Abstract
It has previously been demonstrated that damaged arterial tissue can be acutely modified with protein-reactive polyethylene glycol (PEG) to block undesirable platelet deposition. This concept might be expanded by employing PEG-biotin and its strong interaction with avidin for site-specific targeted delivery. Toward this end, cultured endothelial cells (ECs) were surface modified with PEG-biotin and the available biotin was quantified with flow cytometry. NeutrAvidin-coated microspheres and PEG-biotin modified ECs with NeutrAvidin as a bridging molecule were delivered under arterial shear stress to PEG-biotin modified ECs on a coverslip as well as scrape-damaged bovine carotid arteries. After incubation with a 10 mM solution for 1 min, 8 × 107 PEG-biotin molecules/EC were found and persisted for up to 120 h. Perfused microspheres adhered to NHS-PEG-biotin treated bovine carotid arteries with 60 ± 16 microspheres/mm2 versus 11 ± 4 microspheres/mm2 for control arteries (p < 0.015). Similarly, 22 ± 5 targeted ECs/mm2 adhered to NHS-PEG-biotin treated bovine carotid arteries versus 6 ± 2 ECs/mm2 for control arteries (p < 0.01). The targeting strategy demonstrated here might ultimately find application for drug delivery, gene therapy, or cell therapy where localization to specific labeled vascular regions is desired following catheter-based or surgical procedures.
Keywords: polyethylene glycol, targeted delivery, surface modification, biotin, avidin
INTRODUCTION
Polyethylene glycol (PEG) has been widely applied to modify surfaces or therapeutic proteins for biomedical applications. Attaching PEG molecules to peptides or proteins, commonly referred to as PEGylation, can provide a number of beneficial effects such as increased solubility, enhanced bioactivity, reduced immunogenicity, and improved pharmacokinetics.1-3 Similarly, PEG modification of liposomes and microspheres inhibits nonspecific protein interactions and increases particle circulation time by slowing clearance from the blood.4,5 PEG modification of synthetic surfaces is another common application where PEGylation can decrease protein adsorption and undesirable cell adhesion.6-10 This approach has more recently been extended to tissue and cell surfaces to inhibit acute thrombosis and attenuate immune recognition.11-16
In the current study, N-hydroxysuccinimide-polyethylene glycol-biotin (NHS-PEG-biotin) was employed to covalently modify cellular and vascular surfaces. The active NHS ester of PEG reacts with primary amines, the most common being the epsilon amine of lysine, which can covalently link the PEG-biotin molecule to proteins found on cell membranes or extracellular matrix [Fig. 1(A)].17,18 This reaction can occur rapidly, within one minute, at physiologic temperature and pH.19 As a result, labeling of tissue surfaces with protein-reactive PEG could prove invaluable in time-sensitive applications such as coronary procedures. Modification of vascular surfaces with protein-reactive PEG would be expected to provide a molecular barrier to prevent platelet and leukocyte adhesion [Fig. 1(B)] as demonstrated previously.11,12 However, the addition of biotin to the terminus of the reactive PEG should provide a site for the targeted delivery of agents [Fig. 1(B)] using the high affinity between biotin and avidin (Ka = 1015 M−1), which has been employed in a wide variety of detection, purification, and targeted delivery systems.17,20-22 Specifically, the strong interaction between biotin and avidin has been employed for the targeted delivery of therapeutics in the treatment of malignant neoplasms.20
Figure 1.
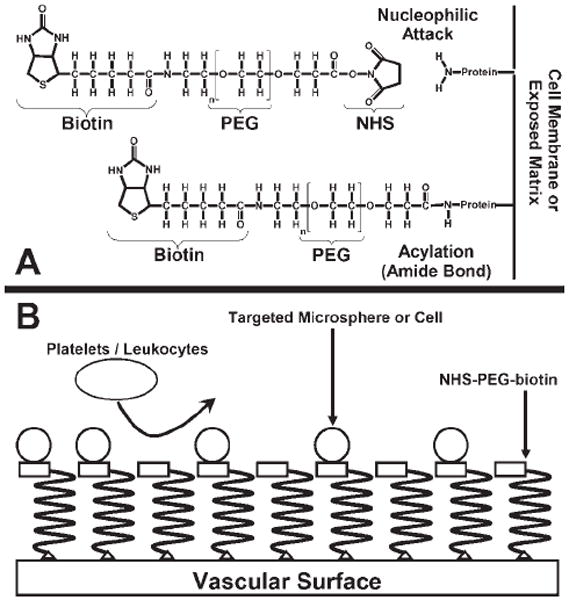
Illustration of the modification reaction for the protein-reactive polymer and schematic of the proposed targeted delivery system. A: Formation of the covalent bond between the protein-reactive polymer and the exposed amine on the vascular surface. B: The reactive polymer is covalently attached to the vascular surface forming a molecular barrier, inhibiting platelet and leukocyte adhesion. Targeted microspheres or cells will specifically adhere to vascular surfaces labeled with the polymer.
In the system under investigation, we utilized NeutrAvidin instead of avidin. NeutrAvidin is a deglycosylated form of avidin with similar binding characteristics and lower nonspecific binding. NeutrAvidin-coated polystyrene microspheres were targeted to PEG-biotin modified vascular surfaces with the microspheres serving as a model for the delivery of particulate drugs, liposomes, or vesicles. Targeting cells was accomplished in a similar manner, but utilizing a two-step process where both cells and the vascular surface were modified with NHS-PEG-biotin. Bridging of the two biotinylated surfaces was accomplished by briefly incubating the biotinylated vascular surfaces with NeutrAvidin. Biotin and avidin mediated binding has previously been shown to enhance endothelial cell (EC) attachment, spreading, and a quiescent phenotype on synthetic surfaces.23-26 These attributes may assist in targeting cells to vascular surfaces exploiting the strong interaction between biotin and avidin. Modification of vascular surfaces with protein-reactive PEG-biotin might thus provide a target for the site-specific delivery of microspheres and cells under physiologic flow conditions following intravascular procedures such as angioplasty or end-arterectomy.
