Abstract
Clear cell renal cell carcinoma (ccRCC) provides a tumor paradigm for the integration of genetics, molecular biology, therapeutic target validation, and the introduction of high impact treatment strategies. Most cases of sporadic as well as familial ccRCC acquire somatic inactivating mutations of the von Hippel-Lindau tumor suppressor gene, VHL. pVHL, VHL gene product and a protein member of the E3 ubiquitin ligase family, acts in normal cells to direct the degradation and clearance of the hypoxia inducible factor (HIFα) transcription factor family, such that in its absence, as in ccRCC, the HIF proteins stabilize, accumulate to supraphysiologic levels, and activate the transcription of genes such as vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF), which contributes substantially to the physiology of the tumor, and has been assessed indirectly as a prognostic factor. Molecularly targeted therapy blocking components of this pathway has been successfully introduced to the clinic with a substantive impact on clinical parameters of RCC. This review will examine the regulation of these molecular pathways in RCC and discuss the impact on the clinical management of patients with RCC.
Keywords: renal cell carcinoma, von Hippel-Lindau, HIF, VEGF, angiogenesis
VHL Mutation and Renal Cell Carcinoma
Germline mutations of the von Hippel-Lindau (VHL) tumor suppressor gene located at chromosome 3p25 cause autosomal dominant hereditary von Hippel-Lindau familial cancer syndrome characterized by an increased risk of tumor development in multiple organs including hemangioblastoma of the central nervous system and retina, pheochromocytoma and paraganglioma, and clear cell renal cell carcinoma (ccRCC) [1-3] The VHL disease is highly penetrant, affecting nearly 90% of individuals by the age of 55 years old. The gene for VHL disease (VHL) was identified in 1993 with the identification of germline carriers of mutations[4*]. Although with rare exception patients inherit one mutant copy of the gene, all manifestations of the disease require the loss or inactivation of the wild type allele, by a classical Knudsen two-hit mechanism [5, 6].
Sporadic ccRCC parallels the genetic mechanism of tumor initiation seen in VHL disease with a high frequency of cytogenetic losses at 3p25 as well as somatic VHL mutations which can be detected in 60 to 90% of patients with this cancer [7-9]. RCC is a heterogeneous disease, and VHL mutations are not observed in nonclear cell (papillary or chromophobe) histologies, however, tumors described as histologically mixed variants including a component of clear cell may harbor these mutations.
VHL Gene Regulation and Activity
VHL: regulation of protein levels
The VHL gene, located on a simple locus and composed of 3 exons, encodes for a short protein (pVHL) with potent tumor suppressive activity as demonstrated by the introduction of a wild type VHL cDNA which represses the growth of tumors in immunocompromised mice [10]. No known mechanisms of alternative splicing occur, but an alternate N-terminal truncated version is produced as a result of in-frame internal ATG 54 amino acid downstream of the traditionally recognized start site, producing a 19kD product in addition to the usual 30kD pVHL product [11]. This second gene product retains the tumor suppressive function of the full length protein, but its specific role is not known. The relative presence of the two pVHL products appears to be of little consequence in the promotion of cancer, as disease causing mutations are rarely if ever identified localizing to this N-terminal 54 amino acid region (Figure 1). Further, although the murine and human genes are highly conserved, the amino terminal region is exclusively quite divergent, adding support to the tumor suppressor activity of the protein to be relegated to the central and C terminal portions [12]. Post-translational modifications are not known to contribute to the stability or overall level of the protein.
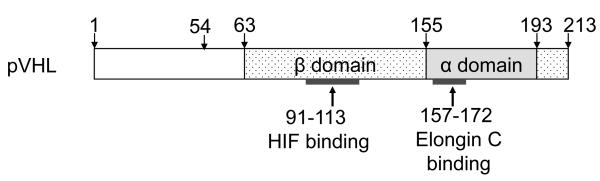
Figure 1. pVHL functional domains.
pVHL has two main domains: β domain and α domain. pVHL contains 213 amino-acids (30 kDa). A second major VHL gene product arises by internal translation initiation from the codon ATG 54 amino acid methionine, producing a 160 amino-acid protein (19 kDa). The α domain contains a subdomain of residues 157-172, a frequent site of missense mutations and binding to Elongin C. The β domain directs the interaction of pVHL with substrates of the E3 ubiquitin ligase, containing residues 91-113 binding to HIF.
The pVHL protein is expressed in the cytoplasm of cells in the tissues broadly affected by VHL disease, specifically kidney tubule, pancreas, adrenal gland, liver parenchyma, and various neural tissues as well as epithelial tissues not characteristically involved in the disease such as the thyroid, intestine, and bronchi [2, 3]. During embryogenesis the protein is expressed in all three germ layers, and later shows strong expression in the kidney, testis, central nervous system, and lung [13].
As mentioned above, the loss of one copy of the VHL allele in ccRCC occurs commonly as a loss of heterozygosity event. This may occur through genetic deletion, either small deletions impacting only a small part of the locus or the large chromosomal aberrations as in the cytogenetic 3p abnormalities. Alternatively, the VHL locus is highly susceptible to methylation, which results in genetic silence and functional loss of pVHL, although the allele is wild type. The majority of primary mutations observed in the VHL gene are gene editing events—frameshift insertions and deletions, nonsense mutations which result in the premature insertion of a stop codon, or missense mutations which may interfere with an important protein interaction domain. Intrinsically, the pVHL protein is quite stable. However, missense mutations may also confer instability, resulting in a functional and/or quantitative loss of pVHL activity [14].
VHL: protein domains
As alluded above, the pVHL protein is structurally simple, composed primarily of protein-protein interaction domains (Figure 1). The N terminus of the protein is largely undefined, and retains no known function essential for the tumor suppressive activity of pVHL. The remainder of the protein is composed of an α domain which promotes the interaction of pVHL with the Elongin B and Elongin C members of the E3 ubiquitin ligase complex [15]. This region, composed of residues 156-193, contains a subdomain of residues 157-172 which is a frequent site of missense mutations in VHL disease and is sufficient to bind Elongin C in vitro, although the remaining residues form additional helices which stabilize this interaction in vivo. The β domain directs the interaction of pVHL with substrates of the E3 ubiquitin ligase. This domain, encompassing variably residues 63-155 has been shown to be essential for the interaction with both atypical protein kinase C and the hydroxylated forms of the hypoxia inducible factor (HIF) [16, 17]. Portions of this domain can also independently mediate tumor growth suppression [18].
Further regulation of pVHL protein interaction occurs through the process of Neddylation which impacts the ability of pVHL to interact with fibronectin, but does not abrogate the activity as an E3 ubiquitin ligase [19]. Indeed, engagement of pVHL with hydroxylated HIF promotes further Neddylation of the Cullin-2 protein and accelerates the activity of the E3 ligase to support HIF ubiquitylation [20].
