Abstract
Dentin matrix protein-1 (DMP1) is a major synthetic product of hypertrophic chondrocytes and osteocytes. Previous in vitro studies showed full-length DMP1 inhibits hydroxyapatite (HA) formation and growth, while its N-terminal fragment (37K) promotes HA formation. Since there are 3 fragments within the mineralized tissues [N-terminal, C-terminal (57K), and a chondroitin-sulfate-linked N-terminal fragment (DMP1-PG)], we predicted that each would have a distinct effect on mineralization related to its interaction with HA. In a gelatin-gel system, 37K and 57K fragments were both promoters of HA formation and growth; DMP1-PG was an inhibitor. The secondary structures of the 3 fragments and the full-length protein in the presence and absence of Ca2+ and HA determined by FTIR showed that the full-length protein undergoes slight conformational changes on binding to HA, while 37K, 57K, and DMP1-PG do not change conformation. These findings indicate that distinct forms of DMP1 may work collectively in controlling the mineralization process.
Keywords: dentin-matrix-protein 1, secondary structure, hydroxyapatite, gelatin gel
Introduction
CDentin matrix protein-1 (DMP1), a member of the SIBLING family of extracellular matrix proteins (Qin et al., 2003), occurs predominantly as (1) a 37K N-terminal fragment, (2) a 57K C-terminal fragment, and (3) a glycosaminoglycan-containing N-terminal fragment with a single chondroitin sulfate (CS) chain, referred to as the dentin matrix protein-1 proteoglycan fragment, DMP1-PG (Qin et al., 2006). Additionally, a small amount of a full-length 106-kDa protein has been found in mineralized tissues (Huang et al., 2008). Based on characterization of DMP-1 knock-out animals (Ye et al., 2004; Ling et al., 2005), descriptions of two individuals with DMP1 mutations (Feng et al., 2006), and in vitro studies of the effects of the parentprotein and the 57K fragment on hydroxyapatite (HA) formation (He et al., 2003a, b; 2005; Tartaix et al., 2004; Gajjeraman et al., 2007), it was suggested that DMP1 plays a crucial role in the regulation of biomineralization. Specifically, we hypothesized that post-translational modification (phosphorylation/dephosphorylation, proteolytic processing, and glycosylation) alter the effects of DMP1 on HA formation and growth, and that these altered effects are due to changes in protein conformation. The objective of this study was to compare the effects of the DMP1 fragments on HA nucleation and growth, and to elucidate the changes in structure of each of these fragments in the presence and absence of HA.
Materials & Methods
Peptide Isolation and Characterization
For isolation of the 37K and 57K fragments, extracellular matrix (ECM) proteins were extracted from rat long bone by standard procedures as described (Qin et al., 2003). Briefly, the rat bone extracts were first subjected to gel chromatography on Sephacryl S-200 (Amersham Biosciences, GE Healthcare Bio-Sciences AB, Uppsala, Sweden) to separate the high-molecular-mass protein fraction (including the 37K and 57K fragments) from smaller-sized proteins (primarily osteocalcin). With Q-sepharose ion-exchange chromatography, the high-molecular-mass protein fraction from the Sephacryl S-200 column was next separated into several subfractions. The subfractions rich in the 37K and 57K fragments were passed over a Bio-Gel A15m column (Bio-Rad, Hercules, CA, USA). Finally, the fractions enriched in 37K and 57K fragments from Bio-Gel A15m chromatography were passed over a MonoQ column (Amersham Biosciences, GE Healthcare Bio-Sciences AB, Sweden) connected to a fast protein liquid chromatography system. Thus, the samples for 37K and 57K fragments of high purity were obtained. The purity of the 37K and 57K fragments was confirmed by SDS-PAGE with Stains-All staining, amino acid analysis, and sequencing of tryptic peptides derived from these fragments (Qin et al., 2003). The isolated and purified C-terminal 57-kDa fragment contained 41 phosphates, while the 37-kDa fragment (core protein) had 12 phosphates (Qin et al., 2003). For isolation of the GAG chain-containing N-terminal fragment (i.e., DMP1-PG), subfractions rich in DMP1-PG were first obtained by Q-sepharose ion-exchange chromatography, which completely eliminated the 37K fragment from DMP1-PG; then the DMP1-PG-rich subfractions were passed over an affinity column composed of monoclonal anti-DMP1 37K fragment antibody (Qin et al., 2006). The identity of DMP1-PG as a protein core of the 37K fragment from DMP1, containing a chondroitin sulfate chain, was elucidated by a series of careful analyses, including Stains-All staining, chondroitinase digestion, amino acid analysis, and tryptic peptide sequencing (Qin et al., 2006). The purity of each of these preparations can be seen in the gels included in the original references (Qin et al., 2003, 2006).