MATERIALS AND METHODS
Cell culture
Human coronary artery endothelial cells (HCAECs) (Cambrex, Walkersville, MD) were cultured in BD Falcon 75 cm2 tissue culture flasks and 12 well tissue culture plates (Fisher Scientific, Pittsburgh, PA). In addition, HCAECs were cultured on 24 × 50 mm Gold Seal cover glasses in Fisherbrand 100 × 15 mm I-Plate compartmentalized petri dishes (Fisher Scientific, Pittsburgh, PA). ECs were grown in EGM-2 BulletKit medium composed of EBM-2 basal medium and SingleQuots with 5% FBS (Cambrex, Walkersville, MD).
Surface modification of ECs
Dry NHS-PEG-biotin (Nektar Therapeutics, San Carlos, CA) at −80°C was warmed to room temperature and phosphate buffered saline (PBS) was added to make 10 mM stock solutions. Aliquots of the stock solution were further diluted with PBS to make 5, 2.5, 1.25, and 0.625 mM solutions of the reactive polymer. Next, culture media on HCAECs in six wells of a 12 well plate were removed and replaced with 0.5 mL of 0, 0.625, 1.25, 2.5, 5, or 10 mM NHS-PEG-biotin solutions. The reactive polymer solutions were incubated on the cells for 1 or 5 min. After the desired incubation time, the reactive solutions were removed from the wells and the cells were washed three times with Dulbecco’s Modified Eagle’s Medium (DMEM) (Cambrex, Walkersville, MD). To measure PEG-biotin persistence on HCAECs, cells were incubated with 0.5 mL of the 10 mM NHS-PEG-biotin solution for 1 min. After this time, the solution was removed and the cells washed three times with DMEM. Cells were collected for flow cytometric analysis at time points of 0.5, 2, 4, 6, 9, 12, 24, 48, 72, 96, and 120 h with untreated HCAECs serving as a control.
Flow cytometric analysis of surface modification
Modified HCAECs were removed from the tissue culture plate with ReagentPack containing trypsin (Cambrex, Walkersville, MD) and individual samples collected in correspondingly labeled 16 × 75 mm round bottom polystyrene tubes (Fisher Scientific, Pittsburgh, PA). The tubes were centrifuged at 220g for 5 min and the supernatants removed. A 15 μL aliquot of a 5 mg/mL solution of fluorescein conjugated NeutrAvidin (Pierce Biotechnology, Rockford, IL) was diluted 1:40 with PBS and 100 μL of the solution added to each tube. Cell pellets were then resuspended in the tube by vortexing and allowed to incubate for 30 min at 4°C. After 30 min, the tubes were centrifuged at 220g for 5 min and the supernatants removed.
Cell pellets were resuspended and fixed with 500 μL of a 1% paraformaldehyde solution (Sigma, St. Louis, MO). Quantum FITC MESF High Level flow cytometry standard beads (Bangs Laboratories, Fishers, IN) were added to 500 μL of a 1% paraformaldehyde solution in another tube to allow for quantitative fluorescence determinations of the cell samples.27 The intensity of the fluorescein conjugated NeutrAvidin signal for the cells modified with the varying PEG-biotin solution concentrations were gathered on a BD FACScan (San Jose, CA). The fluorescence intensity of the Quantum FITC MESF High Level beads was similarly collected to produce a calibration curve (R2 = 0.995–0.999) and then determine the number of PEG-biotin molecules on the cell surface.27
Targeting microspheres and cells in a parallel plate chamber
HCAECs cultured on 24 × 50 mm Gold Seal cover glasses in Fisherbrand 100 × 15 mm I-Plate compartmentalized petri dishes were labeled with a 5 μM CellTracker Orange CMTMR (Molecular Probes, Eugene, OR) solution in EGM-2 media for 45 min in an incubator at 37°C. After the incubation, the CellTracker Orange CMTMR solution was replaced with fresh EGM-2 media. Bovine serum albumin (BSA) (Sigma, St. Louis, MO) at 4.5 g/dL was added to DMEM and warmed to 37°C for the perfusion media.
The coverslip was loaded into a rectangular, parallel plate perfusion chamber to allow assessment of microsphere binding under physiologic flow conditions.28 A silicon gasket and vacuum system secured the coverslip in the chamber creating an air-tight seal. The gap between the coverslip and top of the chamber was 200 μm and 2.65 cm2 of the coverslip area was exposed to the perfusion media. Once the coverslip was loaded into the perfusion chamber, the system was first primed with media using a Harvard PHD 22/200 Infuse/Withdraw Syringe Pump (Harvard Apparatus, Holliston, MA) and then a 1.5 mL solution of 10 mM NHS-PEG-biotin or vehicle (PBS) was injected into the chamber through a stopcock and incubated for 1 min. After the incubation, the coverslip was perfused with media for 10 min.
A 4 μL aliquot of yellow-green fluorescent FluoSpheres NeutrAvidin labeled microspheres (Molecular Probes, Eugene, OR) was mixed with 200 μL of BlockAid Blocking Solution (Molecular Probes, Eugene, OR) and sonicated for 5 min to reduce nonspecific binding. The microsphere suspension was then further diluted to 10 mL with perfusate media to create a 7.28 × 106 microspheres/mL suspension that was drawn through the chamber at a controlled wall shear rate of 100 s−1 for 10 min followed immediately by another 10 min flush with the media alone. The number of adhered microspheres on the coverslip surface was counted following this perfusion using epi-fluorescence microscopy.
