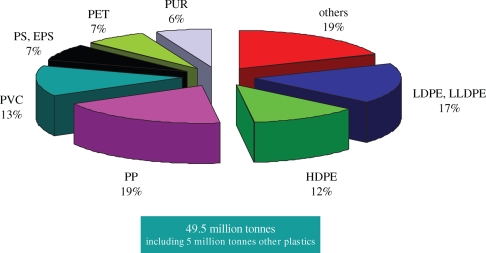
Although literally hundreds of plastic materials are commercially available, only a handful of these qualify as commodity thermoplastics in terms of their high volume and relatively low price. These plastics and their fractional consumption on a global basis are shown below. Low-density polyethylene (LDPE), high-density PE (HDPE), polypropylene (PP), PVC, PS and polyethylene terephthalate (PET) account for approximately 90 per cent of the total demand and will be discussed in more detail (figure 1).
Figure 1.
World plastic materials demand by resin types 2006 (PlasticsEurope 2008).
1. Commodity plastics
This group includes PP and PE. Polypropylene was discovered in 1954 by Giulio Natta, and commercial production of the resin began in 1957. It is the single most widely used thermoplastic globally. It is a very useful cost-effective polymer and can be injection-moulded, blow-moulded, thermoformed, blown film extruded or extruded into a variety of products. Examples of these include flexible barrier film pouches (including the biaxially oriented packaging film used for crisps and nuts); stackable crates for transport and storage, caps and closures for containers, blow-moulded bottles, thin-walled containers (e.g. margarine tubs, yoghurt cups, food trays) used in the food industry; and tree shelters, soil sieves, fork handles, mulch films, and glass replacement, window/door frames, water or sewage pipes and geomembranes used in building applications. Polypropylenes are also used in household goods such as bowls, kettles, cat litter trays; personal goods such as combs, hair dryers, film wrap for clothing; and in other packaged goods.
Polyethylene was discovered in March 1933 by Reginald Gibson and Eric Fawcett, two research chemists at ICI's Winnington Laboratory in the UK, and it was first synthesized as a low-density resin (LDPE) in 1935. Polyethylene manufacturing processes have since become more sophisticated and cost-effective. Currently, there are about 25 different processes for manufacturing the range of PEs and metallocene-catalysed polyethylene (mPE). The latter has superior toughness and is one of the most recent and the fastest growing processes. Polyethylene is presently the second most widely used class of resin globally. There are several different grades of PE classified according to the average density of the resin linear LDPE (LLDPE), 0.925 g cm−3; LDPE, 0.930–0.935 g cm−3; medium density polyethylene (MDPE), 0.93–0.945 g cm−3; HDPE, 0.945–0.965 g cm−3).
About a half of the 35 million tonnes of PE resin produced is used to make plastic film, followed by 13–14% in injection-moulded and blow-moulded products. North American, western European and Asian markets each consume approximately 25–30% of the PE film produced globally. Typical applications of PE are in blow-moulded containers with volumes ranging from a few millilitres such as detergent bottles (200–500 cm3) and milk jugs (0.5–4 l) to hundreds of litres such as water and chemical barrels. Film applications include carrier bags, sandwich bags, freezer bags and cling wrap, and horticultural uses include irrigation pipes, glass replacement and field liners. Polyethylene is also widely used as a dielectric insulator in electrical cables.
Polyvinyl chloride was first created by Eugen Baumann in 1872, but it was not until the late 1920s that the first commercial production of PVC took place in the USA. Large-scale production in Europe followed during the next two decades. Most commodity plastics have carbon and hydrogen as their main component elements, but PVC differs by containing chlorine (around 57% by weight) as well as carbon and hydrogen. PVC as produced is in the form of a white powder. This powder is not used by itself, but blended with other ingredients to give formulations that are suitable for use in a wide range of products. According to Plastics Europe, annual world demand for PVC is around 35 million tonnes, with a predicted growth of approximately 1 per cent per annum until 2010. Approximately 40 per cent of global demand is in Asia (with China accounting for the majority of it). In considering applications for PVC, its chlorine content makes it essentially non-combustible and PVC is therefore used in buildings and furniture, including window shutters, piping and upholstery. It is also used as cling films for both household and various industrial applications.