VHL: regulation of HIF
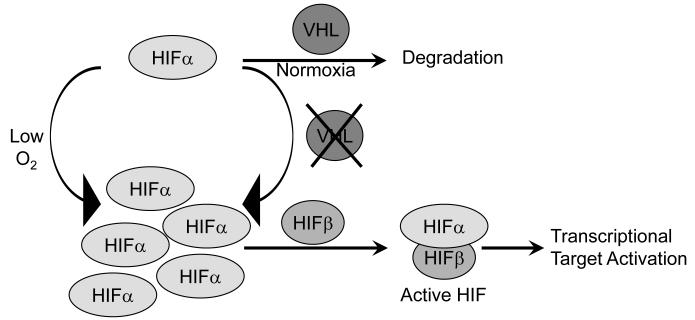
pVHL performs several putative cellular activities, but has been extensively studied for its role in regulating the cellular response to extrinsic oxygen signals. pVHL functions as the substrate recognition component of an E3 ubiquitin ligase complex that also contains Elongin B, Elongin C, Ring box protein 1 (Rbx1), and Cullin 2 (Cul2) [21]. The pVHL protein’s β domain targets substrates for ubiquitination and degradation by the 26S proteasome [7-9]. Known and putative substrates of pVHL E3 ligase activity include atypical protein kinase C (aPKC), hyperphosphorylated Rbp1, VHL deubiquitinating enzymes (VDU)-1 and -2, and HIFα subunits. The latter notable group of proteins targeted in this way by pVHL is a family of potent transcription factors including HIF1α and HIF2α which are induced in response to hypoxia as seen in the deepest tissues where physiologic levels of oxygen fall to 3-5% or in pathological conditions of ischemia [22]. In the presence of oxygen, one (or both) of two prolyl residues in the HIFα oxygen degradation domain (ODD) is hydroxylated by members of the EglN family [23-25]. Hydroxylated HIFα is recognized by pVHL, recruited to the E3 ubiquitin ligase complex, polyubiquitinated, and targeted for proteasomal degradation [26]. When the VHL gene is lost or mutated in such a way that either participation in the E3 ubiquitin ligase complex or interaction with the HIFα substrates is disrupted, these transcription factors are not degraded and accumulate to stably high levels to dimerize with HIFβ to form transcriptionally active HIF (Figure 2).
Figure 2. pVHL activity in physiologic and pathologic states.
HIFα subunit stabilization as a result of either hypoxia or VHL mutation leads to dimerize with HIF1β to form active HIF to transcriptional activate a panel of hypoxia responsive genes.
Other HIF family members regulated by pVHL include HIF3α, a group of alternative spliced isoforms sharing the ODD domain without known physiologic or pathologic roles in either hypoxia regulation or cancer. The specific cellular biological processes or tissue specific contexts which govern the distinctions between HIF1α and HIF2α upregulation remain incompletely understood. Some of this distinction is guided by tissue restriction in the regional expression of these two genes, as observed in mouse tissues [27]. Additionally, the activities of the individual HIF factors may vary in the pathologic state. In VHL null ccRCC, cyclin D1, transforming growth factor α, and vascular endothelial growth factor (VEGF) have been shown to respond specifically to HIF-2α [28]. Thus, the distinct profiles of HIF family members stabilized within a tumor may have important implications for both the origin and the behavior of the tumor.
HIF-related effects of VHL loss
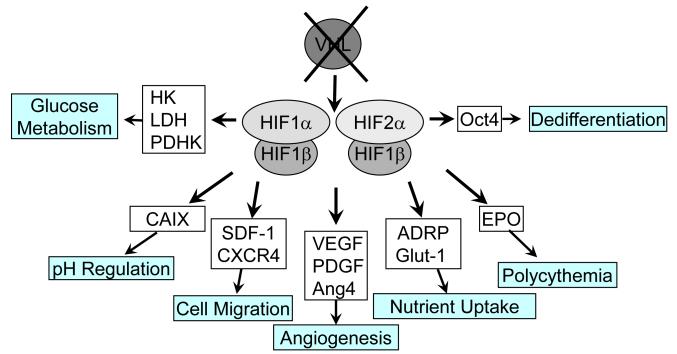
The transcriptional targets of HIF activation are extensive and include essentially the complete set of factors required for a physiologic cellular response to restrictions of oxygen supply [29]. Transcriptionally active HIF is a heterodimer consisting of an unstable α subunit and a stable β subunit which further complex with the CREB binding protein (CBP) and p300 to activate transcription of genes containing specific promoter elements. The repertoire of genes induced by the independent HIF family members is an overlapping, but not identical set of genes involved in the cellular response to oxygen deprivation [30-32]. HIF target gene activation (Figure 3) encompasses targets with a wide range of roles including important tumor promoting actions such as angiogenesis, evasion of cell death, cellular metabolism, and cell differentiation [29, 31]. The activation of these targets contributes to the adaptive cellular survival of a hypoxic insult and tumor-promoting properties underlying most ccRCC.
Figure 3. HIF1a and HIF2a regulate independent, but overlapping panels of target genes.
Common target genes include genes involved in pH regulation, cell migration, angiogenesis, nutrient (glucose and lipid) uptake, and red blood cell production. Exclusive hypoxia response targets of HIF-1 include enzymes involved in glycolysis, whereas Oct4, an important transcription factor in maintaining the differentiation state of the cell, is an independent target of HIF-2. HK, hexokinase; LDH, lactate dehydrogenase; PDHK, pyruvate dehydrogenase kinase; CAIX, carbonic anhydrase IX; SDF-1(CXCL12), stromal cell derived factor-1; CXCR4, chemokine receptor 4; VEGF, vascular endothelial growth factor; PDGF, platelet derived growth factor; Ang4, angiopoietin 4; ADRP, adipose differentiation related peptide; Glut-1, glucose transporter 1; EPO, erythropoietin; Oct4, octamer binding protein 4.
As described above, VHL inactivation in RCC stabilizes the HIF factors within the tumor cells. The HIF transcription factor complex has been demonstrated to transcriptionally induce the expression of genes involved in angiogenesis, anaerobic glucose metabolism, cell motility and metastasis, growth and survival, apoptosis, and telomere maintenance [31]. Notable genes induced by HIF involved in angiogenesis are VEGF, platelet derived growth factor (PDGF), as well as other pro-angiogenic factors such as angiopoietin-4 (Ang4) [33, 34]. These factors promote the proliferation, migration, and maturation of endothelial cells and pericytes supporting the recruitment of vessels or neoangiogenesis necessary to restore blood supply to an ischemic region, or in the case of RCC, this causes the rampant, disorganized proliferation of vessels in this highly vascular tumor. Additional factors include proteins involved in promoting the cellular switch to anaerobic glycolysis such as the glucose transporter, Glut1, enzymes of glucose metabolism such as hexokinase (HK) and lactate dehydrogenase (LDH), and the lactate transporter, MCT-4 [35-37]. This hypoxic repertoire of gene upregulation likely contributes to the highly glycolytic phenotype of RCC, even in the presence of abundant oxygen with which to perform oxidative phosphorylation for energy generation [38].
