Abstract
The therapeutic landscape for renal cell carcinoma has changed drastically over the last several years with the emergence of molecularly-targeted therapies. With previous prognostic and predictive tools based on studies of patients treated with cytokine therapies, confirmation of these prior methods and discovery of new markers in this new era of targeted therapy is of great importance. Alteration of the VHL gene by mutation, loss of heterozygosity, and promoter methylation has been found to be important to renal cell cancer pathogenesis. In this review, we will discuss the potential role of VHL mutation as a prognostic and predictive marker for renal cell cancer.
Introduction
Renal cell carcinoma (RCC) is an important disease with an expected 54,390 new cases predicted in 2008 with over 13,010 deaths.[1] There are several histologic subtypes of RCC including clear cell, papillary, chromophobe, and oncocytoma, with the clear cell variant being the most common, and making up nearly 70% of cases of RCC. Unfortunately, cure for RCC is only available to those with limited stage disease which can be surgically resected as to date systemic therapies cannot eradicate the cancer if it has spread distantly. In advanced stages, systemic therapies are given with the intent to provide debulking and disease stabilization and improvement in symptom control and survival. No conventional cytotoxic chemotherapy agents have demonstrated significant activity in this disease as monotherapy or combinations.[2] Previously systemic therapies consisted only of immune based therapies, such as the cytokines interferon and interleukin-2. However, the utility of cytokine therapy was limited by the small subset of patients with RCC who would derive clinical benefit and the limitation of widespread severe toxicities of these treatments which restricted their use[3]. These issues led to the advent of prognostic and predictive scoring systems to help with improved patient selection for immune-based therapy. One well known system is the Motzer or MSKCC criteria which implements clinical features such as functional status, prior nephrectomy, and blood levels of hemoglobin, calcium, and LDH at the time of presentation with metastatic disease [4]. These criteria and others like them were validated during this era of cytokine therapy and are still widely used today.
However, within the last several years, new molecularly targeted agents for the treatment of RCC have emerged from an improved understanding of the molecular biology of this disease.[5] One crucial finding has been the discovery of the VHL gene and its importance in regulation of the hypoxia pathway via the hypoxia inducible factors (HIFs).[•6] These recently introduced targeted agents which modulate this VHL-HIF pathway include the FDA approved multi-targeted tyrosine kinase inhibitors, sunitinib and sorafenib as well as the mTOR inhibitor, temsirolimus. These agents have shown superiority to previous cytokine therapies and are now part of the arsenal used in the standard treatment of RCC[••7–9]. Additional targeted agents likely to be considered for FDA approval on the immediate horizon include the mTOR inhibitor everolimus and the anti-angiogenic agent, bevacizumab. Many others are in active development. Since there has been a molecularly targeted shift in the RCC treatment paradigm and with multiple treatment choices available, further understanding and discovery of potential prognostic and predictive tools is paramount. Although validation of previous criteria, such as the previously described Motzer criteria is important, a further implementation of RCC molecular biology knowledge is ideal for new prognostic and predictive marker discovery and development in this new age of targeted therapies.