Mineralization Assays
Effects on HA formation and the growth of HA crystals were monitored in the gelatin gel system (Tartaix et al., 2004) in the presence and absence of pre-formed HA seeds. The seeds were prepared from amorphous calcium phosphate as described elsewhere and were characterized by x-ray diffraction before use (Tartaix et al., 2004). The fragment concentrations tested varied depending on the amount of fragment available. Thus, for DMP1-PG, concentrations were 0-21 µg/mL, 57K concentrations were 0-50 µg/mL, and 37K concentrations were 0-100 µg/mL. In brief, 6-cm-long segments of disposable Kimax tubes (Agilent, Lawrence, KS, USA) containing a total of 3 mL of 10% gelatin gel in 0.05 M, pH 7.4, tris-hydroxy-methylamino-methane (Tris) buffer and 0.01% sodium azide to prevent bacterial growth were mounted on an apparatus that circulated 100 mM calcium chloride and 100 mM ammonium acid phosphate through the tubes under constant nitrogen pressure. Diffusion rates have been characterized previously (Boskey, 1989), and in these gels at room temperature and 1 atm pressure, precipitant bands are noted at 3.5 days when the Ca and Pi millimolar product (Ca x Pi) adjacent to the bands is 5.5 mM2. The proteins or protein fragments or the carrier buffer is placed 1.54 mm from the phosphate entrance, the site of precipitant band form in control gels (Boskey, 1989), and the gels were examined for the detection of early or late formation of the initial precipitant band. HA growth was allowed to proceed for 5.0 days. The gels were sliced, and the Ca and Pi contents of the precipitant bands and the 0.3-mm slices adjacent to it were determined by atomic absorption (Willis, 1960) and colorimetry (Heinonen and Lahti, 1981). Results are expressed as experimental/control run at the same time for each concentration. In some cases, the precipitant band was examined by x-ray diffraction (Brucker AX-8 powder diffractometer, Brucker Instruments, Madison, WI, USA) to confirm that HA and no other phase was present. In each experiment, the Ca/Pi ratio of the precipitant band in control and experimental tubes was calculated, and if it differed from a range of 1.5:1 -1.9:1, the experiment was repeated, and the entire experiment where the ratios were not in that range eliminated. In seeded growth experiments, the protein, peptide or carrier, was pre-mixed with 0.5 mg/mL HA seed crystals before 100 µL of the slurry was placed in the position of the precipitant band. Values for multiple experiments for each concentration were pooled following ANOVA; post hoc determination of statistical differences among concentrations was based on the Student’s t test (GraphPad Instat, GraphPad Software Inc., La Jolla, CA, USA).
Fourier Transform Infrared Spectroscopic Protein Secondary Structure Determination
To determine whether there were conformational changes occurring when the fragments or full-length protein interacted with HA, we used Fourier transform infrared spectroscopy. The intact fully phosphorylated human DMP1 was prepared from human marrow stromal cells (provided by L. Fisher, National Institute of Dental and Craniofacial Research, Bethesda, MD, USA) as detailed elsewhere (Jain et al., 2002). 57K C-terminal and 37K N-terminal fragments and DMP1-PG were deuterated by triplicate exchange with D2O prior to secondary structure determination. Tris buffer, HA, and calcium chloride solutions (suspensions) were also prepared by repeated lyophilization against pH 7.4 Tris-buffered D2O. All components were brought into solution or were suspended (HA) in D2O shortly before measurement of the IR spectra, to prevent absorption of atmospheric water. The use of deuterated solutions is a commonly accepted method to circumvent the interference of water absorption bands with the amide I bands of the protein of interest. Attenuated Total Reflection-Fourier Transform Infrared spectroscopy (ATR-FTIR) experiments involved obtaining spectra of the peptide solution (20 mg/mL) alone, then in the presence of HA (5.4 mg/mL) or 3.3 µM Ca2+, both prepared in D2O. Chondroitin-6-sulfate (Sigma Chemicals, St. Louis, MO, USA) was also analyzed in the presence and absence of HA and Ca+2, then buffers were matched and subtracted from all of these protein spectra. Finally, for comparison of the shapes of the amide I bands of each of the resulting spectra, amide-I band envelopes were normalized to the maximum amide I band intensity of the protein-only spectrum, with Origin software (Microcal, GE Healthcare, Uppsala, Sweden).