Commercial production of PS was started in the 1930s by the German company BASF (I G Farben) and was introduced into the USA in 1937. Polystyrene is available in two main forms: a general purpose grade and a high-impact grade in which the PS is modified with polybutadiene. In 1954, The Dow Chemical Company invented expanded PS, which is widely recognized as an excellent insulating medium for buildings (table 1) and as a mouldable packaging material. Expanded PS cups and trays are commonly used for consumer goods, while industrial packaging protects high-value goods such as electronic goods, TVs, washing machines and lighting during transport. This is even true of Ferrari cars on car transporters (author's (M.A.N.) observation on the road from Bologna to Verona, Italy).
Table 1.
A comparison of the thermal conductivity of typical building materials. (EPS, expanded polystyrene.)
| group | material | thermal conductivity (W mK−1) |
|---|---|---|
| metal | aluminium | 204 |
| copper | 372 | |
| steel | 52 | |
| concrete | heavy | 2.0 |
| light | 0.7–0.9 | |
| glass | window | 0.8 |
| foam glass | 0.04 | |
| wood | hardwood | 0.17 |
| chipboard | 0.1–0.3 | |
| cork | 0.06–0.07 | |
| synthetics | EPS foam | 0.035 |
| polyurethane foam | 0.025–0.035 | |
| phenolformaldehyde foam | 0.035 | |
| PP and PE | 0.17 |
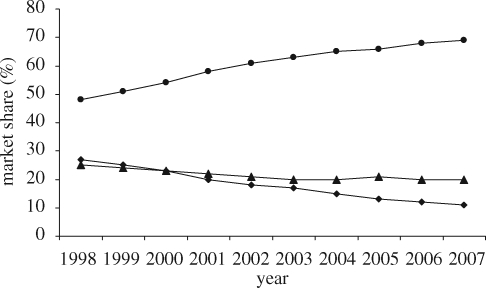
Chemists Whinfield and Dickson, employees of the Calico Printers' Association of Manchester, are credited with the discovery of PET in 1941. It was eventually licensed to DuPont for use in the USA and to ICI for use in the rest of the world. Both DuPont and ICI chemists went on to develop PET fibres. In the 1950s they produced the first polyester films and in the early 1970s the first polyester bottle resins. Of the few polymers that are potentially suitable for bottles, PET is the only plastic with a balance of properties such as transparency (near 100% light transmission in a bottle), gloss, lightweight and resistance to carbon dioxide permeation (table 2). This has resulted in the nearly full replacement of glass in Europe for all but the most demanding applications that require both an oxygen barrier and UV resistance to protect the contents. In the authors' opinion, it will only be a matter of time before oxygen and UV barrier issues are solved for PET, so that glass bottles can be fully replaced. World demand is 14.5 million tonnes per annum (PlasticsEurope 2008) and is increasing. For example, usage of PET for carbonated drink bottles (including beer) will continue to grow at a rate of 8 per cent per annum in the near future (figure 2).
Table 2.
Comparative barrier properties of polymers. Source: Artenius, UK (2008).
| polymer | oxygen (cc/mil/100 sqin/24 h/atm at 23°C and 50% relative humidity) | carbon dioxide (cc/mil/100 sqin/24 h/atm at 23°C and 50% relative humidity) | water vapour (cc/mil/100 sqin/ 24 h/atm at 23°C and 50% relative humidity) |
|---|---|---|---|
| PET | 8 | 20 | 4 |
| PVC | 15 | 40 | 3 |
| oriented PS | 330 | 2000 | 4 |
| PP | 160 | 450 | 0.5 |
Figure 2.
Market share of various materials used for the packaging of soft drinks 1998–2007 (Canadean 2008). Notes: (i) combined total volume for 32 European countries; (ii) packaged water, carbonates, juice, nectars, still drinks, iced tea and coffee, sports drinks, energy drinks, squash/syrups, fruit powders; (iii) ‘others’ include metal (cans), board (cartons), foil (pouch) and other types of plastics. (Diamonds, % glass share; circles, % PET share; triangles, % others.)
2. Plastics additives
Plastics are rarely used by themselves; typically, the resins are mixed with other materials called ‘additives’ to enhance performance. These may include inorganic fillers (e.g. carbon or silica) to reinforce the plastic material, thermal stabilizers to allow the plastics to be processed at high temperatures, plasticizers to render the material pliable and flexible, fire retardants to discourage ignition and burning, and UV stabilizers to prevent degradation when exposed to sunlight. Colorants, matting agents, opacifiers and lustre additives might also be used to enhance the appearance of a plastic product. Additives are often the most expensive component of a formulation, and the minimum quantity needed to achieve a given level of performance is generally used. The additives are intimately mixed with the polymer or ‘compounded’ into a formulation that is processed into the shape of the final product.