The distinction between RCCs which have high levels of only HIF1α, only HIF2α, or a combination of these two HIFα factors is an active area of investigation and not clearly understood. Mutations in VHL can specifically impact the balance of the different HIF factors [39]. Evidence from specific VHL disease-causing mutations which predispose to distinct patterns of tumor development (VHL disease subtypes 2A, 2B, 2C, and Chuvash Polycythemia—each of which carries a high risk for developing pheochromocytoma and greater or lesser penetrance for the hemangioblastoma and RCC phenotypes of the disease) suggests that the degree of HIF2α stabilization contributes substantially to the risk of RCC (Table 1) [40*]. Additionally, increasing evidence suggests that the dosage of HIF factors may influence the disease predisposition as evidenced by individuals who carry a very subtle alteration in the carboxyterminus of the VHL gene are predisposed to the autosomal recessive development of congenital polycythemia induced as a result of a small but not insignificant increase in HIF levels and a steady elevation of serum erythropoietin [41-45]. This prediction is further born out in an animal model of Chuvash polycythemia in which a homozygous mutation in the murine VHL allele confers a limited degree of HIF stabilization and elevated erythropoietin levels with concordant elevations in measured hematocrits in these animals [46].
Table 1. VHL disease subtypes and correlation with HIF 2α levels.
Mutations associated with each subtype of disease have been linked to differential regulation of the HIF2α.
| VHL Disease Subtype |
Hemangioblastoma | Pheochromocytoma | ccRCC | HIF 2α Levels |
|---|---|---|---|---|
| Type 1 | + | Low | + | +++ |
| Type 2A | + | + | Low | + |
| Type 2B | + | + | + | ++ |
| Type 2C | − | + | − | − |
|
Chuvash
Polycythemia |
− | − | − | +/− |
Much evidence points toward VHL associated HIF stabilization as an integral component of the renal cancer phenotype and a key to the maintenance of tumor growth. Indeed, the most significant evidence supporting this comes from an elegant analysis in which renal carcinoma cells lacking VHL and exclusively stabilizing HIF-2α were found to lose the capability to grow as a xenograft when HIF-2α levels were suppressed by shRNA [47]. These observations pinpoint HIF-2α as the potentially critical factor in renal tumorigenesis, and suggest its proclivity for development as a therapeutic target.
VHL: non-HIF activities
A variety of cellular effects caused by the loss of pVHL cannot be explained exclusively by the effects on HIF factor regulation. As an example, a protein involved in cellular polarity and cellular growth, atypical protein kinase C lambda was found to be ubiquitinated by the pVHL-containing E3 ubiquitin ligase complex VCB. This ubiquitination could be observed in both cell free and cellular ubiquitination systems and demonstrated dependence on pVHL [48]. This activity may play a critical role in the development of pheochromocytoma in VHL disease [49].
One interesting activity of pVHL which has recently received a great amount of attention is a role in the formation and maintenance of the primary cilia, a cellular structure essential to the determination of cell polarity. pVHL interacts with a complex that directs the orientation of microtubules and can be localized to the primary cilium. Loss of pVHL results in disorganization of newly formed microtubules and precludes the formation of the primary cilium [50, 51*]. pVHL has been demonstrated to play a role in the formation of the primary cilium, and failure to form this cellular appendage promotes the formation of cysts in the kidney as well as other organs [52, 53].
pVHL is also necessary for the organization of intercellular junctions and control of cellular permeability as well as the maintenance and determination of cell polarity [54, 55]. Similarly, pVHL interactions with various members of extracellular proteins are widely observed largely independent of HIF regulation. Such interactions include directed binding to hydroxylated collagen IV [56, 57]. Evidence suggests additionally that pVHL operates along other pathways promoting extracellular matrix assembly. pVHL interacts with fibronectin and a functional pVHL appears to be required to maintain functions in constructing or maintaining the extracellular matrix. As an example, a wild type copy of VHL is essential for the complete assembly of the fibronectin extracellular matrix [58]. In particular, an analysis of VHL mutants with and without destabilizing effects on HIF regulation demonstrated that pVHL appears to play a role in fibronectin deposition independent of functions regulating HIF stability [39, 59]. In support of this prediction, HIF-directed shRNA, which does abrogate cell growth in xenograft form has minimal impact on the development of the extra cellular matrix [60]. In addition to fibronectin matrix formation, pVHL has been found to have a role in actin and vinculin assembly and functions to restrict cellular motility [61]. This aspect of pVHL activity presents a potentially important tumor suppressive function and efforts are underway to explore the role of disordered extracellular matrix in the development of RCC.
VHL Gene Mutation in RCC
RCC characteristics resulting from VHL inactivation
Histologically, VHL inactivation contributes substantially to characteristic aspects of ccRCC. The defining feature of ccRCC is the histologic appearance of large cells with abundant cytoplasm packed with glycogen and neutral lipids. The high level of glucose metabolism observed in ccRCC likely accounts for the accumulation of immense quantities of glycogen, and further, the neutral lipid may be contributed to by the expression of the HIF target gene adipose differentiation related peptide (ADRP), a cell surface lipid transport molecule which may account for the cytoplasmic neutral lipid accumulation for which ccRCC was named [62]. Additional tumor specific metabolic characteristics can be correlated to HIF target gene activation such as the elevated lactate levels within RCC likely related to the high rates of glucose metabolism, and impairment of the oxidative phosphorylation process for generating ATP.
VHL gene mutation and HIF target gene activation can also contribute to the patient-specific physiology of RCC. Specifically, the paraneoplastic polycythemia can be directly associated with systemic elevated erythropoietin liberated inappropriately from the tumor. Polycythemia is observed only in a small percentage of patients with RCC, likely due to competing factors which contribute to blood loss and iron deficiency anemia or the anemia of chronic disease. This syndrome, however, should not be overlooked as it requires treatment with phlebotomy if the tumor is not amenable to surgical resection, and its presence related to ccRCC provides an indication to initiate systemic therapy for metastatic disease.
Interestingly, the expanding list of HIF target gene activation accounts for much of the unique attributes of conventional RCC: ADRP, neutral lipid accumulation and clear cell histology; VEGF and other angiokines, vascularity; erythropoietin, paraneoplastic polycythemia.
Biomarkers of VHL inactivation
Clinically, the presence of clear cell histology has served as a proxy for VHL inactivation and disregulation of the HIFα factors for several years. However, although this association is common, the rapidly expanding use of sophisticated molecularly targeted therapy demands improved understanding of an individual patient’s disease. As our understanding of the genetics behind RCC expands, so does our ability to use molecular markers as indicators of disease prognosis.