Results
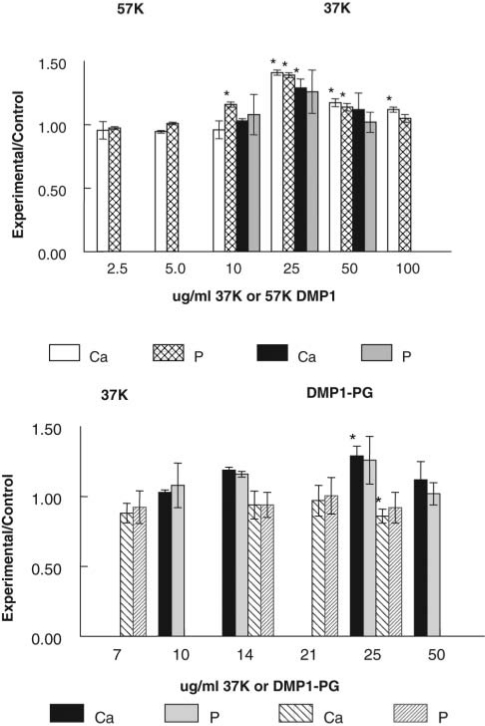
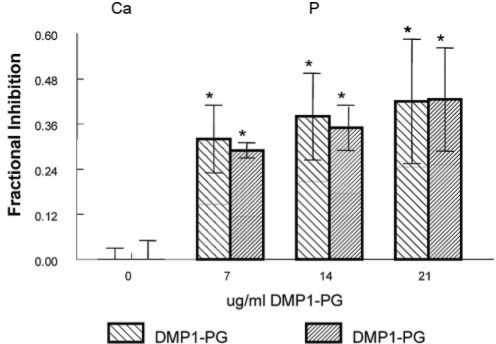
Both the 37K and the 57K fragments promoted de novo HA formation, while DMP1-PG fragment was an inhibitor (Fig. 1). On a weight/weight basis, 57K was slightly more effective as a nucleator than 37K. The effects of DMP1-PG were more apparent for seeded growth experiments (Fig. 2), where DMP1-PG significantly inhibited mineral accumulation in a dose-dependent manner, with 60% inhibition noted at 21 µg/mL.
Figure 1.
Relative accumulation of mineral ions during de novo formation of HA in the gelatin gel diffusion system in the presence of 37K and 57K DMP1 and DMP1-PG fragments. Experimental/control values (mean ± SD), n = 3-6, * p < 0.05, compared with protein-free controls run at the same time. 37K and 57K fragments promoted the formation of HA, and DMP1-PG was an inhibitor in contrast to 37K or protein-free controls.
Figure 2.
DMP1-PG inhibited HA-seeded growth in the gelatin gel system. Values are shown for mean ± SD, n = 4, of ion accumulation as % inhibition relative to both protein-free controls and the 37K fragment. *p < 0.05.
