Abstract
Background
Premature adrenarche (PA) is recognized to be a possible precursor of polycystic ovarian syndrome, type 2 diabetes mellitus and cardiovascular disease. Visceral adiposity and increased intramyocellular lipid (IMCL) are associated with insulin resistance and increased risk of cardiovascular disease.
Aim
To determine whether prepubertal girls with PA have altered visceral adiposity and/or increased muscle lipid content compared to prepubertal girls without PA using proton magnetic resonance imaging (MRI) and spectroscopy (1H MRS).
Patients and Methods
We performed total body dual energy X-ray absorptiometry (DXA) scans, MRI of the trunk, and MRS of the tibialis anterior muscle in the right calf on six girls with PA and eight prepubertal controls.
Results
Amount of visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (SAT), and VAT to SAT ratio did not differ significantly between the PA and control girls. Those with PA, however, had significantly greater IMCL than controls (p = 0.004).
Conclusions
This study adds further evidence that PA is not a benign condition, and future studies investigating early intervention with dietary and exercise counseling may help diminish potential risk for diabetes mellitus and/or cardiovascular disease.
Keywords: premature adrenarche, proton magnetic resonance spectroscopy, intramyocellular lipid, metabolic syndrome, diabetes mellitus, body composition
INTRODUCTION
Whereas adrenarche or ‘puberty of the adrenal gland’ is a normal maturational event, premature adrenarche (PA), defined by the appearance of sexual hair without other signs of puberty before age 8 years in girls and before age 9 years in boys, is secondary to early isolated maturation of the adrenal gland1. Until recently PA was considered a normal variant of puberty, but reports that hyperandrogenism is not benign in women, that metabolic alterations are present in prepubertal PA, that a history of PA is frequent in adolescents with polycystic ovarian syndrome (PCOS), and that postpubertal anovulation and hyperinsulinemic hyperandrogenism are more frequent in prepubertal girls with PA, have led to the recognition that PA may be premonitory of PCOS and cardiovascular disease2–8.
Metabolic abnormalities reported in prepubertal PA include decreased insulin sensitivity and IGFBP-1, hyperinsulinism, and increased free IGF-I and plasminogen activator inhibitor-1 (PAI-1)2,3,5,7,8. To date, only PAI-1 concentrations have been shown to predict progression to PCOS in a small subgroup of prepubertal girls with PA8. PCOS affects 5–10% of reproductive age women and is associated with metabolic syndrome, dyslipidemia, and type 2 diabetes mellitus4. Characterization of the prepubertal phenotype of children with PA could rationalize prospective risk assessment for PCOS, the metabolic syndrome, and associated morbidities.
The purpose of this pilot study was to determine whether prepubertal children with PA have altered adipose tissue partitioning to abdominal depots and/or increased muscle lipid content compared to prepubertal children without PA using proton magnetic resonance imaging (MRI) and spectroscopy (1H MRS). These body composition features which are associated with glucose intolerance and insulin resistance have not been previously investigated in PA.
PATIENTS AND METHODS
Study participants
The study group consisted of six girls with PA and eight girls without evidence of PA, who served as controls. The girls were recruited from the pediatric endocrinology service at Children’s Hospital of New York at Columbia-Presbyterian Medical Center or from St. Luke’s-Roosevelt Hospital Center. Informed consent was obtained from a parent or guardian and all girls above the age of 7 years signed assent. The protocol was approved by the Institutional Review Boards of Columbia-Presbyterian Medical Center and St. Luke’s-Roosevelt Hospital Center. All participants were evaluated by a pediatric endocrinologist. The diagnosis of PA was made based on the following clinical and laboratory criteria: i) pubarche prior to age 8 years; ii) androgen levels consistent with PA, i.e. Tanner II or less for testosterone, Δ4-androstenedione and DHEAS; iii) no evidence of pathological source of increased androgen or defect in adrenal steroidogenesis; iv) breasts Tanner Stage 1. The prepubertal controls had breasts and pubic hair Tanner Stage 1, and age, height, weight, and ethnic background matched as closely as possible to the patients with PA (Tables 1 and 2). Girls were excluded who were born before 37 weeks or were small for gestational age, had the presence or history of medical disorder or medication known to affect body composition, insulin secretion and sensitivity, or the GH–IGF-I axis (i.e. use of steroid or thyroid hormones), the presence of indwelling hardware (inability to undergo imaging studies), a first degree relative with type 1 or type 2 diabetes mellitus, or a maternal history of gestational diabetes.