Rationale of VHL Mutational Status as a Biomarker
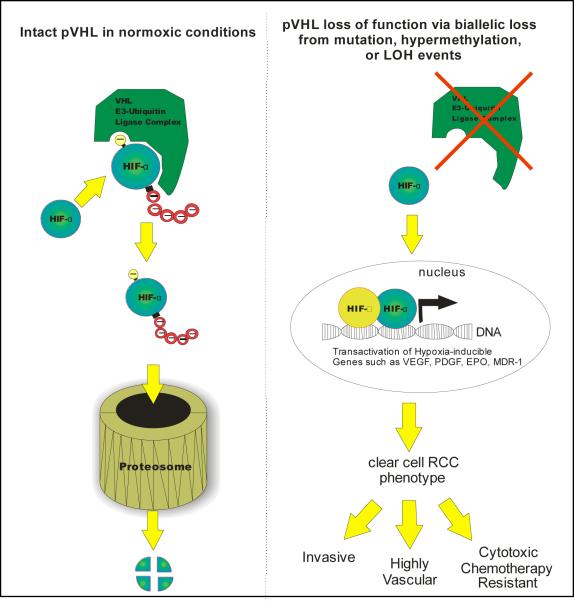
von Hippel-Lindau (VHL) disease is an autosomal dominant hereditary disorder characterized by retinal and CNS hemangioblastomas, pheochromocytoma, and clear cell renal cell carcinoma.[10] With the discovery of the underlying gene linked to this disorder, the VHL gene, a revolution in clear cell RCC cancer biology has followed. The VHL gene which is located on chromosome 3p25, encodes for a 213 amino acid tumor suppressor protein that was found to be a key player in the regulation of the hypoxia response pathway, which is vital to tumor survival in low oxygen conditions.[11, 12] The VHL protein (pVHL) functions as part of an E3 ubiquitin ligase which ubiquitylates a family of proteins known as the hypoxia inducible factors or HIFs and targets them for degradation by the proteasome.[13, 14] In the absence of functional pVHL protein, HIF is allowed to accumulate and translocate to the nucleus where it acts as a transcription factor. Transcriptional targets of HIF include a variety of pro-tumorogenic genes such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), multidrug resistance pump (MDR-1), and erythropoietin (EPO).[15] It is by expression of these and other HIF target genes that RCC receives its phenotype of chemotherapy resistance, invasiveness, and vascularity. Functional loss of pVHL has therefore been linked as a sentinel event in clear cell RCC development and progression, with supportive evidence shown by re-introduction of functional wild-type VHL into a renal cell cancer cell line lacking VHL resulting in an inability to form tumor xenografts[16] as well as renal cancer cell growth inhibition in vitro[17]. Given the tight link to RCC tumorigenesis, the presence or absence of a VHL mutation seems to be a natural object for the development of a predictive or prognostic marker for this disease. In fact, functional loss of pVHL exists in the majority of sporadic cases of clear cell RCC which is the predominant variant of this disease. This functional loss may occur by a variety of methods of VHL gene alterations including somatic mutation events, loss of heterozygosity (LOH) by allele deletion with concomitant alteration of the contralateral gene, and epigenetic interference such as promoter hypermethylation (Figure 1).
Figure 1.
Under normal oxygen conditions (left side), the VHL protein forms a complex that exhibits E3 ubiqtuitin ligase complex activity, recognizing prolyl hydroxylated HIF-α and marking it with a polyubiquitin tail. The cellular proteasome recognizes and degrades polyubiquinated HIF-α. In the absence of functional VHL (right side), HIF-α accumulates and migrates to the nucleus where it joins with HIF-β and CBP to initiate transactivation of hypoxia inducible genes, leading to the characteristic phenotype of clear cell renal cell cancer (RCC).
VHL Alterations in Hereditary and Sporadic RCC
Distinct mutation events in hereditary von Hippel-Lindau disease lead to four separate phenotypes which are classified as types 1, 2A, 2B, and 2C. In this classification system type 1 is distinguished from type 2 due to their lack of pheochromocytoma development. Additionally, type 2A is at low risk of RCC development while type 2B is at increased risk. Type 2C is unique in that this variant has a tendency towards pheochromocytoma formation only. Several studies of families harboring these germline VHL mutations have resulted in the discovery that a predominance of large deletion events with premature protein truncation or complete protein loss occurs in type 1 patients and missense mutations commonly occurs in type 2 patients.[18, 19] Analysis of missense mutations has been difficult due to presence of missense mutations signifying a significant risk of RCC in some cases and no significant risk in others. Additionally, in vitro evidence suggests that missense mutations in VHL such as those associated with the Type 2 subtypes of VHL disease vary significantly with respect to the degree of HIF regulation (Rathmell 2004, Li 2007), with mutations less tightly linked with development of RCC displaying a milder deficiency in HIF regulation.