Similarly, HCAECs cultured on 24 × 50 mm Gold Seal cover glasses in Fisherbrand 100 × 15 mm I-Plate compartmentalized petri dishes were labeled with a 50 μM CellTracker Blue CMAC (Molecular Probes, Eugene, OR) solution in EGM-2 media for 45 min in the incubator. HCAECs grown in a BD Falcon 75 cm2 tissue culture flask were also labeled with a 5 μM CellTracker Orange CMTMR solution in EGM-2 media for 45 min in the incubator. After the incubation, the CellTracker Blue CMAC and CellTracker Orange CMTMR solutions were replaced with fresh EGM-2 media.
Cells grown in the BD Falcon 75 cm2 flask were removed from the flask using ReagentPack with trypsin and collected in a BD Falcon BlueMax Jr. 15 mL Graduated tube (Fisher Scientific, Pittsburgh, PA). The tube was centrifuged at 220g for 5 min and the supernatant removed. The cell pellet was dispersed and modified with 1 mL of a 10 mM NHS-PEG-biotin solution for 1 min. After modification, 9 mL of PBS was added to the cell suspension and the tube vortexed. Again, the cell suspension was centrifuged at 220g for 5 min and the supernatant removed. Next, the cells were resuspended in 10 mL of perfusate media and kept at 37°C in a Fisher Isotemp Economy water bath (Fisher Scientific, Pittsburgh, PA).
The same perfusate media, as was used for microsphere targeting, perfusion system, and wall shear rate was employed for the cell targeting experiments. Once the coverslip was loaded into the perfusion chamber, the system was primed with media using the syringe pump. Next, a 1.5 mL solution of 10 mM NHS-PEG-biotin or the vehicle (PBS) control was injected into the chamber through a stopcock and incubated for 1 min. After the incubation, the coverslip was perfused with media for 10 min and then 1.5 mL of a 2 mg/mL solution of NeutrAvidin Biotin-Binding Protein (Pierce Biotechnology, Rockford, IL) or vehicle (PBS) was injected into the chamber through a stopcock for another 1 min incubation. The coverslip was then perfused with media for 10 min followed by perfusion of the PEG-biotin modified cell suspension for 10 min and an immediate 10 min wash with the media alone. Finally, the number of adherent cells on the coverslip surface was counted using epi-fluorescence microscopy.
Targeting microspheres and cells to damaged arteries ex vivo
Fresh bovine carotid arteries were acquired and placed in a 90 mL specimen cup filled with PBS. Excess fat and tissue were removed from the outer surface of the artery and it was fileted along its length to expose the vessel lumen, which was damaged by scraping with a weighing spatula three times. Vessel segments measuring 2.5 cm in length were cut for use in a tubular perfusion chamber previously described for ex vivo perfusions.29 The upper face of the tubular perfusion chamber had a cylindrical conduit with a window machined into the lower portion of the conduit to allow for introduction of test surfaces. The lower face of the tubular perfusion chamber possessed a raised surface, which was used to press and secure the sample into the window with clamps. Media was drawn through the chamber at a controlled wall shear rate of 100 s−1.
For the microsphere targeting experiments, the scrape-damaged bovine carotid artery was loaded into the tubular perfusion chamber and the system was primed with media using the syringe pump. Next, a 1.5 mL solution of 10 mM NHS-PEG-biotin or vehicle (PBS) was injected into the chamber through a stopcock and incubated for 1 min. After the incubation, the artery was perfused with media for 10 min. A suspension of yellow-green FluoSpheres with NeutrAvidin labeling (7.28 × 106 microspheres/mL) was drawn through the chamber at the specified wall shear rate for 10 min followed immediately by another 10 min wash with the media alone. Finally, the number of bound microspheres on the damaged vessel surface was counted by inverting the vessel segment on a coverslip and utilizing epi-fluorescence microscopy.
For the cell targeting experiments, HCAECs grown in 75cm2 tissue culture flasks were labeled with CellTracker Orange CMTMR, harvested, and modified with NHS-PEG-biotin as described previously. The scrape-damaged bovine carotid artery was loaded into the tubular perfusion chamber and the system was primed with media using the syringe pump. Next, a 1.5 mL solution of 10 mM NHS-PEG-biotin or the vehicle (PBS) control was injected into the chamber through a stopcock and incubated for 1 min. After which, the artery was perfused with media for 10 min. Then, 1.5 mL of a 2 mg/mL NeutrAvidin solution or PBS vehicle was injected into the chamber through a stopcock for another 1 min incubation. After the incubation, the artery was perfused with media for 10 min. The PEG-biotin modified HCAEC suspension was then drawn through the chamber at a wall shear rate of 100 s−1 for 10 min followed immediately by another 10 min wash with the media alone. Finally, the number of adherent cells on the damaged vessel surface was determined using epi-fluorescence microscopy.
Statistical analysis
Data are presented as the mean ± SEM. The number of PEG-biotin molecules per EC as a function of solution concentration and incubation time was compared using a Two-Factor ANOVA for equal sample sizes with p < 0.05 considered statistically significant. Control versus treated group comparisons for microsphere and cell targeting experiments were made using a Student’s t-test with p < 0.05 being considered statistically significant.
RESULTS
Surface modification of ECs
The number of PEG-biotin molecules per EC determined from quantitative flow cytometry is shown as a function of reactive PEG solution concentration and incubation time in Figure 2. A maximum of 110 million PEG-biotin molecules per cell were observed with incubation in a 10 mM solution for 5 min. At an incubation time of 1 min, it was possible to bind ~80 million PEG-biotin molecules per EC. The number of PEG-biotin molecules per cell for each solution concentration tested was always greater after a 5 min incubation when compared to 1 min (p < 0.001). At the higher solution concentrations, with a 5 min incubation time, the number of PEG-biotin molecules per cell begins to plateau. Longer incubation periods were not evaluated due to their limited applicability, especially in coronary procedures. Also, demonstrated in Figure 2 is the covalent nature of the PEG attachment. If the N-hydroxysuccinimide active ester was removed by hydrolysis, no HCAEC modification was observed at 1 or 5 min. The duration of the PEG-biotin modification on the HCAEC surface at time points up to 120 h post-modification is shown in Figure 3. The number of PEG-biotin molecules decreases sharply over the first 24 h post-modification followed by a gradual decrease in the number of remaining molecules for the 120 h study duration. Over half (56%) of the initial number of PEG-biotin molecules per cell were lost in the first 12 h after modification with the reactive polymer. Moreover, just over 20% of the initial PEG-biotin modification persisted at 24 h post-modification. Finally, by the 120 h time point less than 1% of the initial number of molecules remained.