TABLE 1.
Characteristics of MRI study participants
| Premature adrenarche | Controls | P value | |
|---|---|---|---|
| n | 6 | 8 | |
| Age (yr) | 7.98 ± 0.76 | 6.73 ± 0.77 | 0.02 |
| Racea | 2W/1AA/2H/1AH | 2W/2AA/4H | |
| BMI z-score | 0.67 ± 0.68 | 0.49 ± 0.90 | NS |
| Percent body fat by DXA | 26.0 ± 5.32 | 24.4 ± 10.7 | NS |
W = white; AA = African-American; H = Hispanic; AH = African-American and Hispanic.
NS = not significant.
TABLE 2.
Characteristics of MRS study participants
| Premature adrenarche | Controls | P value | |
|---|---|---|---|
| n | 5 | 5 | |
| Age (yr) | 7.84 ± 0.79 | 6.90 ± 0.97 | NS |
| Racea | 1W/1AA/2H/1AH | 1W/3AA/1H | |
| BMI z-score | 0.65 ± 0.77 | 0.78 ± 1.20 | NS |
| Percent body fat by DXA | 26.0 ± 5.95 | 27.0 ± 14.8 | NS |
W = white; AA = African-American; H = Hispanic; AH = African-American and Hispanic.
NS = not significant.
Body composition assessment
Body composition studies were performed in the Body Composition Unit of St. Luke’s-Roosevelt Medical Center. All studies were begun at 07.30 h, after overnight fasting.
MRI
MRI was used to measure visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT). Participants were placed supine on a 1.5 T scanner table (GE, Horizon 6x, Milwaukee, WI), with arms at their sides. Using a body coil and a T1-weighted fast spin echo sequence (TR/TE 300/14 ms, 256×256 data matrix), contiguous 10-mm axial whole-body MRI slices were obtained from T5 to the level of the ischial tuberosity. VAT depots compartmentalized included retro-peritoneal, mesenteric, and omental. All scans were analyzed in the Image Reading Center at St. Luke’s by the same observer, using SliceOMatic, V 4.0 image analysis software (Tomovision, Montreal, Canada) on PC computers (Gateway, PIII 500 MHz).
MRS
Single-voxel MRS was used to assess intramyocellular lipid (IMCL) in the tibialis anterior (TA) muscle in the right calf of each participant using the same 1.5 T GE MRI scanner, with a quadrature lower extremity volume coil9. Three-plane scout images were acquired to enable a 10×10×10 mm3 voxel to be prescribed within the TA muscle and positioned to avoid vascular structures and gross adipose tissue depots (Fig. 1A, inset). Spatially localized 1H spectra for this voxel were recorded using the standard single-voxel PRESS sequence with TE/TR 35/2000 ms, 2048 time-domain data points, a spectral width of 1000 Hz, and 128 excitations, and then processed as recently described9. IMCL and extramyocellular lipid (EMCL) levels were derived from the peak areas of intra- and extramyocellular CH2 resonances, respectively, and expressed as ratios relative to the unsuppressed water peak area (W) in the same voxel10,11.