Concordant with the high risk of RCC associated with VHL disease, alteration in the VHL gene is a common finding in cases of sporadic clear cell RCC. Several studies have linked sporadic cases of clear cell RCC with both somatic VHL gene mutations as well as loss of heterozygosity.[20–23] The frequency of VHL mutation events in these studies ranges from 46 – 82% of sporadic cases, with mutational events including a heterogeneous assortment of frameshift deletions, frameshift insertions, missense, and nonsense mutations. Additionally, in each of these studies the vast majority (85–98%) of cases containing somatic mutations also exhibited contralateral allele loss or loss of heterozygosity, consistent with a two-hit hypothesis of tumorigenesis (Table 1). Other studies have evaluated VHL promoter methylation events in sporadic RCC with hypermethylation rates ranging from 11–19% of cases.[24–26] Together, these data suggest that disruption of the VHL tumor suppressive activity may account for the vast majority of cases of sporadic RCC in many series.
Table 1.
Summary of key clinical trials evaluating VHL gene alterations such as mutation, hypermethylation, or loss of heterozygosity(LOH) and correlation with prognostic or predictive findings. ccRCC=clear cell renal cell carcinoma VEGF=vascular endothelial growth factor
| Study | Number of cases/Histologic Subtype included | Rate of VHL Mutation | Rate of Hypermethylation | Rate of LOH | Prognostic or Predictive Findings |
|---|---|---|---|---|---|
| Brauch, et al | 151/ccRCC | 45% | 17% | 93% | VHL mutation or hypermethylation was significantly associated with pT3 tumor stage (poor risk factor) |
| Yao, et al | 187/ccRCC | 52% | 5.3% | Not reported | VHL alterations correlated with improved clinical outcomes for Stage I–III but not Stage IV in patients treated with immunotherapy |
| Schraml, et al | 113/ccRCC | 34% | Not reported | Not reported | Loss of function mutations associated with worse clinical outcomes |
| Patard, et al | 100/ccRCC | 58% | Not reported | Not reported | Patients with a VHL mutation had a significantly improved 2 year survival |
| Kondo, et al | 240/sporadic RCC cases | 51% | 5% | 90% | No correlations found between mutational status and prognostic factors |
| Gimenez-Bachs, et al | 96/sporadic RCC cases | 21.9% | Not reported | Not reported | No correlations found between mutational status and prognostic factors |
| Smits, et al | 185/ccRCC | 52.4% (with loss of function mutations) | 10.9% | Not reported | No correlations found between mutational status and prognostic factors |
| Kim, et al | 56/ccRCC | 19.6% | 14.2% | Not reported | Loss of function mutations associated with poorer PFS and OS. No correlation between VHL alteration and immunotherapy response |
| Choueiri, et al | 123/ccRCC | 49% | 10% | Not reported | Loss of function mutations were associated with improved response to VEGF targeted therapy. No association with PFS and OS. |
| Gad, et al | 12/ccRCC | 16.6% | Not reported | Not reported | No correlation between VHL mutation and axitinib response |
VHL Mutations as a Prognostic Biomarker
As a broad body of evidence points to VHL as a major tumor suppressor in this disease, it is logical to assume that VHL mutation might have important implications for disease prognosis. Somatic VHL mutation events and their impact on prognosis have been studied in a variety of case series. Findings from these studies have been mixed, demonstrating conflicting results when used to develop prognostic algorithms. The results of this efforts to link VHL mutation to disease prognosis are summarized in Table 1.