The potential adverse health issues associated with the use of specific additives, such as phthalate plasticizers, have been raised (for recent reviews, see Koch & Calafat 2009; Meeker et al. 2009; Oehlmann et al. 2009; Talsness et al. 2009). A major issue in defining the problems associated with these additives is a shared lack of understanding of the available evidence. Industries spends many millions of euros in generating peer-reviewed evidence, which is available to governments and academia. For example consider Lynch et al. (1999), Hext et al. (1999), Elliot et al. (1998), Kennedy et al. (2004) and Andersen et al. (2006), all of whom published industry sponsored research. In the opinion of the authors, rarely is this type of evidence fully explored, discussed and understood before governments, academics and NGOs publicize their findings or concerns. An example of this is the recent UK publication of the SIN list by Chemsec (2008), which is an emotional call for the substitution of certain chemicals without full consultation or discussion of evidence. In considering the use of additives in plastics, it is important to understand the risk to the public of exposure at realistic levels. One should consider Paracelsus—‘the dose is the poison’; a good example of this the recent discussions on antimony catalysts in PET. Following extensive research, a tolerable daily intake (TDI)—a scientifically based estimate of the amount of a substance, expressed on a body weight basis that can be ingested daily over a lifetime without appreciable risk—was established by the World Health Organization (2003) for antimony. Yet Westerhoff et al. (2008) inferred that the minute doses (ng/l) received when consuming water bottled in PET were harmful. The volume of bottled water that would need to be consumed to reach the TDI is unrealistically high (300 l). Hence, the potential risk from excessive intake of water and the associated sodium salts is far higher than that from the antimony.
A further instance where plastics additives were criticized in the absence of adequate evidence is illustrated by bisphenol A (BPA) exposure from polycarbonate products. Bisphenol A has a permanent (lifetime exposure) TDI, which was set by the European Food Safety Authority (EFSA) in January 2007 and announced with a public statement that ‘People's dietary exposure to BPA, including that of infants and children, is estimated to be well below the new TDI1’. However, BPA from drinking water bottles has been cited as a significant source of BPA exposure of individuals (Lea et al. 2008). There have also been instances where a real (as opposed to perceived) risk associated with a plastics additive has been identified based on extensive research, and examples are given below. In such instances, the industry has moved to eliminate or reduce the levels of the identified additive.
Plasticizers are a particular group of additives that has raised concerns; however, there are many types of plasticizer (e.g. adipates, polymerics, trimellitates, 1,2-cyclohexanedicarboxylic acid diisononyl ester, citrates, phthalates, etc.) used in plastics. Of these, about eight different types are in common use. It is not possible to conduct a generalized risk assessment on phthalates as a class of compounds used as plasticizers. Some phthalates, e.g. diisononyl phthalate and diisodecyl phthalate, have been through full European Risk Assessments and have a completely clean bill of health in all applications, whereas with other phthalates such as dibutyl phthalate and diethyl hexyl phthalate, risk-reduction measures are required (described in the ECB published Risk Assessments in the online ORATS (2008) database available from The Phthalates Information Centre Europe) to ensure that safe use has been identified.
In summary, in most countries, the use of additives is strictly controlled, particularly in critical applications such as food contact and packaging of pharmaceuticals and toys; their use is independently monitored by individual government authorities to ensure that consumer health and safety is protected (against exposure to any additive that leaches from the plastic into the packaged product). Examples of standards and controlling authorities are the US Food and Drug Administration, the US Environmental Protection Agency, Environment Canada, the UK Food Standards Agency (DEFRA), the European Chemicals Agency, the EFSA and the World Health Organization (see discussion of policy measures in Shaxson 2009).
3. Plastics consumption
The consumption patterns of the five most widely used types of plastics in their different application sectors appear to be consistent in the developed regions of the world. Well over a third of consumption is in packaging applications (with common products such as containers and plastic bags) and another third or more in building products including common products such as plastic pipes or vinyl cladding. In developing countries, usage patterns may differ slightly; for instance, in India, 42 per cent of resin consumption was reported to be in the packaging sector (Mutha et al. 2006). Automotive applications and toy/furniture manufacture use smaller but significant volumes of plastics. Use of plastics in the developing world is increasing as the lower unit cost and improvements in performance specifications continually promote its substitution for materials such as paper, metals, wood and glass.
