Abstract
Polyphenolic extracts of the principal flavonoid classes present in cranberry were screened in vitro for cytotoxicity against solid tumor cells lines, identifying two fractions composed principally of proanthocyanidins (PACs) with potential anticancer activity. Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry (MALDI-TOF-MS) analysis of the proanthocyanidins (PACs) fractions indicated the presence of A-type PACs with 1–4 linkages containing between 2–8 epicatechin units with a maximum of 1 epigallocatechin unit. PACs exhibited in vitro cytotoxicity against platinum-resistant human ovarian, neuroblastoma and prostate cancer cell lines (IC50 = 79–479 μg/mL) but were non-cytotoxic to lung fibroblast cells (IC50 > 1000 μg/ml). SKOV-3 ovarian cancer cells treated with PACs exhibited classic apoptotic changes. PACs acted synergistically with paraplatin in SKOV-3 cells. Pretreatment of SKOV-3 cells with PACs (106 μg/ ml) resulted in a significant reduction of the paraplatin IC50 value. Similarly, in a BrdU incorporation assay, co-treatment of SKOV-3 cells with PACs and paraplatin revealed reduced cell proliferation at lower concentrations than with either individually. In SKOV-3 cell cultures co-treated with PAC-1 and paraplatin, an HPLC analysis indicated differential quantitative presence of various PAC oligomers such as DP-8, -9, -11 and -14 indicating either selective binding or uptake. Cranberry proanthocyanidins exhibit cell-line specific cytotoxicity, induce apoptotic markers and augment cytotoxicity of paraplatin in platinum-resistant SKOV-3 ovarian cancer cells.
Keywords: proanthocyanidins, anticancer, paraplatin, cranberry, MALDI-TOF
Introduction
The North American cranberry (Vaccinium macrocarpon Ait Ericaceae) is receiving attention as a ‘prophylactic’ food and source of ‘orthomolecular medicines’ (Neto et al., 2006). Cranberry fruit is rich in polyphenolic constituents with flavonols, anthocyanins, and proanthocyanidins (PACs) being the major classes of phytochemicals. In contrast to other species, for example, cocoa, grape, pomegranate, noted for relatively high proanthocyanidin content, cranberries contain proanthocyanidins with A-type linkages between units (Fig. 1). PACs with the A-type linkage are associated with antibacterial adhesion properties (Howell and Vorsa, 1998; Foo et al., 2000), low-density lipoprotein (LDL)-oxidation protective properties (Kruger et al., 2000; Porter et al., 2001; Yan et al., 2002), and decreased oxidative damage to rat neurons in a stroke model (Neto et al., 2005).
Figure 1.
Chemical structure of a representative tetramer (n = 4) proanthocyanidin (DP-4) with A-type linkage.
Potential anticancer and chemopreventive properties of cranberry extracts have been the subject of many recent investigations. Cranberry extracts inhibited the proliferation of MCF-7 and MDA-MB-435 breast cancer cells (Guthrie, 2000; Ferguson et al., 2004). Water-soluble extracts of commercial cranberry powder inhibited the proliferation of several human tumor cell lines (Sreeram et al., 2004). Cranberry phenolic-rich esters of pentacyclic triterpene ursolic acid selectively inhibited the growth of several types of tumor cells in vitro, particularly MCF-7 (Murphy et al., 2003). Cranberry flavonoids may also play a role in chemo-prevention. Flavonoid-rich fractions from Vaccinium species, including cranberry, inhibited ornithine decar-boxylase (ODC) expression in epithelial cells (Kandil et al., 2002) and induced the xenobiotic detoxification enzyme quinone reductase (QR) in vitro (Bomser et al., 1996).
Cranberry proanthocyanidins known to inhibit the growth of various tumor cell lines have not been well characterized, and the effects of cranberry extracts on platinum-resistant ovarian cancer and neuroblastoma cell lines have not been described. Furthermore, the effects of cranberry compounds as adjuvants to paraplatin, the major drug therapy for ovarian cancer, have not been examined. In this study, proanthocyanidin-rich fractions were isolated by chromatographic methods after sub-fractionating a polyphenolic-rich whole-cranberry extract. The fractions were evaluated for their ability to inhibit proliferation in vitro in three solid tumor cell lines, ovarian (SKOV-3), neuroblastoma (SMS-KCNR), and prostate (PC-3), and a normal human lung fibroblast cell line.
The primary objectives of this study were to isolate, characterize, and evaluate the cytotoxicity of cranberry polyphenols in ovarian, neuroblastoma and prostate cancer cell lines. The activity of cranberry constituents was investigated by (i) screening multiple purified and characterized fractions to identify the most active; (ii) screening the most active fractions against several tumor cell lines, and a normal human lung fibroblast cell line; (iii) observing morphological changes associated with apoptosis; (iv) evaluating synergism of PACs when combined with paraplatin treatment in SKOV-3 ovarian cancer cells by measuring inhibition of cell viability and cell proliferation; and (v) measuring the differential uptake of PACs in SKOV-3 cells with and without paraplatin co-treatment using high performance liquid chromatography (HPLC).
Materials and Methods
Plant material
Fresh mature cranberries of the cultivars ‘Stevens’ and ‘Early Black’ were collected from commercial plantings in Burlington Co., New Jersey and maintained frozen at −20 °C until use.