Brauch et al published a series of 227 sporadic clear cell RCC tumors that were analyzed for VHL altering events[27]. The combined rate of VHL mutation and promoter hypermethylation was 45% and the rate of LOH in this study was 93% of cases. This high rate of LOH suggests an even higher rate of VHL disruption, or the presence of an alternate tumor suppressor with tight genetic linkage to VHL. In this series, the presence of VHL mutation or hypermethylation events were significantly correlated with pT3 tumor grade (P=0.009). This was the first publication to demonstrate an association of VHL alteration events with a prognostic factor, in this case linking VHL alteration to a poor risk factor (pT3 tumor stage). In a separate study, 113 cases of sporadic clear cell RCC were evaluated for VHL mutational status as well as the proliferative marker, Ki-67, and microvessel density (via CD-34 staining).[28] Evaluating overall VHL mutations, there was no difference in outcomes for those with or without mutations. However, on multivariate analysis, mutations which were predicted to result in loss of pVHL protein expression or function resulted in poorer cancer specific survival (P=0.02). VHL mutational status was not associated with tumor grade, stage, microvessel density, or proliferative index (Ki-67). Loss of function mutations in this study were defined as events which altered VHL transcriptional read through such as nonsense and frameshift mutations predicted to interfere with protein stability and one missense mutation which altered the VHL start codon. Patients with other unpredictable mutation events that did not fall into these categories showed no significant difference in clinical outcome. These findings, while provocative, are difficult to interpret due to the small number of cases (12) that contained these loss of function mutations. Additionally, as mentioned above, emerging data suggests that subsets of VHL missense mutations will be predicted to have significant effects on HIF regulation, a subtlety which complicates assumptions about the impact of missense mutations on disease prognosis and has not been scrutinized to date in any series.
VHL alterations have also been linked to positive outcomes in several studies. Patard, et al performed VHL mutational analysis and carbonic anhydrase IX (CA-IX) staining on 100 clear cell RCC nephrectomy specimens.[29] Of these, fifty-eight were found to contain VHL mutations. In univariate analysis, patients who had VHL mutations in their tumors had improved 2 year overall survival rates compared to those who did not (76% versus 51%, p=0.037). Cancer specific survival showed a similar positive trend but did not reach significance (p=0.079). Additionally, those that showed high CA-IX staining, in addition to VHL mutation, had an even further improvement in 2 year survival rate (86%). In multivariate analysis, high levels of CA-IX staining continued to remain as a significant independent prognostic factor, while VHL mutation presence did not (HR 1.39 95% CI 0.59–3.31, p=0.455). CAIX, as a target of HIF transcriptional activation, may be an indicator of functional VHL loss, indicating VHL events which impart a significant failure of HIF suppression.
In a separate study, 187 Japanese patients with clear cell RCC were analyzed for VHL alteration events including mutation and promoter hypermethylation.[30] VHL mutation events were present in 98 patients (52%) and hypermethylation in 10 patients (5.3%). VHL alterations were associated with improved outcomes in those with Stage IIII disease, but no correlation was found in those with Stage IV disease, suggesting that VHL mutation may contribute most at the stages at which determinations of metastatic disease potential are made. Also importantly, these metastatic patients were treated with immunotherapy or cytotoxic chemotherapy and therefore it is unclear if the results would have been similar in the current landscape of treatment with targeted agents.
Other clinical trials have shown no correlation between VHL mutational status and prognosis. Kondo et al reported 240 cases of sporadic RCC tumors that were analyzed for mutational status, methylation and LOH.[31] Rates for these events within this cohort were 51%, 5%, and 90% respectively. No correlation was identified between VHL altering events and prognostic features such as tumor size, grade, or rate of metastasis. In another series of 185 cases of clear cell RCC evaluated for VHL mutation and methylation events (57.3% with VHL alteration, 42.7% without), there was no significant difference noted in cancer specific survival.[32] Finally in a series of 96 sporadic RCC cases reported by Gimenez-Bachs, 21.9% of patients were positive for a VHL mutation and there was no correlation between the presence of mutation and tumor stage, size or histological grade.[33]
Ultimately, it is not clear that RCC associated with a VHL gene mutation has specific prognostic value. Studies to date have been limited by the available technology of the time for identifying mutation or methylation patterns in VHL, as well as by potential confounders such as the stage of the tumors analyzed and the potential influence of VHL missense mutations without variable effects on HIF regulation. What is clear is that VHL gene mutation is tightly linked with the clear cell histology subtype, and that not all tumors of this class will display VHL mutation. Correlating VHL mutation with clearly defined prognostic patterns requires highly defined samples within highly annotated data sets which unfortunately exist only rarely. Future studies will likely include VHL mutation as a prospectively defined stratifying factor, and necessary to eventually clarify this issue.