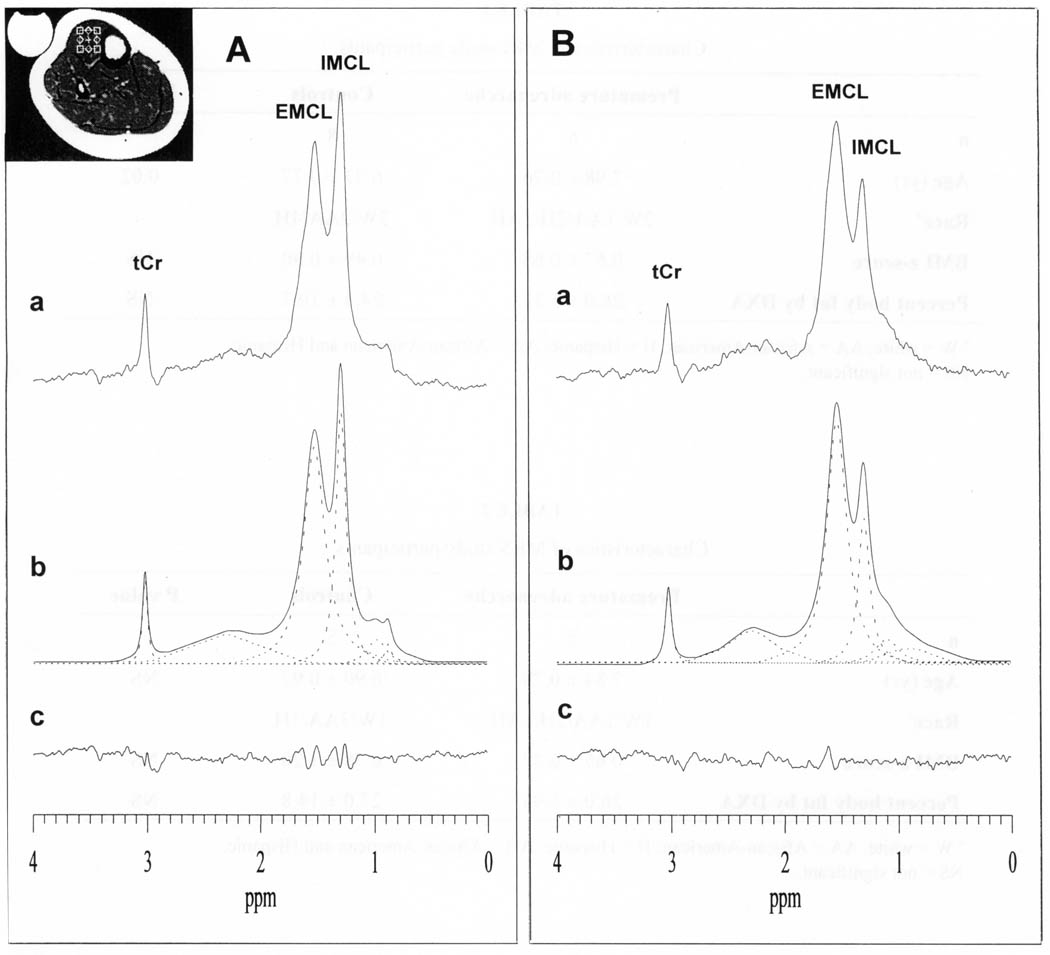
Fig. 1.
Representative 1H spectra from a 10×10×10 mm3 voxel in the tibialis anterior (TA) muscle (see voxel location in inset) of (A) an 8 year-old girl with premature adrenarche and (B) a weight- and age-matched normal control. Detected are resonances for intramyocellular lipid (IMCL), extramyocellular lipid (EMCL) and total muscle creatine (tCr). Sample frequency-domain non-linear least-squares Gauss-Lorentzian lineshape model-fitting of the TA muscle spectra for deriving IMCL, EMCL and tCr peak areas are also shown: (a) measured spectra; (b) calculated ‘best-fit’ spectra and (dashed lines) individual components of the ‘best-fit’ spectra; and (c) residual of the difference between the measured and calculated ‘best-fit’ spectra.
DXA
Each participant had a whole body dual energy X-ray absorptiometry (DXA) scan (GE Lunar Prodigy) for total body fat (kg) and lean body mass (kg) assessment. Pediatric software version 3.8G (Lunar Corp., Madison, WI) was used to estimate non-bone fat-free mass and fat mass in kilograms, percent body fat, total body bone mineral content (BMC) in grams, and total body bone mineral density (BMD) in grams per cm2, as described previously6.
Statistical analysis
Group data are presented as means ± SD. Differences in variables between the PA and control groups were tested for significance using Student’s t-test. Group differences of VAT and SAT were also tested by controlling for age, total abdominal fat by MRI and total body fat by DXA. Group difference in IMCL was also tested controlling for body mass index (BMI) z-score and total body fat by DXA.
RESULTS
Demographic and clinical characteristics
The demographic characteristics for the girls with PA and normal controls with viable MRI and MRS are provided in Tables 1 and 2, respectively. The girls with PA and controls did not differ with respect to race distribution or body weight, BMI z-score or total body fat as assessed by DXA (Table 1); however, the girls with PA were slightly older than the controls (p <0.02). Age was therefore entered as a covariate in comparing the MRI-derived body composition. By contrast, the subset of the girls with PA and controls who also underwent an MRS scan did not differ with respect to any of the clinical or demographic characteristics, including age (Table 2).
Body composition
MRI
Amount of VAT and SAT, as well as the VAT to SAT ratio derived by MRI, did not differ significantly between the girls with PA and controls (Table 3), even after controlling for the slight age difference between the groups.
TABLE 3.