Reagents and instrumentation
Solvents were purchased from Fisher Scientific (Pittsburg, PA, USA) and were HPLC grade. Precoated aluminum plates with silica gel 60 with F254 fluorescent indicator were purchased from Merck, Gibbstown, NJ, USA. Sephadex LH-20 for column chromatography was obtained from Amersham Pharmacia Biotech AB (Uppsala, Sweden). Established standards were used for identification of individual flavonol glycosides (Gregoire et al., 2007) and proanthocyanidins (Duarte et al., 2006; Wilson et al., 2007). Previously described HPLC methods for the identification of proanthocyanidins and flavonols (Wilson et al., 2007, Vvedenskaya et al., 2004) were followed with slight modifications. The calibration matrix 2,5-dihydroxybenzoic acid, α-cyano-4-hydroxycinnamic acid, trans-3-indoleacrylic acid, 2,5-dihydroxybenzoic acid were purchased from Sigma-Aldrich (St Louis, MO, USA). 1H NMR and 13C NMR spectra were recorded with a Bruker AM 500 instrument at room temperature (RT) using 3 mm tubes. Samples (5 mg) were dissolved in MeOH-d4. Chemical shifts were expressed in parts per million (ppm) relative to tetramethyl silane (TMS) as an internal standard.
Extraction and isolation of polyphenolic constituents
Flavonol, anthocyanin and proanthocyanidin-rich fractions were isolated from frozen cranberry fruit with aqueous acetone (80:20 acetone/water, v/v) extraction followed by an ethyl acetate extraction and LH-20 column chromatography (Duarte et al., 2006; Gregoire et al., 2007). PACs containing fractions (1.3 g) obtained from methanol elution were reloaded on a Sephadex column (100 mm × 45 mm) and eluted with increased gradients of methanol and water (10, 20, 30, 40, 60, 80% methanol/water v/v) mixture to remove the flavonols. Oligomeric PACs were eluted with 80% acetone/water (v/v) to isolate PACs consisting of a degree of polymerization (DP) from DP-2 to DP-11, referred to as PAC-1. The PACs obtained from 80% acetone/water (v/v) elution were again chromato-graphed using Sephadex LH-20 and successively eluted with 50%, 80% methanol/water (v/v) and finally eluted with 80% acetone/water (v/v) to obtain polymeric PACs of molecular weight with degrees of polymerization ranging from DP-3 to DP-12, referred to as PAC-2. The fraction obtained from column chromatography containing both dimers (DP-2) and trimers (DP-3) was further purified by preparative HPLC. All fractions were characterized by LC-MS and/or MALDI-TOF.
Cell culture and conditions
SKOV-3 (ovarian epithelial adenocarcinoma), PC-3 (prostate adenocarcinoma), LF (lung fibroblasts) were purchased from ATCC (Manassas, VA, USA). SMS-KCNR (resistant neuroblastoma cell line) was a gift from Dr Giselle Sholler (University of Vermont, Vermont Cancer Center, Burlington, VT, USA). Cells were grown to 80% confluence in T75 cell culture flasks (Corning, New York, NY, USA) in complete medium (Gibco, Rockville, MD, USA) according to the suppliers recommendation. For all assays, cells were allowed to attach overnight and then treated in complete medium.
Cell viability assay
Cell viability was determined using the 96® Aqueous-One-Solution Assay (Promega, Madison, WI, USA) following the manufacturer's recommendations. This colorimetric assay is based on the ability of mitochondria to reduce a substrate (MTS) into a soluble formazan product with an absorbance at 490 nm (ELISA plate reader; Thermo Labsystems, Waltham, MA, USA) that is directly proportional to the number of living cells (Malich et al., 1997). Cells (5 × 103) were plated into 96-well flat bottom plates (Corning, Inc., Corning, NY, USA) and treated with cranberry fractions at concentrations of 0, 31, 62, 125, 250 and 500 μg/mL or co-treated with paraplatin (4.5 μg/ mL). Following incubation for 46 h MTS reagent was added for an additional 2–6 h and absorbance measured at 490 nm. Experiments were performed in triplicate; data are expressed as the mean of the triplicate determinations (X ± SD) of a representative experiment in % of absorbance of samples versus untreated cells [100%].
Morphological studies
Cells were seeded (6 × 104/ chamber) into a Lab-Tek Chamber-Slide System (Nalge Nunc., Naperville, IL, USA) and treated for 24 h with 200 μ/mL PAC-1. Following two wash-steps in PBS, cells were fixed and permeabilized in PBS/2% PFA/0.2% Triton X for 30 min at RT. Fixed cells were stainded and mounted with vectoshield® mounting medium for fluorescence with 4′,6 diamidino-2-phenylindole (DAPI) (Vector Laboratories Burlingame, CA, USA). Representative images were taken with an inverted microscope (Nikon Eclipse TE2000-E, CCD camera) and 20× objective.
Cell proliferation assay
Cell proliferation was determined by a BrdU assay (Roche Applied Science, Indianapolis, IN, USA) that measures the incorporation of a pyrimidine analogue (BrdU) during DNA synthesis. SKOV-3 cells (5 × 103) were plated into 96-well flat-bottom plates (Corning, Inc., Corning, NY, USA), treated for 42 h with cranberry fractions and paraplatin as indicated and the assay carried out as described elsewhere (Singh et al., 2007). Experiments were performed in triplicate; data are expressed as the mean of the triplicate determinations (X ± SD) of a representative experiment in % of absorbance of samples versus untreated cells [100%].