VHL Mutation as a Predictive Biomarker
There have been very few studies regarding VHL gene alteration as a potential predictive marker for response to therapy. Chiefly, this stems from the long dearth of effective therapy for the majority of patients with renal cell carcinoma, leaving the field now in a position of needing to catch up with recent advances in treatment. It was established that clear cell histology, which is most closely associated with VHL mutations, and in particular tumors with alveolar features and no evidence of papillary or granular features, were the most likely of all histologic categories to respond to interleukin-2 (IL-2) therapy.[34] This trend translated a survival benefit as well, and led to the future exclusion of patients with non-clear cell histology from treatment with high dose IL-2. Although suggestive, this data does not directly implicate VHL mutation as a predictive marker of response to cytokine therapy.
Association of VHL mutation with the risk of recurrence following nephrectomy has been evaluated in a series of 56 patients with clear cell RCC who underwent nephrectomy.[35] Sixteen of these patients (29%) had findings of either VHL somatic mutation or methylation events, a surprisingly low percentage compared to other studies, which raises concerns that VHL gene alterations may have been unrecognized among the wild type group. In this investigation, there was no association of VHL mutation or methylation status with either progression-free survival or overall survival. However, further analysis of a subset of these sixteen VHL mutations that predicted for loss of function showed that patients with these more “severe” mutational types had a significantly decreased progression-free survival (P=0.016) and overall survival (p=0.046). Patients that were treated with immunotherapy showed no significant correlation between presence of any VHL mutation and overall survival. When specific types of mutations were evaluated, patients with missense mutations had a tendency toward better cytokine response. Unfortunately, this trial can only be considered hypothesis generating due to the very small number of VHL mutations analyzed.
In a more recent study, Choueiri et al evaluated 123 patients with metastatic clear cell RCC who had undergone treatment with any VEGF targeted therapy (including the receptor tyrosine kinases: sorafenib, sunitinib, axitinib; or the monoclonal VEGF antibody: bevacizumab).[•36] DNA was extracted from archival tumor samples and analyzed for VHL mutations. If wild-type VHL was identified, then analysis of methylation status was performed. In this series, 60 patients (49%) had VHL mutations discovered, with 42% of these events in exon 1, 32% in exon 2, and 27% in exon 3. Additionally 12 patients (10%) had promoter methylation noted in the setting of wild type VHL. Overall the response rate for the entire series of patients was 37% with 2 patients obtaining a complete response and 43 patients with a partial response. In patients with evidence of either promoter methylation or VHL mutation, the response rate was 41% compared to 31% in the wild-type group (p=0.34). Upon multivariate analysis, patients with VHL mutational events which predicted for pVHL loss of function obtained a significant response rate of 52% compared to those with wild-type VHL who had a response rate of 31% (p=0.04). Specifically, the responses seen in patients treated with the very potent VEGF receptor inhibitors sunitinib or axitinib were independent of VHL mutational status. Conversely, no responses were noted in patients with wild-type VHL treated with sorafenib or bevacizumab. The authors hypothesized that these treatment specific findings may be due to off-target effects of sunitinib and axitinib or that they may be more potent VEGF receptor inhibitors, with effects that supercede the VEGF upregulation predicted to be associated with a loss of function VHL mutation. Despite these disease response correlations, presence of a VHL gene mutation was not correlated with either progression free or overall survival. This data set represents an expanded analysis initially reported by Rini, et al which in a small pilot study suggested a trend toward improved progression free survival upon treatment with VEGF targeted therapy in patients with loss of function VHL mutations.[37] Finally, in a much smaller cohort (n=13) of RCC patients treated with axitinib no correlation was seen between somatic VHL mutational status and response.[38] Although each of these investigations has limitations, in terms of VEGF targeted therapy, VHL mutation status as a predictive biomarker of response or survival remains to be established.
