SUMMARY
MUC4 is a heterodimeric membrane mucin, composed of a mucin subunit ASGP-1 (MUC4α) and a transmembrane subunit ASGP-2 (MUC4β), which has been implicated in the protection of epithelial cell surfaces. In the rat stratified corneal epithelium Muc4 is found predominantly in the most superficial cell layers. Since previous studies in other tissues have shown that Muc4 is regulated by TGF-β via a proteosomal degradation mechanism, we investigated the regulation of corneal Muc4 in stratified cultures of corneal epithelial cells. Application of proteosome or processing inhibitors led to increases in levels of Muc4, particularly in the basal and intermediate levels of the stratified cultures. These changes were accompanied by increases in Muc4 ubiquitination, chaperone association and incorporation into intracellular aggresomes. In contrast, treatment with TGF-β resulted in reduced levels of Muc4, which were reversed by proteosome inhibition. The results support a model in which Muc4 precursor is synthesized in all layers of the corneal epithelium, but Muc4 is degraded in basal and intermediate layers by a proteosomal mechanism at least partly dependent on TGF-β inhibition of Muc4 processing.
INTRODUCTION
Rat Muc4/SMC (sialomucin complex) is a heterodimeric membrane mucin composed of a mucin subunit ASGP-1 (called MUC4α in human) and a transmembrane subunit ASGP-2 (MUC4β in human) (Sherblom and Carraway, 1980; Carraway et al., 2002) The mucin in the rat is translated from a 9 kb transcript (Sheng et al., 1992; Wu et al., 1994) into a 300 kDa precursor protein (Sheng et al., 1990), which is cleaved into the two subunits by a proteolytic cleavage (Soto et al., 2003) early in its transit to the cell surface (Sheng et al., 1990). A second cleavage occurs at a similar time in some cells to release a soluble form of the mucin (Komatsu et al., 2002).
Several functions have been attributed to membrane mucins. One important function of the Muc4/SMC is as an anti-adhesive to act as a steric barrier at the cell surfaces of cells by which it is produced (Carraway et al., 2002). The membrane mucin may extend more than a micron from the cell surface. The soluble form of the mucin may aid this protective function by loose adsorption to the membrane mucin (McNeer et al., 1998b; Price-Schiavi et al., 1998b). A second function of the mucin is to regulate signaling from the membrane (Carraway et al., 2002). In this context Muc4/SMC binds the receptor ErbB2 and modulates its localization (Ramsauer et al., 2003), phosphorylation (Carraway et al., 1999; Jepson et al., 2002; Ramsauer et al., 2006) and downstream signaling (Jepson et al., 2002; Ramsauer et al., 2006). The anti-adhesive function of Muc4/SMC has both positive and negative aspects. Though it can protect epithelia from invasion, it also may disrupt normal cell-cell interactions if the mucin is overproduced. Such overproduction appears to occur in some carcinomas (Carraway et al., 2002). To avoid this problem, cells must have stringent mechanisms for controlling membrane mucin production.
An important, but little understood, aspect of Muc4/SMC is its varied distribution in different epithelia (Carraway et al., 2002), including both simple and stratified epithelia, as exemplified by the female reproductive tract, where its localization is cell and hormone dependent (McNeer et al., 1998a; Idris et al., 2000). Muc4/SMC in the corneal epithelium has been proposed to play a role in desquamation and homeostasis (Lomako et al., 2005). Consistent with this proposal immunohistochemical analyses of Muc4/SMC in the cornea indicate that it is limited to the most superficial layers of the stratified epithelium (Swan et al., 2002). Analyses of human MUC4 transcript shows its presence throughout the stratified epithelium. One answer to this discrepancy is that Muc4/SMC is regulated post-transcriptionally in the cornea, as it is in the mammary gland (Lomako et al., 2009). A possible clue to that regulation was our recent observation in tumor cells that Muc4/SMC can be degraded by the proteosome (Lomako et al., 2009). In the tumor cells this degradation is promoted by TGF-β, which blocks processing of the Muc4 precursor (Price-Schiavi et al., 2000), shunting it to proteosomal degradation (Lomako et al., 2009).
To address the mechanism by which Muc4 distribution is regulated in corneal epithelia, we have examined proteosomal degradation of Muc4/SMC in stratified corneal epithelial cell cultures, using immunoblotting and confocal microscopy for the analysis of Muc4/SMC together with proteosome inhibitors and N-glycosylation inhibitors to alter proteosome degradation. We have also used ubiquitin and chaperone analyses to monitor the mechanism leading to degradation. These combined results clearly show that proteosome degradation and TGF-β play roles in regulating the levels of Muc4/SMC in the corneal epithelial layers.