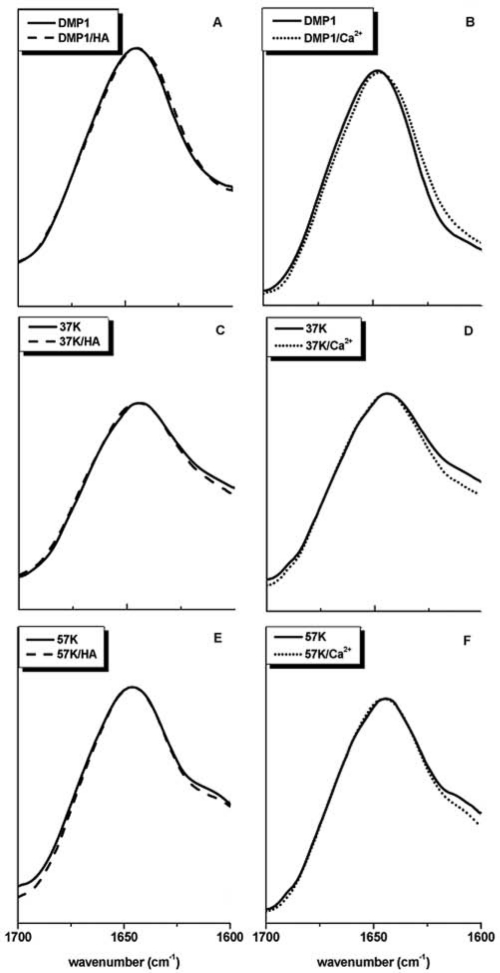
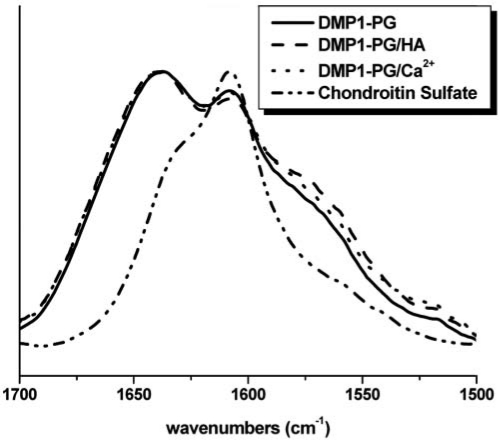
The IR spectra of the full-length protein, the 37K and 57K fragments, are shown in Fig. 3, while the corresponding spectra for DMP1-PG are shown in Fig. 4. The analysis of the structurally sensitive amide-I band envelope (1620-1700 cm-1) reveals a very minor shift in the presence of HA or Ca2+, indicating that the protein assumes a structure that is characterized by a minute increase in β-sheet secondary structure content; however, overall, the protein remained largely disordered. Similarly, we did not observe any significant HA- or Ca2+-induced structural changes for the 37K (Figs. 3C, 3D) or 57K DMP1 (Figs. 3E, 3F) fragments. Dynamic Light Scattering experiments (DLS, not shown) indicated that full-length DMP1 aggregated in the presence of Ca2+, while the 37K fragment showed a particularly strong aggregation (such DLS experiments cannot be used to monitor protein aggregation in the presence of HA, because HA itself scatters too strongly). The amide I band envelope of DMP1-PG (Fig. 4) was essentially unchanged in the presence of Ca2+ or HA and was characteristic of a largely disordered protein. The strong band feature centered at about 1606 cm−1 can be attributed to the glycosaminoglycan moiety of the protein (see, for comparison, the spectrum of chondroitin sulfate).
Figure 3.
Amide I band envelope for DMP1 as well as its 37K and 57K fragments in the presence of HA or Ca2+. (A,B) DMP1. (C,D) 37K. (D,E) 57K.
Figure 4.
Infrared spectra for DMP1-PG between 1500 and 1700 cm−1 for the pure protein and in the presence of Ca2+ and HA. For comparison, the spectrum of chondroitin sulfate is given, indicating that the band feature found at 1606 cm−1 in the DMP1-PG spectra is associated with the glycosaminoglycan moiety of the protein.
Discussion
This study has shown that DMP1 fragments behave differently from the full-length protein and from each other. As previously shown, the full-length protein is a mineralization inhibitor (Tartaix et al., 2004; Tsuji et al., 2008), but it is now recognized that DMP1 generally is rapidly cleaved in situ, ending up in different tissue compartments (Maciejewska et al., 2009). Proteolytic processing is believed to occur due to the action of the enzyme BMP1 (Steiglitz et al., 2004; von Marschall and Fisher, 2008), which releases and activates the fragments. The proteoglycan fragment is also an inhibitor, and it tends to localize in predentin (Maciejewska et al., 2009). The highly phosphorylated C-terminal fragment and the less phosphorylated N-terminal fragment are both HA nucleators (He et al., 2003a,b; Tartaix et al., 2004), although, in agreement with predictions (Lu et al., 2008), the C-terminal fragment was most effective in the gelatin gel system.