VHL gene mutation has also been evaluated as a potential predictive marker for mTOR directed therapy in a recent study. In this report by Cho et al, archival tumor specimens were evaluated from 20 patients who received treatment for advanced RCC with temsirolimus.[39] In this small series, no correlation was seen between VHL mutation and treatment response. However protein expression of phospho-AKT and phospho-S6, two important proteins indicating activity of the mTOR pathway were positively associated with response to mTOR directed treatment.
Conclusions
The discovery of VHL mutation as an integral component of the development of the great majority of sporadic renal cell carcinomas has been a defining feature of the therapeutic revolution that has occurred for this disease. Drugs of multiple classes have been developed which expoit targets tightly linked with the deregulated HIF signaling pathway. With increasing treatment options becoming available for the treatment of RCC, there is now a great need for the development of biomarkers to help guide treatment decisions. Previous prognostic scoring systems have utilized clinical findings such as performance status and simple laboratory findings. Although these scoring systems may continue to be useful in this new era of targeted therapy, improvement upon prognostic and predictive tools is necessary. Molecular targets, such as mutational status, have proven prognostically useful in other disease states, most notably in the case of lung cancer where activating mutations in the EGF receptor have been tightly associated with the therapeutic efficacy of EGFR targeted therapy.[40] As clear cell renal cell cancer is frequently associated with genetic alteration of VHL via somatic mutation, promoter hypermethylation, and/or LOH, the presence or absence of VHL alteration would seem to be an ideal potential biomarker for therapeutic strategies which impinge upon this signaling pathway. Unfortunately, such a correlation has not been easy to discern. Studies to-date have shown mixed results in regards to VHL alterations having a correlation to prognosis, known prognostic factors, or treatment outcomes. Therefore, currently there seems to be no role for screening tumors for VHL mutation outside of the setting of a clinical trial.
Clear limitations have included limited sample size, a heterogeneous population mixed with multiple stages of disease and varied treatments. Additionally, methods of VHL mutation detection are not uniform across the published literature which may explain some discrepancy in VHL detection rates and may skew attempts to draw statistically significant correlations. As methods for detection of VHL gene alteration events have been improved, generally higher rates of events are being discovered. If over time it becomes clear that the vast majority if not all of patients with clear cell RCC have VHL alteration events, then this finding would cease to become a potentially useful predictive or prognostic marker as the finding would be unable to discriminate significantly sized subgroups of patients for treatment efficacy.
An additional confounding factor to the utilization of VHL mutation as a predicitive biomarker of response to therapy may be the targeted nature of drugs currently used to treat kidney cancer. As we have seen for mTOR directed therapy, definitive evidence of pathway activation conveys a greater potential for response to appropriately targeted therapy. Therapy targeting VEGF or the VEGF receptor lies several steps removed from a VHL mutation, and in fact, tumor-specific VEGF expression can be induced by a wide variety of mechanisms in addition to VHL mutation, such as NO levels, p53 activation, and growth factor signaling.[•41] Therefore, such correlations may be limited by alternate genetic events that promote a tumor cell environment that favors treatment with VEGF targeted therapy. This is apparent by the observations that drugs targeting VEGF signaling have been found to be effective in a wide array of tumor types which have never been associated with VHL mutation.[42–44] Conversely, the effects of the tyrosine kinase inhibitors on many alternate targets in addition to the VEGF receptor may negate statistically significant correlations. Relevant to VEGF-targeted therapy are tumor or serum assessments of the activity of that ligand/receptor pair, as are currently ongoing. It may not be until therapeutic strategies specifically achieve successful replacement of pVHL cellular activities that tight correlations with VHL mutation may be detected. At this point VHL mutation remains the hallmark of clear cell RCC, and will continue to be interesting as a prognostic tool. Further evaluations of the role of VHL alterations as a predictive biomarker may be challenging and need to be performed within well designed large clinical trials utilizing state of the art detection methods and modern therapies.