MATERIALS AND METHODS
Reagents
TGFβ was from R&D System, Inc, kifunensine (KIF) from Calbiochem, N-CBZ-ILE-GLU(O-t-BUTYL)ALA-LEUCINAL (PSI) and lactacystin from Sigma, Matrigel from BD Biosciences.
Rat Corneal Epithelium Primary Cultures
Fisher 344 rats were used throughout this study in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals, and corneal epithelial cell culture was performed as described earlier (Lomako et al., 2005). Briefly, dissected corneas were divided into six explants and placed epithelial side up on 12-well plastic plates (Falcon) coated one day earlier with Matrigel (50 μl per well). Explants were allowed to attach at 37°C in a humidified 5% CO2 incubator for 2.5 hours without medium and covered overnight with a drop of medium. Then, one ml of medium was added to the wells. Medium was changed every second day. Migration of epithelial cells from the explants onto the surface of the well was monitored by light microscopy.
Antibodies
Antibodies used during this study were developed in our laboratory: mAb 4F12 recognizing an extracellular epitope of ASGP-2 (Rossi et al., 1996), polyclonal C-pep raised in rabbits and directed against a peptide from the C-terminal cytoplasmic domain of ASGP-2 (Rossi et al., 1996) and polyclonal anti-ASGP-2 against isolated ASGP-2 (Sheng et al., 1989). Monoclonal anti-β-actin and anti-ubiquitin antibodies were from Sigma.
Immunoblotting
To analyze cellular proteins by immunoblotting, cell collection, lysis and immunoblotting were performed as described previously (Lomako et al., 2009). Briefly, Matrisperse-treated cells were washed twice with ice-cold phosphate-buffered saline (PBS) and solubilized for 5 min in boiling 2% SDS. Extracts were clarified by centrifugation at 14,000 × g for 10 min, and 30 μg protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Membranes were probed for 2 h with primary antibodies diluted in 1% bovine serum albumin-Triton tris buffer solution (BSA-TTBS) (Lomako et al., 2009), washed three times with TTBS and subsequently incubated for 1 hr with appropriate secondary antibody-horseradish peroxide-conjugates (Pierce, Rockford, IL) followed by protein visualization by the Renaissance™ Enhanced Chemiluminescence Kit (NEN Life Science Products, Inc., Boston, MA). In each case β-actin was used as a control for protein loading.
Immunoprecipitation
For immunoprecipitation, cells were suspended in RIPA buffer (150 mM NaCl, 1% Nonidet p-40, 0.5% inhibitors (Sigma-Aldrich). Cells were disrupted by four cycles of freezing and thawing, and extracts were clarified by centrifugation at 4000g. The supernatant (1 ml) was added to Protein G/A-agarose beads with bound antibodies. Samples were rotated overnight at 4°C, washed five times with ice-cold RIPA buffer and resuspended and boiled in SDS-PAGE sample buffer for 5 min. Immunoprecipitated proteins were analyzed by SDS-PAGE and immunoblotting with anti-ASGP-2 antibodies.
Immunofluorescence
Single explants were placed on transparent polyethylene terephtalate (PET) 1 μm mesh membranes of 12 well inserts (Falcon). Inserts were placed within the wells over explants seeded on Matrigel, and explants were allowed to attach. Then, 1 ml and 0.2 ml of medium were added, respectively, to each well and insert to assure connection of the medium environment for the inserts and wells. The explants growing under the insert served as a feeder for explants growing in the inserts. The membranes were excised and cultures of epithelial cells were processed for immunofluorescence analyses.
Laser confocal microscopy was performed at the University of Miami Core Analytical Imaging Facility with an LSM microscope (Carl Zeiss, GmbH, Germany) equipped with three laser sources. Cells were analyzed using a 63× water immersion objective. The images were collected and processed using LSM 510 software obtained from Zeiss.
RESULTS
Proteosome inhibitor effect on Muc4 levels in corneal epithelial cells
In recent studies we have shown that the level of Muc4 in a model tumor system can be regulated by proteosomal degradation (Lomako et al., 2009). Treatment of the tumor cells with proteosome inhibitors increased the amount of Muc4 detected by immunoblotting. Immunofluorescence analyses of the cells showed abundant Muc4 sequestered in aggresomes in these cells (Lomako et al., 2009). To determine whether Muc4 might be similarly regulated in rat corneal epithelial cells, we treated stratified 10 day cultures of these cells (Lomako et al., 2005) with the proteosome inhibitor PSI and analyzed for Muc4 by immunoblotting. As shown in Figure 1, proteosomal inhibition increased the level of Muc4 in the corneal epithelial cell cultures, though the increases were modest, less than those found in the tumor cells, probably due to a greater stability of the Muc4 in the corneal epithelial cells.