Proanthocyanidin oligomers recovered from SKOV-3 cells treated with PACs
SKOV-3 (2 × 106) cells were seeded in 100 mm2 petri dishes supplemented with complete Dulbecco's modified Eagle's medium (DMEM) media and treated with PAC-1 (0, 110 μg/mL, 110 μg/ mL + 4.5 μg/mL paraplatin after 3 h pretreatment with PACs) for 24 h. The media with cells were collected, washed with PBS and pooled. Cells were then treated with 2 mL methanol, scraped and centrifuged to collect the methanol layer. Following centrifugation, the cells were extracted with 20:80 acetic acid/methanol (v/v) solution followed by an 80% acetone/water (v/v) (1 mL each) extraction. All methanol and acetone layers were pooled following centrifugation, filtered, and concentrated to dryness with a Speed Vac concentrator (Thermo Model SPD 2010-220; Milford, MA, USA). Residues were dissolved in 0.1 mL of methanol for analysis. PAC-1 proanthocyanidin oligomers were quantified by HPLC at 280 nm (Wilson et al., 2007). The parent compounds and proanthocyanidins were identified by fingerprinting retention time (RT). Quantification of the metabolites was performed by calculating the area under curve (AUC) corresponding to reference standards.
RESULTS
Composition of polyphenolic fractions isolated from American cranberry
HPLC, LC-MS and MALDI-TOF-MS analysis of the aqueous acetone extract of the fruit indicated the polyphenol fraction consisted of A-type proanthocyanidins, anthocyanin glycosides and flavonol glycosides. Characterization of flavonols and PACs based on LC-MS was described previously (Wilson et al., 2007; Krueger et al., 2000) and further structural elaboration of the purified fraction is presented here (Fig. 2 and Table 1). MALDI-TOF mass spectra of the series of PAC-1, recorded as sodium adducts in the positive ion mode, are shown in Fig. 2. The polymeric character is reflected by the periodic occurrence of peak series that distinguish molecular weight differences due to extent of hydroxylation (16 amu) and nature of interflavan bonds (A-type and B-type, 2 amu; Table 1). Our results indicated that cranberry PACs consist of peaks with distances of 288 and 304 Da corresponding to a mass difference of one epicatechin/epicatechin and epicatechin/epigallocatechin between each polymer. An enlarged view of the analytical composition of PAC-1 is displayed in Fig. 2B. Increase in chain length of condensed PACs is due to the addition of epicatechin/epicatechin and epicatechin/ epigallocatechin monomers. The sodium adducts masses corresponding to each peak suggested that they contain only proanthocyanidins and matched well with previously published reports (Howell et al., 2005). Molecular weight of each peak was calculated based on the formula 290 + 288(n − 1) + 23 and 290 + 304(n − 1) + 23. In this equation, 290 indicates the molecular weight of the terminal epicatechin of epicatechin/epicatechin unit, n is the degree of polymerization of epicatechin/epicatechin and epicatechin/epigallocatechin units and 23 is the weight of sodium (Vivas et al., 2004). Similarly, the potassium and lithium adduct formulae were calculated using same equation as above with addition of 38 and 6 as the weights of potassium and lithium as shown in Table 1. Analysis using these equations revealed the presence of novel oligomeric PACs of molecular weight 1181.5, 1183.24, 1187.52, 1474.56, 1763.16, 2035.39, and 2053.21 series. To our knowledge, the existence of these new proanthocyanidin oligomers in cranberry (Table 1) determined by MALDI-TOF techniques has not been described previously.
Figure 2.

(A) MALDI-TOF spectra of the proanthocyanidin fraction in positive ion mode. (B) highly enlarged HPLC profile of PAC-1 used in this study. Peaks 1 to 3 correspond to trimers (DP-3), peaks 4 to 6 correspond to tetramers (DP-4), peak 7 to pentamers (DP-5), peak 8 to hexamers (DP-6), peak 9 to heptamers(DP-7), peak 10 to octamers (DP-8), peak 11 to nonamers (DP-9), peak 12 to decamers (DP-10), peaks 13, 14 and 15 correspond to PACs with DPs of 11, 12, and 13, respectively.
Table 1. Summary of the molecular masses observed by MALDI-TOF-MS (positive ion mode) analysis in salt form. The ratio of A and B type bonds is based on molecular weight and number of epicatechin (EP) and epigallocatechin units. Nd indicates not detected in potassium and lithium salt form.