Carbonic anhydrase IX
Much attention has been focused on a subset of genes induced in response to HIF activation as surrogate markers of VHL inactivation. These transcriptionally regulated genes are selected based on high fidelity with the primary molecular event, either VHL loss or HIFα stabilization, and limited expression in non-cancer cells. These HIF regulated target genes may be developed as indicators of disease prognosis or therapeutic targets in their own right. The antigen carbonic anhydrase IX (CAIX, also called G250) was identified through a massive tissue microarray screen investigating prognostic factors for RCC. CAIX is a transcriptional target of the HIF-mediated transcriptional response to hypoxia or VHL loss [63-65]. CAIX is an immediate target of HIF activation and provides a convenient marker for clear cell histology tumors and has been associated with a favorable prognosis [66]. There may be some limitations to use this biomarker as a general test for RCC due to the high level expression overall, however, as a predictor of response to interleukin-2, this marker shows promise in identifying the subset of patients who are likely to respond well to this treatment and is the subject of a major clinical trial effort [67, 68]. The role this protein plays in ccRCC remains uncertain, although this gene may participate in regulating the tumor intracellular pH [69]. Immunostaining for the CAIX protein in renal carcinomas provides a potentially straightforward molecular prognostication test which could be rapidly implemented into current clinical prognostic schemas. For patients with high risk resected RCC, low CAIX expression predicted a poor outcome measured in rates of disease recurrence. Overall expression of CAIX decreased with development of metastasis and decreased CAIX levels are associated with shorter survival in metastatic RCC [70]. In a specific population of patients undergoing treatment with interleukin-2, CAIX, along with PTEN, was found to be an independent prognostic factor in predicting complete response to this treatment [71]. Further validation of this prognostic biomarker, however, is required before this test can be implemented as a tool for clinical decision making.
Additionally, as a potential tumor marker with cancer cell specificity, CAIX has been considered as a potential therapeutic target. A humanized antibody directed against CAIX has been developed and is currently undergoing investigation in clinical trials.
CXCL12 and CXCR4
A pair of targets of HIF1α transcriptional activation which have been implicated as biomarkers are the chemokine stromal derived factor-1 (SDF-1/CXCL12) and its receptor CXCR4 involved in directing cell migration. CXCR4 expression has been shown to correlate with metastatic potential in xenograft models of human RCC. Further, neutralization of CXCL12 using monoclonal antibodies in mouse models prevented RCC metastasis while sparing effects on tumor cell proliferation, apoptosis, or tumor angiogenesis, suggesting that CXCL12 and CXCR4 may play an important role in directing tumor cell migration and ultimately RCC propensity to form metastasis [72]. As biomarkers of VHL inactivation, CXCL12 and CXCR4 demonstrated an association with poor RCC-specific survival, which is consistent with their association with promoting tumor cell migration[73, 74]. However, reconciling the discrepancy between CXCL12/CXCR4 and CAIX as independently negative and positive predictors of outcome remains difficult. As surrogates of VHL inactivation should not equally predispose to poor or good risk disease, these findings may suggest deviations in the spectrum of HIF target genes activated in an individual tumor or other differences in tumors beyond VHL inactivation and HIF expression. This conundrum may prove impenetrable with the currently available data as the presence of VHL mutation may impact disease prognosis differently for patients with localized versus metastatic disease, and such prognostic markers must ultimately be viewed as independent risk factors for the time being requiring validation in multivariate studies.
VHL Mutation in RCC: Prognostic Implications
VHL mutation: surgical implications
As put forward above, evidence from studies specifically examining prognostic associations with VHL mutations suggests that the presence of an inactivating VHL mutation may associate with improved survival for patients with resectable disease undergoing nephrectomy. Thus, VHL mutation may be associated with a slower growing tumor, with more limited potential to have a priori metastatic disease at the time of resection, but may predispose to a more aggressive or difficult disease course for the later stages of disease. Interestingly, this correlation was most statistically significant for those patients with higher stage tumors going forward with a planned definitive surgical resection (stage III patients) as well as patients whose tumor was of a histologically high grade or those individuals presenting with symptomatic localized disease [75]. In this investigation, patients with stage IV disease undergoing debulking nephrectomy did not have the same positive correlation between VHL mutation and disease outcome. An independent analysis specifically examining of loss of function versus missense mutations in VHL, however, failed to demonstrate significant associations with prognosis except for a potential association of a specific missense mutation with the development of metastatic disease [76].
VHL mutation: implications for the development of targeted therapy
Somatic inactivation of the VHL gene is the most frequent genetic event observed in ccRCC, and it was the elucidation of this signaling pathway that led to the recent revolution in RCC targeted therapy. This review has already discussed the mechanism which directs the dysregulation of VEGF expression. In 2003, an important proof of principle study was undertaken to investigate the potential for clinical activity of therapies directed against one aspect of HIF disregulation, VEGF. In this phase II analysis, a humanized VEGF monoclonal antibody bevacizumab (©Avastin, Genentec, Inc.) was examined in previously treated patients with metastatic RCC. Patients were randomized to placebo or one of two dose levels groups, and patients receiving the higher dosage were found to have an improvement in progression free survival which was statistically significant compared to either the placebo or the low dosage group [77*]. Subsequently, a randomized placebo controlled study investigating the addition of bevacizumab to interferon in comparison to interferon alone (AVOREN) demonstrated an improvement in progression free survival from 5.4 months to 10.2 months as well as an improvement in tumor shrinkage [78]. Results from a similarly designed CALGB investigation are anticipated soon, and will lend additional information in the further exploitation of VEGF as a target for renal cell carcinoma therapy [79].
On the heels of this important study was the development of receptor tyrosine kinase inhibitors which target the VEGF receptor family, effectively neutralizing the signaling pathways activated as a result of growth factor engagement of the cognate receptor. The first of these small molecule inhibitors, sorafenib (©Nexavar, Bayer and Onyx Pharmaceutical), targets a panel of kinases including the VEGF receptor family, PDGF receptor, and raf kinase. This small molecule inhibitor was first investigated in RCC in a randomized discontinuation study which also demonstrated an improvement in progression free survival [80]. This prompted further analysis in the pivotal placebo controlled randomized phase III trial for patients with metastatic ccRCC who had developed progressive disease after prior cytokine therapy showing an improvement in progression free survival which led to the approval of this drug by the Federal Drug Administration (FDA) in December 2005 [81**]. Another VEGF receptor and PDGF receptor inhibitor, sunitinib (©Sutent, Pfizer Oncology) with an overlapping, but distinct portfolio of kinase inhibitory substrates was examined first in a pair of phase II studies in pretreated patients, and subsequently in a randomized phase III study comparing sunitinib to interferon (©Intron A, Shering-Plough) [82**, 83]. This landmark study demonstrated an impressive partial response rate of 31%, prolongation of progression free survival, and a trend in favor of improving overall survival, the benchmark of successful intervention in RCC. Sunitinib was approved for advanced RCC in January 2006. The impact of these new agents in the management of RCC cannot be understated, and the activity in this highly treatment resistant disease underscores the importance of this signaling pathway in RCC.