FIGURE 1.
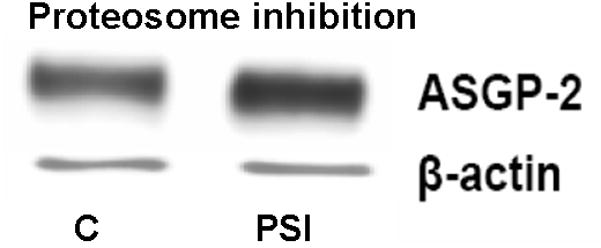
Proteasome inhibition increases the level of Muc4 in rat corneal epithelial cells. Corneal epithelial cells were cultured for 10 days, as previously described (Lomako et al., 2005), then treated with 0.1 μM PSI for 4 hours and analyzed by immunoblotting with anti-ASGP-2 as a surrogate for Muc4. C – untreated control cells, PSI – cells treated with 0.1 μM PSI.
Ubiquitination of Muc4 in corneal epithelial cells
One prediction of the proteosomal degradation model is that Muc4 will be ubiquitinated, and that ubiquitination will be increased in cells treated to block proteosome degradation as a consequence of buildup of undegraded Muc4. Figure 2 shows a low level of ubiquitinated Muc4 in untreated cells, determined by sequential immunoprecipitation and immunoblotting, presumably because the degradation process is very efficient in removing the ubiquitinated protein. However, there is a marked increase in the level of ubiquitinated Muc4 in the cell cultures treated with proteosome inhibitor, as predicted by the proteosomal degradation model.
FIGURE 2.
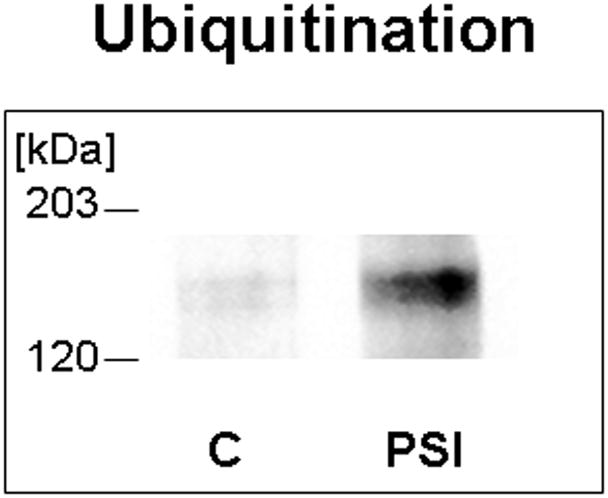
Muc4 is ubiquitinated in rat corneal epithelial cells, and the level of ubiquitinated Muc4 increases after proteasome inhibition. Protein from RIPA extracts of the cells was immunoprecipitated with anti-ASGP-2 polyclonal antibody coupled to A/G-agarose and immunoblotted with anti-ubiquitin antibody. C – untreated control cells, PSI – cells treated for 4 hours with 0.1 μM PSI.
Kifunensin disruption of Muc4 processing reduces its levels in corneal epithelial cells
Proteosome degradation can be altered in cells by specific interference with glycoprotein processing, such as with the mannosidase inhibitor kifunensin, which blocks processing of N-linked oligosaccharides. This block in processing increases proteosome degradation of affected proteins. More importantly, the processing block traps the affected proteins inside the cells. As predicted by the proteosome degradation model, the level of Muc4 in the corneal epithelial cell cultures is reduced by treatment with kifunensin (Fig. 3) as a consequence of the increase in its proteosomal degradation.
FIGURE 3.
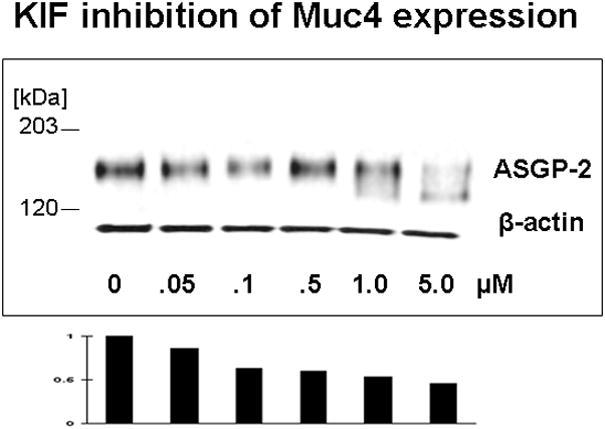
Kifunensin (KIF), a type 1 mannosidase inhibitor which disrupts proper processing of N-glycosylated glycoproteins, reduces the level Muc4 in cornea epithelial cells. Corneal epithelial cells from 10 day cultures were treated with indicated concentrations of KIF for 18 hours and analyzed by immunoblotting.