Figure 2.
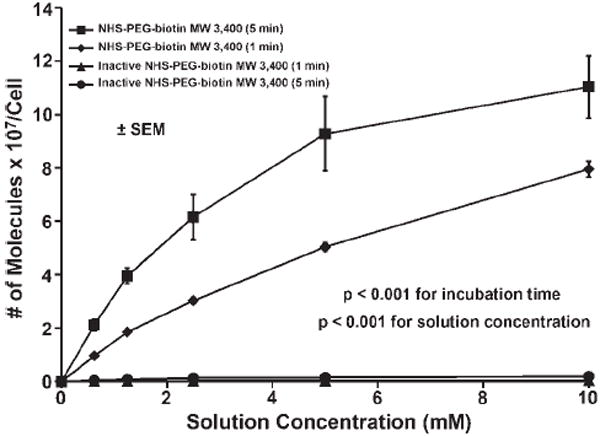
The number of PEG-biotin molecules reacted onto endothelial cell membranes as a function of solution concentration and incubation time. Cells were reacted with NHS-PEG-biotin solutions of 0, 0.625, 1.25, 2.5, 5, and 10 mM and incubation times of 1 or 5 min. The number of PEG-biotin molecules was determined using quantitative flow cytometry. The results are shown ± SEM and are significant with p < 0.001 for the different solution concentrations and incubation times.
Figure 3.
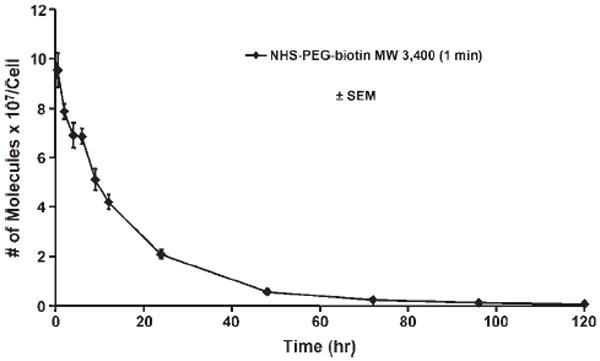
Persistence of PEG-biotin molecules on the cell surface over time. Cells were incubated with a 10 mM solution of NHS-PEG-biotin for 1 min. Samples were taken at 0.5, 2, 4, 6, 12, 24, 48, 72, 96, and 120 h. The number of PEG-biotin molecules was determined using quantitative flow cytometry and the results are shown ± SEM.
Targeting microspheres and cells to cultured HCAECs
For microsphere targeting experiments, the cultured cells were labeled with CellTracker Orange CMTMR, which appear red, and targeted NeutrAvidin-coated microspheres appear green in Figure 4(A,B). The number of microspheres bound to PBS treated coverslips (10 ± 2 per mm2) was significantly less (p < 0.001) than the number bound to NHS-PEG-biotin treated coverslips (115 ± 9 per mm2) as demonstrated in Figure 4(C).
Figure 4.
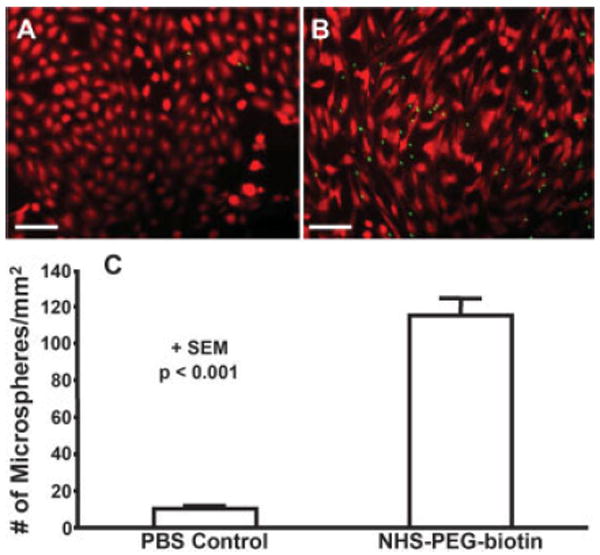
Targeted microspheres adhering to control and treated cells cultured on coverslips in a parallel plate chamber. A: Cultured cells (red) on a coverslip treated with the vehicle (PBS) and then perfused with targeted microspheres (green). B: Cells (red) cultured on a coverslip treated with 10 mM NHS-PEG-biotin and then perfused with targeted microspheres (green). Scale bar is 100 μm. C: Number of adherent microspheres per mm2 on control and PEG-biotin treated coverslips. Data is shown + SEM and the results are significant with p < 0.001. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In the targeted cell delivery experiments, the cultured HCAECs on the coverslip were labeled with CellTracker Blue CMAC and appear this color in Figure 5(A,B); whereas, the targeted HCAECs that deposited from flow were labeled with CellTracker Orange CMTMR and appear red. The number of targeted cells bound to NHS-PEG-biotin treated coverslips (20 ± 5 cells per mm2) was significantly greater (p < 0.005) than the number bound to PBS treated coverslips (1 ± 0.3 cells per mm2) by ~20-fold [Fig. 5(C)].
Figure 5.