As further evidence that HIF activation may play a central role in RCC, a third agent, temsirolimus (©Torisel, Wyeth, Inc.) has been developed and recently approved for RCC based on a randomized phase III study which demonstrated an improvement in overall survival when compared to interferon or interferon in combination with temsirolimus [84**]. This derivative of rapamycin acts as an inhibitor of the mammalian target of rapamycin (mTOR) signaling pathway, which also intersects the HIF pathway by preventing mTOR-promoted protein translation of HIF via the S6 kinase. Thus, in the mTOR inhibited state HIF levels cannot be stabilized because the primary production of HIF is limited, as has been demonstrated elegantly in an in vitro study of HIF accumulation in response to hypoxic stimulation with and without rapamycin [85].
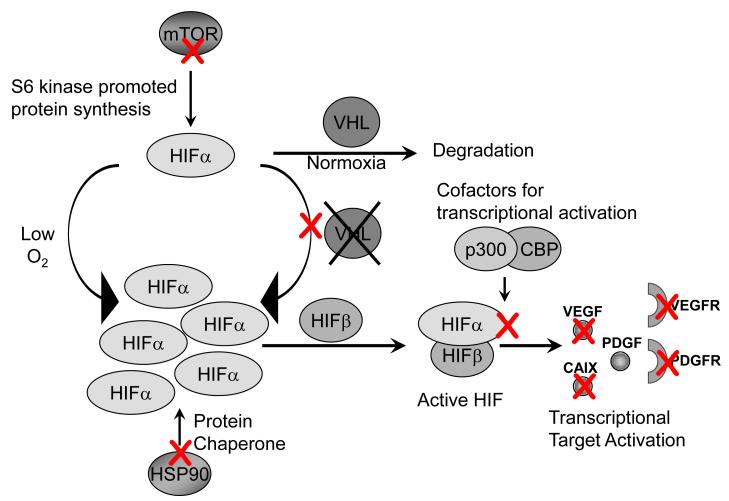
Multiple new drugs are on the horizon for development in RCC based on the biology of VHL inactivation in these tumors. Specific attempts to inhibit HIF accumulation and stability have been explored in a variety of ways (Figure 4). The inhibition of heat shock protein, HSP90, provides an opportunity to eliminate an important chaperone of HIF-1α which can promote its degradation [86, 87]. Other strategies of potential interest include the development of the natural compound, chetomin, as an inhibitor of HIF interaction with the co-factor p300, which is essential for HIF-mediated transcription [88]. A similar approach to high throughput screens of small molecule inhibitors has been applied to targeting cells lacking pVHL expression, with the compound Chromomycin A3 emerging as an agent with specific activity against these cells [89*]. Finally, further approaches to target genes activated on RCC as a result of HIF activation include the development of strategies to target CAIX, a HIF target discussed above as a potential biomarker of good risk disease. Efforts to target cells expressing CAIX have been moved into the clinical arena [90, 91]. These and other approaches to target components of the VHL loss/HIF stabilization/HIF target gene activation pathway have great potential for advancing the care of RCC patients in the near future, but remain to be validated in either accurate animal models or human disease.
Figure 4. Strategies to target the VHL/HIF pathway for therapeutic intervention.
Multiple strategies are currently being employed and explored to inhibit the pathological activation of this pathway. Red X indicates a strategy discussed in the review as a potential or actively used approach for treating patients with disease caused as a result of VHL loss or aberrant HIF activation. These include: inhibition of mTOR, preventing protein synthesis of HIF, inhibition of HSP90, an important chaperone involved in HIF stability during cytoplasmic/nuclear shuttling, inhibition of cell growth in cells lacking VHL, inhibition of HIF interaction with transcriptional cofactors p300 and CBP, direct inhibition of targets of HIF activation, and inhibition of signaling receptors activated downstream of HIF transcriptional activation.
VHL mutation: implications for response to therapy
The implications of RCC histology in each of the current molecularly targeted therapies remains an open question. Only the phase III study with temsirolimus included nonclear cell histologies, and this study found a benefit for patients independent of the histology, and by assumption the genetic history of the tumor. Evidence for activity of the multitargeted kinase inhibitors in the nonclear cell histologies of RCC remains limited to data from either the early phase clinical trials or compassionate use registry studies in place while the drugs were awaiting formal approval. It is clear that all of these new agents have activity in ccRCC, but the weight of evidence is strongest for the use of temsirolimus in those patients with papillary or chromophobe type RCC which are not associated with VHL mutations.
Furthermore, the prognostic relevance of somatic VHL alteration and the resultant stabilization of HIF1α and HIF2α have not been defined in the treatment of metastatic disease. It has long been appreciated that non-clear cell histologies of RCC were poorly responsive to immunotherapies. However, specific associations correlating response to treatment with VHL mutation have long been sought, although only suggestions of increased responsiveness to cytokine therapy among patients with missense VHL mutations as compared to functional VHL deletions have been observed [76].
In the era of targeted therapeutics, an enormously practical question looms. Are patients with VHL mutation-associated RCC tumors more likely to respond to the emerging therapeutic strategies? In one single institution series, 43 patients with metastatic RCC who received therapy with interferon-α plus bevacizumab (©Avastin, Genentech, Inc.), sunitinib (©Sutent, Pfizer, Inc.) or axitinib (Pfizer, Inc.) were examined for the correlation between activation status of the VHL gene and tumor response, time to tumor progression and overall survival, of whom 26 patients (60%) had evidence of VHL mutation or promoter methylation. In this preliminary study, an absolute improvement in objective response from 35% to 48% was observed, and further, a nearly doubling of time to tumor progression (from 7.4 months to 13.3 months) was observed in patients carrying a significant VHL mutation compared to those individuals for whom no VHL mutation was identified [92]. This preliminary observation warrants continued investigation, as VHL mutation status may provide an important predictive marker for response to VEGF-targeted therapy as well as future novel therapeutic strategies in RCC. An expanded set of patients from this series continues to demonstrate a trend in favor of response among tumors with an inactivating VHL mutation, although tumors without VHL mutation can clearly achieve responses to these agents [93].