Complexes of Muc4 with calnexin and calreticulin
Membrane proteins undergoing endoplasmic reticulum-associated degradation, as proposed in our model for Muc4 (Lomako et al., 2009), form complexes with the chaperones calnexin and calreticulum. By blocking the Muc4 oligosaccharide processing, with the inhibitor α-butyl deoxynanojirimycin, which represses proteosomal degradation, increased association of the Muc4 with these chaperones was observed by sequential immunoprecipition and immunoblotting (Fig. 4), as predicted for the Muc4 proteosomal degradation model.
FIGURE 4.
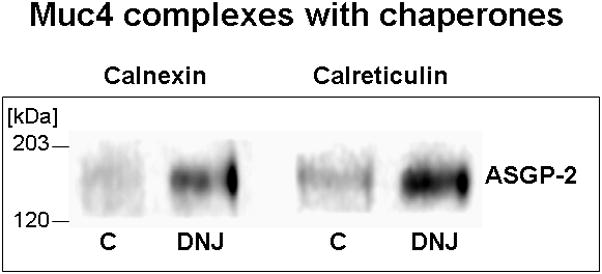
Muc4 complexes with endoplasmic reticulum chaperones calnexin and calreticulin are increased by treatment of cells with α-butyl deoxynanojirimycin (DNJ), the inhibitor of glucose trimming, which represses endoplasmic reticulum degradation. Cells were treated overnight with 0.5 mM DNJ. Protein was extracted in RIPA buffer, immunoprecipitated with anti-calnexin or anti-calreticulin antibodies and immunoblotted with monoclonal 4F12 Abs against ASGP-2 subunit.
Localization of Muc4 in basal and medial layers of stratified corneal epithelial cell cultures with aberrant processing
The key question concerning the proteosome degradation is whether it contributes to the distribution of the Muc4 in the different cell layers of the cornea, i.e. whether it can explain the increased basal to superficial distribution. To address this question, Muc4 in treated and untreated stratified corneal epithelial cell cultures was analyzed by confocal microscopy using a double label procedure. Cell surface Muc4 was analyzed by antibody labeling with the monoclonal antibody 4F12 (Rossi et al., 1996), detected with green-labeled second antibody, without permeabilization of the cells. The cells were then permeabilized and intracellular Muc4 was analyzed with polyclonal antibody c-pep (Pflugfelder et al., 2000), detected with red-labeled second antibody. As shown in panel A of Figure 5, untreated cells primarily exhibit the superficial cell surface Muc4 and show no evidence of Muc4 in the basal layers. In contrast, in cultures treated with kifunensin (panel B), the cells exhibit extensive amounts of intracellular Muc4 in the intermediate levels and even some in the basal layers, distributed in aggesome-like structures, as a consequence of the block in processing and traffic to the cell surface. More importantly, cultures treated with proteosome inhibitor (panel C) also exhibit extensive amounts of intracellular Muc4 in the intermediate levels and basal layers. Little effect is observed on the cell surface Muc4, as this would require internalization and lysosomal degradation, which we have observed (unpublished observations), but which is not affected by these inhibitors. These results are consistent with the proteosome degradation model, in which Muc4 is being synthesized in all layers of the epithelium but is primarily degraded in the basal and intermediate layers.
FIGURE 5.
