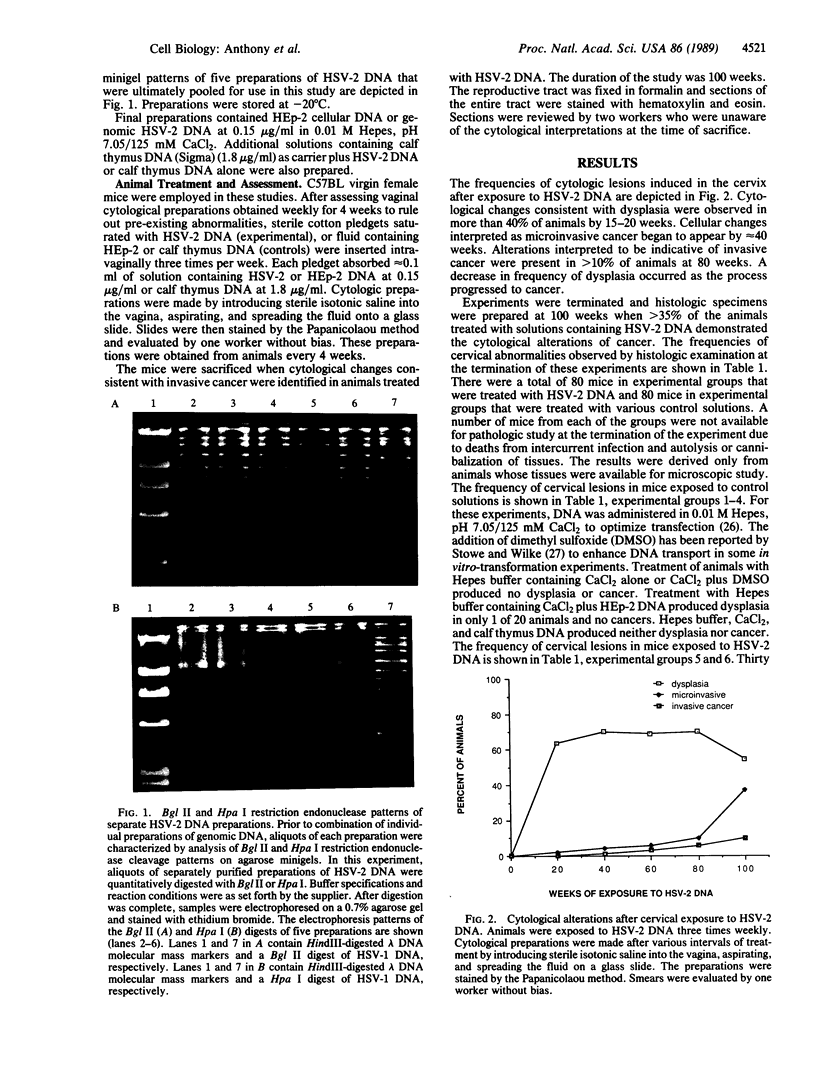
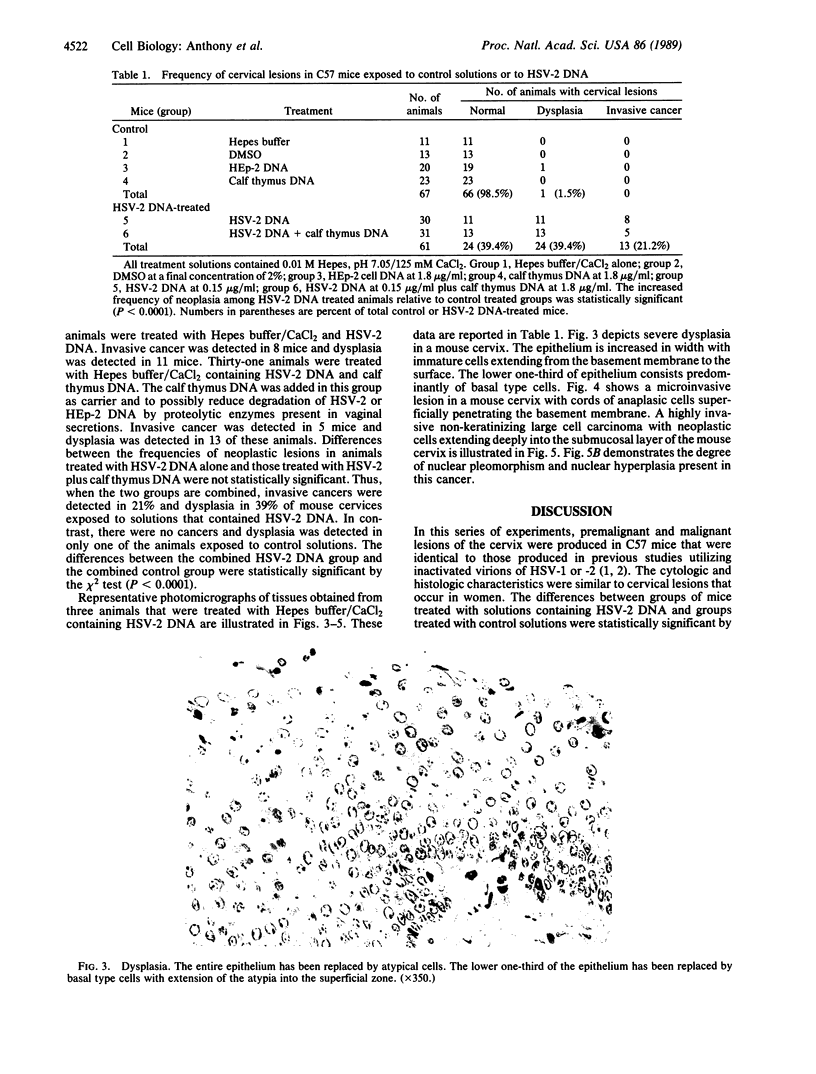
Abstract
Induction of cervical neoplasia in the mouse cervix by herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) has been reported. The present study was done to determine if transfection with DNA of HSV-2 can induce carcinogenesis in this animal model. Genomic HSV-2 DNA was isolated from infected HEp-2 cells and separated from host cell DNA by cesium chloride density gradient centrifugation. The DNA was applied to mouse cervix for periods of 80-100 weeks. Experimental controls were treated with uninfected genomic HEp-2 cell DNA or with calf thymus DNA. Vaginal cytological preparations from all animals were examined monthly to detect epithelial abnormalities. Animals were sacrificed and histopathology studies were done when cellular changes indicative of premalignant or malignant lesions were seen on vaginal smears. Cytologic and histologic materials were coded and evaluated without knowledge of whether they were from animals treated with virus or control DNA. Premalignant and malignant cervical lesions similar to those that occur in women were detected in 61% of the histologic specimens obtained from animals exposed to HSV-2 DNA. The yield of invasive cancers was 21% in animals treated with HSV-2 DNA. No cancers were detected in mice treated with either HEp-2 or calf thymus DNA. Dysplasia was detected in only one of these control animals.