Plastics clearly constitute an important component of the range of materials used in modern society. Almost all aspects of daily life involve plastics or rubber in some form or the other. These include clothing and footwear, together with products for use in food and public health applications. Over 40 million tonnes of plastics were converted into textile fibre (mainly nylon, polyester and acrylics) worldwide for use in apparel manufacture. Polycotton clothing contains high levels of PET plastic; high-performance clothing is almost exclusively plastics—polyesters, fluoropolymers and nylons. Fleece clothing is 100 per cent plastic (PET) and can be made from recycled PET. Most footwear also relies heavily on plastics; the footbed and outsoles are made from polyurethane or other elastomeric material while the uppers might be made of vinyl or other synthetic polymer.
Plastics also deliver many public health benefits. They facilitate clean drinking water supplies and enable medical devices ranging through surgical equipment, drips, aseptic medical packaging and blister packs for pills. They provide packaging that reduces food wastage, for instance in the use of modified atmosphere packaging (Mullan 2002) that prolongs the life of meat and vegetables.
Owing to their light weight, plastics reduce transportation costs and, therefore, atmospheric carbon dioxide emissions. Public and private transportation vehicles can now contain up to 20 per cent plastics typically as parcel shelves, door liners, steering wheels, electrics and electronics, and recent aircraft such as the Boeing Dreamliner is designed from up to 50 per cent plastics. Plastics can also be used to improve the performance and reduce the costs of building materials; examples of this include lightweight fixings, window and door frames, fixtures and insulation materials. Plastics also save energy in a variety of other applications and enhance the quality of many recreational activities, World Cup standard footballs and other equipment such as tennis, squash racquets and golf clubs use nylons, polyether ether ketones, PP and polymeric rubber compounds.
Plastics deliver unparalleled design versatility over a wide range of operating temperatures. They have a high strength-to-weight ratio, stiffness and toughness, ductility, corrosion resistance, bio-inertness, high thermal/electrical insulation, non-toxicity and outstanding durability at a relatively low lifetime cost compared with competing materials; hence plastics are very resource efficient. As reported by PlasticsEurope (2008), plastics can be made from any feedstock containing carbon and hydrogen. Currently, fossil fuels are the preferred feedstock, but plastics are also made from renewable resources such as sugar and corn. Around 4 per cent of global oil and gas production is used as the raw material for plastics production and a similar amount is used as energy in the process. However, plastics by their very nature store carbon, and this energy is retained by reusing and recycling plastics.
4. Plastic litter
In common with all consumer materials, disposal of plastic material contributes to the growth in municipal waste and additionally produces urban litter. Urban litter is increasing and contains large quantities of discarded materials, including thermoplastic products. Thermoplastics are not readily biodegradable in the environment, and plastic litter can persist for extended periods of time (Andrady 2003). The exact lifetime of the discarded plastics depends on the chemical nature of the material, characteristics of the environment in which it is placed and also on how ‘degradation’ is defined or measured, and it is therefore highly variable. Degradation can be by biological breakdown or by environmental breakdown (wind, rain, sunlight); with compostable plastics, this can occur within months but will take considerably longer for conventional plastics (Andrady 2003). In comparing plastics with other discarded materials such as lignocellulosic paper, plastics, because they are chemically resistant, are particularly persistent in the environment and it is this longevity that makes sourcing the origins of plastic litter very difficult. Littering, however, is a behavioural issue that needs to be addressed primarily through education. More attention and resources need to be devoted to increasing the awareness of consumers about the environmental consequences of litter, as this is the most effective solution. For example, in Singapore, a government scheme with large fines and corrective work orders has proved to be a very effective anti-littering measure.
A particularly disconcerting phenomenon is the quantity of plastic litter that enters the world's oceans, mostly from land-based sources (Thompson et al. 2005; for recent reviews, see Barnes et al. (2009); Gregory (2009); Ryan et al. (2009); Teuten et al. (2009)). This litter, estimated by the author at 0.2–0.3% and derived from the report of Greenpeace Allsopp et al. (2006)—Plastics Debris in the World's Oceans (2006) and plastics world production figures (PlasticsEurope 2006) over the last 10 years, is from tourists, sewerage overflows, landfill sites near coastlines, illegal dumping and accidental industrial spillages. The durability of plastics facilitates their use in a wide range of applications (discussed earlier) that also result in ecological concerns when this material ends up as litter. With fishing gear primarily made of plastics and fish packaging boxes and other accessories also made from plastics, it is not surprising that fishing-related debris also end up in the world's oceans.