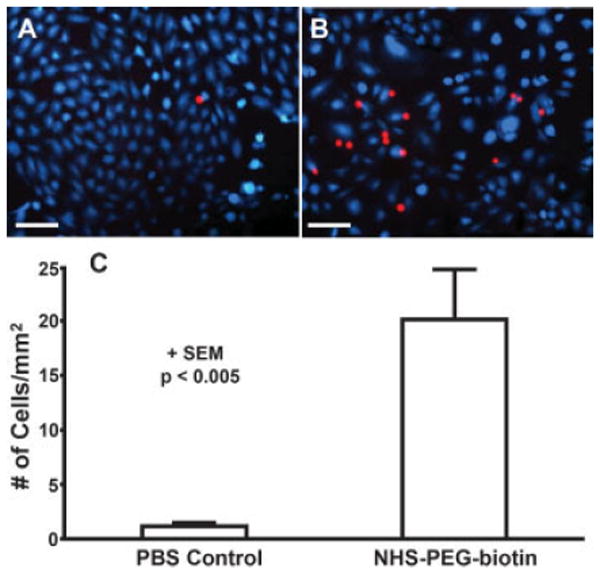
Targeted cells adhering to control and treated cells cultured on coverslips in a parallel plate chamber. A: Cultured cells (blue) on a coverslip treated with the vehicle (PBS) and then perfused with targeted cells (red). B: Cells (blue) cultured on a coverslip treated with 10 mM NHS-PEG-biotin, 2 mg/mL NeutrAvidin, and then perfused with targeted cells (red). Scale bar is 100 μm. C: Number of adherent cells per mm2 on control and PEG-biotin treated coverslips. Data is shown + SEM and the results are significant with p < 0.005. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Targeting of microspheres and cells to damaged arteries ex vivo
In the images of microsphere adherence to scrape-damaged bovine carotid artery segments, the arteries exhibited background auto-fluorescence in green [Fig. 6(A,B)]. Similarly, targeted NeutrAvidin-coated microspheres were yellow-green fluorescent; however, the fluorescence intensity of the microspheres was identifiable over the background auto-fluorescence of the vessel. For clarity, the fluorescent microspheres are presented in purple pseudo-color [Fig. 6(A,B)]. The number of microspheres bound to untreated damaged bovine carotid arteries was significantly less (p < 0.015) than the number bound to NHS-PEG-biotin treated arteries, 11 ± 4 per mm2 versus 60 ± 16 per mm2 [Fig. 6(C)].
Figure 6.
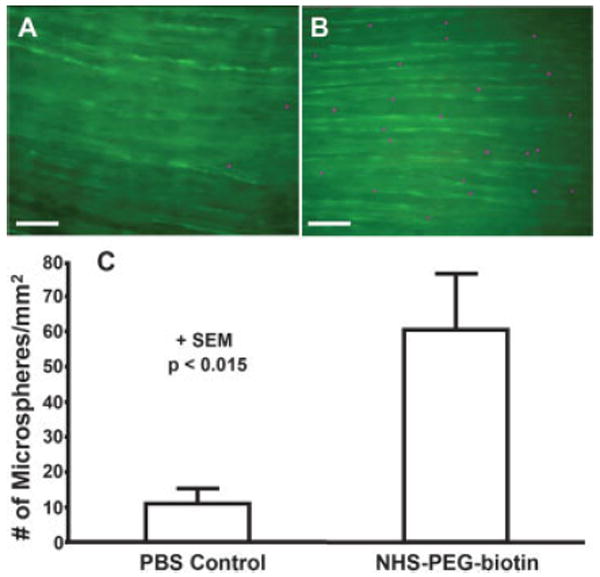
Targeted microspheres adhering to control and treated scrape-damaged bovine carotid arteries in a tubular flow chamber. A: Scrape-damaged bovine carotid artery (green) treated with the vehicle (PBS) and then perfused with targeted microspheres (purple). B: Injured bovine carotid artery (green) treated with 10 mM NHS-PEG-biotin and then perfused with targeted microspheres (purple). Scale bar is 100 μm. C: Number of adherent microspheres per mm2 on control and PEG-biotin treated scrape-damaged bovine carotid arteries. Data is shown + SEM and the results are significant with p < 0.015. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In examining HCAEC adhesion from shear onto PEG-biotin treated scrape-damaged arteries, targeted cells appear red on the auto-fluorescent vessel background [Fig. 7(A,B)]. The number of targeted cells bound to NHS-PEG-biotin treated bovine carotid arteries (22 ± 5 per mm2) was significantly greater (p < 0.01) than the number bound to PBS treated arteries (6 ± 2 per mm2) as shown in Figure 7(C).
Figure 7.