Body composition results for girls with premature adrenarche (PA) and controls
| PA | Controls | P value | |
|---|---|---|---|
| VAT | 0.26 ± 0.08 | 0.33 ± 0.36 | NS |
| SAT | 2.60 ± 0.73 | 2.24 ± 1.51 | NS |
| VAT/SAT | 0.10 ± 0.02 | 0.14 ± 0.06 | NS |
| IMCL/W | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.004 |
VAT = visceral adipose tissue (liters); SAT = abdominal subcutaneous adipose tissue (liters); IMCL = increased intramyocellular lipid; W = water peak area.
MRS
Figure 1 shows 1H spectra from a voxel in the TA muscle (Fig. 1, inset) of a girl with PA (Fig. 1A) and of a control participant (Fig. 1B). Clearly resolved in both spectra are the resonances for IMCL at 1.28 ppm, EMCL at 1.50 ppm and total creatine (tCr) at 3.03 ppm. Also depicted in Figure 1 are the results of model-fitting the experimental spectra by minimizing the residual of the difference (Fig. 1c) between the measured (Fig. 1a) and calculated (Fig. 1b) spectra to obtain the area under each peak. Relative to the tCr peak, which is comparable between the two girls, the IMCL resonance is clearly elevated in the girl with PA compared to the control girl. In the aggregate, the mean IMCL level was significantly higher in the girls with PA than in the controls (p = 0.004) (Table 3). By contrast, levels of EMCL varied unpredictably from individual to individual, reflecting the relative amount of adipose tissue that each voxel contained, which varied randomly.
DISCUSSION
This study revealed that PA is associated with significantly elevated IMCL levels compared with prepubertal controls. Elevated skeletal muscle IMCL levels have been associated with insulin resistance, and have been observed in young and adult insulin-resistant individuals10–13. Our finding of elevated IMCL in PA provides further evidence that PA in girls is not a benign condition, and may be a harbinger of future medical conditions, such as PCOS, diabetes mellitus and the metabolic syndrome.
Events that might trigger the onset of adrenarche are not well understood. It has been suggested that insulin may play a permissive role, and girls with premature adrenarche have been documented with circulating markers of insulin resistance, including hyperinsulinism, decreased IGFBP-1, increased IGF-I and increased PAI-12,3,5,7,8. In earlier studies of girls with PA, elevated concentrations of plasma triglycerides were found compared with controls14,15.
The development of skeletal muscle insulin resistance, which may predate the onset of diabetes mellitus by decades16, has been postulated as one of the initial defects in the progression to type 2 diabetes mellitus. As plasma fatty acid levels rise, as is common in those at risk for diabetes mellitus, insulin stimulated phosphorylation of insulin receptor substrate (IRS)-1 is reduced, decreasing phosphatidylinositol-3 (PI-3) kinase activity and therefore glucose transporter 4 (GLUT4) translocation to the surface of skeletal muscle cells16,17. Mitochondrial ATP production is also decreased in young lean insulin resistant offspring of parents with type 2 diabetes mellitus, which may also predispose to IMCL accumulation12.
Indices of insulin sensitivity calculated from oral glucose tolerance tests correlate with IMCL in children and adolescents18 Two additional studies demonstrated that children and adolescents with insulin resistance or impaired glucose tolerance had increased IMCL compared to adolescents without evidence of insulin resistance10,13. IMCL has been measured previously in healthy 6–9 year-old boys, and found to be inversely related to the fasting glucose to insulin ratio (available in 12 of 41 boys)19.
Although we found IMCL to be increased in PA, this finding is independent of the other measures of body fat (e.g., VAT, SAT, BMI) derived by MRI or DXA, which did not differ significantly between girls with PA and matching controls. The lack of a difference in VAT and SAT between the two groups was unanticipated as our previous (unpublished) observation of an increased trunk to total body fat ratio by DXA in girls with PA would have suggested that they would also have higher abdominal fat.
Study limitations
The relatively small sample size of this study and the heterogeneous ethnic population distribution are potential limitations that may have reduced our ability to detect subtle differences in phenotypes between girls with PA and controls.
CONCLUSION
We report for the first time the presence of increased IMCL in young normal weight girls with PA. Increased IMCL has been closely associated with increased risk for type 2 diabetes mellitus. This study thus adds further evidence that PA is not a benign condition, and future studies need to investigate the effects of appropriate diet and exercise counseling in diminishing this potential risk for diabetes mellitus, PCOS and/or metabolic syndrome.