We suggest that tissue-specific differences may exist in the activities of these fragments, because of their relative abilities to interact with calcium and with HA. The SIBLING proteins in general (Fisher et al., 2001; Huq et al., 2005) and, as shown here, DMP1 in particular are flexible proteins that are largely disordered. Thus, they are typical of the “intrinsically disordered proteins” (Uversky et al., 2005) whose lack of a rigid structure enables them to serve multiple functions, in terms of both signaling and interacting with calcium and HA. Although the secondary and tertiary structures of DMP1 are not known, the primary structure shows that it is a serine-rich acidic protein, containing an integrin-binding (RGD) peptide and multiple serine phosphorylation sites. A mixture of the acidic-containing oligomers from the C- and N-terminal domains was shown by circular dichroism to increase its beta-sheet structures on binding Ca+2 (He et al., 2003b), and a related chimeric protein consisting of the acidic oligomer from the C-terminal domain and silk fibroin, based on FTIR and circular dichroism, also increased its alpha-helix and beta-sheet structure in the presence of Ca+2 (Huang et al., 2007). Since we were interested in the conformational changes associated with the binding of the full-length protein and the fragments to HA, only FTIR was used, because HA scatters light strongly, preventing the use of circular dichroism. We found that the full-length protein undergoes conformational changes with binding to HA, while the structure of the 57K and 37K fragments remained essentially unchanged. The PG fragment also does not undergo such a conformational change, although the PG fragment does interact with Ca+2. These conformational changes are consistent with the proposed mechanism of action—e.g., the flexible 57K and 37K fragments bind to HA nuclei and facilitate the growth and proliferation of HA crystals, enhancing mineralization. The slightly more structured full-length protein presumably blocks access to HA crystals, and prevents their proliferation and growth. The DMP1-PG prevents initial mineral deposition in predentin and, perhaps, in osteoid.
The activities of these fragments may also be regulated by PHEX, which has been shown to degrade the C-terminal peptide of another SIBLING protein, matrix extracellular phosphoglycoprotein, MEPE (Addison et al., 2008). It can also bind to 57K DMP1 (Martin et al., 2008), and may block some of its nucleating activity until needed in the tissue; however, recent studies showed that PHEX had no effect on processing of the 57K C-terminal DMP1 (Lu et al., 2008). DMP1-PG is an inhibitor of HA formation, and may be produced during early mineralized tissue formation to protect the non-mineralized osteoid and pre-dentin from becoming mineralized.
These findings are consistent with the tissue localization of the different fragments. A recent immunolocalization and confocal microscopy study showed that, in rat first molar organs, the NH(2)-terminal fragment localized to predentin, and the COOH-terminal fragment was mainly restricted to mineralized dentin. In the growth plate of bone, the NH(2)-terminal fragment appeared in the proliferation and hypertrophic zones, whereas the COOH-terminal fragment was in the zone of ossification. Chemical analysis of bovine teeth showed that predentin is rich in DMP1-PG, whereas mineralized dentin primarily contains the COOH-terminal fragment (Majiciewski et al., 2009); thus, the tissues that are not destined to become mineralized have more of the form of DMP1, which is a mineralization inhibitor, and the tissues that are mineralized/mineralizing have the forms that can control the rate of nucleation and the extent of growth of HA crystals.
It has been suggested that the C-terminal domain is the essential one, since DMP-1 null mice can be rescued by just the C-terminal 37K protein (Lu et al., 2009). However, some of the SIBLING proteins are functionally and, as suggested here, structurally redundant. It seems more likely that one of the SIBLING proteins, or fragments thereof, may substitute for another in the control of the proper formation of mineralization tissues.
Analysis of the data in this study helps explain the phenotypes of the DMP1 knock-out mice. Both the knock-out animals and a recently generated conditional knock-out of DMP1 have defects in dentin, in teeth, and in the skeleton, including hypophosphatemic rickets, and abnormalities in phosphate homeostasis (Ling et al., 2005; Feng et al., 2008). We suggest that the absence of the 57K as well as the 37K fragments leads to a decrease in the number of proteins/peptides that can act as hydroxyapatite nucleators. This in the face of impaired phosphate-handling (also dependent on DMP1) results in decreased formation of new mineral crystal nuclei, and defective mineralization.