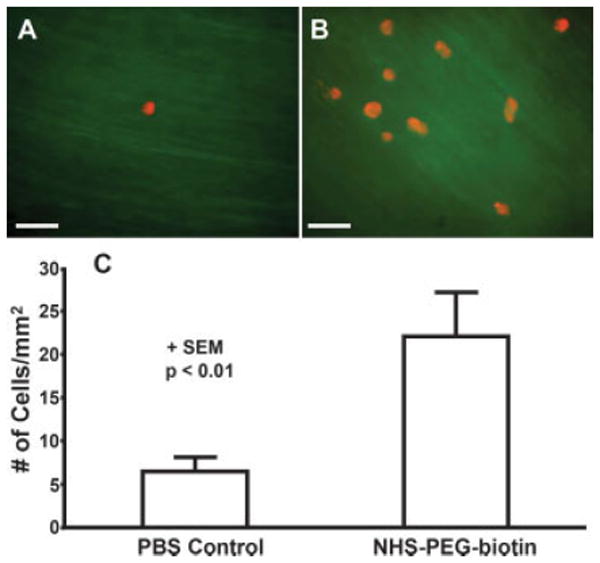
Targeted cells adhering to control and treated scrape-damaged bovine carotid arteries in a tubular flow chamber. A: Scrape-damaged bovine carotid artery (green) treated with the vehicle (PBS) and then perfused with targeted cells (red). B: Injured bovine carotid artery (green) treated with 10 mM NHS-PEG-biotin, 2 mg/mL NeutrAvidin, and then perfused with targeted cells (red). Scale bar is 100 μm. C: Number of adherent cells per mm2 on control and PEG-biotin treated scrape-damaged bovine carotid arteries. Data is shown + SEM and the results are significant with p < 0.01. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The experimental data demonstrate the feasibility of rapidly modifying vascular or endothelial surfaces with protein-reactive PEG-biotin under physiologic conditions for subsequent targeting of microspheres or modified ECs. Even without the targeting biotin molecule, modification of injured vascular surfaces with PEG might provide benefit in preventing platelet deposition following coronary interventions. It has previously been shown that PEG molecular barriers (without biotin) on damaged vascular surfaces can inhibit acute thrombotic deposition.11,12 Several other studies have also employed surface modification of vascular tissue to reduce thrombosis at the sites of vascular injury.30,31 The effectiveness of the polymer coating to serve as a molecular barrier to deposition or anchor for targeting to biotin on biological tissues would depend upon the density and structure of the PEG molecules on the modified surface. PEG molecules might assume pancake, mushroom, or brush conformations depending upon the distance between the covalently linked molecules and the properties of the surface.32,33 Based upon calculations for the expected structure, the number of PEG-biotin molecules per EC observed in this study would occupy 87% or 6% of the cell surface if the PEG molecules were in the mushroom or brush conformation, respectively.32,33
A potential benefit to using the approach of this study would be the ability to target pharmaceuticals or vectors to specific sites within the vasculature labeled with PEG-biotin for therapeutic benefit. Delivery of pharmaceutical agents to sites of vascular injury is another strategy to reduce acute platelet deposition and possibly inhibit intimal hyperplasia. For example, in one recent report anti-restenotic agents, heparin and low molecular weight heparin, were conjugated to antibodies against cross-linked fibrin.34 These modified antibodies were then systemically injected where they putatively targeted to the site of arterial injury.34 The targeting of heparin in this manner was shown to reduce neointimal formation in balloon damaged rabbit carotid arteries. Pharmaceutical targeting to the PEG-biotin labeled site might also allow the localization of anti-proliferative agents for an effect similar to that reported by Thomas and Campbell.34 The protein-reactive PEG targeting approach is also advantageous because of its applicability to healthy endothelium or surfaces where fibrin might not be present, for delivery to locations selected by the clinician at the time of the intervention.
Another approach investigated as a means of inhibiting restenosis following vascular injury seeks to facilitate re-endothelialization of the damaged vessel luminal surface.35-37 Disruption of the endothelial layer following various intravascular procedures is thought to be a major factor in thrombotic occlusions and neointimal formation.38,39 Accelerated replacement of the endothelial layer might abrogate thrombus formation and platelet deposition, as well as act to down-regulate smooth muscle cell proliferation, thereby reducing restenosis. In one study, previously harvested ECs with fibroblast growth factor type 1 (FGF-1) and heparin were delivered to lumens of balloon injured arteries using fibrin glue.37 Administration of the ECs, FGF-1, and heparin in the fibrin glue was associated with an inhibition of neointimal thickening, but required a 8 min incubation for polymerization and an additional step to remove the excess glue.37 Other studies have focused on harvesting endothelial progenitors and incubating them inside the vessel following injury.35,36 The attachment times investigated with the endothelial progenitor incubation technique were 20 min or greater, which would not be well-suited to time sensitive procedures, such as those involving coronary arteries.
The approach with the protein reactive PEG-biotin, where PEG is attached to the vessel lumen for a 1-min period followed by a similar NeutrAvidin incubation period, could be brief enough for consideration of this technique in vivo as a method for directing cells from the circulation to deposit at sites of modification where extended access is not feasible. A constraint inherent in this technique is the requirement that the targeting cells must first be isolated and surface modified with PEG-biotin. A theoretical benefit would be the potential to direct cells to sites selected by the localized application of the protein-reactive PEG-biotin by the clinician. This could be readily accomplished through the use of specialized local delivery balloon catheters, which are designed to allow fluid delivery to a specific region of the blood vessel wall.40,41 Further work would be necessary to characterize the phenotypic behavior and proliferation potential of the ECs following deposition in vivo. We have previously demonstrated that treatment of ECs with a reactive PEG solution resulted in no change in cell viability or proliferation potential when compared to untreated controls at 2, 6, and 9 days post-exposure.12 Furthermore, microscopic visualization of PEG-biotin modified cells displayed no evidence of membrane fusion or a decrease in viability or proliferation potential in the current study.
Several limitations associated with the current report should be noted. Perhaps of greatest significance are the limits associated with the use of in vitro or ex vivo models that employed relatively brief perfusions of modified cell culture medium containing the targeted species. Blood components were not present in the perfusate, and although albumin was added, the numerous plasma proteins and cells that would be encountered in vivo were absent. The perfusion periods were single pass and relatively short (10 min). In vivo studies, or employment of a recirculation loop, would have allowed longer contact times. An increased perfusion time might have led to higher deposition levels for the microspheres and cells, which on an absolute scale were somewhat sparse in the reported experiments. Deposition might also have been increased by not relying entirely upon adhesion from flow, but by employing some period of stagnant incubation. The deposition from flow model was favored, however, since it was believed to have the broadest clinical relevance and would be the more stringent test of the concept.
Another potential limitation of the targeted delivery system could be the loss of the PEG-biotin from the modified surfaces over time. This loss is postulated to be a result of natural cellular processes and not due to cytotoxicity, since the latter was not observed in previous studies PEGylating ECs directly, as mentioned previously.12 The reduction in attached surface molecules would also be expected to occur in vivo for damaged vascular surfaces as healing ensues. The time frame of PEG-biotin loss observed in this report suggests that substantial numbers of PEG-biotin may remain on cellular surfaces for periods of one day, or possibly longer, depending on the surface concentration needed to facilitate targeting. The concentration effect on targeting efficiency remains to be investigated.