Acknowledgments
Disclosures: Bayer/Onyx-consulting, clinical trial support, Wyeth-consulting (WKR)
References and Recommended Reading
Papers of particular interest, having been published recently include:
• Of importance
•• Of high importance
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163(2):408–417. [PubMed] [Google Scholar]
- 3.Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19(2):148–154. [PubMed] [Google Scholar]
- 4.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 5.Cowey C, Rathmell WK. Using molecular biology to develop drugs for renal cell carcinoma. Expert Opinion on Drug Discovery. 2008;3(3):311–327. doi: 10.1517/17460441.3.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 •.Kaelin WG., Jr. The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res. 2007;13(2 Pt 2):680s–684s. doi: 10.1158/1078-0432.CCR-06-1865. [DOI] [PubMed] [Google Scholar]; This review summarizes the key role of VHL gene product loss in the pathogenesis of clear cell RCC via dysregulation of the hypoxia inducible factors, HIF-1α and HIF-2α. Additionally this review highlights the potential for drug development targeting this pathway.
- 7 ••.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]; Multicenter, randomized, controlled phase III trial evaluating 750 previously untreated metastatic RCC patients. Results showed significant increase in progression-free survival and increase in response rate in the sunitinib treated patients compared to interferon control group.
- 8 ••.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]; Multicenter, randomized, controlled phase III trial evaluating 903 patients with advanced clear cell RCC who were treated with sorafenib versus placebo. Sorafenib showed a significantly prolonged progression-free survival compared to placebo.
- 9 ••.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]; Multicenter, randomized, controlled phase III trial evaluating 626 poor risk metastatic RCC patients to either temsirolimus, interferon, or both. Patients treated with temsirolimus had a significantly longer overall survival compared to interferon alone. No survival advantage was seen in the combination group compared to temsirolimus alone.
- 10.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 11.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 12.Iliopoulos O, Levy AP, Jiang C, et al. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 1996;93(20):10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwai K, Yamanaka K, Kamura T, et al. Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci U S A. 1999;96(22):12436–12441. doi: 10.1073/pnas.96.22.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 15.Wiesener MS, Munchenhagen PM, Berger I, et al. Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1alpha in clear cell renal carcinomas. Cancer Res. 2001;61(13):5215–5222. [PubMed] [Google Scholar]
- 16.Iliopoulos O, Kibel A, Gray S, et al. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1(8):822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Kishida T, Duh FM, et al. Suppression of growth of renal carcinoma cells by the von Hippel-Lindau tumor suppressor gene. Cancer Res. 1995;55(21):4804–4807. [PubMed] [Google Scholar]
- 18.Zbar B, Kishida T, Chen F, et al. Germline mutations in the Von Hippel-Lindau disease (VHL) gene in families from North America, Europe, and Japan. Hum Mutat. 1996;8(4):348–357. doi: 10.1002/(SICI)1098-1004(1996)8:4<348::AID-HUMU8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Friedrich CA. Genotype-phenotype correlation in von Hippel-Lindau syndrome. Hum Mol Genet. 2001;10(7):763–767. doi: 10.1093/hmg/10.7.763. [DOI] [PubMed] [Google Scholar]
- 20.Foster K, Prowse A, van den Berg A, et al. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994;3(12):2169–2173. doi: 10.1093/hmg/3.12.2169. [DOI] [PubMed] [Google Scholar]
- 21.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 22.Shuin T, Kondo K, Torigoe S, et al. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994;54(11):2852–2855. [PubMed] [Google Scholar]