5. Societal benefits of plastics
(a) Improved consumer health and safety
Plastics contribute to the health and safety of consumers in food and water packaging applications. Water has become a critical focus in urban areas, and plastics provide the mechanism for the supply and storage of clean drinking water. Additionally, plastics are lightweight, easy to manufacture and are installed in a range of diverse water control and distribution systems (e.g. sewerage, storm water, land drainage and irrigation). Plastic food packaging allows safe, time-dependent storage of fresh produce and other food, using temperature and atmosphere control inside the package (using gas-flush packaging and oxygen scavenger technology). In addition, the quality of packaged foods (especially time–temperature history) can be monitored with low-cost indicator labels built into the packaging (M. A. Neal 1990–1995, personal communication).
6. Energy savings
Using plastics in transportation building and even packaging applications invariably results in very significant savings in materials and in fossil fuel energy. For example, a comprehensive study published in January 2005, GUA (Gesellschaft für umfassende Analysen GmbH) established that packaging beverages in PET versus glass or metal reduces energy consumption by 52% (83.2 GJ yr−1 in Europe alone). Greenhouse gas emissions were reduced 55% on the same basis (4.3 million tonnes CO2 eq yr−1 in Europe). Use of lighter plastic composites in place of metal in the design of newer aircraft results in significant fuel cost savings as well as easier assembly. The new Boeing 787, for instance, will have a skin that is 100 per cent composite and an interior that is 50 per cent plastic composite, allowing it to deliver an expected 20 per cent savings in fuel costs. In the automotive sector, the replacement of metal components by plastic composites that weigh less than 50 per cent of the original contributes to significant energy savings. Aluminium can also be replaced with plastic components that are 50 per cent lighter at a 20–30% saving in cost. For example, the average plastic content of a light vehicle has increased to 110 kg or approximately 12 per cent of its weight (Gehm 2006).
In a recent study, the energy expenditure in manufacturing a disposable foamed polystyrene cup was found to be much lower than that for a ceramic cup or a disposable paper cup. When cleaning is factored in, in terms of energy use, it would take several hundred uses for a reusable ceramic cup to match that associated with a single-use expanded polystyrene cup (Hocking 2006). A similar study by the Dutch Research Institute TNO (2007) confirmed Hocking's findings.
7. Material conservation
Plastics have the advantage of a high strength-to-weight ratio, allowing minimal material usage (and low cost) in packaging design (figure 3b, which illustrates the use of plastics and glass packaging for the same product). On average, plastic packaging accounts for between 1 and 3 per cent of the total product weight. For instance, it takes 2 g of plastic film to package 200 g of cheese; 1.5 l of liquid can be safely stored in a 38 g bottle and a tub containing 125 g of yoghurt weighs only 4.5 g. The ecological balance sheet of plastic packaging, i.e. the sum total of the corresponding energy consumption for production, transport and disposal and other effects on the environment, is often superior to that of competing materials. For example, in one study, in switching from gable-top milk cartons (figure 3a) manufactured from a paper/aluminium/plastic composite to plastic pouches, the energy saving in production of the package was estimated to be 72 per cent, a 50 per cent saving in refrigeration space contributed to further energy savings and the waste stream to landfill was reduced by 90 per cent (API 1996).
Figure 3.
Material and energy savings can be made by replacing composites, such as gable top cartons (a), and glass containers (b, left) with plastic pouches (b, right).
The development of renewable energy resources is likely to rise as a consequence of increasing oil prices. Solar and wind power, geothermal heat and biomass are inexhaustible. Already, some regions in Europe are using renewable energy to meet most of their heating, hot water and electricity requirements, and Iceland exploits its geothermal energy. Plastics as a material can drive innovative designs to support this effort. For instance, modern solar water heaters containing plastics such as PE and PVC can provide up to 65 per cent of a household's annual hot water demand. Photovoltaic collectors that convert solar energy into electricity can cover the remaining energy requirements of a house. The use of these technologies would be impossible without plastics' light weight, mouldability, UV resistance and insulation properties.