Expert Commentary
The discoveries culminated in the elucidation of pVHL regulatory pathway have at this point defined RCC diagnosis, physiology, and treatment. Prior to the breakthroughs linking VHL mutation with HIF activation, angiogenesis, and tumor maintenance, little attention was paid to the histologic description of the tumor, and prognostic algorithms were almost entirely measuring the impact of the tumor on the individual patient rather than that of the genetic lesion on the behavior of the individual tumor. This view has undergone a radical evolution, with tumor histology taking priority status in determining a course of therapy. Knowledge of this pathway has provided a direct avenue for therapeutic development, with now three new FDA approved drugs providing rational therapeutic options, with dramatic shifts in expectations facing patients with both metastatic and limited disease. Currently, oncologists suffer from an embarrassment of riches, with a variety of active compounds and limited data to guide the therapeutic decision for an individual patient. Expanded studies of biomarkers to guide prognosis and therapeutic selection remain essential to bring this new era of RCC to the next level.
Five year view
At this time, the framework and magnitude of these molecular pathways has only just become evident as a diagnostic and therapeutic basis. As we learn more about the important aspects of HIF dysregulation related to such nuances as ratios of the different family members, levels of the stabilized proteins, and the individual portfolios of activated transcriptional targets, we will likely encounter essential biomarkers which impact the prognostic algorithm. Some of these molecular signatures will likely be incorporated into the next generation of prognostic factors. Further, all known molecular aspects of VHL loss are currently undergoing an exhaustive evaluation for the development of novel targeted agents for cancer treatment. Additionally, combinations of the currently approved agents are actively undergoing test to evaluate whether maximal blockade of this signaling pathway can be achieved with available drugs. Although large definitive studies are unlikely to be feasible, smaller clinical trials to address issues of sequential or combination use, and expanded use of biomarkers stands poised to guide therapeutic decision making based on pathologic, genetic, or molecular attributes of an individual tumor in the next step toward the integration of individualized medicine into day-to-day oncology decisions.
Key issues
VHL mutation is a common feature of clear cell renal cell carcinoma.
VHL inactivation imparts all or most of its impact on RCC via HIF stabilization.
VHL inactivation and upregulation of the HIF target gene CAIX is a favorable risk factor for surgically respectable RCC.
Although the recent surge in the development of targeted therapies evolved as a result of understanding the effects of VHL inactivation, it remains unclear what role VHL mutations play in predicting responses to these agents.
References
- 1.Aiello LP, George DJ, Cahill MT, et al. Rapid and durable recovery of visual function in a patient with von hippel-lindau syndrome after systemic therapy with vascular endothelial growth factor receptor inhibitor su5416. Ophthalmology. 2002;109(9):1745–51. doi: 10.1016/s0161-6420(02)01159-4. [DOI] [PubMed] [Google Scholar]
- 2.Seizinger BR, Rouleau GA, Ozelius LJ, et al. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988;332(6161):268–9. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- 3.Los M, Jansen GH, Kaelin WG, et al. Expression pattern of the von Hippel-Lindau protein in human tissues. Lab Invest. 1996;75(2):231–8. [PubMed] [Google Scholar]
- 4.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–20. doi: 10.1126/science.8493574. * This paper describes the pivotal identification of the VHL disease gene.
- 5.Vortmeyer AO, Yuan Q, Lee YS, Zhuang Z, Oldfield EH. Developmental effects of von Hippel-Lindau gene deficiency. Ann Neurol. 2004;55(5):721–8. doi: 10.1002/ana.20090. [DOI] [PubMed] [Google Scholar]
- 6.Vortmeyer AO, Huang SC, Pack SD, et al. Somatic point mutation of the wild-type allele detected in tumors of patients with VHL germline deletion. Oncogene. 2002;21(8):1167–70. doi: 10.1038/sj.onc.1205121. [DOI] [PubMed] [Google Scholar]
- 7.Kamura T, Koepp DM, Conrad MN, et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284(5414):657–61. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- 8.Clifford SC, Astuti D, Hooper L, et al. The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1alpha in renal cell carcinoma. Oncogene. 2001;20(36):5067–74. doi: 10.1038/sj.onc.1204602. [DOI] [PubMed] [Google Scholar]
- 9.Iwai K, Yamanaka K, Kamura T, et al. Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci U S A. 1999;96(22):12436–41. doi: 10.1073/pnas.96.22.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iliopoulos O, Kibel A, Gray S, Kaelin WG., Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1(8):822–6. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 11.Blankenship C, Naglich JG, Whaley JM, Seizinger B, Kley N. Alternate choice of initiation codon produces a biologically active product of the von Hippel Lindau gene with tumor suppressor activity. Oncogene. 1999;18(8):1529–35. doi: 10.1038/sj.onc.1202473. [DOI] [PubMed] [Google Scholar]
- 12.Gao J, Naglich JG, Laidlaw J, et al. Cloning and characterization of a mouse gene with homology to the human von Hippel-Lindau disease tumor suppressor gene: implications for the potential organization of the human von Hippel-Lindau disease gene. Cancer Res. 1995;55(4):743–7. [PubMed] [Google Scholar]
- 13.Richards FM, Schofield PN, Fleming S, Maher ER. Expression of the von Hippel-Lindau disease tumour suppressor gene during human embryogenesis. Hum Mol Genet. 1996;5(5):639–44. doi: 10.1093/hmg/5.5.639. [DOI] [PubMed] [Google Scholar]
- 14.Knauth K, Bex C, Jemth P, Buchberger A. Renal cell carcinoma risk in type 2 von Hippel-Lindau disease correlates with defects in pVHL stability and HIF-1alpha interactions. Oncogene. 2006;25(3):370–7. doi: 10.1038/sj.onc.1209062. [DOI] [PubMed] [Google Scholar]
- 15.Ohh M, Takagi Y, Aso T, et al. Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel-Lindau protein. J Clin Invest. 1999;104(11):1583–91. doi: 10.1172/JCI8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuda H, Hirai S, Takaki Y, et al. Direct interaction of the beta-domain of VHL tumor suppressor protein with the regulatory domain of atypical PKC isotypes. Biochem Biophys Res Commun. 1999;263(2):491–7. doi: 10.1006/bbrc.1999.1347. [DOI] [PubMed] [Google Scholar]
- 17.Ohh M, Park CW, Ivan M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2(7):423–7. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 18.Datta K, Sundberg C, Karumanchi SA, Mukhopadhyay D. The 104-123 amino acid sequence of the beta-domain of von Hippel-Lindau gene product is sufficient to inhibit renal tumor growth and invasion. Cancer Res. 2001;61(5):1768–75. [PubMed] [Google Scholar]