| Degree of Polymerization (DP) | No. of A type bonds | No. of EC units | No. of EGC units | [M+Na]+ | [M+K]+ | [M+Li]+ |
|---|---|---|---|---|---|---|
| 2 | 1 | 2 | 0 | 599.2 | 615.4 | 582.7 |
| 2 | 0 | 2 | 0 | 601.3 | 616.7 | 584.1 |
| 3 | 1 | 3 | 0 | 886.3 | 902.4 | 869.7 |
| 3 | 1 | 3 | 0 | 887.23 | 903.6 | 870.2 |
| 3 | 0 | 3 | 0 | 890.8 | 905.5 | Nd |
| 4 | 0 | 4 | 0 | 1176.23 | 1194.02 | 1161.05 |
| 4 | 1 | 4 | 0 | 1174.66 | 1191.1 | 1157.32 |
| 4 | 2 | 4 | 0 | 1172.95 | 1189.4 | 1156.29 |
| 4 | 0 | 4 | 0 | 1181.5 | 1197.93 | Nd |
| 4 | 0 | 4 | 0 | 1183.24 | 1199.13 | Nd |
| 4 | 2 | 3 | 1 | 1187.52 | 1203.7 | Nd |
| 4 | 2 | 3 | 1 | 1189.05 | 1205.25 | Nd |
| 4 | 1 | 3 | 1 | 1190.46 | 1206.63 | Nd |
| 4 | 0 | 3 | 1 | 1193.69 | 1209.1 | 1215.1 |
| 5 | 3 | 5 | 0 | 1459.68 | Nd | Nd |
| 5 | 2 | 5 | 0 | 1461.66 | 1477.32 | Nd |
| 5 | 1 | 5 | 0 | 1462.46 | 1478.46 | Nd |
| 5 | 0 | 5 | 0 | 1465.07 | 1481.49 | Nd |
| 5 | 3 | 4 | 1 | 1474.56 | 1490.35 | Nd |
| 5 | 2 | 4 | 1 | 1476.24 | 1492.82 | Nd |
| 5 | 1 | 4 | 1 | 1479.62 | 1495.89 | Nd |
| 5 | 0 | 4 | 1 | 1481.02 | 1496.98 | Nd |
| 6 | 3 | 6 | 0 | 1748.33 | Nd | Nd |
| 6 | 2 | 6 | 0 | 1751.04 | 1766.91 | Nd |
| 6 | 1 | 6 | 0 | 1752.79 | 1768.96 | Nd |
| 6 | 0 | 6 | 0 | 1754.92 | 1771.15 | Nd |
| 6 | 4 | 5 | 1 | 1763.16 | 1778.56 | Nd |
| 6 | 3 | 5 | 1 | 1764.44 | 1781.21 | Nd |
| 6 | 2 | 5 | 1 | 1766.11 | 1782.62 | Nd |
| 6 | 1 | 5 | 1 | 1770.64 | Nd | Nd |
| 7 | 4 | 7 | 0 | 2035.39 | Nd | Nd |
| 7 | 3 | 7 | 0 | 2036.76 | 2052.23 | Nd |
| 7 | 1 | 7 | 0 | 2041.01 | Nd | Nd |
| 7 | 2 | 6 | 1 | 2053.21 | 2069.12 | Nd |
| 7 | 1 | 6 | 1 | 2056.79 | 2073.01 | Nd |
| 7 | 0 | 6 | 1 | 2060.39 | Nd | Nd |
| 7 | 3 | 8 | 0 | 2036.76 | 2052.32 | Nd |
| 7 | 2 | 8 | 0 | 2039.12 | Nd | Nd |
| 7 | 1 | 8 | 0 | 2041.01 | 2057.86 | Nd |
| 7 | 1 | 7 | 1 | 2056.79 | 2073.2 | Nd |
| 8 | 2 | 8 | 0 | 2325.77 | 2342.1 | Nd |
| 8 | 1 | 8 | 0 | 2328.1 | Nd | Nd |
| 9 | 1 | 9 | 0 | 2616.85 | 2633.05 | Nd |
| 10 | 3 | 10 | 0 | 2902.23 | 2918.34 | Nd |
| 11 | 3 | 11 | 0 | 3193.40 | 3205.66 | Nd |
| 12 | 3 | 12 | 0 | 3482.23 | Nd | Nd |
Proanthocyanidins (PACs) display differential cytotoxicity among various human cancer cell lines
Fractions PAC-1 and PAC-2 exhibited cytotoxicity against the SMS-KCNR neuroblastoma cell line (IC50 = 0.40 and 0.25 mg/mL) but were relatively less cytotoxic to prostate cancer cell lines (IC50 = 0.48 and 0.53 mg/mL, respectively). PAC-1 and PAC-2, however, were much less cytotoxic against normal human lung fibroblasts (IC50 ≥ 1000 μg/mL), indicating interesting cell selectivities and a potentially large therapeutic index. Fractions which consisted primarily of flavonol glycosides (F-4), dimers (F-5; Fig. 2A), trimers (F-6; data not shown) and peak-19 (F-9; data not shown) did not show cytotoxicity against the ovarian cancer (SKOV-3) cell line (Fig. 3A). Total cranberry extract (F-0) showed modest activity. PAC-1 (F-1) and PAC-2 (F-2) fractions that contained highly purified PACs exhibited the highest activity. Based on these results, we focused our efforts on PAC-1/2 in SKOV-3 ovarian cancer cells.
Figure 3.