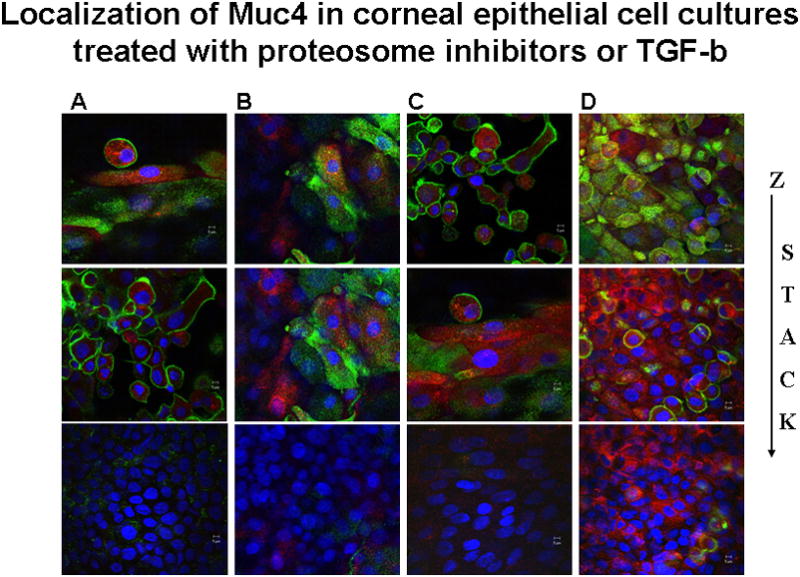
Localization of Muc4 in corneal epithelial cell cultures treated with KIF, proteasome inhibitor or TGF-β. XY- projection of three-color immunofluorescence staining. Cell membrane bound Muc4 was labeled on non-permeabilized cells with 4F12 mAb directed to an extracellular epitope of Muc4, followed by anti-mouse Alexa-Fluor 488 (green). Subsequently cells were permeabilized with 0.2% Triton X100 and intracellular Muc4 was labeled with polyclonal anti C-pep antibodies followed by Alexa-594 anti-rabbit antibodies (red). Nuclei were visualized by DAPI. C – untreated cells; KIF – cells treated for 18 hours with 5 μM KIF, the α-mannosidase inhibitor; PSI – cells treated for 4 hours with 0.01 μM PSI, the proteasome inhibitor; and TGF-β – cells treated with 0.4 nM TGF-β for 18 hours. Horizontal rows are superficial (S), intermediate (I) and basal (B) layers of cells.
The increase in intracellular Muc4 in cultures treated with proteosome inhibitors was further demonstrated by cell surface biotinylation. Cultures treated with increasing concentrations of proteosome inhibitor were biotinylated to detect cell surface Muc4. Biotinylated and unbiotinylated proteins were separated using a streptavidin column procedure, then immunoblotted for quantitation of Muc4. Figure 6 shows the increase in the ratio of intracellular to cell surface Muc4 with KIF, which blocks transit to the cell surface, or with proteosome inhibition by PSI. The results are consistent with the proteosome degradation model for corneal Muc4.
FIGURE 6.
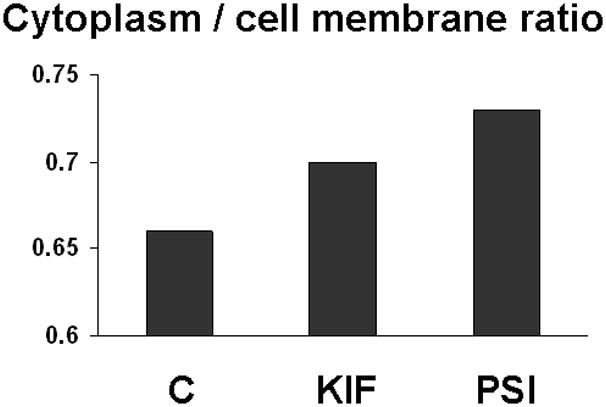
Increased intracellular Muc4 in cells with blocked proteosomal degradation. Cells grown on polycarbonate filters were subjected to simultaneous biotinylation from apical and basal sides by using PinpointTM Cell Surface Protein Isolation Kit (Pierce). Proteins from the biotinylated cells were extracted into lysis buffer, and the extracts were centrifuged for 20 min at 14 000 × g. The pellet was solubilized in sample solubilizing buffer (BioRad). Soluble biotinylated proteins were immobilized on NeutrAvidinTM gel. Protein from extract, pellet, run-through, column wash and eluate from avidin column were subjected to SDS-PAGE and Western blotting with 4F12 mAbs. Blots were scanned and analyzed as previously described and then normalized to initial volume of extract. C – control cells; KIF – cells treated with 5 μM kifunensine; and PSI - cells treated with 0.01 μM PSI.
Endoplasmic reticulum localization of Muc4 in corneal epithelial cell cultures with aberrant processing
The proteosome degradation model requires degradation of Muc4 in the endoplasmic reticulum. Double label confocal analysis of Muc4 in corneal epithelial cultures with the endoplasmic reticulum marker protein disulfide isomerase showed extensive co-localization, particularly in cells treated to repress proteosomal degradation (Fig. 7). Most of the Muc4 not co-localized with the protein disulfide isomerase is present in cytoplasmic particulate structures, presumably aggresomes.
FIGURE 7.