- 19.Stickle NH, Chung J, Klco JM, et al. pVHL modification by NEDD8 is required for fibronectin matrix assembly and suppression of tumor development. Mol Cell Biol. 2004;24(8):3251–61. doi: 10.1128/MCB.24.8.3251-3261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sufan RI, Ohh M. Role of the NEDD8 modification of Cul2 in the sequential activation of ECV complex. Neoplasia. 2006;8(11):956–63. doi: 10.1593/neo.06520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG., Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269(5229):1444–6. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 23.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 24.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98(17):9630–5. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 26.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 27.Jain S, Maltepe E, Lu MM, Simon C, Bradfield CA. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech Dev. 1998;73(1):117–23. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 28.Raval RR, Lau KW, Tran MG, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25(13):5675–86. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maynard MA, Ohh M. The role of hypoxia-inducible factors in cancer. Cell Mol Life Sci. 2007;64(16):2170–80. doi: 10.1007/s00018-007-7082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Covello KL, Simon MC. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37–54. doi: 10.1016/S0070-2153(04)62002-3. [DOI] [PubMed] [Google Scholar]
- 31.Kim WY, Kaelin WG., Jr. Molecular pathways in renal cell carcinoma--rationale for targeted treatment. Semin Oncol. 2006;33(5):588–95. doi: 10.1053/j.seminoncol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23(24):9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamakawa M, Liu LX, Belanger AJ, et al. Expression of angiopoietins in renal epithelial and clear cell carcinoma cells: regulation by hypoxia and participation in angiogenesis. Am J Physiol Renal Physiol. 2004;287(4):F649–57. doi: 10.1152/ajprenal.00028.2004. [DOI] [PubMed] [Google Scholar]
- 34.Igarashi H, Esumi M, Ishida H, Okada K. Vascular endothelial growth factor overexpression is correlated with von Hippel-Lindau tumor suppressor gene inactivation in patients with sporadic renal cell carcinoma. Cancer. 2002;95(1):47–53. doi: 10.1002/cncr.10635. [DOI] [PubMed] [Google Scholar]
- 35.Seagroves TN, Ryan HE, Lu H, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21(10):3436–44. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem. 1995;270(36):21021–7. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- 37.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281(14):9030–7. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 38.Leo C, Giaccia AJ, Denko NC. The hypoxic tumor microenvironment and gene expression. Semin Radiat Oncol. 2004;14(3):207–14. doi: 10.1016/j.semradonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Rathmell WK, Hickey MM, Bezman NA, et al. In vitro and in vivo models analyzing von Hippel-Lindau disease-specific mutations. Cancer Res. 2004;64(23):8595–603. doi: 10.1158/0008-5472.CAN-04-1430. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Zhang L, Zhang X, et al. Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and type 2B VHL mutations. Mol Cell Biol. 2007;27(15):5381–92. doi: 10.1128/MCB.00282-07. * This important paper provides evidence for a direct link between the VHL mutation and degree of HIF 2α activation.
- 41.Ang SO, Chen H, Hirota K, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32(4):614–21. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 42.Pastore YD, Jelinek J, Ang S, et al. Mutations in the VHL gene in sporadic apparently congenital polycythemia. Blood. 2003;101(4):1591–5. doi: 10.1182/blood-2002-06-1843. [DOI] [PubMed] [Google Scholar]
- 43.Ang SO, Chen H, Gordeuk VR, et al. Endemic polycythemia in Russia: mutation in the VHL gene. Blood Cells Mol Dis. 2002;28(1):57–62. doi: 10.1006/bcmd.2002.0488. [DOI] [PubMed] [Google Scholar]
- 44.Pastore Y, Jedlickova K, Guan Y, et al. Mutations of von Hippel-Lindau tumor-suppressor gene and congenital polycythemia. Am J Hum Genet. 2003;73(2):412–9. doi: 10.1086/377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordeuk VR, Sergueeva AI, Miasnikova GY, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103(10):3924–32. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]
- 46.Hickey MMLJ, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2a signaling and splenic erythrocytosis. Journal of Clinical Investigation. 2007 doi: 10.1172/JCI32614. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasuno K, Takabuchi S, Fukuda K, et al. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279(4):2550–8. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 48.Okuda H, Saitoh K, Hirai S, et al. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J Biol Chem. 2001;276(47):43611–7. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Nakamura E, Yang H, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8(2):155–67. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Kuehn EW, Walz G, Benzing T. Von hippel-lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development. Cancer Res. 2007;67(10):4537–40. doi: 10.1158/0008-5472.CAN-07-0391. [DOI] [PubMed] [Google Scholar]
- 51.Lolkema MP, Mans DA, Ulfman LH, et al. Allele-specific regulation of primary cilia function by the von Hippel-Lindau tumor suppressor. Eur J Hum Genet. 2007 doi: 10.1038/sj.ejhg.5201930. * This is a beautiful study demonstrating the association of pVHL protein and the primary cilium.
- 52.Esteban MA, Harten SK, Tran MG, Maxwell PH. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J Am Soc Nephrol. 2006;17(7):1801–6. doi: 10.1681/ASN.2006020181. [DOI] [PubMed] [Google Scholar]
- 53.Lutz MS, Burk RD. Primary cilium formation requires von hippel-lindau gene function in renal-derived cells. Cancer Res. 2006;66(14):6903–7. doi: 10.1158/0008-5472.CAN-06-0501. [DOI] [PubMed] [Google Scholar]
- 54.Kurban G, Hudon V, Duplan E, Ohh M, Pause A. Characterization of a von Hippel Lindau pathway involved in extracellular matrix remodeling, cell invasion, and angiogenesis. Cancer Res. 2006;66(3):1313–9. doi: 10.1158/0008-5472.CAN-05-2560. [DOI] [PubMed] [Google Scholar]
- 55.Calzada MJ, Esteban MA, Feijoo-Cuaresma M, et al. von Hippel-Lindau tumor suppressor protein regulates the assembly of intercellular junctions in renal cancer cells through hypoxia-inducible factor-independent mechanisms. Cancer Res. 2006;66(3):1553–60. doi: 10.1158/0008-5472.CAN-05-3236. [DOI] [PubMed] [Google Scholar]
- 56.Grosfeld A, Stolze IP, Cockman ME, et al. Interaction of hydroxylated collagen IV with the von hippel-lindau tumor suppressor. J Biol Chem. 2007;282(18):13264–9. doi: 10.1074/jbc.M611648200. [DOI] [PubMed] [Google Scholar]
- 57.Kurban G, Duplan E, Ramlal N, et al. Collagen matrix assembly is driven by the interaction of von Hippel-Lindau tumor suppressor protein with hydroxylated collagen IV alpha 2. Oncogene, Epub. 2007 doi: 10.1038/sj.onc.1210709. [DOI] [PubMed] [Google Scholar]