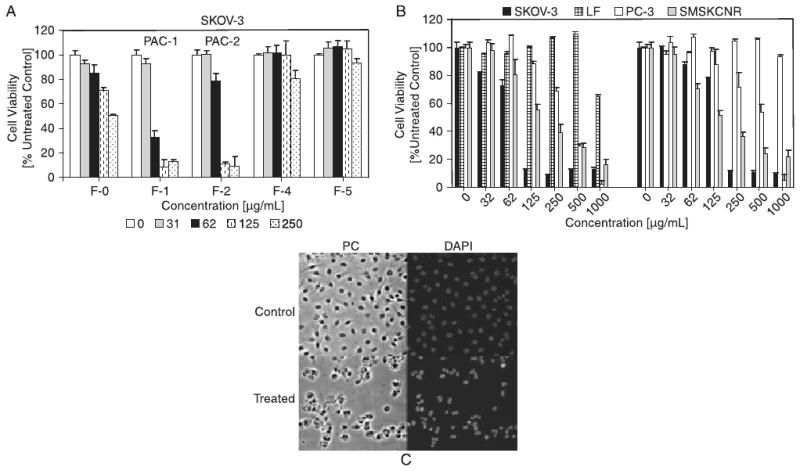
Comparative cytotoxicities of proanthocyanidins in different human cancer and control cell lines. (A) Polyphenolic fractions isolated from cranberry were screened for their cytotoxic potential against SKOV-3 cells. PAC-1 (F-1) exhibited the highest cytotoxicity. (B) PAC-1 was screened against human SKOV-3 (ovarian cancer), PC-3 (prostate cancer), SMS-KCNR (neuroblastoma) cell lines and LF (lung fibroblasts). Cells were treated with various concentrations (0 to 1000 μg/mL) of PAC-1 and PAC-2 for 48 h. The MTS viability assay was used to measure viability. Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X ± SD) of a representative experiment in % cell viability of samples with untreated cells [100%]. (C) SKOV-3 cells treated with PAC-1 (0-top panel or 200 μg/mL-bottom panel) for 24 h before microscopic analysis by phase contrast (PC) or fluorescence analysis after chromatin staining using a DAPI stain. Cells were photographed with a fluorescence microscope (20× objective).
PAC-1 causes morphological hallmarks of apoptosis in SKOV-3 cells
To visualize nuclear changes and apoptotic body formation that are characteristic of apoptosis, SKOV-3 cells treated with PAC-1 (200 μg/mL, 24 h) were stained with DAPI. Untreated cells or cells treated for 24 h with 200 μg/mL of PAC-1 after fixation and chromatin staining with Hoechst dye was analyzed by light and fluorescence microscopy. Treatment of SKOV-3 cells with PAC-1 resulted in cellular changes absent in untreated cells (Fig. 3C). Untreated cells displayed a homogenous morphology with lightly and evenly stained nuclei (Fig. 3C). In contrast, following treatment with PAC-1 (200 μg/mL) very few cells displayed such characteristics. A group of cells exhibited swelling and increased diameter with homogenous staining of the chromatin, indicating necrotic events, whereas the majority of cells (∼80%) displayed fragmented nuclei, densely stained nuclear granular bodies of highly condensed chromatin (‘apoptotic bodies’; Fig. 3C, right panels, typical of apoptosis).
PAC-1 induced cytotoxicity and reduction in cell proliferation is enhanced by co-treatment with subcytotoxic doses of paraplatin
To investigate if PAC-1 causes further reduction in the cell viability of SKOV-3 cells in the presence of sublethal paraplatin concentrations (at concentrations at or below 4.5 mg/mL where viability is not affected or partially reduced by paraplatin alone; Fig. 4A), PAC-1 pretreated cells (for 3 h) were treated with paraplatin (4.5 mg/mL) for 45 h. PAC-1 exhibited an enhanced dose-dependent reduction in the cell viability of SKOV-3 cells in the presence of paraplatin (Fig. 4A). Thus, PAC-1 cytotoxicity is possibly synergistic to paraplatin in SKOV-3 cells.
Figure 4.

Effect of co-treatment of PAC-1 and Paraplatin on cell viability and cell proliferation and quantification of the uptake of PAC-1 in SKOV-3 in the presence or absence of paraplatin. A: SKOV-3 cells were pretreated with (0–150 μg/mL) for 3 h with PACs, followed by subcytotoxic doses of paraplatin (4.5 μg/mL). Cell viability was measured by MTS assay. B: SKOV-3 cells were pretreated with (0– 125 μg/mL) for 3 h with PACs, followed by subcytotoxic doses of paraplatin (4.5 μg/mL). The BrdU proliferation assay was used to measure inhibition of cell proliferation. Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X ± SD) in % cell proliferation of untreated cell. C: SKOV-3 cells were treated with PAC-1 (200 μg/mL) for 48 h and metabolites quantified by HPLC. D: SKOV-3 cells were pretreated with PAC-1 (200 μg/mL, 3 h) followed by subcytotoxic doses of paraplatin (4.5 μg/mL). Cells were incubated for a total of 48 h and proanthocyanidins were quantified by HPLC.
To determine if PAC-1 causes a reduction in the cell proliferation of SKOV-3 cells in the absence and presence of sublethal concentrations of paraplatin (4.5 mg/ mL when viability is only partially reduced; Fig. 4B), we performed BrdU incorporation assays. PAC-1 reduced SKOV-3 proliferation in a dose-dependent manner (Fig. 4B), although the effect was only pronounced at a PAC-1 concentration of 125 μg/mL. However, the addition of paraplatin significantly reduced the concentration of PAC-1 needed to inhibit the proliferation of SKOV-3 cells. Even at PAC-1 concentration 31.2 μg/mL, BrdU incorporation was slightly reduced indicating that the combination PAC-1/paraplatin reduced SKOV-3 proliferation. Thus, PAC-1 in combination with very low concentrations of paraplatin suggests a synergistic effect as determined by both cell viability and cell proliferation assays.
Interaction of PACs in SKOV-3 cells
Phytochemicals have been shown to sensitize tumors to platinum or Taxol therapy and therefore improve the effectiveness of these agents (Yabushita et al., 2004). PAC-1 was cytotoxic to SKOV-3 cells and exhibited synergy with paraplatin in enhancing this cytotoxicity. We next investigated the possible mechanisms responsible for the observed synergy. We hypothesized that the presence of paraplatin may enhance the uptake or retention of particular components within the PAC-1 fraction.