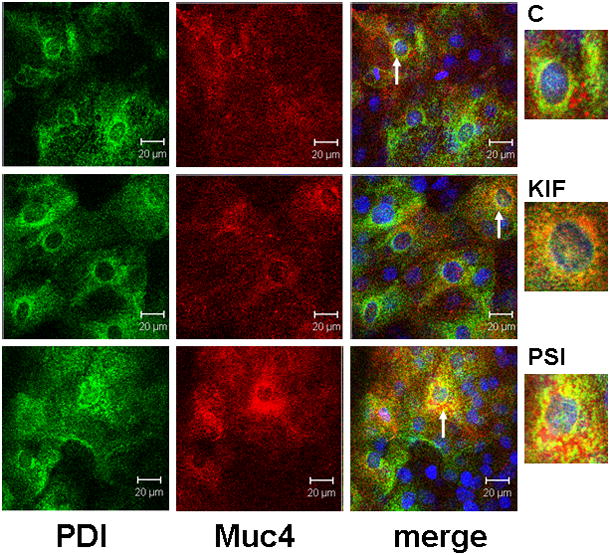
ER localization of Muc4 in cells with blocked proteosomal degradation using XY- projection of three-color immunofluorescence staining. Cells were permeabilized according to the instructions with the SelectFX Alexa Fluor 488 endoplasmic reticulum-labeling kit. Proteins were double-labeled with polyclonal anti-Muc4 and with monoclonal anti protein disulphide isomerase (PDI). Muc4 was visualized with Alexa Fluor 594 (red) and PDI with Alexa Fluor 488 (green) antibodies. Nuclei were stained with DAPI. C – untreated cells; KIF – cells treated for 18 hours with 5 μM KIF, the α-mannosidase inhibitor; and PSI – cells treated for 4 hours with 0.01 μM PSI, the proteasome inhibitor. Insets show areas noted by arrows.
TGF-β effects on Muc4 levels in corneal epithelial cells
Our previous work on mammary epithelial (Price-Schiavi et al., 1998a, 2000) and tumor (Lomako et al., 2009) cells has shown that TGF-β is a potent post-transcriptional regulator of Muc4 expression, blocking processing of the Muc4 precursor and shunting it to the proteosome. Figure 8 shows by immunoblotting that TGF-β also inhibits Muc4 expression in rat corneal epithelial cell cultures. Interestingly, the TGF-β treatment also increases the level of intracellular Muc4, particularly in the basal and intermediate cell layers (Fig. 5, panel D), possibly because the TGF-β effect overloads the proteosome system and shunts some of the Muc4 to aggresomes. These effects clearly demonstrate that Muc4 is being synthesized in all layers of the corneal epithelium, and that TGF-β can serve as a regulator of its expression in the stratified corneal epithelium.
FIGURE 8.
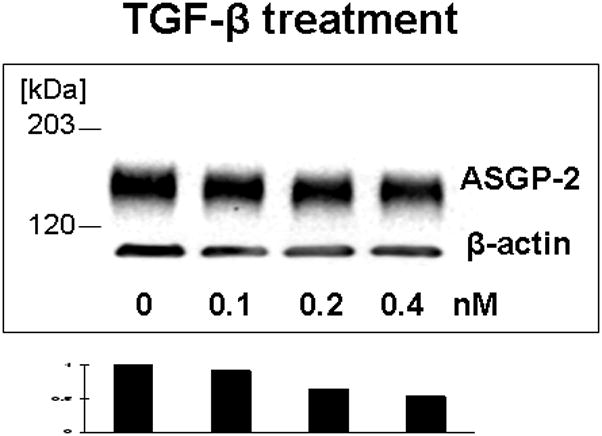
TGF-β reduces Muc4 in corneal epithelial cells. Cell were grown for 10 days in culture, then treated for 18 hours with different concentrations of TGF-β. 20 μg of protein extracted to 2% boiling SDS from each cell type was subjected to SDS-PAGE and Western blotting with 4F12 mAbs recognizing the ASGP-2 subunit of Muc4. Blots were scanned and analyzed by http://rsb.info.nih.govprogram.
DISCUSSION
Muc4 is one of three membrane mucins present at corneal cell surfaces (Gipson, 2004) which may contribute to protection of the cornea. Interestingly, previous studies have shown Muc4 restricted to the most superficial layers of the stratified corneal epithelium (Swan et al., 2002). Based on our previous studies of Muc4 regulation (Price-Schiavi et al., 1998a, 2000; Lomako et al., 2009), we hypothesized that this distribution might be due to post-translational regulation of the Muc4 by proteosomal regulation. Several observations support this hypothesis. 1) Inhibition of proteosomal degradation increases Muc4 in the epithelia. 2) This inhibition also demonstrates the presence of Muc4 protein in the basal and intermediate levels of the stratified epithelia, specifically in intracellular structures. 3) Inhibition of Muc4 processing or proteosomal degradation increases Muc4 ubiquitination and association with chaperones that are important in the endoplasmic reticulum degradation process.