ACKNOWLEDGEMENTS
Funding for this study was provided by unrestricted grants from Genentech and Pfizer.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Korth-Schutz S, Levine LS, New MI. Evidence for the adrenal source of androgens in precocious adrenarche. Acta Endocrinol (Copenh) 1976;82:342–352. doi: 10.1530/acta.0.0820342. [DOI] [PubMed] [Google Scholar]
- 2.Oppenheimer E, Linder B, DiMartino-Nardi J. Decreased insulin sensitivity in prepubertal girls with premature adrenarche and acanthosis nigricans. J Clin Endocrinol Metab. 1995;80:614–618. doi: 10.1210/jcem.80.2.7852529. [DOI] [PubMed] [Google Scholar]
- 3.Ibanez L, Potau N, Zampolli M, Rique S, Saenger P, Carrascosa A. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J Clin Endocrinol Metab. 1997;82:2283–2288. doi: 10.1210/jcem.82.7.4084. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 5.Vuguin P, Linder B, Rosenfeld RG, Saenger P, DiMartino-Nardi J. The roles of insulin sensitivity, insulin-like growth factor I (IGF-I), and IGF-binding protein-1 and -3 in the hyperandrogenism of African-American and Caribbean Hispanic girls with premature adrenarche. J Clin Endocrinol Metab. 1999;84:2037–2042. doi: 10.1210/jcem.84.6.5722. [DOI] [PubMed] [Google Scholar]
- 6.Sopher AB, Thornton JC, Silfen ME, Manibo A, Oberfield SE, Wang J, Pierson RN, Jr, Levine LS, Horlick M. Prepubertal girls with premature adrenarche have greater bone mineral content and density than controls. J Clin Endocrinol Metab. 2001;86:5269–5272. doi: 10.1210/jcem.86.11.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silfen ME, Manibo AM, Ferin M, McMahon DJ, Levine LS, Oberfield SE. Elevated free IGF-I levels in prepubertal Hispanic girls with premature adrenarche: relationship with hyperandrogenism and insulin sensitivity. J Clin Endocrinol Metab. 2002;87:398–403. doi: 10.1210/jcem.87.1.8143. [DOI] [PubMed] [Google Scholar]
- 8.Ibanez L, Aulesa C, Potau N, Ong K, Dunger DB, de Zegher F. Plasminogen activator inhibitor-1 in girls with precocious pubarche: a premenarcheal marker for polycystic ovary syndrome? Pediatr Res. 2002;51:244–248. doi: 10.1203/00006450-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Shen W, Mao X, Wolper C, Heshka S, Dashnaw S, Hirsch J, Heymsfield SB, Shungu DC. Reproducibility of single- and multi-voxel 1H MRS measurements of intramyocellular lipid in overweight and lean subjects under conditions of controlled dietary calorie and fat intake. NMR Biomed. 2008;21:498–506. doi: 10.1002/nbm.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 12.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha R, Dufour S, Petersen KF, LeBron V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 14.Silfen ME, Manibo AM, McMahon DJ, Levine LS, Murphy AR, Oberfield SE. Comparison of simple measures of insulin sensitivity in young girls with premature adrenarche: the fasting glucose to insulin ratio may be a simple and useful measure. J Clin Endocrinol Metab. 2001;86:2863–2868. doi: 10.1210/jcem.86.6.7537. [DOI] [PubMed] [Google Scholar]
- 15.Ibanez L, Potau N, Chacon P, Pascual C, Carrascosa A. Hyperinsulinaemia, dyslipaemia and cardiovascular risk in girls with a history of premature pubarche. Diabetologia. 1998;41:1057–1063. doi: 10.1007/s001250051030. [DOI] [PubMed] [Google Scholar]
- 16.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 Suppl 2:S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage DB, Petersen KF, Shulman GI. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension. 2005;45:828–833. doi: 10.1161/01.HYP.0000163475.04421.e4. [DOI] [PubMed] [Google Scholar]
- 18.Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, Tamborlane WV, Caprio S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 19.Ashley MA, Buckley AJ, Criss AL, Ward JA, Kemp A, Garnett S, Cowell CT, Baur LA, Thompson CH. Familial, anthropometric, and metabolic associations of intramyocellular lipid levels in prepubertal males. Pediatr Res. 2002;51:81–86. doi: 10.1203/00006450-200201000-00015. [DOI] [PubMed] [Google Scholar]