To investigate whether specific oligomers were retained by SKOV-3 cells when treated with PACs, we analyzed and measured the composition of PAC oligomers obtained from cellular extracts of PAC-1-treated, -untreated and paraplatin co-treated SKOV-3 cells by HPLC using reference standards (Figs 4C and 4D). The concentration of octamer [(DP) epicatechin = 8] was found to be higher (6.32 ng) in the paraplatin co-treated cells, while the quantity of unidecamer (DP = 11) was reduced (1 ng). Similarly, the nonamer (DP = 9) and tetradecamer (DP = 14) were not detected in cells treated only with PAC-1, but were found to be present in paraplatin/PAC-1 co-treated cells in significant amount (1.53 and 3.15 ng, respectively). Interestingly, the composition profile of PAC oligomers was altered in paraplatin co-treated cells as compared to PAC-1-treated SKOV-3 cells.
Discussion
Cranberry extracts have shown in vitro inhibitory effects in multiple human cancer cell lines (Gutherie et al., 2000; Fergusan et al., 2004; Neto et al., 2005). However, the structural features of proanthocyanidins as related to their cytotoxic activity are poorly defined. Furthermore, the higher sensitivity of oncogenic cell lines relative to non-oncogenic cell lines to PACs is particularly noteworthy and has not been reported. Understanding the mechanisms underlying these observations is essential to ascertain the true therapeutic potential of cranberry PACs. Our interest in the identification of novel therapies from cranberry plant products specifically for human ovarian cancer arises from the potential consumption-linked health benefits to treat or manage this cancer. In the United States, epithelial ovarian cancer (EOC) is the leading cause of death from gynecologic malignancies and the fourth-most-common cause of death due to cancer among women. In 2007, there were an estimated 22 000 new cases and an estimated 15 000 deaths secondary to ovarian cancer (American Cancer Society, 2007). Progress is being made in treating this devastating cancer but the discovery of new treatments with increased therapeutic activities and decreased dose-related toxicities is needed.
Currently, the development of adjuvant therapies offering additive or synergistic effects is attracting much attention. Dietary phytochemicals could be such agents. They have the potential to increase drug efficacy by either modulating the disease process itself, or affecting specific cellular pathways known to cause resistance to standard cytotoxics. In addition, by acting as chemosensitizers, dietary phytochemicals can reduce the side effects of cytotoxic drugs by simply reducing the dose of drug needed for effect.
The SKOV-3 cell line was chosen for the initial cytotoxicity screening and expanded studies because it is a well-characterized ovarian tumor cell model derived from a highly malignant human ovarian cancer that is platinum-resistant and possesses several key oncogenic characteristics, for example, EGFR over-expression, p53 mutation (Husain et al., 1998; Anderson et al., 2001). The other two cell lines were chosen because they are also highly resistant to known therapies. SMS-KCNR cells represent a resistant phenotype and other features of highly therapy refractory neuroblastoma (Singh et al., 2007). The PC-3 cell line is a well-studied cell line derived from human prostate cancer that is hormone-refractory and resistant to multiple chemotherapeutic agents (Hart et al., 2005).
Our in vitro experiments have shown that PAC-1 and PAC-2 display partially overlapping cytotoxic effects in all cell lines tested. SKOV-3 cancer cells were more sensitive to PAC-1 than to PAC-2 or other flavonoid fractions. Even though other fractions (F1–F5) contained oligomers with type-A structural linkages similar to those present in PAC-1, the PAC-1 composition has relatively greater proportions of higher molecular weight polymers (over PAC-2). Additionally, other structural differences may affect the kinetic behavior of PAC-1 in different cell lines, as suggested by differences in the relative sensitivity to PAC-1 versus other fractions in SKOV-3, SMS-KCNR, PC-3, and lung fibroblasts cells. The altered kinetic behavior of PAC-1 could also partially account for its decreased activity in non-cancerous lung fibroblasts that have a similar metabolic rate than the malignant cell lines tested.
The cytotoxicity profiles of the PAC oligomers tested (PAC-1 and PAC-2) also appeared to be different from previously described PAC activities. These differences may be the result of the specific oligomers in our PAC-1/2 extracts due to differences in our material source and processing vs. PAC oligomeric compositions tested by others. Furthermore, the type of linkages in PACs strongly influences their biological activity. A-type linkage containing dimers and trimers were more cytotoxic than dimers and trimers with only B-type linkages (Kolodziej et al., 1995) against GLC4 lung and COLO 320 colon carcinomas.
Our work also suggests that PACs are effective as chemosensitizers by significantly reducing the dosages of chemotherapeutic agents required to achieve similar or improved efficacy. Pretreatment with PACs allowed lowering of paraplatin dosages while maintaining efficacy. Ultimately, lowering chemotherapeutic drug concentrations should, at the very least, improve toxicity profiles of these drugs.
The unique stereospecific properties of specific components in PAC-1 may allow for more effective targeting of membrane proteins compared with other PAC fractions lacking these properties. Stereospecific properties may account for the apparent synergism with paraplatin in SKOV-3 cells. Qualitatively, PAC-1 appears to impede certain key resistance factors (e.g., affecting platinum drug cellular efflux, decreasing the inactivation of platin by GSH by affecting GSH metabolism, or reversing the inhibition of apoptosis) (Alia et al., 2006; Perez et al., 1998; Siddick, 2003) in SKOV-3 cells. Indeed, even sublethal concentrations of paraplatin achieved significant cytotoxicity and pronounced cell proliferation reduction in SKOV-3 cells in the presence of PAC-1.