Plastics capture around half of the carbon that is used to produce them, and this is a valuable resource. The properties of plastics make them inherently recyclable at several different levels (see the review by Hopewell et al. 2009). Multiple strategies of reuse of products, post-consumer recycling, resource recovery in the form of fuels or chemicals and energy recovery via incineration are all applicable to plastics waste, provided adequate waste management practices can be adopted. Recycling is clearly an energy-saving strategy; most primary recycling and the post-consumer recycling of high-value plastics make economic and environmental sense and greatest benefits are realized when recycling is viewed as a material conservation strategy. Mixed streams of plastics waste can be difficult to recycle. Here waste-to-energy via incineration allows the high heat value of the post-consumer plastics to be recaptured for use. The latter strategy is more advantageous than with most other packaging, as plastics have higher energy content than paper (table 3).
Table 3.
The recoverable energy content of plastics compared with other materials. Sources: Plastics, European Reference Life Cycle Data System (2007); paper, Erdincler & Vesiland (1993); wood, Oregon Department of Agriculture (2008); other materials, Breez (2005).
| polymer | available energy (MJ kg−1) |
|---|---|
| PP | 46 |
| PE (HD, LD etc.) | 46 |
| PS | 42 |
| PET | 25 |
| PVC | 18 |
| paper (mixed) | 16 |
| wood | 18 |
| sub-bituminous coal | 10–15 |
In most countries, as the available landfill space becomes limited, both materials recycling and resource or energy recovery will become increasingly attractive solid-waste management options. For example, in Korea, household waste material from everyday life and economic activities has decreased substantially, from 1.3 kg per person per day in 1994 to 1.04 kg in 2002, and the rate of overall material recycling exceeded that of landfilling for the first time in 2002.
According to industrial sources (PlasticsEurope 2008), in Europe, the collection of waste plastics for conventional recycling was 4.4 million tonnes in 2006, with approximately 12 per cent of this being traded (exported) with Asia. A plastic product with an exceptionally high level of recycling is the plastic bottle. Plastic bottles can be made of PET, PE, PP or PVC, and according to Petcore (2007), 40 per cent of all PET bottles available for collection were recycled in the EU in 2006. This amounts to 1.1 million tonnes yr−1.
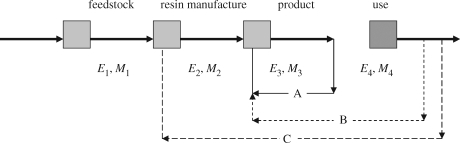
The versatility of the recycling approaches available for post-consumer plastics is summarized in figure 4. The horizontal sequence indicates the main steps in the product chain: feedstock acquisition, resin manufacture and fabrication and use of the product, each associated with an energy Ei. The reuse of manufacturing waste in the same product (regrind use) (A) is a routine cost-saving step presently practised in plastic product fabrication. Post-consumer waste can either be refabricated into other products (usually of lower value) (B) or chemically treated and/or heat-treated to form a feedstock (C). Each recycling strategy, however, has an energy EA, EB, EC associated with it; depending on the comparison of energy demand for recycling with Ei for the main steps in the production and consumption sequence, energy savings via recycling can be readily computed.
Figure 4.
Schematic of various modes of recycling available for plastics. Not shown in the figure is waste-to-energy conversion via incineration. Ei is the energy per unit mass associated with processing Mi units of material. Mi may not be the same for all values of i because of incidental losses.
8. Plastics and the future
As suggested by the futurist Hammond (2007) in his recent publication ‘The World in 2030’, the speed of technological development is accelerating exponentially and, for this reason, by the year 2030, it will seem as if a whole century's worth of progress has taken place in the first three decades of the twenty-first century. In many ways, life in 2030 will be unrecognizable compared with life today. During this time, plastics will play a significantly increased role in our lives. Plastics are already becoming ‘smart’ and will likely serve numerous important roles in future living, including human tissue or even organ transplants, key materials used in ultra-low-emission lightweight cars and aircraft, superior insulation for homes that run on photovoltaic technology based on plastic collectors, reusable electronic graphic media for books or magazines, smart packaging that monitors food content continuously for signs of spoilage and high-efficiency solid-state lighting based on plastic organic diode technology. As petroleum reserves become more limited, new varieties of plastics are likely to increasingly be made from renewable biomass. These will contribute to the already extensive array of mechanical and aesthetic performance properties that plastics are well known for. Any future scenario where plastics do not play an increasingly important role in human life therefore seems unrealistic (see also the discussions in Thompson et al. 2009a,b).
Endnote
One contribution of 15 to a Theme Issue ‘Plastics, the environment and human health’.