Equally important is what regulates this proteosomal degradation. Based on our work in mammary epithelial (Price-Schiavi et al., 1998a, 2000) and tumor (Lomako et al., 2009) cells, we proposed a role for TGF-β. This hypothesis is supported by our observation that TGF-β represses levels of Muc4 in the corneal epithelial cells, an effect which can be blocked by proteosomal inhibitors. Moreover, studies by others have shown that TGF-β is produced primarily by cells of the more basal layers of the corneal epithelium (Tuli et al., 2006). From this work we propose that TGF-β produced by these corneal epithelial cells represses the cleavage of the Muc4 precursor, as we have shown previously (Price-Schiavi et al., 2000). Failure of the uncleaved precursor to fold correctly leads to its degradation by the endoplasmic reticulum-associated proteosomal degradation pathway, thus using a quality control mechanism for quantitative control and preventing production of the mucin in the layers of the stratified epithelium.
Muc4 can act as either an anti-adhesive (Komatsu et al., 1997) or a promoter of cell signaling (Carraway et al., 1999). In the former capacity it might actually damage the integrity of the stratified epithelium if it were highly expressed in the basal or intermediate layers. Nevertheless, it can perform important functions in the protection of the superficial surface. How the signaling function operates in the corneal epithelium is unknown. However, we have shown a role for Muc4 signaling in the damage response of airway epithelium, which is a pseudo-stratified epithelium (Theodoropoulos et al., 2009).
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant numbers: RO1 CA52498 and RO1 EY12343
References
- Carraway KL, III, Rossi EA, Komatsu M, Price-Schiavi SA, Huang D, Guy PM, Carvajal ME, Fregien N, Carraway CAC, Carraway KL. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- Carraway KL, Perez A, Idris N, Jepson S, Arango M, Komatsu M, Haq B, Price-Schiavi SA, Zhang J, Carraway CAC. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acids Res Molec Biol. 2002;171:149–185. doi: 10.1016/s0079-6603(02)71043-x. [DOI] [PubMed] [Google Scholar]
- Gipson IK. Distribution of mucins at the ocular surface. Exp Eyel Res. 2004;78:379–388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- Idris N, Carraway KL. Regulation of Sialomucin Complex/Muc4 expression in rat uterine luminal epithelial cells by transforming growth factor-: implications for blastocyst implantation. J Cell Physiol. 2000;185:310–316. doi: 10.1002/1097-4652(200011)185:2<310::AID-JCP16>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Jepson S, Komatsu M, Haq B, Arango ME, Huang D, Carraway CAC, Carraway KL. Muc4/Sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27kip, but does not activate mitogen-activated kinase or protein kinase B/Akt pathways. Oncogene. 2002;21:7524–7532. doi: 10.1038/sj.onc.1205970. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Carraway CAC, Fregien NL, Carraway KL. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245–33254. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Arango ME, Carraway KL. Synthesis and secretion of Muc4/Sialomucin Complex: Implication of intracellular proteolysis. Biochem J. 2002;368:41–48. doi: 10.1042/BJ20020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomako J, Lomako W, Carraway CAC, Carraway KL. Non-apoptotic desquamation of cells from corneal epithelium: putative role for Muc4/sialomucin complex in cell release and survival. J Cell Physiol. 2005;202:115–124. doi: 10.1002/jcp.20101. [DOI] [PubMed] [Google Scholar]
- Lomako W, Lomako J, Carraway CAC, Carraway KL. Regulationof membrane mucin Muc4 via proteosomal degradation. J Cell Biochem. 2009;107:797–802. doi: 10.1002/jcb.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeer RR, Carraway CAC, Fregien NL, Carraway KL. Characterization of the expression and steroid hormone control of sialomucin complex in the rat uterus: Implications for uterine receptivity. J Cell Physiol. 1998a;176:110–119. doi: 10.1002/(SICI)1097-4652(199807)176:1<110::AID-JCP13>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- McNeer RR, Huang D, Fregien NL, Carraway KL. Sialomucin complex in the rat respiratory tract: a model for its role in epithelial protection. Biochem J. 1998b;330:737–744. doi: 10.1042/bj3300737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Liu Z, Monroy D, Li D-Q, Carvajal ME, Price-Schiavi SA, Idris N, Solomon A, Perez A, Carraway KL. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. 2000;41:1316–1326. [PubMed] [Google Scholar]