The mechanisms by which cranberry proanthocyanidins inhibit tumor cell growth remain unknown. While we were studying PAC-1-induced apoptosis in ovarian cancer cells, published reports revealed that whole cranberry extracts inhibit the expression of matrix metalloproteinases (MMPs) associated with tumor migration and proliferation in the DU-145 prostate cancer cell line (Neto et al., 2005). The authors attributed the observed cytotoxicity partly to PACs but it is likely that other cranberry components, such as quercetin and ursolic acid, contribute to the observed MMP inhibition. Our data indicate that PAC treatment of ovarian cancer cells results in significant morphological changes consistent with apoptosis. It is possible that MMP inhibition may be involved in the mechanism(s) of action of PAC-1/2 in SKOV-3 cells.
Finally, our experiments also demonstrated that subcytotoxic concentrations of paraplatin may alter the absorption, adherence or intracellular metabolism of PAC-1 in SKOV-3 cells. We noted a significant increase in DP-8, and very significant decrease in DP-11 (Figs 4C and 4D), suggesting that DP-11 may be rapidly hydrolyzed, possibly to DP-8 and other PACs but in a non-stoichiometric fashion vs. non-paraplatin-treated cells. Moreover, DP-9 and DP-14 were detected only in cells co-treated with paraplatin/PAC-1, indicating altered oligomer specificity of absorption, which is induced only by combination treatment (Figs 4C and 4D). Thus it would appear that sublethal concentrations of paraplatin have a significant impact on the interaction of the cell membrane with PAC oligomers. The recovery of specific oligomers in SKOV-3 cells following co-treatment suggests that the mechanism(s) of action involve specific drug–drug interactions.
As of now it is unknown to us if the consumption of DP-11 and/or the increased production of DP-8 and the apparent differential formation of DP-9 and DP-14 are essential for cytotoxicity. To answer these questions would require the isolation and/or the chemical synthesis of DP-8, DP-9, and DP-14 PACs and assessing their biological activities. This is beyond the scope of this descriptive study but is the interest of future investigations that will specifically examine the structural differences or key requirements and exact mechanism(s) of action of PACs.
Conclusions
Purified and MALDI-TOF characterized proanthocyanidins (PACs) found in cranberries are polymeric high molecular weight proanthocyanidins with A-type linkages among epicatechins consisting of a mixture of polymeric proanthocyanidins (DP-2 to DP-14). These PACs are selectively cytotoxic to platinum-resistant ovarian cancer cells, hormone-refractory prostate cancer cells, and neuro-blastoma cells but are minimally cytotoxic to lung fibro-blasts. PACs sensitized platinum-resistant ovarian cancer cells to paraplatin and displayed synergism with this chemotherapeutic agent. Finally, paraplatin appeared to affect PAC metabolism in these ovarian cancer cells. These interesting findings warrant the further preclinical/ clinical evaluation of PACs in the treatment of human solid tumors.
Acknowledgments
This work was supported by NIH NCCAM 5R01AT002058 to Dr Vorsa, and an Ovarian Cancer Research Fund Individual Investigator Grant and a NICHD, K12 HD043447 BIRCWH Scholar Grant to Dr Brard.
References
- Alia BH, Moundhri MSA. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: A review of some recent research. Food and Chem Toxicol. 2006;44:1173–1183. doi: 10.1016/j.fct.2006.01.013. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Statistics. 2007 www.cancer.org.
- Anderson NG, Ahmad T, Chan K, Dobson R, Bundred NJ. ZD1839 (Iressa), a novel epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the growth of EGFR-positive cancer cell lines with or without erbB2 overexpression. Int J Cancer. 2001;94:774–782. doi: 10.1002/ijc.1557. [DOI] [PubMed] [Google Scholar]
- Bomser J, Madhavi DL, Singletary K, Smith MAL. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996;62:212–216. doi: 10.1055/s-2006-957862. [DOI] [PubMed] [Google Scholar]
- Duarte S, Gregoire S, Singh AP, Vorsa N, Schaich K, Bowen WH, Koo H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol Lett. 2006;257:50–56. doi: 10.1111/j.1574-6968.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- Ferguson P, Kurowska E, Freeman DJ, Chambers AF, Koropatnick DJ. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J Nutr. 2004;134:1529–1535. doi: 10.1093/jn/134.6.1529. [DOI] [PubMed] [Google Scholar]
- Foo LY, Lu Y, Howell AB, Vorsa N. A-type Proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherischia coli. J Nat Prod. 2000;63:1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- Gregoire S, Singh AP, Vorsa N, Koo H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. J Appl Microbiol. 2007;103:1960–1968. doi: 10.1111/j.1365-2672.2007.03441.x. [DOI] [PubMed] [Google Scholar]