- 58.Ohh M, Yauch RL, Lonergan KM, et al. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell. 1998;1(7):959–68. doi: 10.1016/s1097-2765(00)80096-9. [DOI] [PubMed] [Google Scholar]
- 59.Hoffman MA, Ohh M, Yang H, et al. von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet. 2001;10(10):1019–27. doi: 10.1093/hmg/10.10.1019. [DOI] [PubMed] [Google Scholar]
- 60.Hughes MD, Kapllani E, Alexander AE, Burk RD, Schoenfeld AR. HIF-2alpha downregulation in the absence of functional VHL is not sufficient for renal cell differentiation. Cancer Cell Int. 2007;7:13. doi: 10.1186/1475-2867-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamada M, Suzuki K, Kato Y, Okuda H, Shuin T. von Hippel-Lindau protein promotes the assembly of actin and vinculin and inhibits cell motility. Cancer Res. 2001;61(10):4184–9. [PubMed] [Google Scholar]
- 62.Yao M, Tabuchi H, Nagashima Y, et al. Gene expression analysis of renal carcinoma: adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma. J Pathol. 2005;205(3):377–87. doi: 10.1002/path.1693. [DOI] [PubMed] [Google Scholar]
- 63.Lam JS, Belldegrun AS, Figlin RA. Tissue array-based predictions of pathobiology, prognosis, and response to treatment for renal cell carcinoma therapy. Clin Cancer Res. 2004;10(18 Pt 2):6304S–9S. doi: 10.1158/1078-0432.CCR-sup-040027. [DOI] [PubMed] [Google Scholar]
- 64.Kim HL, Seligson D, Liu X, et al. Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res. 2004;10(16):5464–71. doi: 10.1158/1078-0432.CCR-04-0488. [DOI] [PubMed] [Google Scholar]
- 65.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60(24):7075–83. [PubMed] [Google Scholar]
- 66.Grabmaier K, AdW MC, Verhaegh GW, Schalken JA, Oosterwijk E. Strict regulation of CAIX(G250/MN) by HIF-1alpha in clear cell renal cell carcinoma. Oncogene. 2004;23(33):5624–31. doi: 10.1038/sj.onc.1207764. [DOI] [PubMed] [Google Scholar]
- 67.Leibovich BC, Sheinin Y, Lohse CM, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25(30):4757–64. doi: 10.1200/JCO.2007.12.1087. [DOI] [PubMed] [Google Scholar]
- 68.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11(10):3714–21. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 69.Opavsky R, Pastorekova S, Zelnik V, et al. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics. 1996;33(3):480–7. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 70.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9(2):802–11. [PubMed] [Google Scholar]
- 71.Pantuck AJ, Fang Z, Liu X, et al. Gene Expression and Tissue Microarray Analysis of Interleukin-2 Complete Responders in Patients with Metastatic Renal Cell Carcinoma. Proceedings of the American Society of Clinical Oncology. 2007 Abstr 4535. [Google Scholar]
- 72.Pan J, Mestas J, Burdick MD, et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer. 2006;5:56. doi: 10.1186/1476-4598-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staller P, Sulitkova J, Lisztwan J, et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425(6955):307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 74.Zagzag D, Krishnamachary B, Yee H, et al. Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65(14):6178–88. doi: 10.1158/0008-5472.CAN-04-4406. [DOI] [PubMed] [Google Scholar]
- 75.Yao M, Yoshida M, Kishida T, et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002;94(20):1569–75. doi: 10.1093/jnci/94.20.1569. [DOI] [PubMed] [Google Scholar]
- 76.Kim JH, Jung CW, Cho YH, et al. Somatic VHL alteration and its impact on prognosis in patients with clear cell renal cell carcinoma. Oncol Rep. 2005;13(5):859–64. [PubMed] [Google Scholar]
- 77.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–34. doi: 10.1056/NEJMoa021491. * A landmark study establishing the potential for antiangiogenic therapy to improve outcomes in RCC.
- 78.Escudier B, Koralewski P, Pluzanska A, et al. A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-α2a vs placebo/interferon- α2a as first-line therapy in metastatic renal cell carcinoma. Proc Amer Society Clinical Oncology. 2007 Abstr 3. [Google Scholar]
- 79.Rini BI, Halabi S, Taylor J, Small EJ, Schilsky RL. Cancer and Leukemia Group B 90206: A randomized phase III trial of interferon-alpha or interferon-alpha plus anti-vascular endothelial growth factor antibody (bevacizumab) in metastatic renal cell carcinoma. Clin Cancer Res. 2004;10(8):2584–6. doi: 10.1158/1078-0432.ccr-03-0605. [DOI] [PubMed] [Google Scholar]
- 80.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(16):2505–12. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 81.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. ** A landmark clinical trial demonstrating a clinical benefit of receptor-targeted therapy in RCC
- 82.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. ** A pivotal study demonstrating improved response rate and time to progression of receptor-targeted therapy in RCC.
- 83.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 84.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81. doi: 10.1056/NEJMoa066838. ** Demonstration of improved overall survival with mTOR inhibition in a poor risk group of patients with RCC ** Demonstrated activity in both clear cell and non clear cell RCC.
- 85.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2(10):803–11. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 86.Mabjeesh NJ, Post DE, Willard MT, et al. Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res. 2002;62(9):2478–82. [PubMed] [Google Scholar]
- 87.Isaacs JS, Jung YJ, Mimnaugh EG, et al. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277(33):29936–44. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 88.Kung AL, Zabludoff SD, France DS, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6(1):33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 89.Sutphin PD, Chan DA, Li JM, et al. Targeting the loss of the von Hippel-Lindau tumor suppressor gene in renal cell carcinoma cells. Cancer Res. 2007;67(12):5896–905. doi: 10.1158/0008-5472.CAN-07-0604. * This manuscript describes the potential value of gene specific screens for the identification of new agents for RCC.
- 90.Davis ID, Liu Z, Saunders W, et al. A pilot study of monoclonal antibody cG250 and low dose subcutaneous IL-2 in patients with advanced renal cell carcinoma. Cancer Immun. 2007;7(14) [PMC free article] [PubMed] [Google Scholar]
- 91.Davis ID, Wiseman GA, Lee FT, et al. A phase I multiple dose, dose escalation study of cG250 monoclonal antibody in patients with advanced renal cell carcinoma. Cancer Immun. 2007;7:13. [PMC free article] [PubMed] [Google Scholar]
- 92.Rini BI, Jaeger E, Weinberg V, et al. Clinical response to therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: impact of patient characteristics and Von Hippel-Lindau gene status. BJU Int. 2006;98(4):756–62. doi: 10.1111/j.1464-410X.2006.06376.x. [DOI] [PubMed] [Google Scholar]
- 93.Choueiri TKVS, Rini BI, Elson P, Bhalla I, Jaeger E, Weinberg V, Waldman FM, Zhou M, Bukowski RM. R G. Use of Von-Hippel Lindau (VHL) mutation status to predict objective response to vascular endothelial growth factor (VEGF) -targeted therapy in metastatic renal cell carcinoma (RCC) Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2007 [Google Scholar]