Full text
PDF
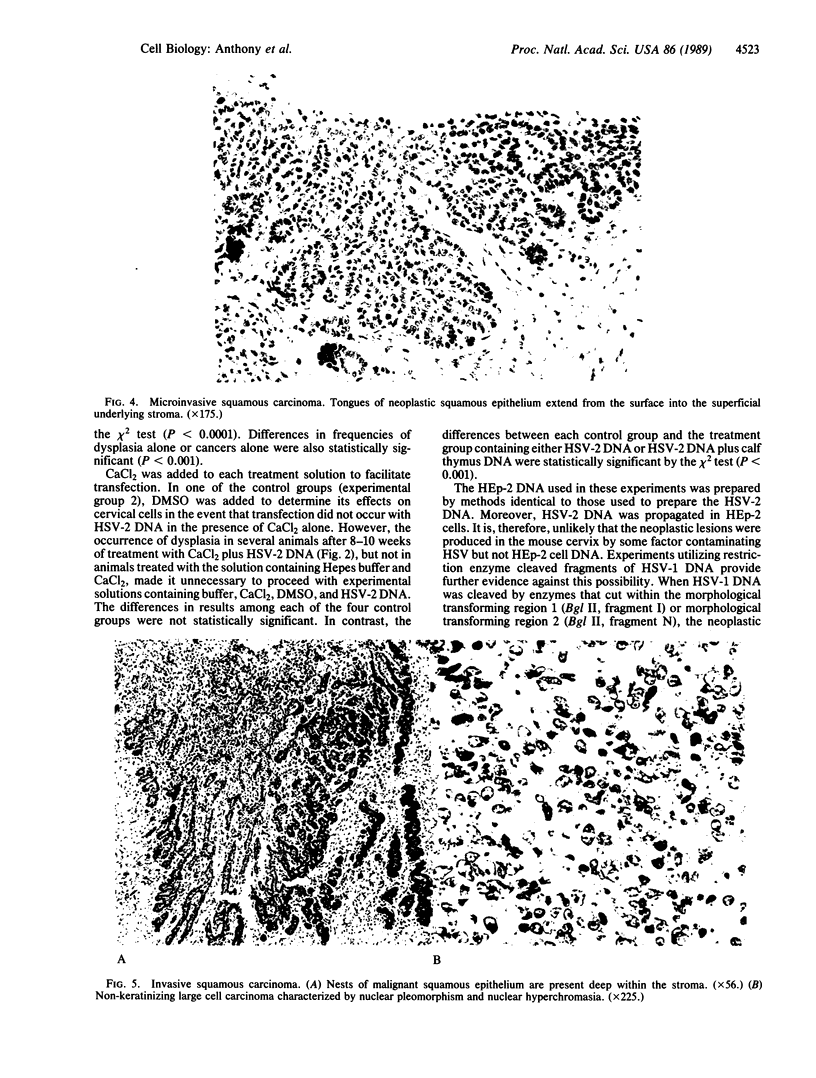

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselin C., Gélinas C., Branton P. E., Bastin M. Polyoma middle T antigen requires cooperation from another gene to express the malignant phenotype in vivo. Mol Cell Biol. 1984 Apr;4(4):755–760. doi: 10.1128/mcb.4.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenisty N., Reshef L. Direct introduction of genes into rats and expression of the genes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9551–9555. doi: 10.1073/pnas.83.24.9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A., Spear G. Transformation of hamster embryo fibroblasts by a specific fragment of the herpes simplex virus genome. Cell. 1978 Nov;15(3):993–1002. doi: 10.1016/0092-8674(78)90283-0. [DOI] [PubMed] [Google Scholar]
- Cameron I. R., Park M., Dutia B. M., Orr A., Macnab J. C. Herpes simplex virus sequences involved in the initiation of oncogenic morphological transformation of rat cells are not required for maintenance of the transformed state. J Gen Virol. 1985 Mar;66(Pt 3):517–527. doi: 10.1099/0022-1317-66-3-517. [DOI] [PubMed] [Google Scholar]
- Chan H. W., Israel M. A., Garon C. F., Rowe W. P., Martin M. A. Molecular cloning of polyoma virus DNA in Escherichia coli: lambda phage vector system. Science. 1979 Mar 2;203(4383):887–892. doi: 10.1126/science.217088. [DOI] [PubMed] [Google Scholar]
- Chen M. H., Dong C. Y., Liu Z. H., Skinner G. R., Hartley C. E. Prevention of type 2 herpes simplex virus induced cervical carcinoma in mice by prior immunization with a vaccine prepared from type 1 herpes simplex virus. Vaccine. 1983 Dec;1(1):13–16. doi: 10.1016/0264-410x(83)90006-3. [DOI] [PubMed] [Google Scholar]
- Dubensky T. W., Campbell B. A., Villarreal L. P. Direct transfection of viral and plasmid DNA into the liver or spleen of mice. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7529–7533. doi: 10.1073/pnas.81.23.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Daniel M. D., Hunt R. D., Werner J., Falk L. A., Mulder C. Tumour induction with DNA of oncogenic primate herpesviruses. Nature. 1978 Jul 6;274(5666):57–59. doi: 10.1038/274057a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Crittenden L. B., Fadly A. M., Kung H. J. Tumor induction by direct injection of cloned v-src DNA into chickens. Proc Natl Acad Sci U S A. 1983 Jan;80(2):353–357. doi: 10.1073/pnas.80.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Copple C. D., McDougall J. K. Analysis of viral DNA sequences in hamster cells transformed by herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1980 Feb;77(2):880–884. doi: 10.1073/pnas.77.2.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. Transformation of rodent cells by a cloned DNA fragment of herpes simplex virus type 2. J Virol. 1981 May;38(2):749–760. doi: 10.1128/jvi.38.2.749-760.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Veldhuisen G., Wilkie N. M. Infectious herpesvirus DNA. Nat New Biol. 1973 Oct 31;245(148):265–266. doi: 10.1038/newbio245265a0. [DOI] [PubMed] [Google Scholar]