Separately in its fact sheet ‘Authorisation under REACH’ (2006) the Polycarbonate/Bisphenol A group of PlasticsEurope state: ‘According to the existing (2003) as well as the recent update (2007) of the European Risk Assessment, BPA is not carcinogenic, mutagenic or toxic for reproduction category 1 or 2, BPA is not very persistent or very bioaccumulative (REACH Terminology—vPvB), BPA is not eligible as a substance of equivalent concern according to the latest available scientific information as BPA does not fulfil the criteria of a substance having endocrine disrupting properties’.
References
- Andersen M. E., Clewell H. J., III, Tan Y.-M., Butenhoff J. L., Olsen G. W.2006Pharmacokinetic modelling of saturable, renal resorption of perfluoroalkylacids in monkeys—probing the determinants of long plasma half-lives. Toxicology 227, 156–164 [DOI] [PubMed] [Google Scholar]
- Andrady A. L.2003. In Plastics and the environment (ed. Andrady A. L.). West Sussex, England: John Wiley and Sons [Google Scholar]
- API 1996Understanding plastic film. American Plastics Council, December 1996. See http://www.americanchemistry.com/s_plastics/bin.asp?CID=1211&DID=4603&DOC=FILE.PDF
- Barnes D. K. A., Galgani F., Thompson R. C., Barlaz M.2009Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998 (doi:10.1098/rstb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breez P. Power generation technologies. 2005 Newnes, ISBN 0750663138. [Google Scholar]
- Canadean 2008Private correspondence—M Neal/Frances Hicks 22 May 2008, PET Market, Data available only from Canadean Head Office, UK [Google Scholar]
- Chemsec. 2008. REACH SIN List 1.0, 17th September 2008. See http://www.chemsec.org/list/
- Elliott B. M., Mackay J. M., Clay P., Ashby J. 1998An assessment of the genetic toxicology of antimony trioxide. Mutat. Res. 415, 109–117 [DOI] [PubMed] [Google Scholar]
- Erdincler A. U., Vesiland P. A.1993Energy recovery from mixed waste paper. Waste Manag. Res. 11, 507–513 [Google Scholar]
- European Commission's Joint Research Centre, Ispra, Italy. See http://ec.europa.eu/dgs/jrc/index.cfm .
- European Food Safety Authority 2006 Statement on Bisphenol A, November 2006. See http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1178620772817.htm .
- European Reference Life Cycle Data System. 2007. v. 1.0.1, See http://lca.jrc.ec.europa.eu/lcainfohub/datasetArea.vm .
- Gehm R.2006Plastic on the outside. Automotive Engineering International. August [Google Scholar]
- GUA Gesellschaft für umfassende Analysen GmbH 2005The Contribution of Plastic Products to Resource Efficiency Sechshauser Straße 83, A-1150 Vienna; pp 57–58
- Greenpeace Allsopp M., Walters A., Santillo D., Johnston P.2006Plastics debris in the worlds oceans. See http://oceans.greenpeace.org/raw/content/en/documents-reports/plastic_ocean_report.pdf
- Gregory M. R.2009Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Phil. Trans. R. Soc. B 364, 2013–2025 (doi:10.1098/rstb.2008.0265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. The world in 2030 Editions Yago. 2007 ISBN: 978-2-916209-18-0. See www.editions-yago.com . [Google Scholar]
- Hext P. M., Pinto P. J., Rimmel B. A.1999Subchronic feeding study of antimony trioxide in rats. J. Appl. Toxicol. 19, 205–209 [DOI] [PubMed] [Google Scholar]
- Hocking M. B.2006Reusable and disposable cups: an energy-based evaluation. Environ. Manag. 18, 889–899 [Google Scholar]
- Hopewell J., Dvorak R., Kosior E.2009Plastics recycling: challenges and opportunities. Phil. Trans. R. Soc. B 364, 2115–2126 (doi:10.1098/rstb.2008.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler D., Burkett S. L., Tarkanian M. J.1999Prehistoric polymers: rubber processing in ancient mesoamerica. Science 284, 1998–1991 (doi:10.1126/science.284.5422.1988) [DOI] [PubMed] [Google Scholar]
- Kennedy G. L., Jr, Butenhoff J. L., Olsen G. W., O'Connor J. C., Seacat A. M., Perkins R. G., Biegel L. B., Murphy S. R., Farrar D. G.2004The toxicology of perfluorooctanoate. Crit. Rev. Toxicol. 34, 351–384 [DOI] [PubMed] [Google Scholar]