Footnotes
This work is supported by NIH grants DE04141, AR046121, and DE005092.
References
- Addison WN, Nakano Y, Loisel T, Crine P, McKee MD. (2008). MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res 23:1638-1649 [DOI] [PubMed] [Google Scholar]
- Boskey AL. (1989). Hydroxyapatite formation in a dynamic gel system: effects of type I collagen, lipids, and proteoglycans. J Phys Chem 93:1628-1633 [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. (2006). Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Scott G, Guo D, Jiang B, Harris M, Ward T, et al. (2008). Generation of a conditional null allele for Dmp1 in mouse. Genesis 46:87-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. (2001). Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun 280:460-465 [DOI] [PubMed] [Google Scholar]
- Gajjeraman S, Narayanan K, Hao J, Qin C, George A. (2007). Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem 282:1193-1204 [DOI] [PubMed] [Google Scholar]
- He G, Dahl T, Veis A, George A. (2003a). Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect Tissue Res 44:240-245 [PubMed] [Google Scholar]
- He G, Dahl T, Veis A, George A. (2003b). Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater 21:552-558 [DOI] [PubMed] [Google Scholar]
- He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, et al. (2005). Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry 44:16140-16148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen JK, Lahti RJ. (1981). A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphates. Anal Biochem 113:313-317 [DOI] [PubMed] [Google Scholar]
- Huang B, Maciejewska I, Sun Y, Peng T, Qin D, Lu Y, et al. (2008). Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tissue Int 82:401-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wong C, George A, Kaplan DL. (2007). The effect of genetically engineered spider silk-dentin matrix protein 1 chimeric protein on hydroxyapatite nucleation. Biomaterials 28:2358-2367 [DOI] [PubMed] [Google Scholar]
- Huq NL, Cross KJ, Ung M, Reynolds EC. (2005). A review of protein structure and gene organisation for proteins associated with mineralised tissue and calcium phosphate stabilisation encoded on human chromosome 4. Arch Oral Biol 50:599-609 [DOI] [PubMed] [Google Scholar]
- Jain A, Karadag A, Fohr B, Fisher LW, Fedarko NS. (2002). Three Small Integrin Binding LIgands N-linked Glycoproteins (SIBLINGs) enhance Factor H’s cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J Biol Chem 277:13700-13708 [DOI] [PubMed] [Google Scholar]
- Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ, Boskey AL. (2005). DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J Bone Miner Res 20:2169-2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Qin C, Xie Y, Bonewald LF, Feng JQ. (2009). Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs 189:175-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewska I, Cowan C, Svoboda K, Butler WT, D’Souza R, Qin C. (2009). The NH2-terminal and COOH-terminal fragments of dentin matrix protein 1 (DMP1) localize differently in the compartments of dentin and growth plate of bone. J Histochem Cytochem 57:155-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, et al. (2008). Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology 149:1757-1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, et al. (2003). Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem 278:34700-34708 [DOI] [PubMed] [Google Scholar]
- Qin C, Huang B, Wygant JN, McIntyre BW, McDonald CH, Cook RG, et al. (2006). A chondroitin sulfate chain attached to the bone dentin matrix protein 1 NH2-terminal fragment. J Biol Chem 281:8034-8040 [DOI] [PubMed] [Google Scholar]
- Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. (2004). Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem 279:980-986 [DOI] [PubMed] [Google Scholar]
- Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, et al. (2004). In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem 279:18115-18120 [DOI] [PubMed] [Google Scholar]
- Tsuji T, Onuma K, Yamamoto A, Iijima M, Shiba K. (2008). Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. Proc Natl Acad Sci USA 105:16866-16870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Oldfield CJ, Dunker AK. (2005). Showing your ID: intrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recognit 18:343-384 [DOI] [PubMed] [Google Scholar]
- von Marschall Z, Fisher LW. (2008). Dentin matrix protein-1 isoforms promote differential cell attachment and migration. J Biol Chem 283:32730-32740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis JB. (1960). Determination of metals in blood serum by atomic absorption spectroscopy. I. Calcium. Spectrochim Acta 16:259-272 [DOI] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, et al. (2004). Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem 279:19141-19148 [DOI] [PubMed] [Google Scholar]