- Inagaki Y., Tsunokawa Y., Takebe N., Nawa H., Nakanishi S., Terada M., Sugimura T. Nucleotide sequences of cDNAs for human papillomavirus type 18 transcripts in HeLa cells. J Virol. 1988 May;62(5):1640–1646. doi: 10.1128/jvi.62.5.1640-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Immortalization and neoplastic transformation of normal diploid cells by defined cloned DNA fragments of herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5902–5906. doi: 10.1073/pnas.80.19.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Neoplastic transformation of cultured Syrian hamster embryo cells by DNA of herpes simplex virus type 2. J Virol. 1979 Apr;30(1):404–409. doi: 10.1128/jvi.30.1.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy I. M., Simpson S., Macnab J. C., Clements J. B. Human papillomavirus type 16 DNA from a vulvar carcinoma in situ is present as head-to-tail dimeric episomes with a deletion in the non-coding region. J Gen Virol. 1987 Feb;68(Pt 2):451–462. doi: 10.1099/0022-1317-68-2-451. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Herpes simplex virus and human cytomegalovirus: their role in morphological transformation and genital cancers. J Gen Virol. 1987 Oct;68(Pt 10):2525–2550. doi: 10.1099/0022-1317-68-10-2525. [DOI] [PubMed] [Google Scholar]
- Macnab J. C., Walkinshaw S. A., Cordiner J. W., Clements J. B. Human papillomavirus in clinically and histologically normal tissue of patients with genital cancer. N Engl J Med. 1986 Oct 23;315(17):1052–1058. doi: 10.1056/NEJM198610233151703. [DOI] [PubMed] [Google Scholar]
- Maitland N. J., Kinross J. H., Busuttil A., Ludgate S. M., Smart G. E., Jones K. W. The detection of DNA tumour virus-specific RNA sequences in abnormal human cervical biopsies by in situ hybridization. J Gen Virol. 1981 Jul;55(Pt 1):123–137. doi: 10.1099/0022-1317-55-1-123. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Cassai E., Deiss L. P., Di Luca D., Segala V., Frenkel N. Sequences homologous to two separate transforming regions of herpes simplex virus DNA are linked in two human genital tumors. Virology. 1986 Nov;155(1):192–201. doi: 10.1016/0042-6822(86)90179-0. [DOI] [PubMed] [Google Scholar]
- McDougall J. K., Galloway D. A., Fenoglio C. M. Cervical carcinoma: detection of herpes simplex virus RNA in cells undergoing neoplastic change. Int J Cancer. 1980 Jan 15;25(1):1–8. doi: 10.1002/ijc.2910250102. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Clements J. B. DNA sequence homology between two co-linear loci on the HSV genome which have different transforming abilities. EMBO J. 1983;2(11):1953–1961. doi: 10.1002/j.1460-2075.1983.tb01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R., Jastreboff M. M., Chiu C. F., Bertino J. R. In vivo expression of a nonselected gene transferred into murine hematopoietic stem cells by electroporation. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1018–1024. doi: 10.1016/s0006-291x(86)80146-2. [DOI] [PubMed] [Google Scholar]