In conclusion, we have demonstrated that a protein-reactive PEG-biotin could be used to modify damaged vascular tissue or cultured ECs in vitro such that it was possible to target microspheres and cells to the modified surfaces under flow conditions. This approach might ultimately find application in the targeting of pharmaceutical agents or cells to vascular sites selected by the controlled application of the protein-reactive PEG-biotin. Such targeted delivery could be of value in vascular interventions where thrombosis and restenosis are of concern, as well as to conditions where it is desirable to target an agent or cell to a user-specified location, such as tumor vasculature or regions where stem cell localization is desirable.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant number: HL58617
Contract grant sponsor: The National Tissue Engineering Center; contract grant number: 02059001
Footnotes
Bovine carotid arteries were obtained from euthanized animals with the assistance of Dr. Kenneth Litwak and Mary Watach.
References
- 1.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 2.Eliason JF. Pegylated cytokines: Potential application in immunotherapy of cancer. BioDrugs. 2001;15:705–711. doi: 10.2165/00063030-200115110-00001. [DOI] [PubMed] [Google Scholar]
- 3.Veronese FM. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials. 2001;22:405–417. doi: 10.1016/s0142-9612(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 4.Managit C, Kawakami S, Nishikawa M, Yamashita F, Hashida M. Targeted and sustained drug delivery using PEGylated galactosylated liposomes. Int J Pharm. 2003;266:77–84. doi: 10.1016/s0378-5173(03)00383-1. [DOI] [PubMed] [Google Scholar]
- 5.Muller M, Voros J, Csucs G, Walter E, Danuser G, Merkle HP, Spencer ND, Textor M. Surface modification of PLGA microspheres. J Biomed Mater Res A. 2003;66:55–61. doi: 10.1002/jbm.a.10502. [DOI] [PubMed] [Google Scholar]
- 6.Elbert DL, Hubbell JA. Surface treatments of polymers for biocompatibility. Ann Rev Mater Sci. 1996;26:365–394. [Google Scholar]
- 7.Xu ZK, Nie FQ, Qu C, Wan LS, Wu J, Yao K. Tethering poly (ethylene glycol)s to improve the surface biocompatibility of poly(acrylonitrile-co-maleic acid) asymmetric membranes. Biomaterials. 2005;26:589–598. doi: 10.1016/j.biomaterials.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Kang ET, Neoh KG, Wang P, Tan KL. Surface modification of stainless steel by grafting of poly(ethylene glycol) for reduction in protein adsorption. Biomaterials. 2001;22:1541–1548. doi: 10.1016/s0142-9612(00)00310-0. [DOI] [PubMed] [Google Scholar]
- 9.Park K, Shim HS, Dewanjee MK, Eigler NL. In vitro and in vivo studies of PEO-grafted blood-contacting cardiovascular prostheses. J Biomater Sci Polym Ed. 2000;11:1121–1134. doi: 10.1163/156856200744228. [DOI] [PubMed] [Google Scholar]
- 10.Deible CR, Petrosko P, Johnson PC, Beckman EJ, Russell AJ, Wagner WR. Molecular barriers to biomaterial thrombosis by modification of surface proteins with polyethylene glycol. Biomaterials. 1999;20:101–109. doi: 10.1016/s0142-9612(98)00001-5. [DOI] [PubMed] [Google Scholar]
- 11.Burchenal JE, Deible CR, Deglau TE, Russell AJ, Beckman EJ, Wagner WR. Polyethylene glycol diisocyanate decreases platelet deposition after balloon injury of rabbit femoral arteries. J Thromb Thrombolysis. 2002;13:27–33. doi: 10.1023/a:1015364024487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deible CR, Beckman EJ, Russell AJ, Wagner WR. Creating molecular barriers to acute platelet deposition on damaged arteries with reactive polyethylene glycol. J Biomed Mater Res. 1998;41:251–256. doi: 10.1002/(sici)1097-4636(199808)41:2<251::aid-jbm10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Chen AM, Scott MD. Current and future applications of immunological attenuation via pegylation of cells and tissue. BioDrugs. 2001;15:833–847. doi: 10.2165/00063030-200115120-00005. [DOI] [PubMed] [Google Scholar]
- 14.Chung HA, Kato K, Itoh C, Ohhashi S, Nagamune T. Casual cell surface remodeling using biocompatible lipid-poly(ethylene glycol)(n): Development of stealth cells and monitoring of cell membrane behavior in serum-supplemented conditions. J Biomed Mater Res A. 2004;70:179–185. doi: 10.1002/jbm.a.20117. [DOI] [PubMed] [Google Scholar]
- 15.Panza JL, Wagner WR, Rilo HL, Rao RH, Beckman EJ, Russell AJ. Treatment of rat pancreatic islets with reactive PEG. Biomaterials. 2000;21:1155–1164. doi: 10.1016/s0142-9612(99)00283-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee DY, Yang K, Lee S, Chae SY, Kim KW, Lee MK, Han DJ, Byun Y. Optimization of monomethoxy-polyethylene glycol grafting on the pancreatic islet capsules. J Biomed Mater Res. 2002;62:372–377. doi: 10.1002/jbm.10246. [DOI] [PubMed] [Google Scholar]
- 17.Savage MD, Mattson G, Desai S, Nielander GW, Morgensen S, Conklin EJ. Avidin-Biotin Chemistry: A Handbook. xxvii. Rockford, IL: Pierce Chemical Company; 1994. p. 467. [Google Scholar]
- 18.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54:459–476. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Kaar JL, Russell AJ, Wagner WR. Characterizing the modification of surface proteins with poly(ethylene glycol) to interrupt platelet adhesion. Biomaterials. 2006;27:3125–3135. doi: 10.1016/j.biomaterials.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilchek M, Bayer EA. Introduction to avidin-biotin technology. Methods Enzymol. 1990;184:5–13. doi: 10.1016/0076-6879(90)84256-g. [DOI] [PubMed] [Google Scholar]