- Price-Schiavi SA, Carraway CAC, Fregien N, Carraway KL. Posttranscriptional regulation of a milk membrane protein, the sialomucin complex (ascites sialoglycoprotein (ASGP)-1/ASGP-2, rat Muc4) by TGFβ. J Biol Chem. 1998a;273:35228–35237. doi: 10.1074/jbc.273.52.35228. [DOI] [PubMed] [Google Scholar]
- Price-Schiavi SA, Meller D, Jing X, Merritt J, Carvajal ME, Tseng SCG, Carraway KL. Sialomucin complex at the rat ocular surface: a new model for ocular surface protection. Biochem J. 1998b;335:457–463. doi: 10.1042/bj3350457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Schiavi SA, Zhu X, Aquinin R, Carraway KL. Sialomucin complex (rat muc4) is regulated by transforming growth factor in mammary gland by a novel post-translational mechanism. J Biol Chem. 2000;275:17800–17807. doi: 10.1074/jbc.275.23.17800. [DOI] [PubMed] [Google Scholar]
- Ramsauer VP, Carraway CAC, Salas PJI, Carraway KL. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, translocates ErbB2 to the apical surface in polarized epithelial cells. J Biol Chem. 2003;2003:30142–30147. doi: 10.1074/jbc.M303220200. [DOI] [PubMed] [Google Scholar]
- Ramsauer VP, Pino V, Farooq A, Carraway CAC, Salas PJI, Carraway KL. Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell. 2006;17:2931–2941. doi: 10.1091/mbc.E05-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA, McNeer R, Price-Schiavi SA, Komatsu M, Van den Brande JMH, Thompson JF, Carraway CAC, Fregien NL, Carraway KL. Sialomucin complex, a heterodimeric glycoprotein complex: Expression as a soluble, secretable form in lactating mammary gland and colon. J Biol Chem. 1996;271:33476–33485. doi: 10.1074/jbc.271.52.33476. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Vanderpuye OA, Hull SR, Carraway CAC, Carraway KL. Topography and microfilament core association of a cell surface glycoprotein of ascites tumor cell microvilli. J Cell Biochem. 1989;40:453–466. doi: 10.1002/jcb.240400406. [DOI] [PubMed] [Google Scholar]
- Sheng ZQ, Hull SR, Carraway KL. Biosynthesis of the cell surface sialomucin complex of ascites 13762 rat mammary adenocarcinoma cells from a high molecular weight precursor. J Biol Chem. 1990;265:8505–8510. [PubMed] [Google Scholar]
- Sheng Z, Wu K, Carraway KL, Fregein N. Molecular cloning of the transmembrane component of the 13762 mammary adenocarcinoma sialomucin complex. A new member of the epidermal growth factor superfamily. J Biol Chem. 1992;267:16341–16346. [PubMed] [Google Scholar]
- Sherblom AP, Carraway KL. A complex of two cell surface glycoproteins from ascites mammary adenocarcinoma cells. J Biol Chem. 1980;255:12051–12059. [PubMed] [Google Scholar]
- Soto P, Price-Schiavi SA, Carraway KL. SMAD2 and SMAD7 involvement in the post-translational regulation of Muc4 via the transforming growth factor-β and interferon-γ pathways in rat mammary epithelial cells. J Biol Chem. 2003;278:20338–20344. doi: 10.1074/jbc.M301886200. [DOI] [PubMed] [Google Scholar]
- Swan JS, Arango ME, Carraway CAC, Carraway KL. An ErbB2-Muc4 complex in rat ocular surface epithelia. Curr Eye Res. 2002;24:397–402. doi: 10.1076/ceyr.24.5.397.8521. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos G, Carraway CAC, Carraway KL. MUC4 involvement in ErbB2/ErbB3 phosphorylation and signaling in reponse to airway cell mechanical injury. J Cell Biochem. 2009;107:112–122. doi: 10.1002/jcb.22106. [DOI] [PubMed] [Google Scholar]
- Tuli SS, Liu R, Chen C, Blalock TD, Goldstein M, Schultz GS. Immunohistochemical localization of EGF, TGF-alpha, TGF-beta, and their receptors in rat corneas during healing of excimer laser ablation. Curr Eye Res. 2006;31:709–719. doi: 10.1080/02713680600837390. [DOI] [PubMed] [Google Scholar]
- Wu K, Fregien N, Carraway KL. Molecular cloning and sequencing of the mucin subunit of a heterodimeric, bifunctional cell surface glycoprotein complex of ascites rat mammary adenocarcinoma cells. J Biol Chem. 1994;269:11950–11955. [PubMed] [Google Scholar]