- Guthrie N. Effect of cranberry juice and products on human breast cancer cell growth. FASEB J. 2000;14:A771. [Google Scholar]
- Hart CA, Brown M, Bagley S, Sharrard M, Clarke NW. Invasive characteristics of human prostatic epithelial cells: understanding the metastatic process. Br J Cancer. 2005;92:503–512. doi: 10.1038/sj.bjc.6602325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Howell A, Vorsa N. Inhibition of adherence of P-fimbricated Escherischia coli to uroepithelial cell surfaces by proanthocyanidin extracts from cranberries. N Engl J Med. 1998;339:1085–1086. doi: 10.1056/NEJM199810083391516. [DOI] [PubMed] [Google Scholar]
- Husain A, Rosales N, Schwartz GK, Spriggs DR. Lisofylline sensitizes p53 mutant human ovarian carcinoma cells to the cytotoxic effects of cis-diamminedichloroplatinum (II) Gynecol Oncol. 1998;70:17–22. doi: 10.1006/gyno.1998.4972. [DOI] [PubMed] [Google Scholar]
- Kandil FE, Smith MAL, Rogers RB, Pepin MF, Song LL, Pezzuto JM, Seigler DS. Composition of a chemopreventive proanthocyanidin-rich fraction from cranberry fruits responsible for the inhibition of TPA-induced ODC activity. J Agric Food Chem. 2002;50:1063–1069. doi: 10.1021/jf011136z. [DOI] [PubMed] [Google Scholar]
- Kolodziej H, Haberland C, Woerdenbag HJ, Konings AWT. Moderate cytotoxicity of proanthocyanidins to human tumour cell lines. Phytother Res. 1995;9:410–415. [Google Scholar]
- Krueger CG, Dopke N, Treichel PM, Folts J, Reed JD. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of polygalloyl polyflavan-3-ols in grape seed extract. J Agric Food Chem. 2000;48:1663–1667. doi: 10.1021/jf990534n. [DOI] [PubMed] [Google Scholar]
- Murphy BT, MacKinnon SL, Yan X, Hammond GB, Vaisberg AJ, Neto CC. Identification of triterpene hydroxycinnamates with in vitro anti-tumor activity from whole cranberry fruit (Vaccinium macrocarpon) J Agric Food Chem. 2003;51:3541–3545. doi: 10.1021/jf034114g. [DOI] [PubMed] [Google Scholar]
- Neto CC, Krueger CG, Lamoureaux TL, Kondo M, Vaisberg AJ. MALDI-TOF MS characterization of proanthocyanidins from cranberry fruit (Vaccinium macrocarpon) that inhibit tumor cell growth and matrix metalloproteinase expression in vitro. J Sci Food Agric. 2006;86:18–25. [Google Scholar]
- Neto CC, Sweeney-Nixon MI, Lamoureaux TL, Solomon F, Kondo M, MacKinnon SL. Symposium Series on Phenolics in Foods and Natural Health Products. ACS Books; Washington, DC: 2005. Cranberry phenolics: effects on oxidative processes, neuron cell death and tumor cell growth; pp. 271–282. [Google Scholar]
- Perez RP. Cellular and molecular determinants of cisplatin resistance. Eur J Cancer. 1998;34:1535–1542. doi: 10.1016/s0959-8049(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Porter ML, Krueger CG, Wiebe DA, Cunningham DG, Reed JD. Cranberry proanthocyanidins associate with low density lipoprotein and inhibit in vitro Cu2+ induced oxidation. J Sci Food Agric. 2001;81:1306–1313. [Google Scholar]
- Sreeam NP, Adams LS, Hardy ML, Heber D. Total cranberry extract versus its phytochemical constituents: anti-proliferative and synergistic effects against human tumor cell lines. J Agric Food Chem. 2004;52:2512–2517. doi: 10.1021/jf0352778. [DOI] [PubMed] [Google Scholar]
- Siddick ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Singh RK, Lange TS, Kim K, Zou Y, Lieb C, Sholler GL, Brard L. Effect of Indole Ethyl Isothiocyanates on Proliferation, Apoptosis and MAPK Signaling in Neuroblastoma Cell Lines. Bioorg Med Chem Lett. 2007;17:5846–5852. doi: 10.1016/j.bmcl.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vvedenskaya IO, Rosen RT, Guido JE, Russell DJ, Mills KA, Vorsa N. Characterization of Flavonols in Cranberry (Vaccinium macrocarpon) Powder. J Agric Food Chem. 2004;52:188–195. doi: 10.1021/jf034970s. [DOI] [PubMed] [Google Scholar]
- Vivas N, Nonier MF, Vivas de Gaulejac N, Absalon C, Bertrand A, Mirabel M. Differentiation of proanthocyanidin tannins from seeds, skins and stems of grapes (Vitis vinifera) and heartwood of Quebracho (Schinopsis balansae) by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and thioacidolysis/liquid chromatography/electrospray ionization mass spectrometry. Anal Chim Acta. 2004;513:247–256. [Google Scholar]
- Wilson T, Singh AP, Vorsa N, Goett CD, Kittleson KM, Roe CM, Kastello GM, Ragsdale FR. Human Glycemic Response and Phenolic Content of Unsweetened Cranberry Juice. J Med Food. 2007 doi: 10.1089/jmf.2007.0531.. [DOI] [PubMed] [Google Scholar]
- Yabushita H, Kishida T, Noguchi M, Masuda T, Tsukada H, Sawaguchi K, Noguchi Y, Noguchi M. Weekly administration of paclitaxel and carboplatin in patients with recurrent ovarian cancer. J App Research. 2004;4:591–599. [Google Scholar]
- Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC. Antioxidant activities and anti-tumor screening of extracts from cranberry fruit (Vaccinium macrocarpon) J Agric Food Chem. 2002;20:5844–5849. doi: 10.1021/jf0202234. [DOI] [PubMed] [Google Scholar]