- Nicolau C., Le Pape A., Soriano P., Fargette F., Juhel M. F. In vivo expression of rat insulin after intravenous administration of the liposome-entrapped gene for rat insulin I. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1068–1072. doi: 10.1073/pnas.80.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Transgenic mice. Cell. 1985 Jun;41(2):343–345. doi: 10.1016/s0092-8674(85)80004-0. [DOI] [PubMed] [Google Scholar]
- Park M., Kitchener H. C., Macnab J. C. Detection of herpes simplex virus type-2 DNA restriction fragments in human cervical carcinoma tissue. EMBO J. 1983;2(7):1029–1034. doi: 10.1002/j.1460-2075.1983.tb01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Prakash S. S., Reeves W. C., Sisson G. R., Brenes M., Godoy J., Bacchetti S., de Britton R. C., Rawls W. E. Herpes simplex virus type 2 and human papillomavirus type 16 in cervicitis, dysplasia and invasive cervical carcinoma. Int J Cancer. 1985 Jan 15;35(1):51–57. doi: 10.1002/ijc.2910350109. [DOI] [PubMed] [Google Scholar]
- Reyes G. R., LaFemina R., Hayward S. D., Hayward G. S. Morphological transformation by DNA fragments of human herpesviruses: evidence for two distinct transforming regions in herpes simplex virus types 1 and 2 and lack of correlation with biochemical transfer of the thymidine kinase gene. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):629–641. doi: 10.1101/sqb.1980.044.01.066. [DOI] [PubMed] [Google Scholar]
- Rotola A., Di Luca D., Monini P., Manservigi R., Tognon M., Virgili A., Segala V., Trapella G., Cassai E. Search for HSV DNA in genital, cerebral and labial tumors. Eur J Cancer Clin Oncol. 1986 Oct;22(10):1259–1265. doi: 10.1016/0277-5379(86)90329-9. [DOI] [PubMed] [Google Scholar]
- Skinner G. R. Transformation of primary hamster embryo fibroblasts by type 2 simplex virus: evidence for a "hit and run" mechanism. Br J Exp Pathol. 1976 Aug;57(4):361–376. [PMC free article] [PubMed] [Google Scholar]
- Sokoloski J. A., Jastreboff M. M., Bertino J. R., Sartorelli A. C., Narayanan R. Introduction of deoxyribonucleoside triphosphates into intact cells by electroporation. Anal Biochem. 1986 Nov 1;158(2):272–277. doi: 10.1016/0003-2697(86)90549-x. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Wentz W. B., Heggie A. D., Anthony D. D., Reagan J. W. Effect of prior immunization on induction of cervial cancer in mice by herpes simplex virus type 2. Science. 1983 Dec 9;222(4628):1128–1129. doi: 10.1126/science.6316503. [DOI] [PubMed] [Google Scholar]
- Wentz W. B., Reagan J. W., Heggie A. D., Fu Y. S., Anthony D. D. Induction of uterine cancer with inactivated herpes simplex virus, types 1 and 2. Cancer. 1981 Oct 15;48(8):1783–1790. doi: 10.1002/1097-0142(19811015)48:8<1783::aid-cncr2820480815>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Vienken J. Electric field-induced cell-to-cell fusion. J Membr Biol. 1982;67(3):165–182. doi: 10.1007/BF01868659. [DOI] [PubMed] [Google Scholar]