- Koch H. M., Calafat A. M.2009Human body burdens of chemicals used in plastic manufacture. Phil. Trans. R. Soc. B 364, 2063–2078 (doi:10.1098/rstb.2008.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea H. H., Carlsona E. M., Chuaa J. P., Belcher S. M.2008Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 176, 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch B. S., Deyo J. A., Capen C. C., Nestmann E. R.1999Review of subchronic/chronic toxicity of antimony potassium tartrate. Regul. Toxicol. Pharmacol. 30, 9–17 [DOI] [PubMed] [Google Scholar]
- Meeker J. D., Sathyanarayana S., Swan S. H.2009Phthalates and other additives in plastics: human exposure and associated health outcomes. Phil. Trans. R. Soc. B 364, 2097–2113 (doi:10.1098/rstb.2008.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. J., Moore S. L., et al. 2002A comparison of neustonic plastic and zooplankton abundance in southern California's coastal waters. Mar. Pollut. Bull. 44, 1035–1038 [DOI] [PubMed] [Google Scholar]
- Mullan W. M. A.2002Science and technology of modified atmosphere packaging. See http://www.dairyscience.info/map-science.asp
- Mutha N., Patel M., Premnath V.2006Plastics materials flow analysis for India. API Resour. Conserv. Recycl. 47, 222–244 [Google Scholar]
- Oehlmann J., et al. 2009A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 364, 2047–2062 (doi:10.1098/rstb.2008.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORATS 2008Phthalate risk assessments, The Phthalates Information Centre Europe. See http://ecb.jrc.ec.europa.eu/esis/index.php?PGM=ora; http://www.phthalates.com/RAs
- Oregon Department of Agriculture 2008Firewood facts. See www.oregon.gov/ODA/MSD/fuel_facts.shtml#Firewood_measure
- Petcore 2007A PCI report for petcore, post consumer PET recycling in Europe 2006 and prospects to 2011, October 2007, available from PCI (PET Packaging Resin and Recycling) Ltd, UK [Google Scholar]
- PlasticsEurope 2008The compelling facts about plastics, analysis of plastics production, demand and recovery for 2006 in Europe, January 2008. Belgium: PlasticsEurope [Google Scholar]
- REACH. 2006 Regulation (EC) no. 1907/2006 of the European Parliament and of the Council, of 18 December 2006, concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) no. 793/93, and Commission Regulation (EC) no. 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. [Google Scholar]
- Ryan P. G., Moore C. J., van Franeker J. A., Moloney C. L.2009Monitoring the abundance of plastic debris in the marine environment. Phil. Trans. R. Soc. B 364, 1999–2012 (doi:10.1098/rstb.2008.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaxson L.2009Structuring policy problems for plastics, the environment and human health: reflections from the UK. Phil. Trans. R. Soc. B 364, 2141–2151 (doi:10.1098/rstb.2008.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsness C. E., Andrade A. J. M., Kuriyama S. N., Taylor J. A., vom Saal F. S.2009Components of plastic: experimental studies in animals and relevance for human health. Phil. Trans. R. Soc. B 364, 2079–2096 (doi:10.1098/rstb.2008.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuten E. L., et al. 2009Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 364, 2027–2045 (doi:10.1098/rstb.2008.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Moore C., Andrady A., Murray G., Hideshige T., Weisberg S.2005New directions in plastic debris. Science 310, 1117. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Swan S. H., Moore C. J., vom Saal F. S.2009aOur plastic age. Phil. Trans. R. Soc. B 364, 1973–1976 (doi:10.1098/rstb.2009.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Moore C. J., vom Saal F. S., Swan S. H.2009bPlastics, the environment and human health: current consensus and future trends. Phil. Trans. R. Soc. B 364, 2153–2166 (doi:10.1098/rstb.2009.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- TNO 2007Single-use versus re-usable (coffee) drinks systems: an environmental comparison. Report RO246/B, October 2007, TNO-Bouw en Ondergrond of Apeldoorn, The Netherlands [Google Scholar]
- Westerhoff P., Prapaipong P., Shock E., Hillaireau A.2008Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 42, 551–556 [DOI] [PubMed] [Google Scholar]
- World Health Organization 2003Antimony trioxide drinking water opinion. See http://www.who.int/water_sanitation_health/dwq/chemicals/antimony.pdf.