- 21.Hoya K, Guterman LR, Miskolczi L, Hopkins LN. A novel intravascular drug delivery method using endothelial biotinylation and avidin-biotin binding. Drug Deliv. 2001;8:215–222. doi: 10.1080/107175401317245895. [DOI] [PubMed] [Google Scholar]
- 22.Huhtinen P, Soukka T, Lovgren T, Harma H. Immunoassay of total prostate-specific antigen using europium(III) nanoparticle labels and streptavidin-biotin technology. J Immunol Methods. 2004;294:111–122. doi: 10.1016/j.jim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Bhat VD, Truskey GA, Reichert WM. Fibronectin and avidin-biotin as a heterogeneous ligand system for enhanced endothelial cell adhesion. J Biomed Mater Res. 1998;41:377–385. doi: 10.1002/(sici)1097-4636(19980905)41:3<377::aid-jbm6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Bhat VD, Truskey GA, Reichert WM. Using avidin-mediated binding to enhance initial endothelial cell attachment and spreading. J Biomed Mater Res. 1998;40:57–65. doi: 10.1002/(sici)1097-4636(199804)40:1<57::aid-jbm7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Chan BP, Bhat VD, Yegnasubramanian S, Reichert WM, Truskey GA. An equilibrium model of endothelial cell adhesion via integrin-dependent and integrin-independent ligands. Biomaterials. 1999;20:2395–2403. doi: 10.1016/s0142-9612(99)00167-2. [DOI] [PubMed] [Google Scholar]
- 26.Chan BP, Reichert WM, Truskey GA. Synergistic effect of shear stress and streptavidin-biotin on the expression of endothelial vasodilator and cytoskeleton genes. Biotechnol Bioeng. 2004;88:750–758. doi: 10.1002/bit.20263. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz A, Fernandez Repollet E, Vogt R, Gratama JW. Standardizing flow cytometry: Construction of a standardized fluorescence calibration plot using matching spectral calibrators. Cytometry. 1996;26:22–31. doi: 10.1002/(SICI)1097-0320(19960315)26:1<22::AID-CYTO4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Villanueva FS, Jankowski RJ, Manaugh C, Wagner WR. Albumin microbubble adherence to human coronary endothelium: Implications for assessment of endothelial function using myocardial contrast echocardiography. J Am Coll Cardiol. 1997;30:689–693. doi: 10.1016/s0735-1097(97)00197-6. [DOI] [PubMed] [Google Scholar]
- 29.Badimon L, Turitto V, Rosemark JA, Badimon JJ, Fuster V. Characterization of a tubular flow chamber for studying platelet interaction with biologic and prosthetic materials: Deposition of indium 111-labeled platelets on collagen, subendothelium, and expanded polytetrafluoroethylene. J Lab Clin Med. 1987;110:706–718. [PubMed] [Google Scholar]
- 30.Geho DH, Smith WI, Jr, Liotta LA, Roberts DD. Fibronectin-based masking molecule blocks platelet adhesion. Bioconjug Chem. 2003;14:703–706. doi: 10.1021/bc034037v. [DOI] [PubMed] [Google Scholar]
- 31.Thierry B, Winnik FM, Merhi Y, Tabrizian M. Nanocoatings onto arteries via layer-by-layer deposition: Toward the in vivo repair of damaged blood vessels. J Am Chem Soc. 2003;125:7494–7495. doi: 10.1021/ja034321x. [DOI] [PubMed] [Google Scholar]
- 32.Efremova NV, Sheth SR, Leckband DE. Protein-induced changes in poly(ethylene glycol) brushes: Molecular weight and temperature dependence. Langmuir. 2001;17:7628–7636. [Google Scholar]
- 33.Allen C, Dos Santos N, Gallagher R, Chiu GN, Shu Y, Li WM, Johnstone SA, Janoff AS, Mayer LD, Webb MS, Bally MB. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol) Biosci Rep. 2002;22:225–250. doi: 10.1023/a:1020186505848. [DOI] [PubMed] [Google Scholar]
- 34.Thomas AC, Campbell JH. Targeted delivery of heparin and LMWH using a fibrin antibody prevents restenosis. Atherosclerosis. 2004;176:73–81. doi: 10.1016/j.atherosclerosis.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: Implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 36.Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 37.Zarge JI, Huang P, Husak V, Kim DU, Haudenschild CC, Nord RM, Greisler HP. Fibrin glue containing fibroblast growth factor type 1 and heparin with autologous endothelial cells reduces intimal hyperplasia in a canine carotid artery balloon injury model. J Vasc Surg. 1997;25:840–848. doi: 10.1016/s0741-5214(97)70213-1. discussion 848–849. [DOI] [PubMed] [Google Scholar]
- 38.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 39.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(Suppl 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 40.Varenne O, Gerard RD, Sinnaeve P, Gillijns H, Collen D, Janssens S. Percutaneous adenoviral gene transfer into porcine coronary arteries: Is catheter-based gene delivery adapted to coronary circulation? Hum Gene Ther. 1999;10:1105–1115. doi: 10.1089/10430349950018102. [DOI] [PubMed] [Google Scholar]
- 41.Westedt U, Barbu-Tudoran L, Schaper AK, Kalinowski M, Alfke H, Kissel T. Effects of different application parameters on penetration characteristics and arterial vessel wall integrity after local nanoparticle delivery using a porous balloon catheter. Eur J Pharm Biopharm. 2004;58:161–168. doi: 10.1016/j.ejpb.2004.03.009. [DOI] [PubMed] [Google Scholar]