- 23.Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14(15):4726–4734. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman JG, Latif F, Weng Y, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994;91(21):9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifford SC, Prowse AH, Affara NA, et al. Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosomes Cancer. 1998;22(3):200–209. doi: 10.1002/(sici)1098-2264(199807)22:3<200::aid-gcc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Dulaimi E, Ibanez de Caceres I, Uzzo RG, et al. Promoter hypermethylation profile of kidney cancer. Clin Cancer Res. 2004;10(12 Pt 1):3972–3979. doi: 10.1158/1078-0432.CCR-04-0175. [DOI] [PubMed] [Google Scholar]
- 27.Brauch H, Weirich G, Brieger J, et al. VHL alterations in human clear cell renal cell carcinoma: association with advanced tumor stage and a novel hot spot mutation. Cancer Res. 2000;60(7):1942–1948. [PubMed] [Google Scholar]
- 28.Schraml P, Struckmann K, Hatz F, et al. VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol. 2002;196(2):186–193. doi: 10.1002/path.1034. [DOI] [PubMed] [Google Scholar]
- 29.Patard JJ, Fergelot P, Karakiewicz PI, et al. Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer. 2008;123(2):395–400. doi: 10.1002/ijc.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao M, Yoshida M, Kishida T, et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002;94(20):1569–1575. doi: 10.1093/jnci/94.20.1569. [DOI] [PubMed] [Google Scholar]
- 31.Kondo K, Yao M, Yoshida M, et al. Comprehensive mutational analysis of the VHL gene in sporadic renal cell carcinoma: relationship to clinicopathological parameters. Genes Chromosomes Cancer. 2002;34(1):58–68. doi: 10.1002/gcc.10054. [DOI] [PubMed] [Google Scholar]
- 32.Smits KM, Schouten LJ, van Dijk BA, et al. Genetic and epigenetic alterations in the von hippel-lindau gene: the influence on renal cancer prognosis. Clin Cancer Res. 2008;14(3):782–787. doi: 10.1158/1078-0432.CCR-07-1753. [DOI] [PubMed] [Google Scholar]
- 33.Gimenez-Bachs JM, Salinas-Sanchez AS, Sanchez-Sanchez F, et al. Determination of vhl gene mutations in sporadic renal cell carcinoma. Eur Urol. 2006;49(6):1051–1057. doi: 10.1016/j.eururo.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Upton MP, Parker RA, Youmans A, et al. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005;28(5):488–495. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Jung CW, Cho YH, et al. Somatic VHL alteration and its impact on prognosis in patients with clear cell renal cell carcinoma. Oncol Rep. 2005;13(5):859–864. [PubMed] [Google Scholar]
- 36 •.Choueiri TK, Vaziri SA, Jaeger E, et al. von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180(3):860–865. doi: 10.1016/j.juro.2008.05.015. discussion 865–866. [DOI] [PubMed] [Google Scholar]; This is an analysis of 123 metastatic clear cell RCC patients for VHL inactivation events who were treated with VEGF-targeted therapy. This report demonstrated a significant correlation between loss of function VHL mutations and response to VEGF-targeted drugs, but no survival advantage.
- 37.Rini BI, Jaeger E, Weinberg V, et al. Clinical response to therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: impact of patient characteristics and Von Hippel-Lindau gene status. BJU Int. 2006;98(4):756–762. doi: 10.1111/j.1464-410X.2006.06376.x. [DOI] [PubMed] [Google Scholar]
- 38.Gad SS-AV, Meric JB, Izzedine H, et al. Somatic von Hippel-Lindau (VHL) gene analysis and clinical outcome under antiangiogenic treatment in metastatic renal cell carcinoma: preliminary results. Targeted Oncology. 2007;2:3–6. [Google Scholar]
- 39.Cho D, Signoretti S, Regan M, et al. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13(2 Pt 2):758s–763s. doi: 10.1158/1078-0432.CCR-06-1986. [DOI] [PubMed] [Google Scholar]
- 40.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 41 •.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]; This comprehensive review highlights VEGF and its essential role in cancer angiogenesis as well as its potential for therapeutic targeting.
- 42.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 43.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 44.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]